Abstract
Streptomyces alfalfae XN-04 has been reported for the production of antifungal metabolites effectively to control Fusarium wilt of cotton, caused by Fusarium oxysporum f. sp. vasinfectum (Fov). In this study, we used integrated statistical experimental design methods to investigate the optimized liquid fermentation medium components of XN-04, which can significantly increase the antifungal activity and biomass of XN-04. Seven variables, including soluble starch, KNO3, soybean cake powder, K2HPO4, MgSO4·7H2O, CaCO3 and FeSO4·7H2O, were identified as the best ingredients based on one-factor-at-a-time (OFAT) method. The results of Plackett–Burman Design (PBD) showed that soluble starch, soybean cake powder and K2HPO4 were the most significant variables among the seven variables. The steepest climbing experiment and response surface methodology (RSM) were performed to determine the interactions among these three variables and fine-tune the concentrations. The optimal compositions of medium were as follows: soluble starch (26.26 g/L), KNO3 (1.00 g/L), soybean cake powder (23.54 g/L), K2HPO4 (0.27 g/L), MgSO4·7H2O (0.50 g/L), CaCO3 (1.00 g/L) and FeSO4·7H2O (0.10 g/L). A verification experiment was then carried out under the optimized conditions, and the results revealed the mycelial dry weight of S. alfalfae XN-04 reaching 6.61 g/L. Compared with the initial medium, a 7.47-fold increase in the biomass was achieved using the optimized medium. Moreover, the active ingredient was purified from the methanol extract of S. alfalfae XN-04 mycelium and then identified as roflamycoin (a polyene macrolide antibiotic). The results may provide new insights into the development of S. alfalfae XN-04 fermentation process and the control of the Fusarium wilt of cotton and other plant diseases.
Keywords: Streptomyces alfalfae, antifungal activity, biomass, optimization, response surface methodology
1. Introduction
The soil-inhabiting fungus Fusarium oxysporum has been reported to infect more than a hundred different crops, causing vascular wilt and even death of the plants [1]. The widely use of chemical pesticides has caused several environmental concerns such as pesticide residues and soil contamination, although it can also control the Fusarium wilt [2]. Therefore, the biocontrol of Fusarium wilt has become an environmentally friendly strategy of the management of plant diseases during the recent years [2]. Among various potential biocontrol methods, the most widely studied are beneficial microorganisms and their metabolites. As one of the most biotechnological and economic value prokaryotes, actinomycetes are responsible for the production of nearly half of the discovered secondary metabolites with economic importance [3]. Currently, more than 75% of bioactive compounds are reported from the members of Streptomyces [4,5,6,7,8,9,10]. Some bioactive compounds showed great activity against phytopathogenic fungi, such as kasugamycin produced by Streptomyces kasugaensis [11], validamycin produced by Streptomyces hygroscopicus [12], ningnanmycin produced by Streptomyces noursei [13] and wuyiencin produced by Streptomyces albulus var. wuyiensis [14].
The optimization of productive conditions is an extremely important step in the fermentation process due to their high complexity. The fermentation processes of microorganisms are influenced by many variables, including physical parameters and the nutritional compositions of culture medium [15]. The yield and metabolic profile of microorganisms are influenced by minor variations in the composition of fermentation medium [16]. The techniques used for the medium optimization of various fermentation parameters including both classical and statistical tools. Classical studies of fermentation optimization are usually performed “one-factor-at-a-time (OFAT)”, in which the level of one factor is changed while keeping the other factors constant [17]. It has the advantage of being simple and easy. However, the conventional OFAT method is tedious, time-consuming and uneconomical. In addition, when plenty of variables are involved, it ignores the combined interactions among various nutritional and physical parameters [18]. Therefore, statistical-based measures have been employed to achieve medium optimization by changing more than one variable at a time. Some of the famous statistics-based experimental designs are used in fermentation optimization, including full factorial design, fractional factorial design (FFD), Plackett–Burman design (PBD), Box–Behnken design (BBD) and central composite design (CCD) [19,20].
Response surface methodology (RSM) is a compilation of mathematical statistical techniques for designing experiments, plotting models and evaluating the effects of various variables, and establishing optimum conditions of a multivariable system to achieve significant responses [18]. This method has been extensively used for optimizing factors of fermentation medium and discovering the interactions among a multitude of fermentation parameters using a minimum of experiments [21]. In many cases, RSM uses statistically based experimental designs such as BBD and CCD to develop empirical models, which mathematically describe the relationships existing among the independent and dependent variables [22]. Further, RSM provides three-dimensional (3D) graphs and two-dimensional (2D) contour plots to clarify the shape of a response surface. Currently, RSM has been successfully applied to optimize variables for fermentation culture across a series of microorganisms including fungi, bacteria and actinomycetes in the production of industrially important metabolites [23,24,25]. For example, Streptomyces sp. WP-1 culture under the optimal medium designed by RSM increased fungichromin yield to 5741.7 mg/L [26]. Medium optimization using RSM contributed to a 45% (220.7 ± 5.7 mg/L) increase in rapamycin production for the Streptomyces hygroscopicus, compared with the unoptimized production medium (151.9 ± 22.6 mg/L) [27].
Streptomyces alfalfae XN-04 is a plant growth-promoting rhizobacterium (PGPR) and an excellent biocontrol strain. A previous study indicated that S. alfalfae XN-04 was able to produce metabolites with significant activity against some plant fungal pathogens [28]. Due to the extremely low production of biomass and long fermentation time of S. alfalfae XN-04, it is necessary to improve S. alfalfae XN-04 yield in submerged fermentation. Moreover, the main antifungal ingredient in the metabolites of S. alfalfae XN-04 remains unclear. In this study, we assessed the following objectives: (1) optimize the fermentation medium of S. alfalfae XN-04 for the maximum biomass at the level of flask fermentation; (2) purify and identify the main antifungal ingredient of S. alfalfae XN-04 using various chromatographic and spectroscopic methods.
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
S. alfalfae XN-04 was originally isolated from the rhizosphere soil sample collected from Qinghai Province, China [28]. The strain XN-04 was cultured on mannitol soybean agar (MS) and incubated at 28 °C for 14 days to produce spores. The phytopathogenic fungus Fusarium oxysporum f. sp. vasinfectum (Fov) was preserved in the Biological Control of Plant Disease Laboratory of Northwest Agriculture and Forestry University. The fungus was cultured on potato dextrose agar (PDA) plates and incubated at 28 °C for 7 days.
2.2. Inoculum Preparation
Inoculum was prepared by inoculating 5 spore cakes (6 mm) of S. alfalfae XN-04 into a 250 mL Erlenmeyer flasks containing 100 mL Gauze’s synthetic No. 1 (GS) medium. Then, the flasks were incubated at 28 °C on a shaker at 180 rpm for 3 days. The inoculum quantity was controlled at 5% (v/v) in all the fermentation experiments.
2.3. Antifungal Activity Assay
Fermentation culture was centrifuged at 5000× g rpm at 4 °C for 10 min, and the precipitate was collected. Three volumes of methanol (MeOH) were added, and the sample was then processed with ultrasonication for 10 min. After filtration using filter paper, the MeOH extract was evaporated to dryness using a rotary vacuum evaporator under reduced pressure at 50 °C. The MeOH extract was redissolved in MeOH to prepare a stock solution with a final concentration of 50 mg/mL. The antifungal activity was estimated with the method of mycelium growth in sealed plates described by previous study [29]. Briefly, 50 μL of the MeOH extract and 50 mL of sterile PDA medium were uniformly mixed and poured into three culture dishes (9 cm in diameter). Inverted mycelial plugs (6 mm in diameter) taken from the active periphery of 7-day-old fungus colonies were placed on the center of these plates. Plates with an appropriate volume of MeOH were used as control. Plates were incubated for 7 days in a growth chamber at 28 °C, and the colony diameter was measured. Each treatment had three plates, and the experiment was repeated three times. The levels of inhibition were calculated by using the equation as follows:
| Inhibition rate (%) = (the colony diameter of control − the colony diameter of treatment)/(the colony diameter of control) × 100 | (1) |
2.4. Single-Factor Optimization of Fermentation Medium Composition
Single-factor experiments were used to screen the most significant medium substrate. GS medium was used as the initial medium. A total of 9 different carbon sources and 9 different nitrogen sources to replace corresponding carbon and nitrogen source in the initial medium while other compositions were kept constant at their original concentration. Glucose, sucrose, xylose, galactose, fructose, millet flour, rice flour, corn steep liquor and soluble starch (at 20 g/L) were investigated as carbon sources. The influence of KNO3, (NH4)2SO4, NH4NO3, NaNO3, urea, yeast extract, beef extract, soybean cake powder and peptone (at 1 g/L) as nitrogen sources were simultaneously investigated. Similarly, K2HPO4, MgSO4·7H2O, CaCO3, KCl, KH2PO4 and NaCl (at 0.1 g/L) were selected to optimize mineral salts. Additionally, CuSO4·5H2O, CoCl2·6H2O, MnCl2·4H2O, FeSO4·7H2O and ZnSO4·7H2O (at 0.01 g/L) were chosen as trace elements. At the end of fermentation, samples obtained from fermentation culture were used to calculate mycelium dry weight and antifungal activity.
2.5. Single-Factor Concentration Screening Test
In order to select the most significant concentration by using the single variable procedure, soluble starch (0, 20, 40, 60, 80 and 100 g/L), KNO3 (0, 1, 2, 3, 4 and 5 g/L), soybean cake powder (0, 20, 40, 60, 80 and 100 g/L), K2HPO4 (0, 0.2, 0.4, 0.6, 0.8 and 1.0 g/L), MgSO4·7H2O (0, 0.2, 0.4, 0.6, 0.8 and 1.0 g/L), CaCO3 (0, 0.2, 0.4, 0.6, 0.8 and 1.0 g/L) and FeSO4·7H2O (0, 0.02, 0.04, 0.06, 0.08 and 0.1 g/L) were added to the initial medium, respectively. The mycelium dry weight and antifungal activity of samples were determined as described above.
2.6. Plackett–Burman Design (PBD)
The Plackett–Burman design (PBD) is an effective method to investigate the effect of medium composition, and is very helpful for screening the most important variables with respect to their main effects. The results of PBD do not describe the interaction among these variables but it is used to screen and evaluate the variables that have a significant impact on response. The total number of experiments performed according to PBD is n + 1, where n is the number of variables. In this study, a range of 12 experiments were constructed using the Design-Expert software version 8.0.6.1 (Stat-Ease, lnc., Minneapolis, MN, USA) for 7 different independent variables including soluble starch, KNO3, soybean cake powder, K2HPO4, MgSO4·7H2O, CaCO3 and FeSO4·7H2O. Each independent variable was tested at 2 levels, high and low, denoted by (+) and (−), respectively (Table 1). A total of 4 dummy variables were designed in these 12 experiments to calculate the standard error. All tests were performed in triplicate, and the average of mycelium dry weight was treated as responses. The effect of medium components on mycelium dry weight was determined by p-values obtained by analysis of variance (ANOVA). A p-value (Prob > F) of less than 0.05 to indicate when factors are mathematically significant.
Table 1.
The level and code of variables chosen for PBD.
| Name | Factors | Code Value (g/L) | |
|---|---|---|---|
| −1 | +1 | ||
| X1 | Soluble starch | 20 | 25 |
| X2 | KNO3 | 1.00 | 1.25 |
| X3 | Soybean cake powder | 20 | 25 |
| X4 | K2HPO4 | 0.20 | 0.25 |
| X5 | MgSO4·7H2O | 0.40 | 0.50 |
| X6 | CaCO3 | 1.00 | 1.25 |
| X7 | FeSO4·7H2O | 0.100 | 0.125 |
2.7. The Steepest Ascent Experiment
The steepest ascent experiment was designed to determine a suitable direction according to the results of PBD, and the change step was determined according to the effect value of each factor. The direction of the steepest ascent experiment and the step of change were determined by the three main influencing factors of soluble starch, soybean cake powder and K2HPO4 according to the results of PBD, and the area of mycelial dry weight maximum production could be approached rapidly.
2.8. Box–Behnken Design (BBD)
Response surface methodology (RSM) based on Box–Behnken design (BBD) was used to optimize optimal levels of medium compositions. Based on the trajectory of the steepest ascent experiments, 3 variables were selected and their concentrations were arranged at 17 levels, with 5 replicates at the center point to study their interaction effect. According to the design, 3 variables with the highest confidence levels were prescribed at 3 levels, coded −1, 0 and +1 for low, middle and high concentrations respectively (Table 2). In order to predicting the maximum value of response, a ternary quadratic equation was established to correlate the relationship between variables and response. The validity of this model was determined based on Student’s t test. Data obtained from the BBD were subjected to first and second order multiple regression analysis using the method of least squares to obtain the parameters of the mathematical models. Model coefficients, R2 values, F values and significance probabilities generated by the Design-Expert software version 8.0.6.1 (Stat-Ease, lnc., Minneapolis, MN, USA) provided a confirmation of the significance of each experimental variable. The optimal medium composition for improving the biomass of S. alfalfae XN-04 were obtained by solving this ternary quadratic equation and by analyzing the response surface contour plots. Combined with the regression equation, the 3D response surfaces and 2D contour plots were plotted by the Design-Expert software to understand the interaction effects of medium components and optimum concentration of each variable required for maximum biomass production. The 2D contour plot is used to determine the interaction strength between the two variables according to the radius of the curved surface of the arc [30]. The elliptic order of contour indicates that the interactions between corresponding variables were significant, while the circular order reveals non-significant interactions [30].
Table 2.
The level and code of variables chosen for BBD.
| Name | Factors | Code Value (g/L) | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1 | Soluble starch | 25.00 | 26.50 | 28.00 |
| X3 | Soybean cake powder | 22.00 | 23.50 | 25.00 |
| X4 | K2HPO4 | 0.250 | 0.265 | 0.280 |
In order to determine the accuracy of this model and verify the results, an experiment under the optimal conditions obtained from BBD was performed, followed by comparing the response value with the predicted data.
2.9. Purification and Identification of Antifungal Compounds
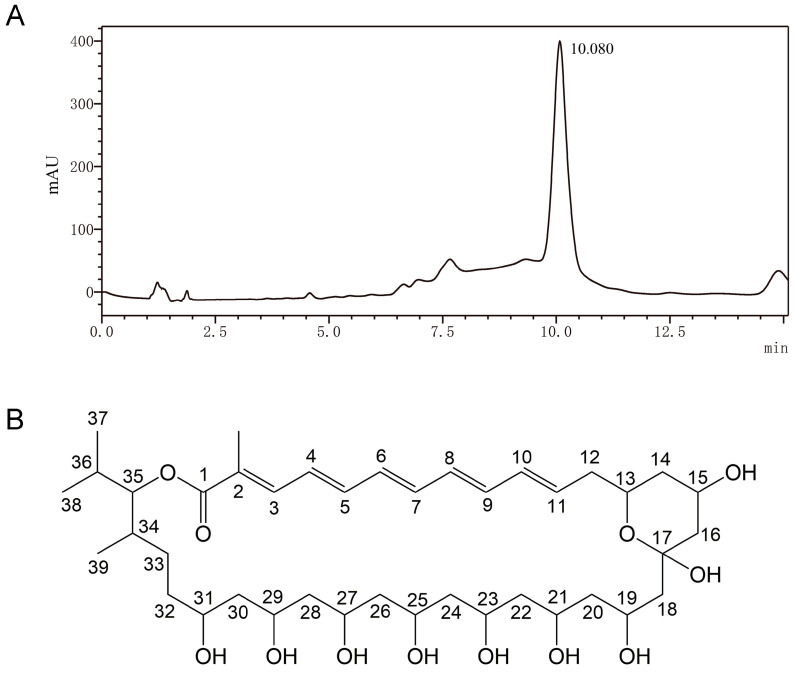
S. alfalfae XN-04 was inoculated into the optimized medium and incubated at 28 °C with shaking at 180 rpm for 14 days. After incubation, a total of 7.5 L fermentation broth was centrifuged at 5000× g rpm at 4 °C for 10 min, and the precipitate was collected. Three volumes of MeOH were added, and the sample was then processed with ultrasonication for 10 min. After filtration using filter paper, the MeOH extract was evaporated to dryness using a rotary vacuum evaporator under reduced pressure at 50 °C to yield a brown crude extract (13.2 g). The crude extract was purified on silica gel column chromatography (300 mesh, Qingdao Marine Chemical Inc., Qingdao, China) and subjected to gradient elution with a mixture solvent consisting of dichloromethane/MeOH from 10:1 to 2:1 (v/v) to obtain 10 fractions (Frs. 1–10). The paper disk (6 mm in diameter) diffusion assay was used to evaluate the antifungal activities of each fraction [31]. Further separation of Fr. 5 (672 mg) by semi-preparative HPLC on a C18 column using acetonitrile/water (45/55, v/v) as the mobile phase with a flow rate of 10 mL/min, collected the peak at 10.08 min (UV detector 363 nm) to yield compound 1 (220 mg, yellow amorphous solid).
The antifungal compound 1 was analyzed by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS; LC-30A + TripleTOF5600+, AB SCIEX, Framingham, MA, USA). Mass spectrometry (MS) and tandem mass spectrometry (MS/MS) were performed using electrospray ionization (ESI) detection in the positive mode. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance III instrument at 600 MHz to elucidate the structure of compound 1.
2.10. Statistical Analysis
All experiments were repeated three times independently and the summary statistics are expressed as mean ± standard deviation (SD). Data were analyzed by one-way ANOVA with Duncan’s post hoc pairwise multiple range test. Differences between sample mean values of p < 0.05 were considered to be significant. Data obtained from the PBD and BBD were analyzed with a statistical software package Design-Expert software (Version 8.0.6.1, Stat-Ease Inc., Minneapolis, MN, USA).
3. Results
3.1. Effect of Different Nutrient Sources on Antifungal Metabolites Production and Biomass
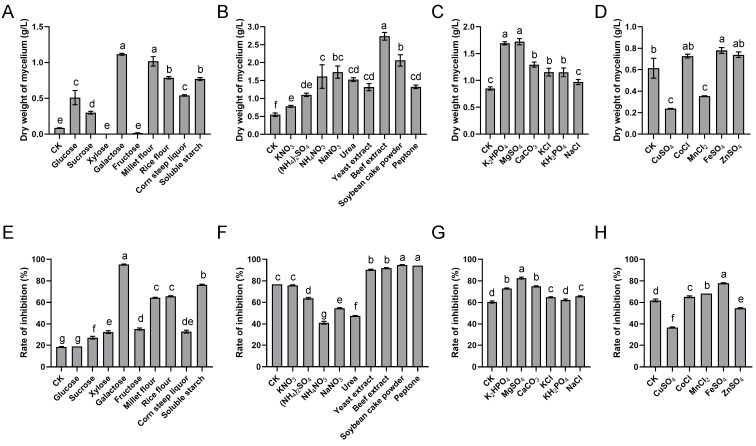
The results showed that different nutrient sources had significant effects on the cell growth and antifungal activity of S. alfalfae XN-04 (Figure 1).
Figure 1.
Effect of different nutrient sources on the biomass yield and antifungal metabolites production of S. alfalfae XN-04. (A,E) Effect of carbon sources. (B,F) Effect of nitrogen sources. (C,G) Effect of mineral salts. (D,H) Effect of trace elements. The dry weight of mycelium was used as indication of biomass, and the rate of inhibition was used as indication of antifungal metabolites production. Bars represent the SD of three replicates. Different lowercase letters indicate a significant difference at p < 0.05 level by Duncan’ s new multiple range test.
3.1.1. Carbon Sources
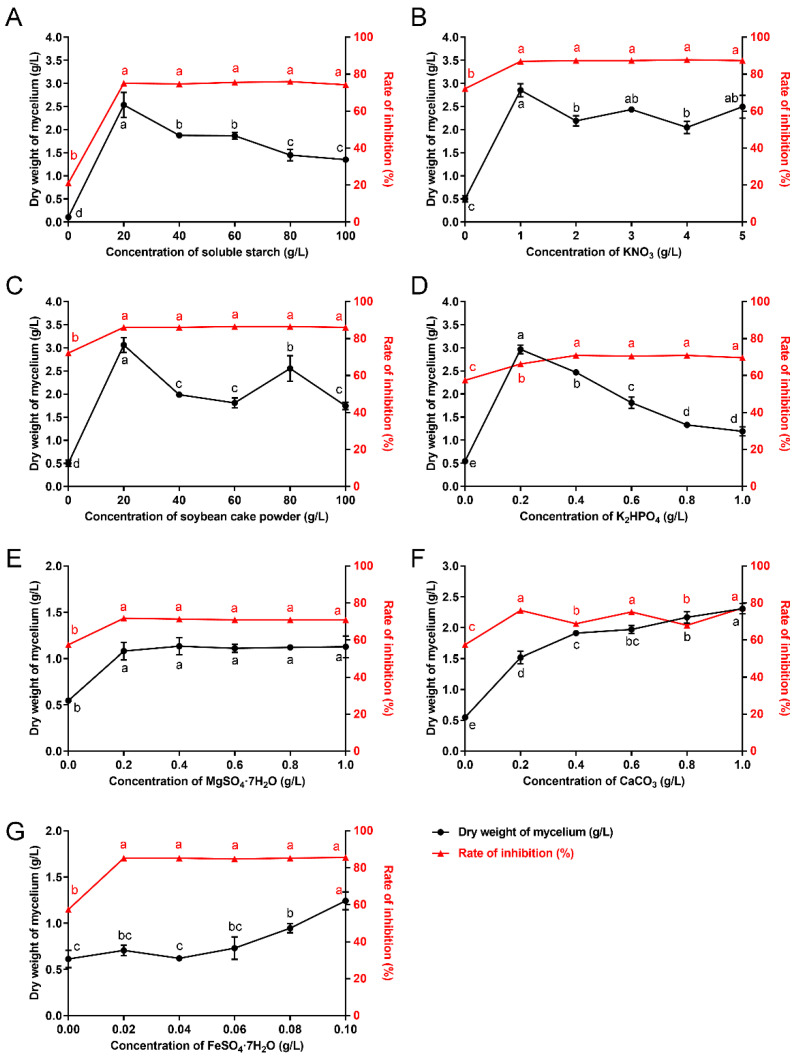
The fermentation medium was supplemented with 20 g/L of different carbon sources to determine their individual influence. The results revealed that the maximum biomass was obtained with galactose (1.12 g/L), followed by millet flour (1.02 g/L) (Figure 1A). Rate of inhibition (%) was used to indicate the production of antifungal metabolites. Maximum inhibition rate was achieved with galactose (95.5%), followed by soluble starch (76.33%) (Figure 1E). Notably, the results showed that the fermentation medium containing millet flour and rice flour increased the levels of biomass but these agents were less efficient in the production of antifungal metabolites (Figure 1A,E). Although maximum biomass and antifungal metabolites was produced by galactose, soluble starch was selected for further studies due to cost consideration. The results of concentration screening test showed that the maximum biomass was produced at the 20 g/L soluble starch level (Figure 2A). However, the antifungal activity remained unchanged between the concentrations of 20 g/L and 100 g/L (Figure 2A). This means the highest antifungal metabolites production was also obtained at the 20 g/L soluble starch level. Therefore, 20 g/L soluble starch was chosen as the carbon source for further experiments.
Figure 2.
Effect of nutrient sources at different concentrations on the biomass yield and antifungal metabolites production of S. alfalfae XN-04. (A) Soluble starch. (B) KNO3. (C) Soybean cake powder. (D) K2HPO4. (E) MgSO4·7H2O. (F) CaCO3. (G) FeSO4·7H2O. The dry weight of mycelium was used as indication of biomass (corresponds to the left X axis), and the rate of inhibition was used as indication of antifungal metabolites production (corresponds to the right Y axis). Bars represent the SD of three replicates. Different lowercase letters indicate a significant difference at p < 0.05 level by Duncan’ s new multiple range test.
3.1.2. Nitrogen Sources
Each organic and inorganic nitrogen source supported growth (Figure 1B). The results showed that the maximum biomass was obtained with beef extract (2.74 g/L), followed by soybean cake powder (2.06 g/L) (Figure 1B). Maximum inhibition rate was achieved with soybean cake powder (94.68%), followed by peptone (94.2%) (Figure 1F). Generally, a higher mycelial growth inhibition rate was achieved when the complex organic nitrogen source was added in fermentation medium (Figure 1F). Notably, the production of antifungal metabolites was negatively affected by nitrogen sources favorable for growth, such as NH4NO3, NaNO3 and urea (Figure 1F). Although the maximum biomass was obtained by beef extract, soybean cake powder was selected for further studies due to cost considerations. Additionally, considering the ratio of organic nitrogen sources to inorganic nitrogen sources, KNO3 was selected as the inorganic nitrogen source. Therefore, KNO3 at 1 g/L and soybean cake powder at 20 g/L were the most suitable nitrogen sources for maximum biomass (Figure 2B,C).
3.1.3. Mineral Salt
Comparative studies on suitable mineral salts were carried out with results shown in Figure 1C,G. The results revealed that K2HPO4, MgSO4·7H2O and CaCO3 had distinct positive effects both on cell growth and antifungal metabolites production of S. alfalfae XN-04. The results of concentration screening test showed that the optimum concentrations of K2HPO4, MgSO4·7H2O and CaCO3 were 0.2 g/L, 0.4 g/L and 1.0 g/L, respectively (Figure 2D–F).
3.1.4. Trace Elements
The results showed that the maximum biomass and inhibition rate was achieved with FeSO4·7H2O (at 0.1 g/L), and was therefore, selected for further studies (Figure 1D,H and Figure 2G).
Taking all this into account, seven factors (soluble starch, KNO3, soybean cake powder, K2HPO4, MgSO4·7H2O, CaCO3 and FeSO4·7H2O) were chosen for further optimization and their initial concentrations were determined.
3.2. Plackett–Burman Design (PBD)
To evaluate the most significant variables for biomass accumulation, PBD design was construed. A total of 12 different medium were prepared and the experimental runs were carried out based on the experimental matrix of PBD, and the observed responses are shown in Table 3. There was a variation from 2.33 to 4.75 g/L in mycelium dry weight (Table 3).
Table 3.
The design and results of PBD.
| Run | Code Number | Mycelial Dry Weight (g/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | ||
| 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 3.39 |
| 2 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 2.75 |
| 3 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 2.52 |
| 4 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 4.75 |
| 5 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 4.54 |
| 6 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 3.73 |
| 7 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 3.14 |
| 8 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 4.34 |
| 9 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 3.54 |
| 10 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 3.28 |
| 11 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 2.33 |
| 12 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 3.50 |
Regression analysis and ANOVA of the PBD were performed and represented in Table 4. The results showed that the regression was significant (p-value 0.0195), with the p-values for soluble starch, soybean cake powder and K2HPO4 were 0.0055, 0.0204 and 0.0261, respectively (Table 4). Values of prob > F less than 0.0500 indicated the model term was significant, and in this case X1, X2 and X4 were significant model terms. Therefore, they were considered to be the most significant variables that affected mycelial dry weight. The other variables had small effects and low confidence levels (p > 0.05) which were considered mathematically insignificant (Table 4). The results of PBD also clearly showed that soluble starch, soybean cake powder and K2HPO4 had coefficient estimates of 0.46, −0.31 and 0.29, respectively, which meant that soluble starch and K2HPO4 exhibited a positive effect, while soybean cake powder acted negatively (Table 4). According to these results, X1, X2 and X4 were selected for further optimization. The next step in the optimization should increase the concentration of soluble starch and K2HPO4 in the fermentation medium, and reduce the concentration of soybean cake powder.
Table 4.
Analysis of variance of PBD.
| Source | Factor | Freedom | Coefficient Estimate | Sum of Squares | Mean Square | F Value | Prob > F |
|---|---|---|---|---|---|---|---|
| Model | 7 | 3.48 | 6.15 | 0.88 | 10.41 | 0.0195 * | |
| X1 | Soluble starch | 1 | 0.46 | 2.51 | 2.51 | 29.77 | 0.0055 ** |
| X2 | KNO3 | 1 | −0.19 | 0.43 | 0.43 | 5.09 | 0.0871 |
| X3 | Soybean cake powder | 1 | −0.31 | 1.17 | 1.17 | 13.89 | 0.0204 * |
| X4 | K2HPO4 | 1 | 0.29 | 1.00 | 1.00 | 11.89 | 0.0261 * |
| X5 | MgSO4·7H2O | 1 | 0.14 | 0.24 | 0.24 | 2.89 | 0.1645 |
| X6 | CaCO3 | 1 | −0.14 | 0.24 | 0.24 | 2.89 | 0.1645 |
| X7 | FeSO4·7H2O | 1 | −0.21 | 0.54 | 0.54 | 6.42 | 0.0644 |
| R2 = 0.9479 | |||||||
* indicates a significant difference at p < 0.05 level; ** indicates a significant difference at p < 0.01 level.
3.3. The Steepest Ascent Experiment
In order to further approach the maximum response value region for each main factor for subsequent response surface analysis, the design of the steepest ascent test was performed. In the steepest ascent experiment, the change range of the concentrations of soluble starch, soybean cake powder and K2HPO4 were selected as 1.5 g/L, 1.5 g/L and 0.015 g/L (Table 5). All other components were fixed at those combinations of the maximum concentration of mycelial dry weight according to the results obtained from PDB (Run 4). The steepest ascent test design and its response values are shown in Table 5. The results revealed that the mycelial dry weight showed a trend of increasing and then decreasing, indicating that the test design was reliable. When the 2nd experimental was run, the mycelial dry weight reached the maximum (5.31 g/L), which was the region of the maximum response value of the three factors. Therefore, this point was chosen to set up basal concentrations for BBD.
Table 5.
The design and results of the steepest ascent experiment.
| Run | X1 (g/L) | X3 (g/L) | X4 (g/L) | Mycelial Dry Weight (g/L) |
|---|---|---|---|---|
| 1 | 25.00 | 25.00 | 0.250 | 4.42 |
| 2 | 26.50 | 23.50 | 0.265 | 5.31 |
| 3 | 28.00 | 22.00 | 0.280 | 5.06 |
| 4 | 29.50 | 20.50 | 0.295 | 4.99 |
| 5 | 31.00 | 19.00 | 0.310 | 4.27 |
| 6 | 32.50 | 17.50 | 0.325 | 4.07 |
| 7 | 34.00 | 16.00 | 0.340 | 3.73 |
3.4. Box–Behnken Design (BBD)
To further increase mycelial dry weight, the interactive effects of the most important variables, i.e., soluble starch (X1), soybean cake powder (X3) and K2HPO4 (X4), were examined by RSM using BBD. The experimental design and the corresponding results are described in Table 6. The response (Y) fits with the ternary quadratic equation (final equation in terms of coded factors):
| Y= +5.99 − 0.54 × X1 + 0.081 × X3 + 0.66 × X4 + 0.71 × X1X3 + 0.93 × X1X4 + 0.58 × X3X4 − 9.92 × X12 − 2.05 × X32 − 1.05X42 | (2) |
Table 6.
The design and results of BBD.
| Run | Code Number | Actual Value (g/L) | Predicted Value (g/L) | ||
|---|---|---|---|---|---|
| X1 | X3 | X4 | |||
| 1 | 0 | 0 | 0 | 6.25 | 5.99 |
| 2 | 0 | 1 | 1 | 4.26 | 4.20 |
| 3 | 0 | 0 | 0 | 5.89 | 5.99 |
| 4 | 0 | 0 | 0 | 5.81 | 5.99 |
| 5 | −1 | −1 | 0 | 4.35 | 4.19 |
| 6 | 1 | 0 | 1 | 5.17 | 5.07 |
| 7 | 0 | 1 | −1 | 1.82 | 1.73 |
| 8 | −1 | 0 | −1 | 5.17 | 5.07 |
| 9 | 0 | −1 | 1 | 2.79 | 2.88 |
| 10 | 0 | 0 | 0 | 5.78 | 5.99 |
| 11 | 1 | −1 | 0 | 1.68 | 1.69 |
| 12 | 1 | 0 | −1 | 1.96 | 1.89 |
| 13 | 1 | 1 | 0 | 3.12 | 3.28 |
| 14 | 0 | −1 | −1 | 2.67 | 2.73 |
| 15 | 0 | 0 | 0 | 6.22 | 5.99 |
| 16 | −1 | 0 | 1 | 4.22 | 4.29 |
| 17 | −1 | 1 | 0 | 2.94 | 2.93 |
Table 6 showed the predicted responses of BBD on the basis of the above equation. This model equation’s importance was statistically evaluated by the F test for the ANOVA, and the results were predicted in Table 7. The ANOVA regression model demonstrated a determination coefficient (R2) of 0.9926, which meant 99.26% variability in the response can be explained by this model (Table 7). The value of the adjusted determination coefficient (R2adj) was 0.9831 (Table 7). The higher value of R2adj showed the strong significance of the model. R2 and R2adj were in close agreement. Moreover, a low value of coefficient of variation (5.11%) indicated the reliability and precision of these experiments executed (Table 7). An adequate precision value (26.776) measured the signal-to-noise ratio, and a ratio > 4.0 was desirable (Table 7). In this model, the very low p-value (<0.0001) for biomass is lower than 0.05, which revealed that the quadratic model we established was significant (Table 7). Based on the results of ANOVA table, it was found that the factors X1, X4, X1X3, X1X4, X3X4, X12, X32 and X42 were all significant model terms (Table 7). The value of lack-of-fit was not significant (p > 0.05) for this model which suggested that the experimental data were in solid agreement with predicted responses (Table 7).
Table 7.
Analysis of variance of BBD.
| Source | Freedom | Sum of Squares | Mean Square | F Value | Prob > F |
|---|---|---|---|---|---|
| Model | 9 | 41.12 | 4.57 | 104.15 | <0.0001 *** |
| X1 | 1 | 2.33 | 2.33 | 53.18 | 0.0002 *** |
| X3 | 1 | 0.053 | 0.053 | 1.20 | 0.3088 |
| X4 | 1 | 3.45 | 3.45 | 78.54 | <0.0001 *** |
| X1X3 | 1 | 2.03 | 2.03 | 46.29 | 0.0003 *** |
| X1X4 | 1 | 3.48 | 3.48 | 79.29 | <0.0001 *** |
| X3X4 | 1 | 1.35 | 1.35 | 30.67 | 0.0009 *** |
| X12 | 1 | 3.53 | 3.53 | 80.36 | <0.0001 *** |
| X32 | 1 | 17.74 | 17.74 | 404.35 | <0.0001 *** |
| X42 | 1 | 4.66 | 4.66 | 106.32 | <0.0001 *** |
| Residual | 7 | 0.31 | 0.044 | ||
| Lack of Fit | 3 | 0.10 | 0.033 | 0.64 | 0.6258 |
| Pure Error | 4 | 0.21 | 0.052 | ||
| C. Total | 16 | 41.43 | |||
| Standard deviation | 0.21 | R-squared | 0.9926 | ||
| Mean | 4.10 | Adjusted R-squared | 0.9831 | ||
| Coefficient of variation (C.V.%) | 5.11 | Predicted R-squared | 0.9535 | ||
| PRESS | 1.92 | Adequate precision | 26.7760 | ||
*** indicates a significant difference at p < 0.001 level.
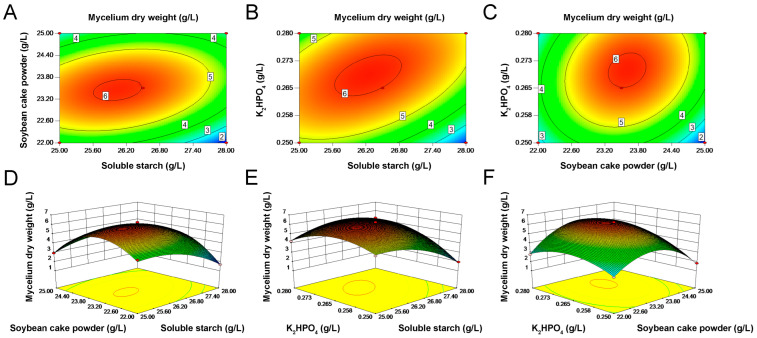
The 3D response surface and the 2D contour plots are generally the graphical representations of the regression equation. The elliptic order of contour in Figure 3A suggested that the interaction between soluble starch and soybean cake powder was significant. Similarly, there was a strong interaction between soluble starch and K2HPO4 (Figure 3B). Moreover, the circular order of contour of Figure 3C suggested that the interaction between soybean cake powder and K2HPO4 had a less significant effect. The 3D response surface aided in the visual determination of optimum levels of each variable as they interact. Three-dimensional response surface plots depicted mycelial dry weight with respect to soluble starch versus soybean cake powder (Figure 3D). Due to the interaction response of soluble starch with soybean cake powder, biomass increased with the increasing concentration of soluble starch and soybean cake powder up to 26.26 g/L and 23.54 g/L, respectively (Figure 3D). Further continuous increases in their concentrations resulted in a decrease in biomass. Figure 3E represented the interaction effect of soluble starch and K2HPO4 on mycelial dry weight. With an increase in soluble starch (25–26.26 g/L) and K2HPO4 (0.25–0.269 g/L) concentration, the biomass increased, and then dropped. Figure 3F revealed that the maximum mycelial dry weight was produced at a high level of soybean cake powder (26.26 g/L) and K2HPO4 (0.269 g/L) in the design range.
Figure 3.
Contour plot described by the model on mycelial dry weight. (A) Effect of soluble starch and soybean cake powder. (B) Effect of soluble starch and K2HPO4. (C) Effect of soybean cake powder and K2HPO4. Response–surface curve of mycelial dry weight showing mutual interactions. (D) Soluble starch and soybean cake powder. (E) Soluble starch and K2HPO4. (F) Soybean cake powder and K2HPO4. Other variables, except for the two in each figure, were maintained at zero level in coded units. The numbers 2–6 in the figure indicate the mycelium dry weight at this location.
3.5. Validation of the Optimized Medium
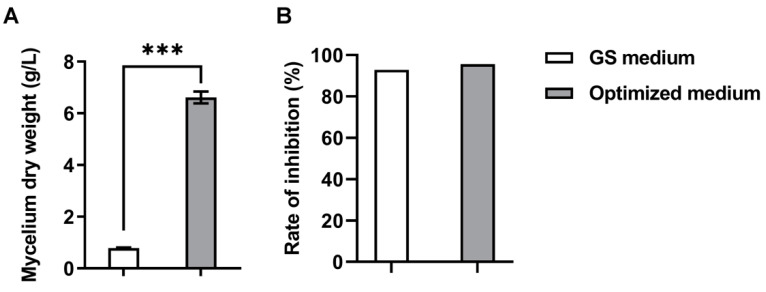
Based on the results of optimized medium composition, the maximum mycelial dry weight of S. alfalfae XN-04 was predicted to be 6.12 g/L when the concentrations of X1, X3 and X4 were 26.26 g/L, 23.54 g/L and 0.269 g/L, respectively. The result predicted by RSM was validated by carrying out an experiment using best-predicted solutions for growth of S. alfalfae XN-04. Under the optimized conditions, the average mycelial dry weight of S. alfalfae XN-04 reached 6.61 g/L, which was close to the RSM predicted value, suggesting that the experimental and the predicted values were in good agreement (Figure 4A). Compared with GS medium, the optimized medium enhanced mycelial dry weight by 7.47-fold (Figure 4A). Furthermore, under optimal conditions, the methanol extracts of cells show a stronger suppressive effect against Fov (95.65%) (Figure 4B). Therefore, we concluded that the model established in this paper was accurate and reliable for predicting the biomass of S. alfalfae XN-04.
Figure 4.
Validation of statistical optimization medium and the initial medium (GS medium). (A) Mycelial dry weight. (B) Rate of inhibition. Bars represent the SD of three replicates. *** indicates statistical significance based on the two-tailed test (p < 0.0001).
3.6. Purification and Structural Elucidation of the Antifungal Compound
A series of chromatographic procedures were performed to purify the main antifungal ingredient in the MeOH extract of S. alfalfae XN-04. A total of 10 fractions were obtained from the silica gel column chromatography (Figure S1), among which Fr. 5–9 exhibited antifungal activity (Table S1). Fr. 5 demonstrated strong antifungal activity, producing a radius of inhibition zone > 14 mm. Therefore, Fr. 5 was subjected to semi-preparative HPLC for further purification. The chromatogram was monitored at 363 nm, and a peak (compound 1) was observed at the retention times of 10.08 (Figure 5A). Finally, compound 1 was obtained as a yellow amorphous solid. The results of LC-MS/MS showed that the most abundant high mass ion [M + H]+ was found at m/z 739.4619 (Figure S2). Compound 1 was identified as roflamycoin (molecular weight, 738; molecular formula, C40H66O12) by comparing its NMR data (Table S1) with the previous study [32].
Figure 5.
HPLC analysis and structure of roflamycoin produced by S. alfalfae XN-04. (A) HPLC analysis of Fr. 5 at 363 nm. (B) The structure of roflamycoin produced by S. alfalfae XN-04.
4. Discussion
Recent studies reported that actinomycetes, particularly Streptomyces spp., acted as effective biocontrol agents against multiple phytopathogens [33]. Streptomyces spp. can directly or indirectly benefit plants due to its ability to produce antibiotics, hydrolytic enzymes, or enzyme inhibitors [34]. A previous study indicated that S. alfalfae XN-04 can colonize the cotton plant root system, inhibiting the mycelial growth of Fov by production of hydrolytic enzymes and antifungal secondary metabolites [28]. However, the extremely low yield of antifungal metabolites and long fermentation period of S. alfalfae XN-04 have restricted its further research and market applications. In order to maximize the yield of antifungal metabolites and to provide data to support the large-scale and low-cost expansion of S. alfalfae XN-04, this study was conducted to optimize the fermentation medium components of S. alfalfae XN-04. Additionally, an antifungal compound was purified from the metabolites of S. alfalfae XN-04 and was identified as roflamycoin.
Carbon sources are essential components in constructing cellular materials and are also used as energy sources [35]. Nitrogen is an important element for nucleic acid and protein, which are the raw materials synthesized by microorganisms to create cellular metabolites [36]. In the process of microorganism fermentation, interaction between cell growth and secondary metabolites secretion is critically influenced by the growth-limiting nutrient at certain concentrations [37]. Therefore, selection as well as optimization of nutritional components is an indispensable step in large-scale production of probiotic biomass at a reasonable cost. Generally, secondary metabolites production in Streptomyces spp. is often stimulated by slowly assimilated complex carbohydrates (e.g., soluble starch, dextrins), but is suppressed by rapidly utilized carbon sources (e.g., glucose). Shakeel et al. (2016) found that soluble starch was the best carbon for the cell growth of Streptomyces platensis 3–10 [37]. Jacob et al. (2014) reported that Streptomyces nogalater NIIST A30 was able to produce antibacterial metabolites at a higher level with starch as carbon source [38]. In this study, the results showed that the biomass and antifungal metabolites production of S. alfalfae XN-04 were significantly improved with the supplementation of soluble starch and soybean cake powder (Figure 1A,B,E,F). Moreover, the results also revealed that glucose had negative effect on the antifungal metabolites production of S. alfalfae XN-04 (Figure 1E). These findings appear to be consistent with the results in related previous studies [39]. A possible explanation of this phenomenon is that glucose may cause catabolite repression, in which the production of enzymes for secondary metabolites biosynthesis might be inhibited [40].
Minerals such as K+, Ca2+, Na+ and Mg2+ have been known to play a crucial role in the metabolic process of all microorganisms, as it is essential for the formation of cell mass and acts as a cofactor for many biosynthetic enzymes to catalyze the necessary reactions [35]. Zhu et al. (2007) made an effort to ameliorate the production of avilamycin using Streptomyces viridochromogenes Tu57–1 and by adopting BBD in which they found a maximum production of 88.33 mg/L (2.80-fold increase) in the optimized medium with the ingredients such as MgSO4·7H2O 0.37 g/L, CaCl2·2H2O 0.39 g/L, soybean flour 21.97 g/L and soluble starch 37.22 g/L [41]. Peng et al. (2020) reported that the fungichromin production in Streptomyces sp. WP-1 was markedly increased in medium supplemented with magnesium phosphate or calcium phosphate [26]. In this study, the results showed that K2HPO4, MgSO4·7H2O and CaCO3 had distinct positive effects both on microorganism growth and on antifungal metabolites production by S. alfalfae XN-04 (Figure 1C,G). Moreover, the results also revealed that the demand for Ca2+ of S. alfalfae XN-04 was at a high level (Figure 2F). This finding suggests that Ca2+ is a key factor in S. alfalfae XN-04 growth. This finding appears to be consistent with the results in related previous studies. A previous study reported that there was a high calcium content in spores of Streptomyces spp. and that for some species it was an essential triggering factor of spore germination [42]. Additionally, CaCO3 used as medium substrate can balance the pH of the fermentation broth by reacting with the acid, which was produced by the process of microorganism fermentation to form neutral salts and CO2, the latter escaping from the medium.
In order to clarify the interactions among factors selected by single-factor experiments, further work will be needed on mixing conditions. Generally, in non-growth associated fermentation, secondary metabolites production was substantially proportional to the quantity of biomass [18]. Therefore, it is essential to increase the total biomass of microorganisms, especially for S. alfalfae XN-04, since its antifungal metabolites were produced inside the cells. Taking in account the above theory, we focused on optimization of medium components, seeking a substantial increase in biomass without reducing the production of secondary metabolites. Therefore, mycelial dry weight was utilized as the response in subsequent experiment designs.
Statistical experimental design was an efficient method for optimizing the effects of each variable. The PBD enables screening for the most significant variables of microorganism fermentation, and that the steepest ascent experiment made the result approximate to the optimal region for easily carrying out the subsequent experiments. Finally, the RSM detected the optimum levels of significant factors. In 2004, Elibol tried to optimize a fermentation medium using a 24 full factorial CCD for the production of benzoisochromanequinone polyketide antibiotic actinorhodin with Strptomyces coelicolor A3 (2), where 200 mg/L of antibiotic yield was attained under the optimized medium composition, which was 32% higher than that of the unoptimized medium (148 mg/L) and was very close to the predicted yield (195 mg/L) [43]. The maximization of olivanic acid production using Streptomyces olivaceus MTCC 6820 in shake flask was executed in which 415 mg/L of yield was achieved in the medium optimized with CCD and it was eight times higher when compared with the normal unoptimized medium (50 mg/L) [44]. The results were in agreement with these results. In this study, the mycelial dry weight of S. alfalfae XN-04 reached 6.61 g/L (7.47-fold increase) in the medium optimized with RSM.
In conclusion, this study optimized the fermentation medium compositions for biomass yield and antifungal metabolites production of S. alfalfae XN-04. This study also reported an antifungal secondary metabolite, roflamycoin, which was purified from S. alfalfae XN-04 metabolites.
Acknowledgments
We are grateful to John Richard Schrock (Emporia State University, USA) for proofreading this manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10091854/s1.
Author Contributions
Conceptualization, J.C. and Y.W.; methodology, J.C. and X.L.; software, J.C. and R.J.; validation, J.C. and L.H.; formal analysis, J.C. and Y.W.; investigation, J.C. and X.L.; resources, J.C.; data curation, J.C.; writing—original draft preparation, J.C. and Y.W.; writing—review and editing, J.C., X.L. and Y.W.; visualization, X.L.; supervision, J.C. and Y.W.; project administration, Y.W. funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Key Industry Chain Innovation Project of Shaanxi province (2020ZDLNY07-02, 2021ZDLNY03-05) and the National Key Research and Development Program of China Grant (2021YFD140100502).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raza W., Ling N., Zhang R.F., Huang Q.W., Xu Y.C., Shen Q.R. Success evaluation of the biological control of Fusarium Wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit. Rev. Biotechnol. 2017;37:202–212. doi: 10.3109/07388551.2015.1130683. [DOI] [PubMed] [Google Scholar]
- 2.Palmieri D., Vitale S., Lima G., Di Pietro A., Turra D. A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat. Commun. 2020;11:5264. doi: 10.1038/s41467-020-18994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang K.S., Kim H.U., Charusanti P., Palsson B.O., Lee S.Y. Systems biology and biotechnology of Streptomyces species for the production of secondary metabolites. Biotechnol. Adv. 2014;32:255–268. doi: 10.1016/j.biotechadv.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Awla H.K., Kadir J., Othman R., Rashid T.S., Hamid S., Wong M.Y. Plant growth-promoting abilities and biocontrol efficacy of Streptomyces sp. UPMRS4 against Pyricularia Oryzae. Biol. Control. 2017;112:55–63. doi: 10.1016/j.biocontrol.2017.05.011. [DOI] [Google Scholar]
- 5.Cho G., Kim J., Park C.G., Nislow C., Weller D.M., Kwak Y.S. Caryolan-1-ol, an antifungal volatile produced by Streptomyces spp., inhibits the endomembrane system of fungi. Open Biol. 2017;7:170075. doi: 10.1098/rsob.170075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faheem M., Raza W., Zhong W., Nan Z., Shen Q.R., Xu Y.C. Evaluation of the biocontrol potential of Streptomyces goshikiensis YCXU against Fusarium oxysporum f. sp. niveum. Biol. Control. 2015;81:101–110. doi: 10.1016/j.biocontrol.2014.11.012. [DOI] [Google Scholar]
- 7.Grahovac J., Grahovac M., Dodic J., Bajic B., Balaz J. Optimization of cultivation medium for enhanced production of antifungal metabolites by Streptomyces hygroscopicus. Crop Prot. 2014;65:143–152. doi: 10.1016/j.cropro.2014.07.020. [DOI] [Google Scholar]
- 8.Worsley S.F., Newitt J., Rassbach J., Batey S., Holmes N.A., Murrell J.C., Wilkinson B., Hutchings M.I. Streptomyces endophytes promote host health and enhance growth across plant species. Appl. Environ. Microbiol. 2020;86:e01053-20. doi: 10.1128/AEM.01053-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong Y., Liu J.Q., Xu M.J., Zhang C.M., Gao J., Li C.G., Xing K., Qin S. Antifungal volatile organic compounds from Streptomyces setonii WY228 control black spot disease of sweet potato. Appl. Environ. Microbiol. 2022;88:e0231721. doi: 10.1128/aem.02317-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntemafack A., Ahmed S., Kumar A., Chouhan R., Kapoor N., Bharate S.B., Hassan Q.P., Gandhi S.G. Plant growth promoting potential of butyl isobutyl phthalate and Streptomyces sp. from Rumex dentatus on rice. Appl. Microbiol. Biotechnol. 2022;106:2603–2617. doi: 10.1007/s00253-022-11862-w. [DOI] [PubMed] [Google Scholar]
- 11.Kasuga K., Sasaki A., Matsuo T., Yamamoto C., Minato Y., Kuwahara N., Fujii C., Kobayashi M., Agematu H., Tamura T., et al. Heterologous production of kasugamycin, an aminoglycoside antibiotic from Streptomyces kasugaensis, in Streptomyces lividans and Rhodococcus erythropolis L-88 by constitutive expression of the biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 2017;101:4259–4268. doi: 10.1007/s00253-017-8189-5. [DOI] [PubMed] [Google Scholar]
- 12.Jiang J., Sun Y.F., Tang X., He C.N., Shao Y.L., Tang Y.J., Zhou W.W. Alkaline pH shock enhanced production of validamycin a in fermentation of Streptomyces hygroscopicus. Bioresour. Technol. 2018;249:234–240. doi: 10.1016/j.biortech.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Yang R., Jiang S.L., Wen X.D., Song X.C., Wang X., Li D.X., Yin Q.X., Wu X., Wang D.L., Chen Z. Antifungal activity and possible mode of action of ningnanmycin against tea gray blight disease pathogen Pseudopestalotiopsis camelliae-sinensis. Phytopathology. 2021;111:1735–1742. doi: 10.1094/PHYTO-09-20-0382-R. [DOI] [PubMed] [Google Scholar]
- 14.Yang M.L., Zhang W., Lv Z.Y., Shi L.M., Zhang K.C., Ge B.B. Evaluation of the inhibitory effects of wuyiencin, a secondary metabolite of Streptomyces albulus CK-15, against Sclerotinia Sclerotiorum in vitro. Plant Dis. 2022;106:156–164. doi: 10.1094/PDIS-05-21-0987-RE. [DOI] [PubMed] [Google Scholar]
- 15.Gomes R.J., Ida E.I., Spinosa W.A. Nutritional supplementation with amino acids on bacterial cellulose production by Komagataeibacter intermedius: Effect analysis and application of response surface methodology. Appl. Biochem. Biotechnol. 2022 doi: 10.1007/s12010-022-04013-4. online ahead of print . [DOI] [PubMed] [Google Scholar]
- 16.Vlajkov V., Anđelić S., Pajčin I., Grahovac M., Budakov D., Jokić A., Grahovac J. Medium for the production of Bacillus-based biocontrol agent effective against aflatoxigenic Aspergillus flavus: Dual approach for modelling and optimization. Microorganisms. 2022;10:1165. doi: 10.3390/microorganisms10061165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyyappan J., Bharathiraja B., Varjani S., PraveenKumar R., Kumar S.M. Anaerobic biobutanol production from black strap molasses using Clostridium acetobutylicum MTCC11274: Media engineering and kinetic analysis. Bioresour. Technol. 2022;346:126405. doi: 10.1016/j.biortech.2021.126405. [DOI] [PubMed] [Google Scholar]
- 18.Latha S., Sivaranjani G., Dhanasekaran D. Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017;43:567–582. doi: 10.1080/1040841X.2016.1271308. [DOI] [PubMed] [Google Scholar]
- 19.Zalila-Kolsi I., Kessentini S., Tounsi S., Jamoussi K. Optimization of Bacillus amyloliquefaciens BLB369 culture medium by response surface methodology for low cost production of antifungal activity. Microorganisms. 2022;10:830. doi: 10.3390/microorganisms10040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santra H.K., Maity S., Banerjee D. Production of bioactive compounds with broad spectrum bactericidal action, bio-film inhibition and antilarval potential by the secondary metabolites of the endophytic fungus Cochliobolus sp. APS1 isolated from the Indian medicinal herb Andrographis paniculata. Molecules. 2022;27:1459. doi: 10.3390/molecules27051459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkateswarulu T.C., Prabhakar K.V., Kumar R.B. Optimization of nutritional components of medium by response surface methodology for enhanced production of lactase. 3 Biotech. 2017;7:186. doi: 10.1007/s13205-017-0802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Huang H., Xu S., Wang B., Ju J., Tan H., Li W. Activation and enhancement of Fredericamycin A production in deepsea-derived Streptomyces somaliensis SCSIO ZH66 by using ribosome engineering and response surface methodology. Microb. Cell. Fact. 2015;14:64. doi: 10.1186/s12934-015-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amid A., Ismail N.A., Yusof F., Salleh H.M. Expression, purification, and characterization of a recombinant stem bromelain from Ananas comosus. Process. Biochem. 2011;46:2232–2239. doi: 10.1016/j.procbio.2011.08.018. [DOI] [Google Scholar]
- 24.Almeida D.G., da Silva R.D.F.S., Luna J.M., Rufino R.D., Santos V.A., Sarubbo L.A. Response surface methodology for optimizing the production of biosurfactant by Candida tropicalis on industrial waste substrates. Front. Microbiol. 2017;8:157. doi: 10.3389/fmicb.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S.K., Singh S.K., Tripathi V.R., Khare S.K., Garg S.K. Comparative one-factor-at-a-time, response surface (statistical) and bench-scale bioreactor level optimization of thermoalkaline protease production from a Psychrotrophic pseudomonasputida SKG-1 isolate. Microb. Cell. Fact. 2011;10:114. doi: 10.1186/1475-2859-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng C., An D.P., Ding W.X., Zhu Y.X., Ye L., Li J.Y. Fungichromin production by Streptomyces sp. WP-1, an endophyte from Pinus dabeshanensis, and its antifungal activity against Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2020;104:10437–10449. doi: 10.1007/s00253-020-10996-z. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.H., Park B.S., Bhatia S.K., Seo H.M., Jeon J.M., Kim H.J., Yi D.H., Lee J.H., Choi K.Y., Park H.Y., et al. Production of rapamycin in Streptomyces hygroscopicus from glycerol-based media optimized by systemic methodology. J. Microbiol. Biotechnol. 2014;24:1319–1326. doi: 10.4014/jmb.1403.03024. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Hu L.F., Chen N., Jia R.M., Ma Q., Wang Y. The biocontrol and plant growth-promoting properties of Streptomyces alfalfae XN-04 revealed by functional and genomic analysis. Front. Microbiol. 2021;12:745766. doi: 10.3389/fmicb.2021.745766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei X.Y., Liu Y.X., Huang H.C., Du F., Huang L.L., Wu J.Q., Li Y.W., Zhu S.S., Yang M. Benzothiazole inhibits the growth of Phytophthora capsici through inducing apoptosis and suppressing stress responses and metabolic detoxification. Pestic. Biochem. Physiol. 2019;154:7–16. doi: 10.1016/j.pestbp.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Yan L., Zhang Z., Zhang Y., Yang H., Qiu G., Wang D., Lian Y. Improvement of tacrolimus production in Streptomyces tsukubaensis by mutagenesis and optimization of fermentation medium using Plackett-Burman design combined with response surface methodology. Biotechnol. Lett. 2021;43:1765–1778. doi: 10.1007/s10529-021-03144-8. [DOI] [PubMed] [Google Scholar]
- 31.Kim J.D., Park M.Y., Jeon B.J., Kim B.S. Disease control efficacy of 32,33-didehydroroflamycoin produced by Streptomyces rectiviolaceus strain DY46 against gray mold of tomato fruit. Sci. Rep. 2019;9:13533. doi: 10.1038/s41598-019-49779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han X., Wang J., Liu L., Shen F., Meng Q., Li X., Li Y., Liu D. Identification and predictions regarding the biosynthesis pathway of polyene macrolides produced by Streptomyces roseoflavus Men-myco-93-63. Appl. Environ. Microbiol. 2021;87:e03157-20. doi: 10.1128/AEM.03157-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olanrewaju O.S., Babalola O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019;103:1179–1188. doi: 10.1007/s00253-018-09577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vurukonda S.S.K.P., Giovanardi D., Stefani E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018;19:952. doi: 10.3390/ijms19040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abhini K.N., Rajan A.B., Zuhara F., Sebastian D. Response surface methodological optimization of l-asparaginase production from the medicinal plant endophyte Acinetobacter baumannii ZAS1. J. Genet. Eng. Biotechnol. 2022;20:22. doi: 10.1186/s43141-022-00309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jose P.A., Jebakumar S.R.D. Phylogenetic appraisal of antagonistic, slow growing actinomycetes isolated from hypersaline inland solar salterns at Sambhar salt Lake, India. Front. Microbiol. 2013;4:190. doi: 10.3389/fmicb.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shakeel Q., Lyu A., Zhang J., Wu M.D., Chen S.W., Chen W.D., Li G.Q., Yang L. Optimization of the cultural medium and conditions for production of antifungal substances by Streptomyces platensis 3–10 and evaluation of its efficacy in suppression of clubroot disease (Plasmodiophora brassicae) of oilseed rape. Biol. Control. 2016;101:59–68. doi: 10.1016/j.biocontrol.2016.06.007. [DOI] [Google Scholar]
- 38.Jacob J., Rajendran R.U., Priya S.H., Purushothaman J., Amma D.K.B.N.S. Enhanced antibacterial metabolite production through the application of statistical methodologies by a Streptomyces nogalater NIIST A30 isolated from Western Ghats forest soil. PLoS ONE. 2017;12:e0175919. doi: 10.1371/journal.pone.0175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messis A., Bettache A., Brahami A., Kecha M., Benallaoua S. Optimization of antifungal production from a novel strain Streptomyces sp. TKJ2 using response surface methodology. Med. Chem. Res. 2014;23:310–316. doi: 10.1007/s00044-013-0627-z. [DOI] [Google Scholar]
- 40.Srivastava A., Singh V., Haque S., Pandey S., Mishra M., Jawed A., Shukla P.K., Singh P.K., Tripathi C. Response surface methodology-genetic algorithm based medium optimization, purification, and characterization of cholesterol oxidase from Streptomyces rimosus. Sci. Rep. 2018;8:10913. doi: 10.1038/s41598-018-29241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu C.H., Lu F.P., He Y.N., Zhang J.K., Du X. Statistical optimization of medium components for avilamycin production by Streptomyces viridochromogenes Tu57-1 using response surface methodology. J. Ind. Microbiol. Biotechnol. 2007;34:271–278. doi: 10.1007/s10295-006-0195-z. [DOI] [PubMed] [Google Scholar]
- 42.González-Quiñónez N., Corte-Rodríguez M., Álvarez-Fernández-García R., Rioseras B., López-García M.T., Fernández-García G., Montes-Bayón M., Manteca A., Yagüe P. Cytosolic copper is a major modulator of germination, development and secondary metabolism in Streptomyces coelicolor. Sci. Rep. 2019;9:4214. doi: 10.1038/s41598-019-40876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elibol M. Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3 (2) with response surface methodology. Process. Biochem. 2004;39:1057–1062. doi: 10.1016/S0032-9592(03)00232-2. [DOI] [Google Scholar]
- 44.Singh V., Tripathi C.K.M. Production and statistical optimization of a novel olivanic acid by Streptomyces olivaceus MTCC 6820. Process. Biochem. 2008;43:1313–1317. doi: 10.1016/j.procbio.2008.07.015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.