Abstract
Detoxification of hydrogen peroxide is a fundamental aspect of the cellular antioxidant responses in which catalases play a major role. Two differentially regulated catalase genes, catA and catB, have been studied in Aspergillus nidulans. Here we have characterized a third catalase gene, designated catC, which predicts a 475-amino-acid polypeptide containing a peroxisome-targeting signal. With a molecular mass of 54 kDa, CatC shows high similarity to other small-subunit monofunctional catalases and is most closely related to catalases from other fungi, Archaea, and animals. In contrast, the CatA (∼84 kDa) and CatB (∼79 kDa) enzymes belong to a family of large-subunit catalases, constituting a unique fungal and bacterial group. The catC gene displayed a relatively constant pattern of expression, not being induced by oxidative or other types of stress. Targeted disruption of catC eliminated a constitutive catalase activity not detected previously in zymogram gels. However, a catalase activity detected in catA catB mutant strains during late stationary phase was still present in catC and catABC null mutants, thus demonstrating the presence of a fourth catalase, here named catalase D (CatD). Neither catC nor catABC triple mutants showed any developmental defect, and both mutants grew as well as wild-type strains in H2O2-generating substrates, such as fatty acids, and/or purines as the sole carbon and nitrogen sources, respectively. CatD activity was induced during late stationary phase by glucose starvation, high temperature, and, to a lesser extent, H2O2 treatment. The existence of at least four differentially regulated catalases indicates a large and regulated capability for H2O2 detoxification in filamentous fungi.
Several studies indicate that reactive oxygen species play crucial roles in various aspects of cell physiology, such as cellular defense (45), life span (38), stress signaling (22), development (19), apoptosis (30), and pathology (33). The hydrogen peroxide formed during aerobic metabolism is capable of generating other reactive oxygen species, which can damage many cellular components (18). Catalases and peroxidases are the most important enzymatic systems used to degrade H2O2. There are three separate families of catalases: Mn-catalases, bifunctional catalase-peroxidases, and monofunctional, or “true,” catalases. The last group is the one best characterized and corresponds to homotetrameric heme-containing enzymes present in eubacteria and eukaryotes and recently also found in the Archaea (34). Within this family of catalases, two clearly distinct classes can be recognized: the small-subunit (50- to 65-kDa) and the large-subunit (∼80-kDa) enzymes. The first class includes a large number of catalases from bacteria, plants, fungi, and animals. An increasing number of catalases of the second class have been identified in bacteria and filamentous fungi (5, 8, 13, 15, 23–25, 27, 37) but not in higher eukaryotes.
The core sequence of the true catalases is composed of 360 to 390 amino acid residues (24, 48), while the large-subunit enzymes typically have ∼70 and ∼150 additional residues at the N and C termini, respectively. These terminal sequences seem to confer increased stability on the enzymes (6, 24).
Our studies focused on the antioxidant response in eukaryotes and its possible connections to cellular development (19), through the detailed analysis of catalase gene regulation in Aspergillus nidulans. Well-characterized sexual and asexual development processes in this filamentous fungus are amenable to genetic analysis (1, 40). In A. nidulans the catalase genes catA and catB have been characterized, both encoding large-subunit (∼84- and ∼79-kDa, respectively) true catalases (23, 27). The catA and catB genes are evolutionarily divergent, as judged from the relatively low similarity among the encoded polypeptides (40% identity) and the different exon structures (23). The catA mRNA accumulates during sporulation as well as in response to multiple types of stress, and its translation is connected to asexual and sexual spore formation, resulting in the high levels of catalase A activity in spores. This regulation is mediated by the catA 5′ untranslated mRNA region (26). In contrast, the catB gene is induced and translated in growing and developing hyphae and in response to oxidative and other types of stress. Both catalases provide protection against H2O2 at different stages of the A. nidulans life cycle, and CatA, and to a lesser extent CatB, protects germlings from heat shock (23, 27, 28). Here, we present the characterization of a third catalase gene, the catC gene, and present evidence for the existence of a fourth catalase (CatD) in A. nidulans. Unlike catA and catB, catC encodes a small-subunit catalase with a peroxisomal targeting sequence which is closely related to catalases from other fungi, animals, and Archaea. The catC gene is not essential for fatty acid and/or purine utilization, and its expression is constitutive, overlapping in time with the expression of the other catalase genes. On the other hand, the CatD activity was induced under a narrow set of conditions, such as the late stationary phase, glucose starvation, high temperature, and H2O2 treatment.
MATERIALS AND METHODS
Strains, media, transformation, and growth conditions.
The A. nidulans strains used in this work are shown in Table 1. All strains were grown in supplemented minimal-nitrate or minimal-ammonium (20 mM ammonium tartrate) medium (21). When carbon sources other than glucose were used, the concentrations were 100 mM (50 mM in solid medium) sodium acetate, 0.5% Tween 80, 200 mM ethanol, 200 mM methanol, 1% glycerol, and 6 mM oleate in 1% Tergitol NP-10. Nitrogen sources other than nitrate or ammonium were 2.2 mM adenine or 0.8 mg of uric acid/ml. Developmental cultures were induced as previously described (2). To disrupt the catC gene, strain RMS011 was transformed with plasmid pLK20 by using standard techniques (46).
TABLE 1.
Strains used in this worka
| Strain | Genotype | Reference or sourceb |
|---|---|---|
| FGSC26 | biA1 veA1 | FGSC |
| RMS011 | pabaA1 yA2 ΔargB::trpCΔB veA1 trpC801 | 36 |
| CLK20 | biA1 ΔcatA::argBΔA metG1 ΔcatB::argBΔB veA1 | Progeny from cross TRN1 × CLK15 (this work) (FGSC strain A1055 and ATCC MYA-116) |
| TLK61 | pabaA1 yA2 ΔcatC::argBΔC ΔargB::trpCΔB trpC801 veA1 | Obtained by transforming strain RMS011 with linear pLK20 (this work) |
| TLK12 | pabaA1 yA2 ΔargB::trpCΔB ΔcatB::argBΔB trpC801 veA1 | 23 (FGSC strain A1054 and ATCC MYA-118) |
| CLK35 | pabaA1 yA2 biA1 ΔcatC::argBΔC ΔcatA::argBΔA ΔcatB::argBΔB veA1 | Progeny from cross CLK20 × TLK61 (this work) |
| CLK14 | biA1 ΔcatA::argBΔA metG1 ΔcatB::argBΔB veA1 | Progeny from cross CLK12 × TLK12 (this work) |
| CLK15 | biA1 metG1 ΔcatB::argBΔB veA1 | Progeny from cross CLK12 × TLK12 (this work) |
| RYC17 | ΔargB::trpCΔB ΔcatA::argB veA1 | Partial genotype 7 |
| RYC16 | ΔargB::trpCΔB ΔcatA::argB ΔcatB::argB veA1 | Partial genotype 7 |
| CLK36 | pabaA1 biA1 ΔcatC::argBΔC ΔcatA::argBΔA metG1 ΔcatB::argBΔB veA1 | Progeny from cross CLK20 × TLK61 (this work) |
To obtain triple catA catB catC mutants, strains CLK20 and TLK61 were crossed. Master plates containing progeny from this cross were screened for the lack of CatB, using 20 mM H2O2, as described previously (23). Of 94 strains, 37 lacked catB and used to extract genomic DNA. DNA samples were screened for catC disruption by PCR, using oligonucleotides catC10 (5′AAGATTGGGTCGAAGCGG3′) and argB1 (5′CATAAGTCCGCCAGCAGG3′). The lack of CatA and CatB activities was confirmed by Zymogram analysis.
FGSC, Fungal Genetics Stock Center.
Catalase induction by different types of stress.
Wild-type strain FGSC26 was used to study catC gene expression under different conditions. Liquid cultures were inoculated with 5 × 105 spores/ml and grown for 12 h (nitrate as the nitrogen source) or 14 h (ammonium as the nitrogen source) at 37°C and 300 rpm. Then mycelia were incubated under different conditions or filtered through Miracloth and transferred to different media. Stress conditions were heat shock (42°C), 5 mM paraquat, 0.5 mM H2O2 (added every 30 min), 1 M sorbitol, and 1 M NaCl. Cultures were incubated under these conditions for 2 to 6 h. Mycelia were harvested and frozen in liquid nitrogen. Total RNA was extracted using Trizol (Gibco-BRL) and Northern blotting analysis was performed using standard techniques using catC as a probe.
Cloning of catC, sequencing, and plasmid construction.
Oligonucleotides catC1 (5′CTAGGTACCGAGCGAGTGGTCCATGCC3′) and catC2 (5′AGTAGATCTCGGGATTCTCGTCAAGG3′) were designed based upon a 1,085-bp A. nidulans genomic sequence (contig ANIC10430), predicting a catalase fragment different from CatA and CatB, provided by Cereon Genomics, LLC. These primers were used to amplify by PCR a 770-bp DNA fragment, using total A. nidulans DNA as the template. This PCR product was cloned into PCRII (pLK12) vector (Invitrogen) and subsequently used to probe an A. nidulans chromosome-specific cosmid library (4). Eight cosmids belonging to chromosome I were identified: L9E07, L28G03, W6C12, W9009, W10009, W11609, W17G01, and W28001. Restriction analysis of cosmids W6C12, W9009, W28001, and W17G01 indicated that they represent the same chromosomal region. Cosmid W17G01 was used as a template to fully sequence both DNA strands of the catC gene, by automatic fluorescent sequencing in an ABI PRISM 310 from Perkin-Elmer. After DNA sequencing was completed, primers catC8 (5′TTCCTCAATGCTTAGTGC3′) and catC9 (5′TCCCGGGAACTTTAAGGCATGTTAG3′) were used to amplify by PCR a 2,200-bp fragment containing the complete catC gene, using cosmid W17G01 as the template. This 2,200-bp fragment was cloned into PCRII to originate plasmid pLK17. The catC KpnI-NotI fragment from pLK17 was ligated into pBluescript II KS(+) (Stratagene) to generate plasmid pLK19. pLK19 was digested with XhoI and HincII and ligated to the argB XhoI-SmaI fragment from plasmid pDC1 to generate pLK20, which was used to transform strain RMS011.
Hybridization analyses and nucleic acid isolation.
Genomic DNA was isolated as reported previously (39). Total RNA was isolated with the Trizol reagent (Gibco-BRL), fractionated in formaldehyde-agarose gels, transferred to Hybond-N nylon membranes (Amersham), and hybridized by using standard techniques. The EcoRI fragment from pLK17 was used as a catC-specific probe, and the BamHI-NruI fragment from pDC1 was used as an argB-specific probe. Both were labeled with 32P using the BRL random priming labeling kit. Transformants containing the desired catC disruption were identified by Southern blotting, using first the catC XhoI-HincII internal fragment from pLK19 and then the entire catC EcoRI fragment from pLK17 as probes.
Catalase activity determination.
Mycelial samples from 50-ml cultures were filtered through Whatman paper, dried by passing ∼200 ml of cold acetone through the mycelia, and stored at −75°C until used. Acetone-dried mycelia were ground with mortar and pestle by using dry ice, until a fine powder was obtained. Ground mycelia were used to prepare protein extracts, which were used to determine catalase activity in zymograms (23) or by O2 evolution, using an oxygen electrode (11).
Nucleotide sequence accession number.
The sequence obtained for catC has been deposited in GenBank under accession number AF316033.
RESULTS
Cloning and characterization of the catC gene.
Using catalase activity zymograms, we detected the presence of a third catalase activity in mycelial samples obtained from several catA catB null mutants grown for 48 h. This novel activity, which was later named CatD (see below), migrated slightly faster than CatB and was not present in asexual spores (Fig. 1). This result led us to contact Cereon Genomics, LLC (Cambridge, Mass.), for possible catalase gene sequences, different from catA and catB, present in their A. nidulans genomic sequence database. We used a 1,085-bp sequence identified in the database to design primers to amplify a 770-bp DNA fragment, using A. nidulans genomic DNA as the template. The cloned PCR product was confirmed by sequencing and used to probe a chromosome-specific library (4), which identified eight cosmid clones from chromosome I. Restriction analysis of four of these cosmids indicated that they represent the same chromosomal region. Using one of the cosmid clones, W17G01, the region of interest was sequenced in both strands. The resulting genomic sequence contains an uninterrupted open reading frame encoding a 475-amino-acid polypeptide. Because the predicted amino acid sequence showed high similarity to known catalases, this newly identified gene and its protein product were named catC and CatC, respectively.
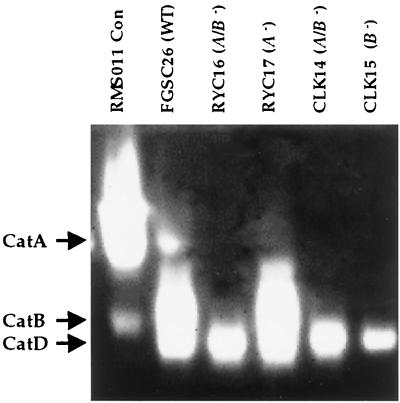
FIG. 1.
A catalase activity is detected in zymogram analysis of mutants carrying different deletions in the catA and catB genes. Protein extracts (55 μg) obtained from mycelia grown for 48 h in minimal-nitrate liquid media were fractionated in a native polyacrylamide gel and stained for catalase activity. Catalase activity from isolated conidia (strain RMS011) is shown as a reference.
catC encodes a small-subunit monofunctional catalase with a putative peroxisomal targeting signal.
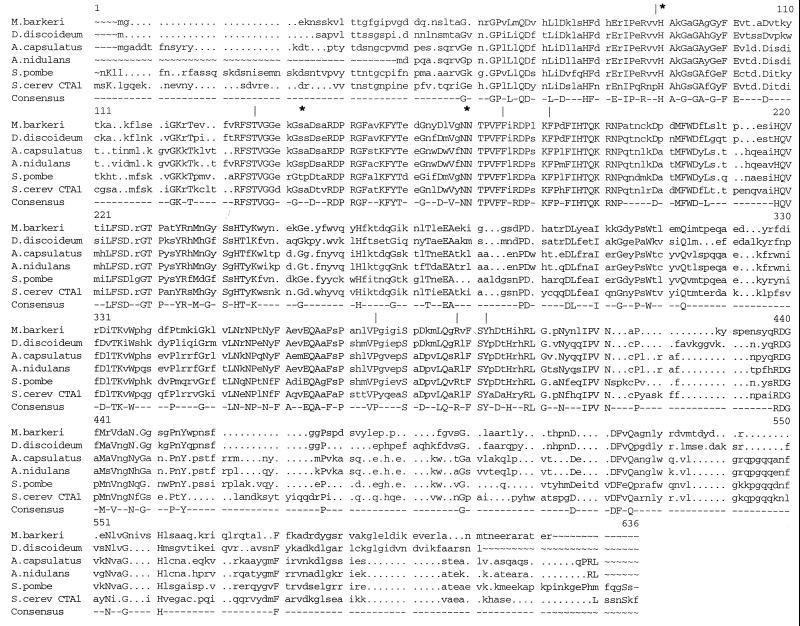
The catC gene predicts a protein with a molecular mass of 54,128 Da. CatC is highly similar to catalase P from the fungal human pathogen Ajellomyces (Histoplasma) capsulatus (84% identity), Ctt1 from Schizosaccharomyces pombe (61% identity), CAT1 (43) from Candida albicans (61% identity), and the peroxisomal catalase Cta1p from Saccharomyces cerevisiae (58% identity). CatC also shows high similarity to CatA (14) from the slime mold Dictyostelium discoideum (52% identity) and catalases from the strict anaerobic methanogenic archaeon Methanosarcina barkeri (52% identity) and animals (data not shown). All of these enzymes belong to the family of small-subunit (50- to 65-kDa) monofunctional catalases. As shown in Fig. 2, amino acid sequences that form part of the active and heme coordination sites are conserved in CatC and these enzymes.
FIG. 2.
Comparison of A. nidulans CatC with the most similar catalases. CatC was aligned with catalases from Ajellomyces capsulatus (GenBank accession number AF189369), Schizosaccharomyces pombe (47), Saccharomyces cerevisiae (10), D. discoideum (14), and M. barkeri (34). Conserved amino acids that form part of the active (*) and heme coordination (|) sites are indicated. The alignment was performed using the programs PILEUP and PRETTY (12).
The CatC carboxy terminus contains the tripeptide ARL, which fits the consensus ([S/C/A][K/R/H]L) peroxisomal targeting signal type I. This signal has been shown to be both necessary and sufficient to direct proteins to peroxisomes (17, 20). It is present in other enzymes from filamentous fungi that very likely are peroxisomal, such as monoamino oxidase (ARL) and urate oxidase (AKL) (29, 32).
catC expression is constant under different conditions.
We have reported that the catA and catB genes are differentially regulated during the A. nidulans life cycle as well as in response to different types of stress (23, 26, 27). With argB mRNA and rRNA staining (not shown) as loading controls, we examined the expression of catC by Northern blot analysis, using RNA samples from the wild-type strain FGSC26 grown under different conditions. As shown in Fig. 3A, the catC mRNA was detected in young hyphae as early as 12 h of growth, and its level was relatively constant up to 48 h of growth. This result suggested either that catC did not encode the catalase activity that appears after 48 h of growth (Fig. 1) or that it was subject to some type of posttranscriptional control. During asexual development, the catC message level showed little change up to 6 h, increased slightly by 12 h, and declined thereafter (Fig. 3B), to become barely detectable in isolated conidia (not shown).
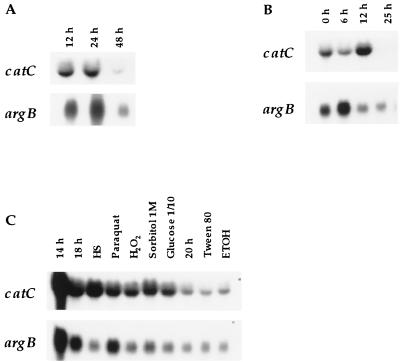
FIG. 3.
catC expression during growth, asexual development, and stress. Total RNA extracted from wild-type strain FGSC26 mycelia, subject to the indicated conditions, was fractionated in a formaldehyde-agarose gel and used for Northern blot analyses. (A) Regulation during growth and stationary phase. RNA samples were from mycelia harvested at 12, 24, and 48 h of growth in liquid minimal-nitrate medium. (B) Expression during asexual development. RNA samples obtained from mycelia grown for 18 h in liquid minimal nitrate medium (0 h) and from mycelia induced to conidiate for the indicated time. (C) catC expression under different nutritional and stress conditions. Strain FGSC26 was grown in liquid minimal-ammonium medium for 14 h and then incubated at 42°C (HS) or in the presence of 5 mM paraquat or 0.5 mM H2O2 for 4 h. In the case of 1 M sorbitol, 0.1% glucose, 0.5% Tween 80, and 200 mM ethanol, the 14-h-grown mycelia were filtered, washed, and transferred to the indicated media for 4 h (sorbitol and glucose) or 6 h. Glucose (0.1% ), Tween 80, and ethanol were used as the sole carbon sources. The EcoRI catC fragment from pLK12 was used as the probe. The same blots probed with argB are shown as RNA loading controls.
The catC mRNA level was virtually unaffected by several stress and nutritional conditions, including oxidative stress, osmotic stress, and growth for 6 h in Tween 80 or ethanol as the sole carbon source (Fig. 3C). A slight induction was noticeable only during heat shock (Fig. 3C) and growth in uric acid as the sole nitrogen source (not shown).
Targeted disruption of catC revealed the existence of an unidentified catalase gene.
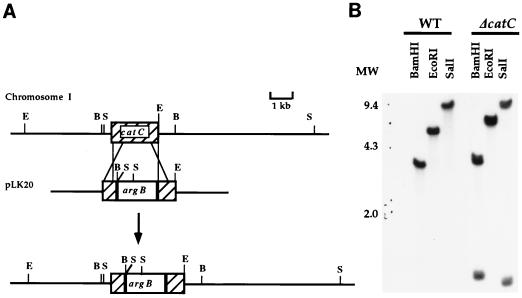
To determine if catC encoded a catalase different from the one previously detected in catA catB double null mutants (Fig. 1), we designed plasmid pLK20 to perform a targeted disruption of catC. In pLK20 a central region of 740 bp from the catC gene was replaced by the argB gene as a selectable marker. This resulted in the deletion of amino acid residues 94 to 341 from CatC. Linear pLK20 was used to transform strain RMS011 to arginine independence. Forty-one Arg+ transformants were analyzed by Southern blotting using the catC internal XhoI-HincII fragment deleted in pLK20. Among these, 12 transformants gave no hybridization signal, indicating deletion of the corresponding catC fragment. Genomic DNA from three of these catC mutants was digested with BamHI, EcoRI, and SalI and analyzed by Southern blotting using the entire catC gene as the probe. All three transformants gave hybridization patterns identical to the one shown in Fig. 4B for strain TLK61. This pattern is consistent with the double recombination event and consequent disruption of the catC gene, depicted in Fig. 4A.
FIG. 4.
catC gene targeted disruption. (A) Plasmid pLK20 was constructed by replacing a central region of 740 bp from the catC gene with the argB gene, used as a selectable marker. The CatC region removed corresponds to amino acids 94 to 341 (Fig. 2). Linear pLK20 was used to transform strain RMS011 to arginine independence. Restriction sites: B, BamHI; E, EcoRI; S, SalI. (B) Total DNA extracted from recipient strain RMS011 and the ΔcatC strain TLK61 was digested with indicated restriction enzymes and used for Southern blot analysis. The probe used was the EcoRI catC fragment from pLK17. The same membrane probed with argB (not shown) gave a hybridization pattern fully consistent with the illustrated integration event. MW, molecular weight (weights are in thousands); WT, wild type.
To analyze zymogram catalase activity patterns in a more conclusive way, we created triple catABC mutant strains (Table 1). Both catA catB (strain CLK20) and catA catB catC (strain CLK36) mutants were grown for 12, 24, and 48 h, and corresponding protein extracts were used to detect catalase activity in zymograms. As shown in Fig. 5, the catalase activity detected previously in 48-h samples from the catAB double mutants (Fig. 1) was unaffected by the deletion of the catC gene, demonstrating that this catalase, which we have designated CatD, is encoded by an as-yet-unidentified gene.
FIG. 5.
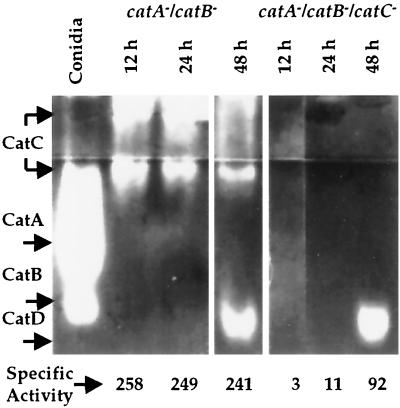
A. nidulans contains at least four different catalases. Protein extracts (40 μg) prepared from strains CLK20 (catA− catB−) and CLK36 (catA− catB− catC−) grown for 12, 24 and 48 h were fractionated in a native polyacrylamide gel that was stained to detect catalase activity. A protein sample from isolated conidia (strain FGSC26) is shown as a catalase A and B reference. Numbers below the zymogram are catalase-specific activities (in units per milligram of protein per milliliter) in each sample, measured by O2 evolution (11). Data are the averages of two determinations, with a maximum variation of 13% with respect to the average.
Samples from the catA catB double mutant showed a catalase activity smear at the gel wells and a more defined band right below the concentrator gel. These activities were totally absent in samples from the catABC triple mutant (Fig. 5), indicating that the catC-encoded catalase was not detected in our previous zymogram gel system. This was supported when catalase-specific activity was assayed by O2 evolution in the samples used for the zymogram. As shown at the bottom of Fig. 5, ∼250 U of catalase activity was detected in samples from strain CLK20 grown for 12 h, which remained constant at 24 h of growth. In contrast, catalase activity was negligible in 12- and 24-h samples from strain CLK36. The 48-h sample of CLK36 contained 92 U of catalase activity, which would correspond to the catalase D activity detected in the zymogram. A slight decrease in CatC activity in the CLK20 48-h sample may explain why the total catalase-specific activity remained around 250 U, despite the contribution of CatD activity. These results confirm that catC encodes a novel catalase activity that remains relatively unchanged during 12 to 48 h of growth. This pattern of CatC activity is consistent with the catC mRNA levels detected during the same period of growth (Fig. 3A).
Catalase C activity is not required for asexual or sexual development or for fatty acid and/or purine utilization.
We observed no obvious defect during asexual development of A. nidulans catC mutants. However, Berteaux-Lecellier et al. (3) reported that peroxisomal function is necessary for caryogamy and sexual development in Podospora anserina. We found that wild-type strain FGSC26 and catAB (CLK20), catB (TLK12), catC (TLK61), and catABC (CLK35) null mutant strains were all able to differentiate sexual fruiting bodies (cleistothecia) in similar amounts and produced viable sexual spores.
A. nidulans can utilize oleate as the sole carbon source and purines as the sole nitrogen source. The degradation of these compounds appears to occur in peroxisomes and involve H2O2 generation (31, 42). We tested the growth response of CLK20, TLK12, TLK61, and CLK35 mutant strains in media containing different carbon and/or nitrogen sources (see Material and Methods). In particular, we tested oleate and Tween 80 as the sole carbon sources, adenine and uric acid as the sole nitrogen sources, and combinations of both carbon and nitrogen sources. All four catalase mutants grew as well as the wild-type strain in all tested media, indicating that CatC function is dispensable for growth in these substrates.
Catalase D is induced during late stationary phase, by glucose starvation, and heat shock.
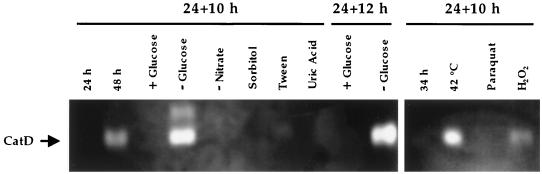
We used catA catB double mutants to examine CatD activity under different conditions. As shown in Fig. 5 and 6, CatD activity was not detectable before 48 h of growth. After 48 h, a slight increase was observed by 72 h (data not shown). Under our growing conditions, glucose in the medium becomes exhausted by 36 h (35). Therefore, 48 to 72 h of growth corresponds to a very late stationary phase under severe nutrient starvation. To analyze CatD activity under different growth and stress conditions, strain CLK20 was grown for 24 h, and then mycelia were shifted to different media for 10 to 12 h. Alternatively, 24-h mycelia were exposed for 10 h to osmotic stress, high temperature, or the oxidative stress caused by paraquat and H2O2 treatments. Figure 6 shows that glucose starvation and incubation at 42°C resulted in a clear induction of CatD activity, while H2O2 produced a modest induction. All other treatments, including nitrate starvation, failed to induce CatD.
FIG. 6.
Catalase D is induced during late stationary phase, by glucose starvation, heat shock, and H2O2. Strain CLK20 (catA− catB−) was grown in minimal-nitrate medium for 24, 34, and 48 h. Mycelia grown for 24 h were incubated at 42°C, in the presence of 1 M sorbitol, 5 mM paraquat, or 0.5 mM H2O2 for 10 h. Where indicated, 24-h mycelia were shifted for 10 to 12 h to fresh media containing or lacking glucose, lacking nitrate, or containing 0.5% Tween 80 or 0.8 mg of uric acid/ml as the sole carbon and nitrogen sources, respectively. A total of 100 μg of protein was loaded in each lane.
DISCUSSION
The catC gene encodes a small-subunit monofunctional catalase, likely localized in peroxisomes.
Here we have shown that A. nidulans catC encodes a catalase more related to small-subunit catalases from other fungi, a slime mold, Archaea, and animals than to catalases from eubacteria and plants. In contrast, CatA and CatB, along with other enzymes up to now found only in filamentous fungi and eubacteria, form the large-subunit catalase family (5, 8, 13, 15, 23, 24, 27, 37, 48). In fact, endosymbiosis and horizontal gene transfer mechanisms have been invoked to explain the grouping of these fungal and bacterial catalases (24). It seems clear that catC and catAB genes have different evolutionary origins, as judged from their sequence and size disparity and the catC lack of introns.
The catC gene was expressed at relatively constant levels under several growth, stress, and nutritional conditions, the most noticeable change being a gradual decrease during conidiation. This constitutive expression correlates well with the CatC activity detected during 12 to 48 h of growth (Fig. 5). CatC activity was not detected previously due to its extremely low migration rate in our zymogram gel system. This can be explained by the high isoelectric point (8.69) predicted for CatC, perhaps the most basic reported for a catalase, with our starting electrophoresis conditions at pH 8.5. A slight change in pH during electrophoresis may account for the CatC activity that enters the zymogram gel. Our attempt to resolve and/or detect CatC using electrofocusing gels was unsuccessful, while CatA and CatB were well separated and detected under the same conditions. This result could be explained by a higher stability of CatA and CatB than of the smaller CatC enzyme. In fact, CatB has been found to be resistant to 9 M urea, 2% sodium dodecyl sulfate, and ethanol-chloroform treatment (6).
Several lines of evidence suggest that CatC may be a peroxisomal enzyme. First, it contains the peroxisome-targeting signal ARL. Second, our preliminary cell fractionation experiments using cell extracts from catA catB double mutant grown for 18 h showed that at least 20% of the total CatC activity is contained within the subcellular particle pellet, along with high activity levels of the peroxisomal marker isocitrate lyase (41) and the mitochondrial marker fumarase. Third, a catalase activity has been cytochemically localized in microbodies from young growing hyphae, and cosedimentation of catalase activity and peroxisome marker enzymes has also been shown in A. nidulans (42). It is unlikely that the reported peroxisome-associated catalase (42) corresponds to CatA, CatB, or CatD. CatA and CatB do not contain peroxisome-targeting signals (23, 27). CatA activity is largely associated with spores (26) and has been immunolocalized in the asexual spore cell wall and cytosol (R. E. Navarro and J. Aguirre, unpublished data), whereas CatB has been immunolocalized in the cell wall and cytosol from hyphae (L. Kawasaki and J. Aguirre, unpublished data). CatD has been shown here to be present in old and high-temperature-grown hyphae.
Multiple catalases and other H2O2 detoxification enzymes in A. nidulans.
Although there is some overlap, CatA and CatB are present at different stages of the A. nidulans life cycle and protect different cell types from H2O2 or other types of oxidative stress (23, 26, 27) and heat shock stress (28). The fact that the catC gene is expressed at relatively constant levels suggests that CatC activity overlaps CatA or CatB activity. However, confirmation of a peroxisomal location for CatC would argue against such functional overlap or redundancy. CatD seems repressed by glucose and is induced during late stationary phase, showing a partial overlap with CatB expression. No other catalase genes besides catA, -B, and -C were found in the A. nidulans genome database, suggesting that the database is not complete or that CatD does not belong to the monofunctional catalase family.
The fact that CatC is dispensable for growth in oleic acid as the sole carbon source and/or in purines as the sole nitrogen sources suggests the presence of alternative peroxisomal H2O2 detoxification systems. A search of an A. nidulans cDNA partial sequence database (http://www.genome.ou.edu/fungal.html) for genes encoding enzymes involved in H2O2 detoxification identified two genes in addition to catA and catB. Clone r2g02a1 predicts a protein with high similarity to fungal and mammalian PMP20 peroxisomal peroxidases (9, 16, 44). Clone c7g02a1 predicts a protein with high similarity to glutathione peroxidases. The existence of two putative thiol-dependent peroxidases and at least four catalases suggests a large and regulated capability for H2O2 degradation in filamentous fungi.
ACKNOWLEDGMENTS
This work was supported by grants IN206097 and IN214199, both from DGAPA-UNAM (PAPIIT).
We are grateful to Thomas Adams and Vicky Gavrias from Cereon Genomics, LLC, for the catC fragment DNA sequence. We thank Fabiola Méndez for experimental support and the IFICE-UNAM Molecular Biology Unit for DNA sequencing and oligonucleotide synthesis. We are indebted to Wilhelm Hansberg for helpful discussions and Kazuhiro Shiozaki for critical reading of the manuscript.
REFERENCES
- 1.Adams T H, Wieser J K, Yu J H. Asexual sporulation in Aspergillus nidulans. Microbiol Mol Biol Rev. 1998;62:35–54. doi: 10.1128/mmbr.62.1.35-54.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre J. Spatial and temporal controls of the Aspergillus brlA developmental regulatory gene. Mol Microbiol. 1993;8:211–218. doi: 10.1111/j.1365-2958.1993.tb01565.x. [DOI] [PubMed] [Google Scholar]
- 3.Berteaux-Lecellier V, Picard M, Thompson-Coffe C, Zickler D, Panvier-Adoutte A, Simonet J M. A nonmammalian homolog of the PAF1 gene (Zellweger syndrome) discovered as a gene involved in caryogamy in the fungus Podospora anserina. Cell. 1995;81:1043–1051. doi: 10.1016/s0092-8674(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 4.Brody H, Griffith J, Cuticchia A J, Arnold J, Timberlake W E. Chromosome-specific recombinant DNA libraries from the fungus Aspergillus nidulans. Nucleic Acids Res. 1991;19:3105–3109. doi: 10.1093/nar/19.11.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calera J A, Paris S, Monod M, Hamilton A J, Debeaupuis J P, Diaquin M, Lopez-Medrano R, Leal F, Latge J P. Cloning and disruption of the antigenic catalase gene of Aspergillus fumigatus. Infect Immun. 1997;65:4718–4724. doi: 10.1128/iai.65.11.4718-4724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calera J A, Sanchez-Weatherby J, Lopez-Medrano R, Leal F. Distinctive properties of the catalase B of Aspergillus nidulans. FEBS Lett. 2000;475:117–120. doi: 10.1016/s0014-5793(00)01637-9. [DOI] [PubMed] [Google Scholar]
- 7.Chang Y C, Segal B H, Holland S M, Miller G F, Kwon-Chung K J. Virulence of catalase-deficient Aspergillus nidulans in p47(phox)−/− mice. Implications for fungal pathogenicity and host defense in chronic granulomatous disease. J Clin Investig. 1998;101:1843–1850. doi: 10.1172/JCI2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho Y H, Lee E J, Roe J H. A developmentally regulated catalase required for proper differentiation and osmoprotection of Streptomyces coelicolor. Mol Microbiol. 2000;35:150–160. doi: 10.1046/j.1365-2958.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 9.Choi H J, Kang S W, Yang C H, Rhee S G, Ryu S E. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat Struct Biol. 1998;5:400–406. doi: 10.1038/nsb0598-400. [DOI] [PubMed] [Google Scholar]
- 10.Cohen G, Rapatz W, Ruis H. Sequence of the Saccharomyces cerevisiae CTA1 gene and amino acid sequence of catalase A derived from it. Eur J Biochem. 1988;176:159–163. doi: 10.1111/j.1432-1033.1988.tb14263.x. [DOI] [PubMed] [Google Scholar]
- 11.del Rio L A, Gomez Ortega M, Leal Lopez A, Lopez Gorge J. A more sensitive modification of the catalase assay with the Clark oxygen electrode. Anal Biochem. 1977;80:409–415. doi: 10.1016/0003-2697(77)90662-5. [DOI] [PubMed] [Google Scholar]
- 12.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler T, Rey M W, Vaha-Vahe P, Power S D, Berka R M. The catR gene encoding a catalase from Aspergillus niger: primary structure and elevated expression through increased gene copy number and use of a strong promoter. Mol Microbiol. 1993;9:989–998. doi: 10.1111/j.1365-2958.1993.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcia X M, Foote C, van Es S, Devreotes P N, Alexander S, Alexander H. Differential developmental expression and cell type specificity of Dictyostelium catalases and their response to oxidative stress and UV-light. Biochim Biophys Acta. 2000;1492:295–310. doi: 10.1016/s0167-4781(00)00063-4. [DOI] [PubMed] [Google Scholar]
- 15.Garre V, Muller U, Tudzynski P. Cloning, characterization, and targeted disruption of cpcat1, coding for an in planta secreted catalase of Claviceps purpurea. Mol Plant-Microbe Interact. 1998;11:772–783. doi: 10.1094/MPMI.1998.11.8.772. [DOI] [PubMed] [Google Scholar]
- 16.Godon C, Lagniel G, Lee J, Buhler J M, Kieffer S, Perrot M, Boucherie H, Toledano M B, Labarre J. The H2O2 stimulon in Saccharomyces cerevisiae J. Biol Chem. 1998;273:22480–22489. doi: 10.1074/jbc.273.35.22480. [DOI] [PubMed] [Google Scholar]
- 17.Gould S J, Keller G A, Hosken N, Wilkinson J, Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989;108:1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halliwell B, Gutteridge J M C. Free radicals in biology and medicine. 2nd ed. Oxford, United Kingdom: Clarendon Press and Oxford University Press; 1989. [Google Scholar]
- 19.Hansberg W, Aguirre J. Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. J Theor Biol. 1990;142:201–221. doi: 10.1016/s0022-5193(05)80222-x. [DOI] [PubMed] [Google Scholar]
- 20.Hettema E H, Distel B, Tabak H F. Import of proteins into peroxisomes. Biochim Biophys Acta. 1999;1451:17–34. doi: 10.1016/s0167-4889(99)00087-7. [DOI] [PubMed] [Google Scholar]
- 21.Käfer E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet. 1977;19:33–131. doi: 10.1016/s0065-2660(08)60245-x. [DOI] [PubMed] [Google Scholar]
- 22.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki L, Wysong D, Diamond R, Aguirre J. Two divergent catalase genes are differentially regulated during Aspergillus nidulans development and oxidative stress. J Bacteriol. 1997;179:3284–3292. doi: 10.1128/jb.179.10.3284-3292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klotz G M, Klassen G R, Loewen P C. Phylogenetic relationships among prokaryotic and eukaryotic catalases. Mol Biol Evol. 1997;14:951–958. doi: 10.1093/oxfordjournals.molbev.a025838. [DOI] [PubMed] [Google Scholar]
- 25.Lledias F, Rangel P, Hansberg W. Oxidation of catalase by singlet oxygen. J Biol Chem. 1998;273:10630–10637. doi: 10.1074/jbc.273.17.10630. [DOI] [PubMed] [Google Scholar]
- 26.Navarro R E, Aguirre J. Posttranscriptional control mediates cell type-specific localization of catalase A during Aspergillus nidulans development. J Bacteriol. 1998;180:5733–5738. doi: 10.1128/jb.180.21.5733-5738.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro R E, Stringer M A, Hansberg W, Timberlake W E, Aguirre J. catA, a new Aspergillus nidulans gene encoding a developmentally regulated catalase. Curr Genet. 1996;29:352–359. [PubMed] [Google Scholar]
- 28.Noventa-Jordao A M, Couto R M, Goldman M H, Aguirre J, Iyer S, Caplan A, Terenzi H F, Goldman G H. Catalase activity is necessary for heat-shock recovery in Aspergillus nidulans germlings. Microbiology. 1999;145:3229–3234. doi: 10.1099/00221287-145-11-3229. [DOI] [PubMed] [Google Scholar]
- 29.Oestreicher N, Scazzocchio C. Sequence, regulation, and mutational analysis of the gene encoding urate oxidase in Aspergillus nidulans. J Biol Chem. 1993;268:23382–23389. [PubMed] [Google Scholar]
- 30.Quillet-Mary A, Jaffrezou J P, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- 31.Scazzocchio C. The purine degradation pathway, genetics, biochemistry and regulation. In: Martinelli S D, Kinghorn J R, editors. Aspergillus: 50 years on. Vol. 29. Amsterdam, The Netherlands: Elsevier; 1994. pp. 221–251. [PubMed] [Google Scholar]
- 32.Schilling B, Lerch K. Cloning, sequencing and heterologous expression of the monoamine oxidase gene from Aspergillus niger. Mol Gen Genet. 1995;247:430–438. doi: 10.1007/BF00293144. [DOI] [PubMed] [Google Scholar]
- 33.Sheikh F G, Pahan K, Khan M, Barbosa E, Singh I. Abnormality in catalase import into peroxisomes leads to severe neurological disorder. Proc Natl Acad Sci USA. 1998;95:2961–2966. doi: 10.1073/pnas.95.6.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shima S, Netrusov A, Sordel M, Wicke M, Hartmann G C, Thauer R K. Purification, characterization, and primary structure of a monofunctional catalase from Methanosarcina barkeri. Arch Microbiol. 1999;171:317–323. doi: 10.1007/s002030050716. [DOI] [PubMed] [Google Scholar]
- 35.Skromne I, Sanchez O, Aguirre J. Starvation stress modulates the expression of the Aspergillus nidulans brlA regulatory gene. Microbiology. 1995;141:21–28. doi: 10.1099/00221287-141-1-21. [DOI] [PubMed] [Google Scholar]
- 36.Stringer A M, Dean R A, Sewall T C, Timberlake W E. Rodletless, a new Aspergillus developmental mutant induced by directed gene inactivation. Genes Dev. 1991;5:1161–1171. doi: 10.1101/gad.5.7.1161. [DOI] [PubMed] [Google Scholar]
- 37.Takasuka T, Sayers N M, Anderson M J, Benbow E W, Denning D W. Aspergillus fumigatus catalases: cloning of an Aspergillus nidulans catalase B homologue and evidence for at least three catalases. FEMS Immunol Med Microbiol. 1999;23:125–133. doi: 10.1111/j.1574-695X.1999.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 38.Taub J, Lau J F, Ma C, Hahn J H, Hoque R, Rothblatt J, Chalfie M. A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-C and clk-1 mutants. Nature. 1999;399:162–166. doi: 10.1038/20208. [DOI] [PubMed] [Google Scholar]
- 39.Timberlake W E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980;78:497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- 40.Timberlake W E, Clutterbuck A J. Genetic regulation of conidiation. In: Martinelli S D, Kinghorn J R, editors. Aspergillus: 50 years on. Vol. 29. Amsterdam, The Netherlands: Elsevier; 1994. pp. 383–427. [PubMed] [Google Scholar]
- 41.Valenciano S, De Lucas J R, Van der Klei I, Veenhuis M, Laborda F. Characterization of Aspergillus nidulans peroxisomes by immunoelectron microscopy. Arch Microbiol. 1998;170:370–376. doi: 10.1007/s002030050655. [DOI] [PubMed] [Google Scholar]
- 42.Valenciano S, Lucas J R D, Pedregosa A, Monistrol I F, Laborda F. Induction of beta-oxidation enzymes and microbody proliferation in Aspergillus nidulans. Arch Microbiol. 1996;166:336–341. doi: 10.1007/s002030050392. [DOI] [PubMed] [Google Scholar]
- 43.Wysong D R, Christin L, Sugar A M, Robbins P W, Diamond R D. Cloning and sequencing of a Candida albicans catalase gene and effects of disruption of this gene. Infect Immun. 1998;66:1953–1961. doi: 10.1128/iai.66.5.1953-1961.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita H, Avraham S, Jiang S, London R, Van Veldhoven P P, Subramani S, Rogers R A, Avraham H. Characterization of human and murine PMP20 peroxisomal proteins that exhibit antioxidant activity in vitro. J Biol Chem. 1999;274:29897–29904. doi: 10.1074/jbc.274.42.29897. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Shah J, Klessig D F. Signal perception and transduction in plant defense responses. Genes Dev. 1997;11:1621–1639. doi: 10.1101/gad.11.13.1621. [DOI] [PubMed] [Google Scholar]
- 46.Yelton M M, Hamer J E, Timberlake W E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci USA. 1984;81:1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshioka S, Kato K, Nakai K, Okayama H, Nojima H. Identification of open reading frames in Schizosaccharomyces pombe cDNAs. DNA Res. 1997;4:363–369. doi: 10.1093/dnares/4.6.363. [DOI] [PubMed] [Google Scholar]
- 48.Zamocky M, Koller F. Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog Biophys Mol Biol. 1999;72:19–66. doi: 10.1016/s0079-6107(98)00058-3. [DOI] [PubMed] [Google Scholar]