Abstract
The Na+/H+ exchanger transporters (NHE) play an important role in various biologic processes including Na+ absorption, intracellular pH homeostasis, cell volume regulation, proliferation, and apoptosis. The wide expression pattern and cellular localization of NHEs make these proteins pivotal players in virtually all human tissues and organs. In addition, recent studies suggest that NHEs may be one of the primeval transport protein forms in the history of life. Among the different isoforms, the most well-characterized NHEs are the Na+/H+ exchanger isoform 1 (NHE1) and Na+/H+ exchanger isoform 3 (NHE3). However, Na+/H+ exchanger isoform 8 (NHE8) has been receiving attention based on its recent discoveries in the gastrointestinal tract. In this review, we will discuss what is known about the physiological function and potential role of NHE8 in the main organ systems, including useful overviews that could inspire new studies on this multifaceted protein.
Keywords: NHE8, eyes, kidney, testis, gastrointestinal tract, lungs
1. Introduction
The presence of the electroneutral Na+/H+ exchanger (NHE) was first proposed by Mitchell in 1966 to describe the exchange of cations and protons across the mitochondrial membrane [1]. The NHE family has different isoforms, and it is identified in all domains of life, suggesting a very early role in evolution. In fact, recent studies suggest that NHEs may be one of the first primeval transport protein forms in the history of life [2]. Given the primitive origin of these NHEs transporters, there is a huge diversity of NHE across various realms thus emphasizing the wide expression pattern, the cellular localization, and the pivotal role they play in virtually all human tissues and organs [2].
The NHE family exploits the electrochemical Na+ concentration gradients that are developed across the cell membrane due to secondary active transport of Na+/K+/ATPase, participating in the regulation of various fundamental biologic processes including Na+ absorption, intracellular pH homeostasis, cell volume regulation, proliferation, and apoptosis. Alteration of NHE function is also implicated in various pathophysiological conditions. Autosomal recessive loss-of-function mutation in the Na+/H+ exchanger isoform 3 (NHE3)-encoding solute carrier family 9 member A3 (SLC9A3) gene has been linked to the pathogenesis of congenital sodium diarrhea characterized by high fecal loss of Na+ [3]. Furthermore, evidence has been shown that mutations in the isoform 6 or 9 of the NHE genes cause X-linked mental retardation and familial autism, respectively [4,5].
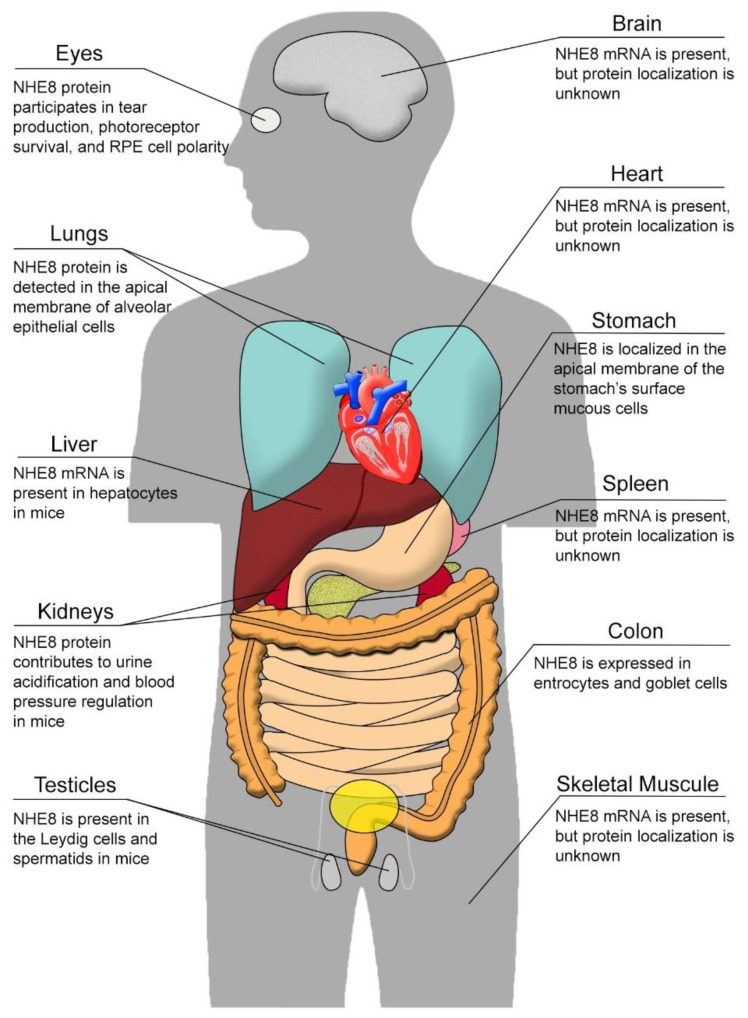
Na+/H+ exchanger isoform 8 (NHE8), encoded by the SLC9A8 gene, is ubiquitously expressed in many tissues. NHE8 protein has been detected in the plasma membrane, Golgi apparatus, and endosomes in different cell types [6,7]. Akin to other NHEs in the NHE family, NHE8 is involved in electroneutral NaCl transport [8]. However, the function of NHE8 extends beyond simple Na+/H+ exchanging. NHE8 also participates in cell volume regulation, cellular adhesion, migration, and proliferation [9]. In addition, NHE8 plays a compensatory role in the absence of other NHE isoforms. In this review paper, we will discuss what is known about the physiological function and potential role of NHE8 since it was discovered. We will also focus on its tissue expression, cellular localization, and unique function in various tissues as summarized in Figure 1, briefly introducing the other NHE isoforms and their roles in each relevant tissue.
Figure 1.
Scheme summarizing the expression of NHE8 in different organs.
1.1. NHEs Classification
As per the most current classification, NHEs are part of the cation proton antiporter (CPA) superfamily that can be further subdivided into CPA1, CPA2, and CPA3 [10,11]. The largest among the three, the CPA1 superfamily, is comprised of three subfamilies: SLC9A1–9 (NHE1–9), SLC9B1–2 (NHA1–2), and SLC9C1–2 (NHE10-11, the so-called sperm NHE, and an orphan-related protein). It also includes transporter proteins from bacteria, archaea, yeast, plants, and mammals. The CPA1 superfamily differs in terms of substrate preferences, kinetics, and tissue localizations [12]. More information about the CPA1 superfamily can be found in Table 1.
Table 1.
The cation proton antiporter 1 (CPA1) superfamily.
| Subdivision | Coding Gene | Molecular Weight | Inhibitors | Localization | Function | Ref. |
|---|---|---|---|---|---|---|
| NHE1 | SLC9A1 | 820aa, ~90 kDa | Amiloride, EIPA, HMA, DMA, HOE-694, SM-20550, S-3226, Clonidine, Cimetidine | It is ubiquitously expressed in nearly all mammalian tissue types and localized basolaterally in every epithelial cell, except in the choroid plexus and syncytio-throphoblast of the human placenta where it expresses apically | It regulates cell volume, intracellular pH, proliferation, differentiation, and apoptosis | [8,13,14,15,16] |
| NHE2 | SLC9A2 | 812aa, ~90 kDa | Amiloride, EIPA, DMA, S-3226 | Highly expressed in intestine and kidney | The physiologic role of NHE2 still remains elusive despite its wide expression pattern with higher expression levels in the intestine and the kidney | [16,17,18,19] |
| NHE3 | SLC9A3 | 834aa, ~92 kDa | Amiloride, Tenapanor, HMA, DMA, HOE-694, Zoniporide, S-3226, Clonidine, Cimetidine | Highly expressed in intestine and kidney | It plays an important role in mediating intestinal electroneutral NaCl absorption in mammals. It also contributes to the absorption of other nutrients, such as amino acids and dipeptides through H+ gradients | [8,16,19,20,21,22] |
| NHE4 | SLC9A4 | 798aa, ~70 kDa | Amiloride, EIPA | NHE4 is highly expressed in the stomach while its expression is low in kidney, intestine, brain, ureter, skeletal muscle, heart, liver, and spleen | It plays a major role in intracellular pH homeostasis | [16,19,23,24] |
| NHE5 | SLC9A5 | 896aa, ~100 kDa | Amiloride, EIPA, HMA, HOE-694, Cimetidine | Predominantly expressed in brain | It regulates growth factor signaling, integrin trafficking, and degradation of glioma cells. It also functions as a negative regulator of dendritic spine growth | [16,19,25,26] |
| NHE6 | SLC9A6 | 669aa, ~85 kDa | Rimeporide | It is highly expressed in skeletal muscles, brain, and heart | It functions to regulate intramitochondrial Na+ and H+ gradients | [19,27,28,29,30] |
| NHE7 | SLC9A7 | 725aa, ~80 kDa | Amiloride | Predominantly localized in the trans-Golgi network | It is prominently expressed in the trans-Golgi network and in the mid-trans-Golgi stacks, and functions as a K+/H+ exchanger in controlling organelle volume through transmembrane K+ flux | [16,19,31,32] |
| NHE8 | SLC9A8 | 581aa, ~65 kDa | HOE-642, S-3226 | NHE8 protein has been detected in the plasma membrane, Golgi apparatus, and endosomes. It is ubiquitously expressed in many tissues | NHE8 is involved in electroneutral NaCl transport, cell volume regulation, cellular adhesion, migration, and proliferation | [6,7,9,19,33,34] |
| NHE9 | SLC9A9 | 645aa, ~72 kDa | Unknown | It is expressed mostly in heart, skeletal muscle, and brain | It transports the protons out of the endosomal lumen in exchange for Na+/K+, thus controlling the pH of endosomal lumens | [35,36] |
| NHE10 | SLC9C1 | 1177aa, ~135 kDa | Unknown | Predominantly expressed in testis and in osteoclasts | NHE10 is associated with sperm motility and fertility, and it is required for osteoclasts differentiation and survivor | [19,37,38,39,40,41] |
| NHE11 | SLC9C2 | 1124aa, ~130 kDa | Unknown | Expressed in the brain, choroid plexus, testis, and uterine tube | Unknown | [19] |
| NHA1 | SLC9B1 | 515aa, ~55 kDa | Unknown | Expressed in testis | It regulates the pH and the cell volume | [19,42] |
| NHA2 | SLC9B2 | 537aa, ~57 kDa | Phloretin | It is ubiquitously expressed in mouse tissues | It regulates the pH and the cell volume | [19,43,44] |
EIPA = 5-N-ethyl-N-isopropyl-amiloride; HMA = 5-N,N-hexamethylene-amiloride; DMA = 5-N,N-dimethyl-amiloride; HOE-694 = 3-methylsulphonyl-4-piperidinobenzoyl, guanidine hydrochloride; SM-20550 = N-aminoiminomethyl-1,4-dimethyl-1H-indole-2-carboxamide methanesulfonic acid; S-3226 = 3-2-3-guanidino-2-methyl-3-oxopropenyl-5-methyl-phenyl-N-isopropylidene-2-methyl-acrylamide dihydrochloride.
1.2. The Molecular Structure of NHEs
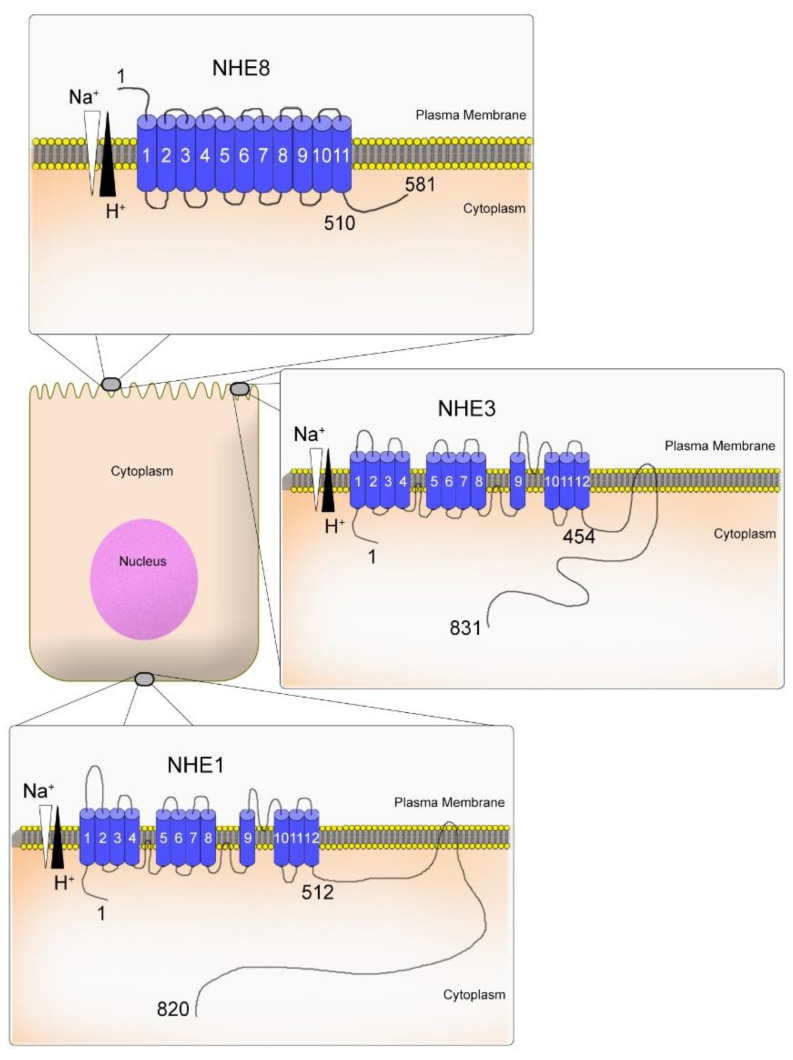
The primary structure of NHE (NHE isoform 1–NHE1) was first discovered by Sardet et al. in 1989 using a genetic complementation approach [45]. Subsequently, other studies discovered more distant related NHE genes that were identified and characterized partially [17,46,47,48]. The molecular structure of NHE8 is still not well known. However, based on the primary structure of NHEs, all isoforms contain a highly conserved 10–12 transmembrane domain that mediates Na+/H+ exchange. The N-terminus region contains approx. 450 amino acids that may be cleaved during protein maturation. The hydrophilic C-terminus region that faces the cytoplasm contains a more divergent domain among the isoforms with a varying length between 70–440 amino acids [46,49]. For example, the two NHE isoforms well studied, NHE1 and NHE3, present a long C-terminus region with 308 amino acids and 377 amino acids, respectively [46]. These long regions have protein interactive sites to bind with multiple phosphorylation sites [46]. However, the NHE8 C-terminus region is short, containing around 70 amino acids with only three phosphorylation sites (Figure 2). According to the UniProt database, NHE8 may interact with 29 proteins [19]. However, none of these protein-protein interactions have been confirmed experimentally. A more detailed molecular structure of the NHE family can be found in the review paper published by John Orlowski and Sergio Grinstein [46].
Figure 2.
Scheme representing the molecular structure of NHE isoform 1 (NHE1), NHE isoform 3 (NHE3), and NHE isoform 8 (NHE8) in the plasma membrane. Data are based on the information from the NCBI website accessed on 1 September 2022 (https://www.ncbi.nlm.nih.gov/nuccore). This illustration was inspired by Orlowski and Grinstein, 2004.
2. NHE8 in the Gastrointestinal Tract
The intestinal epithelia play important roles in barrier function and nutrient absorption. The NHE family is a major player in electroneutral sodium absorption. NHE2, 3, and 8 are expressed in the apical membrane of the intestinal epithelial cells with distinctive patterns. The expression of NHE8 is shown throughout the entire gastrointestinal tract, with high expression detected in the jejunum and colon in both humans and mice [9].
The function of NHE8 was characterized by Xu et al., via transfecting the rat NHE8 cDNA into the Na+/H+ exchanger deficient PS120 cells. It was shown that NHE8 has a relatively higher sodium affinity than NHE2 and a similar proton affinity as NHE3 [9]. Regarding the NHE inhibitor sensitivity, NHE8 displays concentration-dependent sensitivity to HOE694 and S3226 [9]. As a physiological modulator, EGF is known to accelerate intestinal maturation and enhance sodium absorption. While EGF stimulates the activity of NHE2 and NHE3 [50,51], EGF affects NHE8 expression depending on the segment of the intestine. In a study performed in 2010, Xu et al. showed that EGF reduces the endogenous NHE8 gene expression in the ileum of rats (but not in the jejunum) [34]. Mechanistic studies showed that NHE8 inhibition by EGF was mediated by reducing the basal promoter activation of NHE8 [34,52]. In addition, methylprednisolone, a glucocorticoid involved in many physiological processes such as acceleration of intestinal epithelial maturation and enhanced sodium absorption, was shown to inhibit the gene expression and protein abundance of NHE8 in the small intestine of young rats [52].
2.1. The Gender-Specific Compensation in the Intestine
Among the NHE isoforms identified, three (NHE2, 3, and 8) have a significant role in the intestine. NHE2 was initially studied as an NHE isoform responsible for intestinal sodium absorption. However, no significant alteration could be observed in the intestine in the absence of NHE2 [18]. On the other hand, in the absence of NHE3, impaired sodium absorption results in mild diarrhea [53]. Interestingly, NHE3 and NHE2 do not have compensatory roles in the loss of each other [54]. However, NHE8 can compensate for the loss of either NHE2 or NHE3 in a gender-dependent manner. Under normal physiological conditions, the expression of NHE8 in the small intestine is lower in adult male mice compared with adult female mice. In NHE2X3 DKO mice, female NHE2X3 DKO mice have higher survival rates than male NHE2X3 DKO mice. This may be due to the increased NHE8 expression in female NHE2X3 DKO mice. When Caco-2 cells were treated with estradiol or testosterone, the expression of NHE8 was not altered by estradiol treatment, but testosterone treatment led to a significant reduction of NHE8 at both the gene expression and protein level, suggesting a gender-specific regulation is involved in NHE8 expression regulation in the intestine [55].
2.2. NHE8: The Bridge NHE Isoform for the Secretory Lineage?
The intestine is composed of two lineages of cells that are constantly renewed by the leucine-rich repeat-containing G-protein coupled receptor (Lgr5) positive intestinal stem cells: the absorptive and the secretory lineages. Most cell types in the intestine are enterocytes (~85%), which are derived from absorptive lineage. On the other hand, the secretory lineage is represented by goblet cells (~5%), Paneth cells (~5%), enteroendocrine cells (~1%), and tuft cells (<1%) [56,57]. The differentiation and maturation occur from the crypt to the villus, and it is regulated by various signaling pathways. Any breakdown that could cause an imbalance in these cell populations may be implicated in the pathogenesis of inflammatory bowel diseases (IBD) [58].
One of the most studied signaling pathways involved in the developmental process that regulates the fate of intestinal epithelial cells is the Notch pathway [59]. When Notch is stimulated by its ligand, Delta-like Ligand (DLL) or Jagged, it triggers a proteolytic cleavage mediated by γ-secretase that generates a Notch Intracellular domain (NICD). NICD is translocated to the nucleus and promotes gene transcription. One of the transcriptional factors activated by Notch is Hes1, which stimulates the absorptive lineage in the intestine. Hes1 also acts as an inhibitor of AtoH1 (MATH1), which is involved in the stimulation of secretory lineage. When cells migrate out of the Notch pathway, they start to increase the expression of AtoH1 and differentiate toward the secretory lineage [60]. It was shown that loss of NHE8 expression led to reduced mucin production via reduced goblet cells as well as reduced Paneth cell numbers [61,62]. This observation could be associated with the modulation of HES1 and ATOH1 expression levels. However, it must be investigated.
2.3. Lack of NHE8 as a Cause of Dysbiosis
The gut is colonized by trillions of bacteria distributed throughout the gastrointestinal tract that is shaped by diet, the immune system, and genetic factors of the host [63]. It is critical for the organism to be able to identify whether a stimulus is pathogenic or not to respond properly and maintain homeostasis.
Muc2 is a key component of the mucus layer. Without Muc2, the mucin layer that coats the intestinal epithelial cells and separates from luminal contents will be compromised, which would favor the proximity of the commensal and pathogenic microbes, stimulating an intestinal inflammatory response. It has been shown that mice lacking NHE8 have reduced Muc2 expression [61], and they were prone to bacterial adhesion and penetration [62].
Further studies showed that NHE8KO mice also developed dysbiosis [64], which is characterized by the increase of Bacteroidetes and the decrease of Firmicutes, especially the genus Butyricimonas and genus Ruminiclostridium_5 and _6 [65]. In a fecal material transfer study, the composition of microbiota in NHE8KO mice colonized with fecal materials from healthy wild-type control mice was altered with an increase of Bacteroidetes, Lactobacillus, and segmented filamentous bacteria (SFB). Treatment with fecal material from wild-type mice or probiotics is not enough to restore the bacterial population in NHE8KO mice and/or restore mucin production [65]. These observations suggest that NHE8 plays an important role in modulating bacterial diversity [65].
2.4. The Relationship between NHE8 and Diarrhea
The GI tract is lined with various types of ion channels and transporters that function to selectively absorb many nutrients and electrolytes across the intestinal epithelia while flushing toxic chemicals and antigens by maintaining intestinal lumen hydration [66,67]. Any alteration in this dynamic balance between fluid and electrolyte absorption and secretion often leads to diarrhea where either absorption is inhibited, secretion is enhanced or, in a few cases, both processes are affected [68].
Apart from NHE3, NHE8 plays an important role in water and electrolyte transport across the epithelia [69]. In fact, octreotide, an analog of somatostatin has been used as an anti-diarrheal drug for decades [70]. The study by Wang et al. demonstrated that somatostatin, a neuropeptide produced by intestinal D cells, specifically stimulates NHE8 but not NHE3 expression in mouse intestines, probably via the SSTR2-p38 MAPK pathway [71]. Moreover, studies in DSS-induced colitis and Citrobacter rodentium-infected colitis mouse models strongly suggest that upregulation of NHE8 by somatostatin ameliorates diarrheal symptoms in these colitis mouse models [72,73].
2.5. The Relationship between NHE8 and Colitis-Associated Colorectal Cancer
The first line of defense against harmful intestinal luminal contents is the continuously renewing epithelial cells and the inner and outer mucin layer produced by goblet cells [74]. The maintenance of a protective barrier against harmful intestinal luminal contents is pivotal for intestinal homeostasis, and the control of cell renewal depends on the equilibrium between cell proliferation and death. Any imbalance between the two mechanisms is known to result in colorectal cancer (CRC) [75,76].
The majority of human CRC are believed to originate from the activation of multiple signaling pathways, including Wnt signaling, mutation of the APC or β-catenin genes, Hedgehog signaling, p53, and others [77,78,79]. Chronic inflammation is also believed to be involved in the development of CRC [80]. The dysfunction of ion channels, including NHE isoforms, is involved in abnormal proliferation, invasion, and apoptosis, which could lead to CRC [81,82]. For example, NHE1 upregulation in cancer cells stimulates cell proliferation, migration, invasion, and apoptosis, and leads to an intracellular alkalinization and an extracellular acidic tumor environment [83]. NHE3 inhibition is associated with DNA damage and increased local inflammation [84]. In the absence of NHE8, mice have been shown to be susceptible to a colitis-inducible model, dysbiosis, and hyperproliferation [62]. In addition, NHE8 expression is downregulated in colitis animal models and in human UC, and the expression of NHE8 is absent in human colorectal tumors [62,64,85]. NHE8-deficient mice show hyperproliferation and high tumor incidence in colitis-associated CRC via increased expression of Lgr5, β-catenin, and c-Myc [86]. All the data suggested the important roles of NHE8 in intestinal homeostasis and how it contributes to the pathogenesis of colitis-associated CRC.
3. The Role of NHE8 in the Kidney
The kidney is an essential organ responsible for removing waste, controlling electrolytes, and acid-base balance. The structural and functional unit of the kidney is the nephron. Filtration of the blood occurs in the glomerulus, which is wrapped by the Bowman’s capsule in the proximal tubule. Under normal physiological conditions, two-thirds of filtered salt, water, and bicarbonate are reabsorbed in the proximal tubule; pointing to the involvement of ion transporters in the kidney [87].
NHE3 is the most studied NHE isoform involved in proton secretion across the apical membrane of the proximal tubule. However, NHE8 has been shown to be expressed in the same region as NHE3 in the kidney [88]. The function of NHEs in the kidney is further identified using mutant mouse models. NHE2KO mice present similar rates of Na+-dependent proton secretion compared to wild-type mice. On the other hand, NHE3KO mice present approximately 50% Na+-dependent proton secretion compared to wild-type mice. In NHE3KO/NHE2KO mice, the rate of Na+-dependent secretion was the same as that of NHE3KO mice. These observations suggest the existence of another NHE isoform in the proximal tubule participating in acid-base homeostasis regulation [89].
NHE8 was first discovered in the kidney by Sunita Goyal et al. in 2003, as a novel NHE isoform that mediates apical membrane ion transport in the proximal tubules in rats [90]. The same group also showed that neonatal rats have a higher expression level of NHE8 in the brush border membrane vesicles which is decreased 26 days after birth, a pattern opposite to the expression level of NHE3 [88]. Further study suggested that this developmental change of NHE8 expression is mediated by thyroid hormone [91]. In neonatal proximal tubules, metabolic acidosis results in the increased expression of both NHE8 and NHE3 isoforms [92,93]. The acidosis induced NHE8 activity increase was further confirmed in NRK cells [94,95].
In a study using mutant NHE3 and/or NHE8 mouse models, Michel Baum et al., showed that NHE3 protein abundance was increased in the absence of NHE8. Interestingly, the authors observed that serum bicarbonate levels were quite similar between NHE3KO/NHE8KO mice and NHE3KO mice, but the activity of NHE and blood pressure were lower in NHE3KO/NHE8KO mice compared with NHE3KO mice. These observations suggest that NHE8 may play a role in blood pressure regulation [96].
4. NHE8 Expression in the Eyes
NHE8 is highly expressed in the ocular tissue in humans and mice. It plays important roles in protecting the ocular surface. In the absence of NHE8, the tear volume is reduced by approximately 34%, along with increased focal inflammatory infiltration and foamy vacuoles within acinar cells in the lacrimal gland [7]. The conjunctival epithelial layer may also contribute to the reduction of tear volume since abnormal expression of small proline-rich protein 2H (Sprr2h) and transglutaminase 1 (Tgm1) were detected in the eyes of NHE8KO mice [97]. MUC5AC, primarily secreted mucin involved in the lubrication of the ocular surface, is produced by the goblet cells in the conjunctiva [97]. In the absence of NHE8, the expression of Muc5ac is reduced by 60%, suggesting a role of NHE8 in goblet cell function in the eye [97].
The eye is composed of the cornea, iris, pupil, lens, conjunctiva, retina, and other parts to gather light and produce an image that will be transmitted by the optic nerve to the brain. One of the mechanisms that protect the eye against harmful agents is the stimulation of tears by lacrimal glands and conjunctiva to lubricate the ocular surface. Any alteration that leads to the malfunction of tear production may lead to an inflammatory response, which may contribute to the development of dry eye disease. Dry eye is a multifactorial disease that results in tear film instability, discomfort, and visual disturbance [98].
In the back of the eye is the retina. The retinal pigment epithelium (RPE), a monolayer of polarized cells, maintains the homeostasis of the photoreceptor cells [99]. A study performed in rodents showed that an NHE8 mutant led to a resistance of photoreceptor cell death via regulating RPE cell polarity and function. The authors hypothesized that NHE8 protein could regulate pH homeostasis in the protein trafficking pathway [7]. In fact, in a posterior study using the AAV-CRISPR-Cas9 gene editing system to knock down NHE8 in mature retina, the same group showed that NHE8 proteins are localized in the inner segments of the photoreceptor. NHE8 plays a role in photoreceptor survival and in the maintenance of intracellular pH homeostasis in RPE cells [100]. A similar result was also observed by another group showing that mice lacking NHE8 have a noticeable ocular dysfunction, with retinal degeneration and immune cell infiltration associated with abnormal recycling endosomes in the RPE [6].
5. NHE8 in the Testis: Another Fertility Related NHE Isoform
According to the international glossary on infertility and fertility care, fertility is the capacity to establish a clinical pregnancy after one year of regular unprotected sexual intercourse. On the other hand, infertility is a disability to procreation. A third term, subfertility, can be used interchangeably with infertility, and it is related to reduced fertility in unsuccessful sexual intercourse with an aim of procreation [101]. Approximately one in seven couples are infertile, a condition that is well-recognized by the WHO [102]. The etiology of male infertility can vary from genetic mutations, diseases, and/or unhealthy lifestyles. However, testicular dysfunction is the most frequent cause that affects male fertility [103].
Among the five NHE isoforms detected in the testis (NHE1, 2, 3, 8, and 10), only NHE3, NHE8, and NHE10 are known to directly affect male fertility [37,104]. NHE3 is expressed on the apical membrane of epithelial cells in the efferent and epididymal ducts [105]. NHE8 is detected in the cytoplasm, co-localized with the Golgi apparatus in the Leydig cells, and in the developing acrosome of spermatids in the testis in mice [104,106]. NHE10 is the spermatozoa-specific NHE isoform [38]. NHE3KO mice have increased fluid volume in the testis with diminished sperm concentration, which leads to subfertility [107], while NHE10KO mice are infertile due to diminished sperm motility [38].
In NHE8KO mice, infertility is due to a lack of mature sperm in the seminiferous tubules and in the epididymis [104]. Adult NHE8KO male mice have small testis, and low testosterone levels, but normal LH and FSH serum levels. However, LHR protein is approximately 50% reduced in NHE8KO mice compared with wild-type mice. In NHE8 siRNA-transfected Leydig cells (MLTC-1), the expression of LHR is reduced to 35%. Upon stimulation with LH, the cAMP production, which is involved in the production of testosterone in the Leydig cells [108], is only 57% in siRNA-treated Leydig cells compared with control cells [104]. Besides the involvement of NHE8 in testosterone production, NHE8 also contributes to male fertility by affecting spermatogenesis. Karina Oberheide et al. showed that in the absence of NHE8, male mice are infertile due to a disruption of the acrosome formation, producing round-headed spermatozoa lacking the acrosomal cap, which resembles human globozoospermia [106].
6. NHE8 in Other Organs
As a ubiquitous protein, NHE8 is broadly expressed in different organs as represented by the illustration in Figure 1. Besides what we have discussed, NHE8 expression in mice is also detected in other tissues, such as the liver, lung, spleen, and skeletal muscle [90,109]. In humans, the expression of NHE8 is detected in almost all tissues [110]. Recently, NHE8 was localized in the apical membrane of alveolar epithelial cells in the lung, and its expression is subject to the regulation of angiotensin II. However, the precise role of NHE8 in those cells was not elucidated [111].
7. Conclusions
As evidenced in this review article, the function of NHE8 extends beyond a simple Na+/H+ exchanging transporter. Many discoveries have been made by different research groups showing the relevance of NHE8 in different organs. As part of the scientific community, our group has contributed enormously with findings involving the role of NHE8 in the eye, the testis, and the intestine. At present, NHE8 has been well-studied in the colon to show it contributes to mucosal protection by stimulating mucin production, which may be a pivotal molecule for goblet cell differentiation/maturation. However, the mechanisms involved in such processes are still unknown. Therefore, a more advanced investigation of this specific NHE isoform will be needed to discover its role during tissue development/differentiation.
Author Contributions
Conceptualization, C.B.; writing—original draft preparation, C.B. and I.A.S.; writing—review and editing, H.X.; supervision, H.X. and F.K.G.; project administration, H.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK113754.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem. J. 1967;104:588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane N. Proton gradients at the origin of life. Bioessays. 2017;39:1600217. doi: 10.1002/bies.201600217. [DOI] [PubMed] [Google Scholar]
- 3.Janecke A.R., Heinz-Erian P., Yin J., Petersen B.S., Franke A., Lechner S., Fuchs I., Melancon S., Uhlig H.H., Travis S., et al. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum. Mol. Genet. 2015;24:6614–6623. doi: 10.1093/hmg/ddv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronald A., Hoekstra R.A. Autism spectrum disorders and autistic traits: A decade of new twin studies. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B:255–274. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- 5.Kondapalli K.C., Hack A., Schushan M., Landau M., Ben-Tal N., Rao R. Functional evaluation of autism-associated mutations in NHE9. Nat. Commun. 2013;4:2510. doi: 10.1038/ncomms3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jadeja S., Barnard A.R., McKie L., Cross S.H., White J.K., Sanger Mouse Genetics P., Robertson M., Budd P.S., MacLaren R.E., Jackson I.J. Mouse slc9a8 mutants exhibit retinal defects due to retinal pigmented epithelium dysfunction. Investig. Ophthalmol. Vis. Sci. 2015;56:3015–3026. doi: 10.1167/iovs.14-15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia C.H., Liu H., Cheung D., Tang F., Chang B., Li M., Gong X. NHE8 is essential for RPE cell polarity and photoreceptor survival. Sci. Rep. 2015;5:9358. doi: 10.1038/srep09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachos N.C., Tse M., Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu. Rev. Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- 9.Xu H., Chen H., Dong J., Lynch R., Ghishan F.K. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8) Cell Physiol. Biochem. 2008;21:109–116. doi: 10.1159/000113752. [DOI] [PubMed] [Google Scholar]
- 10.Chen J.S., Reddy V., Chen J.H., Shlykov M.A., Zheng W.H., Cho J., Yen M.R., Saier M.H., Jr. Phylogenetic characterization of transport protein superfamilies: Superiority of SuperfamilyTree programs over those based on multiple alignments. J. Mol. Microbiol. Biotechnol. 2011;21:83–96. doi: 10.1159/000334611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saier M.H., Jr., Reddy V.S., Tsu B.V., Ahmed M.S., Li C., Moreno-Hagelsieb G. The Transporter Classification Database (TCDB): Recent advances. Nucleic Acids Res. 2016;44:D372–D379. doi: 10.1093/nar/gkv1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen S.F., Counillon L. The SLC9A-C Mammalian Na(+)/H(+) Exchanger Family: Molecules, Mechanisms, and Physiology. Physiol. Rev. 2019;99:2015–2113. doi: 10.1152/physrev.00028.2018. [DOI] [PubMed] [Google Scholar]
- 13.Damkier H.H., Prasad V., Hubner C.A., Praetorius J. Nhe1 is a luminal Na+/H+ exchanger in mouse choroid plexus and is targeted to the basolateral membrane in Ncbe/Nbcn2-null mice. Am. J. Physiol. Cell Physiol. 2009;296:C1291–C1300. doi: 10.1152/ajpcell.00062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Counillon L., Pouyssegur J., Reithmeier R.A. The Na+/H+ exchanger NHE-1 possesses N- and O-linked glycosylation restricted to the first N-terminal extracellular domain. Biochemistry. 1994;33:10463–10469. doi: 10.1021/bi00200a030. [DOI] [PubMed] [Google Scholar]
- 15.Lin K., Yang N., Luo W., Qian J.F., Zhu W.W., Ye S.J., Yuan C.X., Xu D.Y., Liang G., Huang W.J., et al. Direct cardio-protection of Dapagliflozin against obesity-related cardiomyopathy via NHE1/MAPK signaling. Acta Pharmacol. Sin. 2022 doi: 10.1038/s41401-022-00885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masereel B., Pochet L., Laeckmann D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur. J. Med. Chem. 2003;38:547–554. doi: 10.1016/S0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 17.Orlowski J., Kandasamy R.A., Shull G.E. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J. Biol. Chem. 1992;267:9331–9339. doi: 10.1016/S0021-9258(19)50428-8. [DOI] [PubMed] [Google Scholar]
- 18.Schultheis P.J., Clarke L.L., Meneton P., Harline M., Boivin G.P., Stemmermann G., Duffy J.J., Doetschman T., Miller M.L., Shull G.E. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J. Clin. Investig. 1998;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uniprot.org. 2022. [(accessed on 1 September 2022)]. Available online: https://www.uniprot.org/
- 20.Gurney M.A., Laubitz D., Ghishan F.K., Kiela P.R. Pathophysiology of Intestinal Na(+)/H(+) exchange. Cell Mol. Gastroenterol. Hepatol. 2017;3:27–40. doi: 10.1016/j.jcmgh.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- 22.Johansson S., Rosenbaum D.P., Palm J., Stefansson B., Knutsson M., Lisbon E.A., Hilgendorf C. Tenapanor administration and the activity of the H(+) -coupled transporter PepT1 in healthy volunteers. Br. J. Clin. Pharmacol. 2017;83:2008–2014. doi: 10.1111/bcp.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizzonia J.H., Biemesderfer D., Abu-Alfa A.K., Wu M.S., Exner M., Isenring P., Igarashi P., Aronson P.S. Immunochemical characterization of Na+/H+ exchanger isoform NHE4. Am. J. Physiol. 1998;275:F510–F517. doi: 10.1152/ajprenal.1998.275.4.F510. [DOI] [PubMed] [Google Scholar]
- 24.Chambrey R., Achard J.M., Warnock D.G. Heterologous expression of rat NHE4: A highly amiloride-resistant Na+/H+ exchanger isoform. Pt 1Am. J. Physiol. 1997;272:C90–C98. doi: 10.1152/ajpcell.1997.272.1.C90. [DOI] [PubMed] [Google Scholar]
- 25.Fan S.H., Numata Y., Numata M. Endosomal Na+/H+ exchanger NHE5 influences MET recycling and cell migration. Mol. Biol. Cell. 2016;27:702–715. doi: 10.1091/mbc.E15-04-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diering G.H., Numata M. Endosomal pH in neuronal signaling and synaptic transmission: Role of Na(+)/H(+) exchanger NHE5. Front. Physiol. 2014;4:412. doi: 10.3389/fphys.2013.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garlid K.D. Mitochondrial cation transport: A progress report. J. Bioenerg. Biomembr. 1994;26:537–542. doi: 10.1007/BF00762738. [DOI] [PubMed] [Google Scholar]
- 28.Pescosolido M.F., Stein D.M., Schmidt M., El Achkar C.M., Sabbagh M., Rogg J.M., Tantravahi U., McLean R.L., Liu J.S., Poduri A., et al. Genetic and phenotypic diversity of NHE6 mutations in Christianson syndrome. Ann. Neurol. 2014;76:581–593. doi: 10.1002/ana.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohgaki R., Matsushita M., Kanazawa H., Ogihara S., Hoekstra D., van Ijzendoorn S.C. The Na+/H+ exchanger NHE6 in the endosomal recycling system is involved in the development of apical bile canalicular surface domains in HepG2 cells. Mol. Biol. Cell. 2010;21:1293–1304. doi: 10.1091/mbc.e09-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xian X., Pohlkamp T., Durakoglugil M.S., Wong C.H., Beck J.K., Lane-Donovan C., Plattner F., Herz J. Reversal of ApoE4-induced recycling block as a novel prevention approach for Alzheimer’s disease. Elife. 2018;7 doi: 10.7554/eLife.40048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Numata M., Orlowski J. Molecular cloning and characterization of a novel (Na+,K+)/H+ exchanger localized to the trans-Golgi network. J. Biol. Chem. 2001;276:17387–17394. doi: 10.1074/jbc.M101319200. [DOI] [PubMed] [Google Scholar]
- 32.Kagami T., Chen S., Memar P., Choi M., Foster L.J., Numata M. Identification and biochemical characterization of the SLC9A7 interactome. Mol. Membr. Biol. 2008;25:436–447. doi: 10.1080/09687680802263046. [DOI] [PubMed] [Google Scholar]
- 33.Nikolovska K., Seidler U.E., Stock C. The Role of Plasma Membrane Sodium/Hydrogen Exchangers in Gastrointestinal Functions: Proliferation and Differentiation, Fluid/Electrolyte Transport and Barrier Integrity. Front. Physiol. 2022;13:899286. doi: 10.3389/fphys.2022.899286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H., Zhang B., Li J., Chen H., Tooley J., Ghishan F.K. Epidermal growth factor inhibits intestinal NHE8 expression via reducing its basal transcription. Am. J. Physiol. Cell Physiol. 2010;299:C51–C57. doi: 10.1152/ajpcell.00081.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura N., Tanaka S., Teko Y., Mitsui K., Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. 2005;280:1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 36.Xu H., Ghishan F.K., Kiela P.R. SLC9 Gene Family: Function, Expression, and Regulation. Compr. Physiol. 2018;8:555–583. doi: 10.1002/cphy.c170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kujala M., Hihnala S., Tienari J., Kaunisto K., Hastbacka J., Holmberg C., Kere J., Hoglund P. Expression of ion transport-associated proteins in human efferent and epididymal ducts. Reproduction. 2007;133:775–784. doi: 10.1530/rep.1.00964. [DOI] [PubMed] [Google Scholar]
- 38.Wang D., King S.M., Quill T.A., Doolittle L.K., Garbers D.L. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 2003;5:1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 39.Mine Y., Shuto T., Nikawa H., Kawai T., Ohara M., Kawahara K., Ohta K., Kukita T., Terada Y., Makihira S. Inhibition of RANKL-dependent cellular fusion in pre-osteoclasts by amiloride and a NHE10-specific monoclonal antibody. Cell Biol. Int. 2015;39:696–709. doi: 10.1002/cbin.10447. [DOI] [PubMed] [Google Scholar]
- 40.Lee S.H., Kim T., Park E.S., Yang S., Jeong D., Choi Y., Rho J. NHE10, an osteoclast-specific member of the Na+/H+ exchanger family, regulates osteoclast differentiation and survival [corrected] Biochem. Biophys. Res. Commun. 2008;369:320–326. doi: 10.1016/j.bbrc.2008.01.168. [DOI] [PubMed] [Google Scholar]
- 41.Windler F., Bonigk W., Korschen H.G., Grahn E., Strunker T., Seifert R., Kaupp U.B. The solute carrier SLC9C1 is a Na(+)/H(+)-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat. Commun. 2018;9:2809. doi: 10.1038/s41467-018-05253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye G., Chen C., Han D., Xiong X., Kong Y., Wan B., Yu L. Cloning of a novel human NHEDC1 (Na+/H+ exchanger like domain containing 1) gene expressed specifically in testis. Mol. Biol. Rep. 2006;33:175–180. doi: 10.1007/s11033-006-0010-y. [DOI] [PubMed] [Google Scholar]
- 43.Xiang M., Feng M., Muend S., Rao R. A human Na+/H+ antiporter sharing evolutionary origins with bacterial NhaA may be a candidate gene for essential hypertension. Proc. Natl. Acad. Sci. USA. 2007;104:18677–18681. doi: 10.1073/pnas.0707120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deisl C., Simonin A., Anderegg M., Albano G., Kovacs G., Ackermann D., Moch H., Dolci W., Thorens B., Hediger M.A., et al. Sodium/hydrogen exchanger NHA2 is critical for insulin secretion in beta-cells. Proc. Natl. Acad. Sci. USA. 2013;110:10004–10009. doi: 10.1073/pnas.1220009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sardet C., Franchi A., Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56:271–280. doi: 10.1016/0092-8674(89)90901-X. [DOI] [PubMed] [Google Scholar]
- 46.Orlowski J., Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 47.Tse C.M., Brant S.R., Walker M.S., Pouyssegur J., Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3) J. Biol. Chem. 1992;267:9340–9346. doi: 10.1016/S0021-9258(19)50429-X. [DOI] [PubMed] [Google Scholar]
- 48.Malakooti J., Dahdal R.Y., Schmidt L., Layden T.J., Dudeja P.K., Ramaswamy K. Molecular cloning, tissue distribution, and functional expression of the human Na(+)/H(+) exchanger NHE2. Am. J. Physiol. 1999;277:G383–G390. doi: 10.1152/ajpgi.1999.277.2.G383. [DOI] [PubMed] [Google Scholar]
- 49.Orlowski J., Grinstein S. Na+/H+ exchangers of mammalian cells. J. Biol. Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- 50.Khurana S., Nath S.K., Levine S.A., Bowser J.M., Tse C.M., Cohen M.E., Donowitz M. Brush border phosphatidylinositol 3-kinase mediates epidermal growth factor stimulation of intestinal NaCl absorption and Na+/H+ exchange. J. Biol. Chem. 1996;271:9919–9927. doi: 10.1074/jbc.271.17.9919. [DOI] [PubMed] [Google Scholar]
- 51.Xu H., Collins J.F., Bai L., Kiela P.R., Lynch R.M., Ghishan F.K. Epidermal growth factor regulation of rat NHE2 gene expression. Am. J. Physiol. Cell Physiol. 2001;281:C504–C513. doi: 10.1152/ajpcell.2001.281.2.C504. [DOI] [PubMed] [Google Scholar]
- 52.Xu H., Zhang B., Li J., Chen H., Wang C., Ghishan F.K. Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G921–G927. doi: 10.1152/ajpgi.00227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schultheis P.J., Clarke L.L., Meneton P., Miller M.L., Soleimani M., Gawenis L.R., Riddle T.M., Duffy J.J., Doetschman T., Wang T., et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat. Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 54.Ledoussal C., Woo A.L., Miller M.L., Shull G.E. Loss of the NHE2 Na(+)/H(+) exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1385–G1396. doi: 10.1152/ajpgi.2001.281.6.G1385. [DOI] [PubMed] [Google Scholar]
- 55.Xu H., Li J., Chen R., Zhang B., Wang C., King N., Chen H., Ghishan F.K. NHE2X3 DKO mice exhibit gender-specific NHE8 compensation. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G647–G653. doi: 10.1152/ajpgi.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barker N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 57.Umar S. Intestinal stem cells. Curr. Gastroenterol. Rep. 2010;12:340–348. doi: 10.1007/s11894-010-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 59.Zhang S., Qiang R., Dong Y., Zhang Y., Chen Y., Zhou H., Gao X., Chai R. Hair cell regeneration from inner ear progenitors in the mammalian cochlea. Am. J. Stem Cells. 2020;9:25–35. [PMC free article] [PubMed] [Google Scholar]
- 60.Bray S.J. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 61.Xu H., Zhang B., Li J., Wang C., Chen H., Ghishan F.K. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G335–G343. doi: 10.1152/ajpgi.00146.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang A., Li J., Zhao Y., Johansson M.E., Xu H., Ghishan F.K. Loss of NHE8 expression impairs intestinal mucosal integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309:G855–G864. doi: 10.1152/ajpgi.00278.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernardazzi C., Pego B., de Souza H.S. Neuroimmunomodulation in the Gut: Focus on Inflammatory Bowel Disease. Mediators Inflamm. 2016;2016:1363818. doi: 10.1155/2016/1363818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu C., Xu H., Zhang B., Johansson M.E., Li J., Hansson G.C., Ghishan F.K. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am. J. Physiol. Cell Physiol. 2013;305:C121–C128. doi: 10.1152/ajpcell.00101.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bernardazzi C., Xu H., Tong H., Laubitz D., Figliuolo da Paz V., Curiel L., Ghishan F.K. An indisputable role of NHE8 in mucosal protection. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;319:G421–G431. doi: 10.1152/ajpgi.00246.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiela P.R., Ghishan F.K. Ion transport in the intestine. Curr. Opin. Gastroenterol. 2009;25:87–91. doi: 10.1097/MOG.0b013e3283260900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheikh I.A., Koley H., Chakrabarti M.K., Hoque K.M. The Epac1 signaling pathway regulates Cl- secretion via modulation of apical KCNN4c channels in diarrhea. J. Biol. Chem. 2013;288:20404–20415. doi: 10.1074/jbc.M113.467860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petri W.A., Jr., Miller M., Binder H.J., Levine M.M., Dillingham R., Guerrant R.L. Enteric infections, diarrhea, and their impact on function and development. J. Clin. Investig. 2008;118:1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aronson P.S. Ion exchangers mediating NaCl transport in the renal proximal tubule. Cell Biochem. Biophys. 2002;36:147–153. doi: 10.1385/CBB:36:2-3:147. [DOI] [PubMed] [Google Scholar]
- 70.Lewin M.J. The somatostatin receptor in the GI tract. Annu. Rev. Physiol. 1992;54:455–468. doi: 10.1146/annurev.ph.54.030192.002323. [DOI] [PubMed] [Google Scholar]
- 71.Wang C., Xu H., Chen H., Li J., Zhang B., Tang C., Ghishan F.K. Somatostatin stimulates intestinal NHE8 expression via p38 MAPK pathway. Am. J. Physiol. Cell Physiol. 2011;300:C375–C382. doi: 10.1152/ajpcell.00421.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lei X., Cai L., Li X., Xu H., Geng C., Wang C. Up-regulation of NHE8 by somatostatin ameliorates the diarrhea symptom in infectious colitis mice model. Korean J. Physiol. Pharmacol. 2018;22:269–275. doi: 10.4196/kjpp.2018.22.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X., Cai L., Xu H., Geng C., Lu J., Tao L., Sun D., Ghishan F.K., Wang C. Somatostatin regulates NHE8 protein expression via the ERK1/2 MAPK pathway in DSS-induced colitis mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;311:G954–G963. doi: 10.1152/ajpgi.00239.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hammer A.M., Morris N.L., Earley Z.M., Choudhry M.A. The First Line of Defense: The Effects of Alcohol on Post-Burn Intestinal Barrier, Immune Cells, and Microbiome. Alcohol. Res. 2015;37:209–222. doi: 10.35946/arcr.v37.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koveitypour Z., Panahi F., Vakilian M., Peymani M., Seyed Forootan F., Nasr Esfahani M.H., Ghaedi K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9:97. doi: 10.1186/s13578-019-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pandurangan A.K., Divya T., Kumar K., Dineshbabu V., Velavan B., Sudhandiran G. Colorectal carcinogenesis: Insights into the cell death and signal transduction pathways: A review. World J. Gastrointest. Oncol. 2018;10:244–259. doi: 10.4251/wjgo.v10.i9.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshimoto A.N., Bernardazzi C., Carneiro A.J., Elia C.C., Martinusso C.A., Ventura G.M., Castelo-Branco M.T., de Souza H.S. Hedgehog pathway signaling regulates human colon carcinoma HT-29 epithelial cell line apoptosis and cytokine secretion. PLoS ONE. 2012;7:e45332. doi: 10.1371/journal.pone.0045332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Preisler L., Habib A., Shapira G., Kuznitsov-Yanovsky L., Mayshar Y., Carmel-Gross I., Malcov M., Azem F., Shomron N., Kariv R., et al. Heterozygous APC germline mutations impart predisposition to colorectal cancer. Sci. Rep. 2021;11:5113. doi: 10.1038/s41598-021-84564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aghabozorgi A.S., Bahreyni A., Soleimani A., Bahrami A., Khazaei M., Ferns G.A., Avan A., Hassanian S.M. Role of adenomatous polyposis coli (APC) gene mutations in the pathogenesis of colorectal cancer; current status and perspectives. Biochimie. 2019;157:64–71. doi: 10.1016/j.biochi.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 81.Lin Y., Chang G., Wang J., Jin W., Wang L., Li H., Ma L., Li Q., Pang T. NHE1 mediates MDA-MB-231 cells invasion through the regulation of MT1-MMP. Exp. Cell Res. 2011;317:2031–2040. doi: 10.1016/j.yexcr.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 82.Zhang M., Li T., Zhu J., Tuo B., Liu X. Physiological and pathophysiological role of ion channels and transporters in the colorectum and colorectal cancer. J. Cell Mol. Med. 2020;24:9486–9494. doi: 10.1111/jcmm.15600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cardone R.A., Casavola V., Reshkin S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer. 2005;5:786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 84.Laubitz D., Harrison C.A., Midura-Kiela M.T., Ramalingam R., Larmonier C.B., Chase J.H., Caporaso J.G., Besselsen D.G., Ghishan F.K., Kiela P.R. Reduced Epithelial Na+/H+ Exchange Drives Gut Microbial Dysbiosis and Promotes Inflammatory Response in T Cell-Mediated Murine Colitis. PLoS ONE. 2016;11:e0152044. doi: 10.1371/journal.pone.0152044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu H., Chen H., Dong J., Li J., Chen R., Uno J.K., Ghishan F.K. Tumor necrosis factor-{alpha} downregulates intestinal NHE8 expression by reducing basal promoter activity. Am. J. Physiol. Cell Physiol. 2009;296:C489–C497. doi: 10.1152/ajpcell.00482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu H., Li J., Chen H., Ghishan F.K. NHE8 Deficiency Promotes Colitis-Associated Cancer in Mice via Expansion of Lgr5-Expressing Cells. Cell Mol. Gastroenterol. Hepatol. 2019;7:19–31. doi: 10.1016/j.jcmgh.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakhoul N., Batuman V. Role of proximal tubules in the pathogenesis of kidney disease. Contrib. Nephrol. 2011;169:37–50. doi: 10.1159/000313944. [DOI] [PubMed] [Google Scholar]
- 88.Becker A.M., Zhang J., Goyal S., Dwarakanath V., Aronson P.S., Moe O.W., Baum M. Ontogeny of NHE8 in the rat proximal tubule. Am. J. Physiol. Renal Physiol. 2007;293:F255–F261. doi: 10.1152/ajprenal.00400.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi J.Y., Shah M., Lee M.G., Schultheis P.J., Shull G.E., Muallem S., Baum M. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J. Clin. Investig. 2000;105:1141–1146. doi: 10.1172/JCI9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goyal S., Vanden Heuvel G., Aronson P.S. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am. J. Physiol. Renal Physiol. 2003;284:F467–F473. doi: 10.1152/ajprenal.00352.2002. [DOI] [PubMed] [Google Scholar]
- 91.Gattineni J., Sas D., Dagan A., Dwarakanath V., Baum M. Effect of thyroid hormone on the postnatal renal expression of NHE8. Am. J. Physiol. Renal Physiol. 2008;294:F198–F204. doi: 10.1152/ajprenal.00332.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Twombley K., Gattineni J., Bobulescu I.A., Dwarakanath V., Baum M. Effect of metabolic acidosis on neonatal proximal tubule acidification. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R1360–R1368. doi: 10.1152/ajpregu.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pirojsakul K., Gattineni J., Dwarakanath V., Baum M. Renal NHE expression and activity in neonatal NHE3- and NHE8-null mice. Am. J. Physiol. Renal Physiol. 2015;308:F31–F38. doi: 10.1152/ajprenal.00492.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang J., Bobulescu I.A., Goyal S., Aronson P.S., Baum M.G., Moe O.W. Characterization of Na+/H+ exchanger NHE8 in cultured renal epithelial cells. Am. J. Physiol. Renal Physiol. 2007;293:F761–F766. doi: 10.1152/ajprenal.00117.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joseph C., Twombley K., Gattineni J., Zhang Q., Dwarakanath V., Baum M. Acid increases NHE8 surface expression and activity in NRK cells. Am. J. Physiol. Renal Physiol. 2012;302:F495–F503. doi: 10.1152/ajprenal.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baum M., Twombley K., Gattineni J., Joseph C., Wang L., Zhang Q., Dwarakanath V., Moe O.W. Proximal tubule Na+/H+ exchanger activity in adult NHE8-/-, NHE3-/-, and NHE3-/-/NHE8-/- mice. Am. J. Physiol. Renal Physiol. 2012;303:F1495–F1502. doi: 10.1152/ajprenal.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miyake H., Mori N., Mano H., Imanaka T., Nakamura M. Development of a highly sensitive and reliable enzyme-linked immunosorbent assay for MUC5AC in human tears extracted from Schirmer strips. Clin. Ophthalmol. 2018;12:1571–1580. doi: 10.2147/OPTH.S170552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul. Surf. 2007;5:75–92. doi: 10.1016/S1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 99.Mazzoni F., Safa H., Finnemann S.C. Understanding photoreceptor outer segment phagocytosis: Use and utility of RPE cells in culture. Exp. Eye Res. 2014;126:51–60. doi: 10.1016/j.exer.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xia C.H., Ferguson I., Li M., Kim A., Onishi A., Li L., Su B., Gong X. Essential function of NHE8 in mouse retina demonstrated by AAV-mediated CRISPR/Cas9 knockdown. Exp. Eye Res. 2018;176:29–39. doi: 10.1016/j.exer.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zegers-Hochschild F., Adamson G.D., Dyer S., Racowsky C., de Mouzon J., Sokol R., Rienzi L., Sunde A., Schmidt L., Cooke I.D., et al. The International Glossary on Infertility and Fertility Care, 2017. Hum. Reprod. 2017;32:1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cavallini G. Male idiopathic oligoasthenoteratozoospermia. Asian J. Androl. 2006;8:143–157. doi: 10.1111/j.1745-7262.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 103.Vander Borght M., Wyns C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 104.Xu H., Chen H., Li J., Zhao Y., Ghishan F.K. Disruption of NHE8 expression impairs Leydig cell function in the testes. Am. J. Physiol. Cell Physiol. 2015;308:C330–C338. doi: 10.1152/ajpcell.00289.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hihnala S., Kujala M., Toppari J., Kere J., Holmberg C., Hoglund P. Expression of SLC26A3, CFTR and NHE3 in the human male reproductive tract: Role in male subfertility caused by congenital chloride diarrhoea. Mol. Hum. Reprod. 2006;12:107–111. doi: 10.1093/molehr/gal009. [DOI] [PubMed] [Google Scholar]
- 106.Oberheide K., Puchkov D., Jentsch T.J. Loss of the Na(+)/H(+) exchanger NHE8 causes male infertility in mice by disrupting acrosome formation. J. Biol. Chem. 2017;292:10845–10854. doi: 10.1074/jbc.M117.784108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Q., Clarke L., Nie R., Carnes K., Lai L.W., Lien Y.H., Verkman A., Lubahn D., Fisher J.S., Katzenellenbogen B.S., et al. Estrogen action and male fertility: Roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc. Natl. Acad. Sci. USA. 2001;98:14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Golkowski M., Shimizu-Albergine M., Suh H.W., Beavo J.A., Ong S.E. Studying mechanisms of cAMP and cyclic nucleotide phosphodiesterase signaling in Leydig cell function with phosphoproteomics. Cell. Signal. 2016;28:764–778. doi: 10.1016/j.cellsig.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cao L., Yuan Z., Liu M., Stock C. (Patho-)Physiology of Na(+)/H(+) Exchangers (NHEs) in the Digestive System. Front. Physiol. 2019;10:1566. doi: 10.3389/fphys.2019.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu H., Chen R., Ghishan F.K. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G36–G41. doi: 10.1152/ajpgi.00552.2004. [DOI] [PubMed] [Google Scholar]
- 111.Kinaneh S., Knany Y., Khoury E.E., Ismael-Badarneh R., Hamoud S., Berger G., Abassi Z., Azzam Z.S. Identification, localization and expression of NHE isoforms in the alveolar epithelial cells. PLoS ONE. 2021;16:e0239240. doi: 10.1371/journal.pone.0239240. [DOI] [PMC free article] [PubMed] [Google Scholar]