Highlights
-
•
A community-based cluster randomized controlled trial.
-
•
An FCHV-delivered intervention increased cervical cancer screening uptake.
-
•
Screening uptake among women aged 30–60 years in a semi-urban area of Nepal.
Abbreviations: ANM, Auxiliary Nursing Midwives; CHW, Community Health Worker; COBIN, Community-Based management of non-communicable diseases project In Nepal; CONSORT, Consolidated Standards of Reporting Trials; CRCT, Cluster Randomized Controlled Trial; FCHV, Female Community Health Volunteer; IQR, Interquartile Range; LMIC, Low and Middle-Income Countries; VIA, Visual Inspection with Acetic acid; WHO, World Health Organization
Keywords: Cervical cancer screening, Community-based intervention, Female Community Health Volunteer, Nepal, Cluster randomized controlled trial
Abstract
This study aimed to assess the effect of Female Community Health Volunteer (FCHV)-delivered intervention to increase cervical cancer screening uptake among Nepalese women. A community-based, open-label, 2-group, cluster randomized controlled trial (CRCT) was conducted in a semi-urban setting in Western Nepal. Fourteen clusters (1:1) were randomly assigned to the intervention group, which received a 12-month intervention delivered by FCHVs or the control group (usual care). Between April and June 2019, 690 women aged 30–60 years were recruited for CRCT during the baseline survey. A follow-up assessment was conducted after the completion of the 12 months intervention. The primary outcome was the change in cervical cancer screening from baseline to 12-month follow-up. Of 690 women, 646 women completed the trial. 254 women in the intervention group and 385 women in the control group were included in the primary outcome analysis. There was a significant increase in cervical cancer screening uptake in the intervention group [relative risk (RR), 1.48; 95 % confidence interval (CI) 1.32, 1.66; P < 0.01)], compared to the control group. The secondary outcome was the change in median knowledge score among women that increased from 2 [interquartile range (IQR) 1–4] (baseline) to 6 [IQR 3–9] (follow-up) in the intervention group. However, the median knowledge score remained almost the same among women in the control group 2 [IQR 1–5] to 3 [IQR 2–5]. Our study findings reported that an FCHV-delivered intervention significantly increased cervical cancer screening uptake among women living in a semi-urban setting in Nepal.
Trial registration: ClinicalTrials.gov NCT03808064.
1. Introduction
Globally cervical cancer is the fourth most frequent cancer among women, with approximately 90 % of deaths occurring in low and middle-income countries (LMICs) (Ferlay et al., 2020). The estimated global cervical cancer age-standardized incidence was 13.3 and mortality was 7.3 per 100,000 women in 2020 (Ferlay et al., 2020). According to the World Health Organization (WHO), 40 % of new cervical cancer cases, and 5 million related deaths could be prevented with the successful implementation of vaccination, screening, and treatment of the disease by 2050 (Brisson et al., 2020, Denny et al., 2005, Gaffikin et al., 2003, Sankaranarayanan et al., 2007, World Health Organization, 2020).
Cervical cancer is the major cause of cancer deaths among women in Nepal with an estimated age-standardized incidence of 16.4 and mortality of 11.1 per 100,000 women in 2020 (Ferlay et al., 2020). The national guideline for cervical cancer screening and prevention in 2010 aimed at achieving the goal of screening 50 % of the target population (eligible women aged 30–60 years), which was updated in 2017 to 70 % (Family Health Division, 2010b, Family Health Services, 2017). All Nepalese women aged 30–60 years are recommended to screen for cervical cancer every five years. However, previous studies have shown that cervical cancer screening uptake among Nepalese women is low; 5.4 % among women aged 30–65 years in 2015 and 8 % among women aged 15–49 years in 2019 (Dhimal et al., 2020, Ranjit et al., 2016).
Various barriers to cervical cancer screening – embarrassment, fear, lack of knowledge on screening, lack of trust, gender of health personnel, lack of family support, and inaccessibility can all contribute to low screening uptake (Darj et al., 2019, Dhimal et al., 2020, Greibe Andersen et al., 2020, Ranjit et al., 2016). Mobilizing Community Health Workers (CHWs) could improve the health outcomes of people who are often unable to reach health facilities by visiting door-to-door, linking them with local resources, and encouraging them to access healthcare (Hartzler et al., 2018, Lehmann and Sanders, 2007, Family Health Division, 2010a). Female Community Health Volunteers (FCHVs) are the CHWs introduced by the Government of Nepal in 1988 with an aim to promote maternal, and child health, link communities to health facilities, and provide health education through the monthly mothers’ meeting (Family Health Division, 2010a). There are about 50,000 FCHVs in Nepal; who are married women, selected locally, and have received basic training through the Ministry of Health and Population (Family Health Division, 2010a, Department of Health Services, 2017). FCHVs have a proven record of improving maternal, and child health for almost three decades (Family Health Division, 2010a, Department of Health Services, 2017). Additionally, involving FCHV has proven to be effective in managing hypertension, and type 2 diabetes at the community level in Nepal (Gyawali et al., 2021, Neupane et al., 2018). Therefore, mobilizing FCHV to educate and empower women to increase cervical cancer screening uptake could be a potentially feasible strategy to achieve the target for screening uptake coverage.
The aim of this study was to assess the effect of an FCHV-delivered intervention to increase cervical cancer screening uptake among Nepalese women. We hypothesized that there would be an increase in cervical cancer screening uptake among eligible women aged 30–60 years in the intervention group compared with the control group over the 12 months of the intervention.
2. Methods
2.1. Study design and setting
This study was a community-based, 12-month, open-label, two-arm cluster randomized controlled trial (CRCT). A baseline survey was conducted at the inception and a follow-up survey was conducted after the 12-month intervention. The study was conducted in a semi-urban area of Pokhara Metropolitan City (former Lekhnath municipality) in Kaski district, Gandaki province, situated approximately 180 km west of the capital Kathmandu, Nepal. The Lekhnath municipality area was administratively divided into 15 smaller units called wards. Each ward was considered as one cluster. The total population of the Lekhnath Municipality was 59,498 (males = 27394 and females = 32104), and the number of households was 14,958 according to the 2011 census (CBS, 2012). The study area comprised a 25-bedded hospital, three health posts, and six urban health care centers at the time of the study. There were 123 FCHVs working in the study area during the study period.
2.2. Study participants, inclusion and exclusion criteria
Our study adopted the sampling frame of women aged 30–60 years (as of 31 December 2018) from the COBIN trial (Neupane et al., 2018). A detailed description of the study methodology and design has been provided elsewhere (Shrestha et al., 2021). In brief, we conducted a community-based household survey among women aged 30–60 years (Shrestha et al., 2022). The recruited eligible study participants consented to participate in the study and had no plans to migrate outside the study area during the intervention period. Women who declined to consent, or complete the survey, had had a hysterectomy, were pregnant, or were unlikely to be in the study area throughout the intervention were excluded.
A total of 690 eligible women aged 30–60 years were enrolled in the trial between April and June 2019 during the baseline survey. The recruited study participants provided complete data on ever having been screened for cervical cancer at baseline and the change in screening uptake at the 12-month follow-up (inclusive of any prior screening).
2.3. Baseline and follow-up data collection
Four trained female data enumerators with a health professional background conducted the baseline survey through face-to-face interviews. We adopted a previously validated survey questionnaire, translated into the local language (Nepali), and pretested before conducting the face-to-face interview (Neupane et al., 2017, Shrestha et al., 2022, Thapa et al., 2018, Touch and Oh, 2018, FHD, 2016, MoH, Nepal; New ERA; ICF, 2017, World Health Organization, 2018a). Details about the training of data enumerators, and the questionnaire are described elsewhere (Shrestha et al., 2022). The questionnaire elicited sociodemographic information (age, ethnicity, education, marital status, occupation, and income), questions related to sexual and reproductive health, health-seeking behavior, knowledge, attitude, and cervical cancer screening practice, and knowledge of signs, and symptoms. The follow-up survey for the trial was conducted 12 months after the baseline survey using the same questionnaire (Shrestha et al., 2022).
2.4. Randomization
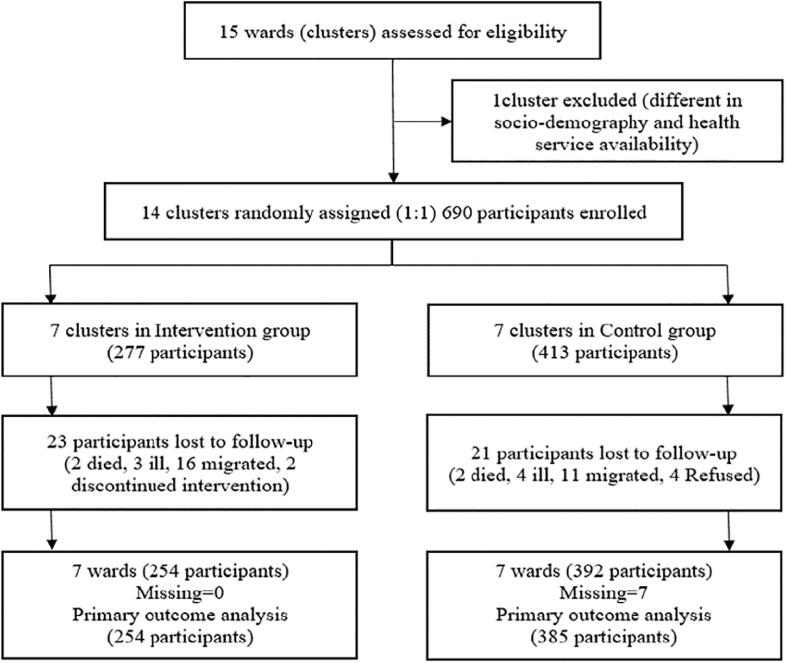
The clusters were randomized to the intervention or control arm after the baseline survey. Out of 15 clusters, 14 were selected for cluster randomization (one cluster comparatively different from others in sociodemographic and health service availability was excluded) as shown in the Consolidated Standards of Reporting Trials CRCT: Cluster Randomized Controlled Trial (CONSORT) diagram (Fig. 1) (Schulz, et al., 2010). Fourteen clusters were randomly assigned (1:1) into two groups – intervention (n = 7), and control (n = 7). To ensure allocation concealment, an independent biostatistician randomly generated the allocation sequence by computer. The participants in the intervention group received a 12-month intervention delivered by FCHVs, and the participants in the control group received usual care as described in the ‘interventions’ section.
Fig. 1.
CONSORT diagram for trial allocation, follow-up, and analysis.
2.5. FCHV training
Thirty-nine FCHVs from the seven randomly assigned intervention clusters attended a one-day orientation and assessment session. FCHVs were informed about their role in the COBIN-C project and introduced to non-communicable diseases, cervical cancer, and signs and symptoms on the first day of training. FCHVs were then assessed for the minimum requirements of reading, writing, motivation, and availability to attend the next two days of training and the one-year intervention period. A total of 16 FCHVs fulfilled the requirements and were enrolled for the next two days of training. The 3-day (approximately 4 h, and 30 min per day) interactive training session was conducted in the local language (Nepali) using PowerPoint presentations, demonstrations, and group exercises. The training covered a wide range of topics, such as cervical cancer causes, and risk factors, cervical cancer screening, and service availability, health education, and counseling eligible women using a flip chart. In addition, FCHVs were trained to properly record, report, refer, and follow up.
Educational sessions were guided by the use of the Health Belief Model (HBM), which is a psychological model that explains individual health-related behaviors, and uptake of health services (Tavafian, 2012). The HBM assumes that health-seeking behavior, such as screening participation, is determined by the individual’s beliefs about the benefits of screening outweighing perceived barriers, and threats (Nutbeam et al., 2010). A register (Fig. 1. Supplement file) was developed, and distributed to FCHVs to record dates, times, and activities along with a referral slip for cervical cancer screening at their local government health facility (hospital/health post/urban health center) (Fig. 2. Supplement file).
The training materials were based on the WHO training for community health workers, developed in consultation with experts, and key stakeholders (WHO-SEARO, 2017). The training was facilitated by the principal investigator (ADS), a health research team from the Nepal Development Society trained in NCD-related training, a gynecologist from a tertiary level hospital, and a nursing officer (focal person for cervical cancer screening, and prevention program of the study area), all of whom had experience in working with the government-run FCHV program.
2.6. Interventions
After the training, FCHVs visited the participants’ households in the intervention group to provide health education, and counsel women about cervical cancer screening using flip charts, and pamphlets. The intervention was delivered from 10 December 2019 to 9 December 2020. One FCHV followed an average of 19 individuals (range, 6–28 individuals) every four months over the following year. The FCHVs maintained mutual communication with the Auxiliary Nursing Midwives (ANM) at their affiliated government health facility (hospital/health post/urban health centers) to arrange Visual Inspection with Acetic acid (VIA) for the participants. A field supervisor was responsible for supervising all 16 FCHVs. A supervision checklist (Fig. 3. Supplement file) was used to track and update the knowledge, and skills of FCHVs. Furthermore, the field supervisor visited all the five health facilities (one 25-bedded hospital, one health post, and three urban health care centers) in the intervention group to collect the referral slips, and confirm the participants’ screening attendance. FCHVs were reimbursed for transport costs and refreshments (US $5) during the training and each household visit. Participants in the control groups continued receiving ‘usual care.’ Usual care means the current health education and promotion practices for cervical cancer screening at the community level provided by the government health system. They did not receive further contact, information, or educational materials from FCHVs until the 12-month assessment. Participants in the control group were originally scheduled to receive the same intervention after the follow-up. However, training of FCHVs became difficult due to COVID situations. Therefore, female data enumerators (with a health professional background) provided services when circumstances allowed.
2.7. Outcomes
The primary outcome was the change in cervical cancer screening uptake (CCSU) from the baseline to the follow-up survey and we calculated the relative risk (RR) and 95 % confidence interval (CI), with a level of statistical significance set at P < 0.05. The secondary outcome was the change in the level of knowledge among women about cervical cancer screening and prevention. Median and interquartile range (IQR) of knowledge scores were calculated for intervention and control groups at both baseline and follow-up surveys.
The correct responses to 14 questions (about knowledge of cervical cancer, signs, and symptoms of cervical cancer, risk factors, Human Papillomavirus (HPV), HPV vaccine, preventive measures, and screening combined to a total score of 36) measured the level of knowledge.
2.8. Statistical analysis
The sample size was calculated by assuming a cervical cancer screening percent change of 10 % between the intervention, and control groups after 12 months of follow-up for participants with cervical cancer screening uptake of 2.8 % (Bruni et al., 2019). With an intraclass correlation coefficient of 0.01, design effect of 1.5, average cluster size of 41, and 90 % power, we determined that we would need 7 clusters with a sample size of 287 in each arm (Killip et al., 2004). Allowing for up to 20 % loss to follow-up, the total sample size of 690 was fixed. Due to unequal cluster size, we had an unequal number of participants in the intervention (2 7 7), and control (4 1 3) groups with a total sample size of 690. All quantitative analyses were performed in STATA version 16.1 software (StataCorp, College Station, TX, USA).
Baseline characteristics are summarized in numbers and percentages with a p-value for the chi2 test. The primary outcome of the study was analyzed using a per-protocol approach (primary analysis), including only participants who completed the follow-up survey with at least one home visit. To check the robustness of the results, we also conducted a secondary analysis with an intent-to-treat approach. The missing information was imputed using the baseline information as the Last Observation Carried Forward (LOCF) method (Horiuchi et al., 2021). The intent-to-treat population consisted of all randomized participants who completed the baseline assessment, irrespective of the number of home visits received. We modeled CCSU at follow-up using mixed-effect multilevel logistic regression with a random intercept for clustering. The estimated intervention effect was controlled for age, literacy, ethnicity, and baseline screening. The estimated intervention effect size was reported as RR, and 95 % CI. The level of statistical significance was set at P < 0.05.
2.9. Ethics
The Ethical Review Board of the Nepal Health Research Council, Kathmandu, Nepal approved the study (Reg. no. 43/2019). All participants gave their written informed consent before enrollment in the surveys as per the Helsinki Declaration of ethical principles for medical research (World Medical Association, 2013). Fingerprints were obtained from the participants who were not able to read and write. Data enumerators read the consent form with the participants and verbally assured them that all the information provided would be kept strictly confidential, and used only for study purposes.
2.10. Safety considerations
The coronavirus (COVID-19) pandemic emerged shortly after the first round of home visits by FCHVs. The government imposed a nationwide lockdown (from 24 March to 26 December 2020) in an effort to curb the COVID-19 pandemic. Therefore, the FCHVs did not make any home visits during this period. FCHVs were insured for COVID-19 and were trained to follow precautionary measures before the second home visit. Standard preventive measures including wearing a facemask, maintaining hand hygiene, and observing social distance were followed to prevent transmission of coronavirus between FCHVs, field supervisors, office staff, data enumerators, and study participants.
3. Results
Of 690 enrolled women, 646 women completed the trial. 254 women in the intervention group, and 392 women in the control group, participated in the follow-up survey. 23 women (34.8 % screened at baseline for cervical cancer) were lost to follow-up in the intervention group, and 21 (38.1 % screened at baseline for cervical cancer) in the control group (Fig. 1). Cervical cancer screening uptake information for 7 women in the control group was missing. Therefore, 254 women in the intervention group and 385 women in the control group were included in the primary outcome analysis following per-protocol analysis. The intent-to-treat analysis was conducted among 690 enrolled women (277 in the intervention group and 413 in the control group). Baseline characteristics of the women in the intervention and control groups are presented as per-protocol and intent-to-treat analyses (Table 1, Table 2). CCSU increased from 42.5 %; 108/254 (baseline) to 73.2 %; 186/254 (follow-up) in the intervention group, and from 45.2 %; 174/385 (baseline) to 51.4 %; 198/385 (follow-up) in the control group. A total of 53.4 % (78/146) women in the intervention group and 11.3 % (24/211) in the control group were screened for cervical cancer during the 12-month study period.
Table 1.
Baseline characteristics of the per-protocol sample.
|
Characteristics |
Participants, No. (%) |
P-valuei |
||
|---|---|---|---|---|
| Intervention group (n = 254) | Control group (n = 385) | |||
| Cluster level, No. | ||||
| Wards | 7 | 7 | ||
| Female community health workers | 16 | 0 | ||
| Sociodemographic characteristics | ||||
| Age | 30–34 years | 21 (8.2) | 25 (6.5) | 0.64 |
| 35–39 years | 37 (14.6) | 72 (18.7) | ||
| 40–44 years | 52 (20.5) | 81 (21.0) | ||
| 45–49 years | 55 (21.7) | 84 (21.8) | ||
| 50–54 years | 41 (16.1) | 64 (16.6) | ||
| 55–60 years | 48 (18.9) | 59 (15.4) | ||
| Literacy | Illiterate | 12 (4.7) | 27 (7.0) | 0.24 |
| Literatea | 242 (95.3) | 358 (93.0) | ||
| Marital status | Unmarriedb | 30 (11.8) | 50 (13.0) | 0.66 |
| Married | 224 (88.2) | 335 (87.0) | ||
| Ethnicity | Dalitc | 32 (12.6) | 63 (16.3) | <0.01 |
| Disadvantaged janajatid | 30 (11.8) | 70 (18.2) | ||
| Relatively advantaged janajatie | 34 (13.4) | 68 (17.7) | ||
| Upper caste groupsf | 158 (62.2) | 184 (47.8) | ||
| Monthly household incomeg | <US$ 256 | 153 (60.2) | 242 (62.9) | 0.51 |
| ≥US$ 256 | 101 (39.8) | 143 (37.1) | ||
| CCSUh at baseline | Yes | 108 (42.5) | 174 (45.2) | 0.51 |
| No | 146 (57.5) | 211 (54.8) | ||
Note: aLiterate = (No formal education – can read and write, Primary, Secondary, Intermediate and equivalent, Graduate and equivalent); bUnmarried: (Unmarried, separated, divorced, widow); cDalit: (Bishwokarma, Gandarba, Pariyar, Sarki, Sunar); dDisadvantaged Janajati: (Kumal, Magar, Sherpa, Tamang); eRelatively Advantaged Janajati: (Gurung, Newar, Thakali); fUpper caste groups: (Brahmin, Chhetri, Sanyasi, Thakuri) (Bhandari et al., 2014); gMonthly household income [US$<256 (NPR 1 = 0.0085 USD, 27 September 2020): 30,121 NPR (Nepal Rastra Bank, 2016)]; hCCSU – Cervical Cancer Screening Uptake; iP-value < 0.05, statistically significant.
Table 2.
Baseline characteristics of the intent-to-treat sample.
|
Characteristics |
Participants, No. (%) |
P-valuei |
||
|---|---|---|---|---|
| Intervention group (n = 277) | Control group (n = 413) | |||
| Cluster level, No. | ||||
| Wards | 7 | 7 | ||
| Female community health workers | 16 | 0 | ||
| Sociodemographic characteristics | ||||
| Age | 30–34 years | 22 (7.9) | 28 (6.8) | 0.87 |
| 35–39 years | 42 (15.2) | 74 (17.9) | ||
| 40–44 years | 56 (20.2) | 87 (21.1) | ||
| 45–49 years | 58 (20.9) | 88 (21.3) | ||
| 50–54 years | 47 (17.0) | 70 (16.9) | ||
| 55–60 years | 52 (18.8) | 66 (16.0) | ||
| Literacy | Illiterate | 14 (5.1) | 28 (6.8) | 0.35 |
| Literatea | 263 (94.9) | 385 (93.2) | ||
| Marital status | Unmarriedb | 36 (13.0) | 57 (13.8) | 0.76 |
| Married | 241 (87.0) | 356 (86.2) | ||
| Ethnicity | Dalitc | 36 (13.0) | 67 (16.2) | 0.01 |
| Disadvantaged janajatid | 36 (13.0) | 77 (18.6) | ||
| Relatively advantaged janajatie | 39 (14.1) | 73 (17.7) | ||
| Upper caste groupsf | 166 (59.9) | 196 (47.5) | ||
| Monthly household incomeg | <US$ 256 | 168 (60.6) | 260 (62.9) | 0.54 |
| ≥US$ 256 | 109 (39.4) | 153 (37.1) | ||
| CCSUh at baseline | Yes | 116 (41.9) | 182 (44.1) | 0.57 |
| No | 161 (58.1) | 231 (55.9) | ||
Note: aLiterate = (No formal education – can read and write, Primary, Secondary, Intermediate and equivalent, Graduate and equivalent); bUnmarried: (Unmarried, separated, divorced, widow); cDalit: (Bishwokarma, Gandarba, Pariyar, Sarki, Sunar); dDisadvantaged Janajati: (Kumal, Magar, Sherpa, Tamang); eRelatively Advantaged Janajati: (Gurung, Newar, Thakali); fUpper caste groups: (Brahmin, Chhetri, Sanyasi, Thakuri) (Bhandari et al., 2014); gMonthly household income [US$<256 (NPR 1 = 0.0085 USD, 27 September 2020): 30,121 NPR (Nepal Rastra Bank, 2016)]; hCCSU – Cervical Cancer Screening Uptake; iP-value < 0.05, statistically significant.
3.1. Primary outcome
The change in CCSU from the baseline to the 12-month follow-up survey in the intervention group was 30.7 % (95 %CI −42 %, −19 %) and in the control group was 6.2 % (95 %CI −16 %, 4 %). The effect of the FCHV delivered intervention resulted in a 48 % increase in CCSU in the intervention group (RR, 1.48; 95 %CI 1.32, 1.66; P < 0.01) compared to the control group in the primary analysis (Table 3). The finding was similar to that of the secondary analysis (RR, 1.45; 95 %CI 1.32, 1.60; P < 0.01), which included the participants who were not followed up (Table 3).
Table 3.
Primary outcome.
| Primary analysis | Intervention | Control | Intervention effect size RRc (95 % CId) | P-valuee |
|---|---|---|---|---|
| CCSUa at baseline | 108/254 (42.5) | 174/385 (45.2) | ||
| CCSUa at Follow-up | 186/254 (73.2) | 198/385 (51.4) | 1.48 (1.32, 1.66) | < 0.01 |
| Change (95 % CId) | 30.7 % (-42 %, −19 %) | 6.2 % (-16 %, 4 %) | ||
| Secondary analysis | ||||
| CCSUa at Follow-up LOCFb | 194/277 (70.0) | 206/413 (49.9) | 1.45 (1.32, 1.60) | < 0.01 |
Note: aCCSU: Cervical Cancer Screening Uptake; bLOCF: Last Observation Carried Forward; The intervention effect size is shown as dRelative Risk (RR), and 95 % dCI (Confidence interval). The estimated intervention effect was controlled for age, literacy, ethnicity, and baseline screening. eP-value < 0.05, statistically significant. P-value was calculated from mixed-effect logistic regression analyses with a random intercept for clusters.
3.2. Secondary outcome
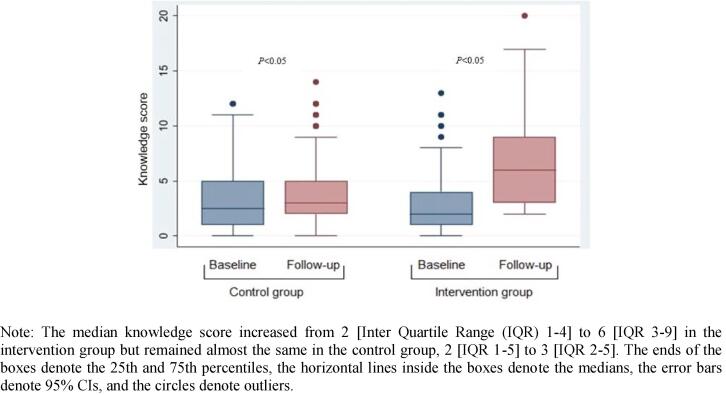
The median knowledge score increased from 2 [Inter Quartile Range (IQR) 1–4] to 6 [IQR 3–9] in the intervention group, and 2 [IQR 1–5] to 3 [IQR 2–5] in the control group after the 12-month intervention (Fig. 2).
Fig. 2.
Unadjusted change in knowledge score of cervical cancer from baseline to follow-up.
4. Discussion
This community-based CRCT mobilizing FCHVs aimed to increase cervical cancer screening among women in a semi-urban area of Pokhara Metropolitan City, Nepal. Our study findings reported a significant increase in CCSU from baseline to follow-up in the intervention group compared to the control group.
In LMICs where the lack of knowledge of cervical cancer is one of the major barriers to screening, (Darj et al., 2019, Greibe Andersen et al., 2020) education interventions are feasible, and effective in increasing screening uptake for cervical cancer (Abiodun et al., 2014, Daryani et al., 2016). Similarly, a CHWs-delivered house-to-house education intervention was associated with a significant increase in cervical cancer screening uptake among Nigerian women (Chigbu et al., 2017). Studies have reported that CHWs have a potentially useful role in promoting the importance of cervical cancer screening, and follow-up through education outreach initiatives (Ghahremani et al., 2016, Tum et al., 2013).
Our study reported a threefold increase in average knowledge score in the intervention group after the 12-month intervention. Similar to our findings, a community-based quasi-experimental study from Iran reported that health education based on the Health Belief Model enhanced women's knowledge and increased screening participation in the intervention group (Shojaeizadeh et al., 2011). Previous studies have reported that education programs for cervical cancer screening have been effective in improving the knowledge regarding cervical cancer (Mary and D'Sa, 2014, Shakya et al., 2016). However, various other barriers (sociocultural barriers, service providers’ behavior, geographical challenges, and limited finances) to screening call for an organized programme targeting eligible Nepalese women to screen for cervical cancer every five years (Darj et al., 2019, Family Health Services, 2017, Greibe Andersen et al., 2020, Shrestha et al., 2021).
The participants in our intervention group received FCHV-delivered, face-to-face individual counseling for cervical cancer screening during home visits. FCHVs performed a key role to liaise, and refer participants for VIA screening at government health facilities in the study area. The intervention was designed using a standardized training package, with a culturally appropriate tailored message and basic healthcare tasks that have important future implications (Shrestha et al., 2021). Therefore, there is a potential to test the intervention in similar settings in other parts of the country. Furthermore, training materials may be improvised using locally developed pictograms and translated into specific ethnic languages in future projects for nationwide expansions.
The WHO has advocated for the deployment of CHWs as a key strategy to reach the most marginalized populations to achieve Universal Health Coverage and reduce health inequities, especially in LMICs (Tulenko et al., 2013, World Health Organization, 2018b). In Nepal, FCHVs are the CHWs who are local community women from various ethnic groups. Because of their geographic and cultural proximity to the populations they serve, FCHVs are described as the liaison between health services and communities, uniquely positioned to extend care to poor, hard-to-access, and underserved groups that fall beyond the reach of institution-based services (Family Health Division, 2010a). In Nepal, FCHVs have proven their contribution to the improvement of maternal and child health (Family Health Division, 2010a). Similarly, community-based intervention mobilizing FCHVs with appropriate training has been proven cost-effective in reducing hypertension in Nepal (Krishnan et al., 2019). Engaging CHWs have been reported to reduce loss to follow-up in cervical cancer screening in a South African cervical cancer screening study (Goldhaber-Fiebert et al., 2005). Additionally, identifying spending levels of CHWs visits to achieve adherence to cervical cancer screening was suggested to be useful for program planning (Goldhaber-Fiebert et al., 2009). Therefore, training FCHVs on cervical cancer could be a cost-effective intervention to pave the way to increase the national CCSU coverage, which could reduce cervical cancer incidence in Nepal. However, studies have also reported that there may be an increase in the workload on FCHVs, and the risk of over-burdening them, as well as an increase in the need for human resources to monitor, and train FCHVs (Khatri et al., 2017, Panday et al., 2017). There is a necessity of assessing the effect of adding components to address non-communicable diseases including cervical cancer to FCHV’s current duties.
FCHVs demonstrated dedication to health promotion even during the COVID-19 pandemic following precautionary measures. However, they did not make any home visits during the lockdown period. Similarly, cervical cancer screening services were halted at the local health facility during the lockdown period, which may have prevented women from screening.
Our study findings provide important evidence to support the integration of an FCHV-delivered intervention with the existing screening guideline to increase cervical cancer screening uptake in Nepal, to be in line with the global strategy to eliminate cervical cancer (Family Health Division, 2010b, Family Health Services, 2017, World Health Organization, 2020). Although Nepal has a national guideline for cervical cancer screening, the current health education and promotion practices for cervical cancer screening at the community level provided by the government health system are still limited in implementation. Additionally, the number of FCHVs required to provide individual-oriented intervention could be a challenge to scale up the intervention at the national level. Community-based self-sample collection for HPV testing followed by treatment for HPV-positive women has the potential to be an effective and cost-effective screening strategy (Mezei et al., 2018). FCHVs could assist in promoting HPV test as a self-sample collection that could be an additional method to be integrated along with this model for cervical cancer prevention (Arrossi et al., 2015, Gök et al., 2010, Johnson et al., 2014).
4.1. Strengths and limitations
This intervention study was tested in a cluster-randomized controlled trial, which is a strong study design where there are practical difficulties in randomizing at the individual level. The risk of contamination was also minimized by the cluster design, whereby the intervention and control clusters were geographically separated and the chance of intervention cluster participants regularly meeting control cluster participants was negligible. FCHVs were instructed not to relay information about the study to other than the ones who were assigned. However, we cannot exclusively deny the risk of exchanging information between FCHVs from intervention and control groups. As the allocated clusters were from one area, the generalizability of the obtained findings may be limited.
5. Conclusions
This CRCT reported a significant increase in cervical cancer screening uptake among women in the intervention group compared to the control group, and proved effective. The findings of this trial suggest that an FCHV-delivered education intervention has a strong potential in increasing cervical cancer screening uptake and can be expanded to similar settings elsewhere.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement/Funding
We would like to thank the FCHVs, study participants, and the Nepal Development Society, team. We would like to acknowledge the data safety and monitoring board members Prof. Dr. Bhagwan Koirala, Prof. Dr. Stephen Hodgins, Prof. Dr. Kedar Baral, Mr. Claus Høstrup Vestergaard, and Prof. Dr. Lochana Shrestha. We particularly thank Abhishek Spakota, Amrita Thapa, Garima Neupane, Sabina Timilsina, Sneha Sharma, Sonam Magar, Tara Ballav Adhikari, Pabitra Babu Soti, and Hari Prasad Pokhrel for their valuable support and contribution in conducting this study. This study is part of research work toward a PhD degree at Aarhus University, Denmark (https://phd.health.au.dk/), and is funded by a university scholarship (Project number: 10439) awarded to ADS. The funding organizations do not have any role in the study design, the data collection, analysis, interpretation, or the reporting of the results.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.101948.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abiodun O.A., Olu-Abiodun O.O., Sotunsa J.O., Oluwole F.A. Impact of health education intervention on knowledge and perception of cervical cancer and cervical screening uptake among adult women in rural communities in Nigeria. BMC Public Health. 2014;14:814. doi: 10.1186/1471-2458-14-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrossi S., Thouyaret L., Herrero R., et al. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob Health. 2015;3(2):e85–e94. doi: 10.1016/S2214-109X(14)70354-7. [DOI] [PubMed] [Google Scholar]
- Bhandari G.P., Angdembe M.R., Dhimal M., et al. State of non-communicable diseases in Nepal. BMC Public Health. 2014;14:23. doi: 10.1186/1471-2458-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson M., Kim J., Canfell K., et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. The Lancet. 2020;395(10224):575–590. doi: 10.1016/S0140-6736(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni, L., Albero, G., Serrano, B., et al., 2019. Human Papillomavirus and Related Diseases in Nepal, Summary Report 17 June 2019. https://hpvcentre.net/statistics/reports/NPL.pdf. Accessed 6 April2021.
- CBS, 2012. National population and housing census 2011 (National Report). Retrieved from https://cbs.gov.np/national-population-and-housing-census-2011national-report/.
- Chigbu C.O., Onyebuchi A.K., Onyeka T.C., et al. The impact of community health educators on uptake of cervical and breast cancer prevention services in Nigeria. Int. J. Gynaecol. Obstet. 2017;137(3):319–324. doi: 10.1002/ijgo.12150. [DOI] [PubMed] [Google Scholar]
- Darj E., Chalise P., Shakya S. Barriers and facilitators to cervical cancer screening in Nepal: A qualitative study. Sexual Reprod. Healthcare. 2019;20:20–26. doi: 10.1016/j.srhc.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Daryani S., Shojaeezadeh D., Batebi A., et al. The effect of education based on a health belief model in women's practice with regard to the Pap smear test. J. Cancer Policy. 2016;8:51–56. doi: 10.1016/j.jcpo.2015.11.001. [DOI] [Google Scholar]
- Denny L., Kuhn L., De Souza M., et al. Screen-and-Treat Approaches for Cervical Cancer Prevention in Low-Resource Settings A Randomized Controlled Trial. JAMA. 2005;294(17):2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- Department of Health Services, 2017. Annual Report - Department of Health Services 2073/74 (2016/17). Ministry of Health and Population, Kathmandu, Nepal. https://dohs.gov.np/wp-content/uploads/2018/04/Annual_Report_2073-74.pdf. Accessed 10 June 2021.
- Dhimal, M., Bista, B., Bhattarai, S., et al., 2020. Report of Non Communicable Disease Risk Factors: STEPS Survey Nepal 2019. Kathmandu: Nepal Health Research Council. https://www.who.int/docs/default-source/nepal-documents/ncds/ncd-steps-survey-2019-compressed.pdf?sfvrsn=807bc4c6_2. Accessed 6 April 2021.
- Family Health Division, 2010a. National Female Community Health Volunteer Program Strategy (Unofficial translation).Ministry of Health and Population, Government of Nepal, Kathmandu. https://www.advancingpartners.org/sites/default/files/nepal_national_female_chv_program_strategy.pdf. Accessed 8 June 2021.
- Family Health Division, 2010b. National Guidelines for Cervical Cancer Screening and Prevention in Nepal. Ministry of Health and Population, Government of Nepal, Kathmandu.
- Family Health Services, 2017. Cervical Cancer Screening and Prevention in Nepal. Implementation Plan 2016-2020. Department of Health Services, Teku, Kathmandu. Accessed 6 April2021.
- Ferlay J., Ervik M., Lam F., et al. International Agency for Research on Cancer; Lyon, France: 2020. Global Cancer Observatory: Cancer Today. 6 April 2021. [Google Scholar]
- Gaffikin L., Blumenthal P.D., Emerson M., et al. Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet. 2003;361(9360):814–820. doi: 10.1016/s0140-6736(03)12707-9. [DOI] [PubMed] [Google Scholar]
- FHD, UNFPA & CMDN, 2016. Study on Selected Reproductive Health Morbidities among Women attending Reproductive Health Camps in Nepal. https://rb.gy/twaaj9. Accessed 6 April 2021.
- Ghahremani, L., Harami, Z.K., Mohammad, H.K., 2016. Investigation of the Role of Training Health Volunteers in Promoting Pap Smear Test Use among Iranian Women Based on the Protection Motivation Theory. Asian Pac J Cancer Prev. 17(3), 1157-62. doi:10.7314/APJCP.2015.17.3.1157. [PubMed]
- Gök M., Heideman D.A., Kemenade F.J., et al. HPV testing on self collectedcervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ. 2010;340:C1040. doi: 10.1136/bmj.c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber-Fiebert J.D., Denny L.E., De Souza M., et al. The costs of reducing loss to follow-up in South African cervical cancer screening. Cost Effectiveness Resour. Allocation: C/E. 2005;3:11. doi: 10.1186/1478-7547-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber-Fiebert J.D., Denny L.A., De Souza M., et al. Program spending to increase adherence: South African cervical cancer screening. PloS one. 2009;4(5):e5691. doi: 10.1371/journal.pone.0005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greibe Andersen, J., Shrestha, A.D., Gyawali, B., et al., 2020. Barriers and facilitators to cervical cancer screening uptake among women in Nepal - a qualitative study. Women & Health. 60(9), 963–974. doi:10.1080/03630242.2020.1781742. [DOI] [PubMed]
- Gyawali B., Sharma R., Mishra S.R., et al. Effectiveness of a Female Community Health Volunteer-Delivered Intervention in Reducing Blood Glucose Among Adults With Type 2 Diabetes: An Open-Label, Cluster Randomized Clinical Trial. JAMA Netw. Open. 2021;4(2):e2035799. doi: 10.1001/jamanetworkopen.2020.35799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler A.L., Tuzzio L., Hsu C., Wagner E.H. Roles and functions of community health workers in primary care. Ann. Fam. Med. 2018;16(3):240–245. doi: 10.1370/afm.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Rattana S., Saysanasongkham B., et al. Effectiveness of self-managed continuous monitoring for maintaining high-quality early essential newborn care compared to supervision visit in Lao PDR: a cluster randomised controlled trial. BMC Health Serv Res. 2021;21(1):460. doi: 10.1186/s12913-021-06481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.C., Bhatta M.P., Smith J.S., et al. Assessment of high-risk human papillomavirus infections using clinician- and self-collected cervical sampling methods in rural women from far western Nepal. PloS one. 2014;9(6):e101255. doi: 10.1371/journal.pone.0101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri R.B., Mishra S.R., Khanal V. Female Community Health Volunteers in Community-Based Health Programs of Nepal: Future Perspective. Front. Public Health. 2017;5:181. doi: 10.3389/fpubh.2017.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killip S., Mahfoud Z., Pearce K. What is an intracluster correlation coefficient? Crucial concepts for primary care researchers. Ann. Family Med. 2004;2(3):204–208. doi: 10.1370/afm.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A., Finkelstein E.A., Kallestrup P., et al. Cost-effectiveness and budget impact of the community-based management of hypertension in Nepal study (COBIN): a retrospective analysis. Lancet. Global Health. 2019;7(10):e1367–e1374. doi: 10.1016/S2214-109X(19)30338-9. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Sanders D., 2007 Community health workers: What do we know about them? The state of the evidence on programmes, activities, costs and impact on health outcomes of using community health workers. Accessed 5 April 2022. Available from: https://www.who.int/hrh/documents/community_health_workers.pdf.
- Mary B., D'Sa J.L. Evaluation of an educational program on cervical cancer for rural women in Mangalore, Southern India. Asian Pac. J. Cancer Prev. 2014;15(16):6603–6608. doi: 10.7314/apjcp.2014.15.16.6603. [DOI] [PubMed] [Google Scholar]
- Mezei A.K., Pedersen H.N., Sy S., et al. Community-based HPV self-collection versus visual inspection with acetic acid in Uganda: a cost-effectiveness analysis of the ASPIRE trial. BMJ Open. 2018;8(6):e020484. doi: 10.1136/bmjopen-2017-020484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoH, Nepal; New ERA; & ICF, 2017. Nepal Demographic and Health Survey 2016 (NDHS). Kathmandu, Nepal: Ministry of Health (MoH), Nepal.
- Nepal Rastra Bank, 2016. Fifth household budget survey Nepal 2014/2015. Kathmandu. https://www.nrb.org.np/contents/uploads/2019/12/Study_Reports-Fifth_Household_Budget_Survey_2014-2015.pdf. Accessed 10 June 2021.
- Neupane D., Shrestha A., Mishra S.R., et al. Awareness, Prevalence, Treatment, and Control of Hypertension in Western Nepal. Am. J. Hypertens. 2017;30(9):907–913. doi: 10.1093/ajh/hpx074. [DOI] [PubMed] [Google Scholar]
- Neupane D., McLachlan C.S., Mishra S.R., et al. Effectiveness of a lifestyle intervention led by female community health volunteers versus usual care in blood pressure reduction (COBIN): an open-label, cluster-randomised trial. Lancet Global Health. 2018;6(1):e66–e73. doi: 10.1016/S2214-109X(17)30411-4. [DOI] [PubMed] [Google Scholar]
- Nutbeam D., Harris E., Wise M. 3rd edition. McGraw-Hill; Sydney, Australia: 2010. Theory in a Nutshell: A practical guide to health promotion theories. [Google Scholar]
- Panday S., Bissell P., van Teijlingen E. The contribution of female community health volunteers (FCHVs) to maternity care in Nepal: a qualitative study. BMC Health Serv. Res. 2017;17(1):623. doi: 10.1186/s12913-017-2567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit A., Gupta S., Shrestha R. Awareness and prevalence of cervical cancer screening among women in Nepal. Int. J. Gynaecol. Obstet. 2016;134(1):37–40. doi: 10.1016/j.ijgo.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R., Esmy P.O., Rajkumar R., et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- Schulz K.F., Altman D.G., Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):1745–6215. doi: 10.1371/journal.pmed.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya S., Karmacharya B.M., Afset J.E., et al. Community-Based Health Education has Positive Influence on the Attitude to Cervical Cancer Screening among Women in Rural Nepal. J. Cancer Educ. 2016;31(3):547–553. doi: 10.1007/s13187-015-0863-7. [DOI] [PubMed] [Google Scholar]
- Shojaeizadeh, D., Hashemi, S.Z., Moeini, B., et al., 2011. The Effect of Educational Program on Increasing Cervical Cancer Screening Behavior among Women in Hamadan, Iran: Applying Health Belief Model. Journal of research in health sciences. 11(1), 20–25.http://jrhs.umsha.ac.ir/index.php/JRHS/article/view/210/312. Accessed 10 June 2021. [PubMed]
- Shrestha A.D., Neupane D., Ghimire S. Community-based intervention for cervical cancer screening uptake in a semi-urban area of Pokhara Metropolitan, Nepal (COBIN-C): study protocol for a cluster-randomized controlled trial. Trials. 2021;22:94. doi: 10.1186/s13063-021-05049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha A.D., Gyawali B., Shrestha A. Knowledge, attitude, preventive practices and utilization of cervical cancer screening among women in Nepal: a community-based cross-sectional study. Eur. J. Cancer Prevent. 2022;31(1):73–81. doi: 10.1097/cej.0000000000000670. [DOI] [PubMed] [Google Scholar]
- Tavafian, S.S., 2012. Predictors of Cervical Cancer Screening: An Application of Health Belief Model. Topics on cervical cancer with an advocacy for prevention. https://www.intechopen.com/chapters/30744. Accessed 20 June 2021.
- Thapa N., Maharjan M., Petrini M.A., et al. Knowledge, attitude, practice and barriers of cervical cancer screening among women living in mid-western rural, Nepal. J. Gynecol. Oncol. 2018;29(4):e57. doi: 10.3802/jgo.2018.29.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touch S., Oh J.K. Knowledge, attitudes, and practices toward cervical cancer prevention among women in Kampong Speu Province, Cambodia. BMC Cancer. 2018;18(1):294. doi: 10.1186/s12885-018-4198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulenko K., Møgedal S., Afzal M., et al. Community health workers for universal health-care coverage: From fragmentation to synergy. Bull. World Health Organ. 2013;91:847–852. doi: 10.2471/BLT.13.118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tum, S.J., E., M. J., Clarke, M., 2013. Creating awareness and facilitating cervical and breast cancer screening uptake through the use of a Community Health Worker: a pilot intervention study. European journal of cancer care. 22, 107–116. doi:10.1111/ecc.12005. [DOI] [PubMed]
- WHO-SEARO, 2017. Cervical cancer screening and management of cervical pre-cancers: training of community health workers. World Health Organization. Regional Office for South-East Asia. https://apps.who.int/iris/handle/10665/279798. Accessed 25 June 2021.
- World Health Organization, 2018. Improving data for decision-making: a toolkit for cervical cancer prevention and control programmes. World Health Organization. https://apps.who.int/iris/handle/10665/279420. Accessed 25 June 2021.
- World Health Organization, 2018. WHO guideline on health policy and system support to optimize community health worker programmes. Geneva. https://www.who.int/publications/i/item/9789241550369. [PubMed]
- World Health Organization, 2020. Draft Global Strategy towards eliminating cervical cancer as a public health problem. https://www.who.int/publications/m/item/draft-global-strategy-towards-eliminating-cervical-cancer-as-a-public-health-problem. Accessed 25 June 2021.
- World Medical Association, 2013. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA, 310(20), 2191-2194. 10.1001/jama.2013.281053. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.