This cohort study describes the incidence of atrial fibrillation in patients with lung cancer and assesses predictive cardiac dosimetric parameters.
Key Points
Question
What is the association between radiation dose exposure to cardiac substructures with cardiac toxic effects and survival in patients who received chemoradiotherapy for lung cancer?
Findings
In this cohort study of 560 patients, individualized dose calculation for various cardiac substructures revealed that the maximum dose delivered to the sinoatrial node was an independent factor associated with atrial fibrillation and overall survival.
Meaning
These findings suggest that incidental irradiation of the sinoatrial node may be associated with the development of atrial fibrillation and increased mortality, and indicate the need to minimize radiation dose exposure to the sinoatrial node during chemoradiotherapy and consider close follow-up for the early detection of atrial fibrillation.
Abstract
Importance
Atrial fibrillation (AF) can develop following thoracic irradiation. However, the critical cardiac substructure responsible for AF has not been properly studied.
Objective
To describe the incidence of AF in patients with lung cancer and determine predictive cardiac dosimetric parameters.
Design, Setting, and Participants
This retrospective cohort study was performed at a single referral center and included 239 patients diagnosed with limited-stage small cell lung cancer (SCLC) and 321 patients diagnosed with locally advanced non–small cell lung cancer (NSCLC) between August 2008 and December 2019 who were treated with definitive chemoradiotherapy.
Exposures
Radiation dose exposure to cardiac substructures, including the chambers, coronary arteries, and cardiac conduction nodes, were calculated for each patient.
Main Outcomes and Measures
Main outcomes were AF and overall survival.
Results
Of the 239 and 321 patients with SCLC and NSCLC, the median (IQR) age was 68 (60-73) years and 67 (61-75) years, and 207 (86.6%) and 261 (81.3%) were men, respectively. At a median (IQR) follow-up time of 32.7 (22.1-56.6) months, 9 and 17 patients experienced new-onset AF in the SCLC and NSCLC cohorts, respectively. The maximum dose delivered to the sinoatrial node (SAN Dmax) exhibited the highest predictive value for prediction of AF. A higher SAN Dmax significantly predicted an increased risk of AF in patients with SCLC (adjusted hazard ratio [aHR], 14.91; 95% CI, 4.00-55.56; P < .001) and NSCLC (aHR, 15.67; 95% CI, 2.08-118.20; P = .008). However, SAN Dmax was not associated with non-AF cardiac events. Increased SAN Dmax was significantly associated with poor overall survival in patients with SCLC (aHR, 2.68; 95% CI, 1.53-4.71; P < .001) and NSCLC (aHR, 1.97; 95% CI, 1.45-2.68; P < .001).
Conclusions and Relevance
In this cohort study, results suggest that incidental irradiation of the SAN during chemoradiotherapy may be associated with the development of AF and increased mortality. This supports the need to minimize radiation dose exposure to the SAN during radiotherapy planning and to consider close follow-up for the early detection of AF in patients receiving thoracic irradiation.
Introduction
Thoracic radiotherapy (RT) can increase the risk of cardiac adverse events.1,2 Radiation-induced cardiac adverse events have been reported, mainly in patients with breast cancer or lymphoma.3,4,5,6 The cardiac effect of RT in patients with lung cancer has also gained interest.7,8,9,10,11 Although lung cancer is well known for its poor prognosis, the median survival has increased with the advancements in treatment strategies, indicating the need for achieving the appropriate balance between tumor control and cardiac toxic effects.12,13,14
Studies conducted on patients before the era of computed tomography (CT)–based planning estimated the cardiac dose based on a representative CT scan using virtual planning.3,4,5 With CT simulation becoming a routine practice in RT planning, individual-based cardiac dose calculations have become possible. Not only the dose delivered to the whole heart, but also radiation dose delivered to cardiac substructures, can be calculated accurately.
Until now, studies on radiation-induced cardiotoxic effects have focused mainly on ischemic heart diseases.1,2 Although the risk of atrial fibrillation (AF) in patients with intrathoracic cancers has been shown to be substantial,7,9,11,15,16 to our knowledge a detailed study of AF in patients with small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC) receiving chemoradiotherapy (CRT) has not been conducted. Moreover, no specific dose constraint of a specific cardiac substructure has been suggested for predicting AF. To this end, we evaluated the dose-volume parameters of diverse cardiac substructures in patients with SCLC and NSCLC who received definitive CRT and investigated critical cardiac substructures associated with development of AF.
Methods
Patients and Treatment
A total of 293 patients diagnosed with histologically confirmed limited-stage SCLC and 412 patients diagnosed with locally advanced NSCLC who were treated with definitive CRT between August 2008 and December 2019 were retrospectively analyzed. Patients with previously diagnosed cancer (n = 75), less than 3 months of follow-up (n = 36), premature termination of RT before reaching 45 Gy (n = 30), and nonrestorable RT plan (n = 4) were excluded, leaving a total of 239 patients with SCLC (SCLC cohort) and 321 patients with NSCLC (NSCLC cohort) for further analysis. None of the 36 patients with less than 3-month follow-up died due to cardiac events. This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Severance Hospital (2021-2365-001), and the requirement for informed consent was waived owing to the retrospective nature of the research. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
All patients with SCLC received either etoposide with cisplatin or carboplatin for 4 to 6 cycles. Patients with NSCLC received weekly paclitaxel with carboplatin for up to 6 cycles. Radiotherapy was delivered using 3-dimensional conformal RT or intensity-modulated RT techniques. A total of 60 to 63 Gy in 1.8 to 2.1 Gy per fraction was delivered. None of the patients received up-front surgery. After treatment, the patients were followed up every 2 to 4 months for the first 3 years and then every 6 months for the next 2 years.
Dosimetric Analysis
The cardiac substructures, including the right atrium, right ventricle, left atrium, left ventricle, left anterior descending artery, right coronary artery, and left circumflex artery, were contoured according to the cardiac contouring atlas17 using a deep-learning based autosegmentation tool of the heart that was developed in-house.18 Next, the contours were reviewed by 3 radiation oncologists (K.H.K., G.Y., and J.L.) who were blinded to the clinical factors. The sinoatrial node (SAN) and atrioventricular node were delineated manually according to the contouring atlas.19 The maximum dose (minimum dose delivered to the hottest 0.035 cc), mean dose, and V5 Gy to V60 Gy in increments of 5 Gy were calculated (V5 Gy to V60 Gy refers to the percentage of the structure receiving at least the indicated dose).
Coronary Artery Calcium Measurement
Coronary artery calcium (CAC) score was automatically determined using a research prototype software (AVIEW CAC, Coreline Soft) and was expressed as Agaston score (eMethods in the Supplement).20,21
Cardiac End Points
Cardiac events were determined through in-depth reviews of the medical records by 2 independent cardiologists (J.O. and S.G.) who were blinded to the dosimetric data. The cardiac end points including cardiac death, unstable anginas, myocardial infarction, coronary revascularization, heart failure (HF), and AF were assessed after the initiation of RT.22 Cardiac events other than AF were categorized as non-AF cardiac events. Patients with preexisting cardiac morbidities were considered to have had a cardiac event if they presented with the same cardiac event, but it was of a greater severity than that experienced during the 6-month interval preceding RT, or if the event was a different class of cardiac event. For AF, the baseline electrocardiogram (ECG) was compared with follow-up ECG to determine new-onset AF. Chest CT scans and echocardiograms were reviewed to evaluate pericardial effusion following CRT.
Statistical Analysis
The predictive power of the dosimetric parameters was evaluated using time-dependent integrated area under the receiver operating characteristic curves (eMethods in the Supplement).23 Time to cardiac event was estimated from the start date of RT to the date of the event or last observation. Overall survival (OS) was defined as the time from the initiation of the treatment to death from any cause or last observation. Fine and Gray regression and Cox proportional hazards regression were performed for the univariable and multivariable analyses (eMethods in the Supplement). A 2-sided P value less than .05 was considered to be statistically significant. All the analyses were performed using R, version 4.0.3 (R Foundation for Statistical Computing).
Results
Patient Characteristics
A total of 239 patients and 321 patients were included in the SCLC and NSCLC cohorts, respectively. The baseline patient characteristics of SCLC and NSCLC cohorts are summarized in Table 1. In the NSCLC cohort, 67 patients (20.9%) received consolidation durvalumab, pembrolizumab, or nivolumab following CRT. Following CRT, 1 patient and 8 patients received chest surgery after CRT in the SCLC and NSCLC cohorts, respectively. The patients underwent surgery for removal of empyema (n = 4) and local recurrence (n = 5).
Table 1. Patient Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| SCLC (n = 239) | NSCLC (n = 321) | |
| Age, median (IQR), y | 68 (60-73) | 67 (61-75) |
| Sex | ||
| Female | 32 (13.4) | 60 (18.7) |
| Male | 207 (86.6) | 261 (81.3) |
| BMI, median (IQR) | 23.7 (21.4-25.8) | 22.7 (20.8-25.0) |
| Hypertension | ||
| No | 124 (51.9) | 187 (58.3) |
| Yes | 115 (48.1) | 134 (41.7) |
| Diabetes | ||
| No | 170 (71.1) | 246 (76.6) |
| Yes | 69 (28.9) | 75 (23.4) |
| ECOG performance status | ||
| 0 | 23 (9.6) | 46 (14.3) |
| 1 | 208 (87.0) | 262 (81.6) |
| 2 | 8 (3.3) | 13 (4.1) |
| Cardiovascular disease | ||
| Atrial fibrillation | 3 (1.3) | 8 (2.5) |
| Valvular heart disease | 1 (0.4) | 1 (0.3) |
| Coronary artery disease | 23 (9.6) | 25 (7.8) |
| Complete atrioventricular block | 1 (0.4) | 0 |
| Stroke | 12 (5.0) | 9 (2.8) |
| Peripheral vascular disease | 3 (1.3) | 5 (1.6) |
| None | 196 (82.0) | 273 (85.0) |
| Coronary artery calcification | ||
| No | 50 (20.9) | 63 (19.6) |
| Yes | 189 (79.1) | 258 (80.4) |
| CAC score, median (IQR) | 165.3 (3.9-712.1) | 108.7 (3.8-428.2) |
| No. of coronary arteries with calcification | ||
| 0 | 50 (20.9) | 63 (19.6) |
| 1 | 50 (20.9) | 64 (19.9) |
| 2 | 21 (8.8) | 38 (11.8) |
| 3 | 34 (14.2) | 51 (15.9) |
| 4 | 94 (39.3) | 98 (30.5) |
| Aortic valve calcification | ||
| No | 174 (72.8) | 236 (73.5) |
| Yes | 65 (27.2) | 85 (26.5) |
| Mitral valve calcification | ||
| No | 226 (94.6) | 294 (91.6) |
| Yes | 13 (5.4) | 27 (8.4) |
| Tobacco use | ||
| Never | 31 (13.0) | 75 (23.4) |
| Current | 54 (22.6) | 57 (17.8) |
| Former | 154 (64.4) | 189 (58.9) |
| Pack-years, median (IQR)a | 40 (25-50) | 40 (27-50) |
| Alcohol use | ||
| Never | 81 (33.9) | 116 (36.1) |
| Current | 69 (28.9) | 96 (29.9) |
| Former | 89 (37.2) | 109 (34.0) |
| Stage | ||
| I-II | 33 (13.8) | 18 (5.6) |
| IIIA | 70 (29.3) | 89 (27.7) |
| IIIB | 84 (35.1) | 137 (42.7) |
| IIIC | 52 (21.8) | 77 (24.0) |
| Chemotherapy regimen | ||
| Etoposide + carboplatin | 102 (42.7) | 0 |
| Etoposide + cisplatin | 137 (57.3) | 0 |
| Paclitaxel + carboplatin | 0 | 291 (90.7) |
| Others | 0 | 30 (9.3) |
| Maintenance IO agent | ||
| None | 0 | 254 (79.1) |
| Durvalumab | 0 | 50 (15.6) |
| Pembrolizumab/nivolumab | 0 | 17 (5.3) |
| RT dose, median (IQR) | 60 (54.0-60.8) | 63 (60.0-64.5) |
| RT fraction, median (IQR) | 30 (30-30) | 30 (30-30) |
| RT modality | ||
| 3D-CRT | 116 (48.5) | 110 (34.3) |
| IMRT | 123 (51.5) | 211 (65.7) |
Abbreviations: 3D-CRT, 3-dimensional conformal radiotherapy; BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CAC, coronary artery calcium; CVD, cardiovascular disease; ECOG, Eastern Cooperative Oncology Group; IMRT, intensity-modulated radiotherapy; IO, immuno-oncology; NSCLC, non–small cell lung cancer; RT, radiotherapy; SCLC, small cell lung cancer; WHO, World Health Organization.
Estimated among patients with history of tobacco use.
Cardiac Events
In the SCLC cohort, 9 patients experienced new-onset AF, and 5 patients experienced non-AF cardiac events during a median (IQR) follow-up time of 25.7 (16.5-47.2) months. The 5 non-AF cardiac events included 2 patients who underwent coronary revascularizations, 1 patient who experienced an ST-segment elevation myocardial infarction, and 2 patients who were hospitalized with HF with reduced ejection fraction. In the NSCLC cohort, 17 patients experienced new-onset AF, and 6 patients experienced non-AF cardiac events during a median (IQR) follow-up time of 36.2 (26.9-60.2) months. The 6 non-AF cardiac events included 5 patients who underwent coronary revascularizations and 1 patient who experienced a non–ST-segment elevation myocardial infarction.
Higher SAN Dmax Predicts AF
Maximum dose (Dmax) delivered to the SAN (SAN Dmax) exhibited the highest C index (0.66; 95% CI, 0.56-0.74) for the prediction of AF in the combined cohort of SCLC and NSCLC (eTable 1 in the Supplement). The top 5 predictive SAN dosimetric variables, in both the SCLC and NSCLC cohorts, are shown in eTable 2 in the Supplement. A representative patient with NSCLC who experienced new-onset AF 7 months post-CRT is illustrated in eFigure 1 in the Supplement. The optimal SAN Dmax cutoff level was 53.5 Gy (95% CI, 48.9-53.7 Gy) in the SCLC cohort. Patients who received a SAN Dmax of 53.5 Gy or greater exhibited a significantly higher 3-year cumulative incidence of AF than those who received a SAN Dmax less than 53.5 Gy (25.0%; 95% CI, 8.4%-74.1% vs 2.7%; 95% CI, 1.1%-6.7%; P < .001; Figure 1A). Patients who received a SAN Dmax of 53.5 Gy or greater and a SAN Dmax less than 53.5 Gy exhibited similar cumulative incidences of non-AF cardiac events (P = .51; Figure 1B). The significance of the SAN Dmax of 53.5 Gy or greater in predicting AF was maintained in multivariable analysis (adjusted hazard ratio [aHR], 14.91; 95% CI, 4.00-55.56; P < .001; Table 2).
Figure 1. Incidence of Cardiac Adverse Events and Overall Survival According to SAN Dmax in the SCLC Cohort.
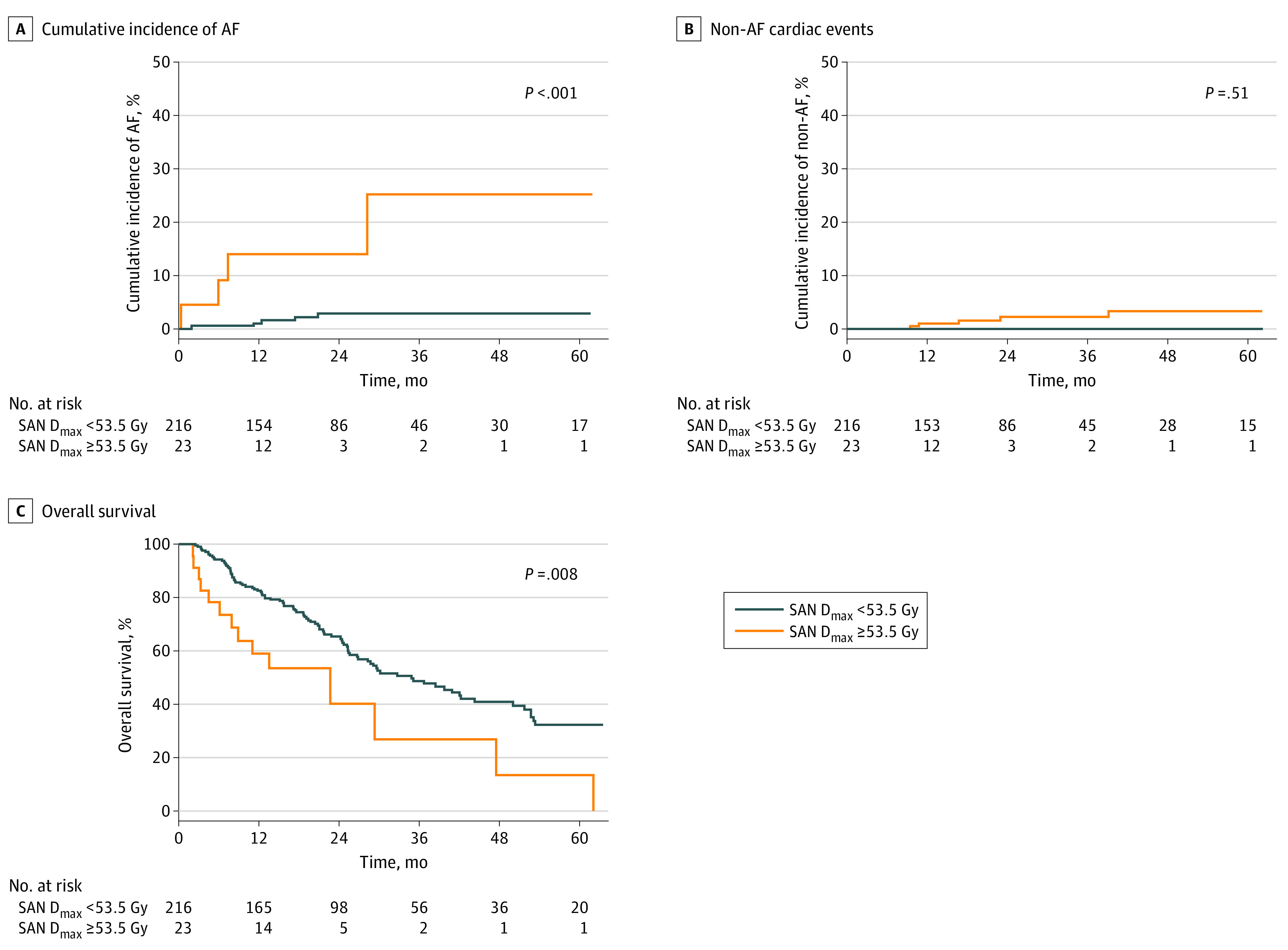
Cumulative incidence of AF (A), non-AF cardiac events (B), and overall survival (C) in patients with SAN Dmax of 53.5 Gy or greater and SAN Dmax less than 53.5 Gy. AF indicates atrial fibrillation; SAN Dmax, maximum radiation dose exposed to sinoatrial node; SCLC, small cell lung cancer.
Table 2. Competing Risk Regression Analysis for Atrial Fibrillation in SCLC Cohort.
| Variablea | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P value | aHR (95% CI) | P value | |
| SAN Dmax, Gy | ||||
| <53.5 | 1 [Reference] | 1 [Reference] | NA | |
| ≥53.5 | 8.90 (2.40-32.96) | .001 | 14.91 (4.00-55.56) | <.001 |
| Age, y | 1.05 (0.99-1.11) | .12 | 1.08 (1.00-1.17) | .04 |
| Tobacco use | ||||
| Never | 1 [Reference] | NA | NA | NA |
| Ever | 2.14 (0.45-10.21) | .34 | NA | NA |
| Alcohol use | ||||
| Never | 1 [Reference] | NA | 1 [Reference] | NA |
| Ever | 4.24 (0.55-32.83) | .17 | 6.09 (0.97-38.47) | .06 |
| Hypertension | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 1.32 (0.35-4.96) | .68 | NA | NA |
| Diabetes | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 0.71 (0.15-3.44) | .67 | NA | NA |
| Cardiovascular disease | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 2.12 (0.53-8.53) | .29 | NA | NA |
| Coronary artery calcium score | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 0.96 (0.20-4.75) | .96 | NA | NA |
| CAC score | 1.00 (1.00-1.00) | .61 | NA | NA |
| No. of coronary arteries with calcification | ||||
| 0 | 1 [Reference] | NA | 1 [Reference] | NA |
| 1-2 | 2.34 (0.45-12.3) | .31 | 1.66 (0.30-9.20) | .56 |
| 3-4 | 0.39 (0.05-2.83) | .35 | 0.21 (0.03-1.55) | .13 |
| Aortic valve calcification | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 1.40 (0.35-5.63) | .64 | NA | NA |
| Mitral valve calcification | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 2.32 (0.28-19.0) | .43 | NA | NA |
| BMI | 1.18 (1.00-1.39) | .05 | 1.37 (1.09-1.72) | .007 |
| AJCC stage | ||||
| I-IIIA | 1 [Reference] | NA | NA | NA |
| IIIB-IIIC | 0.61 (0.16-2.26) | .46 | NA | NA |
| Chemotherapy | ||||
| Etoposide + cisplatin | 1 [Reference] | NA | NA | NA |
| Etoposide + carboplatin | 0.92 (0.25-3.42) | .91 | NA | NA |
| RT dose, Gy | 0.99 (0.89-1.10) | .83 | NA | NA |
| RT modality | ||||
| 3D-CRT | 1 [Reference] | NA | NA | NA |
| IMRT | 0.32 (0.06-1.65) | .18 | NA | NA |
Abbreviations: 3-DCRT, 3-dimensional conformal radiotherapy; AJCC, American Joint Committee on Cancer; aHR, adjusted hazard ratio; BMI, body mass index; CAC, coronary artery calcium; CVD, cardiovascular disease; HR, hazard ratio; HTN, hypertension; IMRT, intensity-modulated radiotherapy; NA, not applicable; RT, radiotherapy; SAN Dmax, maximum radiation dose exposed to sinoatrial node; SCLC, small cell lung cancer; WHO, World Health Organization.
Categorical variables that had no events in 1 of the subgroups and were not applicable for regression analysis were sex, Eastern Cooperative Oncology Group performance status, pericardial effusion after chemoradiotherapy, and chest surgery after chemoradiotherapy.
The optimal cutoff level of SAN Dmax for prediction of AF in the NSCLC cohort was 20.0 Gy (95% CI, 2.5-43.5 Gy). The 3-year cumulative incidence of AF was significantly higher in patients who received a SAN Dmax of 20.0 Gy or greater than in those who received a SAN Dmax less than 20.0 Gy (9.9%; 95% CI, 5.9%-16.4% vs 0.7%; 95% CI, 0.0%-5.1%; P < .001; Figure 2A). Patients who received a SAN Dmax of 20.0 Gy or greater and SAN Dmax less than 20.0 Gy exhibited no significant difference in the cumulative incidence of non-AF cardiac events (P = .13; Figure 2B). The significance of a SAN Dmax of 20.0 Gy or greater in predicting AF was maintained in multivariable analysis (aHR, 15.67; 95% CI, 2.08-118.20; P = .008; Table 3).
Figure 2. Incidence of Cardiac Adverse Events and Overall Survival According to SAN Dmax in the NSCLC Cohort.
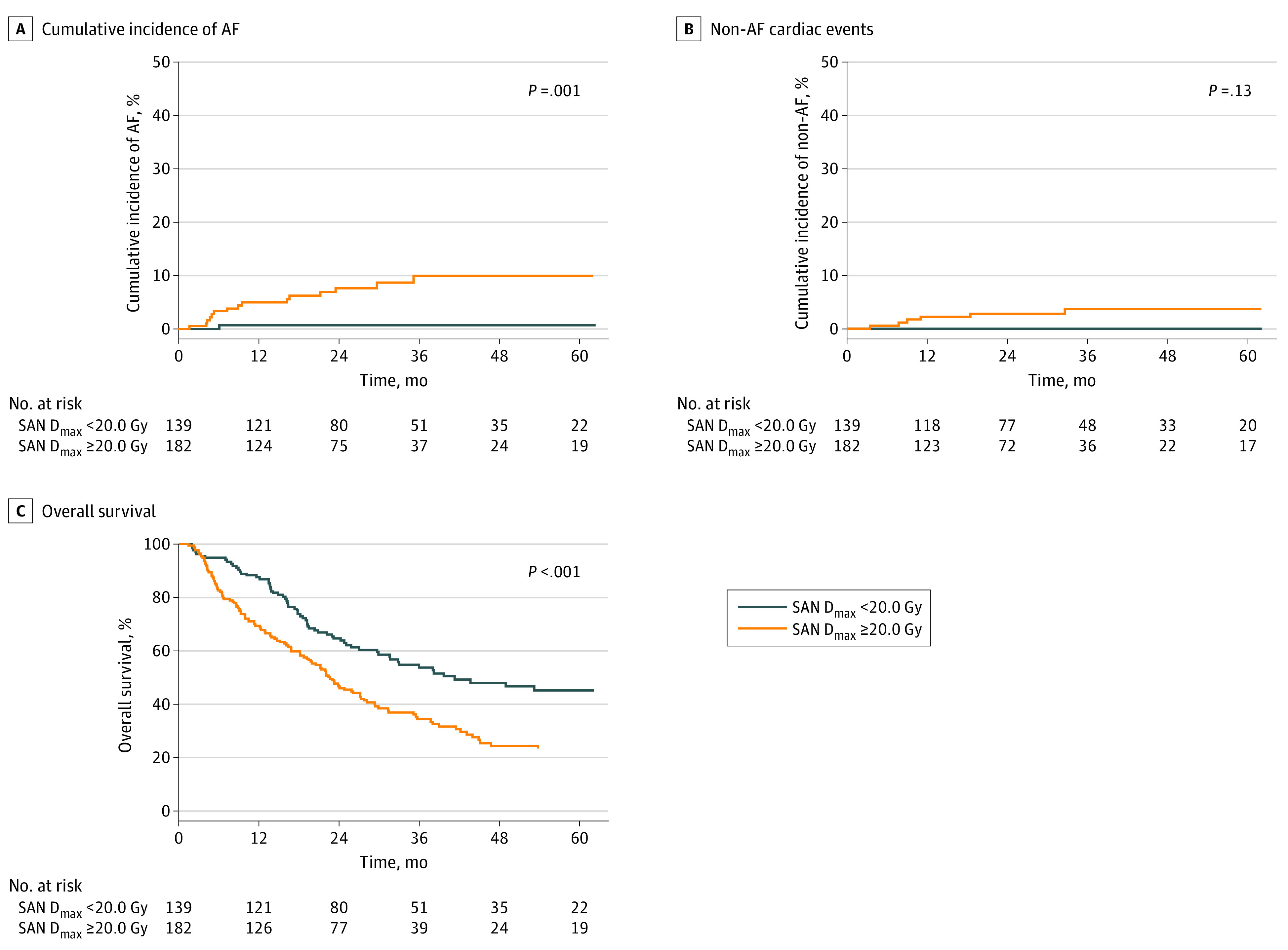
Cumulative incidence of AF (A), non-AF cardiac events (B), and overall survival (C) in patients with SAN Dmax of 20.0 Gy or greater and SAN Dmax less than 20.0 Gy. AF indicates atrial fibrillation; NSCLC, non–small cell lung cancer; SAN Dmax, maximum radiation dose exposed to sinoatrial node.
Table 3. Competing Risk Regression Analysis for Atrial Fibrillation in NSCLC Cohort.
| Variablea | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P value | aHR (95% CI) | P value | |
| SAN Dmax, Gy | ||||
| <20.0 | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥20.0 | 13.15 (1.73-99.82) | .01 | 15.67 (2.08-118.20) | .008 |
| Age, y | 1.03 (0.97-1.09) | .30 | NA | NA |
| Sex | ||||
| Male | 1 [Reference] | NA | NA | NA |
| Female | 0.53 (0.13-2.25) | .39 | NA | NA |
| ECOG performance status | ||||
| 0 | 1 [Reference] | NA | NA | NA |
| 1-2 | 2.64 (0.35-19.96) | .35 | NA | NA |
| Tobacco use | ||||
| Never | 1 [Reference] | NA | 1 [Reference] | NA |
| Ever | 5.15 (0.71-37.7) | .11 | 5.07 (0.74-34.71) | .10 |
| Alcohol use | ||||
| Never | 1 [Reference] | NA | NA | NA |
| Ever | 0.63 (0.25-1.63) | .34 | NA | NA |
| Hypertension | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 3.38 (1.19-9.56) | .02 | 3.74 (1.24-11.20) | .02 |
| Diabetes | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 3.08 (1.18-8.02) | .02 | NA | NA |
| Cardiovascular disease | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 0.69 (0.16-2.95) | .61 | 0.33 (0.09-1.28) | .11 |
| Coronary artery calcium | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 4.16 (0.56-31.1) | .16 | NA | NA |
| CAC score | 1.00 (1.00-1.01) | .03 | NA | NA |
| No. of coronary arteries with calcification | ||||
| 0 | 1 [Reference] | NA | NA | NA |
| 1-2 | 2.97 (0.34-26.3) | .33 | NA | NA |
| 3-4 | 4.96 (0.65-37.8) | .12 | NA | NA |
| Aortic valve calcification | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 4.24 (1.64-11) | .003 | 3.18 (1.17-8.67) | .02 |
| Mitral valve calcification | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 2.45 (0.69-8.64) | .16 | NA | NA |
| Pericardial effusion after CRT | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 0.80 (0.11-5.85) | .82 | NA | NA |
| BMI | 1.01 (0.90-1.12) | .89 | NA | NA |
| AJCC stage | ||||
| II-IIIA | 1 [Reference] | NA | NA | NA |
| IIIB-IIIC | 0.94 (0.35-2.54) | .90 | NA | NA |
| Chemotherapy | ||||
| Others | 1 [Reference] | NA | NA | NA |
| Paclitaxel + carboplatin | 0.71 (0.16-3.11) | .65 | NA | NA |
| Maintenance ICI | ||||
| No | 1 [Reference] | NA | NA | NA |
| Yes | 0.91 (0.26-3.15) | .88 | NA | NA |
| RT dose, Gy | 1.05 (0.94-1.17) | .39 | NA | NA |
| RT modality | ||||
| 3D-CRT | 1 [Reference] | NA | NA | NA |
| IMRT | 1.50 (0.55-4.14) | .43 | NA | NA |
Abbreviations: 3D-CRT, 3-dimensional conformal radiotherapy; AJCC, American Joint Committee on Cancer; aHR, adjusted hazard ratio; BMI, body mass index; CAC, coronary artery calcification; CVD, cardiovascular disease; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; HTN, hypertension; ICI, immune checkpoint inhibitor; IMRT, intensity-modulated radiotherapy; NA, not applicable; NSCLC, non–small cell lung cancer; RT, radiotherapy; SAN Dmax, maximum radiation dose exposed to sinoatrial node; WHO, World Health Organization.
The categorical variable of chest surgery after CRT had no events in 1 of the subgroups and was not applicable for regression analysis.
Pericardial effusion was observed in 14 and 18 patients in the SCLC and NSCLC cohorts, respectively, following CRT. None of the patients with new-onset AF had treatment-related pericarditis prior to AF. Moreover, pericardial effusion, chest surgery after CRT, presence of CAC, and extent of CAC were not significantly associated with new-onset AF in both cohorts (Tables 2 and 3).
Higher SAN Dmax Predicts Poor Survival
The 3-year OS in patients with a SAN Dmax of 53.5 Gy or greater was significantly lower than in those with a SAN Dmax less than 53.5 Gy in the SCLC cohort (30.9%; 95% CI, 13.8%-69.0% vs 48.5%; 95% CI, 41.3%-57.0%; P = .008; Figure 1C). In the NSCLC cohort, the 3-year OS in patients with a SAN Dmax of 20.0 Gy or greater was significantly lower than in those with a SAN Dmax less than 20.0 Gy (35.0%; 95% CI, 28.3%-43.3% vs 54.5%; 95% CI, 46.5%-63.9%; P < .001; Figure 2C). The SAN Dmax maintained a significant association with poorer OS in multivariable analysis in the SCLC cohort (aHR, 2.68; 95% CI, 1.53-4.71; P < .001; eTable 3 in the Supplement) and the NSCLC cohort (aHR, 1.97; 95% CI, 1.45-2.68; P < .001; eTable 4 in the Supplement).
Cardiac Substructures Associated With AF and Survival Other Than SAN
We analyzed the predictive value of maximal radiation dose delivered to the heart (eFigure 2 in the Supplement), left atrium (eFigure 3 in the Supplement), and right atrium (RA Dmax; eFigure 4 in the Supplement), which also exhibited some predictive value (eTable 1 in the Supplement). Patients with higher RA Dmax exhibited higher incidence of new-onset AF and poorer OS in both the SCLC and NSCLC cohorts (eFigure 4 in the Supplement). In multivariable analysis, higher RA Dmax was associated with new-onset AF in the SCLC cohort (aHR, 20.54; 95% CI, 5.57-75.65; P < .001; eTable 5 in the Supplement) and in the NSCLC cohort (aHR, 5.97; 95% CI, 1.34-26.57; P = .02; eTable 6 in the Supplement). The RA Dmax maintained a significant association with poorer OS in the SCLC cohort (aHR, 2.29; 95% CI, 1.34-3.91; P = .002; eTable 7 in the Supplement) and the NSCLC cohort (aHR, 1.57; 95% CI, 1.14-2.17; P = .005; eTable 8 in the Supplement).
Discussion
The present study demonstrated, to our knowledge, a previously undescribed association between the radiation dose exposed to the SAN and new-onset AF in patients with lung cancer who received CRT. Higher SAN Dmax was also associated with an increased risk of mortality. However, it did not predict non-AF cardiac events, indicating the specific association between SAN Dmax and AF.
Studies on radiation-induced cardiac adverse events have mainly focused on ischemic heart diseases.1,3,8,24 However, arrhythmia is one of the most common cardiac adverse events following CRT in patients with lung cancer.7,9,10,16 Nevertheless, dose-volume parameters of cardiac substructures associated with AF have not been properly studied. Most previous studies have focused on the 4 chambers of the heart and coronary arteries with little emphasis on the conduction nodes.8,9,10 In these studies, either the left ventricle doses or left anterior descending artery doses were associated with acute coronary syndromes or HF, which are known to be caused by occlusion of the coronary artery or dysfunction of the left ventricle. However, the pathophysiology of AF is distinct from either acute coronary syndromes or HF, and thus, cardiac substructures other than the chambers or coronary arteries should be evaluated.25
The pathophysiology of AF is complex and has not been fully elucidated. It frequently coexists with SAN dysfunction, which is considered as a predisposing condition for AF.26 Patients with SAN dysfunction are known to be more susceptible to develop AF, and AF may newly develop in 1 of 4 patients receiving cardiac pacing.27,28 More recently, genetic variants significantly associated with AF and SAN dysfunction were identified by genome-wide association studies.29,30,31 The common variants between AF and SAN dysfunction imply a shared genetic background of these 2 conditions. Moreover, in mendelian randomization analysis, AF was associated with SAN dysfunction, suggesting causality.31 Dysfunction of the SAN can be mediated by degenerative fibrosis and electrical remodeling of the atrium, especially in SAN. Radiotherapy has been known to induce fibrosis or remodeling of the heart.32 Therefore, it may be hypothesized that iatrogenic SAN dysfunction by RT may facilitate AF. We took advantage of a recently published contouring atlas for cardiac conduction node delineation to estimate the irradiated dose to the SAN and atrioventricular node in each individual patient.19
The optimal cutoff SAN Dmax values in the SCLC and NSCLC cohorts were different. A lower cutoff of 20.0 Gy was determined in the NSCLC cohort, compared with 53.5 Gy in the SCLC cohort, and the confidence intervals of the cutoff values did not overlap. The incidence of new-onset AF was higher in the NSCLC cohort than in the SCLC cohort despite similar SAN Dmax values between both cohorts (eTable 1 in the Supplement). Therefore, the lower cutoff value of SAN Dmax and higher incidence of AF in the NSCLC cohort than in the SCLC cohort indicate that patients in the NSCLC cohort may have been more sensitized to the effect of radiation compared with the SCLC cohort. Such difference may be partially explained through the different chemotherapy regimen used in the 2 cohorts. Previous studies have demonstrated the arrhythmogenic effect of chemotherapeutic agents and the effect of paclitaxel on AF has been better defined compared with etoposide.33,34 However, whether paclitaxel, compared with etoposide, may further sensitize the cardiac conduction system to RT needs further investigation. Cardiomyocytes generated from human-induced pluripotent stem cell lines may be the proper platform to test the hypotheses.35 Despite the difference in cutoff values, results of the current study indicate a common process involving the SAN in RT-induced AF across different types of cancers.
The SAN Dmax maintained its predictability of AF even after adjustments for well-known clinical risk factors of AF, such as age, body mass index, tobacco use, and hypertension.36 We also incorporated CAC, which has been reported as a risk factor for AF,37,38 as an adjusting factor in the multivariable model. The association between CAC score and cardiac adverse events has been previously demonstrated in patients with breast cancer receiving RT.39 In the current study, a trend of increased risk of new-onset AF in patients with CAC was observed but was not statistically significant. Further studies are needed to confirm the association of CAC with new-onset AF in the context of patients with lung cancer receiving CRT.
The high doses delivered to the SAN were significantly associated not only with AF, but also with poor survival, even after adjustments for other clinical variables. A recent population-based study among patients with breast cancer has also demonstrated that new-onset AF increased all-cause mortality.40 Moreover, numerous studies have demonstrated the association between higher cardiac doses and worse OS.7,8,9,41,42,43,44,45 In the current study, the patients were not tested routinely for arrhythmias, and those with higher SAN doses may have had undiagnosed and untreated AF leading to mortality. The SAN dose also inevitably correlates with mean heart dose, and higher SAN Dmax may serve as a surrogate of higher mean heart dose. Higher mean heart dose is known to correlate with more severe lymphopenia, which leads to inferior survival.46 Although the association seems clear, further studies are needed to elucidate the underlying mechanisms of increased mortality due to higher cardiac doses.
Cardiac parameters other than SAN Dmax, such as RA Dmax, also exhibited comparable C indices, indicating that SAN Dmax is not the only predictor for new-onset AF. Considering the proximity of SAN and RA, the strong correlation between SAN Dmax and RA Dmax may have led to the similar results between the 2 parameters. The current study data imply that if there are limitations in defining SAN, RA Dmax may be able to serve as an alternative. In addition, the radiation dose delivered to the atrium may have caused structural changes in the atrium, such as fibrosis, and facilitate AF. Atrial fibrosis, remodeling, and myopathy can be visualized with cardiac magnetic resonance imaging.47,48 Prospective studies that use cardiac magnetic resonance imaging following thoracic RT may uncover the role of RT-induced structural changes in the development of AF.
Because of the different optimal cutoff points of SAN Dmax in the SCLC and NSCLC cohorts, clear dose constraints cannot be currently recommended. Further validation studies are required to confirm the optimal cutoff value. We suggest keeping SAN Dmax as low as reasonably allowable while satisfying the dose constraints to other organs at risk and maintaining tumor coverage. In addition, we suggest establishing a screening protocol for subclinical or clinical AF in the multidisciplinary team. Patients with cancer receiving thoracic RT may be screened using regular ECG or Holter monitoring. In addition, a recent pragmatic study demonstrated the usefulness of smartwatch application in identifying subclinical AF.49 Monitoring patients using smartwatches may be feasible for early detection of AF.
Limitations
Several limitations of this study stem from its retrospective nature. Patients were not prospectively followed up for cardiac toxic effects, which limited the estimation of the true incidence of cardiac adverse events. In addition, the small number of cardiac events underpowered the results of multivariable analysis. However, 2 cardiologists who were blinded to the dose-volume parameters reviewed the patients’ medical records thoroughly. Prospective studies including proper cardiac screening protocols, such as use of cardiac biomarkers, echocardiogram, and ECG, should be pursued. The results of several ongoing prospective studies (eg, NCT04361240, NCT04674501, NCT04867564, NCT04896242) are awaited.
Conclusions
In this cohort study, results suggest that incidental irradiation of the SAN during CRT may be associated with the development of AF and increased mortality. Although further validation is required, the results indicate that SAN may need to be considered as an organ at risk during RT planning and that patients receiving higher doses to the SAN may need close monitoring for earlier recognition and treatment of AF.
eMethods
eFigure 1. Representative patient who developed AF after completion of definitive CRT
eFigure 2. Incidence of new onset AF and overall survival according to Heart Dmax
eFigure 3. Incidence of new onset AF and overall survival according to LA Dmax
eFigure 4. Incidence of new onset AF and overall survival according to RA Dmax
eTable 1. Time dependent area under the receiver operating characteristic curve analysis of dose variables for cardiac substructures and atrial fibrillation (AF) in total, SCLC, and NSCLC cohorts
eTable 2. C-index of the top 5 dose volume parameters for cardiac substructures predictive for atrial fibrillation in total, SCLC, and NSCLC cohorts
eTable 3. Cox proportional hazards regression analysis for overall survival in SCLC cohort
eTable 4. Cox proportional hazards regression analysis for overall survival in NSCLC cohort
eTable 5. Competing risk regression analysis for atrial fibrillation in SCLC cohort using RA Dmax
eTable 6. Competing risk regression analysis for atrial fibrillation in NSCLC cohort using RA Dmax
eTable 7. Cox proportional hazards regression analysis for overall survival in SCLC cohort using RA Dmax
eTable 8. Cox proportional hazards regression analysis for overall survival in NSCLC cohort using RA Dmax
References
- 1.Banfill K, Giuliani M, Aznar M, et al. ; IASLC Advanced Radiation Technology committee . Cardiac toxicity of thoracic radiotherapy: existing evidence and future directions. J Thorac Oncol. 2021;16(2):216-227. doi: 10.1016/j.jtho.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergom C, Bradley JA, Ng AK, et al. Past, present, and future of radiation-induced cardiotoxicity: refinements in targeting, surveillance, and risk stratification. JACC CardioOncol. 2021;3(3):343-359. doi: 10.1016/j.jaccao.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 4.van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34(3):235-243. doi: 10.1200/JCO.2015.63.4444 [DOI] [PubMed] [Google Scholar]
- 5.van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175(6):1007-1017. doi: 10.1001/jamainternmed.2015.1180 [DOI] [PubMed] [Google Scholar]
- 6.Chung SY, Oh J, Chang JS, et al. Risk of cardiac disease in patients with breast cancer: impact of patient-specific factors and individual heart dose from three-dimensional radiation therapy planning. Int J Radiat Oncol Biol Phys. 2021;110(2):473-481. doi: 10.1016/j.ijrobp.2020.12.053 [DOI] [PubMed] [Google Scholar]
- 7.Atkins KM, Rawal B, Chaunzwa TL, et al. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol. 2019;73(23):2976-2987. doi: 10.1016/j.jacc.2019.03.500 [DOI] [PubMed] [Google Scholar]
- 8.Atkins KM, Chaunzwa TL, Lamba N, et al. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non-small cell lung cancer. JAMA Oncol. 2021;7(2):206-219. doi: 10.1001/jamaoncol.2020.6332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yegya-Raman N, Wang K, Kim S, et al. Dosimetric predictors of symptomatic cardiac events after conventional-dose chemoradiation therapy for inoperable NSCLC. J Thorac Oncol. 2018;13(10):1508-1518. doi: 10.1016/j.jtho.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang BS, Cha MJ, Kim HJ, et al. Heart substructural dosimetric parameters and risk of cardiac events after definitive chemoradiotherapy for stage III non-small cell lung cancer. Radiother Oncol. 2020;152:126-132. doi: 10.1016/j.radonc.2020.09.050 [DOI] [PubMed] [Google Scholar]
- 11.Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387-1394. doi: 10.1200/JCO.2016.70.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faivre-Finn C, Snee M, Ashcroft L, et al. ; CONVERT Study Team . Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116-1125. doi: 10.1016/S1470-2045(17)30318-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogart JA, Wang XF, Masters GA, et al. Phase 3 comparison of high-dose once-daily (QD) thoracic radiotherapy (TRT) with standard twice-daily (BID) TRT in limited stage small cell lung cancer (LSCLC): CALGB 30610 (Alliance)/RTOG 0538. J Clin Oncol. 2021;39(15)(suppl):8505. doi: 10.1200/JCO.2021.39.15_suppl.8505 [DOI] [Google Scholar]
- 14.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301-1311. doi: 10.1200/JCO.21.01308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Palaskas NL, Yusuf SW, et al. Incidence and onset of severe cardiac events after radiotherapy for esophageal cancer. J Thorac Oncol. 2020;15(10):1682-1690. doi: 10.1016/j.jtho.2020.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kravchenko J, Berry M, Arbeev K, Lyerly HK, Yashin A, Akushevich I. Cardiovascular comorbidities and survival of lung cancer patients: Medicare data based analysis. Lung Cancer. 2015;88(1):85-93. doi: 10.1016/j.lungcan.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79(1):10-18. doi: 10.1016/j.ijrobp.2009.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi MS, Choi BS, Chung SY, et al. Clinical evaluation of atlas- and deep learning-based automatic segmentation of multiple organs and clinical target volumes for breast cancer. Radiother Oncol. 2020;153:139-145. doi: 10.1016/j.radonc.2020.09.045 [DOI] [PubMed] [Google Scholar]
- 19.Loap P, Servois V, Dhonneur G, Kirov K, Fourquet A, Kirova Y. A radiation therapy contouring atlas for cardiac conduction node delineation. Pract Radiat Oncol. 2021;11(4):e434-e437. doi: 10.1016/j.prro.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 20.Lee JG, Kim H, Kang H, et al. Fully automatic coronary calcium score software empowered by artificial intelligence technology: validation study using three CT cohorts. Korean J Radiol. 2021;22(11):1764-1776. doi: 10.3348/kjr.2021.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vonder M, Zheng S, Dorrius MD, et al. Deep learning for automatic calcium scoring in population-based cardiovascular screening. JACC Cardiovasc Imaging. 2022;15(2):366-367. doi: 10.1016/j.jcmg.2021.07.012 [DOI] [PubMed] [Google Scholar]
- 22.Hicks KA, Mahaffey KW, Mehran R, et al. ; Standardized Data Collection for Cardiovascular Trials Initiative (SCTI) . 2017 Cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. 2018;71(9):1021-1034. doi: 10.1016/j.jacc.2017.12.048 [DOI] [PubMed] [Google Scholar]
- 23.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61(1):92-105. doi: 10.1111/j.0006-341X.2005.030814.x [DOI] [PubMed] [Google Scholar]
- 24.Atkins KM, Bitterman DS, Chaunzwa TL, et al. Mean heart dose is an inadequate surrogate for left anterior descending coronary artery dose and the risk of major adverse cardiac events in lung cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2021;110(5):1473-1479. doi: 10.1016/j.ijrobp.2021.03.005 [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124(20):2264-2274. doi: 10.1161/CIRCULATIONAHA.111.019893 [DOI] [PubMed] [Google Scholar]
- 26.John RM, Kumar S. Sinus node and atrial arrhythmias. Circulation. 2016;133(19):1892-1900. doi: 10.1161/CIRCULATIONAHA.116.018011 [DOI] [PubMed] [Google Scholar]
- 27.Lamas GA, Lee KL, Sweeney MO, et al. ; Mode Selection Trial in Sinus-Node Dysfunction . Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346(24):1854-1862. doi: 10.1056/NEJMoa013040 [DOI] [PubMed] [Google Scholar]
- 28.Nielsen JC, Thomsen PE, Højberg S, et al. ; DANPACE Investigators . A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32(6):686-696. doi: 10.1093/eurheartj/ehr022 [DOI] [PubMed] [Google Scholar]
- 29.Holm H, Gudbjartsson DF, Sulem P, et al. A rare variant in MYH6 is associated with high risk of sick sinus syndrome. Nat Genet. 2011;43(4):316-320. doi: 10.1038/ng.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorolfsdottir RB, Sveinbjornsson G, Sulem P, et al. A missense variant in PLEC increases risk of atrial fibrillation. J Am Coll Cardiol. 2017;70(17):2157-2168. doi: 10.1016/j.jacc.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorolfsdottir RB, Sveinbjornsson G, Aegisdottir HM, et al. ; DBDS Genomic Consortium . Genetic insight into sick sinus syndrome. Eur Heart J. 2021;42(20):1959-1971. doi: 10.1093/eurheartj/ehaa1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. doi: 10.3389/fonc.2015.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tisdale JE, Chung MK, Campbell KB, et al. ; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142(15):e214-e233. doi: 10.1161/CIR.0000000000000905 [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Li X, Yuan M, et al. Anticancer therapy-induced atrial fibrillation: electrophysiology and related mechanisms. Front Pharmacol. 2018;9:1058. doi: 10.3389/fphar.2018.01058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh J, Lee SH, Choi J, et al. Establishment of a novel human iPSC line (YCMi003-A) from a patient with dilated cardiomyopathy carrying genetic variant LMNA p.Asp364His. Stem Cell Res. 2021;56:102508. doi: 10.1016/j.scr.2021.102508 [DOI] [PubMed] [Google Scholar]
- 36.Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120(9):1501-1517. doi: 10.1161/CIRCRESAHA.117.309732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinter N, Christesen AMS, Mortensen LS, et al. Coronary artery calcium score and the long-term risk of atrial fibrillation in patients undergoing non-contrast cardiac computed tomography for suspected coronary artery disease: a Danish registry-based cohort study. Eur Heart J Cardiovasc Imaging. 2018;19(8):926-932. doi: 10.1093/ehjci/jex201 [DOI] [PubMed] [Google Scholar]
- 38.O’Neal WT, Efird JT, Dawood FZ, et al. Coronary artery calcium and risk of atrial fibrillation (from the multi-ethnic study of atherosclerosis). Am J Cardiol. 2014;114(11):1707-1712. doi: 10.1016/j.amjcard.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gal R, van Velzen SGM, Hooning MJ, et al. Identification of risk of cardiovascular disease by automatic quantification of coronary artery calcifications on radiotherapy planning CT scans in patients with breast cancer. JAMA Oncol. 2021;7(7):1024-1032. doi: 10.1001/jamaoncol.2021.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guha A, Fradley MG, Dent SF, et al. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur Heart J. 2022;43(4):300-312. doi: 10.1093/eurheartj/ehab745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thor M, Deasy JO, Hu C, et al. Modeling the impact of cardiopulmonary irradiation on overall survival in NRG Oncology Trial RTOG 0617. Clin Cancer Res. 2020;26(17):4643-4650. doi: 10.1158/1078-0432.CCR-19-2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McWilliam A, Kennedy J, Hodgson C, Vasquez Osorio E, Faivre-Finn C, van Herk M. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer. 2017;85:106-113. doi: 10.1016/j.ejca.2017.07.053 [DOI] [PubMed] [Google Scholar]
- 43.McWilliam A, Khalifa J, Vasquez Osorio E, et al. Novel methodology to investigate the effect of radiation dose to heart substructures on overall survival. Int J Radiat Oncol Biol Phys. 2020;108(4):1073-1081. doi: 10.1016/j.ijrobp.2020.06.031 [DOI] [PubMed] [Google Scholar]
- 44.Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12(2):293-301. doi: 10.1016/j.jtho.2016.09.134 [DOI] [PubMed] [Google Scholar]
- 45.Bradley JD, Hu C, Komaki RR, et al. Long-term results of NRG Oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38(7):706-714. doi: 10.1200/JCO.19.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abravan A, Faivre-Finn C, Kennedy J, McWilliam A, van Herk M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J Thorac Oncol. 2020;15(10):1624-1635. doi: 10.1016/j.jtho.2020.06.008 [DOI] [PubMed] [Google Scholar]
- 47.Goldberger JJ, Arora R, Green D, et al. Evaluating the atrial myopathy underlying atrial fibrillation: identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132(4):278-291. doi: 10.1161/CIRCULATIONAHA.115.016795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raafs AG, Vos JL, Henkens MTHM, et al. Left atrial strain has superior prognostic value to ventricular function and delayed-enhancement in dilated cardiomyopathy. JACC Cardiovasc Imaging. 2022;15(6):1015-1026. doi: 10.1016/j.jcmg.2022.01.016 [DOI] [PubMed] [Google Scholar]
- 49.Perez MV, Mahaffey KW, Hedlin H, et al. ; Apple Heart Study Investigators . Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909-1917. doi: 10.1056/NEJMoa1901183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Representative patient who developed AF after completion of definitive CRT
eFigure 2. Incidence of new onset AF and overall survival according to Heart Dmax
eFigure 3. Incidence of new onset AF and overall survival according to LA Dmax
eFigure 4. Incidence of new onset AF and overall survival according to RA Dmax
eTable 1. Time dependent area under the receiver operating characteristic curve analysis of dose variables for cardiac substructures and atrial fibrillation (AF) in total, SCLC, and NSCLC cohorts
eTable 2. C-index of the top 5 dose volume parameters for cardiac substructures predictive for atrial fibrillation in total, SCLC, and NSCLC cohorts
eTable 3. Cox proportional hazards regression analysis for overall survival in SCLC cohort
eTable 4. Cox proportional hazards regression analysis for overall survival in NSCLC cohort
eTable 5. Competing risk regression analysis for atrial fibrillation in SCLC cohort using RA Dmax
eTable 6. Competing risk regression analysis for atrial fibrillation in NSCLC cohort using RA Dmax
eTable 7. Cox proportional hazards regression analysis for overall survival in SCLC cohort using RA Dmax
eTable 8. Cox proportional hazards regression analysis for overall survival in NSCLC cohort using RA Dmax


