Abstract
Background
In this study we aimed to assess if a focused lung ultrasound examination predicts the need for mechanical ventilation, admission to an intensive care unit, high-flow oxygen treatment, death from COVID-19 within 30 days and 30-day all-cause mortality in patients with clinical suspicion of COVID-19 or PCR-verified SARS-CoV-2 infection.
Methods
A multicentre prospective cohort trial was performed. Film clips from focused lung ultrasound examinations were recorded and rated by blinded observers using different scoring systems. A prediction model was built and used to test relationship between lung ultrasound scores and clinical outcomes. Diagnostic performance of scoring systems was analysed.
Results
A total of 3889 film clips of 398 patients were analysed. Patients who had any of the outcomes of interest had a significantly higher ultrasound score than those who did not. Multivariable logistic regression analyses showed that lung ultrasound predicts mechanical ventilation (relative risk 2.44, 95% CI 1.32–5.52), admission to intensive care (relative risk 2.55, 95% CI 1.41–54.59) and high-flow oxygen treatment (relative risk 1.95, 95% CI 1.5–2.53) but not survival when adjusting for sex, age and relevant comorbidity. There was no diagnostic difference in area under the receiver operating characteristic curve between a scoring system using only anterolateral thorax zones and a scoring system that also included dorsal zones.
Conclusion
Focused lung ultrasound in patients with clinical suspicion of COVID-19 predicts respiratory failure requiring mechanical ventilation, admission to intensive care units and the need for high-flow oxygen treatment. Thus, focused lung ultrasound may be used to risk stratify patients with COVID-19 symptoms.
Short abstract
Focused lung ultrasound can predict need for intensive care treatment in patients with confirmed or suspected COVID-19. A simple ultrasound examination including only the anterior and lateral sides of the thorax is accurate. https://bit.ly/3CTf3p6
Introduction
The COVID-19 pandemic has put a high load on healthcare systems, as many patients need immediate evaluation for respiratory failure related to pulmonary damage from SARS-CoV-2 infection. In frontline medical facilities like emergency departments or dedicated COVID-19 clinics, the high number of patients requires efficient, fast and reliable management. Most patients with COVID-19 can be managed out of hospital, but around 10–30% need hospitalisation and 7% mechanical ventilation [1–3]. These numbers inflict careful allocation of resources to ensure that patients at high risk of respiratory failure are admitted to a service that can provide life-saving treatment, such as nasal oxygen administration, high-flow oxygen treatment or mechanical ventilation when needed. Conversely, patients at low risk of respiratory failure could be treated out of hospital or admitted to less resource-intensive services.
Assigning COVID-19 patients to the proper treatment and observation intensity requires a precise, safe and applicable clinical tool that can predict the development of respiratory failure. Multiple prediction systems have been developed and validated in large cohorts [4–6]. Most of these include imaging, such as chest radiographs or computed tomography (CT), and scanning of the lungs to assess the degree of damage. While highly relevant, these imaging modalities are problematic in relation to COVID-19, as CT scanning capacity is limited, and in-hospital transportation of isolated infectious patients to scanning facilities poses a risk of contaminating other patients and healthcare personnel. Chest radiographs can be recorded at bedside, but the need for dedicated radiographical staff restricts their use in high-stress situations. Furthermore, supine chest radiograph images are often difficult to interpret. Therefore, there is a clear need for a fast and easy image modality suited to COVID-19 demands.
Focused lung ultrasound (FLUS) is performed at the patient's bedside by the attending physician. Thereby, the risk of the virus spreading is reduced, and the result of the examination is readily available for clinical decision making [7]. FLUS has shown excellent diagnostic accuracy in multiple respiratory conditions, including the diagnosis of COVID-19, but its ability to predict future respiratory failure remains to be studied in a larger study [8, 9].
Consequently, we primarily aimed to study the capability of FLUS to predict the initiation of mechanical ventilation in patients hospitalised with symptoms of, or confirmed, SARS-CoV-2 infection. Secondarily, we aimed to study FLUS's capability to predict initiation of high-flow oxygen therapy, admission to intensive care unit and death in the same population. Thirdly, we wanted to assess the performance of different FLUS scoring systems.
Methods
Study design
The study was designed as a multicentre prospective cohort trial. The study was approved by the local ethical committee, RM: 1-10-72-1-20, and the Danish Health Authority, SST nr. 31-1521-377 and registered on clinical-trails.org, NCT04327674. The manuscript adheres to the STROBE reporting guidelines [10].
Setting
12 hospitals participated in the study. Inclusion spanned March 2020 to mid-June 2020, which was during the first wave of the COVID-19 pandemic in Denmark.
Participants and recruiting
All adult patients (age >18 years) with symptoms of COVID-19 or PCR-confirmed SARS-CoV-2 infection who had a FLUS examination performed when visiting an emergency department or when being admitted to an internal medicine department or a dedicated COVID-19 clinic were eligible for inclusion. Participants were included by convenience, as attendance of a physician able to perform the FLUS examination was not available at all times. Patients were excluded if FLUS was performed after the onset of any of the outcome variables or if they took part in the study at a previous visit or admission.
Data sources and variables
Data on age, sex, vital parameters, comorbidity, mechanical ventilator treatment, 30-day mortality, admission to intensive care facilities, oxygen administration, treatment limitations, laboratory results and results from nasal or tracheal PCR to SARS-CoV-2 were performed as part of normal clinical routine and extracted from electronic patient files. Comorbidity was classified according to the risk of death from COVID-19 [11]. Patients were registered as comorbid if they had COPD, HIV, diabetes, heart failure, hypertension, obstructive sleep apnoea, asthma, atrial fibrillation, ischaemic heart disease, chronic renal failure, dementia, liver cirrhosis, hemiplegia, rheumatoid arthritis, alcohol abuse, hyperthyroidism, metastasised cancer or obesity.
Sonographic data
The FLUS examinations were performed on the anterior and lateral chest zones and, if patients were able to sit, at the dorsal zones according to a standardised generic 14-zone protocol endorsed by the European Respiratory Society [12]. The choice of the ultrasound apparatus, transducer and pre-set was made at the discretion of the operator. Film clips were recorded from each zone and later analysed according to an international standard for lung ultrasound in COVID-19 by observers blinded to any other data [13]. In this scoring system, zones were rated as 0 if the pleural line was intact and if A-lines (horizontal artefacts) were present in the lung parenchyma. A score of 1 was given if the pleural line was indented and vertical white areas were present. A score of 2 was given if the pleural line was broken and subpleural lesions associated with white vertical areas were present. Finally, a score of 3 was given if the scanned area showed dense and large white lung findings. For each patient, all available zones (right and left anterior, lateral, and dorsal zones) were scored (0–3) and summed up in a single mean, mean-FLUS, that reflects the total affection of the lungs. The mean-FLUS score was used as the primary sonographic variable.
Alternative sonographic scoring systems
When only aggregates of the FLUS scores were considered, patients with severe affection in only one lobe would have a low score. It was expected that such patients would have a high risk for respiratory failure, and therefore, the FLUS examinations were reclassified: 1) highest FLUS score in any zone (i.e., a score on two in one zone and zero in all other zones resulted in a maximum score of 2); 2) FLUS score=3 in any zone (i.e., binary, positive if the patient had one or more zones with a score of 3, but negative if the patient did not have any zone scoring 3); 3) count of zones with a score of 3; 4) count of zones with a score of 2; 5) mean-FLUS score of anterior and lateral chest zones only (FLUS data from dorsal zones were expected to be missing in some patients with respiratory distress who were unable to sit; a subgroup analysis was performed on the film results from the anterior and lateral zones only, leaving the posterior zone out); and 6) other studies report a simple sum of zones ignoring any missing zones and to compare with these studies a total sum was analysed.
Prediction analyses and receiver operating characteristic analyses comparing areas under the curve were performed for these different FLUS scoring systems to explore potential differences in the subpopulation that had no missing ultrasound data. Furthermore, the effect of choice of transducer was evaluated.
Outcome variables
The need for COVID-19-related mechanical ventilation was the primary end-point of interest. Secondary outcomes were COVID-19-related admission to intensive care, high-flow oxygen treatment, 30-day mortality and all-cause 30-day mortality.
Bias
The FLUS scoring was blinded to any baseline or outcome variables. Interrater variability in the analysis of FLUS data was blindly assessed on video clips from 25 randomly selected patients and 174 film clips by two observers.
Study size
As all patients were included during the first wave of the COVID-19 pandemic, no prior studies were available for sample size calculation.
Statistical methods
Continuous variables were assessed for parametric distribution with quartile–quartile plots. Medians, interquartile ranges or mean±sd and ranges were reported according to parametric distribution. t-test or Mann–Whitney U-test was used as it was considered appropriate to evaluate differences between groups. The statistical significance was set to 5%. All data were handled in Excel (Microsoft) and REDCap (hosted at Aarhus University). All analyses were performed using Stata 14.2 (StataCorp, College Station, TX, USA).
Building prediction models
Prediction models were based on multivariable logistic regression and preselected variables. The variables were sex, age, relevant comorbidity, oxygen administration and the mean-FLUS score. The selection of these variables was based on published data and clinical experience available to the study group during the study design stage. Explanatory variables were examined to decide cut-points, scales or the need for transformation. Variables were limited and prioritised to avoid overfitting or underfitting, respecting the study population size. Every included variable required at least 15 events.
The dependent variable was the primary outcome of interest: the COVID-19-related need for mechanical ventilation in the main analysis. Secondary outcomes were analysed using the same logistic regression model, except for the outcome on high-flow oxygen treatment. The explanatory variable on oxygen administration was considered closely related to the outcome and was thus excluded from this analysis.
Additional analysis based on COVID-19 status
The entire study population was divided into subgroups based on positive and negative SARS-CoV-2 PCR tests at the time of the FLUS, and all prediction models were performed on these subgroups to evaluate the difference in FLUS prediction capability.
Results
Participants
In total, 417 patients were recruited, but ultrasound film clips were available for analysis in only 398 of these. Some 57% had a positive SARS-CoV-2 PCR test. None of the patients had the outcome of interest before the FLUS examination was performed. Demographic data, baseline clinical data and outcome data for the study population are described in table 1. In total, 17 patients (4.3%) ended up receiving mechanical ventilation due to COVID-19.
TABLE 1.
Demographic, vital parameters, blood test results and end-points in the study population
| Variable | Observations, N | Mean±sd, n (%) or % |
| Study information | ||
| Patients included in study | 415 | |
| Patients excluded due to no lung ultrasound film clips available analysis | 17 | |
| Patients analysed | 398 | 96 |
| Site 1 | 201 | 50.5 |
| Site 2 | 82 | 20.6 |
| Site 3 | 69 | 17.3 |
| Site 4 | 46 | 11.6 |
| Demographics | ||
| Age years | 387 | 67.8±15.8 |
| Weight kg | 242 | 78.4±19.7 |
| Height cm | 246 | 171.6±10.1 |
| Sex, female | 398 | 189 (47.5) |
| Clinical data | ||
| Systolic blood pressure mmHg | 386 | 130.7±22.0 |
| Diastolic blood pressure mmHg | 383 | 74.5±13.5 |
| Peripheral oxygen saturation % | 387 | 95.3±3.3 |
| Heart rate beats·min−1 | 381 | 85.1±18.4 |
| Respiratory frequency breaths·min−1 | 386 | 20.3±5.1 |
| Supplementary oxygen doses L·min−1 | 364 | 1.7±3.3 |
| Biochemical data | ||
| Ferritin μg·L−1 | 175 | 986.0±1183.6 |
| Haemoglobin mmol·L−1 | 384 | 7.7±1.4 |
| C-reactive protein mg·L−1 | 381 | 64±66 |
| Leukocytes, count ×109 L−1 | 385 | 9.0±5.1 |
| Lymphocytes, count ×109 L−1 | 374 | 1.4±1.3 |
| Monocytes, count ×109 L−1 | 299 | 0.6±0.4 |
| Neutrophils, count ×109 L−1 | 379 | 6.7±4.6 |
| Estimated glomerular filtration rate mL·min−1 | 272 | 69.6±23.3 |
| Carbamide mmol·L−1 | 291 | 7.2±5.8 |
| Creatinine μmol·L−1 | 385 | 98.0±92.3 |
| Natrium mmol·L−1 | 385 | 138.5±4.2 |
| Potassium mmol·L−1 | 382 | 3.8±0.5 |
| Fibrin D-dimer mg·L−1 (FEU) | 263 | 2.4±4.6 |
| Pro-brain natriuretic peptide ng·L−1 | 155 | 706.4±2418.1 |
| Troponin I (HS) ng·L−1 | 75 | 211.0±1120.1 |
| Troponin T (HS) ng·L−1 | 93 | 68.9±349.1 |
| Glucose mmol·L−1 | 249 | 7.4±3.0 |
| Haemoglobin A1c mmol·mol−1 | 160 | 44.5±13.2 |
| pH-arterial | 301 | 7.5±0.1 |
| PaCO2 kPa | 297 | 5.0±1.3 |
| PaO2 kPa | 132 | 10.2±3.1 |
| Base excess mmol·L−1 | 133 | 1.3±4.8 |
| Hydrogen carbonate mmol·L−1 | 300 | 25.2±4.7 |
| Lactate-arterial mmol·L−1 | 275 | 1.5±0.9 |
| Ultrasound equipment | ||
| General Electric's Vivid S60 | 398 | 201 (51) |
| General Electric's LogiQ S8 | 398 | 151 (38) |
| Sonosite X-porte | 398 | 46 (12) |
| Phased array transducer with cardiography pre-set | 398 | 201 (51) |
| Curvilinear transducer with abdominal pre-set | 398 | 197 (49) |
| Outcome data | ||
| COVID-19 positive PCR test | 398 | 227 (57) |
| Overall mortality 30 days after admission | 398 | 47 (11.8) |
| COVID-19-related mortality 30 days after admission | 398 | 28 (7) |
| Admission to intensive care | 398 | 21 (5.3) |
| COVID-19-positive admission to intensive care | 398 | 19 (4.8) |
| Ventilator treatment | 398 | 18 (4.9) |
| COVID-19-positive ventilator treatment care | 398 | 17 (4.3) |
| High-flow oxygen treatment | 398 | 56 (14) |
| COVID-19-positive, high-flow oxygen treatment | 398 | 51 (12.8) |
| Nasal oxygen treatment | 398 | 159 (43.3) |
| COVID-19-positive, nasal oxygen treatment | 398 | 117 (29.4) |
FEU: fibrin-equivalent units; HS: high sensitivity; PaCO2: arterial carbon dioxide tension; PaO2: arterial oxygen tension.
FLUS result summary
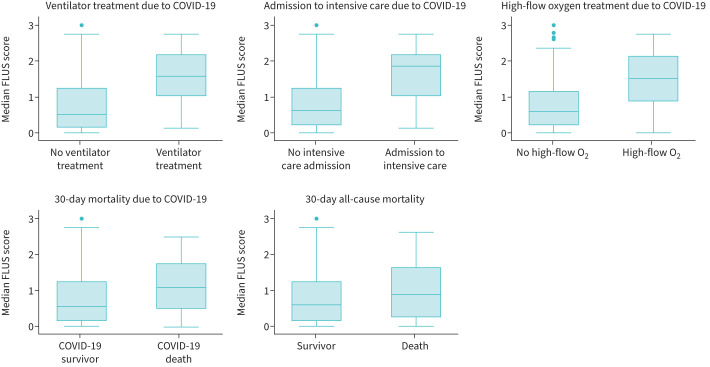
Among the 398 patients, 3889 FLUS film clips were analysed. FLUS was done with a curvilinear transducer and abdominal pre-set with frequency on 4 and focus point at 8 cm in 197 (49.5%) of the patients and with a phased array transducer with a frequency on 3.4 and a focus point at 9 cm in 201 (50.5%) patients. The mean-FLUS findings from the different scanning zones are shown in table 2. The mean-FLUS scores had non-parametric distribution, and the median was 0.59 (IQR 0.14–1.25, n=398). In the SARS-CoV-2-negative patients, the mean-FLUS score was 0.21 (IQR 0–0.71, n=160) versus 0.88 (IQR 0.38–1.63, n=227) in the positive ones (p<0.001). Mean-FLUS scores were higher for patients who received mechanical ventilation, were admitted to intensive care units, were treated with high-flow oxygen and died within 30 days, when compared with patients who did not meet these outcomes (figure 1).
TABLE 2.
Focused lung ultrasound (FLUS) scoring results from the thoracic scannings zones
| Scanning zone | FLUS score | ||||
| 0. The pleural line is continuous and regular; horizontal artefacts are present | 1. The pleural line is indented; below the indent, vertical areas of white are visible | 2. The pleural line is broken; below the breaking point, small-to-large consolidated areas appear with associated areas of white below the consolidated area | 3. The scanned area shows dense and largely extended white lung with or without larger consolidations | Missing; no film recorded or ultrasound not performed in zone | |
| Right 1 | 262 (65.8) | 59 (14.8) | 45 (11.3) | 29 (7.29) | 3 (0.75) |
| Right 2 | 250 (62.8) | 80 (20.1) | 43 (10.3) | 23 (4.8) | 2 (0.5) |
| Right 3 | 185 (45.5) | 67 (16.8) | 99 (24.9) | 22 (8.3) | 14 (3.5) |
| Right 4 | 197 (49.5) | 87 (21.9) | 69 (17.3) | 27 (6.7) | 18 (4.5) |
| Right 5 | 70 (17.6) | 30 (7.5) | 27 (6.8) | 6 (1.5) | 265 (66.6) |
| Right 6 | 98 (24.6) | 21 (5.3) | 11 (2.8) | 2 (0.5) | 266 (66.9) |
| Right 7 | 111 (27.9) | 15 (3.8) | 4 (1) | 2 (0.5) | 266 (66.8) |
| Left 1 | 276 (67.1) | 52 (13.1) | 50 (12.6) | 25 (6.3) | 4 (1) |
| Left 2 | 247 (62.1) | 60 (15.1) | 60 (15.1) | 23(5.8) | 8 (2) |
| Left 3 | 166 (41.7) | 74 (18.6) | 103 (25.9) | 42 (10.6) | 13 (3.3) |
| Left 4 | 200 (50.3) | 57 (14.3) | 81 (20.3) | 39 (9.8) | 21 (5.3) |
| Left 5 | 82 (20.6) | 18 (4.5) | 23 (5.8) | 6 (1.5) | 269 (67.6) |
| Left 6 | 99 (24.9) | 18 (4.5) | 14 (3.5) | 0 (0) | 267 (67.1) |
| Left 7 | 114 (28.6) | 11 (2.8) | 6 (1.5) | 0 (0) | 267 (67.1) |
Data presented as n (%).
FIGURE 1.
Median focused lung ultrasound (FLUS) scores for the different end-points. Statistically significant higher FLUS score was found in patients who received mechanical ventilation (p<0.001), were admitted to intensive care (p<0.001), were treated with high-flow oxygen (p<0.001), who died of COVID-19 (p=0.004) and who died of any cause (p=0.01).
FLUS prediction of outcome
In the primary outcome analysis, a one-unit increase in the continuous mean-FLUS score was the only variable that independently predicted a future event of ventilator treatment (relative risk 2.44, 95% CI 1.32–5.52, per unit increase in FLUS score, p<0.001) when adjusting for age, sex, comorbidity and non-high-flow oxygen administration. In the univariable analysis, the relative risk for ventilator treatment was 2.84 (95% CI 1.69–4.77, p<0.001). In addition, in the univariable analysis, the need for non-high-flow oxygen administration predicted ventilator treatment (relative risk 3.63, 95% CI 1.38–9.53, p=0.009) but age, sex and comorbidity did not (table 3).
TABLE 3.
Uni- and multivariate logistic regression analysis of capability to predict primary outcome and secondary outcomes of median focused lung ultrasound (FLUS) score
| Univariate logistic regression | Multivariate logistic regression | |||||||
| Relative risk | SE | 95% CI | p-value | Relative risk | SE | 95% CI | p-value | |
| Mechanical ventilation | 2.84 | 0.75 | 1.69–4.77 | <0.001 | 2.44 | 0.77 | 1.31–4.52 | 0.005 |
| Intensive care unit | 2.85 | 0.73 | 1.73–4.71 | <0.001 | 2.55 | 0.76 | 1.42–4.6 | 0.002 |
| High-flow oxygen treatment | 2.06 | 0.27 | 1.59–2.66 | <0.001 | 1.95 | 0.26 | 1.5–2.53 | <0.001 |
| 30-day mortality due to COVID-19 | 1.77 | 0.37 | 1.17–2.67 | 0.007 | 1.14 | 0.29 | 0.7–1.88 | 0.601 |
| 30-day all-cause mortality | 1.51 | 0.24 | 1.10–2.06 | 0.01 | 0.95 | 0.22 | 0.61–1.51 | 0.853 |
Age, sex, comorbidity and non-high-flow oxygen treatment are included as covariates in the multivariate analysis. In this analysis FLUS was the only variable that predicted need for mechanical ventilation, admission to intensive care unit and high-flow oxygen treatment. FLUS did not predict 30-day mortality or 30-day mortality due to COVID-19. Age was the only positive predictor for these in a multivariable logistic regression.
Regarding secondary outcomes, a unit increase in the mean-FLUS score independently predicted intensive care admission (relative risk 2.55, 95% CI 1.41–54.59, p<0.001) and high-flow oxygen treatment (relative risk 1.95, 95% CI 1.5–2.53, p<0.001). No other variables included in the multivariable logistic regression models or the univariable analysis were able to predict these events.
Conversely, FLUS did not independently predict all-cause 30-day mortality (relative risk 0.96, 95% CI 0.61–1.51, p=0.85) or 30-day mortality related to COVID-19 (relative risk 1.14, 95% CI 0.7–1.88, p=0.6) in the multivariable analysis. Only age (relative risk 1.07, 95% CI 1.04–1.10, p<0.001, and relative risk 1.08, 95% CI 1.04–1.11, p<0.001) and sex (male sex, relative risk 2.33, 95% CI 1.2–4.54, p=0.013 and relative risk 2.69, 95% CI 1.24–5.83, p=0.012) were statistically significant, predicting variables to all-cause 30-day mortality and 30-day mortality related to COVID-19. Detailed results from the analyses are shown in supplementary table S1.
Interrater variability
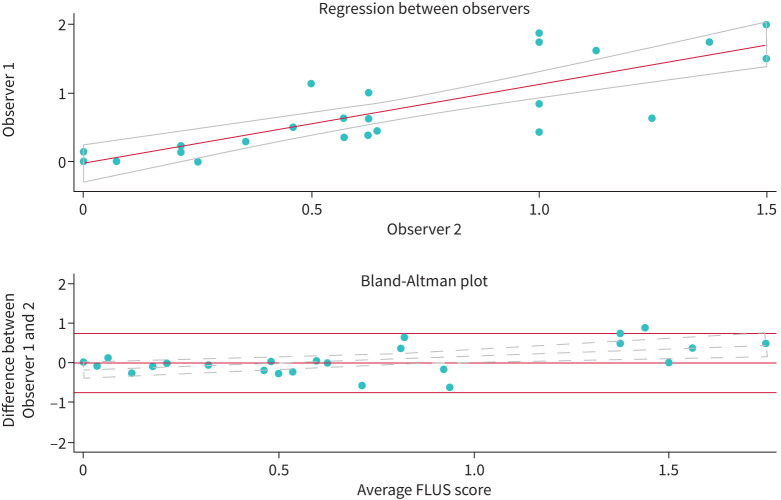
The regression of agreement between observers showed a good linear correlation (R2=0.7) (figure 2). The Bland–Altman plot of the median rating difference in the FLUS score and the average FLUS score showed little discrepancy between the two observers. The variability was statistically larger as the average score increased (y=1.39–0.2, p=0.01). Kappa scores from the individual scanning zones showed moderate agreement, with an average score of 0.4 (supplementary table S9).
FIGURE 2.
Correlation and Bland–Altman plot of median focused lung ultrasound (FLUS) scores in randomly selected patients by two blinded observers. Little and clinically insignificant difference in scores is seen even though there is a trend (α: 0.36, p=0.01) with increasing difference as median FLUS score increases.
Additional analysis of FLUS scoring systems
Uni- and multivariable logistic regression analyses with the maximum FLUS score, number of zones scoring higher than 2 and number of zones scoring higher than 3 predicted the future event of mechanical ventilation, intensive care admission and high-flow oxygen administration (table 4 and supplementary tables S2–S5). The FLUS analysis of the anterior and lateral chest scanning zones (right 1, 2, 3, 4 and left 1, 2, 3, 4), excluding the posterior zones, showed prediction results similar to the entire FLUS scanning that included the posterior zones, as shown in supplementary table S6.
TABLE 4.
Uni- and multivariate logistic regression analysis of different focused lung ultrasound (FLUS) scoring systems’ capability to predict need for mechanical ventilation
| Univariate logistic regression | Multivariate logistic regression | |||||||
| Relative risk | SE | 95% CI | p-value | Relative risk | SE | 95% CI | p-value | |
| Median FLUS score | 2.84 | 0.75 | 1.69–4.77 | <0.001 | 2.44 | 0.77 | 1.31–4.52 | 0.005 |
| Highest FLUS score in any zone | 2.97 | 1.03 | 1.5–5.9 | 0.002 | 2.22 | 0.8 | 1.1–4.51 | 0.028 |
| FLUS score >3 in any of the zones | 5.42 | 2.67 | 2.06–14.25 | <0.001 | 3.54 | 1.84 | 1.27–9.83 | 0.015 |
| Count of FLUS zones scoring 3 | 1.48 | 0.04 | 1.4–1.58 | <0.001 | 1.46 | 0.09 | 1.29–1.66 | <0.001 |
| Count of FLUS zones scoring 2 | 1.31 | 0.09 | 1.14–1.5 | <0.001 | 1.38 | 0.11 | 1.18–1.61 | <0.001 |
| Median FLUS score, anterior and lateral zones only | 2.96 | 0.76 | 1.79–4.89 | <0.001 | 2.63 | 0.77 | 1.47–4.69 | 0.001 |
| Total sum of FLUS | 1.12 | 0.02 | 1.09–1.17 | <0.001 | 1.12 | 0.03 | 1.08–1.18 | <0.001 |
The area under the curve receiver operating characteristics (AUC ROC) of the 129 patients who had all zones scanned are shown in figure 3. For the primary outcome, mechanical ventilation, the AUC ROC for the mean-FLUS score and the total sum FLUS was 0.94 (95% CI 0.85–1). AUC ROC of FLUS score from the anterior and lateral zone only was likewise 0.95 (95% CI 0.86–1). AUC ROC was the lower of the scoring systems that classified the presence of one zone scoring ≥3 (0.79, 95% CI 0.46–1), the number of zones scoring ≥2 (0.88, 95% CI 0.75–1) and for the number of zones scoring >3 (0.79, 95% CI 0.46–1, test for difference, p<0.001). Testing for difference between transducer type, the AUC ROC was 0.93 (95% CI 0.82–1) for the curvilinear transducer and 0.66 (95% CI 0.5–0.81) for the phased array transducer (p=0.005).
FIGURE 3.
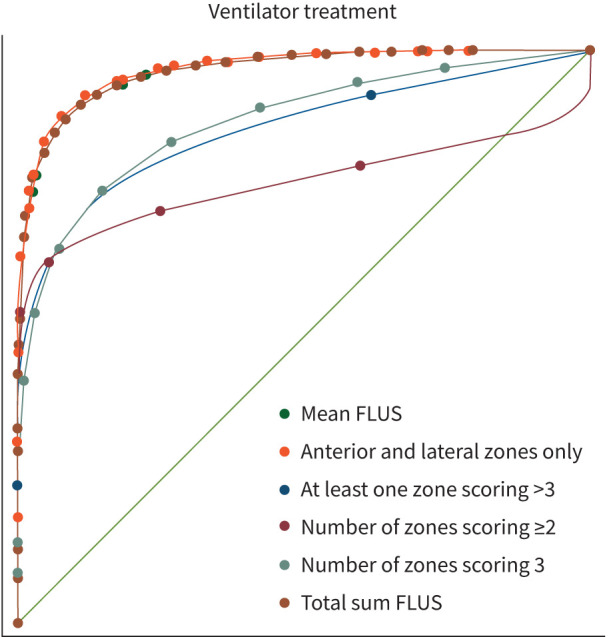
Receiver operating characteristics of different scorings systems. The median focused lung ultrasound (FLUS) score and total sum from all zones and the median FLUS score from the anterior and lateral zones had higher area under the curve than other scoring systems.
Finally, in the patients who were known as SARS-CoV-2-positive at the time of the FLUS examination, the multivariable logistic regression found that FLUS predicted the need for mechanical ventilation (relative risk 1.89, 95% CI 1–3.6, p=0.05), intensive care admission (relative risk 1.98, 95% CI 1.06–3.69, p=0.03) and high-flow oxygen treatment (relative risk 2.17, 95% CI 1.38–3.43, p=0.001) (supplementary table S7). Likewise, in patients with a yet-to-know SARS-CoV-2 status at the FLUS examination timepoint, FLUS predicted future intensive care admission (relative risk 3.56, 95% CI 1.57–8.08, p>0.001) and mechanical ventilation (relative risk 7.07, 95% CI 1.87–31.77, p<0.001). Similar to the main analysis, FLUS did not predict COVID-19-related 30-day mortality or all-cause 30-day mortality regardless of known SARS-CoV-2 status at the scanning time (supplementary table S8).
Discussion
In hospitalised patients with suspected or PCR-verified SARS-CoV-2 infection, a FLUS examination predicted the need for mechanical ventilation, intensive care admission and high-flow oxygen administration when adjusting for sex, age and comorbidity. FLUS did not predict 30-day mortality due to COVID-19 or mortality due to any other cause.
FLUS is performed at bedside by the attending physician. Besides minimising the risk of contamination by eliminating the need for in-hospital patient transport, FLUS provides immediate images of the lungs that are essential in evaluating COVID-19's severity [14]. The affection of the lung may lead to respiratory failure, which is the primary cause of death in COVID-19 cases [4, 6]. Even in patients with a low symptom burden, rapid onset or aggravation of respiratory failure is possible. In a patient with COVID-19 infection without respiratory failure or with low-intensity lung affection, for instance, abnormalities found using FLUS, may lead to a change in management because such a patient is at high risk of developing respiratory failure. In addition to informing the patient and relatives that the risk of COVID-19 infection is severe, the level of patient monitoring could be adjusted accordingly [7], and intensive care or wards that are able to handle patients with respiratory failure could be warned that a patient is likely to arrive. Performing a FLUS examination may thus have substantial impacts for both patients and the healthcare system, while only minimal resources would be used as FLUS is quickly performed [15].
Different FLUS scanning protocols exist. Common to all protocols is that several chest areas are examined and evaluated [13, 16]. Typical COVID-19 FLUS findings include pleural abnormalities, subpleural consolidations and B-lines in the lung parenchyma [17]. A standardised method to analyse and grade findings was developed, and we used this system to analyse film clips [13]. In particular, analysing the anterior and lateral zones seemed to be just as accurate as including the dorsal zones. This finding is surprising, as the lower dorsal parts of the chest are favourite zones for lobar pneumonia and contrasts other findings in COVID-19 [18]. We speculate that a diffuse distribution of lung lesions may resemble what is seen in acute respiratory distress syndrome with the anterolateral zones involvement and may explain the relationship between FLUS and the need for mechanical ventilation.
The use of FLUS during the COVID-19 pandemic has been recommended [15]; multiple trials have studied triage and risk stratification for respiratory failure [9, 19–25]. These studies found results comparable to ours but used smaller samples or were single-centre designs. Other reports have studied FLUS's ability to diagnose COVID-19 or to guide ongoing intensive care treatment [15, 26]. Our study was not designed to assess the diagnostic yield of FLUS compared to the SARS-CoV-2 PCR test because such a test is easily available in our setting. Furthermore, a normal FLUS examination does not exclude COVID-19.
Our study has several limitations. First, the number of events was small, even though the population size is the largest studied so far. Few events potentially led to overfitting the regression models, leading to more conservative estimates, but the signal that FLUS was the only predictor of respiratory deterioration was consistent among all the models we developed. Second, even though our study was prospective and multi-centred in design, COVID-19 restrictions allowed only for inclusion by convenience, which potentially caused selection bias. Third, FLUS was performed according to the operators’ preferences. All FLUS operators were regular users of FLUS or other point-of-care ultrasound examinations, but the selection of transducer and pre-set was at the discretion of the operator. Film clips recorded with a phased array transducer could potentially reduce the image quality of the pleural line and subpleural consolidations, and we saw a difference in ROC AUC between transducers. However, few film clips were unanalysable, and the free selection of transducer is therefore likely to further support generalisability. Fourth, our study included a mixed population of undiagnosed patients with COVID-19 symptoms and a population of patients diagnosed with COVID-19. A subgroup analysis showed lower predicting capability in patients already diagnosed with COVID-19; this is likely due to the disease being more advanced in these patients. Fifth, interrater variability was assessed on a random selection of film clips, not on the entire population. Finally, and most importantly, it must be remembered that FLUS examines pleural and peripheral lung parenchymal lesions only. The findings are not specific to COVID-19, but may also be found in other conditions, such as non-COVID-19 pneumonia, heart failure and acute respiratory distress syndrome caused by other conditions [27, 28]. Conversely, patients can be SARS-CoV-2 PCR-positive without a clinically significant lung pathology and no FLUS findings. Thus, FLUS is not a stand-alone diagnostic test or risk-stratification tool, as many other factors must be included in the decision-making process.
In conclusion, our results demonstrate that FLUS is an independent predictor of respiratory failure requiring mechanical ventilation, admission to intensive care or high-flow oxygen treatment in patients with symptoms of COVID-19 or a positive SARS-CoV-2 PCR test. This establishes FLUS as an important tool to stratify the risk of respiratory failure during the COVID-19 pandemic.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00128-2022.SUPPLEMENT (368.4KB, pdf)
Acknowledgement
We thank Bo Bibby (Department of Biostatistics, Public Health, Aarhus University, Denmark) for statistical support.
Footnotes
Author contributions: S.H. Skaarup is the guarantor of the content of the manuscript including data and analysis. S.H. Skaarup led the study design, data handling, analysis and wrote the first manuscript draft. S.H. Ovesen, J. Weile, H. Kirkegaard and R. Aagaard contributed in study design and cowrote the manuscript. C. Espersen, M.C.H. Lassen, K.G. Skaarup, S. Posth, C.B. Laursen, A. Bock, M.D. Arvig and T. Biering-Sørensen provided clinical advice to support the data analysis and interpretation of data. All authors reviewed and approved the final manuscript.
Submitted article, peer reviewed.
Conflict of interest: None of the authors have any conflicts of interest to report.
Support statement: The trial was sponsored by the Poul Due Jensens Foundation. The sponsors had no role in designing the study or in the collection, management, analysis or interpretation of the data, nor in writing of the manuscript. Together with K.G. Skaarup and M.C.H. Lassen, T. Biering-Sørensen received a research grant from the Novo Nordisk Foundation to conduct part of this study. Europcar Denmark provided cars for K.G. Skaarup and M.C.H. Lassen to transport the equipment from hospital to hospital. T. Biering-Sørensen received funds from Herlev and Gentofte Hospital and the Lundbeck Foundation while conducting part of this study.
References
- 1.Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open 2020; 3: e2022310. doi: 10.1001/jamanetworkopen.2020.22310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei W, Sivapalasingam S, Mellis S, et al. A retrospective study of COVID-19-related urgent medical visits and hospitalizations after outpatient COVID-19 diagnosis in the US. Adv Ther 2021; 38: 3185–3202. doi: 10.1007/s12325-021-01742-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta HB, Li S, Goodwin JS. Risk factors associated with SARS-CoV-2 infections, hospitalization, and mortality among US nursing home residents. JAMA Netw Open 2021; 4: e216315. doi: 10.1001/jamanetworkopen.2021.6315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med 2020; 180: 1081–1089. doi: 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoletti M, Giannella M, Scudeller L, et al. Development and validation of a prediction model for severe respiratory failure in hospitalized patients with SARS-CoV-2 infection: a multicentre cohort study (PREDI-CO study). Clin Microbiol Infect 2020; 26: 1545–1553. doi: 10.1016/j.cmi.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lalueza A, Lora-Tamayo J, Maestro-de la Calle G, et al. A predictive score at admission for respiratory failure among hospitalized patients with confirmed 2019 coronavirus disease: a simple tool for a complex problem. SSRN Electron J 2020; 17: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mongodi S, Orlando A, Arisi E, et al. Lung ultrasound in patients with acute respiratory failure reduces conventional imaging and health care provider exposure to COVID-19. Ultrasound Med Biol 2020; 46: 2090–2093. doi: 10.1016/j.ultrasmedbio.2020.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care 2020; 24: 702. doi: 10.1186/s13054-020-03369-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bock A, Lassen AT, Laursen CB, et al. Lung ultrasound as a prognostic tool in emergency patients clinically suspected of COVID-19. Dan Med J 2021; 68: A07200551. [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elezkurtaj S, Greuel S, Ihlow J, et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep 2021; 11: 4263. doi: 10.1038/s41598-021-82862-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laursen CB, Rahman NM, Volpicelli G, eds. Thoracic Ultrasound. ERS Monograph. Sheffield, UK, European Respiratory Society, 2018. [Google Scholar]
- 13.Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19. J Ultrasound Med 2020; 39: 1413–1419. doi: 10.1002/jum.15285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gravell RJ, Theodoreson MD, Buonsenso D, et al. Radiological manifestations of COVID-19: key points for the physician. Br J Hosp Med 2020; 81: 1–11. doi: 10.12968/hmed.2020.0231 [DOI] [PubMed] [Google Scholar]
- 15.Peixoto AO, Costa RM, Uzun R, et al. Applicability of lung ultrasound in COVID-19 diagnosis and evaluation of the disease progression: a systematic review. Pulmonology 2021; 27: 529–562. doi: 10.1016/j.pulmoe.2021.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millington SJ, Koenig S, Mayo P, et al. Lung ultrasound for patients with coronavirus disease 2019 pulmonary disease. Chest 2021; 159: 205–211. doi: 10.1016/j.chest.2020.08.2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng QY, Wang XT, Zhang LN. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med 2020; 46: 849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demi L, Mento F, Sabatino A Di, et al. Lung ultrasound in COVID-19 and post-COVID-19 patients, an evidence-based approach. J Ultrasound Med 2021; 41: 2203–2215. doi: 10.1002/jum.15902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasukawa K, Minami T, Boulware DR, et al. Point-of-care lung ultrasound for COVID-19: findings and prognostic implications from 105 consecutive patients. J Intensive Care Med 2021; 36: 334–342. doi: 10.1177/0885066620988831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Alencar JCG, de Marchini JFM, Marino LO, et al. Lung ultrasound score predicts outcomes in COVID-19 patients admitted to the emergency department. Ann Intensive Care 2021; 11: 6. doi: 10.1186/s13613-020-00796-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonadia N, Carnicelli A, Piano A, et al. Lung ultrasound findings are associated with mortality and need for intensive care admission in COVID-19 patients evaluated in the emergency department. Ultrasound Med Biol 2020; 46: 2927–2937. doi: 10.1016/j.ultrasmedbio.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahier T, Meuwly J-Y, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study. Clin Infect Dis 2020; 73: e4189–e4196. doi: 10.1093/cid/ciaa1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichter Y, Topilsky Y, Taieb P, et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med 2020; 46: 1873–1883. doi: 10.1007/s00134-020-06212-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrone T, Soldati G, Padovini L, et al. A new lung ultrasound protocol able to predict worsening in patients affected by severe acute respiratory syndrome coronavirus 2 pneumonia. J Ultrasound Med 2021; 40: 1627–1635. doi: 10.1002/jum.15548 [DOI] [PubMed] [Google Scholar]
- 25.Ji L, Cao C, Gao Y, et al. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Crit Care 2020; 24: 700. doi: 10.1186/s13054-020-03416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peyrony O, Marbeuf-Gueye C, Truong V, et al. Accuracy of emergency department clinical findings for diagnosis of coronavirus disease 2019. Ann Emerg Med 2020; 76: 405–412. doi: 10.1016/j.annemergmed.2020.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Islam N, Salameh JP, Leeflang MMG, et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst Rev 2020; 11: CD013639. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Luo X, Wang L, et al. A comparison of lung ultrasound and computed tomography in the diagnosis of patients with COVID-19: a systematic review and meta-analysis. Diagnostics 2021; 11: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00128-2022.SUPPLEMENT (368.4KB, pdf)