Highlights
-
•
Mobile apps are increasingly used in childhood obesity prevention to monitor food intake and PA, and send health messages.
-
•
The studies' small sample size and the heterogeneous interventions prevent this review to conclude on effectiveness.
-
•
The overall picture shows a trend toward improvement in some interventions, while others were essentially ineffective.
-
•
The high acceptability and the widespread use of mobile apps, support new research to develop effective, trustworthy apps.
Abbreviations: PICO(S), Population, Intervention, Comparator, Outcomes, Study design; RCTs, Randomized clinical trials; RoB, risk of bias; BMI, body mass index; WHR, waist-to-hip ratio
Keywords: Childhood obesity prevention, Mobile health, Healthy lifestyles
Abstract
Childhood obesity is a high prevalence condition that causes a high burden of disease in adulthood. Mobile phone app are increasingly used to prevent it. We summarized the evidence on the effectiveness of mobile apps for devices used by parents to prevent and treat childhood and adolescent obesity.
An update of a systematic review of the literature (De Lepeleere et al., 2017) was carried out. PubMed, Embase, Cochrane, CINAHL, PsycINFO, Scopus, and ERIC were searched up to 2020. The included studies should target children 1–18 years, compare an app aimed at preventing or treating overweight and obesity, as stand-alone intervention or as part of a complex program, installed on parents’ mobile devices, to no intervention or an intervention without the app. Outcomes related to weight status, diet, and physical activity (PA) behaviors were considered. Nineteen studies (14 RCTs and 5 non-randomized trials) were included. The app was mainly used to record food consumption and PA, to set goals, to view progress, and send health promotion messages.
One study reported a significant decrease and one a suggestive decrease in anthropometric measures in obese and overweight children, while other studies observed no effect. One study reported a significant increase in PA. Six interventions proved to be effective in changing dietary behaviors. Interventions targeting overweight and/or obese children had the most positive results. All studies reported high acceptability and feasibility of interventions. The differences between interventions and the small sample size of the studies did not allow this review to reach conclusion on effectiveness.
1. Introduction
1.1. Prevalence of obesity in western countries and health consequences
Obesity is highly prevalent among children and adolescents around the world, particularly in industrialized countries. Worldwide, 39 million children under the age of 5 were overweight or obese in 2020 and over 340 million children and adolescents aged 5–19 were overweight or obese in 2016 (World Health Organization.et., 2020).
In Europe, the prevalence of overweight (including obesity) in 2015–2017 was 29 % for boys and 27 % for girls aged 7–9 years, with large differences between countries (WHO et., 2021).
Obesity can have serious organic and psychological consequences in childhood and adolescence, as well as in the long term. Children with obesity have a greater probability of being an adult with obesity, with various clinical complications (Rankin et al., 2016) (Gurnani et al., 2015).
1.2. The behavioral and environmental causes of childhood and adolescent obesity
There are many risk factors for childhood obesity. Some are related to diet, starting in early life (Mameli et al., 2016, Azad et al., 2018, Woo Baidal et al., 2016) and are life-long (Payab et al., 2015, de Ruyter et al., 2012, Pereira, 2014), while others are related to a sedentary lifestyle (Fang et al., 2019, Terrón-Pérez et al., 2021, Pan et al., 2021). Finally, the family and socio-economic context can play an important role. Parents with obesity may unwittingly favor obesogenic behaviors, such as an unhealthy diet and sedentary lifestyles (Weihrauch-Blüher and Wiegand, 2018, Early and Risk, 2018).
Effective Interventions to Prevent and Treat Childhood and Adolescent Obesity and the Role of Mobile Technology as a Support for Health Promotion.
As children and adolescents who are overweight are vulnerable to serious health consequences, early prevention and treatment are essential. Multidisciplinary approaches through lifestyle interventions focusing on both nutrition and physical activity and involving the whole family proved more effective in preventing and treating childhood obesity than one-target, one-setting interventions (Brown et al., 2019, Loveman et al., 2015, Venturelli et al., 2019).
Given the widespread use of mobile technology, health care has also begun to use it to more effectively convey information on healthy lifestyles. (Report, 2020).
The mobile application market for weight loss and management has rapidly expanded in recent years, and app-based interventions targeting adults have been found effective (Mateo et al., 2015).
However, there is still little information on the quality and reliability of these applications. A 2014 review found low adherence to guideline recommendations in existing apps for diet and exercise for children (designed to be used both by parents and by children) (Wearing et al., 2014).
To be consistent with evidence-based recommendations, mobile health apps should be developed according to scientific evidence and on theoretical constructs (Wearing et al., 2014, Schoffman et al., 2013, Rivera et al., 2016). A scientifically reliable assessment of their use as stand-alone interventions as well as an aid in complex intervention programs is also needed (Rivera et al., 2016, Tate et al., 2013, Gittelsohn et al., 2015, Huang et al., 2009, Schoeppe et al., 2016, Wang et al., 2017).
Quelly et al. (Quelly et al., 2016) conducted a systematic review that assessed the impact of mobile apps that are also used by children and adolescents, and Hammersley et al. (Hammersley et al., 2016) conducted a systematic review only on eHealth interventions where parents or caregivers were agents of change in the Body Mass Index (BMI) of overweight and obese children. The results of these reviews provide initial evidence to support the development and implementation of mobile apps in tackling childhood obesity. Since screen time is a strong determinant of child obesity (Fang et al., 2019), several authors criticized interventions that could incentivize the use of mobile devices by children (Tate et al., 2013, Quelly et al., 2016, Hammersley et al., 2016).
The aim of this systematic review was to produce a summary of the evidence on the efficacy and effectiveness of parents’ use of mobile health apps to prevent and treat childhood and adolescent obesity.
2. Methods
2.1. Protocol of the systematic review (PROSPERO)
The protocol of this systematic review was registered on the PROSPERO database: ID CRD42019121430.
2.2. Literature search methods
A previous systematic review that assessed the impact of parents’ use of mobile apps in prevention and treatment interventions for childhood and adolescent obesity was identified (Quelly et al., 2016). We adapted the strategy reported by the authors to our own eligibility criteria. We are confident that no relevant studies published up to 2014 were missed by Quelly’s search, as the only difference we introduced in eligibility— that the app should designed to be installed on parents’ devices— restricted the set of eligible papers. Thus, the strategy was used to search PubMed, Embase, Cochrane, CINAHL, PsycINFO, Scopus, and ERIC for articles published between January 1, 2014, and December 31, 2020.
The references of systematic reviews with objectives similar to ours were used to retrieve further studies. Studies were included without any exclusion regarding the setting where the intervention was carried out and evaluated (school, health care, general population, etc.) and without any geographical limitations.
2.3. Study selection process - criteria and Methods of inclusion and exclusion of studies
The research question was framed as the following PICO:
Population: children and adolescents (ages 1–18 years).
Intervention: Interventions aimed at preventing or treating overweight and obesity through the use of an app, as a stand-alone intervention or as part of a complex program, to be installed on parents' smartphone.
Comparator: no intervention or intervention without the app component.
Outcomes: anthropometric measures: pre-post difference in BMI, BMI z-score, BMI percentile; pre-post difference in waist circumference or waist-to-hip ratio (WHR - absolute, z-score, or percentile), fat mass index (FMI); behaviors: changes in diet, physical activity, and inactivity; usability, acceptability, transferability, and feasibility measures. Participation in the intervention, attitudes, and self-efficacy were considered as secondary outcomes.
Studies with experimental, quasi-experimental, and comparative observational designs were considered, including non-randomized and randomized trials and comparative cohort studies.
Four reviewers (I.P., F.V., L.B, N.P.) screened the title and abstract of the search results. All 4 reviewers agreed on and standardized the selection criteria and screened a first set of 100 titles. The other titles were then screened individually: any case for which a reviewer had doubts was discussed in a consensus meeting. Once the relevant papers were identified, the full texts were retrieved, and their suitability was assessed by 3 reviewers (I.P., F.V., N.P.). Discordant cases were discussed, and a decision was taken by consensus.
2.4. Extraction of data from primary studies
For each primary study, information was extracted concerning the authors, year of publication, country in which the study was carried out, study design, sample characteristics, duration of follow-up, evaluated outcomes, and results. Extraction was conducted by L.B., F.V., and N.P.
2.5. Quality assessment of studies
The quality of the included studies was evaluated using the Risk of Bias 1 (RoB 1) tool for RCTs; for non-randomized studies, an assessment was performed based on the domains addressed in the ROBINS-I tool and was summarized in the text (Methods in Cochrane et., 2021). Two reviewers (I.P. and L.B.) independently assessed the risk of bias. Disagreements were resolved by means of discussion and consensus with other 2 reviewers (F.V. and N.P.).
3. Summary of results
Differences in the interventions made it impossible to perform a meta-analysis. The results of the studies are summarized in narrative form and synopses.
Ethics.
An ethics statement is not applicable because this Systematic Review is based exclusively on published literature.
4. Results
4.1. Included studies
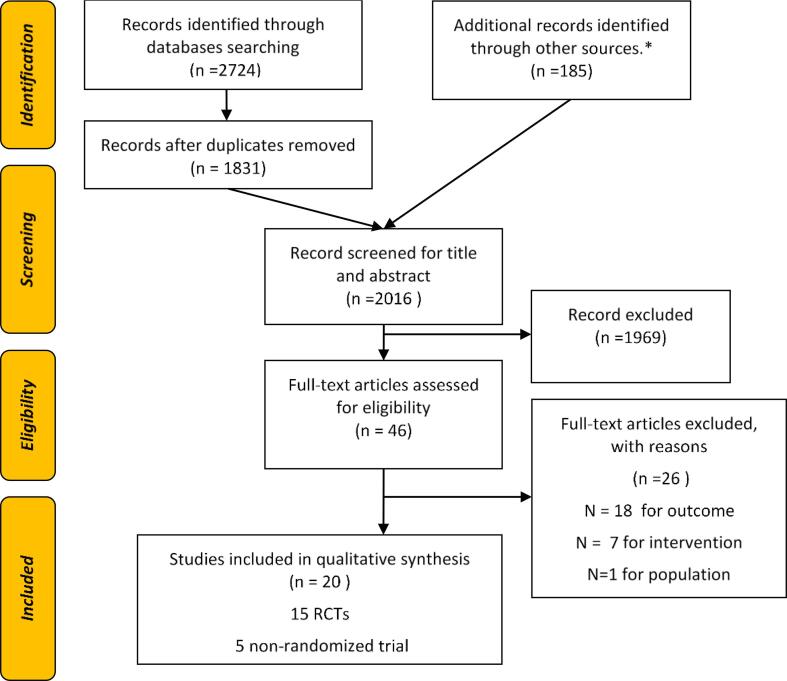
The title and abstract of 2016 papers were screened, with 46 full-text articles assessed for eligibility. At the end of the selection process, 19 interventions (20 papers) were included (Fig. 1) (Wingo et al., 2020, Nyström et al., 2017, Delisle Nyström et al., 2018, Johansson et al., 2020, Nezami et al., 2018, Shen et al., 2020, Wald et al., 2018, Hammersley et al., 2019, De Lepeleere et al., 2017, Vilchis-Gil et al., 2021, Røed et al., 2021, Bakırcı-Taylor et al., 2019, Chai et al., 2019, Clarke et al., 2019, Jake-Schoffman et al., 2018, Pearson et al., 2020, Perdew et al., 2021, Sutherland et al., 2019, Tripicchio et al., 2017, Trost and Brookes, 2021).
Fig. 1.
PRISMA Flow diagram describing the study selection process. Notes: * Articles included in the bibliography of the following reviews: Hammersley 2016 (Hammersley et al., 2016); Quelly 2016 (Quelly et al., 2016), Rose 2017 (Rose et al., 2017); Schoeppe 2016 (Schoeppe et al., 2016), Wang 2017 (Wang et al., 2017), Fowler 2021 (Fowler et al., 2021); Mehdizadeh 2020 (Mehdizadeh et al., 2020); Zarnowiecki 2020 (Zarnowiecki et al., 2020).
The studies were published between 2017 and 2021, while the interventions were conducted from 2014. Follow up ranged from a minimum of 4 weeks (Wingo et al., 2020) to a maximum of 1 year (Nyström et al., 2017, Delisle Nyström et al., 2018). Outcomes were measured at the end of the intervention period in most studies, apart from the studies by Johansson (Johansson et al., 2020), Nezami (Nezami et al., 2018), Shen (Shen et al., 2020), and Wald (Wald et al., 2018), who also did assessments in the middle of the intervention. Nystrom (Nyström et al., 2017, Delisle Nyström et al., 2018), Hammersley (Hammersley et al., 2019), Nezami (Nezami et al., 2018), Johansson (Johansson et al., 2020), De Lepeleere (De Lepeleere et al., 2017), Jenny Vilchis-Gil (Vilchis-Gil et al., 2021), and Røed (Røed et al., 2021) had 2 follow-up times, while Wald (Wald et al., 2018) had 4 follow-up times. (Table 1).
Table 1.
Characteristics of included studies reported by target population and by study design.
|
Interventions targeting children and/or adolescents who are overweight and/or obese | |||||||
|---|---|---|---|---|---|---|---|
|
Randomized Clinical Trials | |||||||
| Author, year and country | Study or app name | Study design | Subjects’ characteristics | Sample size | Follow-up | Primary Outcome | Secondary Outcomes |
| Chai, 2019 (Chai et al., 2019) Australia | Back2Basics Family B2BF website | RCT | Parents and their children aged 4–11 years with BMI above the mid-point of the healthy weight category (21.5) | N = 125 families | 12 weeks | Retention and intervention utilization; BMI; waist circumference; diet | |
| Johansson, 2020 (Johansson et al., 2020) Sweden | Provement | RCT | Parents of 5–12-year-old children with obesity | N = 28 parents | 3 and 6 months | Feasibility of the intervention and changes in BMI SDS | |
| Wald, 2018 (Wald et al., 2018) USA | O-CHESS | RCT | Parents of 3–7-year-old overweight and obese children | N = 73 parents | 3, 6, 9, and 12 months | Feasibility of the intervention to promote healthy behavior changes | BMI z scores, healthy behavior changes; increased parent self-efficacy |
| Non-randomized studies | |||||||
| Perdew, 2021 (Perdew et al., 2021) Canada | Family Healthy Living Early Intervention Program (EIP) | Quasi-experimental design | One parent and his/her healthy children 8–12 years old at or above 85th BMI percentile | N = 71 children | 10 weeks | BMI Z-score | Eating habits and physical activity in children |
| Tripicchio, 2017 (Tripicchio et al., 2017) USA | FITNET | Three cohorts non-randomized comparative study | Parents of 2–18-year-old overweight or obese children | N = 64 parents | 12 weeks | Children’s BMI z-score | Feasibility of the intervention |
| Interventions targeting children and/or adolescents regardless of their BMI | |||||||
| Randomized Clinical Trials | |||||||
| Author, year and country | Study or app name | Study design | Subjects’ characteristics | Sample size | Follow-up | Primary Outcome | Secondary Outcomes |
| Bakırcı-Taylor, 2019 (Bakırcı-Taylor et al., 2019) USA | Jump2Health | RCT | Parents and children (3–8 years old) | N = 30 families | 10 weeks | Feasibility; intake of fruits and vegetables measured through level of skin carotenoid | |
| Clarke, 2019 (Clarke et al., 2019) USA | VeggieBook | Cluster RCT | Household's cook of 9–14-year-old child dyads | N = 15 food pantry distributions including N = 289 pantry clients | 10 weeks | Target-veggie prep scorea and General-veggie prepsb | |
| Hammersley, 2019 (Hammersley et al., 2019) Australia | Time2bHealthy | RCT | Parent-child (2–5 years at risk of overweight) dyads. | N = 86 dyads | 3 and 6 months | BMI | PA, sleep habits, dietary intake, screen time, child feeding, parent modelling and self-efficacy |
| Jake-Schoffman, 2018 (Jake-Schoffman et al., 2018) USA | mFIT | RCT | Parent-child (9–12 years old) dyads. | N = 33 dyads | 12 weeks | Eating habits and physical activity in children and parents | |
| Nezami, 2018 (Nezami et al., 2018) USA | Smart Moms | RCT | Mothers with a BMI 25–50 and a child between the ages of 3–5 years consuming >=12 oz/day of SSB/juice. | N = 51 mothers | 3 and 6 months | Children’s intake of sugary beverages | Mother’s BMI |
| Nyström, 2017 (Nyström et al., 2017); Nyström, 2018 (Delisle Nyström et al., 2018) Sweden | MINISTOP | RCT | Parents of 4-year-old children | N = 315 parents | 6 months and 12 months | Children Fat Mass Index | Eating habits and physical activity in children |
| Pearson, 2020 (Pearson et al., 2020) UK | The Kids FIRST | Cluster four-arm RCT | Parents of 9–11-year-old children | N = 64 parents (75 children) | 13 weeks | Screen-time and eating behaviors | Eating behaviors in children during screen time; Parent screen time and eating behaviors; participation, feasibility, retention. |
| Røed, 2021 (Røed et al., 2021) Norway | Food4toddlers | RCT | Parents of infants and toddlers completing online questionnaire. | N = 298 parents | 6 and 12 months | Child's diet (vegetables, fruits, discretionary food) | Participation |
| Sutherland, 2019 (Sutherland et al., 2019) Australia | SWAP IT | Cluster 2x2 RCT | Parents of 5–12-year-old children from 12 primary schools | N = 948 parents (1915 children) | 10 weeks | Mean kJ content of foods and beverages packed in children’s lunchboxes | Energy from recommended foods packed in school children’s lunchboxes. Feasibility; acceptability. |
| Trost, 2021 (Trost and Brookes, 2021) Australia | Moovisity TM | RCT | Parent-preschool-aged child dyads | N = 34 dyads | 8 weeks | Fundamental Movement Skills proficiency | Child’s PA and parental support for PA |
| Wingo, 2020 (Wingo et al., 2020) USA | POWERS | RCT | Parent-child with mobility disability (6–17 years old) dyads | N = 65 dyads | 12 weeks | Adherence and study completion | Diet and exercise behavior |
| Non-randomized studies | |||||||
| Author, year and country | Study or app name | Study design | Subjects’ characteristics | Sample size | Follow-up | Primary Outcome | Secondary Outcomes |
| De Lepeleere, 2017 (De Lepeleere et al., 2017) Belgium | Movie Models | A quasi-experimental study | Parents of primary schoolchildren (6–12 years old) | N = 207 parents | 1 and 4 months | PA; screen time; diet | Specific parenting practices and parental self-efficacy |
| Shen, 2020 (Shen et al., 2020) China | Measure Your Nutritional Status | Non-randomized parallel-group controlled trial | Parents of 13-year-old students | N = 573 parents | 3 months | Students’ accurate perception of their own nutritional status, accurate parental perception of their children’s nutritional status. | BMI, BMI Z-score, and percentage of students in the contemplation or action stage |
| Vilchis-Gil, 2021 (Vilchis-Gil et al., 2021) Mexico | Alimentate y Activate Sanamente | Non-randomized controlled study | Parents with their children in 4 primary schools (and teachers) | N = 402 children and their parents | 6 and 12 months | Quantity and quality of foods and beverages in the school meals | |
RCT: randomized controlled trial; BMI: body mass index; PA: physical activity.
the number of single preparations cooks made using broccoli, green beans, cauliflower, or zucchini.
frequency of use of a wide assortment of vegetables.
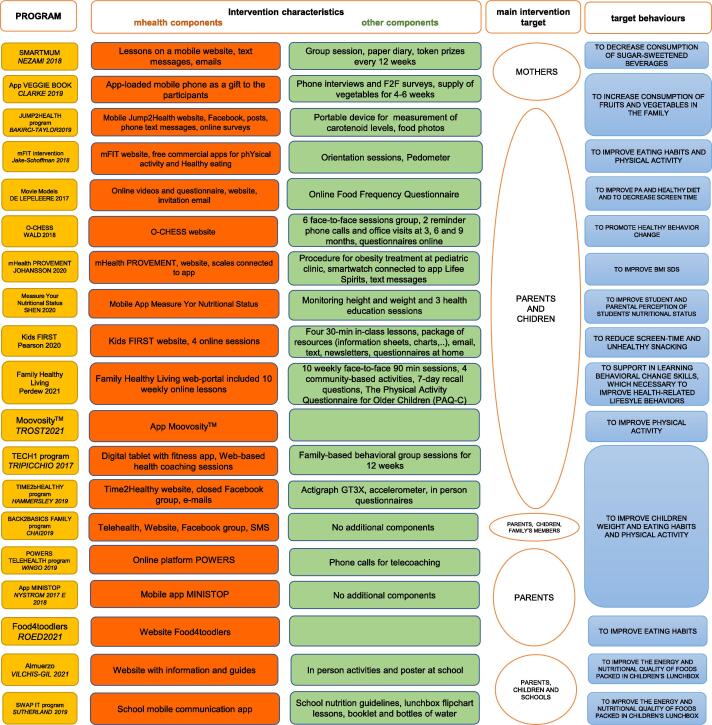
4.2. Characteristics of the interventions
Fig. 2 summarizes the characteristics of the interventions. The MINISTOP, VeggieBook, and Moovosity interventions (Nyström et al., 2017, Delisle Nyström et al., 2018, Clarke et al., 2019, Trost and Brookes, 2021) were delivered via a smartphone app and were among the few interventions without other components. Six interventions targeted children who were overweight or obese (Johansson et al., 2020, Wald et al., 2018, Chai et al., 2019, Perdew et al., 2021, Tripicchio et al., 2017) or children at risk of overweight (Hammersley et al., 2019), while all the other interventions targeted children regardless of their BMI. One intervention was aimed at children with mobility impairments (Wingo et al., 2020).
Fig. 2.
Schematic description of the interventions. The following are highlighted: mHealth and the other components, the main target subjects, and the target behaviors. Note: * All interventions are aimed at reducing childhood overweight and obesity but the agents of change may be different. We indicate the agent of change as the “main target” of the intervention.
The target behavior in 9 interventions (Chai et al., 2019, Perdew et al., 2021, Tripicchio et al., 2017, Nyström et al., 2017, Delisle Nyström et al., 2018, Johansson et al., 2020, Nezami et al., 2018, Shen et al., 2020, Wald et al., 2018, Hammersley et al., 2019) was to improve children’s weight, eating habits, and physical activity, while others focused on specific behavioral changes and did not have any change in anthropometric features as a target.
Three studies assessed the effectiveness of interventions by comparing more than two groups: two intervention groups were compared to a control group in the cohort study conducted by Tripicchio et al. (Tripicchio et al., 2017), as well as in a 3-arms RCT conducted by Chai et al. (Chai et al., 2019); the third study was a pilot four-arm cluster RCT by Pearson et al. (Pearson et al., 2020). For all three studies we considered the intervention group most adherent to our eligibility criteria for interventions vs control group.
Despite the fact that all the mobile health components of the interventions were downloaded onto the parents’ devices this being an inclusion criterion of this systematic review, some interventions aimed directly at changing the child’s behaviors, while others at changing the parents’ behaviors, or both. A detailed description of each intervention is reported in Supplementary file Table A1.
4.3. Theoretical basis of the interventions
The vast majority of interventions (Nezami et al., 2018, Røed et al., 2021, Bakırcı-Taylor et al., 2019, Wald et al., 2018, Hammersley et al., 2019, De Lepeleere et al., 2017) were based on social cognitive theory (Bandura, 1989, Bandura, 1977) and, in many cases (Nyström et al., 2017, Delisle Nyström et al., 2018, Chai et al., 2019, Sutherland et al., 2019), also on theory-based behavior change techniques (Michie et al., 2011). Jack-Schoffmann (Jake-Schoffman et al., 2018) also considered the theory of planned behaviour. Pearson‘s (Pearson et al., 2020) intervention was framed in a social ecological perspective and was theoretically informed also by drawing on constructs derived from Habit Theory (Gardner et al., 2012) and Behavioural Choice.
The interventions were centered around existing guidelines for healthy eating and physical activity.
Some interventions had similar characteristics, but all the interventions had specific features and differed from other in terms of construct and organization.
4.4. Effectiveness of the interventions
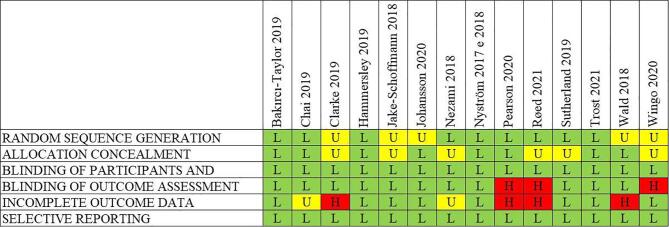
4.4.1. Risk of bias
Fig. 3 shows the risk of bias assessment for each RCT. Overall, the risk of bias was low. However, 8 studies did not clearly report the randomization and allocation process (Wingo et al., 2020, Johansson et al., 2020, Nezami et al., 2018, Wald et al., 2018, Røed et al., 2021, Clarke et al., 2019, Jake-Schoffman et al., 2018, Sutherland et al., 2019). The risk of bias in each study due to the blinding of participants and personnel was considered low since, as the trials were open-label, participant blindness was not possible. On the other hand, the outcome assessment in 3 studies had high risk of bias, particularly in the absence of masking of the intervention (Wingo et al., 2020, Røed et al., 2021, Pearson et al., 2020). Furthermore, the incomplete follow-up in 4 studies was a matter of concern (Wald et al., 2018, Røed et al., 2021, Clarke et al., 2019, Pearson et al., 2020). Detailed information on the risk of bias for each RCT is reported in Supplementary Table A2.
Fig. 3.
Risk of bias summary: review authors' judgements on each risk of bias item for each included RCT.
In the study conducted by Tripicchio (Tripicchio et al., 2017), a non-randomized study, the 3 cohorts differed in their racial/ethnic composition at baseline. The study is at risk of bias due to confounding. The assignment to groups took place according to the recruitment period; the personnel knew in which group the subject was assigned but could not change the assignment, resulting in a low risk for allocation concealment. As follow-up rates were low and unbalanced between groups, the risk of attrition bias was considered high. There was no information on blinding of outcome assessors, leading to an unclear risk of bias in this domain.
In the study by De Lepeleere (De Lepeleere et al., 2017), a quasi-experimental study, parents completed an online questionnaire, from which the variables of the study were evaluated. BMI was calculated by the parents and then reported in the questionnaires. The percentage of dropouts one month after baseline was low but unbalanced.
Shen (Shen et al., 2020) presented a parallel-group controlled trial with a non-randomized design. Schools were allocated to the intervention or control group based on practical considerations, trying to balance the distribution of potential confounders between the two groups. The trained staff conducted baseline and follow-up measurements by using identical protocols and procedures. At the 3-month follow-up the attrition rate was only 5.2 %.
In the non-randomized study by Vilchil-Gil (Vilchis-Gil et al., 2021), anthropometric and dietary habit measurements were standardized. The children were required to open their lunchboxes so that two certified nutritionists could write down what foods and beverages there were inside. At the end of the study, dropout was 13.4 % and 15.9 % in the intervention and control group, respectively.
The outcomes of EIP study (Perdew et al., 2021) were collected by research assistants. Children’s physical activity was measured using a validated questionnaire. The children who dropped out of the program did so during the first 3 weeks (2 children dropped out after 4 weeks).
4.4.2. Effect on anthropometric outcomes
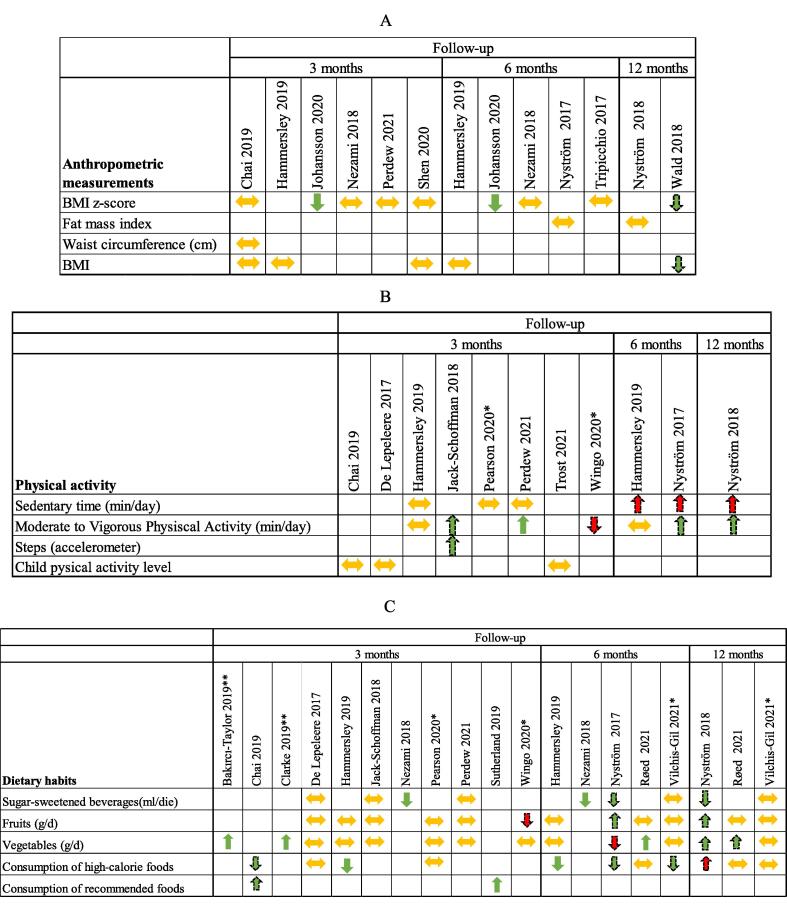
Only 9 studies reported the effect of the intervention on children’s anthropometric measures (BMI or BMI z-score or FMI or waist circumference) (Chai et al., 2019, Perdew et al., 2021, Tripicchio et al., 2017, Nyström et al., 2017, Delisle Nyström et al., 2018, Johansson et al., 2020, Nezami et al., 2018, Shen et al., 2020, Wald et al., 2018, Hammersley et al., 2019), including all the interventions specifically targeting overweight and/or obese children (Johansson et al., 2020, Wald et al., 2018, Hammersley et al., 2019, Chai et al., 2019, Perdew et al., 2021, Tripicchio et al., 2017). Outcome reporting was heterogeneous. Any quantitative synthesis of the results was hampered by the absence of uniform measure reporting (Fig. 4A) and by the differences in intervention characteristics.
Fig. 4.
Anthropometric (A), physical activities (B), and dietary habit (C) outcomes considered in the included studies. Note: Arrows indicate the strength and the direction of the association and the authors' interpretations of their results. Green arrows indicate positive changes, red arrows indicate negative changes. Dashed-line arrows indicate a result that is not statistically significant. *No statistical test performed. ** Data derived from the text.
Two studies reported only small differences between groups in the improvement of BMI z-score. One study targeting overweight children reported mean z-score reductions that were not significantly different between the control and intervention groups (Wald et al., 2018). The other, targeting obese children showed significantly better results in the intervention group compared to the control group: 6-month mean change in BMI z-score intervention −0.23 (95 %CI −0.33, −0.13) vs standard care 0.01 (95 %CI −0.1, 0.11)(p = 0.002) (Johansson et al., 2020). The other studies found no substantial effect on BMI (Shen et al., 2020, Hammersley et al., 2019, Chai et al., 2019), BMI z-score (Nezami et al., 2018, Shen et al., 2020, Chai et al., 2019, Perdew et al., 2021, Tripicchio et al., 2017), waist circumference (Chai et al., 2019), or fat mass index (Nyström et al., 2017, Delisle Nyström et al., 2018) (Fig. 4A).
4.4.3. Effect on behaviors
Physical activity was evaluated using different questions regarding the amount of moderate-to-vigorous activity, the child’s physical activity level score, sedentary time per day in minutes, and the use of different instruments, such as a smartwatch (Johansson et al., 2020), accelerometer (Hammersley et al., 2019), or pedometer (Jake-Schoffman et al., 2018, Tripicchio et al., 2017). Only one study reported the number of steps through a pedometer (Jake-Schoffman et al., 2018). A quantitative synthesis was not possible. Nevertheless, no effect was observed in any of the studies, except for the EIP program (Perdew et al., 2021), targeting overweight children. The EIP program showed a significantly greater increase in moderate-to-vigorous physical activity (MVPA) levels in the intervention group than in the control group after 10 weeks from baseline: mean difference minutes/day of 0.75 vs −0.74, p = 0.001. Sedentary time in the MINISTOP study (Nyström et al., 2017, Delisle Nyström et al., 2018) increased more in the intervention group than in the control group, when measured as minutes-per-day. Conversely, when the sedentary time was measured as percentage of wear time, it decreased in the two groups. All the differences were not statistically significant. Time2bHealthy study only measured the percentage of sedentary time (Hammersley et al., 2019) at the 3- and 6-month follow-up and found a non-significant difference in favor of control at 6 months. Moderate-to-vigorous physical activity tended to increase in all the studies in both the intervention and control groups except in the POWERS study (Wingo et al., 2020), where physical activity, as reported by parents, increased strongly in the control group (Fig. 4B).
Dietary habits were also measured differently. Three studies targeting overweight/obese children or children at risk of overweight reported changes in dietary habits (Hammersley et al., 2019, Chai et al., 2019, Perdew et al., 2021). In the Chai study (Chai et al., 2019), children’s dietary intake improved in the intervention group in terms of a reduced percentage of energy from energy-dense nutrient-poor food and of an increased percentage of energy from nutrient-rich core foods, but differences were not significant. Hammersley (Hammersley et al., 2019) detected a significant change in frequency of intake of takeaway fast food measured with a discretionary food frequency score: estimate −1.36, (95 %CI −2.27, −0.45), p = 0.004.
Eleven studies targeting children regardless of their BMI reported changes in dietary habits (Nezami et al., 2018, Sutherland et al., 2019, Wingo et al., 2020, Nyström et al., 2017, Delisle Nyström et al., 2018, De Lepeleere et al., 2017, Vilchis-Gil et al., 2021, Røed et al., 2021, Bakırcı-Taylor et al., 2019, Clarke et al., 2019, Jake-Schoffman et al., 2018, Pearson et al., 2020).
In the MINISTOP study (Nyström et al., 2017, Delisle Nyström et al., 2018), a statistically non-significant decrease in the consumption of sugar-sweetened beverages (SSB) was observed both at 6 months and 12 months. Nezami (Nezami et al., 2018) observed a reduction in SSB and/or juice consumption in the intervention group at both the 3- and 6-month follow-ups. At 3 months the reduction was 297 ml/die in the intervention group vs 81 ml/die in the control (p < 0.01), similar to the reduction measured at 6 months (291 ml/die vs 51 ml/die, p < 0.01).
Statistically non-significant changes in the consumption of fruits were observed in Wingo study (Wingo et al., 2020) (decrease in the intervention group) and by Nyström et al. (Nyström et al., 2017, Delisle Nyström et al., 2018) (increase in the intervention group in both follow-ups).
In the Røed study (Røed et al., 2021) at follow-up 1, a significantly higher increase of 0.46 (95 %CI 0.06, 0.86) times/day in the frequency of vegetable intake was observed in the intervention group compared to the control group. The Jump2Health intervention (Bakırcı-Taylor et al., 2019) showed improvements in skin carotenoids levels measured by the Veggie Meter, but food photos did not detect any dietary changes. The target-veggie prep score (the number of single preparations made at home using broccoli, green beans, cauliflower, or zucchini) calculated by Clarke (Clarke et al., 2019) was 38 % higher in the intervention group compared to the control group (4.17 vs 3.03; p = 0.03). In the MINISTOP study (Nyström et al., 2017, Delisle Nyström et al., 2018), a decrease in the consumption of vegetables was observed at 6 months, while an increase was observed at 12 months; an opposite direction of effects was observed in the consumption of high-caloric foods, results are not statistically significant.
Sutherland (Sutherland et al., 2019) detected no significant differences between the intervention and control group in mean energy of foods packed within lunchboxes but a significantly higher increase in mean total lunchbox energy from recommended foods in the intervention group compared to the control group (79.21 kJ, 95 %CI 1.99, 156.43; p = 0.04).
Finally, a few studies (De Lepeleere et al., 2017, Vilchis-Gil et al., 2021, Jake-Schoffman et al., 2018, Pearson et al., 2020, Perdew et al., 2021) found no substantial difference in dietary habits between the intervention and control groups (Fig. 4C).
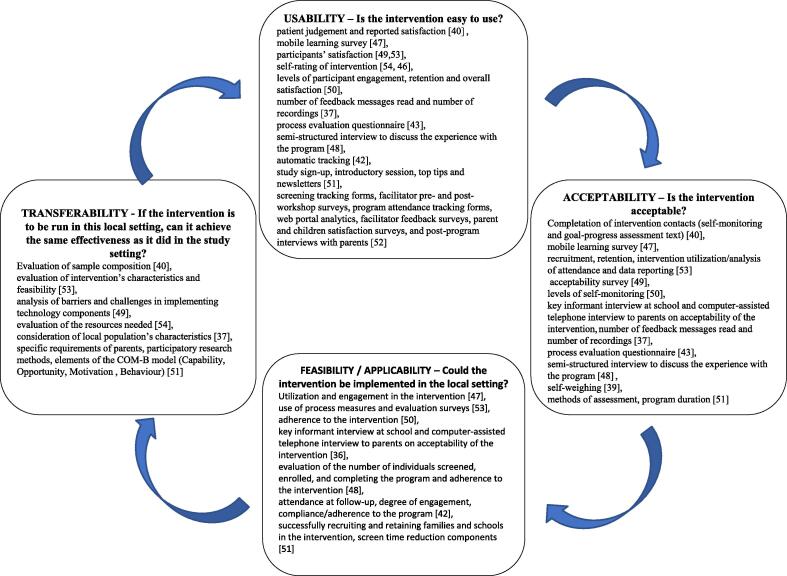
5. Usability, Acceptability, Feasibility, and transferability
All the studies conducted an evaluation of the usability, acceptability, and the feasibility of the intervention; in 3 (Johansson et al., 2020, Wald et al., 2018, Bakırcı-Taylor et al., 2019), this was the primary objective. Transferability was also addressed (Fig. 5, Table A4). The terms usability, acceptability, feasibility, and transferability were not always used consistently; we analyzed them by referring to the definitions shown in Fig. 5 (Wang et al., 2006).
Fig. 5.
Usability, acceptability, applicability, and transferability of interventions in evidence-based public health schemes (adapted by Wang 2006 (Wang et al., 2006) and their measurability for apps and tools in obesity prevention. The four domains can be considered as subsequent steps in the evaluation, although they can be assessed simultaneously. Once assessed the exportability and eventually adapted the intervention to another context, the evaluation in the new context can start again.
Concerning the usability and acceptability of the mHealth intervention as treatment, most of the studies showed positive results and received satisfactory feedback. Adherence in SMART Moms study (Nezami et al., 2018) was high, with mothers completing an average of 81 % of the intervention contacts. Users of the VeggieBook app (Clarke et al., 2019) were trained to use the app; their use of the app seemed increasingly confident. Bakirci-Taylor (Bakırcı-Taylor et al., 2019) observed good engagement, enough to achieve the desired outcome despite the fact that participants did not accept the mobile website as readily as they did the Facebook page. This may have been due to its password-protection, a characteristic that made the website less user-friendly. The high participation rate and study compliance, with consistent usage and low dropout rate (8 %), show that parents were very positive about the MINISTOP app, (Nyström et al., 2017, Delisle Nyström et al., 2018), although some technical skill may be necessary to use it. In the POWERS study (Wingo et al., 2020), the website was deemed user-friendly and easy to navigate. However, the qualitative research indicated that, while the parents had less desire to interact with the platform, the child engaged more readily (parents valued phone calls more than the e-health platform). In the Tripicchio study (Tripicchio et al., 2017), families were guided on how to use the tablets, and a final high acceptability was observed notwithstanding some barriers related to internet connection, the log-in procedure, and technical issues with cameras. De Lepeleere (De Lepeleere et al., 2017) observed a high dropout rate, despite a high proportion of parents watching the videos on the website. In the Johansonn study (Johansson et al., 2020), both parents and clinicians had a positive experience and found the support system accessible. Similarly, high participation and/or completion rates were observed in other interventions including school-based face-to-face interventions (Shen et al., 2020, Pearson et al., 2020, Trost and Brookes, 2021). Other interventions had difficulties achieving high participation or completion rates, despite the fact that the participants usually declared high satisfaction (Wald et al., 2018, Vilchis-Gil et al., 2021, Røed et al., 2021, Perdew et al., 2021); major issues in participation were observed when recruitment was only via web (Wald et al., 2018, Røed et al., 2021).
Feasibility and transferability are more complex to evaluate, and their evaluation may be hampered by different obstacles. In the Nezami (Nezami et al., 2018), Bakirci-Taylor (Bakırcı-Taylor et al., 2019) and Pearson (Pearson et al., 2020) studies, the sample included high-income, highly educated, mostly White families, making it difficult to draw conclusions on transferability. Similar concerns on generalizability were also reported in Nystrom (Nyström et al., 2017, Delisle Nyström et al., 2018), Johansson (Johansson et al., 2020) and De Lepeleere (De Lepeleere et al., 2017).
In 2 studies (Johansson et al., 2020, Clarke et al., 2019), the investigators provided a mobile phone, thereby leaving doubt about the willingness/ability to download the app or about the sustainability of the intervention. The same concerns emerged in the Tripicchio study as well (Tripicchio et al., 2017). Other concerns on technical issues regarding internet connection and app bugs, which could hamper the feasibility of the intervention, were reported in three studies (Johansson et al., 2020, De Lepeleere et al., 2017, Tripicchio et al., 2017). The POWERS web-based telecoaching system (Wingo et al., 2020) was developed specifically for families with children with physical disabilities, but the authors concluded that it might also be used for children with other conditions, including cancer, mental health disorders, substance abuse, and chronic kidney disease.
In the 2019 Chai study (Chai et al., 2019), acceptability and feasibility were both measured through recruitment, retention, and intervention utilization, which were high (Chai et al., 2021). Hammersley (Hammersley et al., 2019), Jake-Shoffman (Jake-Schoffman et al., 2018), and Sutherland (Sutherland et al., 2019) observed good acceptability and feasibility and argued for a possible transferability of the intervention.
6. Discussion
6.1. Main results
We included 20 papers evaluating 19 different interventions. Despite the fact that they were constructed on similar theoretical frameworks, the interventions had no common features or components. Therefore, our synthesis is only narrative.
Only 1 study reported a significant decrease (Johansson et al., 2020) and one a suggestive decrease (Wald et al., 2018) in the BMI z-score of obese and overweight children, while all the other studies observed no effect on anthropometric measures. Concerning physical activity and sedentary behaviors, results indicated mostly no effect (Nyström et al., 2017, Delisle Nyström et al., 2018), with only 1 study reporting a significant increase in moderate-to-vigorous physical activity (Perdew et al., 2021). Positive effects were appreciable in mostly dietary behaviors, where 6 interventions (Nezami et al., 2018, Hammersley et al., 2019, Røed et al., 2021, Bakırcı-Taylor et al., 2019, Clarke et al., 2019, Sutherland et al., 2019) proved to be effective in changing specific aspects of dietary behaviors. Of these, 3 also had an impact on the general diet score (Hammersley et al., 2019, Clarke et al., 2019, Sutherland et al., 2019). Apparently, these interventions did not share any characteristics, with some apps that were not included in prevention programs, and multicomponent interventions; neither the studies had similar methodological characteristics or risk of bias. We can only note that the interventions targeting overweight and/or obese children obtained the most positive results, in particular in the anthropometric measurements.
Few studies (Nyström et al., 2017, Delisle Nyström et al., 2018, Wald et al., 2018, Vilchis-Gil et al., 2021, Røed et al., 2021) followed up the users for more than 6 months; they found that the effects of the interventions rapidly faded. This finding was common to other obesity and overweight control/prevention interventions, which also observed that improvements in BMI or in behaviors did not last beyond one or more years. (Styne et al., 2017, Jones et al., 2011).
It is worth noting that health promotion interventions may have a negative impact on behaviors. In fact, the stages-of-change model suggests, and empirical data confirm, that administering interventions aimed at changing behaviors to subjects that are in the “pre-contemplation” stage may have a negative impact (Diclemente JOP and CC et., 2005). It is possible that parents in that stage perceive some of the app functions as intrusive. Furthermore, some authors (Bakermans-Kranenburg, 2003) have suggested that very intensive approaches may be less effective than less intensive interventions.
In general, all the interventions proved to be usable and acceptable, while feasibility and transferability were more difficult to evaluate, particularly due to the selected populations and the very specific settings.
We excluded interventions aimed directly at children (Dute et al., 2016). Therefore, the theoretical model in the included interventions foresaw two sequential changes: it had to favor a change in parental function, which would then theoretically affect change in the child’s behavior (Abraham and Michie, 2008). Perhaps this is one of the reasons why these interventions struggled to function effectively: from the theoretical approach to the results, they had to intervene not only on mediators of change but also on some environmental aspects. Even if most guidelines recommend developing multitarget, multi-setting interventions, most of the included interventions concentrated on one setting, and the mobile health component was often central; no intervention included actions for societal and contextual change. This limitation may also be the consequence of adopting the RCT design to evaluate the interventions. In fact, randomized trials with individual level randomization to evaluate interventions targeting both the individual and the context are challenging, and cluster randomization should be adopted. However, cluster randomized trials are difficult to conduct as they require a large sample size, particularly when the intervention is implemented simultaneously in large communities, such as schools or neighborhoods. This could explain why only 3 studies in our review were cluster RCTs (Clarke et al., 2019, Pearson et al., 2020, Sutherland et al., 2019). We also found 5 non-randomized studies (Shen et al., 2020, De Lepeleere et al., 2017, Vilchis-Gil et al., 2021, Perdew et al., 2021, Tripicchio et al., 2017). Studies were designed to increase the comparability of the intervention and control groups, and an accurate reporting of possible biases was present.
6.2. Limitations
As we included only mobile health tools to be installed on parents’ devices, many interesting interventions that have recently appeared in the literature were excluded. This choice was made a priori, as this review was conducted as the background work for developing an app targeting children in the age group 0–13 years. Any intervention targeting this age group which might increase the child’s screen time would have a direct negative effect on behaviors and probably on health as well.
Moreover, many interesting interventions are still in a prototypal phase and have not yet reached clinical evaluation. As mobile health technology is rapidly evolving, the interventions reported here may provide an already outdated picture of current opportunities. This limitation must be considered when looking at Fig. 2, which cannot be considered as the exhaustive result of a scoping review.
The language limit in the search of literature in this field may be particularly relevant; in fact, the interventions are often language-based, thus leading to favoring publication of scientific reports in the investigators’ native country and language.
6.3. Implication for research and practice
We found that the apps for preventing and treating childhood and adolescent obesity showed small or no effectiveness. Nevertheless, mobile health applications have the intrinsic potential to reach families, which makes developing them essential.
Considering the universally accepted recommendation to develop interventions addressing different levels and settings, there is a need to construct complex interventions (Waters et al., 2011). Involving the community, including users and stakeholders, could improve the design of such interventions. Co-creation, including co-design, co-constriction, and co-evaluation, has been proposed to overcome problems in making these context-changing interventions acceptable and feasible, but the efficacy of these strategies need to be tested (Giorgi Rossi et al., 2020).
Moreover, we need to explore sound study designs to evaluate complex interventions; we must acknowledge the limitations of individually randomized trials in this field and the difficulties in conducting cluster randomized trials when interventions are provided at the level of large communities and try to overcome the limitations of observational studies. Finally, to increase the comparability and generalizability of evidence generated by these studies, the complex interventions and their results should be described and shared adhering to existing standards of reporting (Eysenbach et., 2011, Agarwal et al., 2016). Comparable evidence is needed to enable a robust effectiveness assessment, and a detailed reporting of complex intervention will facilitate the dissemination of effective interventions.
7. Conclusions
The small sample size of individual studies and the impossibility of conducting meta-analyses due to the differences in the intervention characteristics prevent this systematic review from reaching a high level of certainty on the effectiveness of the interventions. Nevertheless, the overall picture shows a trend toward improvement in some interventions, while others were essentially ineffective. The high acceptability and the importance of mobile phones in family life support further research to develop effective, trustworthy apps.
Funding
The review is part of CoSIE (Co‐creation in Service Innovation in Europe), funded by the European Commission, grant agreement number: 770492‐CoSIE‐127 H2020‐SC6‐CO‐CREATION‐ 2016‐2017/H2020‐SC6‐COCREATION‐2017. This study was partially supported by the Italian Ministry of Health – Ricerca Corrente Annual Program 2023.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank Jacqueline M. Costa for the English language editing.
The review is part of CoSIE (Co-creation in Service Innovation in Europe), funded by the European Commission, grant agreement number: 770492-CoSIE-127 H2020-SC6-CO-CREATION 2016-2017/H2020- SC6-COCREATION-2017. Venturelli F, Ferrari F., Broccoli S., Bonvicini L., Galleli T., Giorgi Rossi P. report grants from European Commission, during the conduct of the study. We would like to thank also the Regione Emilia-Romagna since publication costs of the review are funded through the Regional Council Resolution DGR 912/2020, ATTO DS/20.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.101940.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abraham C., Michie S. A Taxonomy of Behavior Change Techniques Used in Interventions. Heal Psychol. 2008 doi: 10.1037/0278-6133.27.3.379. PMID:18624603. [DOI] [PubMed] [Google Scholar]
- Agarwal S, LeFevre AE, Lee J, L'Engle K, Mehl G, Sinha C, Labrique A; WHO mHealth Technical Evidence Review Group. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ 2016; Mar 17;352:i1174. doi: 10.1136/bmj.i1174. PMID: 26988021. [DOI] [PubMed]
- Azad M.B., Vehling L., Chan D., Klopp A., Nickel N.C., McGavock J.M., Becker A.B., Mandhane P.J., Turvey S.E., Moraes T.J., Taylor M.S., Lefebvre D.L., Sears M.R., Subbarao P. Infant feeding and weight gain: Separating breast milk from breastfeeding and formula from food. Pediatrics. 2018 doi: 10.1542/peds.2018-1092. PMID:30249624. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH, Juffer F. Less is More: Meta-Analyses of Sensitivity and Attachment Interventions in Early Childhood. Psychol Bull 2003; PMID:12696839. [DOI] [PubMed]
- Bakırcı-Taylor A.L., Reed D.B., McCool B., Dawson J.A. mHealth Improved Fruit and Vegetable Accessibility and Intake in Young Children. J Nutr Educ Behav. 2019 doi: 10.1016/j.jneb.2018.11.008. PMID:30638880. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977 doi: 10.1037//0033-295x.84.2.191. PMID:847061. [DOI] [PubMed] [Google Scholar]
- Bandura A. Human agency in social cognitive theory. Am. Psychol. 1989 doi: 10.1037/0003-066x.44.9.1175. PMID:2782727. [DOI] [PubMed] [Google Scholar]
- Brown T., Moore T.H., Hooper L., Gao Y., Zayegh A., Ijaz S., Elwenspoek M., Foxen S.C., Magee L., O’Malley C., Waters E., Summerbell C.D. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2019 doi: 10.1002/14651858.CD001871.pub4. PMID:31332776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai L.K., Collins C.E., May C., Ashman A., Holder C., Brown L.J., Burrows T.L. Feasibility and efficacy of a web-based family telehealth nutrition intervention to improve child weight status and dietary intake: A pilot randomised controlled trial. J Telemed Telecare. 2019 doi: 10.1177/1357633X19865855. [DOI] [PubMed] [Google Scholar]
- Chai L.K., Collins C.E., May C., Brown L.J., Ashman A., Burrows T.L. Fidelity and acceptability of a family-focused technology-based telehealth nutrition intervention for child weight management. J Telemed Telecare. 2021 doi: 10.1177/1357633X19864819. PMID:31390947. [DOI] [PubMed] [Google Scholar]
- Clarke P., Evans S.H., Neffa-Creech D. Mobile app increases vegetable-based preparations by low-income household cooks: A randomized controlled trial. Public Health Nutr. 2019 doi: 10.1017/S1368980018003117. PMID:30472970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lepeleere S., De Bourdeaudhuij I., Cardon G., Verloigne M. The effect of an online video intervention ‘Movie Models’ on specific parenting practices and parental self-efficacy related to children’s physical activity, screen-time and healthy diet: a quasi experimental study. BMC Public Health. 2017 doi: 10.1186/s12889-017-4264-1. PMID:28449658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruyter J.C., Olthof M.R., Seidell J.C., Katan M.B. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 2012 doi: 10.1056/NEJMoa1203034. PMID:22998340. [DOI] [PubMed] [Google Scholar]
- Delisle Nyström C., Sandin S., Henriksson P., Henriksson H., Maddison R., Löf M. A 12-month follow-up of a mobile-based (mHealth) obesity prevention intervention in pre-school children: The MINISTOP randomized controlled trial. BMC Public Health. 2018 doi: 10.1186/s12889-018-5569-4. PMID:29793467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diclemente JOP and CC. The transtheoretical approach. In: John C. Norcross and Marvin R. Goldfried, editor. Handb Psychother Integr 3 edn. New York: Oxford University Press; 2005. p. p 147. [doi: 10.1093/med:psych/9780195165791.001.0001].
- Dute D.J., Bemelmans W.J.E., Breda J. Using mobile apps to promote a healthy lifestyle among adolescents and students: A review of the theoretical basis and lessons learned. JMIR mHealth uHealth. 2016 doi: 10.2196/mhealth.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsson E. Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction, Offspring Distress, and Junk Food Self-Medication. Curr Obes Rep. 2018. PMID:29704182. [DOI] [PMC free article] [PubMed]
- Eysenbach G; CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health interventions. J Med Internet Res 2011 Dec 31;13(4):e126. doi: 10.2196/jmir.1923. PMID: 22209829; PMCID: PMC3278112. [DOI] [PMC free article] [PubMed]
- Fang K., Mu M., Liu K., He Y. Screen time and childhood overweight/obesity: A systematic review and meta-analysis. Child Care Health Dev. 2019 doi: 10.1111/cch.12701. PMID:31270831. [DOI] [PubMed] [Google Scholar]
- Fowler L.A., Grammer A.C., Staiano A.E., Fitzsimmons-Craft E.E., Chen L., Yaeger L.H., Wilfley D.E. Harnessing technological solutions for childhood obesity prevention and treatment: a systematic review and meta-analysis of current applications. Int J Obes. 2021 doi: 10.1038/s41366-021-00765-x. PMID:33627775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner B., Lally P., Wardle J. Making health habitual: The psychology of “habit-formation” and general practice. Br. J. Gen. Pract. 2012 doi: 10.3399/bjgp12X659466. PMID:23211256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi Rossi P, Ferrari F, Amarri S, Bassi A, Bonvicini L, Dall’Aglio L, Giustina C Della, Fabbri A, Ferrari AM, Ferrari E, Fontana M, Foracchia M, Gallelli T, Ganugi G, Ilari B, Scocco S Lo, Maestri G, Moretti V, Panza C, Pinotti M, Prandini R, Storani S, Street ME, Tamelli M, Trowbridge H, Venturelli F, Volta A, Davoli AM. Describing the process and tools adopted to cocreate a smartphone app for obesity prevention in childhood: Mixed method study. JMIR mHealth uHealth 2020; PMID:32357123. [DOI] [PMC free article] [PubMed]
- Gittelsohn J., Mui Y., Adam A., Lin S., Kharmats A., Igusa T., Lee B.Y. Incorporating systems science principles into the development of obesity prevention interventions: Principles benefits, and challenges. Curr Obes Rep. 2015 doi: 10.1007/s13679-015-0147-x. PMID:26069864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnani M., Birken C., Hamilton J. Childhood obesity: Causes, consequences, and management. Pediatr. Clin. North Am. 2015 doi: 10.1016/j.pcl.2015.04.001. PMID:26210619. [DOI] [PubMed] [Google Scholar]
- Hammersley M.L., Jones R.A., Okely A.D. Parent-focused childhood and adolescent overweight and obesity ehealth interventions: A systematic review and meta-analysis. J. Med. Internet. Res. 2016 doi: 10.2196/jmir.5893. PMID:27443862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammersley M.L., Okely A.D., Batterham M.J., Jones R.A. An internet-based childhood obesity prevention program (TIMe2bhealthy) for parents of preschool-aged children: Randomized controlled trial. J. Med. Internet. Res. 2019 doi: 10.2196/11964. PMID:30735139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.T., Drewnowski A., Kumanyika S.K., Glass T.A. A systems-oriented multilevel framework for addressing obesity in the 21st century. Prev Chronic Dis. 2009 PMID:19527584. [PMC free article] [PubMed] [Google Scholar]
- Jake-Schoffman D.E., Turner-McGrievy G., Wilcox S., Moore J.B., Hussey J.R., Kaczynski A.T. The mFIT (Motivating families with interactive technology) Study: a Randomized pilot to promote physical activity and healthy eating through mobile technology. J Technol Behav Sci. 2018 doi: 10.1007/s41347-018-0052-8. [DOI] [Google Scholar]
- Johansson L., Hagman E., Danielsson P. A novel interactive mobile health support system for pediatric obesity treatment: A randomized controlled feasibility trial. BMC Pediatr. 2020 doi: 10.1186/s12887-020-02338-9. PMID:32967638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.A., Sinn N., Campbell K.J., Hesketh K., Denney-Wilson E., Morgan P.J., Lubans D.R., Magarey A. The importance of long-term follow-up in child and adolescent obesity prevention interventions. Int J Pediatr Obes. 2011 doi: 10.3109/17477166.2011.575155. PMID:21612335. [DOI] [PubMed] [Google Scholar]
- Loveman E., Al-Khudairy L., Johnson R.E., Robertson W., Colquitt J.L., Mead E.L., Ells L.J., Metzendorf M.I., Rees K. Parent-only interventions for childhood overweight or obesity in children aged 5 to 11 years. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD012008. PMID:26690844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli C., Mazzantini S., Zuccotti G.V. Nutrition in the first 1000 days: The origin of childhood obesity. Int. J. Environ. Res. Public Health. 2016 doi: 10.3390/ijerph13090838. PMID:27563917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo G.F., Granado-Font E., Ferré-Grau C., Montaña-Carreras X. Mobile phone apps to promote weight loss and increase physical activity: A systematic review and meta-analysis. J Med Internet Res. 2015 doi: 10.2196/jmir.4836. PMID:26554314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdizadeh A., Nematy M., Vatanparast H., Khadem-Rezaiyan M., Emadzadeh M. Impact of parent engagement in childhood obesity prevention interventions on anthropometric indices among preschool children: a systematic review. Child Obes. 2020 doi: 10.1089/chi.2019.0103. PMID:31479311. [DOI] [PubMed] [Google Scholar]
- Methods in Cochrane | Cochrane Methods [Internet]. [cited 2021 Jan 18]. Available from: https://methods.cochrane.org/methods-cochrane.
- Michie S., Ashford S., Sniehotta F.F., Dombrowski S.U., Bishop A., French D.P. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol Heal. 2011 doi: 10.1080/08870446.2010.540664. PMID:21678185. [DOI] [PubMed] [Google Scholar]
- Nezami B.T., Ward D.S., Lytle L.A., Ennett S.T., Tate D.F. A mHealth randomized controlled trial to reduce sugar-sweetened beverage intake in preschool-aged children. Pediatr Obes. 2018 doi: 10.1111/ijpo.12258. PMID:29119719. [DOI] [PubMed] [Google Scholar]
- Nyström C.D., Sandin S., Henriksson P., Henriksson H., Trolle-Lagerros Y., Larsson C., Maddison R., Ortega F.B., Pomeroy J., Ruiz J.R., Silfvernagel K., Timpka T., Löf M. Mobile-based intervention intended to stop obesity in preschool-aged children: The MINISTOP randomized controlled trial. Am. J. Clin. Nutr. 2017 doi: 10.3945/ajcn.116.150995. PMID:28446496. [DOI] [PubMed] [Google Scholar]
- Pan X., Zhao L., Luo J., Li Y., Zhang L., Wu T., Smith M., Dai S., Jia P. Access to bike lanes and childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2021 doi: 10.1111/obr.13042. PMID:32419305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payab M., Kelishadi R., Qorbani M., Motlagh M.E., Ranjbar S.H., Ardalan G., Zahedi H., Chinian M., Asayesh H., Larijani B., Heshmat R. Association of junk food consumption with high blood pressure and obesity in Iranian children and adolescents: The CASPIAN-IV Study. J Pediatr (Rio J) 2015 doi: 10.1016/j.jped.2014.07.006. PMID:25449791. [DOI] [PubMed] [Google Scholar]
- Pearson N., Biddle S.J.H., Griffiths P., Sherar L.B., McGeorge S., Haycraft E. Reducing screen-time and unhealthy snacking in 9–11 year old children: The Kids FIRST pilot randomised controlled trial. BMC Public Health. 2020 doi: 10.1186/s12889-020-8232-9. PMID:31996192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew M., Liu S., Rhodes R., Ball G.D.C., Mâsse L.C., Hartrick T., Strange K., Naylor P.J. The effectiveness of a blended in-person and online family-based childhood obesity management program. Child Obes. 2021 doi: 10.1089/chi.2020.0236. PMID:33370164. [DOI] [PubMed] [Google Scholar]
- Pereira M.A. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk. Adv Nutr. 2014 doi: 10.3945/an.114.007062. PMID:25398745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelly S.B., Norris A.E., Dipietro J.L. Impact of mobile apps to combat obesity in children and adolescents: A systematic literature review. J Spec Pediatr Nurs. 2016 doi: 10.1111/jspn.12134. PMID:26494019. [DOI] [PubMed] [Google Scholar]
- Rankin J., Matthews L., Cobley S., Han A., Sanders R., Wiltshire H.D., Baker J.S. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther. 2016 doi: 10.2147/AHMT.S101631. PMID:27881930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newzoo Global Mobile Market Report 2020 | Light Version | Newzoo [Internet]. [cited 2021 Aug 12]. Available from: https://newzoo.com/insights/trend-reports/newzoo-global-mobile-market-report-2019-light-version/.
- Rivera J., McPherson A., Hamilton J., Birken C., Coons M., Iyer S., Agarwal A., Lalloo C., Stinson J. Mobile apps for weight management: A scoping review. JMIR mHealth uHealth. 2016 doi: 10.2196/mhealth.5115. PMID:27460502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Røed M., Medin A.C., Vik F.N., Hillesund E.R., van Lippevelde W., Campbell K., Øverby N.C. Effect of a parent-focused eHealth intervention on children’s fruit, vegetable, and discretionary food intake (Food4toddlers): Randomized controlled trial. J Med Internet Res. 2021 doi: 10.2196/18311. PMID:33591279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T, Barker M, Maria Jacob C, Morrison L, Lawrence W, Strömmer S, Vogel C, Woods-Townsend K, Farrell D, Inskip H, Baird J. A Systematic Review of Digital Interventions for Improving the Diet and Physical Activity Behaviors of Adolescents. J Adolesc Heal. 2017. PMID:28822682. [DOI] [PMC free article] [PubMed]
- Schoeppe S., Alley S., Van Lippevelde W., Bray N.A., Williams S.L., Duncan M.J., Vandelanotte C. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: A systematic review. Int J Behav Nutr Phys Act. 2016 doi: 10.1186/s12966-016-0454-y. PMID:27927218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffman D.E., Turner-McGrievy G., Jones S.J., Wilcox S. Mobile apps for pediatric obesity prevention and treatment, healthy eating, and physical activity promotion: Just fun and games? Transl Behav Med. 2013 doi: 10.1007/s13142-013-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.H., Liu Z., Li W.H., Zhou S., Xu J.H., Jiang C., Wang H.J. The smartphone-assisted intervention improved perception of nutritional status among middle school students. Int. J. Environ. Res. Public Health. 2020 doi: 10.3390/ijerph17165932. PMID:32824190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styne D.M., Arslanian S.A., Connor E.L., Farooqi I.S., Murad M.H., Silverstein J.H., Yanovski J.A. Pediatric obesity-assessment, treatment, and prevention: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017 doi: 10.1210/jc.2016-2573. PMID:28359099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R., Nathan N., Brown A., Yoong S., Finch M., Lecathelinais C., Reynolds R., Walton A., Janssen L., Desmet C., Gillham K., Herrmann V., Hall A., Wiggers J., Wolfenden L. A randomized controlled trial to assess the potential efficacy, feasibility and acceptability of an m-health intervention targeting parents of school aged children to improve the nutritional quality of foods packed in the lunchbox “SWAP IT”. Int J Behav Nutr Phys Act. 2019 doi: 10.1186/s12966-019-0812-7. PMID:31266506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate E.B., Spruijt-Metz D., O’Reilly G., Jordan-Marsh M., Gotsis M., Pentz M.A., Dunton G.F. mHealth approaches to child obesity prevention: Successes, unique challenges, and next directions. Transl. Behav. Med. 2013:406–415. doi: 10.1007/s13142-013-0222-3. PMID:24294329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrón-Pérez M., Molina-García J., Martínez-Bello V.E., Queralt A. Relationship between the physical environment and physical activity levels in preschool children. A Systematic Review. Curr Environ Heal Reports. 2021 doi: 10.1007/s40572-021-00318-4. PMID:33934294. [DOI] [PubMed] [Google Scholar]
- Tripicchio G.L., Ammerman A.S., Neshteruk C., Faith M.S., Dean K., Befort C., Ward D.S., Truesdale K.P., Burger K.S., Davis A. Technology components as adjuncts to family-based pediatric obesity treatment in low-income minority youth. Child Obes. 2017 doi: 10.1089/chi.2017.0021. PMID:28727927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost S.G., Brookes D.S.K. Effectiveness of a novel digital application to promote fundamental movement skills in 3- to 6-year-old children: A randomized controlled trial. J. Sports Sci. 2021 doi: 10.1080/02640414.2020.1826657. PMID:32985373. [DOI] [PubMed] [Google Scholar]
- Venturelli F., Ferrari F., Broccoli S., Bonvicini L., Mancuso P., Bargellini A., Giorgi Rossi P. The effect of public health/pediatric obesity interventions on socioeconomic inequalities in childhood obesity: a scoping review. Obes. Rev. 2019 doi: 10.1111/obr.12931. PMID:31468647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchis-Gil J., Klünder-Klünder M., Duque X., Martínez-Andrade G., Martínez-Almaráz A., Beristain-Lujano B., Flores-Huerta S. Impact of a nutrition-related community intervention on the quantity and quality of children’s school almuerzo. Life. 2021 doi: 10.3390/life11030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald E.R., Ewing L.J., Moyer S.C.L., Eickhoff J.C. An interactive web-based intervention to achieve healthy weight in young children. Clin Pediatr (Phila) 2018 doi: 10.1177/0009922817733703. PMID:29067819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Moss J.R., Hiller J.E. Applicability and transferability of interventions in evidence-based public health. Health Promot Int. 2006 doi: 10.1093/heapro/dai025. PMID:16249192. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xue H., Huang Y., Huang L., Zhang D. A systematic review of application and effectiveness of mHealth interventions for obesity and diabetes treatment and self-management. Adv Nutr. 2017 doi: 10.3945/an.116.014100. PMID:28507010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E., de Silva-Sanigorski A., Burford B.J., Brown T., Campbell K.J., Gao Y., Armstrong R., . Prosser L, Summerbell C.D. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD001871.pub3. PMID:22161367. [DOI] [PubMed] [Google Scholar]
- Wearing J.R., Nollen N., Befort C., Davis A.M., Agemy C.K. IPhone app adherence to expert-recommended guidelines for pediatric obesity prevention. Child Obes. 2014 doi: 10.1089/chi.2013.0084. PMID:24655230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihrauch-Blüher S., Wiegand S. Risk Factors and Implications of Childhood Obesity. Curr Obes Rep. 2018 doi: 10.1007/s13679-018-0320-0. PMID:30315490. [DOI] [PubMed] [Google Scholar]
- WHO Regional Office for Europe. WHO European Childhood Obesity Surveillance Initiative (COSI): report on the fourth round of data collection, 2015–2017. World Heal Organ -WHO. 2021.
- Wingo B.C., Yang D., Davis D., Padalabalanarayanan S., Hopson B., Thirumalai M., Rimmer J.H. Lessons learned from a blended telephone/e-health platform for caregivers in promoting physical activity and nutrition in children with a mobility disability. Disabil Health J. 2020 doi: 10.1016/j.dhjo.2019.100826. PMID:31416771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo Baidal J.A., Locks L.M., Cheng E.R., Blake-Lamb T.L., Perkins M.E., Taveras E.M. Risk factors for childhood obesity in the first 1,000 Days: A systematic review. Am. J. Prev. Med. 2016 doi: 10.1016/j.amepre.2015.11.010. PMID:26916261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Obesity and overweight World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. April 1. 2020.
- Zarnowiecki D., Mauch C.E., Middleton G., Matwiejczyk L., Watson W.L., Dibbs J., Dessaix A., Golley R.K. A systematic evaluation of digital nutrition promotion websites and apps for supporting parents to influence children’s nutrition. Int J Behav Nutr Phys Act. 2020 doi: 10.1186/s12966-020-0915-1. PMID:32041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.