Abstract
In the present study, the extracts of Cytinus hypocistis (L.) L using both traditional solvents (hexane, ethyl acetate, dichloromethane, ethanol, ethanol/water, and water) and natural deep eutectic solvents (NADESs) were investigated in terms of their total polyphenolic contents and antioxidant and enzyme-inhibitive properties. The extracts were found to possess total phenolic and total flavonoid contents in the ranges of 26.47–186.13 mg GAE/g and 0.68–12.55 mg RE/g, respectively. Higher total phenolic contents were obtained for NADES extracts. Compositional differences were reported in relation to antioxidant potential studied by several assays (DPPH: 70.19–939.35 mg TE/g, ABTS: 172.56–4026.50 mg TE/g; CUPRAC: 97.41–1730.38 mg TE/g, FRAP: 84.11–1534.85 mg TE/g). Application of NADESs (choline chloride—urea 1:2, a so-called Reline) allowed one to obtain the highest number of extracts having antioxidant potential in the radical scavenging and reducing assays. NADES-B (protonated by HCl L-proline-xylitol 5:1) was the only extractant from the studied solvents that isolated a specific fraction without chelating activity. Reline extract exhibited the highest acetylcholinesterase inhibition compared to NADES-B and NADES-C (protonated by H2SO4 L-proline-xylitol 5:1) extracts, which showed no inhibition. The NADES extracts were observed to have higher tyrosinase inhibitory properties compared to extracts obtained by traditional organic solvents. Furthermore, the NADES extracts were relatively better inhibitors of the diabetic enzymes. These findings provided an interesting comparison in terms of total polyphenolic content yields, antioxidant and enzyme inhibitory properties (cholinesterase, amylase, glucosidase, and tyrosinase) between traditional solvent extracts and NADES extracts, used as an alternative. While the organic solvents showed better antioxidant activity, the NADES extracts were found to have some other improved properties, such as higher total phenolic content and enzyme-inhibiting properties, suggesting functional prospects for their use in phytonutrient extraction and fractionation. The obtained results could also be used to give a broad overview of the different biological potentials of C. hypocistis.
Keywords: NADES, total polyphenolic content, antioxidants, enzyme inhibition, functional food, natural medicine, Alzheimer cholinesterase inhibitors
1. Introduction
The genus Cytinus, composed of endophytic parasitic plants (family: Cytinaceae), bears eight recognised species distributed around two centres of diversity: one in southern Africa and Madagascar, and one in the Mediterranean region [1].
Indeed, folkloric medicine has dedicated substantial consideration to this genus. These plants have been used traditionally for treating dysentery, including their ability to soothe inflammations of the eyes and throat. Some ethnobotanical reviews have also noted the use of Cytinus juice as an astringent, a haemostatic, and a tonic substance. They are also used as a scar-healing agent, whereby the scalp pulp is applied daily on corns and calluses, skin and swollen mucous membranes as an astringent and anti-inflammatory therapy [1,2].
Some studies have also pointed out these plants’ beneficial potential and suggested their antimicrobial effects over a range of bacterial strains and antioxidant activities [2,3,4]. Furthermore, they have been highlighted as good sources of biologically active ingredients of cosmeceutical interest [2,3,5]. In fact, their biological activities have been correlated with their high tannin content. For instance, hydrolysable tannins were found to be the active cytotoxic compounds identified in three Cytinus taxa and were assessed against a wide variety of cancer cell lines [6]. In another study, the tested extract of C. hypocistis was found to exhibit anti-inflammatory activity and effective cytotoxicity againcst tumour cells, while it showed the lowest cytotoxicity on a non-tumour cell line, and interestingly, hydrolysable tannins and flavonoids were also identified as the main groups in the extract [7].
Plants possess a diverse range of such bioactive constituents. However, their availability strongly depends on the extraction techniques used, among other factors. Even though to date numerous methods have been developed and upgraded, there is still a need to achieve a standardised solution with high consideration for the extraction of bioactive compounds from plants [8].
In addition, there is growing pressure to investigate alternative solvents that retain the technological advantages of organic solvents while posing less risk to human health and the environment. Deep eutectic solvents (DESs) and their specialised form obtained from compounds of natural origin—natural DESs (NADESs)—have shown the most promise in the field of green chemistry because they are abundant, inexpensive, recyclable, and appealing for a wide range of applications (food, cosmetic, and pharmaceutical). DESs have already proved to have several advantages in separation science, especially in terms of unusual selectivity useful in chromatography [9,10] and extraction [11,12], as well as membrane processes [13,14]. Many studies have effectively employed NADES extraction to gain high-quality extracts from numerous plants, including medicinal plants [15,16,17]. NADESs’ potential biological activity, bioavailability, and the availability of a variety of solvent combinations for its preparation are also intriguing characteristics. Therefore, NADES extraction is a cutting-edge technique that has piqued the interest of researchers and already exhibited great promise in the extraction and isolation of bioactive compounds from plants [17].
Therefore, the aim of the present study is to compare characteristics of extracts obtained by means of NADESs with classic organic solvents. For this purpose, the Cytinus hypocistis (L.) L. extracts were analysed in respect to their LC-MS profiles, the total phenolic (TPC) and flavonoid (TFC) contents, and antioxidant as well as enzyme-inhibitive properties.
2. Results and Discussion
2.1. Phytochemical Profiles
Natural products are important sources for drug development. Thus, it is of crucial importance to develop effective methods to extract and isolate these bioactive products. Indeed, the lab-intensive and laborious extraction and isolation processes have been a major challenge in the application of natural products in drug development. There is an urgent need to develop efficient and selective methods for this [18]. In this respect, different types of solvents have been widely used for the extraction of phytochemicals, whereby dried plant powders are used to extract bioactive phytochemicals and remove the Interference of water concomitantly. The solvents used for the extraction of biomolecules from plants are chosen based on the polarity of the desired solute. For example, a solvent with the same polarity as the solute will effectively dissolve the solute. Several solvents can be used sequentially to limit the number of analogous compounds in the desired yield [19].
Solubility, bioavailability, and stability are all factors in the pharmacological efficacy of plant extracts and their bioactive principles. Natural deep eutectic solvents (NADESs) are considered as green solvents to enhance the extraction performance of plant metabolites [16]. As functional liquid media, NADESs can dissolve both natural and synthetic substances with low water solubility. Hence, they are alternative candidates for applications with some organic solvents, as well as ionic liquids [20], indicating the enormous potential for NADESs to be utilised in the development of pharmaceutical formulations, such as nutraceuticals derived from plant-based metabolites [16]. Thus, in this study, both traditional solvents and NADESs were used to prepare C. hypocistis extracts and to compare their overall performance in terms of their bioactive content yields and biological activities.
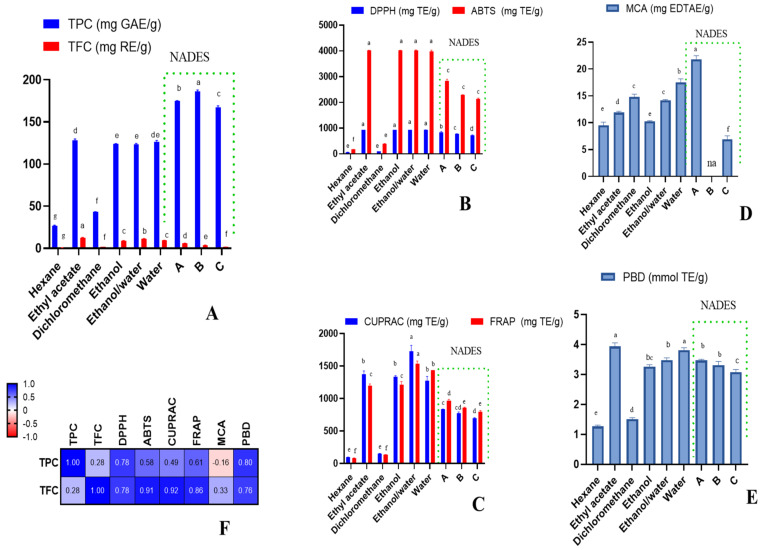
In the phytochemical studies, the investigation of the polyphenol content present in plant extracts is an important part of assessing their biological properties. In this study, the extracts were found to possess TPC and TFC in the range of 26.47–186.13 mg GAE/g and 0.68–12.55 mg RE/g, respectively. Interestingly, the extracts obtained by NADESs yielded higher TPC (167.57–186.13 mg GAE/g), followed by ethyl acetate, water, ethanol, and ethanol/water extracts (123.51–127.83 mg GAE/g). On the other hand, the dichloromethane and hexane extracts yielded low TPC. In the TFC assay, the highest yield was obtained by ethyl acetate and ethanol/water extracts, followed by water and ethanol extracts, while the least TFC was yielded by hexane, NADES-C, and dichloromethane extracts (Figure 1, Table S1). The variation in total polyphenol content clearly varied with the polarity of the solvents used. However, not only the polarity of the solvents, but also other parameters such as pH, extraction time, methods, and temperature can affect the extraction yield and total phenolic content [21,22,23].
Figure 1.
Total phenolic and flavonoid content (A), radical scavenging ability (B), reducing power (C), metal-chelating ability (MCA) (D), total antioxidant ability (by phosphomolybdenum assay (PBD)) (E), and Pearson’s correlations between total bioactive compounds and antioxidant assays (p < 0.05) (F). na: not active; different letters in column for same assays indicate significant differences in the extracts (p < 0.05).
2.2. Characterization of Polar Bioactive Compounds from C. hypocistis Extracts by UPLC-ESI-QTOF-MS
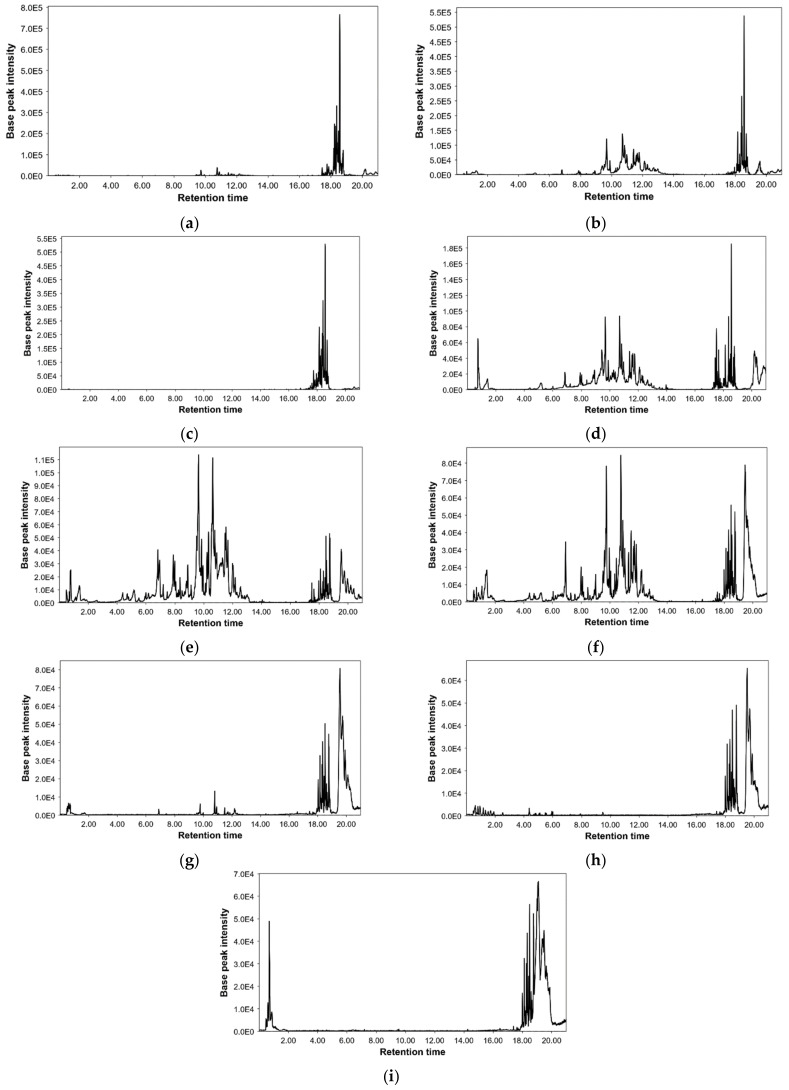
Following the described LC-MS method, all extracts were analysed, resulting in a total of 148 detected compounds. Figure 2 shows the base peak chromatograms performed for each extraction condition and Table S2 summarises all information about detected compounds such as retention time, m/z ratio, error in ppm, molecular formula, and name of each proposed compound. In addition, peak numbers were assigned according to their elution order. The peak areas of the detected compounds are given in Table S3.
Figure 2.
Base peak chromatograms from Cytinus (a) hexane, (b) ethyl acetate, (c) dichloromethane, (d) ethanol, (e) ethanol/water, (f) water, (g) NADES-A, (h) NADES-B, and (i) NADES-C extracts by UPLC-MS.
It is worth to note that to our knowledge, there is little reference to a comprehensive characterisation of C. hypocistis extracts [1]. For this reason, our work is especially relevant. Considering the accurate mass spectra information and data previously reported by literature, 136 compounds were tentatively annotated in this study. Only one common molecular feature was detected among all the different extractions; however, this molecular feature could not be annotated and remained as an unknown compound (Table 1). This could be explained mainly by the differences in the extraction efficiencies of the different solvents used, also considering the different physicochemical properties of the compounds present in the matrix.
Table 1.
Chemical characterisation of the tested extracts.
| Peak | Compound | Hexane | Ethyl Acetate | Dichloromethane | Ethanol | Ethanol/Water | Water | NADES-A | NADES-B | NADES-C |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hydroxy-pseudouric acid | + | + | + | ND | + | + | ND | + | ND |
| 2 | Galloyl-galactarolactone | + | + | ND | ND | + | + | ND | ND | ND |
| 3 | Unknown 1 | + | + | + | + | + | + | + | + | + |
| 4 | Unknown 2 | ND | ND | ND | ND | ND | ND | ND | + | ND |
| 5 | Proline | ND | ND | ND | ND | ND | ND | + | ND | + |
| 6 | Disaccharide | + | + | ND | + | + | + | ND | ND | ND |
| 7 | Unknown 3 | ND | ND | ND | ND | ND | ND | ND | ND | + |
| 8 | Glucose | ND | ND | ND | + | + | ND | ND | ND | ND |
| 9 | Unknown 4 | ND | ND | ND | ND | ND | ND | ND | ND | + |
| 10 | Quinic acid | ND | + | ND | + | + | + | ND | ND | ND |
| 11 | Galloyl-diglucose | ND | ND | ND | + | + | ND | ND | ND | ND |
| 12 | Galloylglucose isomer 1 | + | + | ND | + | + | + | ND | + | ND |
| 13 | Galloylglucose isomer 2 | + | + | ND | + | + | + | ND | + | ND |
| 14 | Galloylglucose isomer 3 | + | + | ND | + | + | + | ND | + | ND |
| 15 | Pyrogallol | ND | + | ND | + | + | + | + | + | + |
| 16 | Gallic acid | ND | + | ND | + | + | + | + | + | + |
| 17 | Galloylglucose isomer 4 | ND | + | ND | + | + | + | ND | + | ND |
| 18 | Fukiic acid | ND | + | ND | ND | ND | + | ND | + | ND |
| 19 | Digalloylglucose isomer 1 | ND | + | ND | + | + | + | ND | + | ND |
| 20 | Digalloylglucose isomer 2 | ND | ND | ND | + | + | + | ND | + | ND |
| 21 | Digalloylglucose isomer 3 | ND | + | ND | + | + | + | ND | + | ND |
| 22 | Digalloylglucose isomer 4 | ND | + | ND | + | + | + | ND | + | ND |
| 23 | Brevifolin carboxylic acid | ND | ND | ND | ND | + | + | ND | ND | ND |
| 24 | Digallate isomer 1 | ND | ND | ND | + | + | + | ND | ND | ND |
| 25 | Unknown 5 | + | + | ND | ND | ND | ND | ND | ND | ND |
| 26 | Trigalloyl-glucoside isomer 1 | ND | + | ND | + | + | + | + | ND | ND |
| 27 | HHDP-galloylglucose isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 28 | Galloylnorbergenin isomer 1 | + | + | ND | + | + | + | + | ND | ND |
| 29 | Brevifolin | ND | ND | ND | + | + | + | + | ND | ND |
| 30 | Galloylnorbergenin isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 31 | Digalloyl-HHDP-glucose isomer 1 | ND | ND | ND | + | + | + | ND | ND | ND |
| 32 | Galloylnorbergenin isomer 3 | + | + | ND | + | + | + | ND | ND | ND |
| 33 | Trigalloyl-glucoside isomer 2 | ND | + | ND | + | + | + | ND | + | ND |
| 34 | HHDP-galloylglucose isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 35 | Trigalloyl-glucoside isomer 3 | ND | + | ND | + | + | + | ND | + | ND |
| 36 | Digalloyl-HHDP-glucose isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 37 | HHDP-galloylglucose isomer 3 | ND | + | ND | + | + | + | ND | ND | ND |
| 38 | Trigalloyl-glucoside isomer 4 | ND | + | ND | + | + | + | ND | ND | ND |
| 39 | Terflavin B isomer 1 | ND | + | ND | + | ND | ND | ND | ND | ND |
| 40 | Galloyl-HHDP-glucose isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 41 | Tetragalloyl-glucoside isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 42 | Balanophotannin E isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 43 | Trigalloyl-HHDP-glucose isomer 1 | ND | ND | ND | ND | + | ND | ND | ND | ND |
| 44 | Terflavin B isomer 2 | ND | + | ND | + | ND | ND | ND | ND | ND |
| 45 | Galloflavin | + | + | ND | ND | ND | ND | ND | ND | ND |
| 46 | Ellagic acid | + | + | ND | + | + | + | + | + | ND |
| 47 | Terflavin B isomer 3 | + | + | ND | + | ND | ND | ND | ND | ND |
| 48 | Trigalloyl-HHDP-glucose isomer 2 | + | + | ND | + | + | + | ND | ND | ND |
| 49 | Digalloyl-lactonised valoneoyl-d-glucose isomer 1 | + | + | ND | + | ND | ND | ND | ND | ND |
| 50 | Trigalloyl-brevifolincarboxyl-glucose isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 51 | Tetragalloyl-glucoside isomer 2 | + | + | ND | + | + | + | + | ND | + |
| 52 | Catechin | ND | + | ND | + | + | + | ND | ND | ND |
| 53 | Tetragalloyl-glucoside isomer 3 | + | + | ND | + | + | + | + | ND | + |
| 54 | Digallate isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 55 | Tetragalloyl-glucoside isomer 4 | + | + | ND | + | + | + | + | ND | ND |
| 56 | Digallate isomer 3 | ND | + | ND | + | + | + | ND | ND | ND |
| 57 | Terflavin B isomer 4 | + | + | ND | + | + | ND | ND | ND | ND |
| 58 | Galloyl-HHDP-glucose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 59 | Tetragalloyl-glucoside isomer 5 | + | + | ND | + | + | + | + | ND | ND |
| 60 | Neochebulagic acid isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 61 | Neochebulagic acid isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 62 | Isorhamnetin glucoside isomer 1 | + | + | ND | + | + | + | ND | ND | ND |
| 63 | Epicatechin | ND | + | ND | + | + | + | ND | ND | ND |
| 64 | Quercetin | ND | + | ND | ND | + | ND | ND | ND | ND |
| 65 | Balanophotannin E isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 66 | Unknown 6 | + | + | ND | + | + | + | ND | ND | ND |
| 67 | Digalloyl-lactonised valoneoyl-d-glucose isomer 2 | ND | + | ND | + | ND | ND | ND | ND | ND |
| 68 | Trigalloyl-brevifolincarboxyl-glucose isomer 2 | + | + | ND | + | + | + | ND | ND | ND |
| 69 | Pentagalloyl-glucose isomer 1 | + | + | ND | + | + | + | ND | ND | ND |
| 70 | Neochebulagic acid isomer 3 | ND | ND | ND | ND | + | ND | ND | ND | ND |
| 71 | Trigalloyl-lactonised valoneoyl glucose isomer 1 | ND | + | ND | + | + | ND | ND | ND | ND |
| 72 | (Galloyl)galloyl-tetragalloylglucose isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 73 | Trigalloyl-brevifolincarboxyl-glucose isomer 3 | + | + | ND | + | + | + | ND | ND | ND |
| 74 | Trigalloyl-DHHDP-glucose isomer 1 | + | + | ND | ND | ND | ND | ND | ND | ND |
| 75 | Digalloyl-lactonised valoneoyl-d-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 76 | Castalagin | + | + | ND | ND | ND | ND | ND | ND | ND |
| 77 | Trigalloyl-HHDP-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 78 | Galloyl-penta-hydroxy-benzoic-brevifolincarboxyl-glucose isomer 1 | + | + | ND | + | + | + | + | ND | ND |
| 79 | Pentagalloyl-glucose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 80 | Trisgalloyl HHDP glucose isome isomer 1 | + | + | ND | ND | ND | ND | ND | ND | ND |
| 81 | Amurensisin | + | + | ND | + | + | + | ND | ND | ND |
| 82 | Trigalloyl-DHHDP-glucose isomer 2 | ND | + | ND | + | ND | + | ND | ND | ND |
| 83 | Digalloyl-lactonised valoneoyl-d-glucose isomer 4 | + | + | ND | + | ND | ND | ND | ND | ND |
| 84 | Pentagalloyl-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 85 | Galloyl-penta-hydroxy-benzoic-brevifolincarboxyl-glucose isomer 2 | + | + | ND | + | + | + | ND | ND | ND |
| 86 | Trigalloyl-brevifolincarboxyl-glucose isomer 4 | + | + | ND | + | + | ND | ND | ND | ND |
| 87 | Phyllanthusiin C isomer 1 | + | + | ND | + | + | + | ND | ND | ND |
| 88 | Trigalloyl-DHHDP-glucose isomer 3 | + | + | ND | ND | + | + | ND | ND | ND |
| 89 | Ethyl gallate | ND | ND | ND | + | + | + | ND | ND | ND |
| 90 | Trigalloyl-brevifolincarboxyl-glucose isomer 5 | + | + | ND | + | + | + | ND | ND | ND |
| 91 | Trisgalloyl HHDP glucose isome isomer 2 | ND | + | ND | ND | ND | + | ND | ND | ND |
| 92 | Trigalloyl-lactonised valoneoyl glucose isomer 2 | + | + | ND | + | ND | ND | ND | ND | ND |
| 93 | (Galloyl)galloyl-tetragalloylglucose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 94 | Digalloyl-HHDP-iso DHDG-glucose isomer 1 | + | + | ND | + | ND | ND | ND | ND | ND |
| 95 | Balanophotannin E isomer 3 | ND | + | ND | + | + | ND | ND | ND | ND |
| 96 | Isorhamnetin glucoside isomer 2 | ND | + | ND | + | + | ND | ND | ND | ND |
| 97 | Hexagalloyl-glucose isomer 1 | + | + | ND | + | + | + | + | ND | ND |
| 98 | Trigalloyl-lactonised valoneoyl glucose isomer 3 | + | + | ND | + | + | ND | ND | ND | ND |
| 99 | Ellagic acid derivative | + | + | ND | ND | ND | + | ND | ND | ND |
| 100 | (Galloyl)galloyl-tetragalloylglucose isomer 3 | + | + | ND | + | + | + | ND | ND | ND |
| 101 | (Galloyl)galloyl-tetragalloylglucose isomer 4 | + | + | ND | + | + | + | ND | ND | ND |
| 102 | Galloyl-penta-hydroxy-benzoic-brevifolincarboxyl-glucose isomer 3 | ND | + | ND | + | + | + | ND | ND | ND |
| 103 | Hexagalloyl-glucose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 104 | Galloyl-HHDP-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 105 | Hexagalloyl-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 106 | Digalloyl-HHDP-iso DHDG-glucose isomer 2 | ND | + | ND | ND | + | + | ND | ND | ND |
| 107 | Tetragalloyl-hydroxybenzoyl-glucopyranoside isomer 1 | ND | + | ND | + | + | ND | ND | ND | ND |
| 108 | Heptagalloyl hexose isomer 1 | + | + | ND | + | + | + | ND | ND | ND |
| 109 | Galloylmyricetin | + | + | ND | + | + | + | + | ND | ND |
| 110 | Heptagalloyl hexose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 111 | Phyllanthusiin C isomer 2 | ND | + | ND | ND | + | + | ND | ND | ND |
| 112 | Digalloyl-HHDP-iso DHDG-glucose isomer 3 | ND | + | ND | ND | + | + | ND | ND | ND |
| 113 | Trigalloyl-brevifolincarboxyl-glucose isomer 6 | ND | + | ND | + | + | ND | ND | ND | ND |
| 114 | Heptagalloyl hexose isomer 3 | + | + | ND | + | + | + | ND | ND | ND |
| 115 | Tetragalloyl-hydroxybenzoyl-glucopyranoside isomer 2 | + | + | ND | + | + | + | ND | ND | ND |
| 116 | Tetragalloyl-hydroxybenzoyl-glucopyranoside isomer 3 | + | + | ND | + | ND | + | ND | ND | ND |
| 117 | Unknown 7 | ND | ND | ND | + | ND | ND | ND | ND | ND |
| 118 | Trihydroxy-octadecenoic acid | ND | + | + | ND | ND | ND | ND | ND | ND |
| 119 | Hydroxyretinoic acid | + | ND | + | ND | ND | ND | ND | ND | ND |
| 120 | Unknown 8 | + | + | + | ND | ND | ND | ND | ND | ND |
| 121 | Hexadecanedioic acid | + | + | + | ND | ND | ND | ND | ND | ND |
| 122 | Hydroxyeicosatrienoic acid | + | + | + | + | ND | ND | ND | ND | ND |
| 123 | Valerenic acid | + | + | + | + | ND | ND | ND | ND | ND |
| 124 | Hydroxylinoleic acid | + | + | + | ND | ND | ND | ND | ND | ND |
| 125 | Linoleic acid | ND | + | + | + | ND | ND | ND | ND | ND |
| 126 | Hydroxylinolenic acid | + | + | + | + | + | ND | ND | ND | ND |
| 127 | Dodecenyl-succinic anhydride | + | + | + | + | ND | ND | ND | ND | ND |
| 128 | Retinoic acid | + | + | + | ND | ND | ND | ND | ND | ND |
| 129 | Oleic acid | + | + | + | + | + | ND | ND | + | ND |
| 130 | Pentadecenoic acid | + | + | + | + | + | ND | ND | ND | ND |
| 131 | Oleiyl glucoside | ND | + | + | ND | ND | ND | ND | ND | ND |
| 132 | Linolenic acid | + | + | + | + | + | + | ND | ND | ND |
| 133 | Stearic acid | + | + | ND | + | ND | ND | ND | ND | ND |
| 134 | Unknown 9 | + | + | + | + | + | ND | ND | ND | ND |
| 135 | Unknown 10 | + | + | + | + | ND | ND | ND | ND | ND |
| 136 | Eicosapentaenoic acid | + | + | + | + | ND | + | ND | ND | ND |
| 137 | Palmitoleic acid | + | + | + | ND | + | + | + | ND | + |
| 138 | Linoleic acid | + | + | + | + | + | + | ND | ND | + |
| 139 | Oxodecanedioic acid | + | ND | ND | ND | ND | ND | ND | ND | ND |
| 140 | Methyl arachidonate | + | + | + | + | ND | ND | ND | ND | ND |
| 141 | Arjungenin | ND | ND | + | ND | ND | ND | ND | ND | ND |
| 142 | Heptadecenoic acid | + | ND | ND | ND | + | + | ND | ND | ND |
| 143 | Unknown 11 | ND | + | + | + | ND | ND | ND | ND | ND |
| 144 | Unknown 12 | + | + | + | + | + | + | ND | ND | ND |
| 145 | Glycerylmonooleate | + | ND | ND | ND | ND | ND | ND | ND | ND |
| 146 | Palmitic acid | + | ND | ND | + | + | + | + | + | + |
| 147 | Hydroxydocosanoic acid | + | + | + | + | + | ND | ND | ND | ND |
| 148 | Dodecenylsuccinic acid | ND | + | ND | ND | + | ND | ND | ND | ND |
+: present; ND: nondetected.
Overall, the tentative characterisation allowed one to classify the compounds in five major groups: gallotannins, ellagitannins, flavonoids, fatty acids, and other compounds, with it being important to note that gallotannins were the most important one, with 61 compounds included. These were mainly annotated as mono-, di-, tri-, tetra-, penta-, hexa-, and hepta-galloyl hexoside. In addition, different isomers were also annotated for each of these types of chemical structures. Silva et al., reported in 2020 from mono- to penta-galloyl hexosides in different parts (petals, stalks, and nectar) of C. hypocistis [24]. However, all these compounds were previously reported in other sources such as Magnifera indica L. kernels and peels [25,26], Rhodiola crenulate roots [27], Rhodiola rosea roots, leaves, stems and flowers [27], Paeonia plants [28], and Pistacia vera leaves [29], among others [30]. Table 1 shows that a major number of these gallotannins were found in ethyl acetate, ethanol, water/ethanol, and water extracts. In recent years, several authors have reported a wide range of biological properties of these galloyl hexoside derivatives [27,29,31,32]. Among other gallic acid derivatives presented in the gallotannins group, three isomers from neochebulagic acid corresponding to peaks 60, 61, and 70 have been also annotated. These compounds were reported in Terminalia chebula Retz. Playing a role in the intestinal glucose transport [33]. Moreover, several compounds previously found in Trapa quadrispinosa pericarps were detected in our extracts, such as peaks 49, 67, 75, and 87 (digalloyl-lactonised valoneoyl-d-glucose isomers); peaks 71, 92, and 98 (trigalloyl-lactonised valoneoyl glucose isomers); and peaks 78, 85, and 102 (galloyl-penta-hydroxy-benzoic-brevifolincarboxyl-glucose isomers) [34].
As the second most important group, ellagitannins contains 30 compounds, which were annotated as different mono-, di-, and tri-galloyl-DHHDP-glucose isomers and digalloyl-HHDP-iso DHDG-glucose isomers. Besides these compounds, other ellagitannins such as terflavin B, phyllanthussin C, geraniin, and balanophlorotannin E isomers have been previously reported in various Terminalia species and in Trapa species [34,35]. In case of our species, only peaks corresponding to m/z 937 and 783 were annotated [24].
Regarding flavonoids, catechin and epicatechin as flavan-3-ols were found; quercetin and two isorhamnetin glucoside isomers as flavonols were also detected, mostly with water, ethanol, their mixture, and ethylacetate.
On the other hand, hexane and dichloromethane extracts presented the major number of fatty acids (Peaks 118, 119, 121–132, 135–141, 144–148). Regarding NADESs, the three extracts showed the lower number of features, and many of the features obtained could not be annotated such as peaks 7 and 9 (unknowns 3 and 4) were only found in NADES-C extract. The same happened for peak 2 (unknown 2); this compound was only detected in NADES-B. Among them, NADES-A presented high number of features corresponding mainly to gallotannins. This highlights the potential of NADESs to obtain extracts containing additional bioactive compounds and to “tailor” the properties of extracts. Secondly, extraction by two NADESs sequentially should allow for the fractionation of bioactive compounds.
Other compounds have been also annotated, although they have not been classified in specific groups due to the low number and the high range of structures. For instance, organic acids (quinic and fukiic acids); simple phenols (gallic and ellagic acids); and two alkyl-phenylketones, namely brevifolin and brevifolin carboxylic acid [36], were included in this group. In addition, three isomers from galloylnorbergenin, antioxidant isocoumarins that were previously found in leaves of Diospyros gilletii De Wild [37] were also included.
2.3. Antioxidant Effects
Antioxidants are important chemical substances that occur naturally in food and can reduce or prevent oxidative stress of the physiological system as the body continuously produces free radicals. Oxidative stress plays a key role in the development of chronic and degenerative diseases such as cancer, autoimmune diseases, and neurodegenerative and cardiovascular diseases. The human body has a variety of mechanisms to counter oxidative stress by producing antioxidants that are either naturally produced in situ or supplied externally through foods such as plants, as a rich source of naturally produced antioxidants. Hence, antioxidants acting as free radical scavengers can eventually help to avert and repair cellular damage generated by these radicals [38,39].
Herein, moderate-to-very-potent antioxidant activity was noted for C. hypocistis extracts. For instance, in the radical scavenging assays, the ethanol/water, ethanol, ethyl acetate, water, and NADES-A extracts demonstrated very high antioxidant potential (DPPH: 829.11–939.35 mg TE/g; ABTS: 2830.66–4026.50 mg TE/g), followed by NADES-C and NADES-B (DPPH: 701.49 and 767.55 mg TE/g; ABTS: 2134.94 and 2285.15 mg TE/g, respectively). On the other hand, a much lower scavenging ability was displayed by the hexane and dichloromethane extracts (DPPH: 70.19 and 93.25 mg TE/g; 172.56 and 398.03 mg TE/g, respectively), compared to the other extracts. The same trend was observed with the reducing assays, whereby the ethanol/water, ethanol, ethyl acetate, water, and NADES-A extracts showed significant reducing capacity in the range of 1730.38–1377.38 mg TE/g and 968.98–1534.85 mg TE/g in CUPRAC and FRAP assays, respectively, whereas a lower reducing activity was noted for NADES-B and NADES-C and a much lower content for hexane and dichloromethane extracts (CUPRAC: 97.41–774.94 mg TE/g; FRAP: 84.11–860.90 mg TE/g) (Figure 1, Table S4). It is clear that most valuable bioactive components are extracted by relatively polar extracts, while low-polarity solvents such as n-hexane and dichloromethane are not effective in this case. In Figure 1, Pearson’s correlation analysis indicates a linear correlation (R > 0.7) between total flavanoid content and radical scavenging and reducing power assays.
While only NADES-B did not possess metal-chelating activity, all the other extracts showed metal-chelating potential in the range of 6.87–21.76 mg EDTAE/g. The extracts also demonstrated total antioxidant capacity in phosphomolybdenum assay (1.27–3.94 mmol TE/g). The lowest total antioxidant capacity was revealed for hexane and dichloromethane extracts (Figure 1). This part of the research reveals a possibility to isolate metal-chelating components by step extraction using NADES-B at the first stage, followed by a second extractant that is effective to extract this specific group of compounds present in the studied plant.
2.4. Enzyme Inhibitory Effects
Low levels of the neurotransmitter acetylcholine, oxidative stress, and inflammation in the central nervous system (CNS) are hallmarks of Alzheimer’s disease (AD), a progressive neurodegenerative disease. To date, patients diagnosed with AD are only offered enzyme inhibitors (acetylcholinesterase/butyrylcholinesterase, or AChE/BChE) for treatment [40]. Hence, as mechanism of pharmacological action, these cholinesterase inhibitors are able to modify cholinergic signalling by disrupting the degradation of acetylcholine [41].
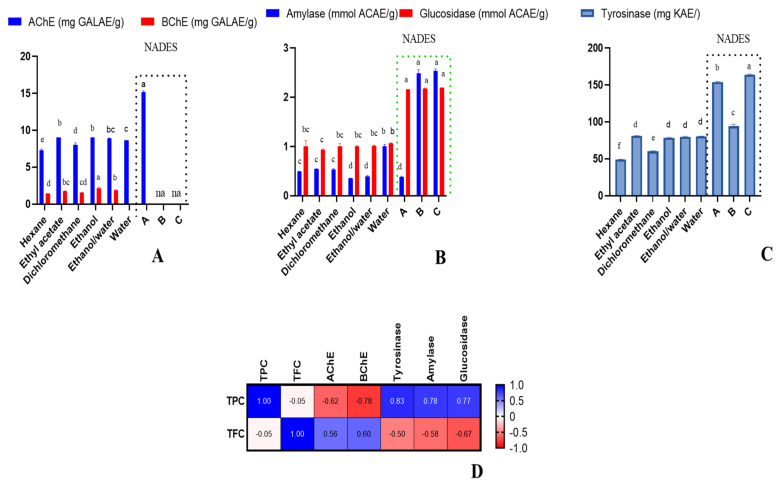
All extracts, except NADES-B and NADES-C extracts, possessed anti-AChE activity (7.32–15.16 mg GALAE/g). Interestingly, the highest anti-AChE activity was revealed for NADES-A. It is particularly important observation, as components of NADES-A—choline chloride and urea—are nontoxic; thus, such obtained extracts could be further used without removal of NADES. On the other hand, only the extracts prepared in the traditional way, with solvents hexane, ethyl acetate, dichloromethane, ethanol, and ethanol/water, displayed anti-BChE potential (1.39–2.13 mg GALAE/g). The water extract and the NADES extracts showed no anti-BChE activity. It follows from the ionic nature of the used NADESs, which in this case was not favourable for extraction. The ethanol extract demonstrated relatively higher BChE inhibitory effect compared to the other extracts (Figure 3).
Figure 3.
Cholinesterase inhibitory effects (A), amylase and glucosidase inhibitory effects (B), tyrosinase inhibitory effects (C), Pearson’s correlations between total bioactive compounds and enzyme inhibitory assays (p < 0.05) (D). na: not active. Different letters in column for same assays indicate significant differences in the extracts (p < 0.05).
Tyrosinase is the rate-limiting enzyme in melanin synthesis. Melanin is synthesised in human melanocytes when tyrosine is hydroxylated to l-DOPA, which is then oxidised to dopaquinone and polymerises to form melanin. Melasma, melanoma, and freckles are just some of the dermatological conditions that can develop when melanin production increases too rapidly. However, tyrosinase in plant-based foods oxidises phenolic compounds into quinones. The former reacts with amino acids and proteins to produce brown/black pigments, a process known as enzymatic browning, which is one of the most pressing problems in the food industry and the source of 50 percent of the industry’s economic losses. In addition, browning reduces the food’s nutritional value and safety because it leads to the loss of vitamin C, antioxidants, and other nutrients, and can even lead to the production of antinutritional and toxic substances. Consequently, tyrosinase inhibition is seen as an efficient method for preventing hyperpigmentation in the pharmaceutical industry and delaying enzymatic browning, which is helpful in the food industry [42].
In the current investigation, all the studied extracts were found to possess antityrosinase activity (49.14–153.97 mg KAE/g). However, NADES-A exhibited the highest inhibitory activity against tyrosinase, while the hexane extract displayed the lowest (Figure 3). On this basis, it is clear that polar NADES-A, as well as other polar solvents, should be preferred for the extraction of bioactive compounds responsible for tyrosinase activity.
Interestingly, in the study by Zucca et al. [2], the ethanolic extract of C. hypocistis showed the highest tyrosinase inhibition activity, compared to cyclohexane and water extracts, probably due to being predominantly rich in polyphenols, in most part hydrolysable tannins. C. hypocistis aerial part extract was also reported to show tyrosinase inhibition of 80%, when tested at 50 μg/mL [43]. Furthermore, a linear correlation was obtained between enzymatic activities and increasing TPC and TFC. The reason could be a specific class of polyphenols acting against tyrosinase through a competitive inhibition mechanism, thus interfering with the biological function of tyrosinase, which is a polyphenoloxidase [43].
The inhibition of the carbohydrate-digesting enzymes alpha-glucosidase and alpha-amylase is an important strategy for controlling blood glucose levels in patients with Type 2 diabetes and borderline diabetes, because it significantly reduces the postprandial rise in blood glucose [44]. Even though drugs such as voglibose, acarbose, and miglitol are commercially available as those enzymes’ inhibitors and are also used in practice, they produce undesirous effects such as abdominal discomfort, bloating, and diarrhoea.
In addition, many chronic diseases such as diabetes are associated with oxidative stress, during which reactive oxygen species (O2−, H2O2 and OH−) are generated. The role of free radicals in the onset and development of diabetes has also been established. Therefore, compounds that possess both antidiabetic and antioxidant properties without causing serious side effects would be of great value [45].
In the present investigation, all extracts were found to inhibit both carbohydrate-hydrolysing enzymes (Amylase: 0.35–2.54 mmol ACAE/g; glucosidase: 0.93–2.20 mmol ACAE/g). Remarkably, the NADES extracts were found to be better inhibitors of amylase and glucosidase compared the other extracts (Figure 3). This could be due to the higher TPC in the NADES extracts, and this fact was also confirmed by Pearson’s correlation analysis (Figure 3). In fact, it has been previously suggested that phenolics are involved in the modulation of the activity of starch digestive enzymes [46].
2.5. Data Mining
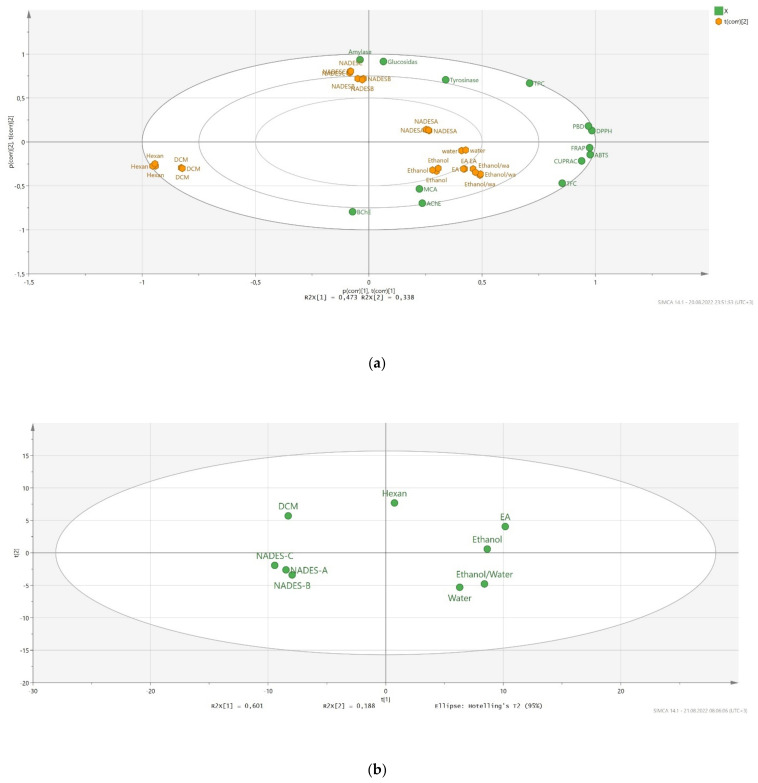
To gain more insight into the tested extracts and biological activity assays, we performed PCA analysis. The results are given in Figure 4. Firstly, we examined the relationship between the tested extracts based on the biological activity results. We obtained a good distribution, and the tested extracts were very well-separated based on the biological activity results. Two components (PC1: 47.3% and PC2: 33.8) accounted for 811% of the total components. Two extracts (hexane and dichloromethane) exhibited the lowest biological abilities and were distributed in the same axis. In addition, polar extracts (ethanol, ethanol/water, water) and NADES-A had similar biological abilities and were placed in the same group. The PCA plot also confirmed a strong correlation between total flavonoid and antioxidant properties, which were very close to each other on the PCA plot. In addition to biological activity results, we investigated the similarities/differences of the tested extracts based on their chemical profiles. Two components were used in the analysis to determine the distribution of the tested solvents (PC1: 60.1% and PC2: 18.8%). In Figure 4b, the used nonpolar and polar solvents and NADESs were clearly separated, and these results were very similar to the distribution from the biological activity results. Taken together, we concluded that there is a good connection between chemical compounds and biological activities of Cytinus extracts.
Figure 4.
Principal component analysis between tested extracts and biological activities (a). Distribution of the tested extracts in principal component analysis by using chemical compound peak areas (b).
3. Materials and Methods
3.1. Materials
The investigated DESs were prepared from reagents of >99% purity (Sigma Aldrich, Burlington, VT, USA), while hydrochloric and sulphuric acid for DES protonation were of analytical reagent grade (POCH, Gliwice, Poland).
3.2. Apparatus
DES: chemicals were precisely prepared on a weight basis using a AS.310.R2 analytical balance (Radwag, Radom, Poland). A 06-MSH-PRO-T magnetic stirrer (Chemland, Stargard, Poland) was used to prepare DESs.
3.3. Preparation of NADESs
Three NADESs having different properties were used. NADES-A—choline chloride—urea 1:2; NADES-B protonated by HCl L-proline-xylitol 5:1; NADES-C, protonated by H2SO4 L-proline-xylitol 5:1.
Two synthesis routes were used.
NADES-A was prepared by simple mixing of compounds at 60 °C. The synthesis of the deep eutectic solvents based on protonated L-proline (NADES-A and -B) involved dissolving L-proline in an acid solution (the amount of acid with respect to L-proline was equimolar). Next, a predetermined amount of xylitol was added to the solution. Thus, prepared solution was placed in a rotary evaporator model Rotavapor R-300 (Buchi, Flawil, Switzerland) and water was distilled off under reduced pressure. Studies on the synthesis of this type of DESs and their physicochemical characteristics were the subject of a separate paper [47].
3.4. Plant Material and Preparation of Extracts
Cytinus hypocistis samples were collected at the flowering season in June 2020 (Anamur, Mersin, Turkey). The plants were identified by one of the authors (Dr. Evren Yildiztugay) and voucher specimens were deposited at the herbarium of Selcuk University, Konya, Turkey. The samples’ aerial parts were dried in the shade at room temperature for about 7 days, and then ground into a powder using a mill. All of the samples were kept in a dark place.
In this study, the extracts were prepared using traditional solvents (n-hexane, ethyl acetate, dichloromethane, ethanol, ethanol/water (70%), and water) and NADEs. Maceration was employed as the extraction method to obtain n-hexane, ethyl acetate, dichloromethane, EtOH, and EtOH/water extracts. The plant materials (10 g) were macerated overnight at room temperature with 200 mL of these solvents. Finally, the solvents were evaporated from the mixtures. To obtain water extracts, the plant materials (10 g) were kept with 200 mL of boiled water, and then the extracts were filtered and lyophilised. In the preparation of NADE extracts, the plant materials (10 g) were mixed with the NADESs for 20 min at 25 °C in an ultrasonic bath. The extracts were filtered and all extracts were stored at 4 °C until further analysis was required.
3.5. Chemical Reagents
All chemicals were of HPLC-MS grade and used as received. Acetic acid and acetonitrile for UPLC were purchased from Fluka (Sigma-Aldrich, Steinheim, Germany) and Lab-Scan (Gliwice, Sowinskiego, Poland), respectively. For solutions, ultrapure water was obtained with a Milli-Q system Millipore (Bedford, MA, USA), and absolute ethanol was purchased from VWR chemicals (Radnor, PA, USA).
3.6. UPLC-ESI-QTOF-MS Conditions
Cytinus extracts were redissolved at 5 mg/mL in the same extraction solvent and filtered by 0.22 µm. The compounds were separated using an ACQUITY UPLC H-Class System (Waters Corp., Milford, MA, USA) with a reversed-phase column (ACQUITY UPLC BEH Shield RP18, 130Å, 1.7 µm, 2.1 mm × 150 mm) at a flow rate of 0.7 mL/min and using a injection volume of 10 μL. The mobile phases were acidified water (0.5% acetic acid, v/v) and acetonitrile as solvents A and B, respectively. The following multi-step linear gradient was used in order to achieve an efficient separation: 0.00 min [A:B 99/1], 2.33 min [A:B 99/1], 4.37 min [A:B 93/7], 8.11 min [A:B 86/14], 12.19 min [A:B 76/24], 15.99 min [A:B 60/40], 18.31 min [A:B 2/98], 21.03 min [A:B 2/98], 22.39 min [A:B 99/1] and 25.00 [A:B 99/1].
The UPLC was coupled to an electrospray-quadrupole-time of flight mass spectrometer (ESI-QTOF-MS) Synapt G2 (Waters Corp., Milford, MA, USA) working in negative-ionisation mode in a m/z range from 50 to 1200 m/z. The MS acquisition was based on two parallel scan functions switching between them continuously. The first function was operated at low collision energy in the gas cell (4 eV) and the other at an elevated collision energy (MSE energy linear ramp: from 20 to 60 eV). Leu-enkephalin was injected for mass calibration continuously. Other MS parameters were, as follows, source temperature 100 °C; scan duration 0.1 s; resolution 20,000 FWHM; desolvation temperature 500 °C; desolvation gas flow 700 L/h; capillary voltage 2.2 kV; cone voltage 30 V; cone gas flow 50 L/h. Finally, the acquired data were processed using MZmine 2.53 open-source software and Sirius 4.4.29.
3.7. UPLC-ESI-QTOF-MS Data Processing
Firstly, the raw data files were transformed to. mzML format using MSConvert software. The converted data were processed using the open-source software MZmine 2.53 (Pluskal et al., 2020). A noise level of 1.0 × 103 was selected. ADAP chromatogram builder method was used under the following parameters: MS level: 1; min number of scans: 9; group intensity threshold: 1.0 × 103; min highest intensity: 1.0 × 104; m/z tolerance: 10 ppm. After that, the chromatogram was deconvoluted using the Wavelets (ADAP) algorithm and the following parameters: S/N threshold: 50; min feature height: 5E4; coefficient/area threshold: 110; peak duration range: 0.05–0.3 min; RT wavelet range: 0–0.30. An isotopic peak grouper algorithm was also applied (m/z tolerance: 10 ppm, RT tolerance: 0.02 min, maximum charge: 2). The obtained features were aligned between samples using the “Join Aligner” algorithm using a m/z tolerance of 10 ppm and a RT tolerance of 0.1 min. The molecular features, which were also detected in blank samples, were filtered from the final dataset. Finally, the molecular formulas of the final features were predicted using Sirius 4.4.29 (Dührkop et al., 2019) and the biological identities were annotated by comparing the MS/MS spectra of different databases (e.g., MoNA, Massbank, HMDB, FoodDB, etc.), with the fragments detected in the MSE scans.
3.8. Determination of Total Polyphenol and Flavonoids Contents
Total phenolic and flavonoid contents were calculated with the Folin-Ciocalteu and AlCl3 assays, respectively [48]. Gallic acid equivalents (mg GAEs/g dry extract) and rutin equivalents (mg REs/g dry extract) were used to describe the outcomes of the two tests.
3.9. Antioxidant and Enzyme Inhibitory Assays
The antioxidant and enzyme inhibitory activity of comfrey root extracts was assessed according to methods presented previously [49,50]. Data were expressed as: mg Trolox equivalents (TE)/g extract in ferric ion reducing antioxidant power (FRAP), cupric ion reducing antioxidant capacity (CUPRAC), ABTS and DPPH radical scavenging activity; mg EDTA equivalents (EDTAE)/g extract in the metal chelating ability (MCA), mmol TE/g extract in the phosphomolybdenum assay (PBD); mg galanthamine equivalents (GALAE)/g extract in AChE and BChE assays; mg kojic acid equivalents (KAE)/g extract in tyrosinase inhibitory assay; and mmol acarbose equivalents (ACAE)/g extract in amylase and glucosidase assays.
3.10. Data Analysis
All analyses were performed in triplicate and results were reported as means ± SD. Pearson’s correlation coefficients were calculated between total bioactive components and biological activity parameters. Pearson’s correlation was performed by GraphPad version 9.0. The relationship between species, chemical compounds and bioactivities was also assessed using principal component analysis (PCA). PCA analysis was performed by SIMCA version 14.0.
4. Conclusions
In the present study, nine extracts of C. hypocistis obtained using traditional solvents and NADESs were investigated for their total polyphenolic contents, antioxidant and enzyme inhibitory properties. The extracts were found to be richer in TPC than TFC. In particular, the NADES extracts were found to yield higher TPC compared to the other extracts. On the other hand, generally, ethanol/water, ethanol, and water showed very potent and better antioxidant potential compared to the other extracts, whereas hexane and dichloromethane exhibited weaker antioxidant potential in almost all antioxidant assays. While NADES-A extract displayed the highest anti-AChE activity, none of the NADES extracts displayed inhibition against the butylcholinesterase. With the exception of the water extract, all the other traditional solvent extracts showed dual cholinesterase inhibitory properties. Remarkably, the NADES extracts were found to have an enhanced antityrosinase effect compared to the traditional solvent extracts. Similarly, the NADES extracts were found to be better glucosidase inhibitors, and the NADES-B and -C extracts showed higher antiamylase activity in comparison with the other studied extracts. It is worth highlighting that this study demonstrated NADESs to have brought some improved abilities in bioactive content yields and bioactivity compared to the traditional extracts, especially in terms of TPC and some of the enzyme inhibitory activities. However, the traditional solvents were much better in extracting the antioxidant compounds. Hence, the current investigation enabled a comparison between traditional solvents and NADESs and suggested the potential of NADESs as an alternative to traditional organic solvents for higher extraction of phytonutrients and some better biological performance, although the traditional solvents were found to be more effective in yielding higher antioxidant activity. The paper proved the importance of natural medicine where sources of pro-health components are taken from plants. NADESs as mixtures obtained from compounds of natural origin, in respect to the studied examples, are nontoxic; thus, their extracts could be used as components of functional foods as well as food additives without the need of NADES removal.
Acknowledgments
The authors M.d.l.L.C.-G. and Á.F.-O. would like to thank the Regional Ministry of Economy, Knowledge, Enterprise and Universities of Andalusia for the contract for Young Researchers (PAIDI) at the University of Granada. F.J.L.-J. thanks the Spanish Ministry of Science and Innovation for the postdoctoral contract Juan de la Cierva-Formación (FJC2020-044298-I).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27185788/s1, Table S1: Total phenolic (TPC) and flavonoid content (TFC) of the tested extracts; Table S2: Proposed annotated compounds by UPLC-ESI-QTOF-MS in all Cytinus extracts; Table S3: Compound areas extracted for each Cytinus extract; Table S4: Antioxidant properties of the tested extracts; Table S5: Enzyme inhibitory of the tested extracts.
Author Contributions
Conceptualisation, G.Z., M.d.l.L.C.-G. and G.B.; methodology, G.Z., M.d.l.L.C.-G., Á.F.-O., F.J.L.-J., M.M., E.Y., R.K. and G.B.; software, G.Z., M.d.l.L.C.-G. and G.B.; validation, R.K., S.J. and M.F.M.; formal analysis, G.Z.; investigation, G.Z., S.J. and M.F.M.; resources, G.Z., E.Y. and R.K.; data curation, G.Z., M.d.l.L.C.-G. and M.F.M.; writing—original draft preparation, G.Z., M.d.l.L.C.-G., S.J. and M.F.M.; writing—review and editing, M.M. and G.B.; visualisation, G.Z.; supervision, G.B., A.S.C.; project administration, G.Z. and G.B.; funding acquisition, G.Z. and A.S.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
M. Momotko and G. Boczkaj gratefully acknowledge the financial support from the National Science Centre, Warsaw, Poland—decision no. UMO-2018/30/E/ST8/00642.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sanjust E., Rinaldi A.C. Cytinus under the Microscope: Disclosing the Secrets of a Parasitic Plant. Plants. 2021;10:146. doi: 10.3390/plants10010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucca P., Pintus M., Manzo G., Nieddu M., Steri D., Rinaldi A.C. Antimicrobial, antioxidant and anti-tyrosinase properties of extracts of the Mediterranean parasitic plant Cytinus hypocistis. BMC Res. Notes. 2015;8:562. doi: 10.1186/s13104-015-1546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisetta G., Batoni G., Caboni P., Esin S., Rinaldi A.C., Zucca P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complementary Altern. Med. 2019;19:82. doi: 10.1186/s12906-019-2487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandrone M., Bonvicini F., Lianza M., Sanna C., Maxia A., Gentilomi G., Poli F. Sardinian plants with antimicrobial potential. Biological screening with multivariate data treatment of thirty-six extracts. Ind. Crops Prod. 2019;137:557–565. doi: 10.1016/j.indcrop.2019.05.069. [DOI] [Google Scholar]
- 5.Silva A.R., Pinela J., García P.A., Ferreira I.C., Barros L. Cytinus hypocistis (L.) L.: Optimised heat/ultrasound-assisted extraction of tannins by response surface methodology. Sep. Purif. Technol. 2021;276:119358. doi: 10.1016/j.seppur.2021.119358. [DOI] [Google Scholar]
- 6.Magiatis P., Pratsinis H., Kalpoutzakis E., Konstantinidou A., Davaris P., Skaltsounis A.-L. Hydrolyzable tannins, the active constituents of three Greek Cytinus taxa against several tumor cell lines. Biol. Pharm. Bull. 2001;24:707–709. doi: 10.1248/bpb.24.707. [DOI] [PubMed] [Google Scholar]
- 7.Silva A.R., Ayuso M., Pereira C., Dias M.I., Kostić M., Calhelha R.C., Soković M., García P.A., Ferreira I.C., Barros L. Evaluation of parasite and host phenolic composition and bioactivities—The Practical Case of Cytinus hypocistis (L.) L. and Halimium lasianthum (Lam.) Greuter. Ind. Crops Prod. 2022;176:114343. doi: 10.1016/j.indcrop.2021.114343. [DOI] [Google Scholar]
- 8.Koçak E., Pazır F. Effect of Extraction Methods on Bioactive Compounds of Plant Origin. Turk. J. Agric. Food Sci. Technol. 2018;6:663–675. [Google Scholar]
- 9.Momotko M., Łuczak J., Przyjazny A., Boczkaj G. A natural deep eutectic solvent-protonated L-proline-xylitol-based stationary phase for gas chromatography. J. Chromatogr. A. 2022;1676:463238. doi: 10.1016/j.chroma.2022.463238. [DOI] [PubMed] [Google Scholar]
- 10.Momotko M., Łuczak J., Przyjazny A., Boczkaj G. First deep eutectic solvent-based (DES) stationary phase for gas chromatography and future perspectives for DES application in separation techniques. J. Chromatogr. A. 2021;1635:461701. doi: 10.1016/j.chroma.2020.461701. [DOI] [PubMed] [Google Scholar]
- 11.Faraz N., Haq H.U., Balal Arain M., Castro-Muñoz R., Boczkaj G., Khan A. Deep eutectic solvent based method for analysis of Niclosamide in pharmaceutical and wastewater samples—A green analytical chemistry approach. J. Mol. Liq. 2021;335:116142. doi: 10.1016/j.molliq.2021.116142. [DOI] [Google Scholar]
- 12.Serna-Vázquez J., Ahmad M.Z., Boczkaj G., Castro-Muñoz R. Latest Insights on Novel Deep Eutectic Solvents (DES) for Sustainable Extraction of Phenolic Compounds from Natural Sources. Molecules. 2021;26:5037. doi: 10.3390/molecules26165037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khajavian M., Vatanpour V., Castro-Muñoz R., Boczkaj G. Chitin and derivative chitosan-based structures—Preparation strategies aided by deep eutectic solvents: A review. Carbohydr. Polym. 2022;275:118702. doi: 10.1016/j.carbpol.2021.118702. [DOI] [PubMed] [Google Scholar]
- 14.Castro-Muñoz R., Msahel A., Galiano F., Serocki M., Ryl J., Hamouda S.B., Hafiane A., Boczkaj G., Figoli A. Towards azeotropic MeOH-MTBE separation using pervaporation chitosan-based deep eutectic solvent membranes. Sep. Purif. Technol. 2022;281:119979. doi: 10.1016/j.seppur.2021.119979. [DOI] [Google Scholar]
- 15.Grozdanova T., Trusheva B., Alipieva K., Popova M., Dimitrova L., Najdenski H., Zaharieva M.M., Ilieva Y., Vasileva B., Miloshev G. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020;14:73. doi: 10.1186/s13065-020-00726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hikmawanti N.P.E., Ramadon D., Jantan I., Mun’im A. Natural deep eutectic solvents (Nades): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants. 2021;10:2091. doi: 10.3390/plants10102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlić B., Mrkonjić Ž., Teslić N., Kljakić A.C., Pojić M., Mandić A., Stupar A., Santos F., Duarte A.R.C., Mišan A. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules. 2022;27:1508. doi: 10.3390/molecules27051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q.-W., Lin L.-G., Ye W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Friesen J.B., McAlpine J.B., Lankin D.C., Chen S.-N., Pauli G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018;81:679–690. doi: 10.1021/acs.jnatprod.7b00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewi S.R., Stevens L.A., Pearson A.E., Ferrari R., Irvine D.J., Binner E.R. Investigating the role of solvent type and microwave selective heating on the extraction of phenolic compounds from cacao (Theobroma cacao L.) pod husk. Food Bioprod. Process. 2022;134:210–222. doi: 10.1016/j.fbp.2022.05.011. [DOI] [Google Scholar]
- 23.Belwal T., Ezzat S.M., Rastrelli L., Bhatt I.D., Daglia M., Baldi A., Devkota H.P., Orhan I.E., Patra J.K., Das G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018;100:82–102. doi: 10.1016/j.trac.2017.12.018. [DOI] [Google Scholar]
- 24.Silva A.R., Pinela J., Dias M.I., Calhelha R.C., Alves M.J., Mocan A., García P.A., Barros L., Ferreira I.C. Exploring the phytochemical profile of Cytinus hypocistis (L.) L. as a source of health-promoting biomolecules behind its in vitro bioactive and enzyme inhibitory properties. Food Chem. Toxicol. 2020;136:111071. doi: 10.1016/j.fct.2019.111071. [DOI] [PubMed] [Google Scholar]
- 25.Luo F., Fu Y., Xiang Y., Yan S., Hu G., Huang X., Huang G., Sun C., Li X., Chen K. Identification and quantification of gallotannins in mango (Mangifera indica L.) kernel and peel and their antiproliferative activities. J. Funct. Foods. 2014;8:282–291. doi: 10.1016/j.jff.2014.03.030. [DOI] [Google Scholar]
- 26.Namngam C., Pinsirodom P., Boonyuen S. Fractionation, antioxidant and inhibitory activity of Thai mango seed kernel extracts. Czech J. Food Sci. 2018;36:8–15. doi: 10.17221/225/2017-CJFS. [DOI] [Google Scholar]
- 27.Olennikov D.N., Chirikova N.K., Vasilieva A.G., Fedorov I.A. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of Rhodiola rosea herb metabolites: A comparative study with subterranean organs. Antioxidants. 2020;9:526. doi: 10.3390/antiox9060526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong N.-N., Zhou X.-Y., Peng L.-P., Liu Z.-A., Shu Q.-Y. A comprehensive study of three species of Paeonia stem and leaf phytochemicals, and their antioxidant activities. J. Ethnopharmacol. 2021;273:113985. doi: 10.1016/j.jep.2021.113985. [DOI] [PubMed] [Google Scholar]
- 29.Gok H.N., Pekacar S., Orhan D.D. Investigation of Enzyme Inhibitory Activities, Antioxidant Activities, and Chemical Properties of Pistacia vera Leaves Using LC-QTOF-MS and RP-HPLC. Iran. J. Pharm. Res. 2022;21:e127033. doi: 10.5812/ijpr-127033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gan R.-Y., Kong K.-W., Li H.-B., Wu K., Ge Y.-Y., Chan C.-L., Shi X.-M., Corke H. Separation, identification, and bioactivities of the main gallotannins of red sword bean (Canavalia gladiata) coats. Front. Chem. 2018;6:39. doi: 10.3389/fchem.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho K.-V., Roy A., Foote S., Vo P.H., Lall N., Lin C.-H. Profiling anticancer and antioxidant activities of phenolic compounds present in black walnuts (Juglans nigra) using a high-throughput screening approach. Molecules. 2020;25:4516. doi: 10.3390/molecules25194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quintana S.E., Salas S., García-Zapateiro L.A. Bioactive compounds of mango (Mangifera indica): A review of extraction technologies and chemical constituents. J. Sci. Food Agric. 2021;101:6186–6192. doi: 10.1002/jsfa.11455. [DOI] [PubMed] [Google Scholar]
- 33.Wang H., Fowler M.I., Messenger D.J., Ordaz-Ortiz J.J., Gu X., Shi S., Terry L.A., Berry M.J., Lian G., Wang S. Inhibition of the intestinal postprandial glucose transport by gallic acid and gallic acid derivatives. Food Funct. 2021;12:5399–5406. doi: 10.1039/D1FO01118A. [DOI] [PubMed] [Google Scholar]
- 34.Liang X., Jiang Y., Guo Z., Fang S. Separation, UPLC-QTOF-MS/MS analysis, and antioxidant activity of hydrolyzable tannins from water caltrop (Trapa quadrispinosa) pericarps. LWT. 2020;133:110010. doi: 10.1016/j.lwt.2020.110010. [DOI] [Google Scholar]
- 35.Chang Z., Zhang Q., Liang W., Zhou K., Jian P., She G., Zhang L. A Comprehensive Review of the Structure Elucidation of Tannins from Terminalia Linn. Evid.-Based Complement. Altern. Med. 2019;2019:8623909. doi: 10.1155/2019/8623909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fidelis M., de Moura C., Kabbas Junior T., Pap N., Mattila P., Mäkinen S., Putnik P., Bursać Kovačević D., Tian Y., Yang B. Fruit seeds as sources of bioactive compounds: Sustainable production of high value-added ingredients from by-products within circular economy. Molecules. 2019;24:3854. doi: 10.3390/molecules24213854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tameye N.S.J., Akak C.M., Happi G.M., Frese M., Stammler H.-G., Neumann B., Lenta B.N., Sewald N., Nkengfack A.E. Antioxidant norbergenin derivatives from the leaves of Diospyros gilletii De Wild (Ebenaceae) Phytochem. Lett. 2020;36:63–67. doi: 10.1016/j.phytol.2020.01.012. [DOI] [Google Scholar]
- 38.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008;4:89. [PMC free article] [PubMed] [Google Scholar]
- 40.Szwajgier D., Baranowska-Wójcik E., Winiarska-Mieczan A., Gajowniczek-Ałasa D. Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients. 2022;14:2969. doi: 10.3390/nu14142969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pope C., Karanth S., Liu J. Pharmacology and toxicology of cholinesterase inhibitors: Uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005;19:433–446. doi: 10.1016/j.etap.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 42.Yu Z.-Y., Xu K., Wang X., Wen Y.-T., Wang L.-J., Huang D.-Q., Chen X.-X., Chai W.-M. Punicalagin as a novel tyrosinase and melanin inhibitor: Inhibitory activity and mechanism. LWT. 2022;161:113318. doi: 10.1016/j.lwt.2022.113318. [DOI] [Google Scholar]
- 43.Chiocchio I., Mandrone M., Sanna C., Maxia A., Tacchini M., Poli F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018;122:498–505. doi: 10.1016/j.indcrop.2018.06.029. [DOI] [Google Scholar]
- 44.Tundis R., Loizzo M., Menichini F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010;10:315–331. doi: 10.2174/138955710791331007. [DOI] [PubMed] [Google Scholar]
- 45.Alqahtani A.S., Hidayathulla S., Rehman M.T., ElGamal A.A., Al-Massarani S., Razmovski-Naumovski V., Alqahtani M.S., El Dib R.A., AlAjmi M.F. Alpha-amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules. 2019;10:61. doi: 10.3390/biom10010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aleixandre A., Gil J.V., Sineiro J., Rosell C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022;372:131231. doi: 10.1016/j.foodchem.2021.131231. [DOI] [PubMed] [Google Scholar]
- 47.Janicka P., Przyjazny A., Boczkaj G. Novel “acid tuned” deep eutectic solvents based on protonated L-proline. J. Mol. Liq. 2021;333:115965. doi: 10.1016/j.molliq.2021.115965. [DOI] [Google Scholar]
- 48.Zengin G., Aktumsek A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014;11:481–488. doi: 10.4314/ajtcam.v11i2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uysal S., Zengin G., Locatelli M., Bahadori M.B., Mocan A., Bellagamba G., De Luca E., Mollica A., Aktumsek A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017;8:290. doi: 10.3389/fphar.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grochowski D.M., Uysal S., Aktumsek A., Granica S., Zengin G., Ceylan R., Locatelli M., Tomczyk M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017;20:365–372. doi: 10.1016/j.phytol.2017.03.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.