Abstract
Introduction
Using data from the ertugliflozin cardiovascular outcomes trial in patients with type 2 diabetes mellitus (VERTIS CV; NCT01986881), associations between the initial estimated glomerular filtration rate (eGFR) “dip” with eGFR slope, glucosuria/natriuresis-related measures, and safety were investigated.
Methods
Patients were categorized into tertiles based on change in eGFR at week 6: >+1.00 mL/min/1.73 m<sup>2</sup> (tertile 1), >−5.99 and ≤+1.00 (tertile 2), and ≤−6.00 (tertile 3). eGFR slope after week 6 and week 18 was assessed by tertile. Glucosuria/natriuresis-related measures were also determined. Adverse events (AEs) were analyzed in the acute (baseline–week 6) and chronic periods (week 6–30 days after last dose of trial medication).
Results
In the ertugliflozin group, chronic eGFR slopes (95% CI, mL/min/1.73 m<sup>2</sup>/year; weeks 6–156) were −0.76 (−1.03, −0.50), −0.29 (−0.51, −0.07), and −0.05 (−0.26, 0.17) in tertiles 1, 2, and 3, respectively (p value <0.001), and approximately −1.5 mL/min/1.73 m<sup>2</sup>/year across tertiles in the placebo group (p value = 0.79). At week 18, least squares mean (LSM) changes from baseline in glycated hemoglobin (%) were −0.77, −0.71, and −0.67 in tertiles 1, 2, and 3, respectively, in the ertugliflozin group; a similar tertile-associated trend was observed for uric acid. At week 18, LSM changes from baseline in hematocrit (%) were 2.07, 2.33, and 2.55 in tertiles 1, 2, and 3, respectively, in the ertugliflozin group; similar tertile-associated trends were observed for blood pressure. All p<sub>interaction</sub> values were <0.0001 for glucosuria- and natriuresis-related measures. Kidney-related AEs were reported more frequently in tertiles 3 and 2 in the chronic period for both placebo- and ertugliflozin-treated groups. In both periods and in all tertiles, incidences of AEs did not differ between placebo- and ertugliflozin-treated groups.
Conclusion
With ertugliflozin, the tertile with the largest initial dip in eGFR had a slower rate of chronic eGFR decline. Initial eGFR changes were associated with changes in both glucosuria- and natriuresis-related measures.
Keywords: Kidney protection, Kidney function decline, Glomerular filtration rate, Diabetic nephropathy, Type 2 diabetes mellitus, Clinical trial, Sodium-glucose cotransporter 2 inhibitor
Introduction
Sodium-glucose cotransporter 2 (SGLT2) inhibitors induce a transient natriuresis leading to a characteristic “dip” in estimated glomerular filtration rate (eGFR) [1, 2, 3]. The overall mean eGFR “dip” with SGLT2 inhibitors is generally 3–8 mL/min per 1.73 m2 [3], followed by a gradual return towards baseline over 4–52 weeks [4, 5]. The eGFR “dip” is associated with hemodynamic effects on the basis of natriuresis and activation of tubuloglomerular feedback leading to preglomerular vasoconstriction [6]. Approximately 30% of patients treated with SGLT2 inhibitors do not experience the initial eGFR “dip” [7, 8, 9]. Analogous acute declines in eGFR with renin-angiotensin-aldosterone system (RAAS) blockers attributable to glomerular hemodynamics and not acute kidney injury (AKI) have been associated with long-term reduced eGFR decline [10]. However, the association of the initial “dip” in previous SGLT2 inhibitor studies has not been analyzed according to the “tertile” definition used in the RAAS inhibitor literature [7, 9]. As a result, the interaction between the initial “dip” and subsequent chronic eGFR decline is not completely understood.
Accordingly, the objective of the present analyses was to understand the clinical relationship of ertugliflozin, based on change in eGFR in the first 6 weeks of therapy, and subsequent outcomes. Similar to RAAS blockers [10], we hypothesized that patients treated with ertugliflozin with the largest initial eGFR reduction (or “dip”) would have a slower eGFR decline.
Materials and Methods
The VERTIS CV trial (NCT01986881; protocol MK8835-004) was an event-driven trial in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease comparing ertugliflozin (5 mg and 15 mg) with placebo; ertugliflozin dose groups were pooled for all analyses. The protocol, design, and primary results have been previously published [11, 12].
Initial Change in eGFR Tertiles
Regardless of treatment allocation, the entire trial population was categorized into tertiles defined by week 6 change in eGFR from baseline (mL/min per 1.73 m2), tertile 1: change in eGFR >+1.00; tertile 2: change in eGFR >−5.99 and ≤+1.00; and tertile 3: change in eGFR of ≤−6.00.
We report the baseline characteristics by the eGFR “dip” tertile. We also evaluated the odds ratio for the ertugliflozin group of having a decrease in eGFR ≥6 mL/min/1.73 m2 at week 6 for the overall population and by baseline subgroups.
Kidney Function, Glucosuria, and Natriuresis Measures by Tertile
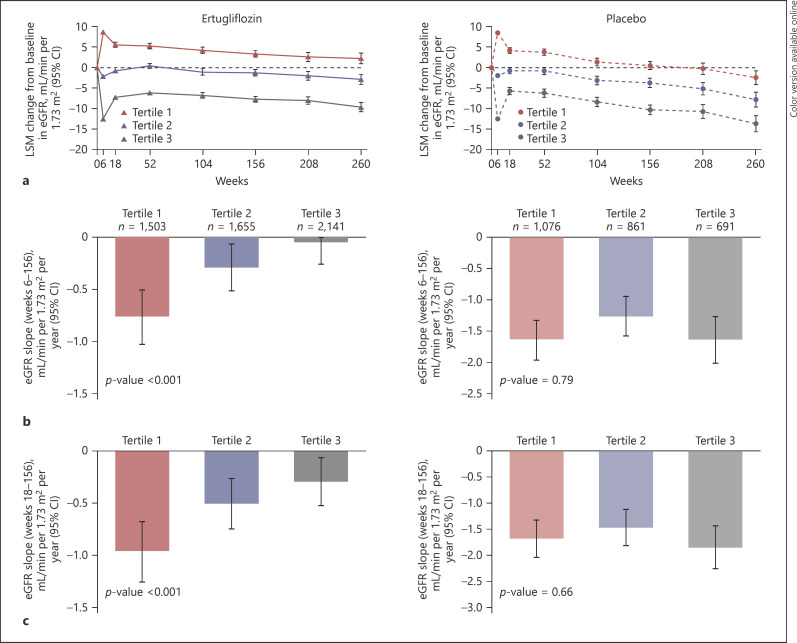
The eGFR was calculated using the Modification of Diet in Renal Disease formula. Chronic eGFR slope analyses evaluated the rate of change in eGFR per year from week 6 to weeks 104, 156, 208, and 260. As there is a tendency for a return towards baseline at week 18 (possible regression to the mean in both ertugliflozin and placebo groups, Fig. 1a), the chronic eGFR slope was also evaluated from week 18 to weeks 104, 156, 208, and 260. For the analyses of urinary albumin-to-creatinine ratio (UACR) over time, the percent change from baseline in the geometric mean of UACR is reported. UACR analyses in the overall population and in patients with baseline normoalbuminuria (UACR <30 mg/g) or elevated albuminuria (UACR ≥30 mg/g) were performed.
Fig. 1.
LSM change from baseline in eGFR over time by tertile of 6-week change in eGFR (a) and chronic yearly eGFR slope from week 6 to 156 by tertile of 6-week change in eGFR (b). Chronic yearly eGFR slope from week 18 to 156 by tertile of 6-week change in eGFR (c). CI, confidence interval; eGFR, estimated glomerular filtration rate; LSM, least squares mean.
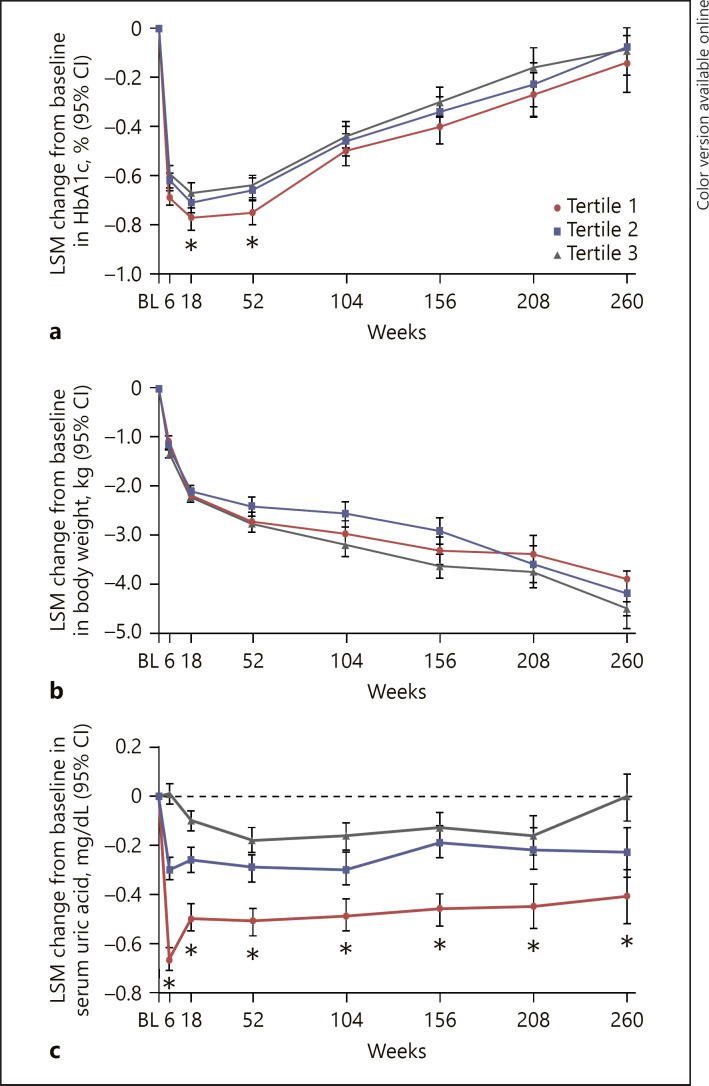
Change from baseline at weeks 6, 18, 52, 104, 156, 208, and 260 in glucosuria-dependent measures (glycated hemoglobin [HbA1c], serum uric acid, and body weight), and natriuresis/volume-related measures (UACR [not measured at week 6], hematocrit, sitting systolic blood pressure [SBP], and diastolic BP) were analyzed for each tertile by treatment.
Placebo-adjusted differences were not performed for these analyses, as tertile categories were dependent on an ertugliflozin treatment effect occurring after randomization, representing a nonrandomized comparison, an approach consistent with similar prior studies [7, 8, 9]. Data from those randomized to placebo are presented to contrast with the results.
Safety by Tertile
Safety evaluation was based on adverse event (AE) reporting by trial personnel at investigational sites. The number and percentage of participants with AEs during the acute phase (baseline–week 6) and chronic phase (week 6–30 days after last dose of trial medication) are presented by tertile by treatment. AEs, serious AEs (SAEs), AEs leading to discontinuation of study medication, and kidney-related AEs were assessed. Kidney-related AEs were evaluated using the acute renal failure standardized Medical Dictionary for Regulatory Activities (MedDRA) query, and the single MedDRA Preferred Term (MedDRA PT) AKI. Hyperkalemia AEs were evaluated using the single MedDRA PTs hyperkalemia and blood potassium increased. The incidence of patients with a sustained ≥40% decline in eGFR was tabulated.
Statistical Analyses
The analyses were performed in the full analysis set (randomized patients who received ≥1 dose of trial medication and had ≥1 post-baseline measurement) for the eGFR, UACR, and kidney-related measures. Data obtained after adjustments of glycemic therapy were included. Data obtained more than 2 days after the last dose of trial medication were excluded for the change from baseline of the eGFR and UACR analyses. Analyses in subgroups by tertile required patients to have baseline and week 6 eGFR values. Mean changes from baseline over time by tertile were estimated using the repeated measures analysis of covariance method.
The repeated measures analysis of covariance model was adjusted for the baseline value of the outcome variable, baseline HbA1c, treatment, tertile, treatment-by-tertile interaction, and treatment-by-tertile-by-visit interaction. Visit was treated as a categorical variable. An unstructured covariance matrix was used to model the correlation among repeated measurements. The p values (pInteraction) of tertile difference during the entire treatment period in the ertugliflozin-treated group were estimated from treatment-by-tertile-by-visit interaction by slice statement in SAS software. Owing to the non-normal distribution of UACR, log-transformation of UACR data was performed. The mean percentage changes derived from the exponentiation of adjusted estimates are presented.
Slopes for change in eGFR over time were analyzed by generalized random coefficient models. Models included eGFR values as a response variable, with treatment, time, baseline HbA1c, baseline eGFR, and treatment-by-time interaction as linear covariates. Time was treated as the continuous variable. The models allowed individual participant slopes to vary by random effects of intercept and time. The p value of eGFR slopes by tertile in the ertugliflozin or placebo groups was estimated by models including eGFR values as a response variable, with treatment, time, tertile, baseline HbA1c, baseline eGFR, treatment-by-time interaction, treatment-by-tertile interaction, and treatment-by-tertile-by-time interaction as linear covariates. Time and tertile were treated as the continuous variables.
For the safety analyses of AEs, Fisher's exact test was performed for testing the relationship of AE occurrence and eGFR change tertile at week 6 separately for the treatment groups based on the safety population. Except for the safety analyses, the analyses were prespecified and exploratory which were not adjusted for multiple comparisons. The safety analyses were post hoc and not adjusted for multiple comparisons. Baseline characteristics are summarized using descriptive statistics for all randomized patients. Logistic regression analyses were performed to evaluate the association of having a decrease in eGFR ≥6.0 mL/min/1.73 m2 from baseline at week 6 (Yes/No) by selected baseline subgroups. The logistic regression model included baseline subgroup, treatment, and baseline subgroup-by-treatment interaction. Missing data were not imputed. All analyses were performed using SAS version 9.4 (SAS Institute Inc.).
Results
Distribution by Tertiles
At week 6, the distribution by tertile for all participants with an eGFR value at baseline and week 6 (n = 7,927) was 32.5%, 31.7%, and 35.7% in tertiles 1, 2, and 3, respectively. Of the 5,299 patients randomized to the pooled ertugliflozin group, 28.4%, 31.2%, and 40.4% were in tertiles 1, 2, and 3, respectively. Of the 2,628 patients randomized to placebo, 40.9%, 32.8%, and 26.3% were in tertiles 1, 2, and 3, respectively (online suppl. Fig. 1; see www.karger.com/doi/10.1159/000524889 for all online suppl. material).
Baseline Characteristics by Week 6 eGFR Change by Tertile in the Pooled Ertugliflozin Group
Most baseline characteristics were similar between the three tertiles in the ertugliflozin group (Table 1). Mean eGFR (mL/min per 1.73 m2) at baseline in the ertugliflozin group was 74, 71, and 81 in tertiles 1, 2, and 3, respectively. Mean SBP (mm Hg) at baseline in the ertugliflozin group was 132, 133, and 135 in tertiles 1, 2, and 3, respectively.
Table 1.
Baseline characteristics by 6-week change in eGFR tertile for both randomized treatments
| Tertile (change in eGFR from baseline at week 6; mL/min per 1.73 m2) | Ertugliflozin |
Placebo |
||||
|---|---|---|---|---|---|---|
| Tertile 1 (change in eGFR >+1.00) | Tertile 2 (change in eGFR >−5.99 and ≤+1.00) | Tertile 3 (change in eGFR of ≤–6.00) | Tertile 1 (change in eGFR >+1.00) | Tertile 2 (change in eGFR >−5.99 and ≤+1.00) | Tertile 3 (change in eGFR of ≤–6.00) | |
| N (%) | 1,503 (28.4) | 1,655 (31.2) | 2,141 (40.4) | 1,076 (40.9) | 861 (32.8) | 691 (26.3) |
| LSM in eGFR from baseline at week 6, mL/min per 1.73 m2 (95% CI) | 8.69 (8.38, 9.00) | −2.17 (−2.47,–1.88) | −12.46 (−12.72,–12.20) | 8.55 (8.18,8.92) | −1.91 (−2.32,–1.50) | −12.49 (−12.95,–12.03) |
| Female, n (%) | 461 (30.7) | 472 (28.5) | 627 (29.3) | 319 (29.6) | 262 (30.4) | 219 (31.7) |
| Age, years | 63.7 (8.0) | 64.9 (8.1) | 64.3 (8.1) | 64.2 (8.2) | 65.2 (7.6) | 63.5 (8.1) |
| Duration of DM, years | 12.5 (8.3) | 12.8 (8.1) | 13.1 (8.2) | 13.4 (8.5) | 13.4 (8.3) | 12.3 (8.1) |
| HbA1c, % | 8.3 (1.0) | 8.2 (0.9) | 8.2 (1.0) | 8.3 (1.0) | 8.2 (0.9) | 8.2 (0.9) |
| Body weight, kg | 90.9 (17.8) | 91.2 (17.7) | 92.8 (19.5) | 91.4 (17.5) | 93.3 (19.3) | 91.0 (17.8) |
| Body mass index, kg/m2 | 31.8 (5.2) | 31.7 (5.1) | 32.2 (5.6) | 31.8 (5.4) | 32.5 (5.6) | 31.5 (5.3) |
| Median UACR, mg/g (IQR) | 17.0 (6.0–59.0) | 18.0 (6.0–68.0) | 20.0 (7.0–78.5) | 21.0 (6.0–66.5) | 19.0 (6.0–71.0) | 17.0 (7.0–63.0) |
| SBP, mm Hg | 131.9 (13.5) | 133.3 (13.6) | 134.6 (13.6) | 132.3 (14.0) | 133.4 (13.9) | 133.6 (13.6) |
| Hematocrit, % | 43.6 (4.2) | 43.1 (4.1) | 42.7 (4.0) | 43.1 (4.0) | 42.7 (4.3) | 42.9 (4.0) |
| eGFR, mL/min per 1.73 m2 | 74.2 (19.2) | 71.3 (20.0) | 81.1 (21.5) | 72.4 (19.7) | 71.7 (18.7) | 86.2 (21.3) |
| Serum uric acid, mg/dL | 5.6 (1.6) | 5.7 (1.6) | 5.6 (1.5) | 5.8 (1.7) | 5.8 (1.6) | 5.4 (1.5) |
| Serum albumin, mg/dL | 4.5 (0.3) | 4.4 (0.3) | 4.4 (0.3) | 4.4 (0.3) | 4.4 (0.3) | 4.4 (0.3) |
| CKD stage 3 (eGFR <60 mL/min per 1.73 m2), n (%) | 335 (22.3) | 487 (29.4) | 332 (15.5) | 279 (25.9) | 235 (27.3) | 66 (9.6) |
| CKD stage 2 (eGFR ≥60 and <90 mL/min per 1.73 m2), n (%) | 863 (57.4) | 867 (52.4) | 1,096 (51.2) | 587 (54.6) | 470 (54.6) | 337 (48.8) |
| CKD stage 1 (eGFR ≥90 mL/min per 1.73 m2), n (%) | 305 (20.3) | 301 (18.2) | 713 (33.3) | 210 (19.5) | 156 (18.1) | 288 (41.7) |
| Normoalbuminuria (UACR <30 mg/g), n (%) | 900 (61.3) | 960 (59.7) | 1,209 (58.1) | 623 (59.2) | 499 (59.1) | 407 (60.5) |
| Elevated albuminuria (UACR ≥30 mg/g), n (%) | 568 (38.7) | 649 (40.3) | 871 (41.9) | 429 (40.8) | 346 (40.9) | 266 (39.5) |
| KDIGO low risk,a n (%) [20] | 720 (49.0) | 732 (45.4) | 1,066 (51.3) | 472 (45.0) | 396 (46.8) | 383 (56.9) |
| KDIGO moderate risk,b n (%) [20] | 488 (33.2) | 501 (31.1) | 657 (31.6) | 358 (34.1) | 263 (31.1) | 207 (30.8) |
| KDIGO high/very high risk, c n (%) [20] | 261 (17.8) | 378 (23.5) | 357 (17.2) | 220 (21.0) | 187 (22.1) | 83 (12.3) |
| Insulin use, n (%) | 636 (42.3) | 813 (49.1) | 1,013 (47.3) | 529 (49.2) | 440 (51.1) | 311 (45.0) |
| Metformin use, n (%) | 1,172 (78.0) | 1,209 (73.1) | 1,652 (77.2) | 842 (78.3) | 658 (76.4) | 541 (78.3) |
| RAAS inhibitor use, n (%) | 1,192 (79.3) | 1,343 (81.1) | 1,757 (82.1) | 879 (81.7) | 707 (82.1) | 558 (80.8) |
| Diuretic use, n (%) | 616 (41.0) | 692 (41.8) | 956 (44.7) | 459 (42.7) | 391 (45.4) | 292 (42.3) |
Data are mean (standard deviation) unless otherwise specified. CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; IQR, interquartile range; KDIGO CKD, Kidney Disease: Improving Global Outcomes in Chronic Kidney Disease; LSM, least squares mean; RAAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure; UACR, urinary albumin-to-creatinine ratio.
Baseline eGFR ≥60 mL/min per 1.73 m2 and UACR <30 mg/g.
Baseline eGFR ≥60 mL/min per 1.73 m2 and UACR ≥30 and ≤300 mg/g or if baseline eGFR ≥45 and <60 mL/min per 1.73 m2 and UACR <30 mg/g.
Baseline eGFR ≥60 mL/min per 1.73 m2 and UACR >300 mg/g or if baseline eGFR ≥45 and <60 mL/min per 1.73 m2 and UACR >30 mg/g or if baseline eGFR <45 mL/min per 1.73 m2.
Overall, the odds ratio (95% confidence interval [CI]) for the ertugliflozin-treated group to have a decrease in eGFR ≥6.0 mL/min/1.73 m2 from baseline at week 6 was 1.90 (1.71, 2.11). The baseline subgroups associated with significantly higher odds of having a decrease in eGFR ≥6.0 mL/min/1.73 m2 from baseline at week 6 were eGFR <60 mL/min/1.73 m2, age ≥ the median (65.0 years), serum uric acid ≥ the median (5.5 mg/dL), the high or very high Kidney Disease: Improving Global Outcomes in Chronic Kidney Disease (KDIGO CKD) risk category, use of insulin, body weight ≥ the median (90.1 kg), duration of diabetes ≥ the median (11.6 years), and SBP ≥ the median (133.3 mm Hg) (Pinteraction < 0.05; online suppl. Fig. 2).
Kidney Function Measures by Tertile
At week 6, least squares mean (LSM) changes from baseline in eGFR {mL/min per 1.73 m2 (95% CI)} were 8.69 (8.38, 9.00), −2.17 (−2.47, −1.88), and −12.46 (−12.72, −12.20) in tertiles 1, 2, and 3, respectively, in the ertugliflozin group (Fig. 1a; online suppl. Table 1). Similar changes from baseline in eGFR at week 6 were observed in the tertiles in the placebo group. Following the initial eGFR change at week 6, there was a tendency towards a return to baseline at week 18 in all groups, followed by an apparent slower rate of eGFR decline over time in all tertile groups when comparing ertugliflozin versus placebo (Fig. 1a). Chronic eGFR slopes from weeks 6 to 156 (mL/min per 1.73 m2 per year [95% CI]) were −0.76 (−1.03, −0.50), −0.29 (−0.51, −0.07), and −0.05 (−0.26, 0.17) in tertiles 1, 2, and 3, respectively, in the ertugliflozin group (p value <0.001; Fig. 1b; online suppl. Table 1). Chronic eGFR slopes were approximately −1.5 mL/min per 1.73 m2/year across tertiles in the placebo group (p value = 0.79).
Chronic eGFR slopes from weeks 18 to 156 (mL/min per 1.73 m2 per year [95% CI]) were −0.97 (−1.25, −0.68), −0.50 (−0.74, −0.27), and −0.29 (−0.52, −0.07) in tertiles 1, 2, and 3, respectively, in the ertugliflozin group (p value < 0.001; Fig. 1c; online suppl. Table 1). Chronic eGFR slopes from weeks 18 to 156 (mL/min per 1.73 m2 per year [95% CI]) were −1.68 (−2.03, −1.34), −1.47 (−1.80, −1.13), and −1.85 (−2.24, −1.45) in tertiles 1, 2, and 3, respectively, in the placebo group (p value = 0.66).
Ertugliflozin and Glucosuria-Mediated Measures by Tertile
At week 18, LSM changes from baseline in HbA1c (% [95% CI]) were −0.77 (−0.82, −0.73), −0.71 (−0.75, −0.67), and −0.67 (−0.70, −0.63) in tertiles 1, 2, and 3, respectively, in the ertugliflozin group. Reductions from baseline in HbA1c decreased over time, but the difference between tertiles remained (pinteraction < 0.0001; Fig. 2a).
Fig. 2.
LSM change from baseline in glucosuria-related measures by tertile of 6-week eGFR change in the pooled ertugliflozin group: HbA1c (a), body weight (b), and serum uric acid (c). BL, baseline; CI, confidence interval; HbA1c, glycated hemoglobin; LSM, least squares mean.*95% CIs between tertiles 1 and 3 do not overlap. PInteraction < 0.001 for all measures.
Body weight decreased in all tertiles in the ertugliflozin group; at week 260, LSM changes from baseline in body weight (kg [95% CI]) were −3.89 (−4.36, −3.42), −4.19 (−4.65, −3.74), and −4.50 (−4.91, −4.09) in tertiles 1, 2, and 3, respectively (Pinteraction < 0.0001; Fig. 2b). At week 6, the LSM changes in serum uric acid (mg/dL [95% CI]) from baseline were −0.67 (−0.71, −0.62), −0.30 (−0.34, −0.25), and 0.01 (−0.03, 0.05) in tertiles 1, 2, and 3, respectively (pinteraction < 0.0001; Fig. 2c). The differences followed a similar pattern for the duration of the trial.
Ertugliflozin and Natriuresis-Related Measures by Tertile
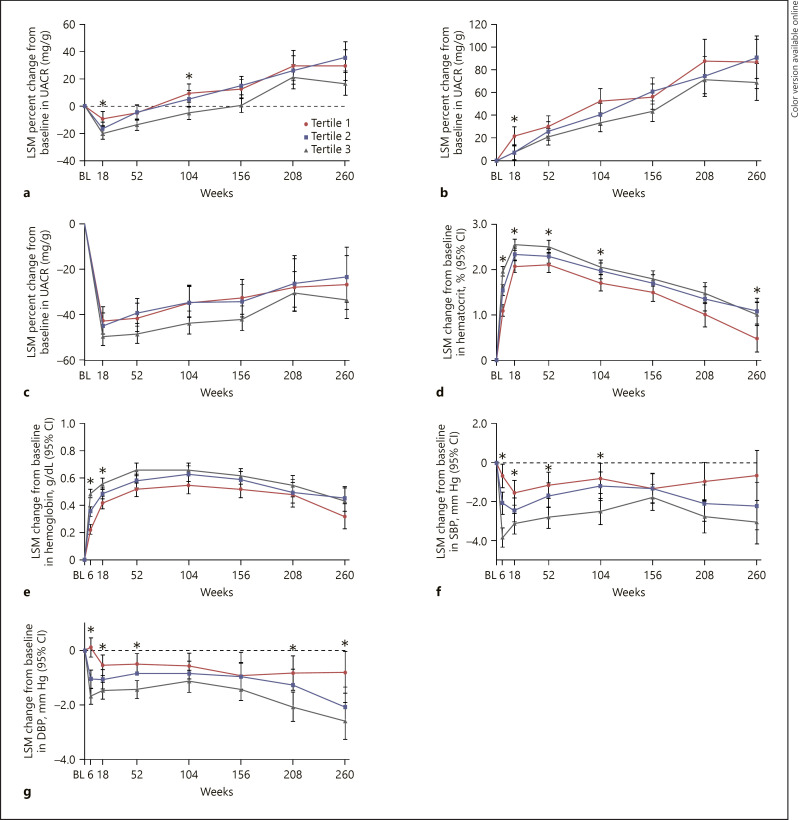
At week 18, the LSM percent changes from baseline in geometric mean UACR (95% CI) were −9.4 (−14.5, −4.0), −16.6 (−21.1, −11.9), and −20.1 (−23.9, −16.2) in tertiles 1, 2, and 3, respectively, in the ertugliflozin group (Fig. 3a). Similar trends with respect to changes from baseline in UACR were observed in the ertugliflozin group across tertiles in patients with normoalbuminuria or elevated albuminuria at baseline (Fig. 3b and c).
Fig. 3.
LSM percent change from baseline in UACR by tertile of 6-week change in eGFR in the pooled ertugliflozin group in all patients (a), patients with normoalbuminuria at baseline (b), patients with elevated albuminuria at baseline and LSM change from baseline in natriuresis-related measures by tertile of 6-week eGFR change from baseline in the pooled ertugliflozin group (c): hematocrit (d), hemoglobin (e), SBP (f), and DBP (g). BL, baseline; CI, confidence interval; DBP, diastolic blood pressure; LSM, least squares mean; SBP, systolic blood pressure; UACR, urinary albumin-to-creatinine ratio. *95% CIs between tertiles 1 and 3 do not overlap.
At week 6, LSM changes from baseline in hematocrit (% [95% CI]) were 1.09 (0.97, 1.22), 1.54 (1.43, 1.66), and 1.96 (1.86, 2.06) in tertiles 1, 2, and 3, respectively, in the ertugliflozin group (pinteraction < 0.0001; Fig. 3d). The differences followed a similar pattern for the duration of the trial. Similar findings were observed for the changes in hemoglobin (pinteraction < 0.0001; Fig. 3e).
At week 6, LSM changes from baseline in SBP (mm Hg [95% CI]) were −0.68 (−1.27, −0.10), −2.06 (−2.62, −1.51), and −3.83 (−4.32, −3.35) in tertiles 1, 2, and 3, respectively, in the ertugliflozin group; these differences between the tertiles were observed up to week 104 (Pinteraction < 0.0001; Fig. 3f). Similar findings were observed for the changes in DBP (Pinteraction < 0.0001; Fig. 3g).
Safety by Tertile
There were no significant differences by tertile in both the ertugliflozin and placebo cohorts during the acute phase (week 0 to week 6; online suppl. Table 2). During the chronic phase (week 6 to 30 days post-study drug discontinuation), kidney-related AEs for ertugliflozin were lower in tertile 1 than in tertiles 2 and 3; 2.9%, 4.8%, and 4.5%, respectively (Fisher-exact p value = 0.015; online suppl. Table 3). In the placebo cohort, kidney-related AEs were 3.8%, 5.8%, and 5.1% for tertiles 1, 2, and 3, respectively (Fisher-exact p value = 0.108). The incidence of kidney-related SAEs and discontinuations due to kidney-related events were low and similar across all tertiles in both ertugliflozin and placebo cohorts. No significant differences between tertiles were observed for other AE assessments.
The proportion of patients with a sustained ≥40% decrease from baseline in eGFR was highest in tertile 3 and lowest in tertile 1 in the ertugliflozin and placebo groups (online suppl. Table 4). The proportions were lower in the ertugliflozin groups than in the placebo groups: 0.86% and 1.67% for ertugliflozin and placebo in tertile 1, respectively; 1.45% and 2.90%, for the ertugliflozin and placebo groups in tertile 2, respectively; and 3.36% and 5.79%, for the ertugliflozin and placebo groups in tertile 3, respectively.
Discussion
Although the eGFR “dip” observed after SGLT2 inhibitor initiation is a recognized phenomenon, it does not occur in all patients. In previous work, about one-fifth to one-third of patients did not experience an eGFR “dip” [7, 9]. The clinical relevance of the eGFR “dip” has not been well described. In line with the mechanism responsible for the eGFR “dip” consistent with a decline in glomerular pressure, our main observation was that those with the largest eGFR “dip” had the most stable eGFR slope over time. In addition, the degree of the eGFR “dip” was associated with changes in clinical parameters related to natriuresis and glucosuria.
The eGFR “dip” may represent a decline in glomerular hypertension via vasoconstriction of the glomerular afferent arteriole [13], although other pathways may exist [14]. Regardless of the physiological factors responsible, the eGFR “dip” should be understood both in the context of efficacy and safety. RAAS inhibitors also induce an initial eGFR “dip,” an effect linked with subsequent kidney protection in people with albuminuric diabetic kidney disease (DKD) [10]. Consistent with RAAS inhibitor data, tertile 3 in the ertugliflozin group (with the largest eGFR “dip”) had the slowest chronic yearly eGFR slope decline after week 6 compared with other tertiles in the ertugliflozin group. This finding was also observed when evaluating the chronic slope after week 18, despite the potential effect of regression to the mean observed in all tertiles in both ertugliflozin and placebo between week 6 and week 18 (Fig. 1a), where patients randomized to ertugliflozin in tertile 3 (the tertile with the largest initial eGFR dip) still had a slower rate of eGFR decline from week 18 to weeks 104, 156, 208 and 260 (online suppl. Table 1). In the CREDENCE trial involving patients with type 2 diabetes mellitus and established albuminuric DKD, eGFR decrease groups had numerically lower rates of eGFR decline from baseline than other patients randomized to canagliflozin, although between-group differences were not significant [9]. However, chronic slope from week 13 onward was attenuated in patients treated with canagliflozin compared with placebo.
In the current analysis, the proportion of patients with a sustained ≥40% eGFR decrease was higher in tertile 3 for both ertugliflozin and placebo groups. The placebo tertile groups had 1.7- to 2-fold higher frequencies than the corresponding ertugliflozin tertile groups, suggestive of a consistent treatment effect on eGFR preservation across eGFR “dip” tertiles.
The eGFR “dip” may reflect the natriuretic response to SGLT2 inhibition [15]. SGLT2 inhibition induces a transient natriuresis; however, effects on other natriuresis measures persist over a longer term [16]. Consistent with this hypothesis was the observation that patients randomized to ertugliflozin in tertile 3 (those with a greater decrease in eGFR at week 6) exhibited larger reductions in BP and UACR, and greater increases in hematocrit, compared with patients in tertiles 1 and 2, suggesting kidney, hemodynamic, and volume-related effects. Although these differences were of a small magnitude and of uncertain clinical significance, they reflect underlying physiological mechanisms that result in differential clinical parameter changes.
There were also differences in glucosuria-related parameters between patients in the various tertiles in the ertugliflozin group. Tertile 1 (those with an increase in eGFR at week 6) had significantly greater reductions in HbA1c and serum uric acid. Although the relationship between increased glucosuria and greater HbA1c reduction seems self-evident, the observation that patients in tertile 1 exhibited more HbA1c lowering may be based on more glucose filtration and hence excretion compared with the other tertiles. Similarly, as tubular glucose filtration leads to more uric acid secretion in the proximal tubule, the increase in GFR in tertile 1 may account for the exaggerated reduction in uric acid levels [17, 18, 19]. Finally, body weight reduction was greater in tertile 3. As body weight reductions can be influenced by volume or by fat loss, the initial greater weight loss in tertile 3 is suggestive of enhanced volume loss related to natriuresis, although cannot be further elucidated based on the clinical data available from VERTIS CV.
It is not known whether the direction and magnitude of the eGFR “dip” reflects underlying comorbidities or other factors, such as dietary sodium intake. At baseline, patients treated with ertugliflozin with stage 3 CKD, older age, higher uric acid, and in the high/very high KDIGO CKD risk category had a higher likelihood of having the largest decrease in eGFR at baseline (Pinteraction < 0.01); other baseline characteristics with a significant Pinteraction (<0.05) included use of insulin, greater body weight, longer duration of diabetes, and higher SBP. In a previous analysis from EMPA-REG OUTCOME, the baseline predictors of the largest dip were diuretic use, higher KDIGO CKD risk categories, and lower eGFR categories [7]. Regardless of the mechanisms responsible for eGFR dipping, this change in kidney function is unlikely to represent AKI but instead seems to identify those individuals who exhibit a slower rate of eGFR decline over time.
The incidence of AEs was generally similar in the ertugliflozin and placebo groups. In the acute period, the safety review of kidney-related AEs did not identify a safety concern. In the chronic period, a significant Fisher-exact p value in kidney-related AEs was observed in the ertugliflozin groups, which was due to a difference in incidence of AEs in tertile 1 (lower incidence) compared with tertiles 2 and 3; however, AEs (including SAEs) were similar between tertiles 2 and 3 in both the ertugliflozin and placebo cohorts. Of the cases of AKI SAEs in the ertugliflozin-treated subjects, nearly all seemed to be secondary to other concomitant illnesses (e.g., heart failure, myocardial infarction, malignancy) that could increase AKI risk.
The population in this analysis, like the population in the analysis from EMPA-REG OUTCOME [7], has a lower risk for kidney disease than the population from the CREDENCE study [9]. These analyses have limitations. As “dipping” status was defined based on eGFR change at 6 weeks, the analyses were not based on patients being randomized to eGFR categories. The classification of “dipping status” is a post-randomization stratification by a factor directly affected by the intervention, with all associated limitations of such stratified analyses. Although “dipping” status was associated with differential statistically significant effects on natriuresis- and glucosuria-related parameters, these changes may not have been clinically relevant. These were prespecified exploratory measures but were not controlled for type 1 error, while analyses of safety measures were post hoc and also not controlled for type 1 error. The number of participants decreased through the observation period, limiting the interpretability of the results at later time points. Finally, UACR does have inherent limitations due, in part, to variability of single UACR measures. Nevertheless, VERTIS CV included a large sample size, which mitigated some of the inherent limitations around the use of single UACR measurements at each time point. This analysis highlights the heterogeneity in responses seen in clinical trials, and the importance of characterizing these differences to further understand the efficacy and safety of medications.
In conclusion, in these analyses of data from VERTIS CV, the early eGFR “dip” was associated with kidney function preservation after week 6, possibly due to changes in glomerular hemodynamics due to treatment with ertugliflozin. eGFR “dipping” was also associated with larger natriuresis-related clinical effects and attenuation of glucosuria-linked factors. The degree of eGFR “dip” was not associated with meaningful safety findings. In VERTIS CV, the eGFR “dip” does not affect the sustained ≥40% decline in eGFR outcome compared with placebo.
Statement of Ethics
In collaboration with a group of academic investigators comprising the scientific advisory committee, representatives of the sponsors (Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and Pfizer Inc.) designed and oversaw the conduct of the trial in accordance with the ethical principles that have their origin in the Declaration of Helsinki, and that are consistent with Good Clinical Practice and the applicable regulatory requirements. The study was approved by the appropriate institutional review boards and regulatory agencies, with all participants providing written informed consent.
Conflict of Interest Statement
D.Z.I. Cherney has received consulting fees or speaking honoraria, or both, from Bristol Myers Squibb, Novo Nordisk, Mitsubishis-Tanabe, MAZE, Janssen, Bayer, Boehringer Ingelheim–Eli Lilly, AstraZeneca, Merck & Co., Inc., Prometic, and Sanofi, and has received operating funds from Janssen, Boehringer Ingelheim–Eli Lilly, Sanofi, AstraZeneca, and Merck & Co., Inc. F. Cosentino has received fees from Abbott; AstraZeneca; Bayer; Boehringer Ingelheim; Bristol Myers Squibb; Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc, Rahway, NJ, USA; Novo Nordisk; and Pfizer, as well as research grants from Swedish Research Council, Swedish Heart & Lung Foundation, and the King Gustav V and Queen Victoria Foundation. S. Dagogo-Jack has led clinical trials for AstraZeneca, Novo Nordisk, Inc., and Boehringer Ingelheim, has received fees from AstraZeneca, Boehringer Ingelheim, Janssen, Merck & Co. Inc., and Sanofi, and holds equity interests in Jana care, Inc. and Aerami Therapeutics. D.K. McGuire has received honoraria for leadership roles in clinical trials for AstraZeneca, Boehringer Ingelheim, Eisai, Esperion, GlaxoSmithKline, Janssen, Lexicon, Merck & Co., Inc., Novo Nordisk, CSL Behring, and Sanofi USA, and has received consultancy fees from AstraZeneca, Boehringer Ingelheim, Lilly USA, Merck & Co., Inc., Bayer, Pfizer, Novo Nordisk, Metavant, Afimmune, Lexicon, Applied Therapeutics, and Sanofi USA. R.E. Pratley has received the following (directed to his institution): speaker fees from Novo Nordisk; consulting fees from Merck, Novo Nordisk, Pfizer, Sanofi, Scohia Pharma Inc., and Sun Pharmaceutical Industries; and grants from Lexicon Pharmaceuticals, Hanmi Pharmaceuticals Co., Novo Nordisk, Poxel SA, and Sanofi. R. Frederich is an employee and shareholder of Pfizer Inc. M. Maldonado is an employee of MSD UK, who may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. C.-C. Liu and A. Pong are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who may own stock and/or stock options in the Company. C.P. Cannon reports research grants and consulting fees from Pfizer Inc. and Merck & Co., Inc.; and research grants and consulting fees from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, and Janssen, research grants from Better Therapeutics, Daiichi Sankyo, and Novo Nordisk, and consulting fees from Aegerion/Amryt, Alnylam, Amarin, Applied Therapeutics, Ascendia, Lexicon, Sanofi, Eli Lilly, and Rhoshan, and reports serving on the Data and Safety Monitoring Boards for the Veteran's Administration, Applied Therapeutics and NovoNordisk, outside the submitted work.
Funding Sources
The VERTIS CV trial and these analyses were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, in collaboration with Pfizer Inc.
Author Contributions
D.Z.I.C., S.D.-J., D.K.M., M.M., and C.P.C. substantially contributed to the conception, design, or planning of the study. D.K.M. and R.F. substantially contributed to the acquisition of the data. D.Z.I.C., S.D.-J., M.M., C.-C.L., A.P., and C.P.C. substantially contributed to the analysis of the data. D.Z.I.C., F.C., S.D.-J., D.K.M., R.E.P., R.F., M.M., C.-C.L., and C.P.C. substantially contributed to the interpretation of the data. All authors substantially contributed to the drafting of the manuscript and/or critically reviewing or revising the manuscript for important intellectual content.
Data Availability Statement
Data sharing policy of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical trial data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Supplementary Material
Supplementary data
Acknowledgments
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, in collaboration with Pfizer Inc., New York, NY, USA. The authors would like to thank the patients, their families, and all investigators involved in the VERTIS CV trial. Medical writing and/or editorial assistance was provided by Moamen Hammad, PhD, and Ian Norton, PhD, both of Scion, London, UK. This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and Pfizer Inc., New York, NY, USA. The authors would like to acknowledge Jie Liu, MD, PhD, of Merck & Co Inc., Rahway, NJ, USA, for his review of an early draft of the manuscript, Ira Gantz, MD, of Merck & Co Inc., Rahway, NJ, USA, for his input and discussions with the authors, Ingrid Adamson of Merck & Co Inc., Rahway, NJ, USA, for her support with the safety analyses, and Philip Jones of Pfizer Inc., New York, NY, USA, for his support with the safety analyses. Some of these results have been presented at the American Society of Nephrology Kidney Week 2020 Reimagined congress. The sponsor was involved in the trial design and collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
References
- 1.Cherney DZI, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014 Feb 4;129((5)):587–97. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 2.Heyman SN, Bursztyn M, Szalat A, Muszkat M, Abassi Z. Fasting-induced natriuresis and SGLT: a new hypothesis for an old enigma. Front Endocrinol. 2020 May 7;11:217. doi: 10.3389/fendo.2020.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornstad P, Laffel L, Tamborlane WV, Simons G, Hantel S, von Eynatten M, et al. Acute effect of empagliflozin on fractional excretion of sodium and egfr in youth with type 2 diabetes. Diabetes Care. 2018 Aug;41((8)):e129–30. doi: 10.2337/dc18-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020 May;31((5)):1128–39. doi: 10.1681/ASN.2019111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol. 2018 Nov;29((11)):2755–69. doi: 10.1681/ASN.2018010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lytvyn Y, Bjornstad P, van Raalte DH, Heerspink HL, Cherney DZI. The new biology of diabetic kidney disease-mechanisms and therapeutic implications. Endocr Rev. 2020 Apr 1;41((2)):202–31. doi: 10.1210/endrev/bnz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, et al. Characterization and implications of the initial estimated glomerular filtration rate “dip” upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021 Mar;99((3)):750–62. doi: 10.1016/j.kint.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Kohagura K, Yamasaki H, Takano H, Ohya Y, Seino Y. Luseogliflozin, a sodium-glucose cotransporter 2 inhibitor, preserves renal function irrespective of acute changes in the estimated glomerular filtration rate in Japanese patients with type 2 diabetes. Hypertens Res. 2020 Sep;43((9)):876–83. doi: 10.1038/s41440-020-0426-0. [DOI] [PubMed] [Google Scholar]
- 9.Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021 Apr;99((4)):999–1009. doi: 10.1016/j.kint.2020.10.042. [DOI] [PubMed] [Google Scholar]
- 10.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80((3)):282–7. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, McGuire DK, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV) Am Heart J. 2018 Dec;206:11–23. doi: 10.1016/j.ahj.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020 Oct 8;383((15)):1425–35. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 13.Kidokoro K, Cherney DZI, Bozovic A, Nagasu H, Satoh M, Kanda E, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019 Jul 23;140((4)):303–15. doi: 10.1161/CIRCULATIONAHA.118.037418. [DOI] [PubMed] [Google Scholar]
- 14.van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020 Jan;97((1)):202–12. doi: 10.1016/j.kint.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018 Jul;94((1)):26–39. doi: 10.1016/j.kint.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 16.van Raalte DH, Bjornstad P, Persson F, Powell DR, de Cassia Castro R, Wang PS, et al. The impact of sotagliflozin on renal function, albuminuria, blood pressure, and hematocrit in adults with type 1 diabetes. Diabetes Care. 2019 Oct;42((10)):1921–9. doi: 10.2337/dc19-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lytvyn Y, Perkins BA, Cherney DZI. Uric acid as a biomarker and a therapeutic target in diabetes. Can J Diabetes. 2015 Jun;39((3)):239–46. doi: 10.1016/j.jcjd.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Lytvyn Y, Škrtić M, Yang GK, Lai V, Scholey JW, Yip PM, et al. Plasma uric acid effects on glomerular haemodynamic profile of patients with uncomplicated type 1 diabetes mellitus. Diabet Med. 2016 Aug;33((3)):1102–11. doi: 10.1111/dme.13051. [DOI] [PubMed] [Google Scholar]
- 19.Lytvyn Y, Škrtić M, Yang GK, Yip PM, Perkins BA, Cherney DZI. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015 Jan 15;308((2)):F77–83. doi: 10.1152/ajprenal.00555.2014. [DOI] [PubMed] [Google Scholar]
- 20.Kidney Disease Improving Global Outcomes (KDIGO) Chapter 1: definition and classification of CKD. Kidney Int Suppl. 2013;3((1)):19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
Data sharing policy of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical trial data can be submitted through the EngageZone site or via email to dataaccess@merck.com.