Abstract
Theobromine is a caffeine metabolite most abundant in dark chocolate, of which consumption is linked with a lower risk of cognitive decline. However, the mechanisms through which theobromine affects neuronal function remain ill-defined. Using electrophysiological recordings in mouse hippocampal synapses, we now characterized the impact of a realistic concentration of theobromine on synaptic transmission and plasticity. Theobromine (30 μM) facilitated synaptic transmission while decreasing the magnitude of long-term potentiation (LTP), with both effects being blunted by adenosine deaminase (2 U/mL). The pharmacological blockade of A1R with DPCPX (100 nM) eliminated the theobromine-dependent facilitation of synaptic transmission, whereas the A2AR antagonist SCH58261 (50 nM), as well as the genetic deletion of A2AR, abrogated the theobromine-induced impairment of LTP. Furthermore, theobromine prevented LTP deficits and neuronal loss, respectively, in mouse hippocampal slices and neuronal cultures exposed to Aβ1–42 peptides, considered a culprit of Alzheimer’s disease. Overall, these results indicate that theobromine affects information flow via the antagonism of adenosine receptors, normalizing synaptic plasticity and affording neuroprotection in dementia-related conditions in a manner similar to caffeine.
Keywords: theobromine, caffeine, adenosine receptors, synaptic transmission, synaptic plasticity, Alzheimer’s disease
1. Introduction
Caffeine is the most widely consumed psychoactive drug, enhancing well-being, alertness and attention [1]. Epidemiological studies have also shown that the regular consumption of a moderate amount of coffee (3–5 cups per day) increases memory consolidation [2,3] and decreases cognitive decline and, in particular, the risk of developing AD (e.g., [4,5,6]). Accordingly, animal studies indicate that caffeine triggers pro-cognitive adaptive changes in the brain [7] and attenuates memory deficits in the animal models of different brain disorders (reviewed in [8]), including AD (e.g., [9,10]). Caffeine modulates information flow in neuronal circuits, enhancing basal synaptic transmission through the antagonism of adenosine A1 receptors (A1R) [11,12] and dampens hippocampal long-term potentiation (LTP) [11,13], a form of synaptic plasticity considered the neurophysiological basis of memory [14], through the antagonism of adenosine A2A receptors (A2AR). The neuroprotective effect of caffeine in different animal models of neuropsychiatric disorders, including AD [9,10], has been linked to the ability of caffeine to normalize synaptic plasticity via A2AR (reviewed in [15]), since the overactivation of hippocampal A2AR is sufficient to trigger memory impairment [16,17,18] and is critically necessary for the emergence of synaptic and memory deficits in different animal models of early AD [19,20,21,22].
In a recent attempt to correlate the levels of caffeine with the currently established neurochemical markers of AD, we were surprised to conclude that it was the levels of theobromine rather than caffeine that correlated inversely with the altered cerebrospinal fluid levels of Aβ1–42 and modified tau proteins [23]. This was particularly surprising, since the 1-N-demethylation of caffeine into theobromine only accounts for approximately 7 to 8% of caffeine metabolism, although its liver metabolism through CYP1A2 and CYP2E1 is slower than that of caffeine in humans [24] and different in different mammals [25], making it a toxic compound for some animals (e.g., [26,27]). The association between theobromine and AD was further bolstered by a recent study [28], showing that the aging-associated cognitive decline was attenuated in consumers of chocolate [29], whose main psycho-pharmacologically active constituent is theobromine. Accordingly, in animal models, theobromine has been associated with memory improvement [30,31].
The pharmacological characterization of the targets of xanthines prompted the conclusion that theobromine has a low potency (in the hundreds of micromolar range) to antagonize A1R and A2AR, compared to caffeine [32,33,34], making it a lesser homologue of caffeine. This has contributed to the poor interest dedicated to possible central effects of theobromine, which is instead clinically used as a vasodilator, a diuretic, and heart stimulant, with renewed interest in tooth decay prevention [35] and as an antitussive [36], possibly due to the particular ability of theobromine to interfere with targets such as poly(ADP-ribose)polymerase-1 [37] or sirtuin-1 [38].
This apparently different pharmacokinetic and pharmacodynamic profile of caffeine and theobromine prompts the need for a clarification of their mechanisms of action in the nervous system to grasp this surprising inverse association between cognitive deterioration and chocolate consumption [28] or theobromine levels [23]. Thus, we now hypothesized and tested if theobromine also acted on neuronal function and viability through the antagonism of adenosine receptors, as previously defined for caffeine [11].
2. Results
2.1. Theobromine Bolsters Basal Synaptic Transmission and Impairs Long-Term Potentiation
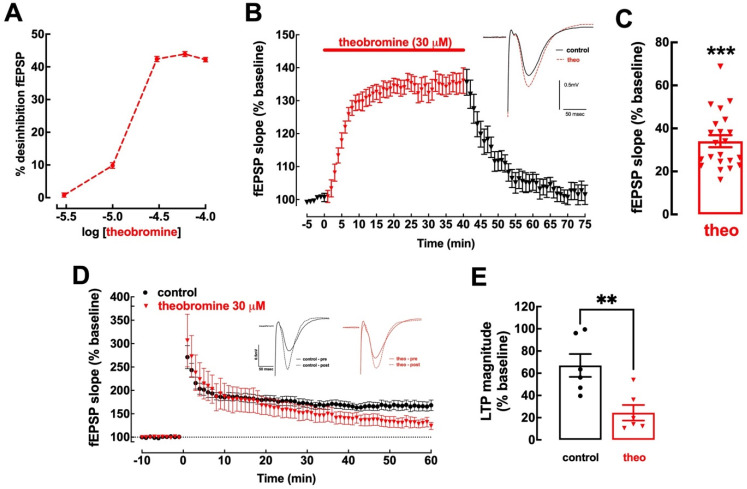
Theobromine enhances basal synaptic transmission in mouse hippocampal slices in a concentration-dependent manner, reaching a plateau after 30 μM (Figure 1A). This is within the range of concentrations of theobromine found in the cerebral cortex of rats that consumed theobromine-supplemented chow for 40 days (1.94 ± 0.16 μg/mL or 10.77 ± 0.89 μM) [39]. Exposure to theobromine (30 μM) increased basal synaptic transmission by 34.04 ± 2.80% (p < 0.001 vs. baseline (0%), n = 22), reaching a plateau after approximately 10 min, which remained stable up to 30 min; upon washout, synaptic transmission returned to near-baseline values after 30 min (2.13 ± 2.77%; p > 0.05 vs. baseline, n = 22, one sample t test) (Figure 1B,C).
Figure 1.
Theobromine alters basal synaptic transmission and synaptic plasticity. Theobromine increased basal synaptic transmission in Schaffer fibers-CA1 pyramid synapses of mouse hippocampal slices in a concentration-dependent manner, reaching a plateau after 30 μM (A). Theobromine (theo, 30 μM) rapidly and reversibly increased basal synaptic transmission (B,C), and the insert in (B) displays representative field extracellular postsynaptic potential (fEPSP) recorded in ACSF medium (control conditions, black filled line) and in the presence of theobromine (red dashed line). Additionally, theobromine significantly decreased the magnitude of long-term potentiation (LTP): the time course of fEPSP recordings (D) shows a sustained increase in fEPSP slope after delivering a high-frequency stimulation train (HFS: one train of 100 pulses of 1 Hz for 1 s), which is larger in ACSF (black symbols) than in the presence of 30 μM theobromine (red symbols), as quantified in (E). The inserts in (D) show two superimposed pairs fEPSP collected before (filled traces) and 60 min after HFS (dashed lines) in control conditions (black traces on the left) and in the presence of theobromine (red traces on the right). Data are mean ± S.E.M. of 5–22 (A), 22 (B,C) or 6 (D,E) experiments. The asterisks denote significant differences: ** p < 0.01 vs. control, Student’s t test (E); *** p < 0.001 vs. baseline, one-sample t test (C).
Contrariwise, mouse hippocampal slices superfused with 30 μM theobromine displayed an impairment of synaptic plasticity with a significant decrease in long-term potentiation (LTP), with a magnitude reaching 66.99 ± 10.29% in the absence of theobromine vs. 24.39 ± 7.03% in its presence (p < 0.01; n = 6; Figure 1D,E). Remarkably, these effects of theobromine on synaptic transmission and plasticity are analogous to those recorded upon the exposure of mouse hippocampal slices to an identical concentration of caffeine [11].
2.2. The Effect of Theobromine on Synaptic Transmission and Plasticity Is Lost upon Removal of Extracellular Adenosine
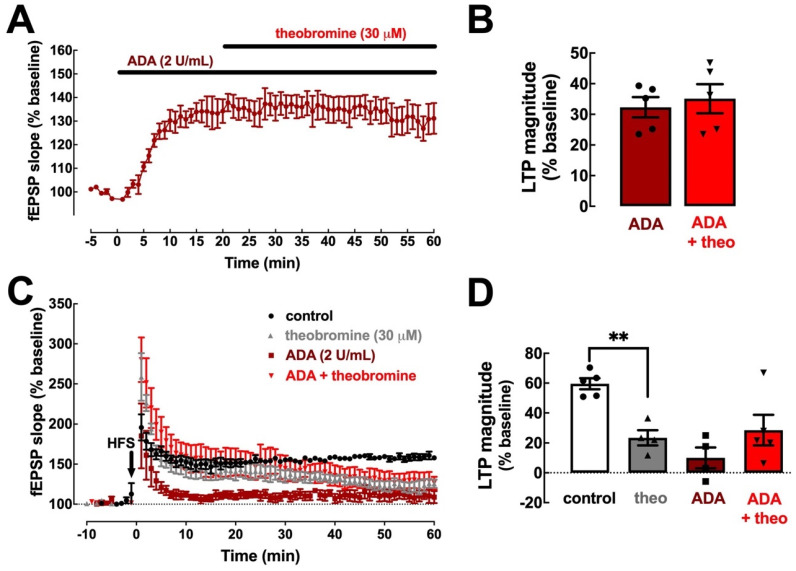
In order to assess if theobromine relies on adenosine neuromodulation to alter synaptic transmission and plasticity, as previously shown for caffeine [11], we probed for the impact of adenosine deaminase (ADA), an enzyme that converts adenosine to inosine, on the synaptic changes elicited by theobromine (30 μM). Clearing endogenous extracellular adenosine increased basal synaptic transmission (32.30 ± 3.31%, p < 0.001 vs. baseline, n = 5) and occluded the effect of theobromine on basal synaptic transmission (32.30 ± 3.31% in the presence of ADA vs. 35.10 ± 4.74% in the presence of ADA and theobromine; p > 0.05, n = 5; Figure 2A,B). Likewise, ADA also hindered the decrease of LTP magnitude induced by theobromine (23.51 ± 5.09% in the presence of ADA vs. 28.57 ± 10.21% in the presence of ADA and theobromine; p > 0.05, n = 4–5; Figure 2C,D).
Figure 2.
Clearance of endogenous extracellular adenosine abolishes the effects of theobromine on synaptic transmission and plasticity. Adenosine deaminase (ADA, 2 U/mL) enhanced hippocampal basal synaptic transmission (A,B) and abrogates the effects of theobromine (30 μM). ADA (2 U/mL) also decreased the magnitude of long-term potentiation (C,D) induced by a high-frequency stimulation train (HFS: one train of 100 pulses of 1 Hz for 1 s) in Schaffer fiber-CA1 pyramid synapses of mouse hippocampal slices and abrogates the effects of theobromine (theo, 30 μM). This shows that these measured effects of theobromine are strictly dependent on the presence of endogenous extracellular adenosine. Data are mean ± S.E.M. of 5 (A,B) and 4–5 (C,D) experiments. The asterisks denote significant differences: ** p < 0.01 vs. control, two-way ANOVA with Tukey’s post hoc test.
2.3. Theobromine Bolsters Synaptic Transmission through the Antagonism of A1R
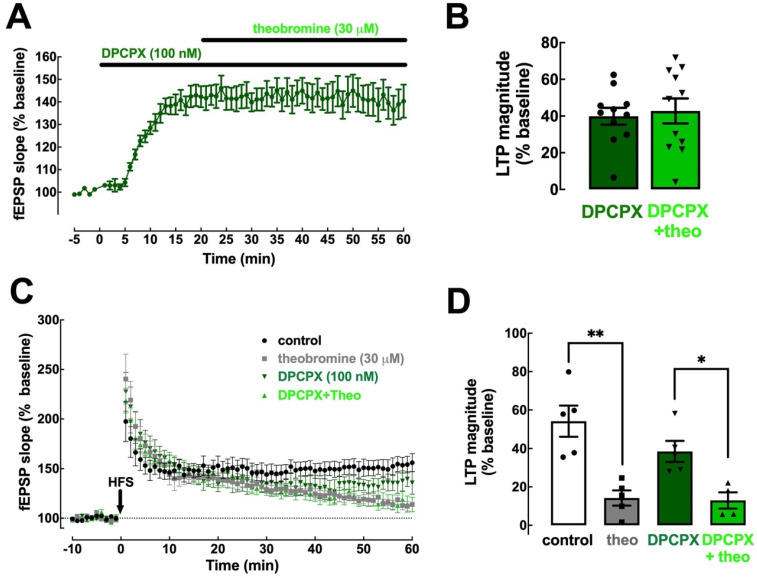
We next determined the contribution of the two main adenosine receptor subtypes (A1R and A2AR) to the effects of theobromine on basal synaptic transmission and synaptic plasticity. To detail the involvement of A1R we used the selective A1R antagonist DPCPX (100 nM). DPCPX increased basal synaptic transmission by 39.91 ± 4.59% (p < 0.001 vs. baseline, n = 11; Figure 3A,B) but had no significant effect on LTP (54.16 ± 8.12% in the absence and 38.47 ± 5.44% in the presence of DPCPX; p > 0.05, n = 5–6; Figure 3C,D), which is in line with the modest impact of A1R antagonism on synaptic plasticity in adult rodents [11,40]. Analogously to what we previously showed for caffeine [11], DPCPX occluded the effect of theobromine (30 μM) on basal synaptic transmission (2.87 ± 8.23% vs. the baseline in the presence of DPCPX; p > 0.05, n = 11; Figure 3A,B). However, the antagonism of A1R did not alter the impact of theobromine on LTP magnitude (38.47 ± 5.44% in the presence of DPCPX vs. 12.94 ± 4.23% in the presence of DPCPX + theobromine, p < 0.05, n = 4–5; Figure 3C,D).
Figure 3.
Blockade of A1R eliminates the impact of theobromine on basal synaptic transmission but not on synaptic plasticity. The selective A1R antagonist DPCPX (100 nM) enhanced basal synaptic transmission and occluded the effect of theobromine (theo, 30 μM) on synaptic transmission (A,B). In contrast, DPCPX did not modify the magnitude of long-term potentiation (LTP), induced with a high-frequency stimulation train (HFS: one train of 100 pulses of 1 Hz for 1 s), recorded in Schaffer fiber-CA1 pyramid synapses of mouse hippocampal slices and failed to modify the effects of theobromine on LTP magnitude (C,D). Data are mean ± S.E.M. of 11 (A,B) and 5–6 (C,D) experiments. The asterisks denote significant differences: * p < 0.05 and ** p < 0.01, two-way ANOVA with Tukey’s post hoc test.
2.4. Theobromine Impairs Synaptic Plasticity through the Antagonism of A2AR
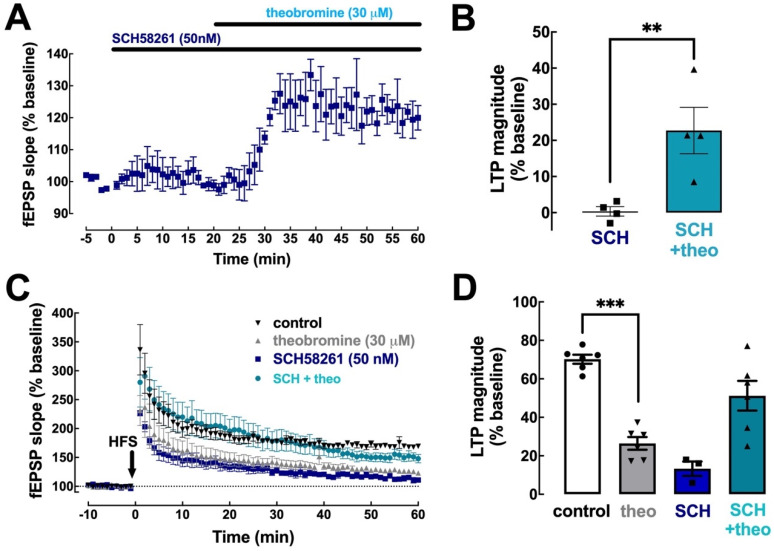
To define the importance of A2AR in the effects of theobromine on synaptic transmission and plasticity, we first used the selective A2AR antagonist SCH58261 (50 nM) [40]. SCH58261 did not alter basal synaptic transmission (0.35 ± 1.29% vs. baseline, p > 0.05, n = 4) and did not affect the increase in basal synaptic transmission triggered by theobromine (30 μM) (22.74 ± 6.41% vs. baseline in the presence of SCH58261; p < 0.05, n = 4) (Figure 4A,B).
Figure 4.
Blockade of A2AR prevents the effect of theobromine on long-term potentiation. The selective A2AR antagonist SCH58261 (SCH, 50 nM) did not modify basal synaptic transmission nor altered the effect of theobromine (theo, 30 μM) on basal synaptic transmission in mouse hippocampal slices (A,B). In the presence of SCH58261 (50 nM), theobromine (30 μM) failed to decrease LTP magnitude (C,D), induced with a high-frequency stimulation train (HFS: one train of 100 pulses of 1 Hz for 1 s) in Schaffer fiber-CA1 pyramid synapses of mouse hippocampal slices. Data are mean ± S.E.M. of 4 (A,B) and 3–6 (C,D) experiments. The asterisks denote significant differences: ** p < 0.05 vs. SCH58261, Student’s t test (B); *** p < 0.001 vs. control, two-way ANOVA with Tukey’s post hoc test.
Since A2AR are selectively involved in the control of hippocampal synaptic plasticity [40,41], we next tested if SCH58261 prevented the effects of theobromine on hippocampal LTP. In agreement with previous observations [11,20,40], SCH58261 (50 nM) significantly decreased LTP magnitude (13.31 ± 3.74% with SCH58261 vs. 70.21 ± 2.36% without SCH58261, p < 0.001, n = 3–6; Figure 4C,D). Remarkably, the blockade of A2AR with SCH58261 (50 nM) prevented the inhibitory effect of theobromine on hippocampal LTP (13.31 ± 3.74% in the presence of SCH58261 vs. 51.22 ± 7.72% in the presence of SCH58261 + theobromine; p < 0.05, n = 3–6), an increase probably related to the disrupted interaction of A2AR and A1R to reveal and A1R-medated effect of theobromine upon blockade of A2AR [42,43].
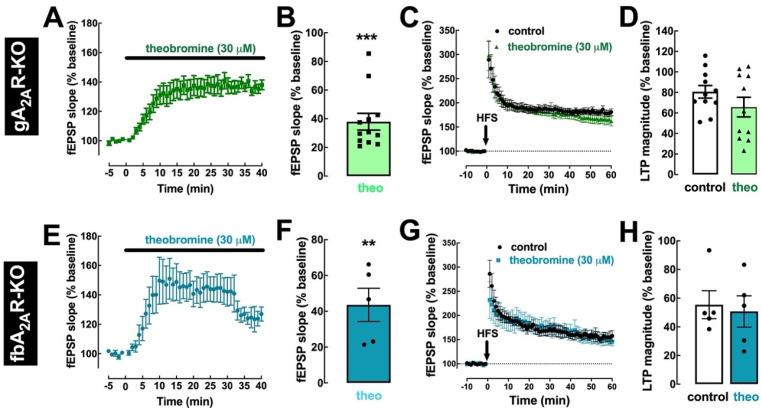
To further confirm the involvement of A2AR in the effect of theobromine on hippocampal LTP, we tested the effects of theobromine in hippocampal slices from mice where A2AR were selectively deleted, either from the whole animal (gA2AR-KO mice) or only from forebrain neurons (fbA2AR-KO mice). In hippocampal slices from both mouse strains, the enhancement of basal synaptic transmission by 30 μM theobromine was maintained (37.93 ± 5.80% for gA2AR-KO and 43.59 ± 9.27% for fbA2AR-KO vs. baseline; p < 0.001, n = 12 and p < 0.01, n = 5, respectively; Figure 5A,B,E,F). However, theobromine was unable to significantly alter LTP magnitude in slices from gA2AR-KO mice (65.75 ± 9.59% in the absence vs. 80.66 ± 6.17% in the presence of theobromine; p > 0.05, n = 12; Figure 5C,D) and from fbA2AR-KO mice (50.69 ± 10.95% in theobromine-treated slices vs. 55.40 ± 9.72% in control slices; p > 0.05, n = 5; Figure 5G,H).
Figure 5.
Genetic deletion of A2AR prevents the effect of theobromine on long-term potentiation. In slices from knockout mice where A2AR were deleted globally (gA2AR-KO, (A–D)) or selectively in forebrain neurons (fbA2AR-KO, (E–H)), theobromine (theo, 30 μM) still enhanced basal synaptic transmission (A,B,E,F), but did not modify the magnitude of LTP (C,D,G,H), induced with a high-frequency stimulation train (HFS: one train of 100 pulses of 1 Hz for 1 s) in Schaffer fiber-CA1 pyramid synapses of mouse hippocampal slices. Data are mean ± S.E.M. of 12 (A,B), 11 (C,D), and 5 (E–H) experiments. The asterisks denote significant differences: ** p < 0.01, *** p < 0.001 vs. baseline, one-sample t test.
2.5. Theobromine and Caffeine Abrogate the Impairment of Synaptic Plasticity and Neuronal Viability Triggered by β-Amyloid Peptides
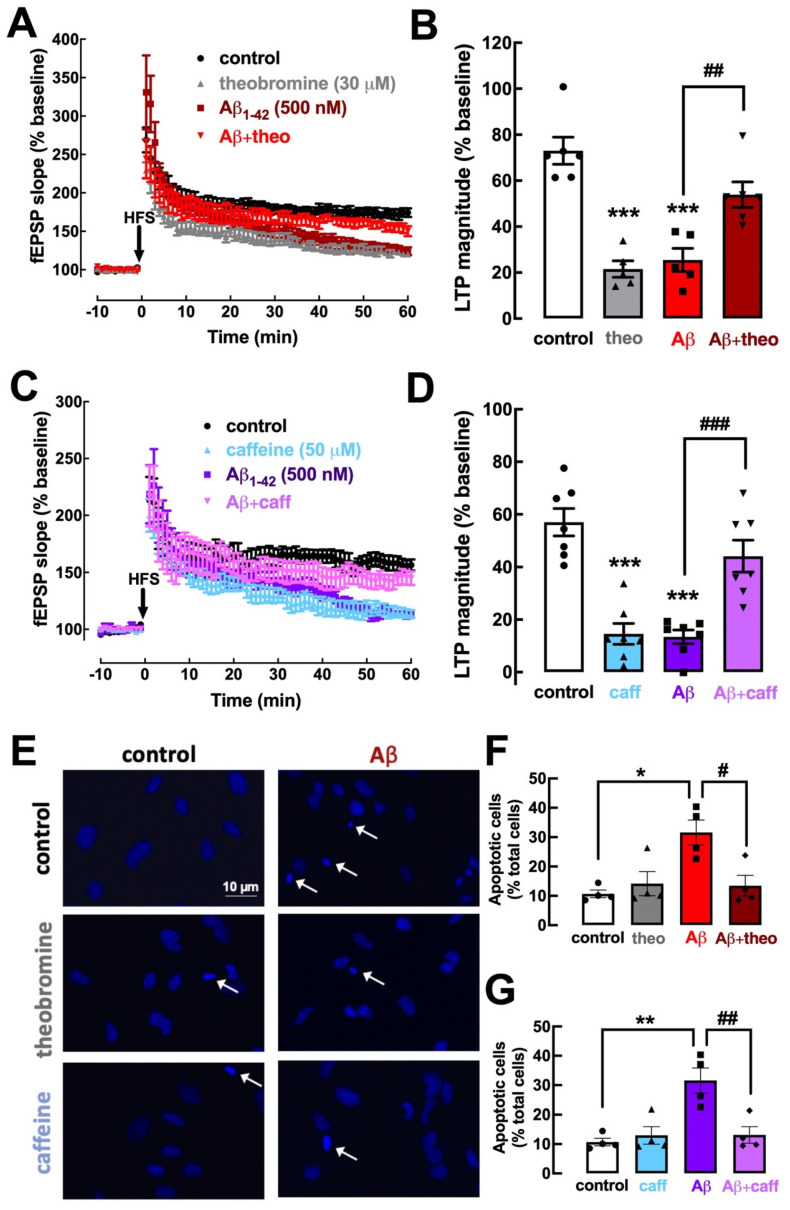
Synaptic dysfunction is at the core of initial changes in early AD [44,45]. Modelling early AD, a two-week exposure to oligomeric Aβ1–42 significantly impairs synaptic plasticity, typified by a decrease in LTP magnitude [20]. This is mimicked by the acute exposure of mouse hippocampal slices to oligomeric Aβ1–42 (50 nM during 40 min) (unpublished preliminary data), which replicates the decrease in LTP magnitude observed in the Aβ-injection model [20]. We now report that a previous superfusion of mouse hippocampal slices with theobromine (30 μM) prevented the noxious impact of Aβ1–42 on hippocampal LTP magnitude (25.50 ± 5.05% without vs. 53.88 ± 5.58% with theobromine; p < 0.01, n = 5–6; Figure 6A,B). Remarkably, the exposure to caffeine (50 μM) also prevented the noxious impact of Aβ1–42 on hippocampal LTP magnitude (13.40 ± 2.61% without vs. 44.12 ± 6.07% with caffeine; p < 0.001, n = 7; Figure 6C,D).
Figure 6.
Theobromine and caffeine prevent synaptic plasticity deficits and neurotoxicity induced by Aβ1–42 modelling early Alzheimer’s disease. The superfusion of the mouse hippocampal slices with Aβ1–42 (50 nM) during 40 min induced a significant decrease in LTP magnitude (A–D) that was prevented by the presence of either theobromine (theo, 30 μM) (A,B) or caffeine (caff, 50 μM) (C,D). Likewise, primary neuronal cultures treated with Aβ1–42 (500 nM) during 24 h exhibit a significant increase in the number of apoptotic neurons (E–G), which were identified as cells with condensed nuclei with an irregular form often fragmented, displaying a more intense light blue staining with DAPI, as indicated by the arrows in the photographs (E). This neurotoxicity of Aβ1–42 was prevented both by theobromine (30 μM) (E,G) or caffeine (50 μM) (F,G). Data are mean ± S.E.M. of 5–6 (A,B), 7 (C,D), and 4 (E–G) experiments, where the counting of apoptotic-like neurons versus the total number of neurons was calculated in 4 field per coverslip. The scale bar (10 μm) of the first photograph in (E) applies to all other photographs. The asterisks denote significant differences: * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control, two-way ANOVA with Tukey’s post hoc test; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. Aβ1–42, two-way ANOVA with Tukey’s post hoc test.
Aβ peptides were previously demonstrated to have a neurotoxic effect, triggering an apoptotic response in neuronal cultures, which was characterized with different methods [19,46]. Accordingly, the exposure of primary cultures of mouse cortical neurons to Aβ1–42 (500 nM during 24 h) triggered the appearance of 31.58 ± 4.25% (n = 4) of apoptotic-like cells, displaying condensed and fragmented nuclei stained more intensely with DAPI (Figure 6E). This noxious effect was prevented by theobromine (30 μM) (13.46 ± 3.52% of apoptotic cells; p < 0.05, n = 4; Figure 6E,F) as well as by 50 μM caffeine (13.13 ± 2.81% of apoptotic cells; p < 0.05, n = 4; Figure 6E,G). Overall, this similar prevention of Aβ-induced synaptic deficits and neuronal toxicity by theobromine and caffeine indicates an analogous mechanism operated by both xanthines to afford a prophylactic neuroprotection against early AD.
3. Discussion
The present study clarifies how theobromine, a caffeine derivative and the major psychoactive component of cocoa, controls synaptic function in concentrations close to those monitored in the blood and brain parenchyma [39]. This involves the modulation of adenosine A1 and A2A receptors in a manner similar to the effects of caffeine. The data also show that theobromine and caffeine can equally prevent the deficits of synaptic plasticity and of neuronal viability, induced by β-amyloid peptides that are purported culprits of AD.
Although it has been proposed that theobromine might affect different molecular targets [37,38,39,47], we now observed that the effects of theobromine on synaptic transmission and plasticity were completely occluded upon removal of endogenous extracellular adenosine, a neuromodulator known to mainly operate A1R and A2AR in the brain [48]. Indeed, the pharmacological blockade with DPCPX of A1R, which inhibits excitatory synaptic transmission in central glutamatergic synapses, namely in Schaffer fiber-CA1 pyramid synapses [49], fully prevents the effect of theobromine on basal synaptic transmission. This points to the conclusion that theobromine acts via A1R to control basal synaptic transmission. Additionally, the conversion of adenosine to inosine by ADA, which abolishes the excitatory effect of tonic A2AR activation in Schaffer fiber-CA1 pyramid synapses [40], abrogated the effect of theobromine on LTP. This effect is mimicked by the pharmacological inhibition of A2AR with the selective antagonist SCH58261, as well as by the genetic elimination of A2AR. This shows that theobromine acts via neuronal A2AR to modulate synaptic plasticity.
The present conclusion that theobromine seems to act solely through A1R and A2AR to control information flow in neuronal circuits is rather surprising, since the reported affinities of theobromine for adenosine receptors are in the high micromolar range [32,33,34,50]; in contrast, the impact of theobromine on hippocampal synaptic transmission and plasticity is strictly dependent on adenosine receptors, and these effects of theobromine are superimposable to these previously reported for caffeine [11]. This may partially result from particular binding kinetics to adenosine receptors of these two xanthines, a factor recently highlighted to be of crucial importance to understand the efficiency of drugs to activate adenosine receptors [51]. This similar efficiency of theobromine and caffeine to modulate synaptic function through adenosine receptors justifies the observations that the acute exposure to theobromine has discrete central stimulant effects [52] and mostly affects peripheral physiology with lower psychomotor properties, compared to caffeine [53,54], although the effects of theobromine are longer lasting, due to its longer half-life [55].
Notably, the regular consumption of theobromine has neuroprotective action, revealed both in animal and human studies (reviewed in [56,57,58]). Indeed, recent studies indicate that chocolate consumption associates with a lower risk of developing age-related cognitive decline [28]; also, there is a positive correlation between theobromine and Aβ1–42 levels in the plasma of AD patients [23] and that theobromine blocks the increase in Aβ levels induced by lard-enriched diet in rodents [30]. We now report that theobromine is equi-effective with caffeine to prevent Aβ-induced neurotoxicity, as well as Aβ-induced impairment of synaptic plasticity, one of the earliest alterations found in AD [59]. Since previous studies have identified that the synaptoprotective role of caffeine is mediated by A2AR (e.g., [9,60,61]) and we now observed that theobromine controls hippocampal synaptic plasticity through the antagonism of A2AR, it is reasonable to assume that theobromine prevents Aβ-induced dysfunction through the antagonism of A2AR. Notably, the previously proposed involvement of the CaMKII/CREB/BDNF pathway in the effects of theobromine on memory performance [31,62] is compatible with the involvement of A2AR that are well known to control this pathway (reviewed in [15]). This understanding of how theobromine impacts on synaptic plasticity is relevant to mechanistically understand the benefits provided by chocolate and other cocoa-based substances for memory and cognitive function upon ageing and brain disorders, such as AD [28,58,63]. Furthermore, the near equi-effective neuroprotection afforded by caffeine and theobromine is supportive of the long-lasting beneficial effects, resulting from the consumption of caffeinated beverages, namely of caffeinated coffee, which may involve an initial protection afforded by caffeine that is prolonged by the protective effect of caffeine metabolites, such as theobromine, which has a longer half-life [55]. Moreover, although caffeine-mediated neuroprotection seems to be mostly accounted by the control of neuronal A2AR, it is possible that the neuroprotective effects of methylxanthines might also involve glia (astrocytes and/or microglia)-mediated effects, which remains to be adequately characterized.
One question that remains unsolved is the toxicity of theobromine in most animals (e.g., [26,27]), which is not present in humans. Since both theobromine and caffeine, which has a limited toxicity, can antagonize adenosine receptors, it is unlikely that adenosine receptors are involved in chocolate toxicosis in animals, which might instead involve other molecular targets, such as poly(ADP-ribose)polymerase-1 [37] or sirtuin-1 [37,38,39,45]. These theobromine-operated molecular targets involved in chocolate toxicosis are likely to have a greater peripheral rather than central impact, since we now observed that the exposure of neurons to theobromine did not cause a direct neurotoxicity. However, further studies are required to directly identify the targets operated by theobromine to trigger toxicosis in animals.
In conclusion, our results demonstrate that theobromine, analogously to caffeine, can alter synaptic functioning through the control of adenosine receptors, modulating synaptic transmission via A1R and synaptic plasticity by acting as an A2AR antagonist. Furthermore, theobromine is equi-effective with caffeine to prevent the impairment of synaptic plasticity and the neuronal loss in conditions designed to replicate what occurs in early AD, which provides a new mechanistic insight on the prophylactic benefits, resulting from the consumption of chocolate and other cocoa-derived products.
4. Methods and Materials
4.1. Ethical Approval
Animal procedures were performed in accordance with the guidelines of the European community guidelines (EU Directive 2010/63/EU) and the Portuguese law on animal care (1005/92) and approved by the Ethical Committee of the Center for Neuroscience and Cell Biology of Coimbra.
All efforts were made to reduce the number of animals used and to minimize their stress and discomfort. Thus, the animals were anesthetized in halothane atmosphere before decapitation, and, whereas the hippocampus was used in this study, other tissues from these animals were collected for use in different projects at our research center.
4.2. Animals
We used C57bl\6j mice with 8–10 weeks of age, obtained from Charles River (Barcelona, Spain), global A2AR knockout mice (gA2ARKO), and forebrain A2AR knockout mice (FbA2ARKO) with the same age and genetic background, which were generated in our colony, as previously described [64,65]. The animals were housed under controlled temperature (23 ± 2 °C), subject to a fixed 12 h light/dark cycle, with free access to food and water.
Pregnant female C57bl\6j mice for embryo collection were obtained from parallel breeding at CNC and maintained on a 12 h light/dark cycle with food and water ad libitum. The mating procedure was limited to 12 h, and the day of vaginal plug observation was considered embryonic day 0.5 (E0.5).
4.3. Drugs
Theobromine, caffeine, adenosine deaminase (ADA), and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) were from Sigma (St. Louis, MI, USA). SCH58261 was obtained from Tocris (Bristol, UK). The Aβ1–42 peptide was purchased from Bachem (Bubendorf, Germany).
Theobromine was freshly prepared in milliQ water to a stock concentration of 1 mM. Caffeine was prepared in milliQ H2O to a stock concentration of 100 mM. Stock solutions of DPCPX (5 mM) and SCH58261 (5 mM) were prepared in dimethylsulfoxide (Sigma) and dilutions were prepared in ACSF or Krebs solution, controlling for the impact of the residual amount of dimethylsulfoxide. ADA, DPCPX, and SCH58261 were used in supramaximal but selective concentrations, respectively, 2 U/mL [66], 100 nM [67], and 50 nM [68]. Aβ1–42 was dissolved in water to obtain a solution mostly composed of Aβ low molecular weight oligomers [19,69].
All other chemical substances used, unless stated otherwise, were from Sigma (St. Louis, MI, USA).
4.4. Extracellular Electrophysiological Recordings
Following decapitation, the mouse brain was quickly removed and placed in ice-cold, oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF; in mM: 124.0 NaCl, 4.4 KCl, 1.0 Na2HPO4, 25.0 NaHCO3, 2.0 CaCl2, 1.0 MgCl2, 10.0 glucose). Using a McIlwain tissue chopper (Brinkmann Instruments, Long Island, NY, USA), slices (400 µm-thick) from the dorsal hippocampus were cut transverse to the long axis of the hippocampus and placed in a holding chamber with oxygenated ACSF at room temperature (RT). Slices were allowed to recover for at least 1 h prior to recording, when they were transferred to a submerged recording chamber and superfused at 3 mL/min with oxygenated ACSF kept at 30.5 °C.
The configuration of the extracellular recordings was as previously described [8,29] with the stimulating bipolar concentric electrode placed in the proximal CA1 stratum radiatum for stimulation of the Schaffer fibers and the recording electrode, filled with 4 M NaCl (2–5 MΩ resistance), was placed in the CA1 stratum radiatum, targeting the distal dendrites of pyramidal neurons. Stimulation was performed using either a Grass S44 or a Grass S48 square pulse stimulator (Grass Technologies, West Warwick, RI, USA) and every 20 s with rectangular pulses of 0.1 ms. After amplification (ISO-80, World Precision Instruments, Hitchin, Hertfordshire, UK), the recordings were digitized (BNC-2110, National Instruments, Newbury, UK), averaged in groups of 3, and analyzed using the WinLTP version 2.10 software (WinLTP Ltd., Bristol, UK) [70]. The intensity of stimulation was chosen between 50–60% of maximal fEPSP response, determined on the basis of input/output curves, in which the percentage of maximum fEPSP slope was plotted versus stimulus intensity.
Alterations of synaptic transmission were quantified as the % modification of the average value of the fEPSP slope taken from 25 to 30 min after beginning theobromine application, depending on the experiment or from 10 to 15 min, after beginning the application of modifying drugs (adenosine deaminase, DPCPX or SCH58261), in relation to the average value of the fEPSP slope during the 5 min that preceded application of each modifying drug. To quantify the effect of theobromine applied after other modifying drugs, we compared fEPSP slope values in the 5 min that preceded application of theobromine (baseline) in relation to the average values from 25 to 30 min after theobromine exposure.
Long-term potentiation (LTP) was induced by a high-frequency stimulation train (100 Hz for 1 s). LTP was quantified as the percentage change between two values: the average slope of the 10 averaged potentials taken between 50 and 60 min, after LTP induction, in relation to the average slope of the fEPSP, measured during the 10 min that preceded LTP induction. The effect of drugs on LTP was assessed by comparing LTP magnitude in the absence and presence of the drug in experiments carried out in different slices from the same animal.
4.5. Cell Culture
Primary cultures of cortical neurons were prepared as previously described [71]. Briefly, cortices from C57BL/6 E17.5 mouse embryos were dissected and digested for 15 min at 37 °C with trypsin (0.125%; type II-S, from porcine pancreas; Sigma) and DNAse (50 μg/mL; DNAse I from bovine pancreas; Sigma) in calcium- and magnesium-free sterile Hank’s balanced salt solution (137 mM NaCl, 5.36 mM KCl, 0.44 mM KH2PO4, 4.16 mM NaHCO3, 0.34 mM Na2HPO4, 5 mM glucose, 5 mM sodium pyruvate, 10 mM HEPES, pH 7.2). An equal amount of DMEM (Sigma) supplemented with 0.44 mM NaHCO3, 5 mM sodium pyruvate, 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), and 1% penicillin/streptomycin (Thermo Fisher Scientific), pH 7.3, was added, and cells were mechanically dissociated, centrifuged at 170× g for 5 min at RT, and resuspended in fresh DMEM, prior to seeding at a density of 2.0 × 104 cells/cm2 on poly-D-lysine- (100 μg/mL; Sigma) coated coverslips. After 2 h, the medium was replaced by Neurobasal medium (Thermo Fisher Scientific) with 2% B27 supplement (Thermo Fisher Scientific), 50 U/mL penicillin, 50 μg/mL streptomycin (Thermo Fisher Scientific), and 2 mM glutamine (GlutaMAX-I, Gibco, Life Technologies, Carlsbad, CA, USA), and cells were grown at 37 °C in an atmosphere of 95%/5% air/CO2 until they were used 5 days later.
4.6. Peptide Treatment and Immunocytochemistry
Based on our previous characterization of Aβ1–42-induced neurotoxicity [19], cultured cortical neurons in glass coverslips were treated with oligomeric Aβ1–42 (500 nM) for 24 h in the presence/absence of either theobromine (30 μM) or caffeine (50 μM). The drugs were added into the culture medium at the 5th culturing day. After a 24 h exposure period, cells were washed with PBS and fixed with a 4% paraformaldehyde solution (pH 7.4), for 30 min at RT. The cells were permeabilized with 0.2% Triton X-100/PBS for 10 min at RT and blocked with 3% bovine serum albumin and 5% horse serum for 1 h before incubation with 4′,6-diamidino-2-phenylindole (DAPI) (1:5000). After being washed in PBS to remove the excess staining, the cells were mounted with the DakoCytomation fluorescent medium and visualized in a fluorescence microscope. Apoptotic-like neurons were identified as cells with condensed nuclei with an irregular form, often fragmented, displaying a more intense light blue staining with DAPI. The counting of apoptotic-like neurons versus the total number of neurons was calculated in 4 fields per coverslip.
4.7. Statistics
The values presented are mean ± S.E.M. with the number of determinations (n, i.e., slices or cells from different mice) in each experimental condition, indicated in the legend to the figures. The comparison of two experimental conditions was performed using either a paired or unpaired Student’s t test. Otherwise, statistical analysis was performed by a two-way analysis of variance (ANOVA), followed by a Tukey’s post hoc test. p < 0.05 was considered to represent statistical significance. Statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA).
Author Contributions
Conceptualization, R.A.C. and J.P.L.; Methodology, R.A.C. and J.P.L.; Formal Analysis, P.V., S.A.-M. and J.P.L.; Resources, R.A.C.; Writing—Original Draft Preparation, J.P.L.; Writing—Review and Editing, R.A.C. and J.P.L.; Supervision, R.A.C. and J.P.L.; Funding Acquisition, R.A.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was performed in accordance with the guidelines of the European Community guidelines (EU Directive 2010/63/EU) and the Portuguese law on animal care (1005/92) and approved by the Ethical Committee of the Center for Neuroscience and Cell Biology of Coimbra.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
R.A.C. is a scientific advisor of the Institute for Scientific Information on Coffee (ISIC). All other authors declare no competing financial interests.
Funding Statement
This work was supported by “la Caixa” Banking Foundation (project LCF/PR/HP17/52190001), by Centro 2020 (CENTRO-01-0246-FEDER-000010) and by Fundação para a Ciência e Tecnologia (FCT, POCI-01-0145-FEDER-031274, UIDB/04539/2020).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith A. Effects of caffeine on human behavior. Food Chem. Toxicol. 2002;40:1243–1255. doi: 10.1016/S0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 2.Angelucci M.E.M., Cesário C., Hiroi R.H., Rosalen P.L., Da Cunha C. Effects of caffeine on learning and memory in rats tested in the Morris water maze. Braz. J. Med. Biol. Res. 2002;35:1201–1208. doi: 10.1590/S0100-879X2002001000013. [DOI] [PubMed] [Google Scholar]
- 3.Borota D., Murray E., Keceli G., Chang A., Watabe J.M., Ly M., Toscano J.P., Yassa M.A. Post-study caffeine administration enhances memory consolidation in humans. Nat. Neurosci. 2014;17:201–203. doi: 10.1038/nn.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong X., Li S., Sun J., Li Y., Zhang D. Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients. 2020;12:840. doi: 10.3390/nu12030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eskelinen M.H., Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimers Dis. 2010;20((Suppl. S1)):S167–S174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- 6.Maia L., de Mendonça A. Does caffeine intake protect from Alzheimer’s disease? Eur. J. Neurol. 2002;9:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 7.Paiva I., Cellai L., Meriaux C., Poncelet L., Nebie O., Saliou J.M., Lacoste A.S., Papegaey A., Drobecq H., Le Gras S., et al. Caffeine intake exerts dual genome-wide effects on hippocampal metabolism and learning-dependent transcription. J. Clin. Investig. 2022;132:e149371. doi: 10.1172/JCI149371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha R.A., Agostinho P.M. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J. Alzheimers Dis. 2010;20((Suppl. S1)):S95–S116. doi: 10.3233/JAD-2010-1408. [DOI] [PubMed] [Google Scholar]
- 9.Dall’Igna O.P., Fett P., Gomes M.W., Souza D.O., Cunha R.A., Lara D.R. Caffeine and adenosine A2a receptor antagonists prevent β-amyloid (25–35)-induced cognitive deficits in mice. Exp. Neurol. 2007;203:241–245. doi: 10.1016/j.expneurol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Laurent C., Eddarkaoui S., Derisbourg M., Leboucher A., Demeyer D., Carrier S., Schneider M., Hamdane M., Müller C.E., Buée L., et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol. Aging. 2004;35:2079–2090. doi: 10.1016/j.neurobiolaging.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Lopes J.P., Pliassova A., Cunha R.A. The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A1 and A2A receptors. Biochem. Pharmacol. 2019;166:313–321. doi: 10.1016/j.bcp.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Simons S.B., Caruana D.A., Zhao M., Dudek S.M. Caffeine-induced synaptic potentiation in hippocampal CA2 neurons. Nat. Neurosci. 2011;15:23–25. doi: 10.1038/nn.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaise J.H., Park J.E., Bellas N.J., Gitchell T.M., Phan V. Caffeine consumption disrupts hippocampal long-term potentiation in freely behaving rats. Physiol. Rep. 2018;6:e13632. doi: 10.14814/phy2.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin S.J., Grimwood P.D., Morris R.G. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 15.Cunha R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016;139:1019–1055. doi: 10.1111/jnc.13724. [DOI] [PubMed] [Google Scholar]
- 16.Li P., Rial D., Canas P.M., Yoo J.H., Li W., Zhou X., Wang Y., van Westen G.J., Payen M.P., Augusto E., et al. Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol. Psychiatry. 2015;20:1339–1349. doi: 10.1038/mp.2014.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagnussat N., Almeida A.S., Marques D.M., Nunes F., Chenet G.C., Botton P.H., Mioranzza S., Loss C.M., Cunha R.A., Porciúncula L.O. Adenosine A2A receptors are necessary and sufficient to trigger memory impairment in adult mice. Br. J. Pharmacol. 2015;172:3831–3845. doi: 10.1111/bph.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Temido-Ferreira M., Ferreira D.G., Batalha V.L., Marques-Morgado I., Coelho J.E., Pereira P., Gomes R., Pinto A., Carvalho S., Canas P.M., et al. Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol. Psychiatry. 2020;25:1876–1900. doi: 10.1038/s41380-018-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canas P.M., Porciúncula L.O., Cunha G.M., Silva C.G., Machado N.J., Oliveira J.M., Oliveira C.R., Cunha R.A. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by β-amyloid peptides via p38 mitogen-activated protein kinase pathway. J. Neurosci. 2009;29:14741–14751. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonçalves F.Q., Lopes J.P., Silva H.B., Lemos C., Silva A.C., Gonçalves N., Tomé Â.R., Ferreira S.G., Canas P.M., Rial D., et al. Synaptic and memory dysfunction in a β-amyloid model of early Alzheimer’s disease depends on increased formation of ATP-derived extracellular adenosine. Neurobiol. Dis. 2019;132:104570. doi: 10.1016/j.nbd.2019.104570. [DOI] [PubMed] [Google Scholar]
- 21.Laurent C., Burnouf S., Ferry B., Batalha V.L., Coelho J.E., Baqi Y., Malik E., Marciniak E., Parrot S., Van der Jeugd A., et al. A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol. Psychiatry. 2016;21:97–107. doi: 10.1038/mp.2014.151. [DOI] [PubMed] [Google Scholar]
- 22.Viana da Silva S., Haberl M.G., Zhang P., Bethge P., Lemos C., Gonçalves N., Gorlewicz A., Malezieux M., Gonçalves F.Q., Grosjean N., et al. Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat. Commun. 2016;7:11915. doi: 10.1038/ncomms11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Travassos M., Santana I., Baldeiras I., Tsolaki M., Gkatzima O., Sermin G., Yener G.G., Simonsen A., Hasselbalch S.G., Kapaki E., et al. Does Caffeine Consumption Modify Cerebrospinal Fluid Amyloid-β Levels in Patients with Alzheimer’s Disease? J. Alzheimers Dis. 2015;47:1069–1078. doi: 10.3233/JAD-150374. [DOI] [PubMed] [Google Scholar]
- 24.Arnaud M.J. Methylxanthines: Handbook of Experimental Pharmacology. Volume 200. Springer; Berlin/Heidelberg, Germany: 2011. Pharmacokinetics and metabolism of natural methylxanthines in animal and man; pp. 33–91. [DOI] [PubMed] [Google Scholar]
- 25.Miller G.E., Radulovic L.L., DeWit R.H., Brabec M.J., Tarka S.M., Cornish H.H. Comparative theobromine metabolism in five mammalian species. Drug Metab. Dispos. 1984;12:154–160. [PubMed] [Google Scholar]
- 26.Johnston J.J. Evaluation of cocoa- and coffee-derived methylxanthines as toxicants for the control of pest coyotes. J. Agric. Food Chem. 2005;53:4069–4075. doi: 10.1021/jf050166p. [DOI] [PubMed] [Google Scholar]
- 27.Soffietti M.G., Nebbia C., Valenza F., Amedeo S., Re G. Toxic effects of theobromine on mature and immature male rabbits. J. Comp. Pathol. 1989;100:47–58. doi: 10.1016/0021-9975(89)90089-3. [DOI] [PubMed] [Google Scholar]
- 28.Moreira A., Diógenes M.J., de Mendonça A., Lunet N., Barros H. Chocolate consumption is associated with a lower risk of cognitive decline. J. Alzheimers Dis. 2016;53:85–93. doi: 10.3233/JAD-160142. [DOI] [PubMed] [Google Scholar]
- 29.Smit H.J., Gaffan E.A., Rogers P.J. Methylxanthines are the psycho-pharmacologically active constituents of chocolate. Psychopharmacology. 2004;176:412–419. doi: 10.1007/s00213-004-1898-3. [DOI] [PubMed] [Google Scholar]
- 30.Mendiola-Precoma J., Padilla K., Rodríguez-Cruz A., Berumen L.C., Miledi R., García-Alcocer G. Theobromine-induced changes in A1 purinergic receptor gene expression and distribution in a rat brain Alzheimer’s disease model. J. Alzheimers Dis. 2017;55:1273–1283. doi: 10.3233/JAD-160569. [DOI] [PubMed] [Google Scholar]
- 31.Yoneda M., Sugimoto N., Katakura M., Matsuzaki K., Tanigami H., Yachie A., Ohno-Shosaku T., Shido O. Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice. J. Nutr. Biochem. 2017;39:110–116. doi: 10.1016/j.jnutbio.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Bruns R.F., Daly J.W., Snyder S.H. Adenosine receptors in brain membranes: Binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc. Natl. Acad. Sci. USA. 1980;77:5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daly J.W., Butts-Lamb P., Padgett W. Subclasses of adenosine receptors in the central nervous system: Interaction with caffeine and related methylxanthines. Cell Mol. Neurobiol. 1983;3:69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fredholm B.B., Persson C.G. Xanthine derivatives as adenosine receptor antagonists. Eur. J. Pharmacol. 1982;81:673–676. doi: 10.1016/0014-2999(82)90359-4. [DOI] [PubMed] [Google Scholar]
- 35.Amaechi B.T., Porteous N., Ramalingam K., Mensinkai P.K., Ccahuana Vasquez R.A., Sadeghpour A., Nakamoto T. Remineralization of artificial enamel lesions by theobromine. Caries Res. 2013;47:399–405. doi: 10.1159/000348589. [DOI] [PubMed] [Google Scholar]
- 36.Usmani O.S., Belvisi M.G., Patel H.J., Crispino N., Birrell M.A., Korbonits M., Korbonits D., Barnes P.J. Theobromine inhibits sensory nerve activation and cough. FASEB J. 2005;19:231–233. doi: 10.1096/fj.04-1990fje. [DOI] [PubMed] [Google Scholar]
- 37.Geraets L., Moonen H.J., Wouters E.F., Bast A., Hageman G.J. Caffeine metabolites are inhibitors of the nuclear enzyme poly(ADP-ribose)polymerase-1 at physiological concentrations. Biochem. Pharmacol. 2006;72:902–910. doi: 10.1016/j.bcp.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Papadimitriou A., Silva K.C., Peixoto E.B., Borges C.M., Lopes de Faria J.M., Lopes de Faria J.B. Theobromine increases NAD⁺/Sirt-1 activity and protects the kidney under diabetic conditions. Am. J. Physiol. 2015;308:F209–F225. doi: 10.1152/ajprenal.00252.2014. [DOI] [PubMed] [Google Scholar]
- 39.Sugimoto N., Katakura M., Matsuzaki K., Sumiyoshi E., Yachie A., Shido O. Chronic administration of theobromine inhibits mTOR signal in rats. Basic Clin. Pharmacol. Toxicol. 2019;124:575–581. doi: 10.1111/bcpt.13175. [DOI] [PubMed] [Google Scholar]
- 40.Costenla A.R., Diógenes M.J., Canas P.M., Rodrigues R.J., Nogueira C., Maroco J., Agostinho P.M., Ribeiro J.A., Cunha R.A., de Mendonça A. Enhanced role of adenosine A2A receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur. J. Neurosci. 2011;34:12–21. doi: 10.1111/j.1460-9568.2011.07719.x. [DOI] [PubMed] [Google Scholar]
- 41.Rebola N., Lujan R., Cunha R.A., Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Lopes L.V., Cunha R.A., Ribeiro J.A. Cross talk between A1 and A2A adenosine receptors in the hippocampus and cortex of young adult and old rats. J. Neurophysiol. 1999;82:3196–3203. doi: 10.1152/jn.1999.82.6.3196. [DOI] [PubMed] [Google Scholar]
- 43.Ciruela F., Casadó V., Rodrigues R.J., Luján R., Burgueño J., Canals M., Borycz J., Rebola N., Goldberg S.R., Mallol J., et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferreira S.T., Klein W.L. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol. Learn. Mem. 2011;96:529–543. doi: 10.1016/j.nlm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh D.M., Selkoe D.J. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Lopes J.P., Oliveira C.R., Agostinho P. Role of cyclin-dependent kinase 5 in the neurodegenerative process triggered by amyloid-β and prion peptides: Implications for Alzheimer’s disease and prion-related encephalopathies. Cell Mol. Neurobiol. 2007;27:943–957. doi: 10.1007/s10571-007-9224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang M.H., Mukherjee S., Choi M.J., Kang N.H., Pham H.G., Yun J.W. Theobromine alleviates diet-induced obesity in mice via phosphodiesterase-4 inhibition. Eur. J. Nutr. 2020;59:3503–3516. doi: 10.1007/s00394-020-02184-6. [DOI] [PubMed] [Google Scholar]
- 48.Fredholm B.B., Chen J.F., Cunha R.A., Svenningsson P., Vaugeois J.M. Adenosine and brain function. Int. Rev. Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 49.Thompson S.M., Haas H.L., Gahwiler B.H. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J. Physiol. 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwabe U., Ukena D., Lohse M.J. Xanthine derivatives as antagonists at A1 and A2 adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1985;330:212–221. doi: 10.1007/BF00572436. [DOI] [PubMed] [Google Scholar]
- 51.IJzerman A.P., Jacobson K.A., Müller C.E., Cronstein B.N., Cunha R.A. International Union of Basic and Clinical Pharmacology. CXII: Adenosine receptors: A further update. Pharmacol. Rev. 2022;74:340–372. doi: 10.1124/pharmrev.121.000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baggott M.J., Childs E., Hart A.B., de Bruin E., Palmer A.A., Wilkinson J.E., de Wit H. Psychopharmacology of theobromine in healthy volunteers. Psychopharmacology. 2013;228:109–118. doi: 10.1007/s00213-013-3021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carney J.M., Holloway F.A., Modrow H.E. Discriminative stimulus properties of methylxanthines and their metabolites in rats. Life Sci. 1985;36:913–920. doi: 10.1016/0024-3205(85)90386-8. [DOI] [PubMed] [Google Scholar]
- 54.Judelson D.A., Preston A.G., Miller D.L., Muñoz C.X., Kellogg M.D., Lieberman H.R. Effects of theobromine and caffeine on mood and vigilance. J. Clin. Psychopharmacol. 2013;33:499–506. doi: 10.1097/JCP.0b013e3182905d24. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell E.S., Slettenaar M., vd Meer N., Transler C., Jans L., Quadt F., Berry M. Differential contributions of theobromine and caffeine on mood, psychomotor performance and blood pressure. Physiol. Behav. 2011;104:816–822. doi: 10.1016/j.physbeh.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 56.Camandola S., Plick N., Mattson M.P. Impact of coffee and cacao purine metabolites on neuroplasticity and neurodegenerative disease. Neurochem. Res. 2019;44:214–227. doi: 10.1007/s11064-018-2492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cova I., Leta V., Mariani C., Pantoni L., Pomati S. Exploring cocoa properties: Is theobromine a cognitive modulator? Psychopharmacology. 2019;236:561–572. doi: 10.1007/s00213-019-5172-0. [DOI] [PubMed] [Google Scholar]
- 58.Martínez-Pinilla E., Oñatibia-Astibia A., Franco R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front. Pharmacol. 2015;6:30. doi: 10.3389/fphar.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Selkoe D.J. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 60.Cognato G.P., Agostinho P.M., Hockemeyer J., Müller C.E., Souza D.O., Cunha R.A. Caffeine and an adenosine A2A receptor antagonist prevent memory impairment and synaptotoxicity in adult rats triggered by a convulsive episode in early life. J. Neurochem. 2010;112:453–462. doi: 10.1111/j.1471-4159.2009.06465.x. [DOI] [PubMed] [Google Scholar]
- 61.Kaster M.P., Machado N.J., Silva H.B., Nunes A., Ardais A.P., Santana M., Baqi Y., Müller C.E., Rodrigues A.L., Porciúncula L.O., et al. Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc. Natl. Acad. Sci. USA. 2015;112:7833–7838. doi: 10.1073/pnas.1423088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Islam R., Matsuzaki K., Sumiyoshi E., Hossain M.E., Hashimoto M., Katakura M., Sugimoto N., Shido O. Theobromine improves working memory by activating the CaMKII/CREB/BDNF pathway in rats. Nutrients. 2019;11:888. doi: 10.3390/nu11040888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cimini A., Gentile R., D’Angelo B., Benedetti E., Cristiano L., Avantaggiati M.L., Giordano A., Ferri C., Desideri G. Cocoa powder triggers neuroprotective and preventive effects in a human Alzheimer’s disease model by modulating BDNF signaling pathway. J. Cell Biochem. 2013;114:2209–2220. doi: 10.1002/jcb.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J.F., Huang Z., Ma J., Zhu J., Moratalla R., Standaert D., Moskowitz M.A., Fink J.S., Schwarzschild M.A. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Augusto E., Matos M., Sévigny J., El-Tayeb A., Bynoe M.S., Müller C.E., Cunha R.A., Chen J.F. Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 2013;33:11390–11399. doi: 10.1523/JNEUROSCI.5817-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cunha R.A. Release of ATP and Adenosine and Formation of Extracellular Adenosine in the Hippocampus. In: Okada Y., editor. The Role of Adenosine in the Nervous System. Elsevier; Amsterdam, Netherlands: 2007. pp. 135–142. [Google Scholar]
- 67.Sebastião A.M., Cunha R.A., de Mendonça A., Ribeiro J.A. Modification of adenosine modulation of synaptic transmission in the hippocampus of aged rats. Br. J. Pharmacol. 2000;131:1629–1634. doi: 10.1038/sj.bjp.0703736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopes L.V., Halldner L., Rebola N., Johansson B., Ledent C., Chen J.F., Fredholm B.B., Cunha R.A. Binding of the prototypical adenosine A2A receptor agonist CGS 21680 to the cerebral cortex of adenosine A1 and A2A receptor knockout mice. Br. J. Pharmacol. 2004;141:1006–1014. doi: 10.1038/sj.bjp.0705692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Resende R., Ferreiro E., Pereira C., Resende de Oliveira C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-β peptide 1–42: Involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience. 2008;155:725–737. doi: 10.1016/j.neuroscience.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 70.Anderson W.W., Collingridge G.L. The LTP Program: A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Meth. 2001;108:71–83. doi: 10.1016/S0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- 71.Alçada-Morais S., Gonçalves N., Moreno-Juan V., Andres B., Ferreira S., Marques J.M., Magalhães J., Rocha J., Xu X., Partidário M., et al. Adenosine A2A receptors contribute to the radial migration of cortical projection neurons through the regulation of neuronal polarization and axon formation. Cereb. Cortex. 2021;31:5652–5663. doi: 10.1093/cercor/bhab188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.