Abstract
Lupin varieties with a low content of quinolizidine alkaloids (QAs) like blue sweet lupin (BSL) have long been used as a protein source for dairy cows. A health concern for humans may arise from the transfer of acute toxic QAs from feed into cow’s milk. This study is the first to quantify the transfer of QAs from BSL into cow’s milk with experimental and modeling methods. Four lactating dairy cows were subjected to two 7 day feeding periods with 1 and 2 kg/d BSL, respectively, each followed by a depuration period. BSL contained 1774 mg/kg dry matter total QAs. Individual milk samples were taken twice daily and QA contents in feed and milk determined with liquid chromatography–tandem mass spectrometry. Transfer of QAs into the milk was already seen with the administration of 1 kg/d BSL, with differences in transfer rates (TRs) between individual QAs. A toxicokinetic model was derived to quantify and predict QA feed-to-food transfer. For the four most prominent QAs, our model shows an α-half-life of around 0.27 d. TRs were obtained for six QAs and were between 0.13 (sparteine) and 3.74% (multiflorine). A toxicological assessment of milk containing QAs as measured in this study indicated a potential health concern.
Keywords: carry-over, secondary plant metabolites, plant alkaloids, cattle, lupins
Introduction
Lupins have a long tradition as a protein source in animal nutrition because of their high crude protein (CP) content (up to 40% in dry matter, DM), and they are further gaining importance in Europe, especially in organic animal husbandry. While several secondary plant metabolites in lupins have been shown to have beneficial effects (e.g., antidiabetic or antioxidant activity),1 some alkaloids are known to have detrimental effects on human and animal health. The latter is the case for quinolizidine alkaloids (QAs), which constitute the main secondary plant metabolites occurring in lupins, offering protection against insects and herbivores.2 To date, more than 300 lupine species are known, with varying QA contents. Depending on their alkaloid content, lupins are commonly classified into bitter lupins (with a total QA content of up to 8% in DM) and sweet lupins with a low alkaloid content.3 This low alkaloid content should not exceed 0.05% in DM (500 mg/kg DM) in agricultural practice, while levels <0.02% in DM (<200 mg/kg DM) are recommended by health authorities for lupin seeds used for food production.4−7
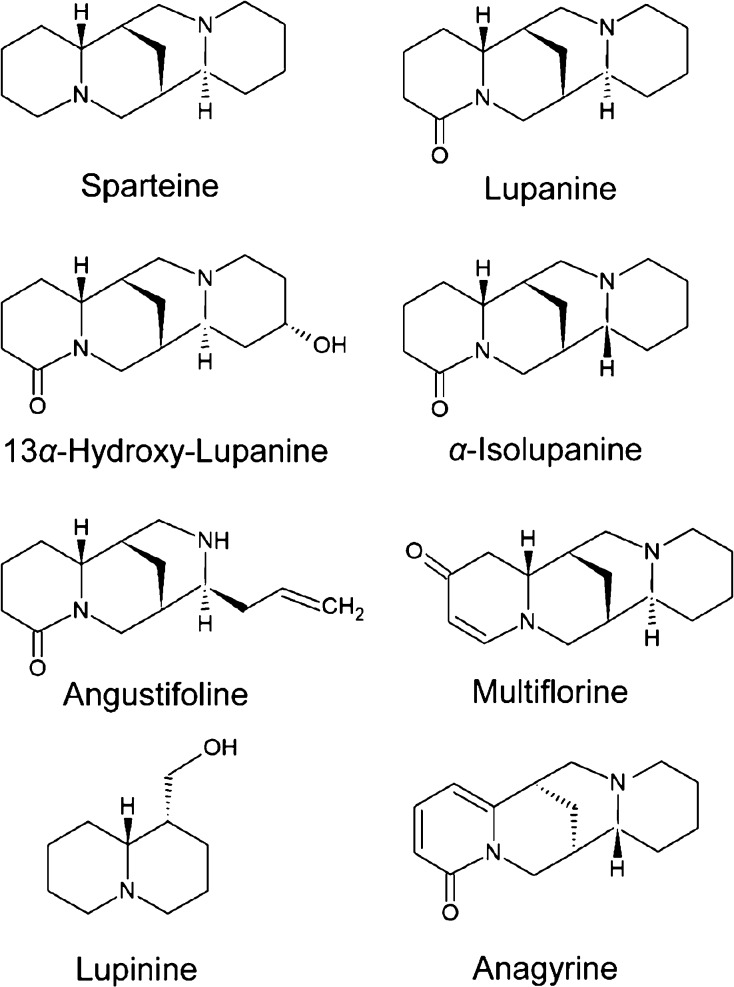
The synthesis of QAs occurs mainly in the leaves, but they are distributed via the phloem into other parts of the plant including the seeds, causing a bitter taste as a protection against herbivores.8 More than 170 QAs have been identified among lupin species, with lupanine, 13α-hydroxylupanine, and sparteine being the most abundant ones.9 Depending on their chemical structure, QAs can be chemically divided into, for example, sparteine and its derivatives, lupanine and its derivatives, angustifoline and its derivatives, multiflorine and its derivatives, lupinine, and anagyrine(Figure 1).
Figure 1.
Chemical structures of selected QAs.
The QAs exert their toxicity by inhibiting acetylcholine receptors and voltage-dependent ion channels in the central nervous system, on motor endplates and the peripheral autonomic nervous system, where the individual QAs appear to have different levels of toxicity.10 Common acute toxic exposure symptoms in humans and mammals include respiratory depression, vomiting, and tachycardia.10,11 Some QAs, such as anagyrine, also show teratogenic properties and have been associated with congenital skeletal malformations (crooked calf disease) in calves.12 Thus, to minimize the risk of QA intoxication in livestock animals, only sweet lupins are listed as feed for livestock species in the catalogue of feed materials.13
However, mutations, cross-breeding, or recombination can result in descendants with higher QA contents despite their original classification as sweet lupins.14,15
Most of the toxicological data originate from research on lupanine and sparteine, the latter compound was used as a pharmaceutical in the past.10 The European Food Safety Authority (EFSA) stated that “anticholinergic effects and changes in cardiac electric conductivity” are the relevant endpoints for risk assessment. A dose of 0.16 mg sparteine/kg bodyweight (bw) was identified as the ‘lowest single oral effective dose’ in humans for such acute effects, while no reference point could be identified for risks potentially resulting from chronic exposure. Due to similar modes of action of QAs, the EFSA assumed dose additivity for all derivatives. Furthermore, due to the limited overall data basis and the associated uncertainties, no health-based guidance value could be derived. Therefore, the EFSA applied the margin of exposure (MoE) approach for a preliminary risk characterization using the dose of 0.16 mg sparteine/kg bw as an appropriate reference point. The authority concluded that an MoE >1 would not indicate a health concern. However, the assessment revealed the possibility of exposures for some consumer groups, resulting in MoE values <1, indicating a potential risk for these consumers. Additionally, the EFSA stated that there is indirect evidence of a possible transfer of QAs from feed into milk, due to the QAs’ weak basic nature, which makes milk a possible additional exposure source.10 However, until now, there has only been one published case report of possible QA intoxication in a human infant after its mother drank goat milk in early pregnancy.16 Lambs from the same goats showed skeletal deformations as described for crooked calf disease, indicating QA intoxication.17 In the present study, we tested the hypothesis that QAs from lupin in the diet of dairy cows are transferred into cow’s milk. We determined the profiles of six QAs in milk and quantified the TRs of the four most prominent QAs from lupin seeds into the milk of four lactating dairy cows fed with increasing amounts of QA-containing sweet lupin seeds. We conducted a toxicological assessment in order to evaluate the potential risk resulting from the sole exposure to QAs via milk containing QA levels as measured in the present study.
Materials and Methods
Ethics Approval Statement
All experimental procedures involving animals were approved by the local authority (Regional Office for Health and Social Affairs, Berlin—LAGESO, Germany) under registration number StN010/19.
Animals, Housing, and Sampling
Four Holstein-Friesian dairy cows (3 primiparous, 1 multiparous, 58 ± 11 days in milk) with an average milk yield of 30.4 ± 4.12 kg/day were housed in one group in an open barn stable with free access to water. During the experiment, which lasted 46 days in total, cows were milked twice daily at 6.00 a.m. and 4.30 p.m. in a tandem milking parlor (Lemmer Fullwood). Milk samples were taken during each milking and stored at −20 °C until being analyzed for QA contents.
Lupin Seeds and Diets
Lupin seeds (whole grain, untoasted, Lupinus angustifolius var. Boregine [blue sweet lupine, BSL]) harvested in Brandenburg, Germany, approximately 52°6′ N 12°7′ E, in August 2019 were milled in a common hammer mill (Siemens) to pass a screen of 3 mm, divided into four subsamples of 25 kg each and stored in a container under dry, cool, and dark conditions prior to use. Forages, beet pulp, and minerals were offered as a partial mixed ration (27.7% grass silage, 29.5% maize silage, 6.0% straw, 30.1% hay, 6.0% beet pulp, and 0.61% minerals) ad libitum in feeding troughs. A concentrate mixture was provided in separate feeding troughs, transponder-controlled one for each cow, to meet the energy requirements for a milk yield of 25 kg/d energy-corrected milk (Table 1).
Table 1. Composition of Experimental Diets.
| experimental
dietsa |
|||
|---|---|---|---|
| BSL-free | BSL-1 | BSL-2 | |
| ingredients (g/kg DM) | |||
| concentrate mixture | 569.6 | 569.6 | 569.6 |
| rapeseed meal | 430.4 | 289.6 | 140.8 |
| BSL | 0 | 140.8 | 289.6 |
| chemical composition (g/kg DM) | |||
| CP | 288 | 275 | 262 |
| crude ash | 69.6 | 63.7 | 57.4 |
| NDFb | 274 | 271 | 267 |
Blue sweet lupin seeds (BSL), BSL-free, blue sweet lupin-free feeding; BSL-1, blue sweet lupin seeds 1 kg; BSL-2, blue sweet lupin seeds 2 kg.
Neutral Detergent Fiber (NDF).
The feeding trial, carried out in July to September 2020, started with a 7-day adaptation period without lupin seed meal [BSL-free (AP)]. Afterward, 1 kg of rapeseed meal was replaced by 1 kg of BSL for 7 days (BSL-1). Therefore, a corresponding mixture of rapeseed meal, BSL, and dairy concentrate was prepared and offered in two equal portions daily at 7 a.m. after the morning milking and 2 p.m. before the evening milking to ensure total uptake. The period was followed by a 10-day depuration period [BSL-free (DP1)], without BSL in the diet. Afterward, 2 kg of rapeseed meal was replaced by 2 kg of BSL (BSL-2). Therefore, again a corresponding mixture of rapeseed meal and BSL was prepared and fed twice daily for 7 days, which was followed again by a 10-day depuration period [BSL-free (DP2)].
Analysis of Feed Ingredients
Feed components were analyzed for DM, crude ash, CP, and neutral detergent fiber (NDF) according to VDLUFA (Association of German Agricultural Analytic and Research Institutes) standard methods.18−20
Analysis of Milk Ingredients
Milk yield was recorded daily. Milk samples were taken twice daily during each milking and stored at −20 °C for analysis of QAs. In regular intervals, milk samples were taken for proximate analysis of milk protein, fat, and lactose according to § 64 L01.00-78 of the German Food and Feed Code (LFGB), and milk urea according to directive 1.13 of the German Association for Performance and Quality Testing e.V. (DLQ).21,22
Solvents and Chemicals
All organic solvents used in this work were of at least analytical grade. Solvents used for liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis were of LC–MS grade.
Analytical Standards
For identification and quantification, the following analytical standards were used: (+)-13α-hydroxylupanine (purity 97%, TRC), (+)-lupanine perchlorate (purity 97%, TRC), (+)-α-iso-lupanine perchlorate (purity 97%, TRC), (−)-angustifoline (purity 97%, CfmOT), (−)-lupinine (purity 96%, Sigma-Aldrich), multiflorine (purity 99%, CfmOT), and (−)-sparteine-sulfate·5 H2O (purity, 98%; Targetmol), respectively.
QAs in BSL and Milk
For determination of the QAs in ground BSL, representative samples of about 100 g each were collected (samples from four storage containers of 25 kg). Subsequently, the samples were ground with an Ultra Centrifugal Mill passing a sieve of 1 mm. QA analyses were performed at the National Reference Laboratory (NRL) for Feed Additives at the German Federal Institute for Risk Assessment (BfR). Samples were analyzed for nine QAs (anagyrine, cytisine, angustifoline, 13α-hydroxylupanine, isolupanine, lupanine, lupinine, multiflorine, and sparteine), which were also used to calculate the sum of the QAs. Analysis and quantification of all samples was done using high-performance liquid-chromatography–tandem mass spectrometry with electrospray ionization in positive ion mode (LC-ESI-MS/MS; API 6500 Sciex). Each measurement was performed in duplicate.
Two in-house validated sample preparation methods were utilized, one for solid (feed) and the other for liquid matrices (milk). Briefly, BSL or milk samples were mixed and the QAs were extracted with an acidified acetonitrile/water solution. For this purpose, 5 g of BSL was extracted with 5 mL of extraction solution (0.1% formic acid, acetonitrile/water, 50:50, v/v) or 2 mL of milk was extracted with 25 mL of extraction solution (0.1% formic acid, acetonitrile/water, 90:10, v/v). After 15 min extraction time in an overhead-shaker, the samples were frozen (−80 °C) to precipitate proteins. After thawing, samples were centrifuged (4000 × g) for 5 min to separate precipitated proteins from the solution.
For milk samples, additionally a degreasing step of the supernatant was included by using n-hexane. The n-hexane layer was discarded.
The sample extracts must be diluted with ultrapure water and injection solution. The dilution factor depends on the concentration of the analytes in the respective sample and must be within the concentration range of the standard curve used. The concentration ranges of the standard curves are between 0.5 mg/kg and 5.5 mg/kg for BSL and between 34 and 370 μg/kg for milk samples. After centrifugation (4000 × g for 5 min), the final supernatant was decanted into a 2 mL crimp vial for injection into the LC-ESI-/MS–MS. Measurement results were evaluated with the software Analyst 1.6.
For identification (examples of chromatograms are given in Figures S1–S5 in the Supporting Information pages S1–S4), a retention time window of ±0.1 min around the expected retention time of the corresponding QA was set. Furthermore, the QAs were identified by using two multiple reaction monitoring (MRM) transitions (at least 1 precursor and 2 product ions detected) and calculating the relative ion ratio between both MRM transitions according to regulation (EU) 2021/808.23 Quantification was performed by preparing a matrix-matched external calibration curve using the analytical standards mentioned before. Briefly, the obtained validation parameters of both methods (milk and BSL) are summarized here for the assessment of the transfer study.
For the analysis of QA in BSL, the recovery was determined by analyzing soybean meal fortified at two different QA concentrations 5 mg/kg and 50 mg/kg (n = 6), respectively. The recoveries for all determined QAs ranged between 80 and 110%. The coefficient of variation (CV) as measure for the repeatability of the applied methods was below 10%. The inter-laboratory reproducibility determined by analyzing samples on different days, by different operators, and with different LC–MS/MS instruments was below 10%.
For the BSL, the limit of detection (LOD) ranged between 0.01 mg/kg (lupanine) and 0.36 mg/kg (multiflorine), and the limit of quantitation (LOQ) ranged between 0.03 mg/kg (lupanine) and 1.19 mg/kg (multiflorine).
For the analysis of milk, the recovery was determined by analyzing milk samples fortified at two different QA concentrations 6 and 60 μg/kg (n = 6), respectively. The mean recovery for the determined QAs ranged between 85 and 105%. The CV as measure for the repeatability is below 10%. The inter-laboratory reproducibility determined by analyzing samples on different days, by different operators, and with different LC–MS/MS instruments was below 8%. For milk, the LOD ranged between 0.02 μg/kg (13α-hydroxylupanine) and 0.41 μg/kg (multiflorine), and the LOQ ranged between 0.06 μg/kg (13α-hydroxylupanine) and 1.36 μg/kg (multiflorine).
Statistical Analysis
Statistical analyses were carried out using the MIXED procedure of SAS (version 9.4, 2016, SAS Institute Inc., Cary, NC, USA). Days and periods were included as fixed effects in the model for milk yield, fat, protein, urea, and lactose concentration. Measurements taken on the same cow but at different times were considered as repeated measures. Multiple comparisons among periods were evaluated by Tukey’s post hoc test. A p-value of <0.05 was considered as indicative for significant difference between periods.
Toxicokinetic Modeling of QA Transfer into the Milk
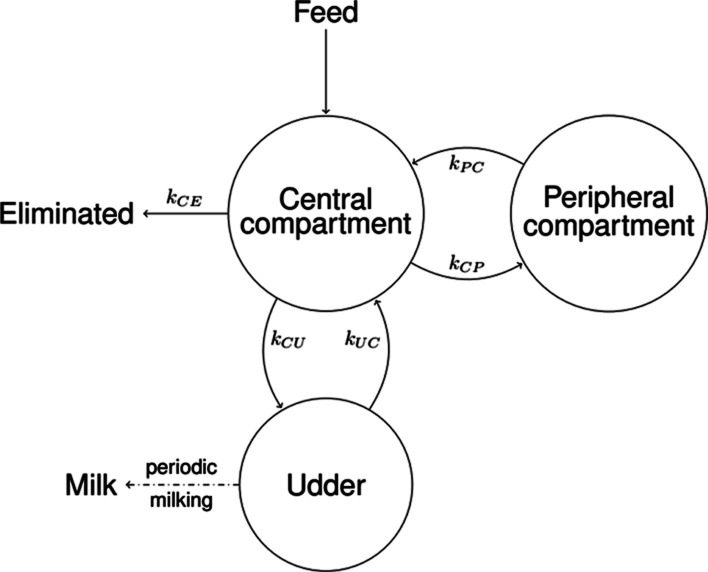
To derive transfer parameters relevant for risk assessment and to allow the prediction of the transfer of QAs from feed into cow’s milk, a mathematical model was developed based on the data, specifically a 3-compartment physiologically based toxicokinetic (PBTK) model (Figure 2 and eqs 1 and 2). The model was fitted for the four most prevalent QAs, for which enough data were available: lupanine, 13α-hydroxylupanine, isolupanine, and angustifoline.
Figure 2.
Schema of the 3-compartment model of QA toxicokinetics in dairy cows. The central compartment represents the entry point for QAs into the cow and the output site for elimination (grouping unabsorbed as well as putatively metabolized and/or excreted QAs). The peripheral compartment acts as a small storage. The udder compartment is where the milk is produced, stored, and periodically emptied at milking events (together with the QAs contained). The parameter kij represents the transition rate from compartment i to compartment j for the compartments: i,j = C, Central; P, Peripheral; U, Udder; and E, Elimination.
The PBTK model in Figure 2 was compared to other similar models (with different arrangements of compartments) using the Bayesian information criterion (BIC), where the chosen model performed best (data not shown). The chosen model consists of 3 compartments. The first one is the central compartment, the entry point for QAs with feed into the cow, as well as the place where QAs are eliminated. These elimination groups together unabsorbed as well as putatively metabolized and/or excreted QAs. The central compartment represents both blood plasma and a biological component (e.g., groups of cells, proteins, or lipids) that is in rapid equilibrium with plasma regarding QAs. The second compartment is the peripheral compartment, which acts as a small storage for QAs; it is a biological component that more slowly exchanges QAs with the central compartment. The third and last is the udder compartment, which can also exchange QAs with the central compartment, while producing and storing milk and, critically, excreting QAs with that milk at periodic milking events. Since only milk data were available, the exact biological nature of all the components of each compartment could not be established, which does not undermine the predictive ability of the model. The PBTK model (Figure 2) is described by the following differential equations between milking events
| 1 |
where A(t)=(AC(t), AU(t), AP(t))T is the amount vector containing the amount of the respective compartment at time t; I(t) is the input vector at time t; and M is the transition matrix given by
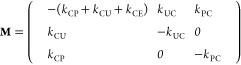 |
2 |
here the model parameter kij represents the transition rate from compartment i to compartment j for the following compartments: i,j = C, Central; i,j = P, Peripheral; i,j = U Udder; and i,j = E, Eliminated (conceptually lumping any metabolization and excretion). Here, the complete emptying of the udder compartment occurs twice daily during the periodic morning and evening milking events.
A peripheral compartment was included based on the shape of the data from the depuration period (Figure S6, Days 14–17 and 31–34), where a biphasic behavior (two half-lives) was apparent. A very dominant short α-half-life, reflecting elimination of QAs from the central compartment, and a second less prevalent longer β-half-life, reflecting elimination of QAs from the peripheral compartment, were identified. The model mechanics assume complete and uniform absorption of QAs into the central compartment distributed uniformly across 5 h after feeding; this does not imply that the effective physiological absorption is 100%; the effective absorption from feed and bioavailability for milk excretion is included via the interplay of rate constant kij. The last piece of the model is the implementation of the periodic emptying of the udder at each milking time, which is performed algorithmically as detailed in the Supporting Information Section Complete Toxicokinetic Model.
An optimization approach was used to obtain model parameter kij by minimizing the log squared error for the best fit.24 In addition, the tails of the depuration (10 days after start of feeding for each feeding period) was weighted with only 25% in order not to overvalue the more irrelevant β-phase of elimination. Data below LOQ or LOD were also considered for the fit by interpreting them as an interval in which the true values lie, so that the error function does not penalize values within that interval. A permutation test was applied to check the hypothesis of a dose-dependent transfer into the milk.25 Confidence intervals were derived using the delete-two jackknife method.26 In addition, the optimized model for each QA was used to estimate transfer parameters: the α- and β-half-lives of the respective elimination phases as well as the steady-state TR from feed to milk, defined as
| 3 |
Lastly, the relative transition amount (RTA) was determined for each QA. RTA is helpful to understand at what point there is a transition from the α- to the β-elimination phase. Specifically, RTA tells us at what amount in milk (as a percentage of steady state or maximum) the slope of the depuration is better approximated by the β-half-life rather than the α-half-life. A more detailed description of the derivation of transfer parameters can be found in the Supporting Information Sections 2.1–2.3.
Assessment of Consumer Exposure to QAs Using the EFSA RACE Tool
The EFSA Rapid Assessment of Contaminant Exposure (RACE) software tool was used to estimate the exposure to QAs resulting from milk consumption, considering the determined QA levels.27 With the help of food consumption information from the EFSA Comprehensive European Food Consumption Database, RACE provides an estimate of acute and chronic exposure from single foods. These values can then be compared with relevant toxicological reference points. For the assessment, maximum QA levels in milk during the exposure phases were used. As in the EFSA opinion on QAs, risk characterization was performed by applying the MoE approach using the dose of 0.16 mg sparteine/kg bw as reference point.
Results and Discussion
Feed Intake and Milk Yield
Throughout the experiment, the whole concentrate proportion was ingested, indicating no obvious adverse effect of BSL on concentrate intake. The forage mixture was provided ad libitum, and individual intake was not recorded. Milk yield slightly declined over the course of the experiment from 31.6 ± 4.7 kg/d to 29.1 ± 4.5 kg/d (p < 0.001) (Table 2).
Table 2. Milk Yield and Milk Composition of the Cowsa.
| experimental periodsb |
|||||||
|---|---|---|---|---|---|---|---|
| BSL-free (AP1) | BSL-1 | BSL-free (DP1) | BSL-2 | BSL-free (DP2) | SEM | p-value period | |
| milk yield (kg) | 31.6c | 31.4c,d | 30.3c,d,e | 29.9d,e | 29.1e | 1.04 | 0.002 |
| fat (%) | 3.85c,d | 4.13c | 3.91c,d | 3.50d | 3.77c,d | 0.23 | 0.039 |
| protein (%) | 3.01 | 2.90 | 2.85 | 2.86 | 2.96 | 0.07 | 0.144 |
| lactose (%) | 4.83 | 4.84 | 4.84 | 4.81 | 4.66 | 0.08 | 0.136 |
| urea (mg/L) | 668 | 626 | 632 | 675 | 520 | 62.1 | 0.128 |
SEM, standard error of the mean.
BSL-free (AP1), Adaptation period, blue sweet lupin-free feeding; BSL-1, experimental period 1, blue sweet lupin seeds 1 kg; BSL-free (DP1), depuration period 1, blue sweet lupin-free feeding; BSL-2, experimental period 2, blue sweet lupin seeds 2 kg; BSL-free (DP2), depuration period 2, blue sweet lupin-free feeding.
Means in the same row with different letters differ significantly.
Means in the same row with different letters differ significantly.
Means in the same row with different letters differ significantly.
The period had a significant effect on the fat content in milk (p = 0.039), with highest contents found in BSL-1 with 4.13% and lowest contents in BSL-2 with 3.5%. Contents of protein, lactose, and urea in milk did not differ between periods (p > 0.05). The lactation stage of the individual cows and external influences, such as the outside temperature, which exceeded 25 °C throughout the present experiment, can have an impact on the performance parameters like milk production.28 Additionally, other authors previously reported decreases in milk yield due to the feeding of lupin seeds in comparison to feeding rapeseed meal- or soybean meal-based concentrates, which might be related to the lower CP content in lupin seeds (Table 1).29−31 In addition to a generally lower CP content in lupin seeds, the CP of unprocessed lupin seeds is known to be extensively degraded in the rumen, causing a reduction in amino acid flux to the duodenum.30,32 Joch suggested that decreases in milk yield may be due to the lower methionine content of lupin protein, although the addition of ruminally protected methionine did not increase the milk yield in that study.31
Milk fat represents the most variable component in milk and can be influenced by nutritional as well as physiological aspects.33 Froidmont showed increased levels of milk fat after protein replacement of soybean meal with lupin and attributed increased milk fat to the higher fiber content in lupin seeds, with a concomitant increase in acetate liberation in the rumen as a precursor for milk fat.34 This was not observed in the present study and may depend on other dietary effects and lactation stage of the cows. A reduced milk fat content due to the feeding of lupin seeds has also been observed by others.35,36
QAs in BSL and Transfer into the Milk
The sum of determined QAs in the present BSL ranged between 0.17 and 0.19% in DM and was higher than the commonly reported <0.05% for “sweet” lupins.4 Higher levels of QAs in L. angustifolius have been reported before and are most likely due to abiotic influences, cross mutation, and backcrossing with wild varieties. Higher outdoor temperatures or lower soil pH values during the growing season can also lead to higher levels of QAs in sweet lupins.37,38
The literature reports the main alkaloids for L. angustifolius: lupanine, 13α-hydroxylupanine, isolupanine, angustifoline, 13-angeloyloxylupanine, and 13-tigloyloxylupanine.39,40 We found a slightly different set in the present study, where levels of 13α-hydroxylupanine, lupanine, angustifoline, and isolupanine in BSL were higher than levels of multiflorine and sparteine, resulting in high intakes of 13α-hydroxylupanine and lupanine (Table 3). The intake of total QAs was 1774 mg/d during the BSL-1 feeding period and 3549 mg/d during the BSL-2 feeding period (Table 3).
Table 3. Intake of QAs with BSL in mg/d.
| experimental dietsb | ||
|---|---|---|
| QA intake with BSL (mg/d) | BSL-1 kg | BSL-2 kg |
| totala | 1774 | 3549 |
| angustifoline | 223 | 446 |
| 13α-hydroxylupanine | 702 | 1404 |
| isolupanine | 129 | 257 |
| lupanine | 715 | 1430 |
| multiflorine | 2.45 | 4.89 |
| sparteine | 3.03 | 6.06 |
QAs as analyzed in BSL.
Blue sweet lupine (BSL), BSL-1, blue sweet lupin seeds 1 kg/d; BSL-2, blue sweet lupin seeds 2 kg/d.
Toxicity of QAs has been more thoroughly studied for sparteine and lupanine in humans and rats, while effects of other QAs have not yet been systematically investigated.10,12,41,42 In rat studies, a lower toxicity was observed for lupanine and 13α-hydroxylupanine than for sparteine.42,43 Until now, there are only few studies evaluating the toxicity of QAs in cattle. For instance, cattle showed reduced voluntary feed intake when intact lupin seeds were fed in contrast to lupin seeds that were previously detoxified by boiling and soaking in water.44 However, QA intake with Lupinus albus used in that study was considerably higher than that in the present one, reaching estimated levels of 60 g/d of lupanine and 21 g/d 13α-hydroxylupanine with L. albus. Increased levels of QAs therefore appear to result in decreased appetite, confirming observations made by others.30,45 No negative effects on animal health were seen in the present study with intakes of 1.27–2.54 mg lupanine/kg bw and 1.24–2.49 mg 13α-hydroxylupanine/kg bw. However, other studies with cattle observed symptoms like reduced general condition, frothing at the mouth, and protrusion of the nictating membrane with higher total QA intake levels of 57.6 g QAs/kg bw.46 Severe toxic effects of QA in cattle have been described only for the teratogenic QA anagyrine.47−49 During critical times of gestation, the ingestion of several lupin species by pregnant cattle has been associated with the so-called crooked calf syndrome.12,50,51 Anagyrine has been identified as a main causative QA, but anagyrine was not detected in BSL used in the present study.50,51 So far, possible intoxication of calves by anagyrine or other QA in milk has not been reported, but according to the present results, this has to be taken into consideration.
There are currently no maximum levels of QAs for animal or human nutrition in the EU. Nevertheless, gathering knowledge concerning the transfer of QAs from feed to animal food is vital. Although animals showed no adverse health effects in the present study, it was demonstrated that with the administration of only 1 kg sweet lupin seeds, a transfer of QAs into the milk occurs, resulting in a total QA concentration of 2.81 mg/kg milk. Although the quantities of QAs excreted via milk differed slightly between the cows, the QA excretion pattern was similar (Figure 3).
Figure 3.
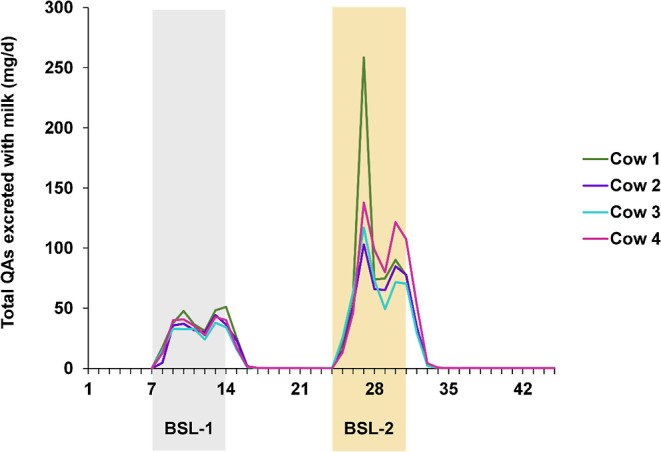
Total QAs excreted with milk daily. Shaded are the feeding periods BSL–1 (blue sweet lupin seeds 1 kg/d) and BSL–2 (blue sweet lupin seeds 2 kg). Unshaded are periods with no QA feeding: the adaptation periods before BSL-1 as well as the depuration periods following BSL-1 and BSL-2.
The concentrations of individual QAs quantified in morning and evening milk during steady state are shown in Figure 4.
Figure 4.
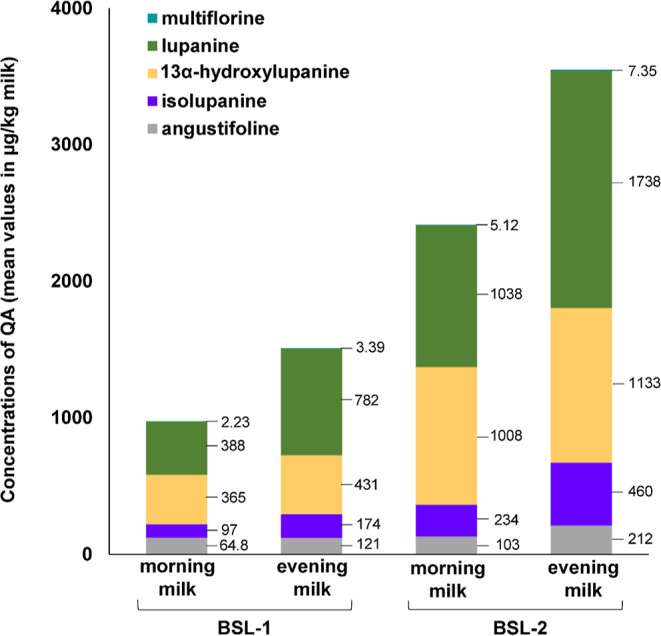
QA contents in morning and evening milk during BSL-1 and BSL-2 feeding (mean values in steady state in μg/kg). Feeding periods, BSL–1 (blue sweet lupin seeds 1 kg/d) and BSL–2 (blue sweet lupin seeds 2 kg/d).
As in BSL, 13α-hydroxylupanine and lupanine were found to be the most abundant QA in milk. Despite of average contents in BSL of 3.03 mg/kg, concentrations in milk of sparteine were near or below the LOQ of <0.10 μg/kg milk.
Concentrations of multiflorine, angustifoline, and especially lupanine were noticeably higher in the evening milk than in the morning milk (Figure 4). This effect was also reflected in the higher TRs of multiforine, angustifoline, and lupanine for evening milk (Table 4). In the evening, cows were fed with lupin seeds 2 h before milking, while in the morning, cows were milked before feeding. It follows that QAs both from morning feeding and in part from evening feeding were excreted in the evening milk, while in the morning milk, only the remainder was found. Interestingly and in contrast to the other QAs, the TR of 13α-hydroxylupanine was higher in the morning than in the evening milk (Table 4). An explanatory hypothesis is the possible biotransformation of QAs in the cow. So far, metabolism of individual QAs has been investigated only in rats, pigs, rabbits, and humans.10,52,53 Studies in rats showed that sparteine was oxidized to lupanine, which was found in the urine of orally dosed rats in vivo (suspected microsomal metabolization), while lupanine was found to be presumably transformed to a hydroxyl derivative through a yet unknown pathway.10,54 Until now, there exists no information regarding the possible metabolization of lupanine into 13α-hydroxylupanine in cows. However, conversion could explain its higher values in the morning milk.
Table 4. Estimated TRs of QAs from Feed into Milk, which is made up out of morning + evening Milk.
| mean [%] = (morning + evening) | 95% confidence interval [%] | |
|---|---|---|
| 13α-hydroxylupanine | 1.74 (0.95 + 0.79) | 1.34–2.16 |
| lupanine | 2.31 (0.96 + 1.35) | 1.85–2.77 |
| isolupanine | 2.92 (1.21 + 1.71) | 2.57–3.35 |
| angustifoline | 1.05 (0.43 + 0.62) | 0.93–1.18 |
| multiflorinea | 3.74 (1.79 + 1.95) | |
| sparteinea | 0.13 (0.06 + 0.06) |
Marks the QAs for which no model was developed but nevertheless a rough approximation of the TRs from the data was made.
It is known that ruminants can render certain plant toxins harmless via microbial metabolization in the rumen. However, an in vitro rumen fermentation study conducted in our department (data not shown) did not find ruminal degradation of lupanine, which confirms the previous results of Aguiar.55 Accordingly, metabolization of QAs in the liver might be the cause for the observed differences in QA excretion, but further research is needed in this regard. Other metabolites were not investigated with the current analytical method, therefore, an occurrence of possible metabolites in milk cannot be excluded.
Toxicokinetic Modeling and TRs for QAs
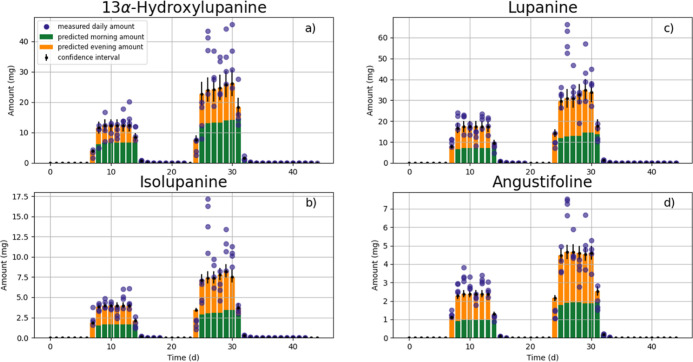
As a first step, the hypothesis of dose-dependent QA transfer into milk24 was tested. A permutation test was applied to verify whether the experimental data allow rejection of the hypothesis. With the exception of angustifoline, the permutation test provided no indication of a non-linear dose-dependent TR for the QAs studied. The apparent non-linearity for angustifoline was neglected because it can be attributed to the small sample size. Therefore, all QAs were fitted to the 3-compartment PBTK model (eqs 1 and 2, Figure 2) using the data for all cows and all experimental periods (both doses BSL-1 and BSL-2) simultaneously to obtain the optimized model parameters (Table S4). Results of the PBTK model for QA excretion via milk are shown in Figure 5.
Figure 5.
Daily amounts excreted via milk for four QAs. Bars denote the toxicokinetic model results plotted together with their confidence intervals across animals (divided into morning—yellow—and evening excretion—green). Blue dots represent the daily amount excreted obtained from the feeding experiment.
In Figure 5, QA excretion from morning and evening milk was lumped together as daily excretion (total bar for model and dots for experiment). During the first BSL feeding period (BSL-1), the concentration profiles of QAs could be adequately predicted. Concerning the second feeding period (BSL-2), the model was only able to reproduce the average behavior, as the measured QA contents in milk displayed higher variability. In particular, the model was unable to reproduce the apparent peak (Figure 5, day 27) in the analyzed QA contents in milk at the beginning of BSL-2 feeding, which might indicate more complex underlying kinetics. Since the PBTK model could nevertheless reproduce the average behavior, it was used to calculate transfer parameters, namely TRs (Table 4) and milk excretion α- and β-half-lives (Tables S1, S2).
All four investigated QAs showed fast and dominant milk excretion α-half-lives of around 0.27 d (Table S1), which are similar to the literature plasma half-lives for lupanine of 0.29 and 0.23 d in cows.48,49 In contrast, the half-life of lupanine in beef cattle reported in another study was 0.48 d with a mean residence time of 50–61 h, equivalent to half-lives of 1.44 and 1.76 d, respectively, in a 1-compartment setting.49,56,57 Those values are considerably higher than the derived values of the present study (Table S1), suggesting that there are differences in the kinetic behavior of lupanine between different breeds or production purposes and may be attributable to the lack of excretion with milk.49
The shape of the data profile from the depuration period (Figure S6, days 14–17 and 31–34) shows a biphasic behavior (two half-lives). The chosen model (Figure 2) reproduces this behavior; from it, β-half-lives of 2.48–5.18 d for the four QAs were estimated (Table S2). The intake of QAs from sources other than measured feed can be excluded. Additionally, the QA analysis showed values above the LOQ in the depuration periods contrary to the adaptation period. This suggests that small amounts of QAs remained in the peripheral compartment after exposure, resulting in an extended β-half-life during the depuration period. But how relevant are these β-half-lives for risk analysis? The answer comes from the postulated parameter RTA (eq S17, Table S3) that quantifies the relative importance of the α- and β-half-lives. RTA indicates when the system moves from the α-phase to the β-phase of depuration. The RTAs found for QAs (Table S3) range from 0.11 to 0.33% of the steady state amounts, which means that more than 99.67% of the depuration occurs in the α-phase. Therefore, the β-phase of depuration is practically irrelevant, provided it happens at amounts that are toxicologically of no concern. Combined with knowledge of a very short α-half-life (Table S1), we conclude that in most cases, it is possible to rely on a simple multiplicative TR calculation. The TRs into morning + evening milk of individual modeled QAs (Table 4) range from 1.05% for angustifoline to 2.92% for isolupanine. Furthermore, although the data did not allow the development of a PBTK model for sparteine, its TR can be roughly estimated directly from the data by averaging
| 4 |
for all days in apparent steady state with measurements above LOQ, resulting in a TR of 0.12%. The same method for multiflorine yields a TR of 3.74%. These results may partly be explained by the fact that, so far, it cannot be ruled out that individual QAs are metabolized to other QAs, resulting in higher TR for individual QA. Although the use of simple multiplicative calculations using TR should suffice for most cases of risk analysis, the full predictive toxicokinetic model code is included as part of the Supporting Information as it can help understanding how these contaminants are transported into the milk.
Assessment of Consumer Exposure to QAs Using the EFSA RACE Tool
A preliminary estimation of the dietary acute exposure by using the EFSA RACE tool showed that the sole consumption of milk containing QAs at a level as measured in the present study might result in intakes above 0.16 mg/kg bw for high milk consumers.8 This, in turn, means that the corresponding MoE is <1, reflecting an exposure in the effect level and consequently a health concern (Table 5).
Table 5. Comparison of the Exposure of High (P95) Milk Consumers to the Lowest Single Oral Effective Dose for QAa.
| population group | high consumer (P95) |
|
|---|---|---|
| QA content in cow’s milk (μg/kg) | ||
| BSL-1 | BSL-2 | |
| max | max | |
| 19607.3 | 90186.5 | |
| comparison of exposure to toxicological reference point expressed as MoE | ||
| infants | 0.04 | 0.01 |
| toddlers | 0.11 | 0.02 |
| other children | 0.18 | 0.04 |
| adolescents | 0.41 | 0.09 |
| adults | 0.72 | 0.16 |
| elderly | 0.93 | 0.20 |
| very elderly | 0.96 | 0.21 |
| pregnant woman | 0.77 | 0.17 |
| lactating woman | 0.92 | 0.20 |
The EFSA Rapid Assessment of Contaminant Exposure (RACE) tool was used for calculation of different exposure scenarios. Maximum QA in the milk during BSL-1 and BSL-2 of the feeding experiment and a lowest single oral effective dose of 0.16 mg sparteine/kg bw/d were taken as a basis. Bold numbers: exceedance of MoE 1.
Already in BSL-1, MoEs <1 were measured for all population groups. Additionally, in BSL-2, maximum levels in milk also represent an exposure in the effect level for all population groups.
The calculated MoE values refer only to the sole consumption of raw milk containing QA levels as measured in the present study. Therefore, it cannot be excluded that dilution, processing of milk, as well as the production of dairy products may have consequences for the content of QA and, consequently, for the exposure level and the resulting MoE values.
However, QA contents and QA profiles differ considerably between lupin breeds and even within the same variety. The different excretion patterns of individual QAs also show that further investigations are necessary to understand the metabolism of QAs within dairy cows. In conclusion, the present study proves the transfer of QAs from BSL into milk of dairy cows already at low inclusion levels of lupin seeds in the ruminant diet.
Acknowledgments
The authors would like to thank the animal care staff of the German Federal Institute for Risk Assessment for assistance during the feeding experiment. The support of Mandy Schwieters from the National Reference Laboratory (NRL) for Feed Additives of the BfR is highly appreciated.
Glossary
Abbreviations used
- AP
adaptation period
- BIC
Bayesian information criterion
- BfR
German Federal Institute for Risk Assessment
- BSL
blue sweet lupin
- DLQ
German Association for Performance and Quality Testing e.V.
- DM
dry matter
- DP
depuration period
- EFSA
European Food Safety Authority
- LC/MS–MS
high-performance liquid-chromatography–tandem mass spectrometry
- LFGB
German Food and Feed Code
- LOQ
limit of quantification
- MoE
margin of exposure
- MRM
multiple reaction monitoring
- NDF
neutral detergent fiber
- NRL
national reference laboratory
- PBTK
physiologically based toxicokinetic model
- QAs
quinolizidine alkaloids
- TR
transfer rate
- VDLUFA
Association of German Agricultural Analytic and Research Institutes
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.2c02517.
LC–MS/MS chromatograms; overlay LC–MS/MS chromatogram of the quantifier MRM transitions of nine QAs in a standard solution with a concentration of 2.5 ng/mL each; overlay LC–MS/MS chromatogram of the quantifier MRM transitions of five QAs analyzed in lupin seeds (whole grain, untoasted) used for feeding; overlay LC–MS/MS chromatogram of the quantifier MRM transitions of nine QAs in a matrix matched calibration by utilizing cow milk (dilution 1:20) fortified at a level of 2.5 ng/mL; overlay LC–MS/MS chromatogram of the quantifier MRM transitions of four QAs analyzed in a cow milk sample (dilution 1:20); overlay LC–MS/MS chromatogram of the quantifier MRM transitions of four QAs analyzed in a cow milk sample (dilution 1:200); parameters for risk assessment (TRs, half-lives, and RTA); α-Half-lives; β-half-lives; RTA relative transition amount; complete toxicokinetic model; optimized model parameters; logarithmic plot to show biphasic behavior during depuration and computer code for running the predictive toxicokinetic model including instructions (PDF)
The study was funded by the German Federal Institute for Risk Assessment grant BfR-SiN-1322–789.
The authors declare no competing financial interest.
Supplementary Material
References
- Mazumder K.; Biswas B.; Kerr P. G.; Blanchard C.; Nabila A.; Golder M.; Aziz M. G.; Farahnaky A. Comparative assessment of nutritional, thermal, rheological and functional properties of nine Australian lupin cultivars. Sci. Rep. 2021, 11, 2151. 10.1038/s41598-021-00838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M. Chemical Defense of Leguminosae. Are Quinolizidine Alkaloids part of the antimicrobial defense system of lupins?. Z. Naturforsch. 1984, 39, 548–552. 10.1515/znc-1984-0607. [DOI] [Google Scholar]
- Gresta F.; Wink M.; Prins U.; Abberton M.; Capraro J.; Scarafoni A.; Hill G.. Lupins in European Cropping Systems. Legumes in Cropping Systems; CAB International, 2017; pp 88–108. 10.1079/9781780644981.0088 [DOI] [Google Scholar]
- Von Baer D.; Perez I.. Quality standard propositions for commercial grain of white lupin (Lupinus albus). Proceeding of the 6th International Lupin Conference; Temuco-Pucon: Chile, 1991; pp 158–167. [Google Scholar]
- Board of Food Standards Australia New Zealand . Schedule 19 – Maximum Levels of Contaminants and Natural Toxicants – Food Standards (Proposal P1025 – Code Revision) Variation – Australia New Zealand Food Standards Code – Amendment No. 154, Rev. 01/2016, last revised June 2021 .https://www.legislation.gov.au/Details/F2021C00628 (accessed Jan 19, 2022).
- ACNFP . Report on Seeds from Narrow Leafed Lupin. Appendix IX; MAFF Publications: London, GB. 1996, p 107. [Google Scholar]
- EU Commission (Direction Générale de la. 1998. Avis du 17 mars 1998 du Conseil supérieur d’hygiène publique de France (section de l’alimentation et de la nutrition) relatif à l’emploi de farine de lupin en alimentation humaine. Bulletin Officiel; EU, 1998. n 98/27. [Google Scholar]
- Wink M.; Hartmann T.; Witte L.; Rheinheimer J. Interrelationship between Quinolizidine Alkaloid Producing Legumes and Infesting Insects: Exploitation of the Alkaloid- Containing Phloem Sap of Cytisus scoparius by the Broom Aphid Aphis cytisorum. Z. Naturforsch. 1982, 37, 1081–1086. 10.1515/znc-1982-11-1206. [DOI] [Google Scholar]
- Boschin G.; Resta D. C.. Alkaloids Derived from Lysine: Quinolizidine (a Focus on Lupin Alkaloids). In Natural Products; Ramawat K. G., Mérillon J. M.; Springer: Berlin, Heidelberg, 2013; pp 381–403. [Google Scholar]
- Schrenk D.; Schrenk L.; Bodin J. K.; Chipman J.; del Mazo B.; Grasl-Kraupp C.; Hogstrand L. R.; Hoogenboom J. C.; Leblanc C. S.; Nebbia E.; et al. Scientific opinion on the risks for animal and human health related to the presence of quinolizidine alkaloids in feed and food, in particular in lupins and lupin-derived products. EFSA J. 2019, 17, 5860. 10.2903/j.efsa.2019.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovo K.; Huguet F.; Pothier J.; Durand M.; Breteau M.; Narcisse G. Comparative Pharmacological Study of Sparteine and its Ketonic Derivative Lupanine from Seeds ofLupinus albus. Planta Med. 1984, 50, 420–424. 10.1055/s-2007-969753. [DOI] [PubMed] [Google Scholar]
- Keeler R. F. Lupin alkaloids from teratogenic and nonteratogenic lupins. III. Identification of anagyrine as the probable teratogen by feeding trials. J. Toxicol. Environ. Health 1976, 1, 887–898. 10.1080/15287397609529391. [DOI] [PubMed] [Google Scholar]
- Commission Regulation (EU) No 1017/2017 of 15 June 2017 Amending Regulation (EU) No 68/2013 on the Catalogue of Feed Materials; Off J Eur Union, 2017. L 159/48. [Google Scholar]
- Wink M. Quinolizidine and Pyrrolizidine Alkaloid Chemical Ecology - a Mini-Review on their similarities and differences. J. Chem. Ecol. 2019, 45, 109–115. 10.1007/s10886-018-1005-6. [DOI] [PubMed] [Google Scholar]
- Böhme A.; Dietze M.; Gefrom A.; Priepke A.; Schachler B.; Struck C.; Wehling P.. Lupinen - Anbau und Verwertung; Publisher: Universität Rostock, Agrar- und Umweltwissenschaftliche Fakultät - Phytomedizin: Gesellschaft zur Förderung der Lupine (G.F.L.), 2016. [Google Scholar]
- Ortega J. A.; Lazerson A. Anagyrine-induced red cell aplasia, vascular anomaly, and skeletal dysplasia. J. Pediatr. 1987, 111, 87–89. 10.1016/s0022-3476(87)80349-9. [DOI] [PubMed] [Google Scholar]
- Shupe JL.; Binns W.; James LF.; Keeler R. F. A congenital deformity in calves induced by the maternal consumption of lupin. Aust. J. Agric. Res. 1968, 19, 335–340. 10.1071/AR9680335. [DOI] [Google Scholar]
- VDLUFA (Association of German Agricultural Analytic and Research Institutes) . 3.1 Bestimmung von Feuchtigkeit. In: VDLUFA. Method Book Volume III of VDLUFA: The Chemical Analysis of Feedstuffs; VDLUFA-Verlag: Darmstadt, 1976. [Google Scholar]
- VDLUFA (Association of German Agricultural Analytic and Research Institutes) . 4.1.1 Bestimmung von Rohprotein. In: VDLUFA. Method Book Volume 3 of VDLUFA: The Chemical Analysis of Feedstuffs; VDLUFA-Verlag: Darmstadt, 1993. [Google Scholar]
- VDLUFA (Association of German Agricultural Analytic and Research Institutes) . 6.5.1 Bestimmung der Neutral-Detergenzien-Faser nach Amylasebehandlung (aNDF) sowie nach Amylasebehandlung und Veraschung (aNDFom). In: VDLUFA; Method Book Volume 3 of VDLUFA: The Chemical Analysis of Feedstuffs; VDLUFA-Verlag: Darmstadt, 2012. [Google Scholar]
- Lebensmittel- und Futtermittelgesetzbuch (LFGB) as amended by the announcement of September 15, 2021 (BGBl. I S. 4253) established by Article 7 of the Law of 27 in September 2021 (BGBl. I S. 4530).
- DLQ . DLQ Directive 1.13 on DLQ Reference Method for the Determination of Urea Content in Milk Continuous Flow Analysis; Deutscher Verband für Leistungs- und Qualitätsprüfung e. V.; 2013. [Google Scholar]
- EU Commission Regulation (EU) No 2021/808 of March 2021 Amending Regulation (EC) No 657/2007 and No 179/98 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing; Off J Eur Union, 2021. L 180/84. [Google Scholar]
- LMFIT . Non-Linear Least-Square Minimization and Curve-Fitting for Python (0.8.0); Newville M., Stensitzki T., Allen D. B.; Ingargiola A.; Zenodo, 2014. [Google Scholar]
- Collingridge D. S. A Primer on Quantitized Data Analysis and Permutation Testing. J. Mix. Methods Res. 2012, 7, 81–97. 10.1177/1558689812454457. [DOI] [Google Scholar]
- Shao J. Consistency of jackknife variance estimators jun. Statistics 1991, 22, 49–57. 10.1080/02331889108802282. [DOI] [Google Scholar]
- EFSA, European Food Safety Agency . Risk Evaluation of Chemical Contaminants in Food in the Context of RASFF Notifications: Rapid Assessment of Contaminant Exposure Tool (RACE); EFSA Supporting publication, 2019: EN-1625. 2019. [Google Scholar]
- West J. W.; Mullinix B. G.; Bernard J. K. Effects of Hot, Humid Weather on Milk Temperature, Dry Matter Intake, and Milk yield of lactating dairy cows. J. Dairy Sci. 2003, 86, 232–242. 10.3168/jds.S0022-0302(03)73602-9. [DOI] [PubMed] [Google Scholar]
- Boguhn J.; Kluth H.; Bulang M.; Engelhard T.; Spilke J.; Rodehutscord M. Effects of using thermally treated lupins instead of soybean meal and rapeseed meal in total mixed rations onin vitromicrobial yield and performance of dairy cows. J. Anim. Physiol. Anim. Nutr. 2008, 92, 694–704. 10.1111/j.1439-0396.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- Guillaume B.; Otterby D. E.; Linn J. G.; Stern M. D.; Johnson D. G. Comparison of Sweet White Lupin Seeds with Soybean Meal as a Protein Supplement for Lactating Dairy Cows. J. Dairy Sci. 1987, 70, 2339–2348. 10.3168/jds.S0022-0302(87)80294-1. [DOI] [PubMed] [Google Scholar]
- Joch M.; Kudrna V. Partial replacement of soybean meal by white lupine seeds in the diet of dairy cows. Asian-Australas. J. Anim. Sci. 2020, 33, 957–964. 10.5713/ajas.19.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchaar C.; Moncoulon R.; Bayourthe C.; Vernay M. Effects of a supply of raw or extruded white lupin seeds on protein digestion and amino acid absorption in dairy cows. J. Anim. Sci. 1994, 72, 492–501. 10.2527/1994.722492x. [DOI] [PubMed] [Google Scholar]
- Bauman D. E.; Griinari J. M. Regulation and nutritional manipulation of milk fat: low-fat milk syndrome. Livest. Prod. Sci. 2001, 70, 15–29. 10.1016/s0301-6226(01)00195-6. [DOI] [PubMed] [Google Scholar]
- Froidmont E.; Bartiaux-Thill N. Suitability of lupin and pea seeds as a substitute for soybean meal in high-producing dairy cow feed. Anim. Res. 2004, 53, 475–487. 10.1051/animres:2004034. [DOI] [Google Scholar]
- Kuoppala K.; Jaakkola S.; Garry B.; Ahvenjärvi S.; Rinne M. Effects of faba bean, blue lupin and rapeseed meal supplementation on nitrogen digestion and utilization of dairy cows fed grass silage-based diets. Animal 2021, 15, 100300. 10.1016/j.animal.2021.100300. [DOI] [PubMed] [Google Scholar]
- Mendowski S.; Chapoutot P.; Chesneau G.; Ferlay A.; Enjalbert F.; Cantalapiedra-Hijar G.; Germain A.; Nozière P. Effects of replacing soybean meal with raw or extruded blends containing faba bean or lupin seeds on nitrogen metabolism and performance of dairy cows. J. Dairy Sci. 2019, 102, 5130–5147. 10.3168/jds.2018-15416. [DOI] [PubMed] [Google Scholar]
- Jansen G.; Jürgens H. U.; Ordon F. Effects of Temperature on the Alkaloid Content of Seeds of Lupinus angustifolius Cultivars. J. Agron. Crop Sci. 2009, 195, 172. 10.1111/j.1439-037X.2008.00356.x. [DOI] [Google Scholar]
- Jansen G.; Jürgens H.-U.; Schliephake E.; Ordon F. Effect of the Soil pH on the Alkaloid Content ofLupinus angustifolius. Int. J. Agron. 2012, 2012, 1–5. 10.1155/2012/269878. [DOI] [Google Scholar]
- Wink M.; Meißner C.; Witte L. Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry 1995, 38, 139–153. 10.1016/0031-9422(95)91890-D. [DOI] [Google Scholar]
- Boschin G.; Annicchiarico P.; Resta D.; D’Agostina A.; Arnoldi A. Quinolizidine alkaloids in seeds of lupin genotypes of different origins. J. Agric. Food Chem. 2008, 56, 3657–3663. 10.1021/jf7037218. [DOI] [PubMed] [Google Scholar]
- Robbins M. C.; Petterson D. S.; Brantom P. G. A 90-Day Feeding Study of the Alkaloids of Lupinus angustifolius in the Rat. Food Chem. Toxicol. 1996, 34, 679–686. 10.1016/0278-6915(96)00040-3. [DOI] [PubMed] [Google Scholar]
- Pothier J.; Cheav S.-L.; Galand N.; Dormeau C.; Viel C. A Comparative Study of the Effects of Sparteine, Lupanine and Lupin Extract on the Central Nervous System of the Mouse. J. Pharm. Pharmacol. 2011, 50, 949–954. 10.1111/j.2042-7158.1998.tb04013.x. [DOI] [PubMed] [Google Scholar]
- Petterson D. S.; Ellis Z. L.; Harris D. J.; Spadek Z. E. Acute toxicity of the major alkaloids of cultivated Lupinus angustifolius seed to rats. J. Appl. Toxicol. 1987, 7, 51–53. 10.1002/jat.2550070109. [DOI] [PubMed] [Google Scholar]
- Mukisira E. A.; Phillip L. E.; Mitaru B. N. The effect of feeding diets containing intact or partially detoxified lupin on voluntary intake and milk production by Friesian dairy cows. Anim. Sci. 1995, 60, 169–175. 10.1017/s1357729800008316. [DOI] [Google Scholar]
- Johnson J. C.; Miller J. D.; Bedell D. M. Tifwhite-78 Lupine Seed as a Feedstuff for Cattle. J. Dairy Sci. 1986, 69, 142–147. 10.3168/jds.S0022-0302(86)80378-2. [DOI] [Google Scholar]
- Gardner D. R.; Panter K. E. Comparison of blood plasma alkaloid levels in cattle, sheep and goats fed Lupinus caudatus. J. Nat. Toxins 1993, 2, 1. [Google Scholar]
- Kennelly E. J.; Flynn T. J.; Mazzola E. P.; Roach J. A.; McCloud T. G.; Danford D. E.; Betz J. M. Detecting potential teratogenic alkaloids from blue cohosh rhizomes using an in vitro rat embryo culture. J. Nat. Prod. 1999, 62, 1385–1389. 10.1021/np9901581. [DOI] [PubMed] [Google Scholar]
- Green B. T.; Lee S. T.; Welch K. D.; Gardner D. R.; Stegelmeier B. L.; Davis T. Z. The serum concentrations of lupine alkaloids in orally-dosed Holstein cattle. Res. Vet. Sci. 2015a, 100, 239–244. 10.1016/j.rvsc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Green B. T.; Panter K. E.; Lee S. T.; Welch K. D.; Pfister J. A.; Gardner D. R.; Stegelmeier T. Z.; Davis T. Z. Differences between Angus and Holstein cattle in the Lupinus leucophyllus induced inhibition of fetal activity. Toxicon 2015b, 106, 1–6. 10.1016/j.toxicon.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Panter K. E.; Welch K. D.; Gardner D. R.; Green B. T. Poisonous plants: effects on embryo and fetal development. Birth Defects Res., Part C 2013a, 99, 223–234. 10.1002/bdrc.21053. [DOI] [PubMed] [Google Scholar]
- Panter K. E.; Gay C. C.; Clinesmith R.; Platt T. E. Management practices to reduce lupine-induced crooked calf syndrome in the Northwest. Rangelands 2013b, 35, 12–16. 10.2111/RANGELANDS-D-12-00074.1. [DOI] [Google Scholar]
- Jurima M.; Inaba T.; Kalow W. Sparteine oxidation by the human liver: absence of inhibition by mephenytoin. Clin. Pharmacol. Ther. 1984, 35, 426–428. 10.1038/clpt.1984.54. [DOI] [PubMed] [Google Scholar]
- Ebner T.; Meese C. O.; Eichelbaum M. Mechanism of cytochrome P450 2D6-catalyzed sparteine metabolism in humans. Mol. Pharmacol. 1995, 48, 1078–1086. [PubMed] [Google Scholar]
- Chaudhuri A.; Keller W. J. New mammalian metabolites of sparteine. Life Sci. 1990, 47, 319–325. 10.1016/0024-3205(90)90590-n. [DOI] [PubMed] [Google Scholar]
- Aguiar R.; Wink M. Do Naïve Ruminants Degrade Alkaloids in the Rumen?. J. Chem. Ecol. 2005, 31, 761–787. 10.1007/s10886-005-3543-y. [DOI] [PubMed] [Google Scholar]
- Gay C. C.; Panter E. P.; Mealey K. L.; Gay J. M.; Hjartarson S. W.; Tibary E. S.; Motteram T.; Wierenga L. F.; James L. F. Comparison of plasma disposition of alkaloids after lupine challenge in cattle that had given birth to calves with lupine-induced arthrogryposis or clinically normal calves. Am. J. Vet. Res. 2004, 65, 1580–1583. 10.2460/ajvr.2004.65.1580. [DOI] [PubMed] [Google Scholar]
- Veng-Pedersen P. Mean Time Parameters in Pharmacokinetics. Clin. Pharmacokinet. 1989, 17, 424–440. 10.2165/00003088-198917060-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.