Abstract
We report the synthesis of molecular prime and composite knots by social self-sorting of 2,6-pyridinedicarboxamide (pdc) ligands of differing topicity and stereochemistry. Upon mixing achiral monotopic and ditopic pdc-ligand strands in a 1:1:1 ratio with Lu(III), a well-defined heteromeric complex featuring one of each ligand strand and the metal ion is selectively formed. Introducing point-chiral centers into the ligands leads to single-sense helical stereochemistry of the resulting coordination complex. Covalent capture of the entangled structure by ring-closing olefin metathesis then gives a socially self-sorted trefoil knot of single topological handedness. In a related manner, a heteromeric molecular granny knot (a six-crossing composite knot featuring two trefoil tangles of the same handedness) was assembled from social self-sorting of ditopic and tetratopic multi-pdc strands. A molecular square knot (a six-crossing composite knot of two trefoil tangles of opposite handedness) was assembled by social self-sorting of a ditopic pdc strand with four (S)-centers and a tetratopic strand with two (S)- and six (R)-centers. Each of the entangled structures was characterized by 1H and 13C NMR spectroscopy, mass spectrometry, and circular dichroism spectroscopy. The precise control of composition and topological chirality through social self-sorting enables the rapid assembly of well-defined sequences of entanglements for molecular knots.
Introduction
Knots and entanglements are found at all length scales, from spontaneous random tangling of polymer chains to well-defined specialized climbing and sailing knots.1 Self-entanglement of a molecular strand can cause changes in properties such as molecular volume and shape,2 strain,3 chiral expression,4 and photophysical characteristics.5 Relatively simple synthetic molecular knot topologies have proven efficacious in areas as diverse as catalysis,6 mechanical barrier formation,7 dopants for chiral materials,8 and nanotherapeutics.9
However, accessing different molecular knot scaffolds remains challenging.10−14 Single enantiomer15 trefoil knots have been synthesized by coordination of three 2,6-pyridinedicarboxamide (pdc) ligands14 containing asymmetrically substituted benzyl groups around a lanthanide(III) ion; the point chirality leads to stereoselective assembly of the ligands around the metal center.14a,15 This, in turn, directs the topological chirality of the closed-loop knot that results from covalent capture of the complex by ring-closing olefin metathesis16 (RCM).
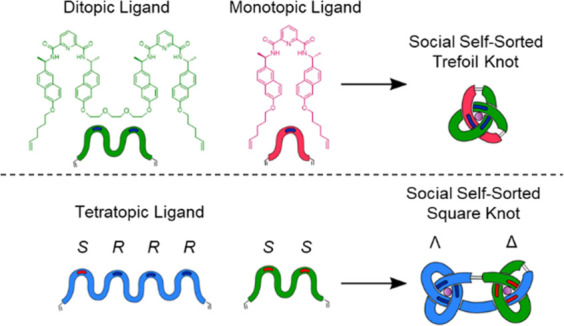
Molecular trefoil knots have been synthesized by the folding and threading of a single tritopic ligand strand17,18 (including those containing three pdc units18) around a metal ion template, reminiscent of the familiar way that knots are tied in our everyday world. This generates robust trefoil knot precursors (“tangles”1a,1c) with unjoined strand ends, so-called overhand knots.17−20 Enantiopure overhand knots18 can be used for the synthesis20 of composite19−21 knots, joining together two tangles of either the same (31#31 granny knots) or opposing (31#*31 square knots) handedness.20 However, such syntheses require linear synthetic schemes that may be lengthy and result in low overall yields.19,20
A useful synthetic strategy for rapidly assembling complexity from simple building blocks is self-sorting.22 Self-sorting systems can either be narcissistic23—each component preferring to interact with others like themselves—or social,24 whereby a compound has greater affinity for components within a system that are different from itself. Narcissistic self-sorting is more common in artificial supramolecular systems, where it tends to yield simpler, high symmetry, homomeric assemblies.25
The synthesis of two 12-crossing composite triskelion knots via a Vernier template approach was recently reported.18b By using a coordinative mismatch of lanthanide(III) ions and pdc ligands of varying topicity, entangled assemblies containing the lowest common multiple total binding sites were formed. This allowed for the synthesis of large composite knots from comparatively simple ligand precursors in relatively few synthetic steps.18b However, it also suggests a more general strategy for rapidly accessing complex higher-order entanglements. Here, we report the social self-sorting synthesis of prime and composite molecular knots, exemplified by the entropically driven synthesis of heteromeric trefoil, granny, and square knots.26
Results and Discussion
Social Self-Sorting Synthesis of a Trefoil Knot Precursor Using Achiral Monotopic and Ditopic Building Blocks
A 1:1:1 mixture of monotopic ligand L1, ditopic ligand L2, and lutetium(III) ions could potentially form any of three distinct complexes: two narcissistically self-sorted homomeric helicates comprising three identical ligands, or a socially self-sorted heteromeric helicate featuring one of each ligand (Figure 1). In contrast to homomeric complexes L13·[Lu] and L23·[Lu]2, heteromeric complex {L1,L2·[Lu]} requires only two ligands per metal ion to satisfy the Lu(III) coordination requirements and should therefore be favored.
Figure 1.

Social self-sorting of ligand strands of different topicity leads to favored heteromeric open knot complexes. Achiral monotopic ligand L1 and achiral ditopic ligand L2 form racemic heteromeric trefoil knot precursor {L1,L2·[Lu]} upon coordination to Lu(III), as this complex requires only two ligand strands to satisfy the lanthanide coordination sphere.
To assess this concept, first of all, homomeric complexes of each type of ligand coordinated to lutetium(III) were prepared (Scheme 1). Achiral monotopic ligand L1 and ditopic ligand L2 were synthesized as described in the Supporting Information (Scheme S2). Complexation of three equivalents of L1 with one equivalent of lutetium(III) trifluoromethanesulfonate in acetonitrile afforded helicate L13·[Lu] upon heating to 80 °C for 2 h (Schemes 1i and S4).14 The progress of the coordination process was monitored by electrospray ionization (ESI) mass spectrometry (Figure S77) and 1H nuclear magnetic resonance (NMR) spectroscopy (Figure S48). Under similar reaction conditions, ditopic ligand L2 was complexed to Lu(III) in a 3:2 ratio, generating complex L23·[Lu]2 over 48 h (Schemes 1iii and S5). The 1H NMR spectrum (Figure S2) and ESI mass spectrum (Figure S79) confirmed that an entangled species with 3:2 ligand:metal ratio had been formed.18b
Scheme 1. Assembly of Achiral Complexes L13·[Lu], L23·[Lu]2, and Socially Self-Sorted Complex {L1,L2·[Lu]}.
Reagents and conditions: (i) 3× L1, Lu(CF3SO3)3, MeCN, 80 °C, 2 h, 85%. (ii) L1, L2, Lu(CF3SO3)3, MeCN, 80 °C, 20 h, 92%. (iii) 3× L2, 2× Lu(CF3SO3)3, MeCN, 80 °C, 48 h, 82%.
We then investigated the propensity of L1 and L2 to self-sort into heteromeric coordination complexes. An acetonitrile solution containing a 1:1:1 mixture of L1, L2 and lutetium(III) trifluoromethanesulfonate was heated at 80 °C (Scheme 1ii). After 20 h, the major ions in the ESI mass spectrum corresponded to the heteromeric complex {L1,L2·[Lu]}, with no evidence of homomeric complexes L13·[Lu] or L23·[Lu]2 (Figure S81), indicating that the mixture does, indeed, socially self-sort to form the favored complex. Given the similarity in coordination chemistry of different lanthanides, it appears likely that the high selectivity observed originates mainly from entropic effects and, in particular, the number of species in each complex. Next, in order to use social self-sorting to control the crossing sequences more generally using this strategy, directors for entanglement stereochemistry were also introduced (Figure 2).14,18
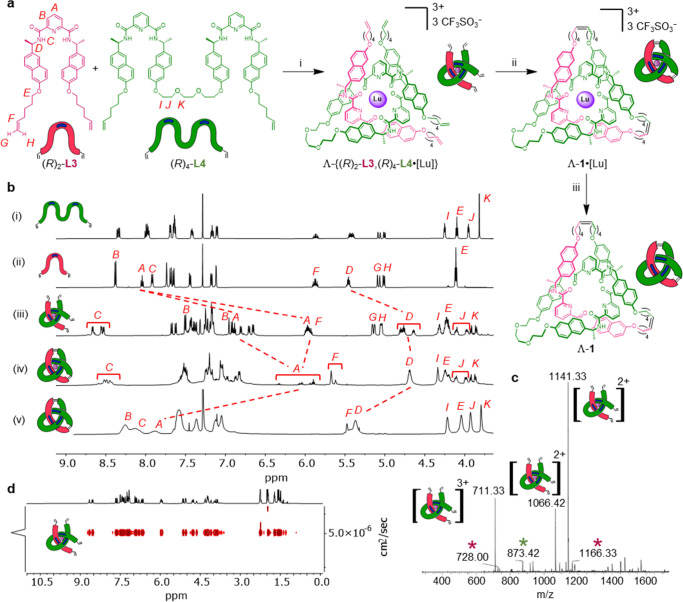
Figure 2.
Socially self-sorted assembly of a trefoil knot of single topological handedness. (a) Synthesis of trefoil knot Λ-1. Reagents and conditions: (i) Lu(CF3SO3)3, MeCN, 80 °C, 24 h. (ii) Hoveyda–Grubbs second generation catalyst,16 CH2Cl2/CH3NO2 1:1 (v/v), 50 °C, 24 h, 53% over two steps. (iii) Et4NF, MeCN, r.t., 0.5 h, 35% over three steps. Stereochemistry indicated by dark blue bars (R-stereocenters) on the cartoon representations of the ligand strands. (b) Partial 1H NMR spectral stack plot of trefoil knot Λ-1 and precursors (600 MHz, 298 K): (i) ligand (R)4-L4 (CDCl3), (ii) ligand (R)2-L3 (CDCl3), (iii) open trefoil knot complex Λ-{(R)2-L3,(R)4-L4·[Lu]} (MeCN-d3), (iv) metalated knot Λ-1·[Lu] (MeCN-d3), and (v) metal-free knot Λ-1 (CDCl3). Proton assignments refer to atom labels in part (a). For full assignments, see Supporting Information. (c) Low resolution ESI-MS(+) of a crude open trefoil knot complex mixture, showing major signals corresponding to Λ-{(R)2-L3,(R)4-L4·[Lu]}, and small signals corresponding to homomeric complexes Λ-((R)2-L3)3·[Lu] (pink *) and (Λ,Λ)-((R)4-L4)3·[Lu]2 (green *). (d) DOSY 1H NMR spectrum of open trefoil knot complex Λ-{(R)2-L3,(R)4-L4·[Lu]} (600 MHz, 298 K, MeCN-d3), showing a single species is present in solution.
Social Self-Sorting Synthesis of Trefoil Knots with Chiral Monotopic and Ditopic Building Blocks
To use point chirality to direct tangle stereochemistry within the self-sorted complexes, ditopic ligand (R)4-L4 and monotopic ligand (R)2-L3 were synthesized as described in the Supporting Information (Scheme S3). Homomeric complexes Λ-((R)2-L3)3·[Lu] and (Λ,Λ)-((R)4-L4)3·[Lu]2 were prepared in an analogous manner to the reactions featuring the achiral building blocks (Scheme S7).15 Addition of lutetium(III) trifluoromethanesulfonate (1 equiv) to a 1:1 mixture of (R)2-L3 and (R)4-L4 in acetonitrile resulted in the formation of heteromeric complex Λ-{(R)2-L3,(R)4-L4·[Lu]} after 24 h at 80 °C (Figure 2a).
The progress of the assembly process was monitored by 1H NMR spectroscopy (Figure 2(bi–iii)) and ESI-MS (Figure 2c). The most abundant ions in the ESI mass spectrum (m/z Λ-{(R)2-L3,(R)4-L4·[Lu]}3+ 711.3, Λ-{(R)2-L3,(R)4-L4·[Lu]}-H2+ 1066.4, Λ-{(R)2-L3,(R)4-L4·[Lu]}[CF3SO3]2+ 1141.3) correspond to the heteromeric complex, while smaller signals arise from the homomeric circular helicate (m/z Λ-((R)2-L3)3·[Lu]3+ 728.0, Λ-((R)2-L3)3·[Lu][CF3SO3]2+ 1166.3) and open granny knot complex (m/z (Λ,Λ)-((R)4-L4)3·[Lu]2[CF3SO3]5+ 873.4) (Figure 2c). High-resolution mass spectrometry displays isotopic distributions for each complex consistent with the calculated values (Figure S84).
Diffusion-ordered NMR spectroscopy (DOSY) indicates a single species is present in solution (Figure 2d). The CD spectrum showed exciton couplings and signal intensities consistent with previously reported tangled pdc complexes (Figure S121).14,15,18 Substantial upfield shifts of protons HA and HD in Λ-{(R)2-L3,(R)4-L4·[Lu]} (Figure 2b(iii)) result from shielding by the naphthalene rings and are consistent with an entangled conformation.14 The splitting of the HA signals into different regions (∼7.0 and 6.0 ppm) reflects the difference in the environments of their positions in the coordination complex. The pyridine protons at the open side of the complex (green pyridine sites) are less shielded than the more tightly bound (pink) sites internal to the structure.4b Additional splitting of each set of protons for HB, HC, and HD into chemically distinct environments also reflects the formation of the low-symmetry coordination complex, Λ-{(R)2-L3,(R)4-L4·[Lu]}.
The entangled complex Λ-{(R)2-L3,(R)4-L4·[Lu]} was covalently captured by RCM using a Hoveyda–Grubbs second generation catalyst to give the closed-loop trefoil knot Λ-1·[Lu] (Figure 2a). The ESI mass spectrum of the crude reaction mixture after RCM showed only ions corresponding to the desired knot, Λ-1·[Lu] (m/z Λ-1·[Lu]3+ 692.7, Λ-1·[Lu]-H2+ 1038.4, Λ-1·[Lu][CF3SO3]2+ 1113.4), with no trace of assemblies derived from narcissistic self-sorting (Figure S3). The DOSY spectrum indicated a single species (Figure S72), and the CD spectrum confirmed that the entanglement stereochemistry is conserved in the closed-loop knot (Figure S109). Purification by size exclusion chromatography removed small amounts of unreacted starting material and larger molecular weight species to give trefoil knot Λ-1·[Lu] in 53% yield over two steps (Scheme S8). The modest isolated yield results from the oligomeric and polymeric side products from alkene metathesis and the loss of some of the poorly soluble knot during chromatography.
The 1H NMR spectrum of Λ-1·[Lu] shows the absence of terminal alkenes (Figure 2b(iv)). The narrower range of chemical shifts of HA with respect to the open complex (Λ-{(R)2-L3,(R)4-L4·[Lu]}) reflects the similarity between the glycol-linked and alkyl chain-linked environments after closure by RCM.
Knot Λ-1·[Lu] was readily demetalated by tetraethylammonium fluoride to give wholly organic knot Λ-1 (Scheme S9). To confirm the absence of alternative entangled species, the reaction was also carried through all three steps from the ligand precursors to Λ-1 without purification of the intermediates or final product. The matrix-assisted laser desorption/ionization (MALDI) spectrum of the crude reaction mixture after the final step features only ions corresponding to Λ-1 (m/z [Λ-1 + Na]+ 1926.3, [Λ-1 + K]+ 1942.0, Figure S6). The absence of homomeric side products after kinetic trapping of the compound by RCM highlights the effectiveness of entropy-driven error correction in the system.
The demetalated knot Λ-1 was subsequently isolated by size exclusion chromatography in overall 35% yield over three steps (Figure 2a). The 1H NMR spectrum of Λ-1 is broad and typical of other molecular knots that have no single well-defined conformation (Figure 2b(v)).2 The metalated knot Λ-1·[Lu] could be smoothly regenerated (94% yield) by treatment of Λ-1 with one equivalent of lutetium(III) trifluoromethanesulfonate in acetonitrile at 80 °C (Scheme S9).
Social Self-Sorting Synthesis of Composite Knots with Chiral Ditopic and Tetratopic Building Blocks
The applicability of the social self-sorting approach to more complex systems was then explored in the formation of a granny knot complex directly from two different chiral ligands. Ditopic ligand (R)4-L4 and tetratopic ligand (R)8-L5 were treated with lutetium(III) trifluoromethanesulfonate in a 1:1:2 ratio (Figure 3a). After 24 h, the ESI mass spectrum showed several ions corresponding to a heteromeric complex (m/z (Λ,Λ)-{(R)4-L4,(R)8-L5·[Lu]2}6+ 694.8, (Λ,Λ)-{(R)4-L4,(R)8-L5·[Lu]2}[CF3SO3]5+ 863.4, (Λ,Λ)-{(R)4-L4,(R)8-L5·[Lu]2}[CF3SO3]24+ 1116.4, (Λ,Λ)-{(R)4-L4,(R)8-L5·[Lu]2}[CF3SO3]33+ 1538.2, Figure S89). Other ions in the spectrum correspond to overhand knot fragments containing a single non-coordinated ligand site, as observed previously for related open Vernier lanthanide complexes.18b The complex consisting of just one tetratopic ligand (m/z Λ-(R)8-L5·[Lu]3+ 901.3, Λ-(R)8-L5·[Lu][CF3SO3]2+ 1425.9) can result from fragmentation of either the heteromeric granny knot or the Vernier template triskelion assembly. However, the complex consisting of two ditopic ligands (m/z Λ-((R)4-L4)2·[Lu]3+ 918.0, Λ-((R)4-L4)2·[Lu][CF3SO3]2+ 1450.8) can only arise from fragmentation of the heteromeric granny complex. The 1H NMR spectrum shows shifts characteristic of strand entanglement [Figure 3(bi–iii)) and additional small signals which correspond to residual unbound ligand even after prolonged reaction times (signals marked *).
Figure 3.
Socially self-sorted assembly of a granny knot of single topological handedness. (a) Synthesis of granny knot (Λ,Λ)-2. Reagents and conditions: (i) Lu(CF3SO3)3, MeCN, 80 °C, 72 h. (ii) Hoveyda–Grubbs second generation catalyst, CH2Cl2/CH3NO2 1:1 (v/v), 50 °C, 24 h, 26% over two steps. (iii) Et4NF, MeCN, r.t., 0.5 h, 13% over three steps. (b) Partial 1H NMR spectral stack plot of granny knot (Λ,Λ)-2 and precursors (600 MHz, 298 K): (i) ligand (R)8-L5 (CDCl3), (ii) ligand (R)4-L4 (CDCl3), (iii) open granny knot complex (Λ,Λ)-{(R)4-L4,(R)8-L5·[Lu]2} (MeCN-d3), (iv) metalated knot (Λ,Λ)-2·[Lu]2 (MeCN-d3), and (v) metal-free granny knot (Λ,Λ)-2 (CDCl3). Uncoordinated ligand impurities are indicated *. For full assignments, see Supporting Information. (c) High-resolution ESI-MS(+) of closed-loop granny knot (Λ,Λ)-2·[Lu]2, comparing the observed spectrum (above) to calculated isotopic distribution of [M – 5(CF3SO3)]5+ (below).
The alkene end groups of complex (Λ,Λ)-{(R)4-L4,(R)8-L5·[Lu]2} were joined by RCM and the resulting closed-loop knot demetalated by treatment with Et4NF (Figure 3a).14b,15 As with trefoil knot Λ-1, the three steps to granny knot (Λ,Λ)-2 were also undertaken without isolation of the intermediates to examine the efficacy of the self-sorting. We found no evidence of alternative homomeric products in the mass spectrum of the metalated (Figure S8) or demetalated (Figure S11) crude reaction mixtures, although a trefoil-entangled side product derived from intramolecular closure of fragment Λ-(R)8-L5·[Lu] was observed throughout. The demetalated granny knot was isolated by size exclusion chromatography, yielding (Λ,Λ)-2 in 13% yield over three steps (Scheme S11). The metalated knot (Λ,Λ)-2·[Lu]2 could be isolated either after purification by size exclusion chromatography in 26% yield in two steps from (R)4-L4 and (R)8-L5 (Figure 3a) or in 68% yield by remetalation of (Λ,Λ)-2 (Scheme S11).
The molecular masses of (Λ,Λ)-2·[Lu]2 and (Λ,Λ)-2 were confirmed by HRMS (Figure 3c) and MALDI-TOF (Figure S97), respectively. The 1H NMR spectrum of (Λ,Λ)-2·[Lu]2 (Figure 3b(iv)) is similar to that of previously reported composite knots,20,21 and the 1H NMR spectrum of the demetalated knot (Λ,Λ)-2 is broad (Figure 3b(v)).
Social Self-Sorting of Pre-Coordinated Entangled Strands under Thermodynamic Control
The dynamic conversion of entangled complexes was also investigated (Figure 4a). Equimolar solutions of homomeric helicate Λ-((R)2-L3)3·[Lu] and open granny knot complex (Λ,Λ)-((R)4-L4)3·[Lu]2 in MeCN were mixed, and the evolution of heteromeric complex Λ-{(R)2-L3,(R)4-L4·[Lu]} was monitored by 1H NMR spectroscopy and ESI mass spectrometry. Within 10 min at room temperature, ions corresponding to Λ-{(R)2-L3,(R)4-L4·[Lu]} became apparent by mass spectrometry, becoming the dominant species after 4 h at 80 °C (Figure 4b). Near-complete conversion to the heteromeric socially self-sorted complex was qualitatively confirmed by 1H NMR spectroscopy (Figure 4c). The dynamic rearrangement of pre-formed complexes to an entropically favored heteromeric compound was also qualitatively shown by an equimolar mixture of granny knot complex (Λ,Λ)-((R)4-L4)3·[Lu]2 and triskelion knot complex (Λ3,Λ)-((R)8-L5)3·[Lu]4 (see Section S4.7).
Figure 4.
Socially self-sorted rearrangement of complexes to give a heteromeric complex of single entanglement stereochemistry. (a) Synthesis of heteromeric complex Λ-{(R)2-L3,(R)4-L4·[Lu]}. Reagents and conditions: MeCN, 80 °C, 4 h. (b) Comparison of precursor complexes and (qualitative) in situ monitoring of rearrangement by ESI(+) mass spectrometry. (c) Comparison of precursor complexes and (qualitative) in situ monitoring of rearrangement by 1H NMR spectroscopy (600 MHz, 298 K, MeCN-d3), including a reference sample of pristine heteromeric complex Λ-{(R)2-L3,(R)4-L4·[Lu]} (top).
Control of Entanglement Stereochemistry with Building Block Point Chirality
Monotopic pdc lanthanide helicates containing either (R)- or (S)-stereogenic centers do not self-sort on the basis of chirality.15 However, covalently tethered tritopic ligands containing four (R)- and two (S)-stereocenters do not self-entangle upon coordination to lanthanide(III) ions because of steric clashes arising from the strand stereochemistry.18b We therefore explored combining stereochemical discrimination and entropically driven social self-sorting in order to prepare a composite knot containing tangles of opposing handedness. Ditopic ligand (S)4-L4 and tetratopic ligand (S)2(R)6-L5 were synthesized as previously reported.18b The combination of six stereocenters of (R)-chirality and six of (S)-chirality across the two ligands is required for forming two trefoil tangles of opposing handedness, a 31#*31 “square knot” (Λ,Δ)-2, and a diastereomer of granny knot (Λ,Λ)-2 (Schemes S10 and S12).
To assemble square knot (Λ,Δ)-2, a 1:1:2 mixture of tetratopic ligand (S)2(R)6-L5, ditopic ligand (S)4-L4 and lutetium(III) trifluoromethanesulfonate in acetonitrile afforded open square knot complex (Λ,Δ)-{(S)4-L4,(S)2(R)6-L5·[Lu]2} after heating at 80 °C for 72 h (Figure 5a). Its mass spectrum was similar to that of the diastereomeric granny knot complex (Λ,Λ)-{(R)4-L4,(R)8-L5·[Lu]2} (Figure S93). Complex (Λ,Δ)-{(S)4-L4,(S)2(R)6-L5·[Lu]2} is pseudo-achiral, particularly in terms of the environment and stereochemistry of the point-chiral centers around each coordinated Lu(III) ion, and accordingly gives a near baseline CD spectrum (Figure S122). Joining of the terminal alkenes of (Λ,Δ)-{(S)4-L4,(S)2(R)6-L5·[Lu]2} by RCM gave square knot (Λ,Δ)-2·[Lu]2 (Scheme S12). Subsequent demetalation by Et4NF afforded square knot (Λ,Δ)-2 in 7% yield over three steps (Scheme S13).
Figure 5.
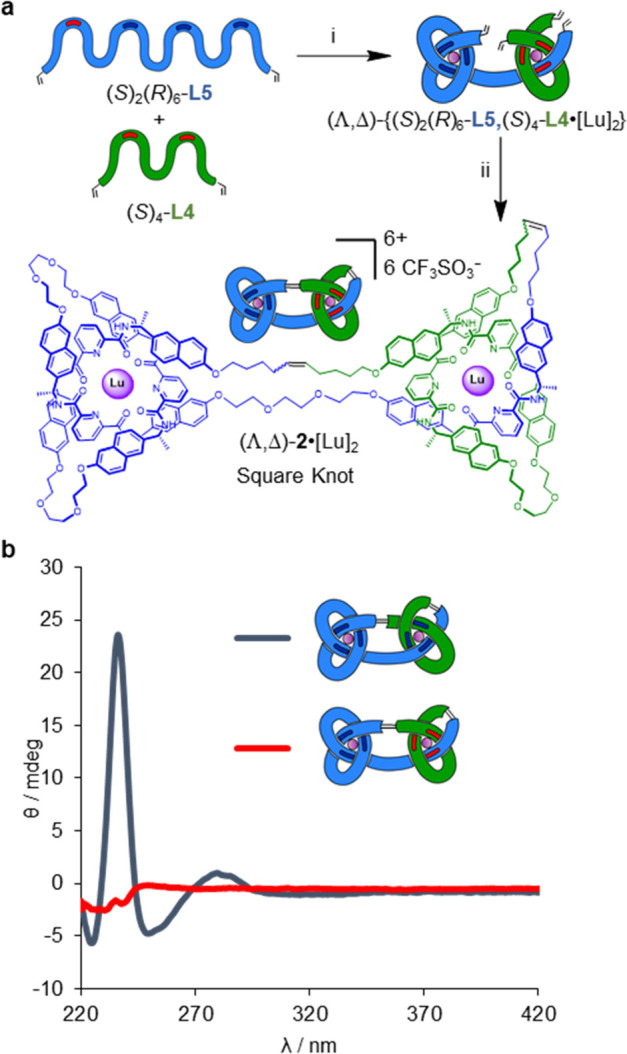
Socially self-sorted assembly of a square knot (meso-topological handedness). (a) Synthesis of square knot (Λ,Δ)-2·[Lu]2. Reagents and conditions: (i) Lu(CF3SO3)3, MeCN, 80 °C, 72 h. (ii) Hoveyda–Grubbs second generation catalyst,16 CH2Cl2/CH3NO2 1:1 (v/v), 50 °C, 24 h, 29% over two steps. (b) CD spectral stack plot (5 × 10–5 M, MeCN, normalized for absorbance) showing comparison of granny knot (Λ,Λ)-2·[Lu]2 (blue) and square knot (Λ,Δ)-2·[Lu]2 (red).
Square knot (Λ,Δ)-2·[Lu]2 and granny knot (Λ,Λ)-2·[Lu]2 have virtually indistinguishable 1H NMR spectra (Figures S60 and S64) but strikingly different CD responses (Figure 5b). The small deviations from the baseline in the CD spectrum of (Λ,Δ)-2·[Lu]2 are likely the result of the different connectivities of the point-chiral groups on the strand.
Conclusions
Our findings demonstrate that molecular prime and composite knots can be rapidly assembled by social self-sorting using 2,6-pyridinedicarboxamide-containing strands of different topicity. A 1:1 ratio of monotopic and ditopic pdc ligands coordinates to Lu(III) to selectively generate a heteromeric precursor complex to a trefoil knot. Molecular granny and square knots can be assembled through social self-sorting of chiral ditopic and tetratopic ligand strands. The pdc-Ln(III) social self-sorting is dynamic, with pre-coordinated lanthanide complexes of the ligand strands rapidly rearranging to the entropically preferred self-sorted structures. Social self-sorting of programed pdc-ligand strands is a highly effective new addition to the strategies10−13,18 available for the rapid assembly of well-defined sequences of orderly molecular entanglements. The ability to access low-symmetry knots with simpler synthetic strategies provides new avenues to explore the functions and properties associated with molecular entanglements.
Acknowledgments
We thank the Engineering and Physical Sciences Research Council (EPSRC; EP/P027067/1), the European Research Council (ERC; Advanced Grant 786630), the Marie Skłodowska-Curie Actions of the European Union (Individual Postdoctoral Fellowship to F.S., EC 746993), and the German Research Foundation (DFG, Individual Postdoctoral Fellowship to E.K.) for funding. We thank the University of Manchester Mass Spectrometry Service Center for high-resolution mass spectrometry and MALDI-TOF. D.A.L. is a Royal Society Research Professor.
Glossary
Abbreviations
- OTf
trifluoromethanesulfonate
- pdc
2,6-pyridinedicarboxamide
- MALDI
matrix-assisted laser desorption/ionization
- RCM
ring-closing olefin metathesis
- r.t.
room temperature.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c07682.
Additional experimental details including synthetic procedures and characterization data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Adams C. C.The Knot Book: An Elementary Introduction to the Mathematical Theory of Knots; American Mathematical Society: Providence, RI, 2004. [Google Scholar]; b Lim N. C. H.; Jackson S. E. Molecular knots in biology and chemistry. J. Phys.: Condens. Matter 2015, 27, 354101 10.1088/0953-8984/27/35/354101. [DOI] [PubMed] [Google Scholar]; c Fielden S. D. P.; Leigh D. A.; Woltering S. L. Molecular knots. Angew. Chem., Int. Ed. 2017, 56, 11166–11194. 10.1002/anie.201702531. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Guo Q.; Jiao Y.; Feng Y.; Stoddart J. F. The rise and promise of molecular nanotopology. CCS Chem. 2021, 3, 1542–1572. 10.31635/ccschem.021.202100975. [DOI] [Google Scholar]; e Ashbridge Z.; Fielden S. D. P.; Leigh D. A.; Pirvu L.; Schaufelberger F.; Zhang L.. Knotting matters: Orderly molecular entanglements. Chem. Soc. Rev. 2022, Advance Article 10.1039/d2cs00323f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh D. A.; Schaufelberger F.; Pirvu L.; Halldin Stenlid J.; August D. P.; Segard J. Tying different knots in a molecular strand. Nature 2020, 584, 562–568. 10.1038/s41586-020-2614-0. [DOI] [PubMed] [Google Scholar]
- Saitta A. M.; Soper P. D.; Wasserman E.; Klein M. L. Influence of a knot on the strength of a polymer strand. Nature 1999, 399, 46–48. 10.1038/19935. [DOI] [PubMed] [Google Scholar]
- a Zhang L.; Lemonnier J.-F.; Acocella A.; Calvaresi M.; Zerbetto F.; Leigh D. A. Effects of knot tightness at the molecular level. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 2452–2457. 10.1073/pnas.1815570116. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Song Y.; Schaufelberger F.; Ashbridge Z.; Pirvu L.; Vitorica-Yrezabal I. J.; Leigh D. A. Effects of turn-structure on folding and entanglement in artificial molecular overhand knots. Chem. Sci. 2021, 12, 1826–1833. 10.1039/D0SC05897A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprice K.; Aster A.; Cougnon F. B. L.; Kumpulainen T. Untying the photophysics of quinolinium-based molecular knots and links. Chem. – Eur. J. 2020, 26, 1576–1587. 10.1002/chem.201904456. [DOI] [PubMed] [Google Scholar]
- a Marcos V.; Stephens A. J.; Jaramillo-Garcia J.; Nussbaumer A. L.; Woltering S. L.; Valero A.; Lemonnier J.-F.; Vitorica-Yrezabal I. J.; Leigh D. A. Allosteric initiation and regulation of catalysis with a molecular knot. Science 2016, 352, 1555–1559. 10.1126/science.aaf3673. [DOI] [PubMed] [Google Scholar]; b Prakasam T.; Devaraj A.; Saha R.; Lusi M.; Brandel J.; Esteban-Gómez D.; Platas-Iglesias C.; Olson M. A.; Mukherjee P. S.; Trabolsi A. Metal–organic self-assembled trefoil knots for C—Br bond activation. ACS Catal. 2019, 9, 1907–1914. 10.1021/acscatal.8b04650. [DOI] [Google Scholar]
- Leigh D. A.; Pirvu L.; Schaufelberger F.; Tetlow D. J.; Zhang L. Securing a supramolecular architecture by tying a stopper knot. Angew. Chem., Int. Ed. 2018, 57, 10484–10488. 10.1002/anie.201803871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsonis N.; Lancia F.; Leigh D. A.; Pirvu L.; Ryabchun A.; Schaufelberger F. Knotting a molecular strand can invert macroscopic effects of chirality. Nat. Chem. 2020, 12, 939–944. 10.1038/s41557-020-0517-1. [DOI] [PubMed] [Google Scholar]
- a Benyettou F.; Prakasam T.; Ramdas Nair A.; Witzel I.-I.; Alhashimi M.; Skorjanc T.; Olsen J.-C.; Sadler K. C.; Trabolsi A. Potent and selective in vitro and in vivo antiproliferative effects of metal–organic trefoil knots. Chem. Sci. 2019, 10, 5884–5892. 10.1039/c9sc01218d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b August D. P.; Borsley S.; Cockroft S. L.; della Sala F.; Leigh D. A.; Webb S. J. Transmembrane ion channels formed by a Star of David [2]catenane and a molecular pentafoil knot. J. Am. Chem. Soc. 2020, 142, 18859–18865. 10.1021/jacs.0c07977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dietrich-Buchecker C. O.; Sauvage J.-P. A synthetic molecular trefoil knot. Angew. Chem., Int. Ed. 1989, 28, 189–192. 10.1002/anie.198901891. [DOI] [Google Scholar]; b Dietrich-Buchecker C. O.; Guilhem J.; Pascard C.; Sauvage J.-P. Structure of a synthetic trefoil knot coordinated to two copper(I) centers. Angew. Chem., Int. Ed. 1990, 29, 1154–1156. 10.1002/anie.199011541. [DOI] [Google Scholar]; c Safarowsky O.; Nieger M.; Fröhlich R.; Vögtle F. A molecular knot with twelve amide groups — one-step synthesis, crystal structure, chirality. Angew. Chem., Int. Ed. 2000, 39, 1616–1618. . [DOI] [PubMed] [Google Scholar]; d Feigel M.; Ladberg R.; Engels S.; Herbst-Irmer R.; Fröhlich R. A trefoil knot made of amino acids and steroids. Angew. Chem., Int. Ed. 2006, 45, 5698–5702. 10.1002/anie.200601111. [DOI] [PubMed] [Google Scholar]; e Barran P. E.; Cole H. L.; Goldup S. M.; Leigh D. A.; McGonigal P. R.; Symes M. D.; Wu J.; Zengerle M. Active-metal template synthesis of a molecular trefoil knot. Angew. Chem., Int. Ed. 2011, 50, 12280–12284. 10.1002/anie.201105012. [DOI] [PubMed] [Google Scholar]
- a Ponnuswamy N.; Cougnon F. B. L.; Clough J. M.; Pantoş G. D.; Sanders J. K. M. Discovery of an organic trefoil knot. Science 2012, 338, 783–785. 10.1126/science.1227032. [DOI] [PubMed] [Google Scholar]; b Cougnon F. B. L.; Caprice K.; Pupier M.; Bauzá A.; Frontera A. A strategy to synthesize molecular knots and links using the hydrophobic effect. J. Am. Chem. Soc. 2018, 140, 12442–12450. 10.1021/jacs.8b05220. [DOI] [PubMed] [Google Scholar]
- a Prakasam T.; Lusi M.; Elhabiri M.; Platas-Iglesias C.; Olsen J. C.; Asfari Z.; Cianférani-Sanglier S.; Debaene F.; Charbonnière L. J.; Trabolsi A. Simultaneous self-assembly of a [2]catenane, a trefoil knot, and a Solomon Link from a simple pair of ligands. Angew. Chem., Int. Ed. 2013, 52, 9956–9960. 10.1002/anie.201302425. [DOI] [PubMed] [Google Scholar]; b Prakasam T.; Bilbeisi R. A.; Lusi M.; Olsen J.-C.; Platas-Iglesias C.; Trabolsi A. Post-synthetic modifications of cadmium-based knots and links. Chem. Commun. 2016, 52, 7398–7401. 10.1039/c6cc02423h. [DOI] [PubMed] [Google Scholar]; c Bilbeisi R. A.; Prakasam T.; Lusi M.; El Khoury R.; Platas-Iglesias C.; Charbonnière L. J.; Olsen J. C.; Elhabiri M.; Trabolsi A. [C-H···Anion] interactions mediate the templation and anion binding properties of topologically non-trivial metal-organic structures in aqueous solutions. Chem. Sci. 2016, 7, 2524–2531. 10.1039/c5sc04246a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. A synthetic molecular pentafoil knot. Nat. Chem. 2012, 4, 15–20. 10.1038/nchem.1193. [DOI] [PubMed] [Google Scholar]; b Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. Pentameric circular iron(II) double helicates and a molecular pentafoil knot. J. Am. Chem. Soc. 2012, 134, 9488–9497. 10.1021/ja303355v. [DOI] [PubMed] [Google Scholar]; c Danon J. J.; Krüger A.; Leigh D. A.; Lemonnier J.-F.; Stephens A. J.; Vitorica-Yrezabal I. J.; Woltering S. L. Braiding a molecular knot with eight crossings. Science 2017, 355, 159–162. 10.1126/science.aal1619. [DOI] [PubMed] [Google Scholar]; d Zhang L.; August D. P.; Zhong J.; Whitehead G. F. S.; Vitorica-Yrezabal I. J.; Leigh D. A. A molecular trefoil knot from a trimeric circular helicate. J. Am. Chem. Soc. 2018, 140, 4982–4985. 10.1021/jacs.8b00738. [DOI] [PubMed] [Google Scholar]; e Leigh D. A.; Danon J. J.; Fielden S. D. P.; Lemonnier J.-F.; Whitehead G. F. S.; Woltering S. L. A molecular endless (74) knot. Nat. Chem. 2021, 13, 117–122. 10.1038/s41557-020-00594-x. [DOI] [PubMed] [Google Scholar]; f Carpenter J. P.; McTernan C. T.; Greenfield J. L.; Lavendomme R.; Ronson T. K.; Nitschke J. R. Controlling the shape and chirality of an eight-crossing molecular knot. Chem 2021, 7, 1534–1543. 10.1016/j.chempr.2021.03.005. [DOI] [Google Scholar]
- a Leonard J. P.; Jensen P.; McCabe T.; O’Brien J. E.; Peacock R. D.; Kruger P. E.; Gunnlaugsson T. Self-assembly of chiral luminescent lanthanide coordination bundles. J. Am. Chem. Soc. 2007, 129, 10986–10987. 10.1021/ja073049e. [DOI] [PubMed] [Google Scholar]; b Ayme J.-F.; Gil-Ramírez G.; Leigh D. A.; Lemonnier J.-F.; Markevicius A.; Muryn C. A.; Zhang G. Lanthanide template synthesis of a molecular trefoil knot. J. Am. Chem. Soc. 2014, 136, 13142–13145. 10.1021/ja506886p. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Gil-Ramírez G.; Markevicius A.; Browne C.; Vitorica-Yrezabal I. J.; Leigh D. A. Lanthanide template synthesis of trefoil knots of single handedness. J. Am. Chem. Soc. 2015, 137, 10437–10442. 10.1021/jacs.5b07069. [DOI] [PubMed] [Google Scholar]
- Garber S. B.; Kingsbury J. S.; Gray B. L.; Hoveyda A. H. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J. Am. Chem. Soc. 2000, 122, 8168–8179. 10.1021/ja001179g. [DOI] [Google Scholar]
- a Adams H.; Ashworth E.; Breault G. A.; Guo J.; Hunter C. A.; Mayers P. C. Knot tied around an octahedral metal center. Nature 2001, 411, 763. 10.1038/35081143. [DOI] [PubMed] [Google Scholar]; b Guo J.; Mayers P. C.; Breault G. A.; Hunter C. A. Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template. Nat. Chem. 2010, 2, 218–222. 10.1038/nchem.544. [DOI] [PubMed] [Google Scholar]
- a Gil-Ramírez G.; Hoekman S.; Kitching M. O.; Leigh D. A.; Vitorica-Yrezabal I. J.; Zhang G. Tying a molecular overhand knot of single handedness and asymmetric catalysis with the corresponding pseudo-D3-symmetric trefoil knot. J. Am. Chem. Soc. 2016, 138, 13159–13162. 10.1021/jacs.6b08421. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ashbridge Z.; Kreidt E.; Pirvu L.; Schaufelberger F.; Halldin Stenlid J.; Abild-Pedersen F.; Leigh D. A. Vernier template synthesis of molecular knots. Science 2022, 375, 1035–1041. 10.1126/science.abm9247. [DOI] [PubMed] [Google Scholar]
- Carina R. F.; Dietrich-Buchecker C.; Sauvage J.-P. Molecular composite knots. J. Am. Chem. Soc. 1996, 118, 9110–9116. 10.1021/ja961459p. [DOI] [Google Scholar]
- Leigh D. A.; Pirvu L.; Schaufelberger F. Stereoselective synthesis of molecular square and granny knots. J. Am. Chem. Soc. 2019, 141, 6054–6059. 10.1021/jacs.9b01819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Danon J. J.; Leigh D. A.; Pisano S.; Valero A.; Vitorica-Yrezabal I. J. A six-crossing doubly interlocked [2]catenane with twisted rings, and a molecular granny knot. Angew. Chem., Int. Ed. 2018, 57, 13833–13837. 10.1002/anie.201807135. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang L.; Stephens A. J.; Nussbaumer A. L.; Lemonnier J.-F.; Jurček P.; Vitorica-Yrezabal I. J.; Leigh D. A. Stereoselective synthesis of a composite knot with nine crossings. Nat. Chem. 2018, 10, 1083–1088. 10.1038/s41557-018-0124-6. [DOI] [PubMed] [Google Scholar]
- a Safont-Sempere M. M.; Fernández G.; Würthner F. Self-sorting phenomena in complex supramolecular systems. Chem. Rev. 2011, 111, 5784–5814. 10.1021/cr100357h. [DOI] [PubMed] [Google Scholar]; b Ludlow R. F.; Otto S. Systems chemistry. Chem. Soc. Rev. 2008, 37, 101–108. 10.1039/b611921m. [DOI] [PubMed] [Google Scholar]
- a Kramer R.; Lehn J.-M.; Marquis-Rigault A. Self-recognition in helicate self-assembly: Spontaneous formation of helical metal complexes from mixtures of ligands and metal ions. Proc. Natl. Acad. Sci. U. S. A. 1993, 90, 5394–5398. 10.1073/pnas.90.12.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ayme J.-F.; Beves J. E.; Campbell C. J.; Leigh D. A. The self-sorting behavior of circular helicates and molecular knots and links. Angew. Chem., Int. Ed. 2014, 53, 7823–7827. 10.1002/anie.201404270. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhong J.; Zhang L.; August D. P.; Whitehead G. F. S.; Leigh D. A. Self-sorting assembly of molecular trefoil knots of single handedness. J. Am. Chem. Soc. 2019, 141, 14249–14256. 10.1021/jacs.9b06127. [DOI] [PubMed] [Google Scholar]
- a Mukhopadhyay P.; Wu A.; Isaacs L. Social self-sorting in aqueous solution. J. Org. Chem. 2004, 69, 6157–6164. 10.1021/jo049976a. [DOI] [PubMed] [Google Scholar]; b He Z.; Jiang W.; Schalley C. A. Integrative self-sorting: A versatile strategy for the construction of complex supramolecular architecture. Chem. Soc. Rev. 2015, 44, 779–789. 10.1039/c4cs00305e. [DOI] [PubMed] [Google Scholar]; c Bloch W. M.; Clever G. H. Integrative self-sorting of coordination cages based on ‘naked’ metal ions. Chem. Commun. 2017, 53, 8506–8516. 10.1039/c7cc03379f. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Greenaway R. L.; Santolini V.; Pulido A.; Little M. A.; Alston B. M.; Briggs M. E.; Day G. M.; Cooper A. I.; Jelfs K. E. From concept to crystals via prediction: multi-component organic cage pots by social self-sorting. Angew. Chem., Int. Ed. 2019, 58, 16275–16281. 10.1002/anie.201909237. [DOI] [PubMed] [Google Scholar]; e Han K.; Go D.; Tigges T.; Rahimi K.; Kuehne A. J. C.; Walther A. Social self-sorting of colloidal families in co-assembling microgel systems. Angew. Chem., Int. Ed. 2017, 129, 2208–2214. 10.1002/ange.201612196. [DOI] [PubMed] [Google Scholar]; f Coubrough H. M.; van der Lubbe S. C. C.; Hetherington K.; Minard A.; Pask C.; Howard M. J.; Fonseca Guerra C.; Wilson A. J. Supramolecular self-sorting networks using hydrogen-bonding motifs. Chem. – Eur. J. 2018, 25, 785–795. 10.1002/chem.201804791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A.; Isaacs L. Self-sorting: the exception or the rule?. J. Am. Chem. Soc. 2003, 125, 4831–4835. 10.1021/ja028913b. [DOI] [PubMed] [Google Scholar]
- De S.; Mahata K.; Schmittel M. Metal-coordination-driven dynamic heteroleptic architectures. Chem. Soc. Rev. 2010, 39, 1555–1575. 10.1039/b922293f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.