Abstract
A total of 603 samples of selected spices from different seasons (winter and summer) were analyzed for the occurrence of aflatoxin B1 (AFB1), total AFs, and tocopherols. The findings revealed that 120 (38.7%) samples from the summer and 136 (46.4%) samples from the winter season were observed to be infected with AFB1 and a large amount of AFs. The highest means of both AFB1 and total Afs were observed in red pepper, i.e., 15.5 ± 3.90 µg/kg and 22.90 ± 4.10 µg/kg, respectively. The minimum averages of AFB1 and total AFs were observed in cloves of 6.32 ± 1.8 and 8.40 ± 1.60 µg/kg, respectively (from the winter season). The seasonal variations in the levels of the total AFs in selected spices were observed to be nonsignificant (p ≥ 0.05), except for the levels in red pepper and ginger samples, which showed significant differences (p ≤ 0.05). The maximum average of the dietary intake of Afs, 4.80 µg/day/kg, was found in ginger from the winter season in individual females. Furthermore, the findings document that the maximum level of total tocopherol, i.e., 44.8 ± 9.3 mg/100 g, was observed in black pepper from the winter season. A significant difference in the concentration of total tocopherols was observed in selected spices from the summer and the winter seasons (p ≤ 0.05).
Keywords: AFB1, AFs, selected spices, dietary intake, tocopherol levels, seasonal variations
1. Introduction
Spices are essential food ingredients and defined as “the eatable parts of plants which are usually mixed with food items to create color, aroma, appearance, and also for flavoring purposes” [1]. Spices such as paprika, oregano, clove, onion, and garlic have characteristic antimicrobial activities [2]. The essential spices such as chili, coriander, turmeric, cinnamon, cloves, ginger, black pepper, and nutmeg are famous for their food products. In a report from 2016–17, 2.8 million tons of spices were produced worldwide, and 95% of production was contributed by Asia, 0.5% by Europe, 2.9% by Africa, and a 1.5% share from America [3]. In a report by the Food and Agriculture Organization, spices were harvested from an area of 16,653 ha in 2019, as compared to 17,153 ha in 2018 [4], with a production of 76.08 tons as compared to the production of 77.54 tons in 2018. Chilies and dry peppers were harvested in 2018 and 2019 from areas of 47,349 and 65,275 ha, with a production of 101.66 thousand tons and 148.14 thousand tons, respectively. Spices are exposed to various microflora during harvesting, processing, transportation, and storage, e.g., normally open grounds are used for sun-drying due to this being a cost-efficient and inexpensive approach. However, it creates substantial losses in terms of product quality and quantity [5]. Fungal infection in spices leads to mycotoxin formation, with temperature ranges from 25 to 30 °C and moisture contents greater than 16% [6]. Furthermore, the storage of crops under inadequately maintained on-farm or in-house requirements could also lead to damages to the value of crops, which results from insect infestation and fungal contamination [7]. Mycotoxins are recognized as toxic compounds that are formed by different types of foodborne fungi. They have shown poisonous and carcinogenic effects in animals and humans [7]. The fungi can attack fruits, cereals, grains, animal forages, and spices. The species Fusarium, Alternaria, Aspergillus, Penicillium, and Claviceps genera are important fungal species that produce mycotoxins [8]. The significant types of mycotoxins are aflatoxins (AFs), fumonisins, ochratoxin A, sterigmatocystin, zearalenone, patulin, and trichothecenes (HT-2 and T-2 toxins, nivalenol, and deoxynivalenol) [9]. The Food and Agriculture Organization documented that 25% of food and food crops are infected by these mycotoxins [10]. The main producers of AF fungi are Aspergillus flavus and Aspergillus parasiticus, and rarely by A. nomious [11,12]. AFs are odorless, tasteless, and invisible chemical compounds with polar solubility [13]. International organizations, such as The International Agency for Research on Cancer [14], have categorized these fungal metabolites as group 1 carcinogens to humans. The main subclass of AFs is aflatoxin B1 (AFB1), which is identified as the most toxic of all [15]. The other classes include aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2). The exposure of aflatoxins to humans occurs in two ways: directly by consuming contaminated food or indirectly using produce from an animal or plant origin (e.g., eggs, meat, or vegetables) [16]. AFB1 consumed by animals through feed could cause lower blood production and anorexia [17]. It has been observed that chronic exposure to AFs could cause liver cancer. If the carrier has the hepatitis B virus, it synergistically deteriorates the health and functions of the liver. Acute aflatoxicosis causes episodic poisoning outbreaks and death [18,19,20]. The exposure of AFB1 in humans is found to increase the cellular calcium in mitochondria, which is responsible for increasing the levels of reactive oxygen species in cells [21,22]. However, aflatoxin M1 (AFM1) is the metabolite of AFB1, and it is mainly excreted in milk and the urine of milking animals [23]. A limit of 5 µg/kg for AFB1, and 10 µg/kg for total AFs in spices was implemented by the European Union (EU) [24].
Pakistan is a tropical country with severe environmental conditions. Therefore, the routine analysis for the presence of such toxins is highly desirable and recommended. Previous findings have documented a considerable concentration of ochratoxin A (OTA) and AFs in chilies [25,26,27,28,29,30,31,32,33]. Therefore, the current research is focused on investigating the seasonal variation and prevalence of AFB1 and total AFs in selected spices, comparing the amounts of AFB1 and total AFs with the European Union (EU) regulations, and investigating the daily intake of aflatoxins in spices and determining the levels of tocopherols in spices. The results will be helpful for law enforcement agencies and food regulation agencies to execute rigorous guidelines for these mycotoxins in spices.
2. Results and Discussion
2.1. Validation of Analytical Method
The HPLC method validation parameters were performed by evaluating the limit of detection (LOD) and the quantification limit (LOQ). The LOD and LOQ for AFB1 and AFG1 were 0.05 and 0.15 μg/kg, and 0.07 and 0.22 μg/kg for AFB2 and AFG2, respectively. The LOQ was assessed as having a signal-to-noise ratio S/N = 10 and an LOD as (S/N) = 3. The accuracy and precision of the method was determined using recovery analysis. The fortified amounts of 2, 10, and 25 μg/kg of AFB1 and AFG1 were mixed into spices, and the levels of 2, 8 and 20 μg/kg of AFB2 and AFG2 were combined in unaffected spice samples. The recovery values of all fortified samples ranged from 78.8% to 108.6%, with the RSD varying from 10 to 22%, as shown in Table 1. Reproducibility refers to producing the same results using different labs, and repeatability refers to producing the same results using the same analytical conditions.
Table 1.
Analytical parameters for the analysis of AFs and tocopherols.
| Target Compound | Linearity µg/kg | LOD µg/kg | LOQ µg/kg |
R2 | Precision (%RSD) | |
|---|---|---|---|---|---|---|
| Reproducibility * | Repeatability * | |||||
| AFB1 | 0.5–120 | 0.05 | 0.15 | 0.9940 | 10 | 15 |
| AFB2 | 0.5–50 | 0.07 | 0.22 | 0.9920 | 14 | 17 |
| AFG1 | 0.5–120 | 0.05 | 0.15 | 0.9870 | 13 | 9 |
| AFG2 | 0.5–50 | 0.07 | 0.22 | 0.9890 | 15 | 17 |
RSD: relative standard deviation; LOQ: limit of quantification; LOD: limit of detection. * Reproducibility = (mean of 7 replicates); * Repeatability = (mean of 7 replicates).
2.2. Occurrence of AFs in Spices
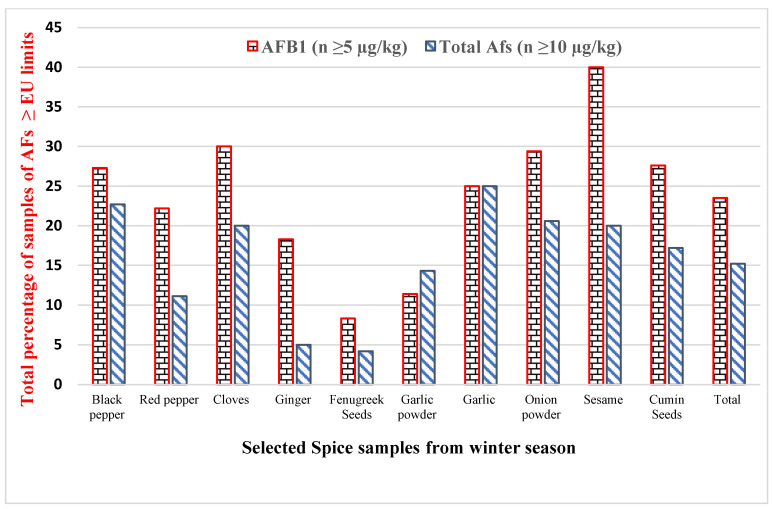
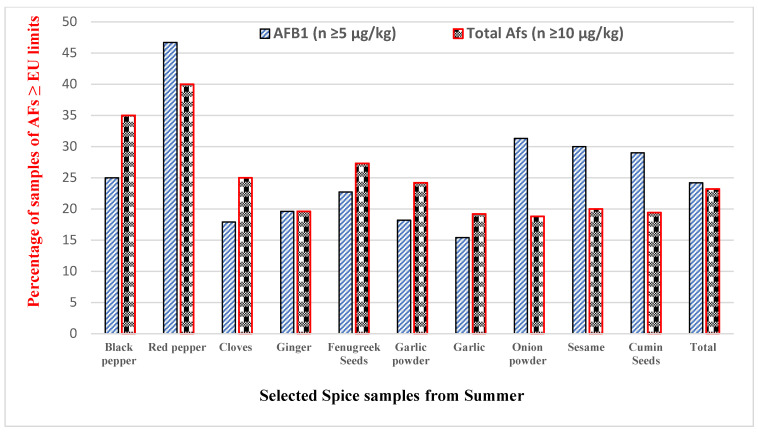
The incidence and co-occurrence of AFs in different spices were examined in 293 samples (winter season) and 310 samples (summer season), as shown in Table 2. The research findings revealed that the highest averages of AFB1 and total AFs were observed in red pepper, i.e., 15.50 ± 3.90 µg/kg and 22.90 ± 4.10 µg/kg, respectively, and the minimum average levels were observed in cloves, 6.32 ± 1.80 and 8.40 ± 1.60 µg/kg, respectively, from the winter season. During the summer season, the highest average amounts of AFB1 and total AFs were discovered in red pepper, i.e., 9.80 ± 2.10 and 12.50 ± 1.90 µg/kg, respectively, and the minimum average amounts were documented in garlic, 4.10 ± 1.60 and 8.50 ± 1.60 µg/kg, respectively. The outcomes of the current survey show that the levels of total AFs in selected spices from different seasons were nonsignificant (p ≥ 0.05), except for the red pepper and ginger samples, which showed a significant difference (p ≤ 0.05). Furthermore, 136 (46.4%) samples from the winter season and 120 (38.7%) samples from the summer season were examined and shown to be positive with AFs. The percentages of samples above the mentioned limit of the EU for AFB1 (≥5 µg/kg) and total AFs (≥10 µg/kg) in selected spices were 24.2% and 23.2%, respectively, from the winter season, as shown in Figure 1. Similarly, the percentages of samples greater than the set limits (the EU) for AFB1 and total AFs from the summer season were 23.5% and 15.2%, respectively, as presented in Figure 2.
Table 2.
Occurrence of AFB1 and total AFs (µg/kg) in selected spices from summer and winter season from Punjab, Pakistan.
| Summer Season | Winter Season | |||||||
|---|---|---|---|---|---|---|---|---|
| Spices | Samples | Positive Sample | AFB1 | Total AFs | Samples | Positive Sample | AFB1 | Total AFs |
| n | n (%) | µg/kg ± SD | µg/kg ± SD | n | n (%) | µg/kg ± SD | µg/kg ± SD | |
| Black pepper | 22 | 10 (45.4) | 5.60 ± 1.80 | 8.80 ± 2.10 NS | 20 | 12 (60.0) | 7.90 ± 2.10 | 11.20 ± 2.50 NS |
| Red pepper | 18 | 8 (44.4) | 9.80 ± 2.10 | 12.50 ± 1.90 ** | 15 | 10 (53.3) | 15.50 ± 3.90 | 22.90 ± 4.10 ** |
| Cloves | 30 | 12 (40.0) | 4.32 ± 1.00 | 6.50 ± 1.50 NS | 28 | 9 (32.1) | 6.32 ± 1.80 | 8.40 ± 1.60 NS |
| Ginger | 60 | 15 (25.0) | 9.42 ± 2.50 | 11.45 ± 2.10 ** | 56 | 19 (33.9) | 14.50 ± 2.00 | 20.60 ± 4.40 ** |
| Fenugreek Seeds | 24 | 6 (25.0) | 5.80 ± 1.90 | 8.60 ± 1.80 NS | 22 | 8 (36.3) | 7.40 ± 1.95 | 10.20 ± 1.80 NS |
| Garlic powder | 35 | 10 (28.6) | 6.72 ± 1.50 | 9.20 ± 1.90 NS | 33 | 12 (36.4) | 8.90 ± 3.20 | 11.10 ± 3.40 NS |
| Garlic | 28 | 12 (42.8) | 4.10 ± 1.60 | 8.50 ± 1.60 NS | 26 | 13 (50.0) | 5.90 ± 2.40 | 11.20 ± 2.10 NS |
| Onion powder | 34 | 15 (44.1) | 6.10 ± 1.20 | 10.20 ± 2.10 NS | 32 | 18 (56.2) | 7.30 ± 1.90 | 11.90 ± 2.30 NS |
| Sesame | 30 | 18 (60.0) | 7.90 ± 1.30 | 9.10 ± 2.20 NS | 30 | 19 (63.3) | 9.50 ± 1.90 | 12.40 ± 2.0 NS |
| Cumin Seeds | 29 | 14 (48.3) | 4.50 ± 1.40 | 10.50 ± 1.40 NS | 31 | 18 (58.1) | 6.40 ± 1.80 | 11.90 ± 2.10 NS |
| Total Samples | 310 | 120 (38.7) | 293 | 136 (46.4) | ||||
N = number of samples. NS = nonsignificant (the samples show nonsignificant difference between summer and winter seasons (α = 0.05). ** = the samples show significant difference in AF levels in summer and winter seasons.
Figure 1.
The percentage of samples higher than the recommended limits for AFB1 and total AFs in winter season.
Figure 2.
The percentage of samples higher than the recommended limits for AFB1 and total AFs in summer season.
Earlier studies [34,35,36,37,38,39,40,41,42,43,44] from different countries (Table 3) showed the levels of AFs in spices. Our earlier study, Iqbal et al. [27], from Pakistan, reported that AFB1 levels were found in 39.7% samples of ground chili samples and 33.3% in the whole chili samples, i.e., with mean levels of AFB1 of 18.5 and 17.4 µg/kg, respectively. These results are in agreement with the findings of the current research.
Table 3.
Incidence and occurrence of AFs in different spices from different countries.
| Food Type | Samples | Positive | Mean (µg/kg) | Range (µg/kg) | Country | Author |
|---|---|---|---|---|---|---|
| Black pepper | AFB1 (69.28 | 84.09 | Qatar | Hammami et al. [34] | ||
| Chili powder | 66.6% | AFB1 (37.44) | 5.60 to 69.28 | China | Zhao, et al. [35] | |
| Black cumin | 40.0% | AFB1 (25) | 20 to 30 | |||
| Chili | 100% | AFs (33.8) | 1.9 to 65.7 | Spain | Santos et al. [36] | |
| Chili | AFB1 (41) | 1.6 to 80.4 | Turkey | Bircan [37] | ||
| Red pepper | 75 | 72 (96%) | AFB1 (12.405) | 0.11 to 24.7 | Turkey | Ardic et al. [38] |
| Pepper | 11 | 5 (54.5%) | AFB1 (13.74) | 0.57 to 26.9 | Italy | Romagnoli et al. [39] |
| Pepper | 30 | 13 | 18.7 | 1.9 to 35.5 | Turkey | Colak et al. [40] |
| Ground chili | 39.7% | 18.5 | Pakistan | Iqbal et al. [27], | ||
| Whole chili | 33.3% | 17.4 | ||||
| Ginger (Dry season) | 46% | AFs (1.18) | 0.17 to 12.02 | Nigeria | Lippolis et al. [41] | |
| Ginger (rainy season) | 81% | AFs (3.13) | 0.11 to 9.52 | |||
| Cayenne | 25% | 0.06 | Ireland | O’Riordan and Wilkinson [42] | ||
| Coriander | 11.1% | 0.31 | ||||
| Paprika | 20% | 0.32 | ||||
| Turmeric | 40% | 1.90 | ||||
| Ginger | 7 | 1 (14.2%) | 0.295 | China | Zhao et al. [35] | |
| Mixed Spices | 6 | 5 (83.3) | 2.64 | |||
| Spices | 130 | 20 | 0.96 | 0.59 to 5.38 | Italy | Prelle et al. [43] |
| Chili | 37.5% | 3 | 1 to 5 | Portugal | Martins et al. [44] |
The variations and fluctuations in the levels of AFs in spices are dependent on various factors. For example, temperature and moisture are the two main factors affecting the growth of mold, while factors such as dry, hot surface areas and the microbial resistance of crops against the toxigenic species are vital [6]. The toxicity level depends upon the difference in the toxin structure during farming seasons [45]. The high occurrence of AFs in spices might be due to the climatic conditions in Pakistan, which are conducive for the growth of aflatoxigenic fungi [39]. Furthermore, the old traditional technology and drying techniques used with spices are also one other reason for the high incidence of AFs in spices [46]. Pakistan is the major contributor in chili production and has tropical climate conditions, i.e., a hot and humid environment, for the growth of spices. Therefore, the production and postharvest conditions such as the processing, transportation, and storage of spices can lead to significant challenges in the quality of the food. To avoid or minimize the levels of AFs in food stuffs, adopting good harvesting practices and good storage practices, and implementing critical control point (HACCP) principles will be decisive for controlling the proliferation of fungi [30,32]. The following points should be kept in mind to avoid fungal attacks on spices.
The proper handling of spices during storage and transportation with skilled practices.
Drying process should be carried out under controlled conditions of temperature and moisture.
Humidity levels should be controlled during preharvest and postharvest steps.
2.3. Estimation of Dietary Intake
Assessments of the dietary intake of the total AFs in spices were determined from different seasons and appear in Table 4. The highest mean dietary intake of AFs was observed in onion powder, 3.71 µg/day/kg, in individual females. However, the maximum average dietary daily intake of total AFs was found to be 2.91 µg/day/kg in onion powder in individual males. The highest average dietary intake of AFs was documented in ginger, i.e., 4.80 µg/day/kg, and the lowest in cloves from the winter season, i.e., 0.76 µg/day/kg, in the female participants. The maximum dietary intake levels were noticed in ginger, i.e., 3.77 µg/day/kg, and the lowest, 0.60 µg/day/kg, in cloves, in individual males.
Table 4.
Estimation of dietary intake for AFs in spices in local population from Punjab, Pakistan.
| Product Type | Consumption kg/day | Winter | Summer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Afs’ Mean Level µg/kg | Dietary Intake ng/kg/day | MOE | Cancer Risk (Per 105 Individuals Per Year) | Afs’ Mean Level µg/kg | Dietary Intake (ng/kg/day) | MOE | Cancer Risk (Per 105 Individuals Per Year) | ||
| Black pepper | 0.005 | 11.2 | 0.80 | 0.002 | 8.4 × 10−3 | 8.8 | 0.63 | 0.002 | 6.6 × 10−3 |
| Red pepper | 0.01 | 17.9 | 2.56 | 0.006 | 2.68 × 10−2 | 12.5 | 1.79 | 0.004 | 1.88 × 10−2 |
| Cloves | 0.005 | 8.4 | 0.60 | 0.002 | 6.3 × 10−3 | 6.5 | 0.46 | 0.001 | 4.87 × 10−3 |
| Ginger | 0.015 | 17.6 | 3.77 | 0.009 | 3.96 × 10−3 | 11.45 | 2.45 | 0.006 | 2.58 × 10−2 |
| Fenugreek Seeds | 0.005 | 10.2 | 0.73 | 0.002 | 7.65 × 10−3 | 8.6 | 0.61 | 0.002 | 6. 45 × 10−3 |
| Garlic powder | 0.01 | 11.1 | 1.59 | 0.004 | 1.76 × 10−2 | 9.2 | 1.31 | 0.003 | 1.38 × 10−2 |
| Garlic | 0.015 | 11.2 | 2.40 | 0.006 | 2.52 × 10−2 | 8.5 | 1.82 | 0.005 | 1.91 × 10−2 |
| Onion powder | 0.02 | 11.9 | 3.40 | 0.009 | 3.57 × 10−2 | 10.2 | 2.91 | 0.007 | 3.06 × 10−2 |
| Sesame | 0.01 | 12.4 | 1.77 | 0.004 | 1.86 × 10−2 | 9.1 | 1.30 | 0.003 | 1.37 × 10−2 |
| Cumin Seeds | 0.015 | 11.9 | 2.55 | 0.006 | 2.68 × 10−2 | 10.5 | 2.25 | 0.006 | 2.36 × 10−2 |
The average weight of male = 70 kg (mean age, 30.5 years).
The levels of dietary assessment from Pakistan were documented in another study, Akhtar et al. [47]. They documented the dietary intake of AFs in branded and nonbranded spices, and the observed dietary intake of AFs was 0.72 ng/kg bw/day, ranging from 0.31 to 1.30 ng/kg bw/day, in branded spices, and 1.59 ng/kg bw/day, ranging from 0.66 to 3.29 ng/kg bw/day, in nonbranded spices. Iqbal et al. [30] documented the maximum dietary intakes of AFB1, OTA, and total Afs, i.e., 3.67, 3.629, and 3.82 µg/day/kg in chili sauces, respectively, from Pakistan, which are comparable to the results of the dietary intake levels documented in the present study.
3. Conclusions
The study documents the high levels of AFB1 and total AFs in selected spices. The results show a nonsignificant difference between the total amount of AFs from the summer season to the winter season, except for the red pepper and ginger samples, which show a significant difference (p ≤ 0.05). The estimated dietary intake of total AFs is higher in individual females as compared to individual males. The results show that there is a significant difference in the tocopherol levels in selected spices from the winter season compared to the summer season. The results are useful for farmers, consumers, local traders, and law enforcement agencies to execute rigorous rules in the country.
4. Materials and Methods
4.1. Sampling
A total of 603 samples of selected spices (black pepper, red pepper, cloves, ginger, fenugreek seeds, garlic, garlic powder, onion powder, sesame, and cumin seeds) were collected from central cities of Punjab, Pakistan. Due to uneven distribution of fungi, a size of at least half kg was selected, and the samples were stored in polyethylene zip bags at room temperature in lab.
4.2. The Regents and Chemicals
The standards, including of Afs, tween® 20, phosphate-buffered saline (PBS), acetonitrile (HPLC grade), and methanol (HPLC grade), were obtained from Sigma-Aldrich (Steinheim, Germany). The cleanup columns, immunoaffinity columns (IAC), AflaOchra (IAC) were acquired from VICAM (Watertown, MA, USA). Furthermore, other chemicals and reagents used during research were freshly prepared and of high purity (≥95%). Water (double-distilled) was preferred throughout the study.
4.3. Extraction Process for AFs
The samples were kept in a vacuum oven for 24 h till dryness, and then ground to a particle size (<0.5 mm) with the help of a grinding mill (Retsch, Haan, Düsseldorf, Germany). The process of extraction of AFs in spices was carried out following the method (AOAC official method 2008.02). The sample of 5 g was weighed, and 1 g of NaCl was added to a 25 mL solution of methanol in a beaker after adding 0.5% of NaHCO3 (70:30 v/v). Then the mixture was centrifuged (4500 rpm) for 10 min. After centrifugation, supernatant (7 mL) was diluted in 28 mL of 0.1% phosphate buffer saline (PBS) containing Tween® 20 (1%). The solution (25 mL) was passed at a speed of 60 drops per minute through an IAC. Then washing was carried out on a column with a solution of 5 mL of PBS (10 mM), at a flow rate of one drop per second. Finally, the AFs were eluted by passing 2 mL of methanol with a speed, as mentioned above. The eluted solution was then moved in a glass vial and diluted with 1 mL of mobile phase before final analysis.
4.4. Analytical Method
The HPLC instrument used for analysis was a Shimadzu (LC-10A, Shimadzu, Kyoto, Japan). The HPLC column was nonpolar of C18 (Discovery HS) and the detector was a fluorescence detector (RF-530) used for AFs and tocopherols analysis. For tocopherols, gradient mobile phase with two solvents were used, i.e., solvent A (50% acetonitrile) and solvent B (50% methanol), at 1 mL/min flow rate. The detector (fluorescence) was used for tocopherol analysis with emission and excitation wavelengths of 325 nm and 295 nm, respectively. However, isocratic mobile phase with a combination of acetonitrile–methanol–water (20:20:60, v/v/v) was utilized for the evaluation of AF levels in selected spices with a flow rate of 1 mL/min. The wavelengths of excitation and emission were set at 360 and 440 nm, respectively. The final sample used for HPLC injection was 20 µL.
4.5. Estimation of Exposure
The estimated daily intake (EDI) was estimated as mentioned by FAO/WHO [48], with given formula
| (1) |
The consumption data were obtained from 500 individuals (males and females) including a food frequency questionnaire and asking them the portion of each spice used in their daily food dishes. The questionnaire was evaluated considering the uses of spice consumption in different food dishes during each day, in a week, and food dishes cooked occasionally. This questionnaire analyzed the exact amount of spices used in food products. The average weight of male and female participants was 70.
4.6. Margin of Exposure Characterization
The carcinogenic and toxic effects of AFs were determined based on margin of exposure Assessment (MOE), which was calculated by dividing the benchmark lower dose limit (BMDL) for aflatoxins as 400 ng/kg bw/day as expressed in Equation (2).
| (2) |
Health risk of exposure to pollutants was determined using MOE (margin of exposure) [49,50]. MOE can be estimated using benchmark dose (BMD) or benchmark dose lower confidence limit 10% (BMDL10). BMD is defined as a dose that causes a low but measurable effect and BMDL10 is the dose that does not cause more than 10% cancer with 95% confidence. This was determined by dividing the benchmark dose lower limit (BMDL) for aflatoxins 400 ng/kg−1 bw/day−1 (reference value for AFB1 in control group) [51]. When MOE value is equal or greater than 10,000, it poses fewer health concerns, and if its value is less than 10,000, it indicates that exposure to that pollutant has high health risks [52].
4.7. Estimation of Liver Cancer Risk
The ingestion of AFs and the synergistic effects on liver cancer are well established. The liver cancer risk in Pakistani population were calculated by JECFA [53]. According to JECFA, the potency value for AFB1 corresponds to 0.3 cancers/year/100,000 population ng/kg bw/day, and the hepatitis positive rate is 2.4% [54]. The mean potency can be calculated as
| Mean potency = [0.03 × HBsAg – negative individuals in Pakistan] + [0.01 × HBsAg − positive individuals/prevalence rate in Pakistan] = (0.03 × 0.024) + (0.01 × 0.976) = 0.00072 + 0.00976 = 0.0105 |
(3) |
The formula for cancer risk (cancer per year per 105 individuals per ng/kg bw/day was estimated using formula
| Cancer risk = EDI × Mean potency | (4) |
A carcinogenic risk of <10−4 is deemed acceptable (tolerable) by the US Environmental Protection Agency. However, if the risk is greater than 10−4, it is declared carcinogenic [54].
4.8. Statistical Analysis
The findings were given as mean levels using standard deviations. The standard curve (seven point) was created, and the equation of straight line was achieved. Using linear regression the coefficient of regression/correlation was obtained. To evaluate the significant difference among AFs in selected spices from seasonal variation, one-way ANOVA (α = 0.05) was used (SPSS, IBM SPSS Statistics 25, USA), and LSD was applied to obtain the significant difference among groups.
Author Contributions
Conceptualization, F.N., F.N. and N.N.; methodology, F.N.; software, F.N. and M.R.A.; validation, F.N., M.R.A. and F.N.; formal analysis, F.N. and N.N.; investigation, M.R.A.and S.Z.I.; resources, F.N.; data curation, F.N. and M.R.A.; writing—original draft preparation, F.N.; writing—review and editing, S.Z.I. and F.V.; visualization, S.Z.I.; supervision, S.Z.I. and M.R.A.; project administration, F.N.; funding acquisition, F.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data will be available for request.
Conflicts of Interest
The authors have no conflict of interest.
Key Contribution
A total of 603 samples of selected spices were analyzed for AFB1, and total Afs. In total, 120 and 136 samples from summer and winter, respectively, were observed to be infected with total AFs. The highest means of AFB1 (15.5 µg/kg) and AFs (22.90 µg/kg) were found in red pepper. The maximum dietary intake of AFs (4.80 µg/day/kg) was found in ginger in females.
Funding Statement
F.N. postdoc fellow appreciates the funding of Shenzhen University, China. SZI acknowledge the funding (NRPU 5574) of Higher Education Commission, Islamabad Pakistan.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clemenson S. Swainson’s Handbook of Technical and Quality Management for the Food Manufacturing Sector. Woodhead Publishing Books—Elsevier; Sawston, UK: 2019. Herbs and Spices. [DOI] [Google Scholar]
- 2.Liu Q., Meng X., Li Y., Zhao C.N., Tang G.Y., Li H.B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017;18:1283. doi: 10.3390/ijms18061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thanushree M.P., Sailendri D., Yoha K.S., Moses J.A., Anandharamakrishnan C. Mycotoxin contamination in food: An exposition on spices. Trends Food Sci. Technol. 2019;93:69–80. doi: 10.1016/j.tifs.2019.08.010. [DOI] [Google Scholar]
- 4.FAO. IFAD. UNICEF. WFP. WHO . The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition. Food and Agriculture Organization of the United Nations Rome; Rome, Italy: 2018. [Google Scholar]
- 5.Moses J.A., Karthickumar P., Sinija V.R., Alagusundaram K., Tiwari B.K., Cruz C., Barcelon E.U. Effect of microwave treatment on drying characteristics and quality parameters of thin layer drying of coconut. Asian J. Food Agro Ind. 2013;6:72–85. [Google Scholar]
- 6.Paterson R.R.M., Lima N. How will climate change affect mycotoxins in food? Food Res. Int. 2010;43:1902–1914. doi: 10.1016/j.foodres.2009.07.010. [DOI] [Google Scholar]
- 7.Vithu P., Moses J.A. Machine vision system for food grain quality evaluation: A review. Trends Food Sci. Technol. 2016;56:13–20. doi: 10.1016/j.tifs.2016.07.011. [DOI] [Google Scholar]
- 8.Ashiq S. Natural occurrence of mycotoxins in food and feed: Pakistan perspective. Comp. Rev. Food Sci. Food Saf. 2015;14:159–175. doi: 10.1111/1541-4337.12122. [DOI] [PubMed] [Google Scholar]
- 9.Zinedine A., Fernández-Franzón M., Mañes J., Manyes L. Multi-mycotoxin contamination of couscous semolina commercialized in Morocco. Food Chem. 2017;214:440–446. doi: 10.1016/j.foodchem.2016.07.098. [DOI] [PubMed] [Google Scholar]
- 10.FAO. 2018. [(accessed on 17 October 2020)]. Available online: http://www.fao.org/faostat/en/#data/QC.
- 11.Ruadrew S., Craft J., Aidoo K. Occurrence of toxigenic Aspergillus spp. and aflatoxins in selected food commodities of Asian origin sourced in the West of Scotland. Food Chem. Toxicol. 2013;55:653–658. doi: 10.1016/j.fct.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 12.De Roma A., Rossini C., Ritieni A., Gallo P., Esposito M.A. survey on the Aflatoxin M1 occurrence and seasonal variation in buffalo and cow milk from Southern Italy. Food Contr. 2017;81:30–33. doi: 10.1016/j.foodcont.2017.05.034. [DOI] [Google Scholar]
- 13.Klingelhöfer D., Zhu Y., Braun M., Bendels M.H., Brüggmann D., Groneberg D.A. Aflatoxin—Publication analysis of a global health threat. Food Contr. 2018;89:280–290. doi: 10.1016/j.foodcont.2018.02.017. [DOI] [Google Scholar]
- 14.IARC . Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. Volume 82. World Health Organ; Geneva, Switzerland: 2002. Working Group on the Evaluation of Carcinogenic Risks to Humans, & International Agency for Research on Cancer. [PMC free article] [PubMed] [Google Scholar]
- 15.Iqbal S.Z., Asi M.R., Jinap S. Aflatoxins in dates and dates products. Food Contr. 2014;43:163–166. doi: 10.1016/j.foodcont.2014.03.010. [DOI] [Google Scholar]
- 16.Akbar N., Nasir M., Naeem N., Ahmad M.U.D., Saeed F., Anjum F.M., Iqbal S., Imran M., Tufail T., Shah F.H., et al. Assessment of aflatoxin in milk and feed samples and impact of seasonal variations in the Punjab, Pakistan. Food Sci. Nutr. 2020;8:2699–2709. doi: 10.1002/fsn3.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Blanco M., Ramos A.J., Prim M., Sanchis V., Marín S. Usefulness of the analytical control of aflatoxins in feedstuffs for dairy cows for the prevention of aflatoxin M1 in milk. Mycotoxin Res. 2020;36:11–22. doi: 10.1007/s12550-019-00362-y. [DOI] [PubMed] [Google Scholar]
- 18.Eskola M., Kos G., Elliott C.T., Hajšloá J., Mayar S., Krska R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate ‘of 25%. Crit. Rev. Food Sci. Nutr. 2020;60:2773–2789. doi: 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- 19.Ülger T.G., Uçar A., Çakıroglu F.P., Yilmaz Š. Genotoxic effects of mycotoxins. Toxicon. 2020;185:104–113. doi: 10.1016/j.toxicon.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Weidenborner M. Encyclopedia of Food Mycotoxins. Springer Science & Business Media; Berlin, Germany: 2001. [DOI] [Google Scholar]
- 21.Dey D.K., Kang J.I., Bajpai V.K., Kim K., Lee H., Sonwal S., Simal-Gandara J., Xiao J., Ali S., Huh Y.S., et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. Nut. 2022;21:1–22. doi: 10.1080/10408398.2022.2059650. [DOI] [PubMed] [Google Scholar]
- 22.Dey D.K., Chang S.N., Kang S.C. The inflammation response and risk associated with aflatoxin B1 contamination was minimized by insect peptide CopA3 treatment and act towards the beneficial health outcomes. Environ. Pollut. 2021;268:115713. doi: 10.1016/j.envpol.2020.115713. [DOI] [PubMed] [Google Scholar]
- 23.Bilandžić N., Tanković S., Jelušić V., Varenina I., Kolanović B.S., Luburić Đ.B., Cvetnić Ž. Aflatoxin M1 in raw and UHT cow milk collected in Bosnia and Herzegovina and Croatia. Food Contr. 2016;68:352–357. doi: 10.1016/j.foodcont.2016.04.022. [DOI] [Google Scholar]
- 24.European Commission (EU) Commission regulation (EC) No. 1881/2006 of 19. European Commission; Brussels, Belgium: Official Journal of the European Union; Maastricht, Netherlands: 2006. L345/5. [Google Scholar]
- 25.Iqbal S.Z., Paterson R.R.M., Bhatti I.A., Asi M.R., Sheikh M.A., Bhatti H.N. Aflatoxin B1 in chilies from the Punjab region, Pakistan. Mycotoxin Res. 2010;26:205–209. doi: 10.1007/s12550-010-0055-6. [DOI] [PubMed] [Google Scholar]
- 26.Iqbal S.Z., Paterson R.R.M., Bhatti I.A., Asi M.R. Survey of aflatoxins in chilies from Pakistan produced in rural, semi-rural and urban environments. Food Addit. Contam. Part-B. 2010;3:268–274. doi: 10.1080/19393210.2010.520341. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal S.Z., Paterson R.R.M., Bhatti I.A., Asi M.R. Aflatoxin concentrations in chilies vary depending on variety. Mycoscience. 2011;52:296–299. doi: 10.1007/S10267-011-0106-7. [DOI] [Google Scholar]
- 28.Iqbal S.Z., Paterson R.R.M., Bhatti I.A., Asi M.R. Comparing aflatoxins contamination in chilies from Punjab, Pakistan, produced in summer and winter. Mycotoxin Res. 2011;27:75–80. doi: 10.1007/s12550-010-0078-z. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal S.Z., Bhatti I.A., Asi M.R., Bhatti H.N., Sheikh M.A. Aflatoxin contamination in chilies from Punjab Pakistan with reference to climate change. Int. J. Agric. Biol. 2011;13:261–265. [Google Scholar]
- 30.Iqbal S.Z., Asi M.R., Zuber M., Akhtar J., Saif M.J. Natural occurrence of aflatoxins and ochratoxin A in commercial chilli and chilli sauce samples. Food Contr. 2013;30:621–625. doi: 10.1016/j.foodcont.2012.09.003. [DOI] [Google Scholar]
- 31.Iqbal S.Z., Mustafa H.G., Asi M.R., Jinap S. Variation in vitamin E level and aflatoxins contamination in different rice varieties. J. Cereal Sci. 2014;60:352–355. doi: 10.1016/j.jcs.2014.05.012. [DOI] [Google Scholar]
- 32.Iqbal S.Z., Asi M.R., Mahmood Z., Mumtaz A., Malik N. Survey of aflatoxins and Ochratoxin A in retail market chilies and chili sauce samples. Food Contr. 2017;81:218–223. doi: 10.1016/j.foodcont.2017.06.012. [DOI] [Google Scholar]
- 33.Iqbal S.Z., Mumtaz A., Mehmood Z., Waqas M., Gaffar A., Ismail A., Perviz W. Assessment of aflatoxins and ochratoxin A in chili sauce samples and estimation of dietary intake. Food Contr. 2021;121:107621. doi: 10.1016/j.foodcont.2020.107621. [DOI] [Google Scholar]
- 34.Hammami W., Fiori S., Al-Thani R., Kali N.A., Balmas V., Migheli Q., Jaoua S. Fungal and aflatoxin contamination of marketed spices. Food Contr. 2014;37:177–181. doi: 10.1016/j.foodcont.2013.09.027. [DOI] [Google Scholar]
- 35.Zhao X., Schaffner D.W., Yue T. Quantification of aflatoxin risk associated with Chinese spices: Point and probability risk assessments for aflatoxin B1. Food Contr. 2013;33:366–377. doi: 10.1016/j.foodcont.2013.03.012. [DOI] [Google Scholar]
- 36.Santos L., Marín S., Mateo E.M., Gil-Serna J., Valle-Algarra F.M., Patiño B., Ramos A.J. Mycobiota and co-occurrence of mycotoxins in Capsicum powder. Int. J. Food Microbiol. 2011;151:270–276. doi: 10.1016/j.ijfoodmicro.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Bircan C. The determination of aflatoxins in spices by immunoaffinity column extraction using HPLC. Int. J. Food Sci. Technol. 2005;40:929–934. doi: 10.1111/j.1365-2621.2005.01025.x. [DOI] [Google Scholar]
- 38.Ardic M., Karakaya Y., Atasever M., Durmaz H. Determination of aflatoxin B1 levels in deep-red ground pepper (isot) using immunoaffinity column combined with ELISA. Food Chem. Toxicol. 2008;46:1596–1599. doi: 10.1016/j.fct.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 39.Romagnoli B., Menna V., Gruppioni N., Bergamini C. Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. Food Contr. 2007;18:697–701. doi: 10.1016/j.foodcont.2006.02.020. [DOI] [Google Scholar]
- 40.Colak H., Bingol E.B., Hampikyan H., Nazli B. Determination of aflatoxin contamination in red-scaled, red and black pepper by ELISA and HPLC. J. Food Drug Anal. 2006;14:292–296. doi: 10.38212/2224-6614.2476. [DOI] [Google Scholar]
- 41.Lippolis V., Irurhe O., Porricelli A.C.R., Cortese M., Schena R., Imafidon T., Pascale M. Natural co-occurrence of aflatoxins and ochratoxin A in ginger (Zingiber officinale) from Nigeria. Food Contr. 2017;73:1061–1067. doi: 10.1016/j.foodcont.2016.10.026. [DOI] [Google Scholar]
- 42.O’Riordan M.J., Wilkinson M.G.A. Survey of the incidence and level of aflatoxin contamination in a range of imported spice preparations on the Irish retail market. Food Chem. 2008;107:1429–1435. doi: 10.1016/j.foodchem.2007.09.073. [DOI] [Google Scholar]
- 43.Prelle A., Spadaro D., Garibaldi A., Gullino M.L. Co-occurrence of aflatoxins and ochratoxin A in spices commercialized in Italy. Food Contr. 2014;39:192–197. doi: 10.1016/j.foodcont.2013.11.013. [DOI] [Google Scholar]
- 44.Martins M.L., Martins H.M., Bernardo F. Aflatoxins in spices marketed in Portugal. Food Addit. Contam. 2001;18:315–319. doi: 10.1080/02652030120041. [DOI] [PubMed] [Google Scholar]
- 45.Obonyo M.A., Salano E.N. Perennial and seasonal contamination of maize by aflatoxins in eastern Kenya. Int. J. Food Contam. 2018;5:6. doi: 10.1186/s40550-018-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hierro J.M.H., Villanova R.J.G., Torrero P.R., Fonseca I.T. Aflatoxins and ochratoxin A in red paprika for retail sale in Spain: Occurrence and evaluation of a simultaneous analytical method. J. Agric. Food Chem. 2008;56:751–756. doi: 10.1021/jf073002c. [DOI] [PubMed] [Google Scholar]
- 47.Akhtar S., Riaz M., Naeem I., Gong Y.Y., Ismail A., Hussain M., Akram K. Risk assessment of aflatoxins and selected heavy metals through intake of branded and non-branded spices collected from the markets of Multan city of Pakistan. Food Contr. 2020;112:107132. doi: 10.1016/j.foodcont.2020.107132. [DOI] [Google Scholar]
- 48.FAO/WHO (2014) Guidelines for the Simple Evaluation of Dietary Exposure to Food Additives CAC/GL 3-1989 Adopted 1989. Revision 2014 (Formerly Guidelines for the Simple Evaluation of Food Additive Intake). Volume 2014. [(accessed on 17 August 2022)]. Available online: www.fao.org/input/download/standards/6/cxg_003e.pdf/
- 49.EFSA Opinion of the scientific committee on a request from EFSA related to A harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic; opinion of the scientific committee on a request from EFSA related to A harmonised app. EFSA J. 2005;282:1–31. doi: 10.2903/j.efsa.2005.282. [DOI] [Google Scholar]
- 50.EFSA Opinion of the scientific panel on contaminants in the food chain. EFSA J. 2007;5:478. doi: 10.2903/j.efsa.2007.446. [DOI] [Google Scholar]
- 51.Hooshfar S., Khosrokhavar R., Yazdanpanah H., Eslamizad S., Kobarfard F., Nazari F., Tsitsimpikou C. Health risk assessment of aflatoxin M1 in infant formula milk in IR Iran. Food Chem. Toxicol. 2020;142:111455. doi: 10.1016/j.fct.2020.111455. [DOI] [PubMed] [Google Scholar]
- 52.JECFA. 2005. [(accessed on 10 August 2022)]. Available online: http://www.who.int/ipcs/food/jecfa/summaries/en/summary_report_64_final.pdf.
- 53.Ali A., Donahue R.M., Qureshi H., Vermund S.H. Hepatitis B and hepatitis C in Pakistan: Prevalence and risk factors. Int. J. Infect. Dis. 2009;13:9–19. doi: 10.1016/j.ijid.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.USEPA . Risk Assessment Guidance for Superfund-Part A, Process for Conducting Probabilistic Risk Assessment (Vol. III) DC; Burbank, CA, USA: US Environmental Protection Agency; Washington, WA, USA: 2001. EPA 540-R-02–002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available for request.