Abstract
Overexpression of evgA, a response regulator of a two-component system, increased multidrug efflux in Escherichia coli. Since overexpression of the emrKY operon, which is controlled by evgAS, could account only for deoxycholate resistance, the evgAS locus apparently controls expression of at least one other multidrug efflux operon.
A currently favored mechanism underlying antibiotic resistance involves the extrusion of compounds by an efflux pump or carrier. The most intriguing drug extrusion mechanisms are those that involve a wide variety of structurally unrelated compounds as substrates for multidrug resistance (MDR) transporters. MDR transporters are found in a variety of bacterial species (4, 9, 12, 15). Many genes coding for MDR transporters have been mapped on Escherichia coli chromosomal DNA (16), but the transport capabilities of most of them have not been established. Recently we cloned all of them and investigated their drug resistance phenotypes (K. Nishino and A. Yamaguchi, unpublished data). During the course of that study, we found that the DNA locus including a putative transporter emrKY gene system (21) and two-component signal transduction evgA and -S genes (22) conferred multidrug resistance of E. coli. The emrK and -Y genes exhibit sequence similarity to the emrA and -B genes encoding a multidrug exporter of E. coli (10), although the transport capabilities of EmrK and Y have not been elucidated. EvgA exhibits sequence similarity to the BvgA gene regulator, which controls the expression of adhesins, toxins, and other virulence factors in Bordetella pertussis (1, 18). Kato et al. reported that evgAS regulates the expression of emrKY (7).
To investigate the transport capabilities of EmrK and -Y, we amplified emrK and -Y genes with peripheral evgA and -S genes from the chromosomal DNA of E. coli W3104 by PCR using primers containing restriction enzyme SphI and BamHI sites, respectively. The DNA fragment was ligated into the SphI and BamHI sites of pUC118 to produce pUCSAKY. E. coli KAM3 (11), a derivative of K-12 that lacks multidrug transporter genes acrA and -B, was used for drug susceptibility testing. Resistance to various compounds was tested by plating diluted samples of overnight cultures on YT (17) agar containing various concentrations of compounds. E. coli KAM3 showed hypersensitivity to various drugs and toxic compounds (Table 1). E. coli KAM3 harboring pUCSAKY exhibited elevated resistance to several drugs and toxic compounds, including doxorubicin, novobiocin, erythromycin, phosphomycin, crystal violet, rhodamine 6G, ethidium, acriflavine, methylviologen, benzalkonium, and sodium dodecyl sulfate (SDS) (Table 1). To identify an MDR gene(s), several combinations of these four genes were individually subcloned. The resulting pUCAKY carries complete evgA and emrKY but not evgS. pUCKY carries emrKY but not evgAS. pUCY carries only complete emrY. pUCAK carries complete evgA and emrK but neither evgS nor emrY. pUCA carries only complete evgA, i.e., neither evgS nor emrKY. As a result, all plasmids that conferred multidrug resistance were found to contain evgA. pUCA, which contains only evgA, also exhibited multidrug resistance comparable to that of pUCSAKY. These observations indicated that evgA seems to be solely responsible for the multidrug resistance of the evgSA and emrKY loci. Thus, the results presented here indicate that the bacterial two-component signal transduction system may regulate multidrug resistance. Neither pUCKY nor pUCY conferred resistance to any compounds. Since emrKY might not be expressed from the native promoter, we cloned emrKY into the pQE30 expression vector to produce histidine-tagged proteins under the control of the T5 promoter (pQE30emrKY). Protein expression could be detected with antipolyhistidine antibodies (data not shown). pQE30emrKY conferred to E. coli KAM3 cells high-level resistance only to deoxycholate, not to the other compounds tested (Table 2). The deoxycholate resistance of EmrKY is in agreement with previous observations (7).
TABLE 1.
Drug resistance of E. coli KAM3 cells harboring plasmids carrying evgSA and/or emrKY
| Drug | MIC (μg/ml) for:
|
||||||
|---|---|---|---|---|---|---|---|
| KAM3 (host) | KAM3 harboring:
|
||||||
| pUCSAKY | pUCAKY | pUCKY | pUCY | pUCAK | pUCA | ||
| Doxorubicin | 3.13 | 200 | 200 | 3.13 | 3.13 | 200 | 200 |
| Novobiocin | 0.78 | 1.56 | 1.56 | 0.78 | 0.78 | 1.56 | 1.56 |
| Erythromycin | 3.13 | 12.5 | 12.5 | 3.13 | 3.13 | 12.5 | 12.5 |
| Phosphomycin | 1.56 | 3.13 | 3.13 | 1.56 | 1.56 | 3.13 | 3.13 |
| Crystal violet | 1.56 | 6.25 | 6.25 | 1.56 | 1.56 | 6.25 | 12.5 |
| Rhodamine 6G | 6.25 | 50 | 50 | 6.25 | 6.25 | 50 | 50 |
| Ethidium | 12.5 | 25 | 25 | 12.5 | 12.5 | 25 | 25 |
| Acriflavine | 25 | 50 | 50 | 25 | 25 | 50 | 50 |
| Methylviologen | 100 | 200 | 200 | 100 | 100 | 200 | 200 |
| Benzalkonium | 3.13 | 25 | 25 | 3.13 | 3.13 | 25 | 25 |
| SDS | 100 | 200 | 200 | 100 | 100 | 300 | 300 |
| Deoxycholate | 1,250 | 5,000 | 10,000 | 1,250 | 1,250 | >40,000 | >40,000 |
TABLE 2.
Drug resistance of E. coli KAM3 cells harboring a plasmid carrying emrKY under control of the T5 promoter
| Drug | MIC (μg/ml) for:
|
||
|---|---|---|---|
| KAM3 | KAM3/pQE30emrKY
|
||
| +IPTG | −IPTG | ||
| Doxorubicin | 3.13 | 3.13 | 3.13 |
| Erythromycin | 3.13 | 3.13 | 3.13 |
| Tetracycline | 0.78 | 0.78 | 0.78 |
| Crystal violet | 0.78 | 0.78 | 0.78 |
| Rhodamine 6G | 6.25 | 6.25 | 6.25 |
| Benzalkonium | 3.13 | 3.13 | 3.13 |
| SDS | 100 | 100 | 100 |
| Deoxycholate | 1,250 | 10,000 | 1,250 |
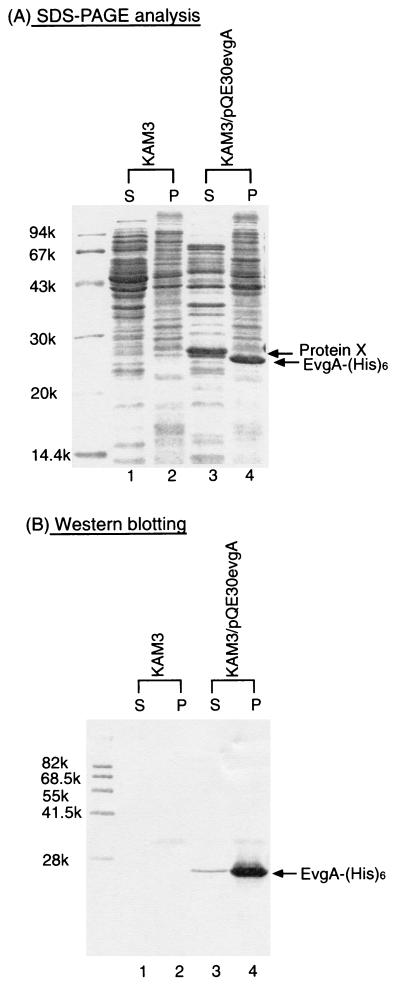
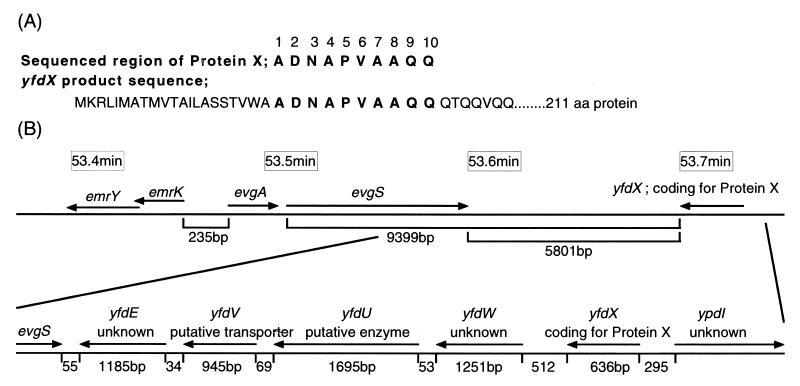
Since emrKY did not confer evgA-induced multidrug resistance, there was a possibility that evgA regulates other MDR system(s). When histidine-tagged EvgA expression was induced by isopropyl-β-d-thiogalactopyranoside in E. coli cells harboring pQE30evgA, a dense band corresponding to 23.5 kDa was observed on SDS-polyacrylamide gel electrophoresis (PAGE) of the membrane fraction (Fig. 1A, lane 4). This band was also detected on Western blotting with antipolyhistidine antibodies (Fig. 1B, lane 4). Thus, it is clear that this band represents EvgA-His6. This protein was transferred to a supernatant on 5 M urea washing (data not shown), indicating that EvgA is a peripheral membrane protein. For the cytoplasmic fraction of E. coli KAM3/pQE30evgA cells (Fig. 1A and B, lanes 3), only a very faint band at 23.5 kDa was observed. On the other hand, a very dense band at 25 kDa was observed for the cytoplasmic fraction. Since this band was not detected on Western blotting with antipolyhistidine antibodies, it is clear that this protein is different from EvgA. This protein, named protein X, was induced by EvgA because E. coli KAM3 cells did not yield this protein band (Fig. 1A, lane 1). Protein X was eluted from the SDS-polyacrylamide gels, and its partial 10-amino-acid sequence was determined with a peptide sequencer (Fig. 2A). The resulting sequence, ADNAPVAAQQ, was completely consistent with the sequence of residues 22 to 31 of a putative protein encoded by the yfdX open reading frame (ORF). This ORF encodes a protein composed of 211 amino acids, but its function is not known. The yfdX ORF is close to the evgA and -S loci, and there are four ORFs (yfdE, yfdV, yfdU, and yfdW) between evgS and yfdX (Fig. 2B). The functions of these four ORFs are also not known. We cloned the yfdX ORF into the pQE70 expression vector. E. coli KAM3 cells harboring pQE70yfdX exhibited high level expression of 26-kDa histidine-tagged protein X, as observed on Coomassie brilliant blue staining (data not shown). However, protein X did not confer resistance to doxorubicin (data not shown). Thus, it seems that protein X represents an EvgSA-induced signal transduction pathway other than drug resistance. The ORFs, yfdE, yfdV, yfdU, and yfdW, between evgS and yfdX were also individually cloned into pUC118 under the control of the corresponding native promoters. None of the E. coli KAM3 cells harboring pUCyfdE, pUCyfdV, pUCyfdU, or pUCyfdW exhibited resistance to any compounds tested (data not shown), although the possibility that these genes were not expressed from their native promoters cannot be excluded.
FIG. 1.
SDS-PAGE and Western blotting analysis of cells expressing EvgA and protein X. Membrane and cytoplasmic fractions of E. coli KAM3 and KAM3/pQE30evgA were prepared. The membrane (P) and cytoplasmic (S) fractions were then subjected to SDS-PAGE on 15% polyacrylamide gels. After electrophoresis, the gels were stained with Coomassie brilliant blue (A). Then proteins were electroblotted onto poly-vinylidene difluoride membranes. Histidine-tagged EvgA was detected with antipolyhistidine antibodies (B).
FIG. 2.
Amino acid sequences of protein X and the corresponding ORF on the E. coli chromosome. EvgA-induced protein X was eluted from an SDS polyacrylamide gel, and then its amino acid sequence was determined with a peptide sequencer. (A) The 10 sequenced amino acid residues are depicted as bold capital letters. The corresponding sequence encoded by the yfdX ORF is shown in the lower row. (B) Series of ORFs close to evgAS and yfdX in E. coli chromosomal DNA.
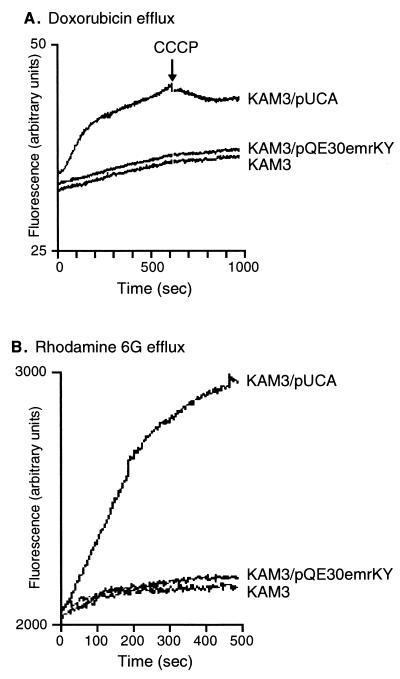
Doxorubicin and rhodamine 6G were chosen as representative of drugs and toxic dyes to which E. coli KAM3 cells became resistant with a plasmid carrying the evgA gene. These compounds can be detected by their fluorescence. To determine whether the evgA-induced multidrug resistance is due to multidrug efflux of these compounds from the cells, we measured the efflux of these compounds from cells preloaded with these compounds. Exponential cultures of E. coli KAM3, KAM3/pUCA, or KAM3/pQE30emrKY were harvested and washed twice with 100 mM potassium phosphate buffer (pH 7.5) containing 5 mM MgSO4. For maximal accumulation of the fluorophore, the cells (optical density at 600 nm of 20 for doxorubicin or 1.0 for rhodamine 6G) were incubated with 1 μM rhodamine 6G or 11.5 μM doxorubicin and 40 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP) at 37°C for 1 h. The cells were then centrifuged, resuspended in the same medium containing 25 mM glucose to energize them, and subjected to fluorescence measurement. As shown in Fig. 3, rapid efflux of doxorubicin and rhodamine 6G from E. coli KAM3/pUCA cells was observed as an increase in the fluorescence. On the other hand, no significant efflux was observed from KAM3 and KAM3/emrKY cells. Plasmid pQE30emrKY did not affect the rate of efflux, indicating that EmrKY exported neither doxorubicin nor rhodamine 6G. The addition of the proton conductor CCCP inhibited the doxorubicin efflux from E. coli KAM3/pUCA cells (Fig. 3A), indicating that the active efflux is driven by a proton motive force. These observations clearly indicate that EvgA induces a doxorubicin and rhodamine 6G active efflux system(s) which is different from EmrKY.
FIG. 3.
Active efflux of doxorubicin (A) and rhodamine 6G (B) from E. coli KAM3 cells harboring no plasmid, pUCA, or pQE30emrKY. Energy-starved cells of E. coli KAM3, KAM3/pUCA, and KAM3/pQE30emrKY cells were loaded with doxorubicin (A) or rhodamine 6G (B). The fluorescence of the compounds was continuously monitored with a Hitachi model F-2000 fluorescence spectrophotometer. Doxorubicin transport was measured with excitation at 478 nm and emission at 591 nm. Rhodamine 6G transport was measured with excitation at 529 nm and emission at 553 nm.
Two-component systems are general signal transduction pathways in prokaryotic organisms responding to changes in environmental conditions (5, 14). They have also been found in some eukaryotes (8). A typical two-component system consists of two types of signal transducers, a sensory kinase and a response regulator (20). The sensory kinase monitors some environmental parameters and accordingly modulates the functions of the response regulator through its phosphorylation (6, 19). The response regulator mediates gene expression and/or cell behavior. Recently, it was found that a two-component system regulates bacterial drug resistance. VncRS in Streptococcus pneumoniae (13) and VanRS in enterococci (2) regulate vancomycin resistance. It was subsequently reported that another two-component system, ArIRS, in Staphylococcus aureus regulates the expression of multidrug transporter NorA (3). In this study, we found that overexpression of the response regulator evgA of the two-component signal transduction system modulates multidrug resistance conferred by MDR transporters in E. coli. The findings in this study indicate that two-component system-controlled multidrug resistance via multidrug exporter(s) may be another general way for bacteria to acquire multidrug resistance.
REFERENCES
- 1.Arico B, Scarlato V, Monack D M, Falkow S, Rappuoli R. Structural and genetic analysis of the bvg locus in Bordetella species. Mol Microbiol. 1991;5:2481–2491. doi: 10.1111/j.1365-2958.1991.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 2.Arthur M, Molinas C, Courvalin P. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1992;174:2582–2591. doi: 10.1128/jb.174.8.2582-2591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fournier B, Aras R, Hooper D C. Expression of the multidrug resistance transporter NorA from Staphylococcus aureus is modified by a two-component regulatory system. J Bacteriol. 2000;182:664–671. doi: 10.1128/jb.182.3.664-671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George A M. Multidrug resistance in enteric and other gram-negative bacteria. FEMS Microbiol Lett. 1996;139:1–10. doi: 10.1111/j.1574-6968.1996.tb08172.x. [DOI] [PubMed] [Google Scholar]
- 5.Hock J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. [Google Scholar]
- 6.Inouye M. His-Asp phosphorelay: two components or more? Cell. 1996;85:13–14. [Google Scholar]
- 7.Kato A, Ohnishi H, Yamamoto K, Furuta E, Tanabe H, Utsumi R. Transcription of emrKY is regulated by the EvgA-EvgS two-component system in Escherichia coli K-12. Biosci Biotechnol Biochem. 2000;64:1203–1209. doi: 10.1271/bbb.64.1203. [DOI] [PubMed] [Google Scholar]
- 8.Kennelly P J, Potts M. Fancy meeting you here! A fresh look at “prokaryotic” protein phosphorylation. J Bacteriol. 1996;178:4759–4764. doi: 10.1128/jb.178.16.4759-4764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis K. Multidrug resistance pumps in bacteria: variations on a theme Trends Biochem. Sci. 1994;19:119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 10.Lomovskaya O, Lewis K. Emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci USA. 1992;89:8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother. 1998;42:1778–1782. doi: 10.1128/aac.42.7.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson J S. Signal transduction schemes of bacteria. Cell. 1993;73:857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- 15.Paulsen I T, Brown M H, Skurrav R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulsen I T, Sliwinski M K, Saier M H., Jr Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J Mol Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 18.Stibitz S, Yang M S. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–4296. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stock J B, Surette M G, Levit M, Stock A M. Two-component signal transduction systems: structure-function relationships and mechanisms of catalysis. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 25–51. [Google Scholar]
- 21.Tanabe H, Yamasaki K, Furue M, Yamamoto K, Kato A, Yamamoto M, Yoshioka S, Tagami H, Aiba H, Utsumi R. Growth phase-dependent transcription of emrKY, a homolog of multidrug efflux emrAB genes of Escherichia coli, is induced by tetracycline. J Gen Appl Microbiol. 1997;43:257–263. doi: 10.2323/jgam.43.257. [DOI] [PubMed] [Google Scholar]
- 22.Utsumi R, Katayama S, Taniguchi M, Horie T, Ikeda M, Igaki S, Nakagawa H, Miwa A, Tanabe H, Noda M. Newly identified genes involved in the signal transduction of Escherichia coli K-12. Gene. 1994;140:73–77. doi: 10.1016/0378-1119(94)90733-1. [DOI] [PubMed] [Google Scholar]