Key Points
Questions
How is pembrolizumab extended-interval dosing being adopted, and are there differences between it and standard-interval dosing regarding time-to-treatment discontinuation, a real-world measure of clinical effectiveness?
Findings
In this retrospective cohort study of a nationwide, single-payer health system population, approximately 35% of single-agent pembrolizumab regimens used extended-interval dosing. No differences in time-to-treatment discontinuation were observed between extended- and standard-interval dosing.
Meaning
The findings of this cohort study suggest that adoption of extended-interval dosing has plateaued despite its potential advantages to individual patients and health systems; retrospective real-world data augment pharmacokinetic, pharmacodynamic, and existing clinical data to suggest the near-equivalence of standard- vs extended-interval pembrolizumab.
Abstract
Importance
Extended-interval dosing of pembrolizumab (400 mg every 6 weeks) was approved by US Food and Drug Administration (FDA) in April 2020 as an alternative to standard-interval dosing (200 mg every 3 weeks). Extended-interval dosing may enhance access, alleviate patient and health system financial toxicity, and improve patient quality of life, particularly during the COVID-19 pandemic. Neither adoption nor effectiveness of extended interval in the US has been adequately described.
Objective
To describe adoption of extended-interval dosing of pembrolizumab since its FDA approval and to measure its preliminary real-world effectiveness compared with standard-interval dosing.
Design, Setting, and Participants
This was a retrospective cohort study that used data from the Veterans Health Administration (VHA), a US-based, nationwide single-payer health system. Participants were veterans who were prescribed single-agent pembrolizumab within the VHA between April 1, 2020, and July 1, 2021. Patients receiving combinations of pembrolizumab and cytotoxic chemotherapy or tyrosine kinase inhibitors were excluded. A subcohort of veterans with non−small cell lung cancer (NSCLC) was also identified using claims-based codes.
Exposures
Single-agent pembrolizumab at extended or standard intervals.
Main Outcomes and Measures
The number and proportion of single-agent pembrolizumab prescriptions that were extended compared with standard interval. Effectiveness was described in terms of time-to-treatment discontinuation (TTD) and extended- to standard-interval pembrolizumab prescriptions were compared using Cox proportional hazards regression.
Results
A total of 835 veterans (mean age [SD], 70.9 [8.7] years; 809 [96.9%] men) began single-agent pembrolizumab during the study period (all-diseases cohort), and of these, 234 (mean [SD] age, 71.6 [7.3] years; 225 [96.2%] men) had NSCLC (NSCLC cohort). Extended-interval adoption reached its steady state plateau of approximately 35% by January 2021; 65% of participants who began standard-interval single-agent pembrolizumab received only standard-interval dosing during the treatment course. In analysis consistent with the intention-to-treat principle, no differences in TTD were observed between standard- and extended-interval dosing in either the all-diseases cohort (HR, 1.00; 95% CI, 1.00-1.00) or the NSCLC cohort (HR, 1.00; 95% CI, 1.00-1.00).
Conclusions and Relevance
This retrospective cohort study found that extended-interval dosing comprised a minority of single-agent pembrolizumab prescriptions despite the FDA approval and its potential health system and public health benefits. The findings support the TTD equivalence of standard- and extended-interval pembrolizumab across indications, complementing clinical pharmacology and single-arm clinical trial data in melanoma. This study provides further support for extended-interval pembrolizumab dosing.
This retrospective cohort study examines adoption and real-world effectiveness of extended-interval pembrolizumab among a cohort of more than 800 US veterans being treated with single-agent pembrolizumab.
Introduction
The immune checkpoint inhibitor pembrolizumab, first approved by the US Food and Drug Administration (FDA) in 2014, is a standard treatment for nearly 20 diseases. Registrational pembrolizumab trials administered doses every 3 weeks (standard interval) of 200 mg (flat) or 2 mg per kg (weight based).1 Pharmacokinetic modeling2 led the European Medicines Agency to approve 400 mg every 6 weeks dosing (extended interval) of pembrolizumab in March 2019.1 The FDA rejected a supplemental biologics license application for extended-interval pembrolizumab in February 2020 owing to concern that lower trough concentrations would reduce efficacy.3 However, the FDA reversed course in April 2020, approving extended-interval pembrolizumab after preliminary nonprespecified analysis of KEYNOTE-555 cohort B—a single-arm cohort of patients outside of the US (n = 44) receiving extended-interval pembrolizumab for melanoma—demonstrated response and immune-related adverse event rates approximately equivalent to historic standard-interval controls.4
Extended-interval dosing has potential health system advantages that the COVID-19 pandemic heightened. As referenced by the FDA, “given current public health considerations…there is a different risk-benefit calculus.”3 Extended-interval dosing may improve access, mitigate out-of-pocket expenses, and reduce time to therapy initiation for patients.5,6 Insofar as prescribers believe that (1) a patient’s COVID-19 exposure risk and pembrolizumab administration-related financial burdens are issues and that (2) extended- and standard-interval dosing are equivalent, brisk adoption of extended-interval dosing would be expected.
To date, the comparative effectiveness of extended- to standard-interval dosing has been evaluated in only 1 small single-center study7 and its adoption is undescribed. In this retrospective analysis of data from the Veterans Health Administration (VHA), the largest single-payer health system in the US, we describe extended-interval pembrolizumab adoption and examine its real-world comparative effectiveness vs standard-interval dosing.
Methods
Because this study was a nonresearch operations activity and proceeded under a Memorandum of Understanding between the VA Center for Clinical Management and Research and the VA National Oncology Program, it was exempt from review. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Data Sources and Record Selection
Using the US Department of Veterans Affairs (VA) Corporate Data Warehouse, we extracted pembrolizumab administrations occurring between April 1, 2020 and August 24, 2021, along with pembrolizumab start dates, age, sex, codes per the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10), and noncancer Charlson comorbidity index (CCI) components.8 We excluded records showing pembrolizumab coadministered with chemotherapy or tyrosine kinase inhibitors (eMethods and eFigure 1 in the Supplement). To ensure adequate follow-up, we excluded records with incident pembrolizumab after September 6, 2020.
We generated a primary cohort (all diseases; eFigure 2A in the Supplement) and 1 subcohort (non−small cell lung cancer [NSCLC]; eFigure 2B in the Supplement). To generate the NSCLC cohort, we excluded records that in the 6 months before April 1, 2020, had a diagnostic code(s) for cancer(s) other than NSCLC for which pembrolizumab was FDA approved (eTable 1 in the Supplement). Cohort derivation graphical representations were generated with a web-based application.9
Primary Outcome Measures
We evaluated the number and proportion of extended-interval pembrolizumab prescriptions. We used time-to-treatment discontinuation (TTD)—a commonly reported measure available from prescription records, defined as the time between incident prescription and end of the most recent prescription—for comparative effectiveness analysis. The TTD of immune checkpoint inhibitors in NSCLC is correlated with progression-free survival (PFS) and overall survival (OS).10
Secondary Outcome Measures
Incident immune-related adverse events were assessed indirectly using incident levothyroxine and prednisone prescriptions. We assessed the frequency of response assessment using per-patient rates of computed tomography, magnetic resonance imaging, and positron emission tomography orders.
Statistical Analyses
Extended and standard intervals were compared according to incident prescription in a manner consistent with the intention-to-treat principle. Descriptive statistics, Kaplan-Meier statistic and curves, and Cox proportional hazards regression analyses were performed from January 28 to June 22, 2022, using Stata version 17.0 (StataCorp). Tests were 2-tailed and P = .05 was defined as having statistical significance.
Results
Adoption of Extended-Interval Pembrolizumab
After the FDA approval of extended-interval pembrolizumab in April 2020, its adoption rose steadily to 32.6% by January 2021, and this rate was maintained through the end of the analysis period in August 2021 (Figure 1A). Adoption of the extended interval among first pembrolizumab doses followed a similar pattern, reaching peak adoption in June 2020 and maintaining a steady 20% to 25% share of prescriptions from November 2020 onward (Figure 1B). Of patients who began treatment with the standard-interval dose, 65% continued with it for the entire treatment duration (Figure 1C). Of those who began the extended-interval dosing, 95% to 100% continued with it (Figure 1D).
Figure 1. Pembrolizumab Dosing Practices After FDA Approval of Extended-Interval Dosing.
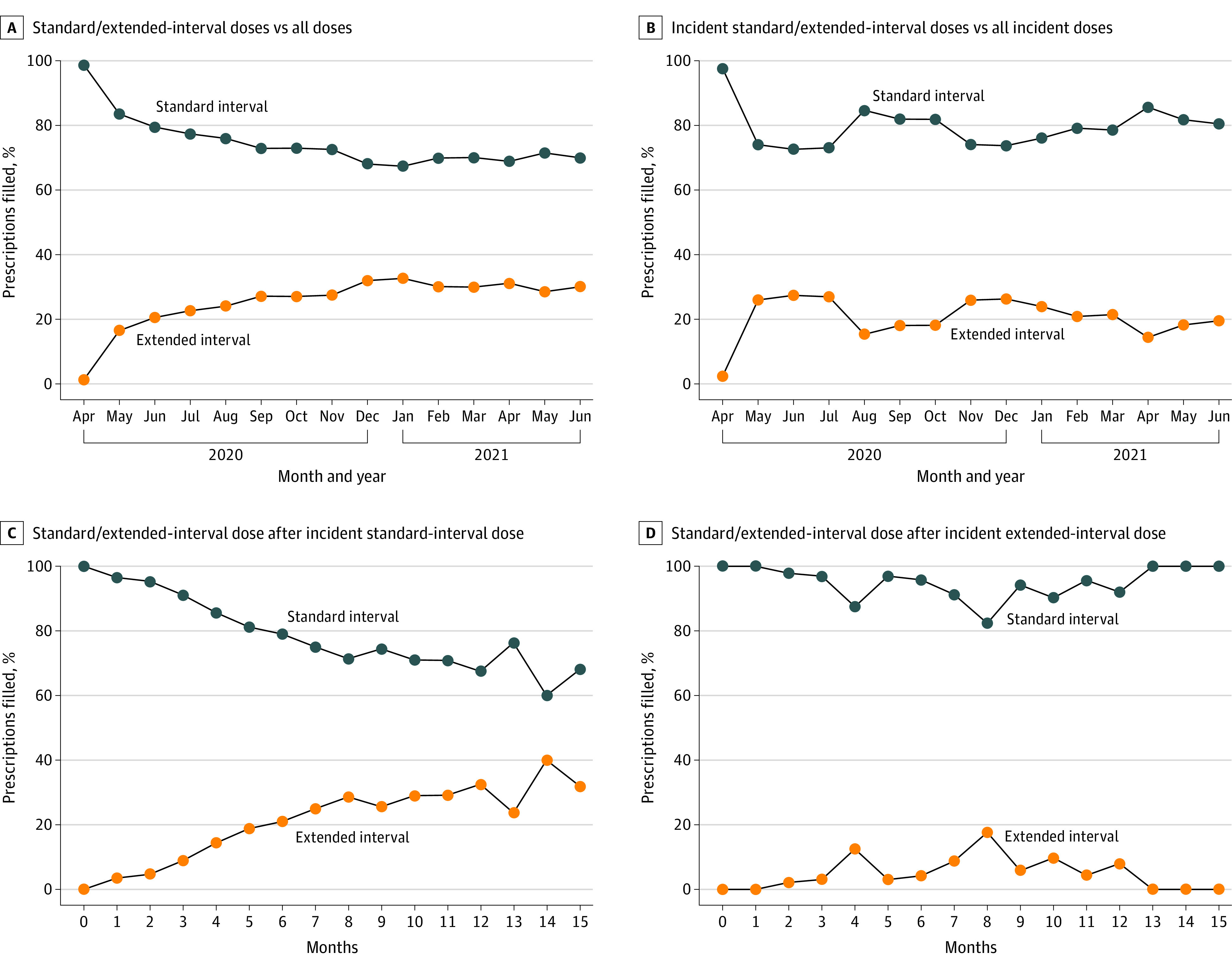
A and B, the x-axis corresponds to the month and year of the study. C and D, the x-axis corresponds to time since incident pembrolizumab. FDA refers to the US Food and Drug Administration.
TTD of Extended vs Standard Intervals
The all-diseases cohort included 835 patients (mean [SD] age, 70.9 (8.7) years; 809 [96.9%] men; data on race and ethnicity were not considered); 676 patients (81.0%) received standard-interval dosing and 159 patients (19.0%) received extended-interval dosing alone (Table). Age, sex, CCI, and pembrolizumab indication were well balanced between the subpopulations. After a median follow-up of 373 days, median TTD was 127.5 days for standard interval dosing and 168 days for extended interval (HR, 1.00; 95% CI, 1.00-1.00; P = .08; Figure 2A; eTable 2 in the Supplement). The TTD outcomes by primary site of disease are summarized in eTable 3 in the Supplement. We did not observe differences in either incident levothyroxine or prednisone prescriptions or response assessment rate (eTables 4 and 5 in the Supplement).
Table. Characteristics of the Comparative Effectiveness Analysis Cohorts of Standard-Interval vs Extended-Interval Dosing of Pembrolizumab.
| Characteristic | No. (%) | P value for differencesa | ||||
|---|---|---|---|---|---|---|
| Standard interval | Standard interval to extended interval | Pooled standard interval | Extended interval | Cohort | ||
| All-diseases cohort | ||||||
| No. | 534 | 142 | 676 | 159 | 835 | |
| Age, mean (SD) | 70.8 (8.5) | 71.1 (10.0) | 70.9 (8.8) | 70.9 (8.5) | 70.9 (8.7) | .99 |
| Male sex | 514 (96.3) | 139 (97.9) | 653 (96.7) | 156 (98.1) | 809 (96.9) | .32 |
| Noncancer CCI | 2.3 (2.0) | 2.2 (1.9) | 2.3 (2.0) | 2.1 (1.8) | 2.3 (2.0) | .25 |
| Primary site | ||||||
| Lung | 238 (44.6) | 59 (41.5) | 297 (43.9) | 76 (47.8) | 373 (44.7) | .38 |
| Bladder | 82 (15.4) | 27 (19.0) | 109 (16.1) | 28 (17.6) | 137 (16.4) | .65 |
| Kidney | 64 (12.0) | 19 (13.4) | 83 (12.3) | 11 (6.9) | 94 (11.3) | .05 |
| Head and neck | 58 (10.9) | 16 (11.3) | 74 (10.9) | 20 (12.6) | 94 (11.3) | .56 |
| Melanoma | 44 (8.2) | 19 (13.4) | 63 (9.3) | 13 (8.2) | 76 (9.1) | .65 |
| Gastroesophageal | 54 (10.1) | 8 (5.6) | 62 (9.2) | 8 (5.0) | 70 (8.4) | .09 |
| Colorectal | 25 (4.7) | 10 (7.0) | 35 (5.2) | 10 (6.3) | 45 (5.4) | .58 |
| Hepatocellular | 26 (4.9) | 4 (2.8) | 30 (4.4) | 9 (5.7) | 39 (4.7) | .51 |
| NSCLC cohort | ||||||
| Age, mean (SD) | 71.8 (7.2) | 71.5 (9.2) | 71.8 (7.5) | 71.2 (6.6) | 71.6 (7.3) | .59 |
| Male sex | 144 (95.4) | 30 (100) | 174 (96.1) | 51 (96.2) | 225 (96.2) | .98 |
| Noncancer CCI | 2.5 (1.9) | 2.3 (1.7) | 2.4 (1.9) | 2.0 (1.7) | 2.3 (1.9) | .12 |
Abbreviations: CCI, Charlson Comorbidity Index; NSCLC, non−small cell lung cancer.
Tests of statistical significance compared pooled standard- and extended-interval cohorts. Summary statistics of patients included in the all-diseases and NSCLC cohorts. No meaningful between-groups differences were identified.
Figure 2. Survival Curves of Extended- and Standard-Interval Pembrolizumab Time-to-Treatment Discontinuation (TTD).
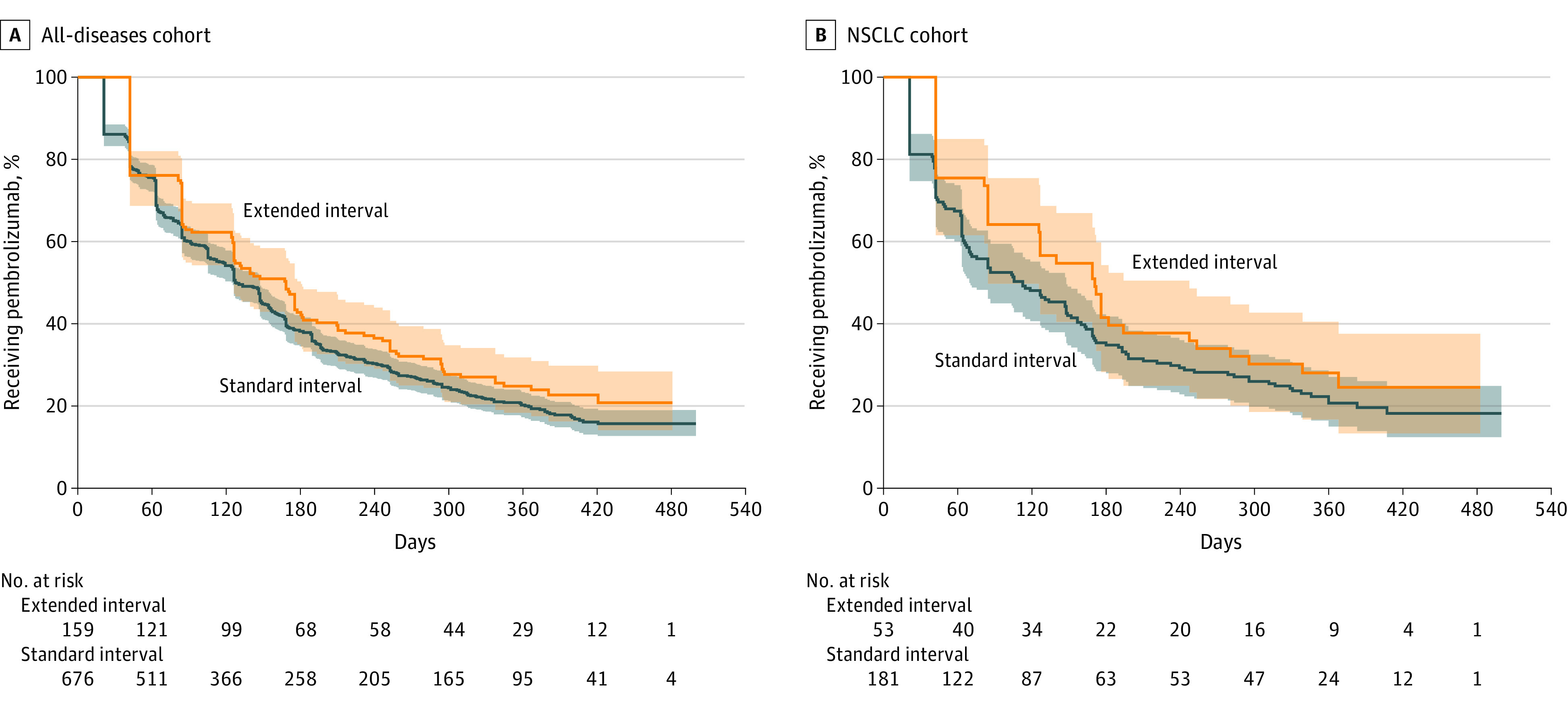
A, median TTD was 127.5 days for standard interval and 168 days for extended interval (HR, 1.00; 95% CI, 1.00-1.00; P = .08). B, Median TTD was 112 days for standard interval and 170 days for extended interval (HR, 1.00; 95% CI, 1.00-1.00; P = .15). In both, the y-axis is the proportion of patients still receiving pembrolizumab and the x-axis is number of days since incident pembrolizumab. In both panels, standard interval is blue and extended interval is orange. Shaded portions indicate 95% CIs.
The NSCLC cohort included 234 patients (mean [SD] age, 71.6 [7.3] years; 225 [96.2%] men; data on race and ethnicity were not considered); 181 patients (77.4%) received standard interval and 53 patients (22.6%) received extended interval alone (Table). Age, sex, and CCI were well balanced between the subpopulations. After a median follow-up of 377 days, median TTD was 112 days for standard interval and 170 days for extended interval (HR, 1.00; 95% CI, 1.00-1.00; P = .15; Figure 2B; eTable 6 in the Supplement). We did not observe differences in incident levothyroxine or prednisone prescriptions or response assessment rate (eTables 7 and 8 in the Supplement).
Discussion
In this cohort study, we observed low and stagnant adoption of FDA approved extended-interval pembrolizumab dosing in the largest single-payer health care system in the US. This study provides what is, to our knowledge, the first health system-level, real-world comparative effectiveness data for standard- and extended-interval pembrolizumab. Consistent with pharmacokinetic, pharmacodynamic, and single-arm clinical trial data, we observed no differences between extended and standard intervals in terms of TTD as a measure of real-world effectiveness.
Because of an absence of direct financial incentives for physicians to prescribe frequently and patients’ lower drug-related financial burdens, the VHA is an appealing setting for evaluating the adoption of an alternative dosing practice. Low extended-interval adoption, even in the face of the COVID-19 pandemic, seems attributable to prescribers’ therapeutic inertia and doubts about extended-interval dosing’s efficacy, given its approval history. The real-world near-equivalence of extended and standard intervals shown in these findings is, therefore, reassuring. We observed no differences in baseline characteristics or response assessment rate in the extended- and standard-interval groups, suggesting true TTD equivalence between the regimens. Supporting the study’s external validity, the approximately 3.25 months TTD in the NSCLC cohort was consistent with previous NSCLC clinical trial estimates of TTD (approximately 3.5 months).10 We observed similar rates of incident levothyroxine or prednisone prescriptions in the extended- and standard-interval groups, suggesting equivalent immune-related adverse events incidence.
Limitations
This study had limitations. Beneficiaries using the VA health system are disproportionately men. Disentangling small cell lung cancer from NSCLC using claims-based measures is challenging, but our chemotherapy exclusions likely eliminated all patients with SCLC.11 Categorization of this study as nonresearch operations activity precludes chart-by-chart analysis of electronic health record data. The TTD of immune checkpoint inhibitors is correlated with overall survival in NSCLC.10 Despite the frequent use of TTD, it has not been clearly validated in other cancers. Both cohorts demonstrated median ratios that exceeded their HRs, consistent with the discontinuation or continuation decision being made after clinical evaluation.
Conclusions
The findings of this cohort study suggest that extended-interval dosing merits consideration as a best practice for single-agent pembrolizumab.12 Clinical guideline promotion may help overcome some of the barriers to the adoption of extended-interval pembrolizumab. A broader point for oncologic medicine emerges: when different dosing regimens are equally effective, patient-centered dosing should be encouraged, particularly when it is consistent with patient-centered care.13
eMethods
eFigure 1. Flow of records through study
eFigure 2. Derivation of the cohorts used in comparative effectiveness analysis
eTable 1. Inclusionary and exclusionary ICD-9 and -10 codes for the NSCLC cohort
eTable 2. Time-to-treatment discontinuation of standard- and extended-interval pembrolizumab in the All Diseases cohort
eTable 3. Time-to-treatment discontinuation of standard- and extended-interval pembrolizumab in the All Diseases Cohort, by primary site of disease
eTable 4. Incident prescriptions for levothyroxine and prednisone in the All Diseases Cohort
eTable 5. Frequency of response assessment imaging ordering in the All Diseases Cohort
eTable 6. Time-to-treatment discontinuation of standard- and extended-interval pembrolizumab in the NSCLC cohort
eTable 7. Incident prescriptions for levothyroxine and prednisone in the NSCLC Cohort
eTable 8. Frequency of response assessment imaging ordering in the NSCLC Cohort
eReferences
References
- 1.Goldstein DA, Ratain MJ, Saltz LB. Weight-based dosing of pembrolizumab every 6 weeks in the time of COVID-19. JAMA Oncol. 2020;6(11):1694-1695. doi: 10.1001/jamaoncol.2020.2493 [DOI] [PubMed] [Google Scholar]
- 2.Lala M, Li TR, de Alwis DP, et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer. 2020;131:68-75. doi: 10.1016/j.ejca.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration . Review Memo–Supplemental Biologics License Application 125514. Accessed August 21, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s59-64,69,76-83SumR.pdf
- 4.Lala M, Akala O, Chartash E, et al. Abstract CT042: pembrolizumab 400mg Q6W dosing: first clinical outcomes data from Keynote-555 cohort B in metastatic melanoma. Cancer Res. 2020;80(16)(suppl):CT042l. doi: 10.1158/1538-7445.AM2020-CT042 [DOI] [Google Scholar]
- 5.Serritella AV, Strohbehn GW, Goldstein DA, Lichter AS, Ratain MJ. Interventional pharmacoeconomics: a novel mechanism for unlocking value. Clin Pharmacol Ther. 2020;108(3):487-493. doi: 10.1002/cpt.1853 [DOI] [PubMed] [Google Scholar]
- 6.Hsieh PH, Kacew AJ, Dreyer M, et al. Alternative trastuzumab dosing strategies in HER2-positive early breast cancer are associated with patient out-of-pocket savings. NPJ Breast Cancer. 2022;8(1):32. doi: 10.1038/s41523-022-00393-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hijmering-Kappelle LBM, Hiltermann TJN, Bensch F. Safety and efficacy of extended interval dosing for immune checkpoint inhibitors in non-small cell lung cancer during the COVID-19 pandemic. Clin Lung Cancer. 2022;23(2):143-150. doi: 10.1016/j.cllc.2021.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 9.Schneeweiss S, Rassen JA, Brown JS, et al. Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med. 2019;170(6):398-406. doi: 10.7326/M18-3079 [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal GM, Gong Y, Kehl K, et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small cell lung cancer. Ann Oncol. 2019;30(5):830-838. doi: 10.1093/annonc/mdz060 [DOI] [PubMed] [Google Scholar]
- 11.Turner RM, Chen YW, Fernandes AW. Validation of a case-finding algorithm for identifying patients with non-small cell lung cancer (NSCLC) in administrative claims databases. Front Pharmacol. 2017;8:883. doi: 10.3389/fphar.2017.00883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peer CJ, Goldstein DA, Goodell JC, Nguyen R, Figg WD, Ratain MJ. Opportunities for using in silico-based extended dosing regimens for monoclonal antibody immune checkpoint inhibitors. Br J Clin Pharmacol. 2020;86(9):1769-1777. doi: 10.1111/bcp.14369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah M, Rahman A, Theoret MR, Pazdur R. The drug-dosing conundrum in oncology—when less is more. N Engl J Med. 2021;385(16):1445-1447. doi: 10.1056/NEJMp2109826 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Flow of records through study
eFigure 2. Derivation of the cohorts used in comparative effectiveness analysis
eTable 1. Inclusionary and exclusionary ICD-9 and -10 codes for the NSCLC cohort
eTable 2. Time-to-treatment discontinuation of standard- and extended-interval pembrolizumab in the All Diseases cohort
eTable 3. Time-to-treatment discontinuation of standard- and extended-interval pembrolizumab in the All Diseases Cohort, by primary site of disease
eTable 4. Incident prescriptions for levothyroxine and prednisone in the All Diseases Cohort
eTable 5. Frequency of response assessment imaging ordering in the All Diseases Cohort
eTable 6. Time-to-treatment discontinuation of standard- and extended-interval pembrolizumab in the NSCLC cohort
eTable 7. Incident prescriptions for levothyroxine and prednisone in the NSCLC Cohort
eTable 8. Frequency of response assessment imaging ordering in the NSCLC Cohort
eReferences


