Abstract
Objective:
To examine the role of physical function impairments on the change in urinary incontinence symptoms after pelvic floor muscle training in older women.
Methods:
This is a prospective cohort study of 70 community-dwelling participants, older than 70 years, with at least moderate incontinence symptoms. A comprehensive pelvic floor and physical function assessment was done at baseline. Individualized pelvic floor muscle training prescriptions with behavioral management strategies to reduce incontinence episodes were provided for 12 weeks. Baseline physical function was determined using the Short Physical Performance Battery: total score ≤9/12 defined impaired physical function and scores >9 defined normal physical function. A 3-day bladder diary established daily incontinence episodes. The between group difference in the change in number of urinary incontinence episodes from baseline to 6 weeks was our primary outcome. Descriptive analyses compared important demographic and clinical characteristics. Longitudinal mixed model linear regression analyses determined the change in incontinence episodes and estimates of improvement based on the presence of impaired physical function and adjusted for age, race, and body mass index (BMI).
Results:
Participants’ mean ± SD age was 76.9 ± 5.4 years and 15.7% were African American with no significant differences in age or race between groups. Participants with impaired physical function had higher mean ± SD BMI (33.6 ± 14.5 vs 27.4 ± 5.8 kg/m2; p=0.03) and more baseline incontinence episodes (4.5 ± 2.9 vs. 2.7 ± 2.1 episodes per day; p=0.005) than in women without functional impairment. After 6 weeks of pelvic floor exercises, the change in number of incontinence episodes per day was not different between participants with physical functional impairment compared to women with normal physical function (mean [95%CI], −1.2 [−2.0,−0.5] vs −0.4 [−1.1, 0.3], p=0.31). Overall, after 12 weeks of pelvic floor muscle training, complete satisfaction with incontinence symptom improvement was low for both groups (41.8% with physical function impairments vs. 44.8% with normal physical function; p=0.90).
Conclusions:
Behavioral therapy including pelvic floor muscle training may not significantly decrease urinary incontinence symptoms to a degree that is satisfactory in women older than 70 years seeking treatment for urinary incontinence, regardless of the presence of physical function impairments.
Precis:
Pelvic floor muscle training and behavioral therapy may not significantly reduce urinary incontinence symptoms in women older than 70 years.
INTRODUCTION
Urinary incontinence (UI) is a prevalent pelvic floor condition presenting in up to 50% of adult women. However, with aging beyond 70 years, UI becomes heterogeneous, often evolving into a multi-factorial geriatric syndrome. Geriatric syndromes are defined as prevalent conditions present in older adults with shared risk factors such as physical function impairments, mobility disability, and cognitive decline.[1] Urinary incontinence and impaired physical function are inter-related geriatric conditions that result, in part, from skeletal muscle dysfunction with aging. Growing evidence supports that physical function impairment is a cause and consequence of UI in aging adults.[1–3]
Pelvic floor weakness is a central cause of UI. Pelvic floor muscles have slow- and fast-twitch fibers that have both tonic and reflexive function during routine daily activities. However, voluntary contractions are required for strengthening and training.[4] In the 1940s, pelvic floor muscle training developed to treat UI in young post-partum women under the premise that improved skeletal muscle bulk and functional efficiency of the pelvic floor and urethra sphincter would improve urethral closure and bladder support. Currently, pelvic floor muscle training focuses muscle strengthening, endurance, and coordination summarized in a patient specific exercise program. Pelvic floor muscle training is the crux of first-line therapy for treatment of UI in women, regardless of age, based on data suggesting significant reductions in daily incontinence episodes after 6 or 12 weeks of training. [5] However, as women age beyond 70 years and develop UI symptoms, there is higher risk of concomitant development of skeletal muscle weakness and subsequent impairments in physical performance to include mobility disability and falls.[6] Impairments in physical function may decrease enrollment into clinical trials as there is increased concern for adverse events.[7] Further, women older than 70 years often have more severe and refractory UI symptoms.[8] To date, health care professionals treating geriatric urinary incontinence have not considered the potential broader impact of aging-related changes in skeletal muscle health on the treatment of UI.
Healthy skeletal muscle structure and function are imperative to success of pelvic floor muscle training. With aging beyond 70 years, up to 60% of older women will live with physical function impairments and mobility disability; consequently, they may lack sufficient skeletal muscle function to allow for successful pelvic floor muscle training.[6, 9] We hypothesized that presence of impaired physical function may decrease the efficacy of pelvic floor muscle training in older women with UI. The objective of this prospective study was to examine the effectiveness of behavioral and pelvic floor muscle training in older women with moderate-to-severe incontinence concomitant with physical function impairments.
METHODS
We conducted a prospective cohort study of 70 community-dwelling older women with a diagnosis of UI from January 1, 2017 to June 1, 2020 and who were interested in treatment for their symptoms. Potential participants were identified using and ICD-10 code search for a UI diagnosis within 6 months of the query [R32 (unspecified UI), N39.81 (functional UI), N39.41 (urge UI), N39.46 (mixed UI), and N39.3 (stress UI)]. Fourteen-hundred and seventy-three women were targeted through introductory letters that included the 6-item Questionnaire for Urinary Incontinence Diagnosis (QUID); a valid measure to establish urinary incontinence diagnosis, type, and severity.[10] Women who desired treatment for their UI symptoms underwent pre-screening by telephone. Eligible participants were: age ≥ 70 years with a diagnosis of at least moderate incontinence symptoms based on the QUID subscale score for stress ≥4; urge ≥ 6; or total ≥ 10 [10]; willing and able to be compliant with pelvic floor muscle training and log adherence; and willing and able to undergo an extensive physical function evaluation. Participants were excluded if they had any of the following: recent history of a surgical intervention for incontinence or hysterectomy; pelvic organ prolapse beyond the hymenal ring; neurogenic overactive bladder; measured post void residual volume greater than 150 ml (measured by bladder scan); inability to ambulate without single point cane; and those with current treatment for dementia. This protocol was approved by our institutional review board and written informed consent was obtained. All study visits were conducted at the Geriatric Clinical Research Unit in our Claude D. Pepper Older Americans Independence Center.
At baseline, clinical characteristics were assessed using validated measures. Race was documented to determine its influence on urinary incontinence symptoms. Overall health status and 10-year mortality risk was assessed using the Charlson Comorbidity Index. [11] The Montreal Cognitive Assessment (MoCA) was used to assess cognitive function.[12] The MoCA scoring ranges from 0–30; mild cognitive impairment is defined by scores between 19–25. Emotional health was assessed using the Center for Epidemiologic Studies Depression (CESD-10), a validated depression-screening tool that assesses depressive symptoms in the past week. A cut-off score of ≥10 represents significant depressive symptoms. [13]
Pelvic floor symptom assessment was performed through clinical interview and validated questionnaires at baseline, 6, and 12 weeks. A 3-day bladder diary established daily voiding and incontinence episodes.[14] The mean total incontinence episodes was the sum of the total number of stress and urgency incontinence episodes over a 24-hour period and averaged from the 3-day bladder diary. Urinary incontinence symptom impact on daily life was assessed using the Urinary Distress Inventory (UDI-6); scores greater than 33.3 indicate high distress from UI symptoms.[15, 16] Bowel habits were assessed using the Bristol stool chart.
At baseline, each participant underwent a supervised pelvic floor muscle assessment and training session performed by a certified pelvic floor physical therapist or a Urogynecologist (PI) with extensive formal training in pelvic floor physical therapy. Each participant was taught how to effectively contract their pelvic floor through digital vaginal exam and pelvic floor muscle function was assessed objectively using the PERFECT scheme. PERFECT is an acronym used to ensure assessment of the main components of pelvic floor contractility[17]: P= power (a measure of maximal strength using vaginal digital exam or manometric perineometer), E=endurance (how long can women hold the contraction up to a max of 10 seconds), R=repetitions (how many maximal hold strength repetitions can women sustain up to 10 rep), F=fast contractions (how many fast maximal contractions can be repeated), ECT = every contraction timed (how long do women hold the fast contractions). Pelvic floor power was determined objectively using the Peritron® perineometer. At week 2, all participants returned for repeat pelvic floor assessment and to ensure their confidence in the execution of pelvic floor muscle.
At baseline, each participant was assigned an individualized behavioral therapy and pelvic floor muscle training prescription that included a minimum of 3-sets of daily pelvic floor exercises with each set followed by rapid contractions for 12 weeks. Urgency and stress suppression strategies were included as appropriate to isolate the pelvic floor through rapid contractions when urinary urgency occurred, or a stress-provoked UI episode was anticipated. Behavioral therapy included recommendations for fluid and bowel management as appropriate.
The Short Physical Performance Battery was used to determine lower extremity physical function. It is a standard and robust predictor for disability that includes progressively more challenging standing balance tasks held for 10 seconds each (side-by-side, tandem, and semi-tandem), the faster of two 4-m courses at usual pace, and time to complete five repeated chair stands. [18, 19] Each of the three performance measures was assigned a score ranging from 0 (inability to perform the task) to 4 (the highest level of performance) and summed to create an SPPB score ranging from 0 to 12 (best). A low performance score (≤ 9) is a strong risk factor of decreased mobility and activities of daily living (ADL) disability in nondisabled older adults and was used in this study to define impaired physical function.[20]
Participants were asked to perform leg-extensions at 60 degrees per second on the isokinetic dynamometer (Biodex®) as an objective measure of maximal isokinetic lower extremity strength.[21] A whole-body DEXA scan was performed to determine appendicular lean muscle mass; the lean muscle mass index was used as an indicator for sarcopenia based on validated cut-offs.[22, 23]
Our primary outcome was the between group difference in the change in urinary incontinence episodes from baseline to 6 weeks. In clinical practice, it is common to perform an assessment of urinary incontinence outcomes after 6 weeks of PFM training.[24] Based on physical therapy and exercise science literature, 8–12 repetitions of 3–4 sets over 6 weeks typically increases strength and efficiency of muscle recruitment.[25] Therefore, we used this time point to strengthen the pragmatic impact of our observations. Secondarily, we examined the change in strength of pelvic floor muscles, the estimated global impression of improvement, and satisfaction rates at 6 and 12 weeks.
Baseline means and standard deviations for continuous variables and frequencies and percentages for categorical variables were used to describe the sample by physical function status. Tests of difference utilized Student’s t-test, Wilcoxon rank sum non-parametric test, or Chi-square for continuous and categorical measures, respectively. Mixed effect model analyses were used to assess for change in urinary incontinence episodes, overall satisfaction, global impression of improvement, and estimate of improvement based on the presence of physical function impairment adjusted for age, race, and body mass index (BMI). Generalized estimating equations models were used to compare overall satisfaction and global impression of improvement between women with and without physical function impairment adjusted for age, race, and BMI. We estimated that a sample size of 60 women (30 per physical function group) would provide 82% power at a 0.05 significance level applying a 2-tailed test to detect a decrease of at least one urinary incontinence episode per day at 6 weeks.
RESULTS
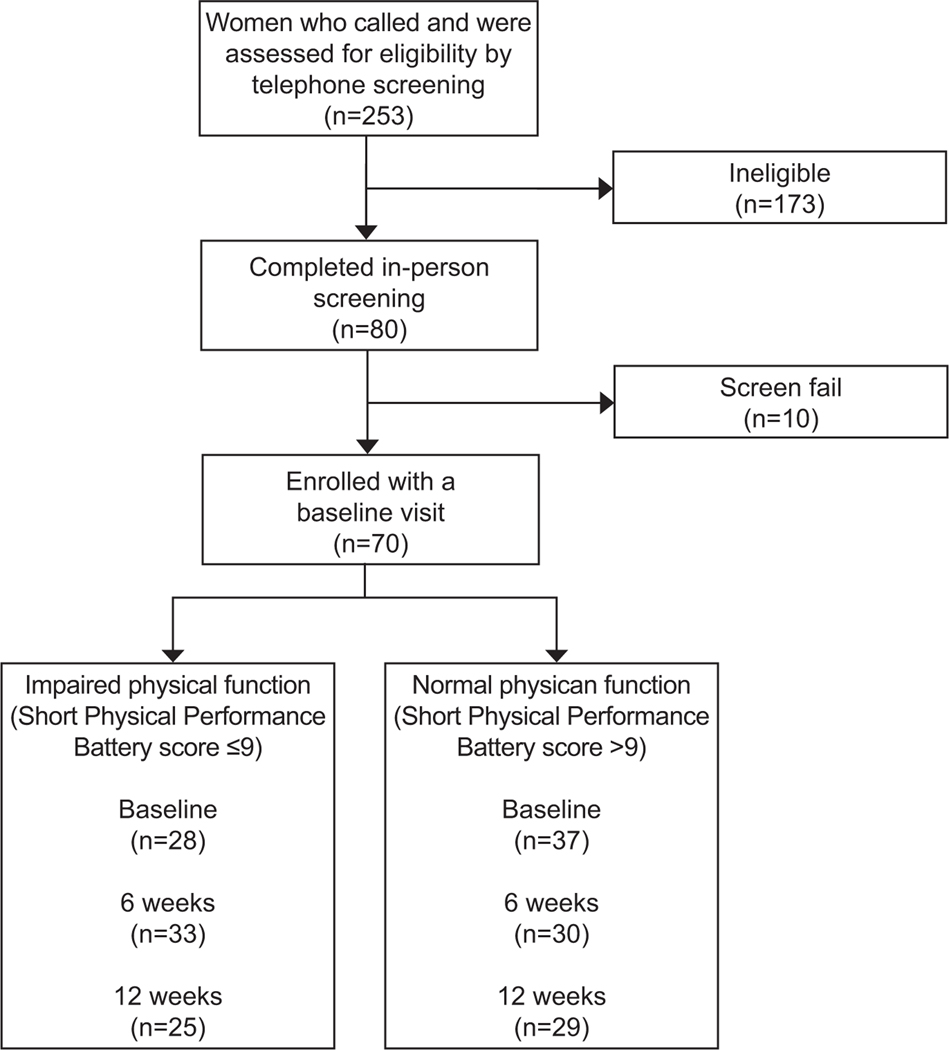
Two-hundred fifty-three women were phone screened between January 2018 and January 2020. One-hundred and seventy-three individuals were ineligible on phone screening leaving 80 individuals who completed in-person baseline screening. Ten individuals screen-failed at the baseline visit leaving 70 participants included in our analysis (Figure 1). At baseline, 33 participants had impaired physical function (low performance score of ≤ 9) and 37 had normal physical function (Table 1). The mean age was 76.9 ± 5.4 years and our sample was 15.7% African American. There were no differences in mean age or race between physical function groups at baseline. Participants with physical function impairment had a higher mean BMI than those without (P=0.032). Ten-year survival based on the mean ± SD Charlson Comorbidity Index of 4.5 ± 0.8 was approximately 50%. Depressive symptoms were uncommon. Mean MoCA scores for the cohort was 24.6 ± 3.0, indicating the presence of mild cognitive impairment in the 50% of participants, with no differences based on physical function status. (Table 1)
Figure 1.
Flow diagram of participant identification, recruitment, and enrollment stratified based on baseline physical function status.
Table 1.
Demographic and clinical characteristics of the cohort based on physical function group
| Overall | Impaired N=33 |
Normal N=37 |
P-value | |
|---|---|---|---|---|
| Age, mean ± SD, years | 76.9 ± 5.4 | 77.9 ± 6.4 | 76.0 ± 4.1 | 0.14 |
| Race, No. (%) | 0.59 | |||
| African American | 11 (16%) | 6 (18.%) | 5 (14%) | |
| White | 59 (84%) | 27 (82%) | 32 (87%) | |
| Vaginal parity, median (IQR) | 2.0 (1.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (1.0, 3.0) | 0.16 |
| Body Mass Index (BMI), mean ± SD, kg/m2 | 30.4 ± 11.2 | 33.6 ± 14.5 | 27.4 ± 5.8 | 0.032 |
| Total Medications, mean ± SD | 6.4 ± 3.5 | 7.2 ± 3.3 | 5.7 ± 3.6 | 0.08 |
| Charlson Comorbidity Index, mean ± SD | 4.5 ± 0.8 | 4.6 ± 0.9 | 4.4 ± 0.7 | 0.14 |
| CESD-10 total score category, No. (%) | 0.58 | |||
| CESD-10<10 | 63 (90%) | 29 (88%) | 34 (92%) | |
| CESD-10≥10 | 7 (10%) | 4 (12%) | 3 ( 8%) | |
| MoCA Score, mean ± SD | 24.6 ± 3.0 | 24.4 ± 2.6 | 24.7 ± 3.3 | 0.63 |
| Bristol Stool, No. (%) | 0.92 | |||
| Type 1–3 | 14 (20%) | 6 (18%) | 8 (22%) | |
| Type 4 | 49 (71%) | 24 (73%) | 25 (69%) | |
| Type 5–7 | 6 ( 9%) | 3 ( 9%) | 3 ( 8%) |
Charlson Comorbidity Index indicates the role of multiple morbidities on lifespan/risk of death within 10 years
CES-D, The Center for Epidemiological Studies - Depression
MoCA, Montreal Cognitive Assessment detects mild cognitive impairment and Alzheimer’s disease in adults
IQR is the interquartile ratio
SD is the standard deviation
When we examined pelvic floor and skeletal muscle outcomes based on baseline physical function status (Table 2), we observed that participants with impaired physical function had significantly more urinary incontinence episodes compared to women with normal physical function [4.5 ± 2.9 vs. 2.7 ± 2.1 UI episodes per day, p=0.005]. Urgency urinary incontinence was predominant in participants with physical function impairments compared to those without (p=0.037). Further, skeletal muscle strength was globally weaker among participants with impaired physical function. Compared to participants with normal physical function, those with impaired physical function had significantly weaker pelvic floor maximal strength measured with the perineometer (p=0.007), lower extremity strength as indicated by Biodex scores (p=0.05), and lower appendicular lean muscle mass (p=0.007).
Table 2.
Examining associations between pelvic floor and skeletal muscle outcomes based on baseline physical function status
| Overall | Impaired | Normal | P-value | |
|---|---|---|---|---|
| Pelvic floor outcomes | ||||
| 3 day voiding diary, mean±SD | ||||
| Day time voiding episodes, mean ± SD, per day | 7.1 ± 2.9 | 7.3 ± 3.2 | 6.9 ± 2.7 | 0.64 |
| Night time voiding episodes, mean ± SD, per day | 2.5 ± 1.5 | 2.5 ± 1.8 | 2.5 ± 1.2 | 0.94 |
| Total incontinence episodes, mean ± SD, per day | 3.6 ± 2.7 | 4.5 ± 2.9 | 2.7 ± 2.1 | 0.005 |
| Questionnaire for Urinary Incontinence Diagnosis | ||||
| Total Score, mean ± SD | 15.4 ± 4.8 | 15.6 ± 5.6 | 15.2 ± 4.0 | 0.76 |
| Stress Score, mean ± SD | 6.5 ± 3.5 | 6.8 ± 3.5 | 6.3 ± 3.5 | 0.53 |
| Urgency Score, mean ± SD | 9.1 ± 2.5 | 9.3 ± 3.2 | 8.9 ± 1.9 | 0.59 |
| Urinary Distress Index, mean ± SD | 16.7 ± 4.9 | 16.2 ± 4.5 | 17.1 ± 5.3 | 0.42 |
| Pelvic Floor Impact Questionnaire Score, mean ± SD | 38.2 ± 25.6 | 40.9 ± 20.2 | 35.7 ± 29.6 | 0.39 |
|
| ||||
| Skeletal muscle health outcomes | ||||
| PERFECT pelvic floor muscle assessment | ||||
| Objective strength (perineometer), mean ± SD | 24.4 ± 15.5 | 19.2 ± 13.3 | 29.3 ± 16.0 | 0.007 |
| Digital strength (Brink scale), mean ± SD | 3.0 ± 1.0 | 2.8 ± 0.9 | 3.1 ± 1.0 | 0.24 |
| Endurance, mean ± SD | 4.6 ± 2.0 | 4.4 ± 2.0 | 4.7 ± 1.9 | 0.49 |
| Repetitions of max power, mean ± SD | 9.0 ± 1.9 | 9.1 ± 1.9 | 8.8 ± 1.9 | 0.53 |
| Number of fast contractions in 10 seconds, mean ± SD | 4.5 ± 1.6 | 4.3 ± 1.7 | 4.7 ± 1.5 | 0.32 |
| Appendicular Lean Mass Adjusted for BMI, mean ± SD, m2 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.007 |
| Lower extremity strength (Biodex assessment), mean ± SD, kg | 72.3 ± 20.6 | 66.2 ± 23.4 | 77.0± 17.2 | 0.05 |
SD represents standard deviation
PERFECT acronym represents the standardized pelvic floor assessment previously defined as Power, Endurance, Repetitions, Fast contractions with Every Contraction Timed
At 6 weeks, we retained 28/33 (85%) of participants with physical function impairments and 30/37 (81%) with normal physical function (Figure 1). After 6 weeks of pelvic floor muscle training and behavioral therapy, there was no difference in the between group decrease in number of urinary incontinence episodes between participants with impaired physical function (mean [95%CI]: −1.2[−2.0,−0.5] UI episodes per day] or normal physical function (−0.4[−1.1, 0.3]; p=0.31);. However, within the cohort of participants with impaired physical function, incontinence episodes did decline at 6 weeks (mean [95%CI], −1.2 [−2.0,−0.5], p= 0.002). After 12 weeks of pelvic floor muscle training and behavioral therapy, we retained 25/33 (76%) of participants with impaired physical function and 29/37 (78%) with normal physical function. Observations in change in incontinence episodes remained consistent. Women in both groups had a sustained decrease in their incontinence episodes from baseline, but there was no difference in the between group change (Table 3; p = 0.15).
Table 3.
Examining the effect of impaired physical performance on reduction of urinary incontinence episodes after 6 and 12 weeks of pelvic floor physical therapy from mixed effect models adjusting for age, BMI, and race.
| Baseline | 6 Week Follow-Up Visit | 12 Week Follow-Up Visit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Impaired1 | Normal2 | P-value4 | Impaired | Normal | P-value4 | Impaired | Normal | P-value4 | ||||||
| LSM±SE3 | LSM±SE | Week 6 LSM±SE | Change from Baseline LSM (95% CI) | Week 6 LSM±SE | Change from Baseline LSM (95% CI) | Week 12 LSM±SE | Change from Baseline LSM±SE | Week 12 LSM±SE | Change from Baseline LSM (95% CI) | |||||
| Primary Outcomes | ||||||||||||||
| 3 day bladder diary | Incontinence episodes | 4.3±0.6 | 2.7±0.6 | 0.028 | 3.4±0.6 | −1.2 (−2.0, −0.5) | 2.3±0.6 | −0.4 (−1.1, 0.3) | 0.31 | 3.5±0.6 | −0.8 (−1.6, 0.0) | 2.4±0.6 | −0.2 (−1.0, 0.5) | 0.15 |
| Urgency with voids | 1.1±0.3 | 1.9±0.3 | 0.037 | 1.4±0.3 | 0.3 (−0.3, 0.8) | 1.3±0.3 | −0.6 (−1.1, −0.1) | 0.90 | 0.8±0.3 | −0.3 (−0.8, 0.3) | 1.3±0.3 | −0.6 (−1.1, −0.1) | 0.28 | |
| Secondary Outcomes | ||||||||||||||
| UDI-6 Score | 15.7±1.1 | 17.4±1.1 | 0.20 | 15.0±1.1 | −0.6 (−2.4, 1.1) | 14.6±1.1 | −2.9 (−4.5, −1.2) | 0.75 | 14.6±1.2 | −1.1 (−2.9, 0.8) | 13.7±1.1 | −3.7 (−5.4, −2.1) | 0.55 | |
| Peak Pelvic Floor Muscle Strength | 21.1±3.3 | 27.5±3.3 | 0.12 | 23.3±3.3 | 2.2 (−1.1, 5.6) | 26.8±3.4 | −0.8 (−4.1, 2.6) | 0.42 | 22.9±3.4 | 1.9 (−1.7, 5.4) | 26.7±3.4 | −0.9 (−4.4, 2.7) | 0.39 | |
Impaired: impaired physical function
Normal: normal physical function
LSM: Least squares mean from mixed effect model; SE: standard error
Comparison between physical function groups at each visit
Bold data are statistically significant
Pelvic floor muscle training in participants with impaired physical function led to an increase in pelvic floor muscle strength from baseline to 6 weeks with mean peak strength over 10 trials being +2.2(−1.1, 5.6) cm H2O compared to a slight decline in peak strength of −0.8(−4.1, 2.6) among participants with normal physical function (p=0.42). After 12 weeks of training, participants with impaired physical function had a sustained improvement in their pelvic floor muscle strength [+1.9(−1.7, 5.4) cm H2O] compared to a slight decline in strength in women with normal function [−0.9(−4.4, 2.7)], with no between group differences (p = 0.21).
Overall, after 12 weeks of pelvic floor muscle training complete satisfaction with incontinence symptom improvement was low for both groups (41.8% of participants with physical function impairments vs. 44.8% of participants with normal physical function; p=0.90). The majority (93%) of participants with normal physical function self-reported being better or much better after 6 weeks of pelvic floor muscle training and behavioral therapy compared to 69% of participants with impaired physical function (p=0.25; Table 4) Women with normal physical function had 2.8 (95% CI 0.5, 16.6) higher odds of rating their impression of improvement as better or much better compared to women with physical function impairments (p=0.25). There were no significant differences in the percent estimated improvement based on physical function status at 6 week. However, by 12 weeks, participants with physical function impairments had a lower percent estimate of improvement as compared to those with normal physical function (49.7 ± 5.9 vs. 67.6 ± 5.9, p=0.023, OR 18, 95% CI (2.5, 33.5).
Table 4.
Examining satisfaction and improvement rates regarding reduction of incontinence episodes after 6 and 12 weeks of behavioral therapy and pelvic floor muscle training from mixed effect models (for continuous variables) and GEE (for categorical variables) adjusting for age, BMI, and race.
| Subjective improvement/satisfaction @ 6 weeks | Subjective improvement/satisfaction @ 12 weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| Impaired | Normal | Normal vs Impaired OR(95%CI) | P-value | Impaired | Normal | Normal vs Impaired OR(95%CI) | P-value | |
| Overall satisfaction | 0.97 | 0.90 | ||||||
| Completely N (%) | 10 (38.5%) | 13 (44.8%) | 1.0 (0.3,3.3) | 10 (41.8%) | 13 (44.8%) | 1.1 (0.3,3.6) | ||
| Not at all/Somewhat N (%) | 16 (61.5%) | 16 (55.2%) | REF | 14 (58.3%) | 16 (55.2%) | REF | ||
| Global impression of improvement | 0.25 | 0.08 | ||||||
| Better/Much better N (%) | 18 (69.2%) | 27 (93.1%) | 2.8 (0.5,16.6) | 18 (75.0%) | 27 (93.1%) | 4.1 (0.8,20.3) | ||
| About the same or Worse/Much worse N (%) | 8 (30.8%) | 2 (6.9%) | REF | 6 (25.0%) | 2 (6.9%) | REF | ||
DISCUSSION
Pelvic floor muscle training, a primary recommended intervention strategy for urinary incontinence, did not provide a significant reduction in urinary incontinence symptoms in this cohort of women older than 70 years with moderate to severe incontinence symptoms, regardless of baseline global physical function impairment. Further, satisfaction rates were overall low with behavioral therapy and pelvic floor muscle training. Older women with impaired physical function did achieve small (−1.3 episodes), but significant improvement in their incontinence episodes at 6 weeks and this was sustained to 12 weeks after pelvic floor muscle training and behavioral therapy. However, this decline in incontinence episodes per day did not translate into clinically important improvements in bother from their urinary incontinence symptoms based on the urinary distress inventory-6 scores, despite reports of “being better” on the satisfaction survey. The high percentage of women who reported a favorable global impression of improvement may reflect a desire to appease the study staff regarding the intervention. Women with normal physical function did not experience a significant decline in incontinence episodes after 6 and 12 weeks of pelvic floor muscle training and behavioral therapy.
Our original hypothesis that impaired physical function would result in significant differences in efficacy of pelvic floor muscle training was not proven. Contradictory to our stated hypothesis, women with impaired physical function actually demonstrated greater improvement in their pelvic floor strength after directed pelvic floor muscle training compared to women with normal physical function. This observation may be due to the observed improvement in pelvic floor muscle strength not observed among women with normal physical function. In our cohort, we noted a strong corollary between pelvic floor strength and global physical function performance metrics. This may reflect a global weakness in skeletal muscle as evidenced by lower appendicular lean mass, weaker lower extremities, and higher incidence of sarcopenia among women with concomitant physical function impairment.[6] It is plausible that they experienced greater improvement in their pelvic floor muscle strength because they had greater baseline weakness compared to women with normal physical function. Therefore, this low-risk intervention may remain a reasonable treatment option in elderly women with evidence of frailty and mobility disability especially if it is introduced before symptoms advance in severity.
While meta-analyses have confirmed the utility of pelvic floor muscle exercises for all types of urinary incontinence compared to placebo, Cammu et al identified several key predictors of treatment failure most notably of which was baseline severity of incontinence (>2 episodes per day).[26, 27] It is plausible that our cohort represents a select population of women whose urinary incontinence symptoms have crossed the threshold of severity for which pelvic floor muscle therapy might be efficacious, regardless of age or global physical function. Further, baseline pelvic floor muscle strength and global physical function have not previously been considered when recommending pelvic floor muscle training to older incontinent women.
Several studies have affirmed the association between poor physical function and urinary incontinence. Tinetti et al studied a cohort of older community-dwelling adults to assess predisposing factors associated with urinary incontinence, falling, and functional dependence. Lower extremity weakness (poor performance on timed chair stands) showed the strongest relationship with these factors.[1] In a secondary cross-sectional analysis among women with daily urinary incontinence, 24% reported specific difficulty or dependence with using the toilet, and were 3.3 times more likely to have functional difficulty or dependence compared to continent older women. [28] Nearly a quarter of women older than 65 years who participated in the California Health Interview Survey reported symptoms; this was significantly associated with poorer overall health (adjusted OR 3.43), decreased mobility (OR, 1.81), and history of falls (OR, 1.53).[29] Current practice guidelines should consider these important age-related changes when considering incontinence treatment.[30]
The greatest strength of this work is in the population studied. Women with urinary incontinence who are older than 70 years are rarely included in clinical trials, thus our knowledge regarding the efficacy of our standard urinary incontinence treatments is under explored. In addition, the comprehensive evaluation for physical function impairments is a novel occurrence in clinical trials designed to observe effectiveness of non-surgical treatment for urinary incontinence. Our findings are strengthened by our use of validated measures to determine changes in urinary incontinence episodes, pelvic floor muscle strength, and for assessment of satisfaction with treatment. The use of subjective and objective outcome measures for urinary incontinence further strengthens this work as it provides a more wholistic assessment of the impact of physical function impairments on the success of pelvic floor muscle exercise to treat urinary incontinence in older women. Lastly, we were able to successfully recruit 70 women older than 70 years into this prospective study with a retention rate of 77% at 12 weeks.
While robust in design and implementation, there are several important limitations to this study that may influence the conclusions. First, our criterion for incontinence severity upon enrollment targeted women with at least moderate symptoms. It is plausible that the impact of pelvic floor muscle exercises may only be in women with mild symptoms; thus explaining the decreased efficacy observed in this cohort overall. Had we chosen women with mild incontinence, it is plausible that pelvic floor muscle training may have resulted in significant improvement and satisfaction, particularly in the absence of physical function impairment. However, we documented that greater severity of urinary incontinence is associated with physical function impairments.[8] Therefore, targeting women with more severe symptoms to understand the role of pelvic floor muscle training in this cohort of understudied women was the purpose of this work. A criticism to our findings is in the pragmatic design of the intervention implementation. The majority of older women with urinary incontinence symptoms seeking treatment are not referred to pelvic floor physical therapy for the conduct of pelvic floor muscle training as the first-line treatment for their urinary incontinence symptoms [31]. Access to a pelvic floor physical therapist is limited by a cost, access to qualified health care professionals, and need to travel to appointments multiple times per week. However, supervised weekly pelvic floor muscle training programs with a certified pelvic floor physical therapist may have superior results compared to home prescriptions as used in this study. Our pragmatic design of progressive, home-based pelvic floor training and behavioral therapy is significantly more generalizable compared to a more supervised approach.
In conclusion, aging and physical function impairment strongly affect urinary incontinence rates and conservative behavioral and pelvic floor muscle interventions appear to have limited impact in this population. The demonstrated positive impact on pelvic floor muscle strength, however, even in women with poor global physical function, raises the question of utilization of these exercises as a more effective prevention than treatment strategy. It is also possible that pelvic floor muscle exercises could be an important intervention for limiting progression of incontinence severity in women with declining physical function. However, advancements in non-surgical treatments are needed to more significantly influence urinary incontinence severity and to improve treatment satisfaction for older incontinent women.
Supplementary Material
FUNDING:
Supported by the National Institute on Aging grant R03 AG056460 and contracts N01-AG-6-2101, N01- AG-6-2103, and N01-AG-6-2106; National Institute on Aging grant R01 G028050; and the Wake Forest Older Americans Independence Center (P30 AG021332).
Financial Disclosure
Catherine A. Matthews disclosed that money was paid to her institution from Boston Scientific and Neomedic. She received funding from Boston Scientific and has been an expert witness for Ethicon.
Footnotes
The other authors did not report any potential conflicts of interest.
Before submission to Obstetrics & Gynecology, this article was posted to a preprint server at https://www.medrxiv.org/content/10.1101/2021.10.21.21265353v1.
Each author has confirmed compliance with the journal’s requirements for authorship.
Authors’ Data Sharing Statement
Will individual participant data be available (including data dictionaries)? No.
What data in particular will be shared? None
What other documents will be available? None
When will data be available (start and end dates)? Not applicable.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Not applicable.
Clinical Trial Registration: Clinical Trials.gov, NCT03057834.
REFERENCES
- 1.Tinetti ME, et al. , Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA, 1995. 273(17): p. 1348–53. [PubMed] [Google Scholar]
- 2.Erekson EA, et al. , Functional disability and compromised mobility among older women with urinary incontinence. Female Pelvic Med Reconstr Surg, 2015. 21(3): p. 170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker-Autry C, et al. , Characterizing the physical function decline and disabilities present among older adults with fecal incontinence: a secondary analysis of the health, aging, and body composition study. Int Urogynecol J, 2021. [DOI] [PubMed]
- 4.Laycock J. and Jerwood D, Pelvic floor muscle assessment: the PERFECT scheme. Physiotherapy, 2001. 87(12): p. 631–642. [Google Scholar]
- 5.Abrams P, et al. , 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: EVALUATION AND TREATMENT OF URINARY INCONTINENCE, PELVIC ORGAN PROLAPSE AND FAECAL INCONTINENCE. Neurourol Urodyn, 2018. 37(7): p. 2271–2272. [DOI] [PubMed] [Google Scholar]
- 6.Parker-Autry C, et al. , Characterizing the Functional Decline of Older Women With Incident Urinary Incontinence. Obstet Gynecol, 2017. 130(5): p. 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocha F, et al. , Evaluation of the pelvic floor muscles training in older women with urinary incontinence: a systematic review. Porto biomedical journal, 2018. 3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker-Autry C, et al. , The geriatric incontinence syndrome: Characterizing geriatric incontinence in older women. J Am Geriatr Soc, 2021. [DOI] [PubMed]
- 9.Erekson EA, et al. , Frailty, cognitive impairment, and functional disability in older women with female pelvic floor dysfunction. International Urogynecology Journal, 2015. 26(6): p. 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley CS, et al. , The questionnaire for urinary incontinence diagnosis (QUID): validity and responsiveness to change in women undergoing non-surgical therapies for treatment of stress predominant urinary incontinence. Neurourol Urodyn, 2010. 29(5): p. 727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson M, et al. , Validation of a combined comorbidity index. J Clin Epidemiol, 1994. 47(11): p. 1245–51. [DOI] [PubMed] [Google Scholar]
- 12.Nasreddine ZS, et al. , The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 2005. 53(4): p. 695–9. [DOI] [PubMed] [Google Scholar]
- 13.Andresen EM, et al. , Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med, 1994. 10(2): p. 77–84. [PubMed] [Google Scholar]
- 14.Madill SJ, et al. , Effects of PFM rehabilitation on PFM function and morphology in older women. Neurourol Urodyn, 2013. 32(8): p. 1086–95. [DOI] [PubMed] [Google Scholar]
- 15.Skorupska K, et al. , Identification of the Urogenital Distress Inventory-6 and the Incontinence Impact Questionnaire-7 cutoff scores in urinary incontinent women. Health Qual Life Outcomes, 2021. 19(1): p. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber MD, et al. , Further validation of the short form versions of the Pelvic Floor Distress Inventory (PFDI) and Pelvic Floor Impact Questionnaire (PFIQ). Neurourol Urodyn, 2011. 30(4): p. 541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laycock J JD, Pelvic Floor Muscle Assessment: The PERFECT Scheme. Physiotherapy, 2001. 87(12): p. 631–642. [Google Scholar]
- 18.Guralnik JM, et al. , Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med, 1995. 332(9): p. 556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahor M, et al. , Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci, 2006. 61(11): p. 1157–65. [DOI] [PubMed] [Google Scholar]
- 20.Simonsick EM, et al. , Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci, 2001. 56(10): p. M644–9. [DOI] [PubMed] [Google Scholar]
- 21.Drouin JM, et al. , Reliability and validity of the Biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur J Appl Physiol, 2004. 91(1): p. 22–9. [DOI] [PubMed] [Google Scholar]
- 22.Fielding RA, et al. , Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc, 2011. 12(4): p. 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean RR, et al. , Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci, 2014. 69(5): p. 576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaya S, et al. , Short-term effect of adding pelvic floor muscle training to bladder training for female urinary incontinence: a randomized controlled trial. Int Urogynecol J, 2015. 26(2): p. 285–93. [DOI] [PubMed] [Google Scholar]
- 25.Radaelli R, et al. , Dose-response of 1, 3, and 5 sets of resistance exercise on strength, local muscular endurance, and hypertrophy. J Strength Cond Res, 2015. 29(5): p. 1349–58. [DOI] [PubMed] [Google Scholar]
- 26.Cammu H, et al. , Who will benefit from pelvic floor muscle training for stress urinary incontinence? Am J Obstet Gynecol, 2004. 191(4): p. 1152–7. [DOI] [PubMed] [Google Scholar]
- 27.Cacciari LP, Dumoulin C, and Hay-Smith EJ, Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: a cochrane systematic review abridged republication. Braz J Phys Ther, 2019. 23(2): p. 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browne WJ, et al. , Urinary incontinence in 9–16 year olds with cystic fibrosis compared to other respiratory conditions and a normal group. J Cyst Fibros, 2009. 8(1): p. 50–7. [DOI] [PubMed] [Google Scholar]
- 29.Bresee C, et al. , Prevalence and correlates of urinary incontinence among older community-dwelling women. Female Pelvic Med Reconstr Surg, 2014. 20(6): p. 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gynecologists, A.C.o.O.a., Urinary Incontinence in women. Obstet Gynecol, 2015. 126: p. e66–81. [DOI] [PubMed] [Google Scholar]
- 31.Brown HW, et al. , Better together: multidisciplinary approach improves adherence to pelvic floor physical therapy. International urogynecology journal, 2020. 31(5): p. 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.