Abstract
Alleles of glpR associated with the same GlpR− phenotype produce substitutions in different conserved portions of the glycerol 3-phosphate repressor which are not part of the helix-turn-helix motif. Analysis of the effects on growth and enzyme expression show that glucose repression of glycerol utilization is not dependent on a functional repressor.
In the course of strain construction for studies of allosteric regulatory properties of glycerol kinase (EC 2.7.1.30; ATP-glycerol 3-phosphotransferase), we identified a novel allele for the glycerol 3-phosphate repressor. It confers a GlpR− phenotype that is indistinguishable from that seen for the glpR2 allele, which contains an undetermined defect (5). We report here that the sequences of these two alleles are different. Phenotypes associated with these alleles reveal that a functional glycerol 3-phosphate repressor is not required for glucose inhibition of glycerol utilization.
Strain KH11 was constructed to serve as a prototrophic strain with a wild-type glycerol kinase. Characterization of KH11 showed high levels of glycerol kinase enzymatic activity when cells are grown in Luria-Bertani (LB) medium, i.e., noninducing conditions (Table 1). The specific activities of glycerol kinase were determined in extracts of cells that were grown overnight in LB medium at 37°C, collected by centrifugation, and disrupted by sonication after being resuspended in 0.1 M triethanolamine-HCl, 2 mM glycerol, and 2 mM β-mercaptoethanol (pH 7.0). Glycerol kinase activities were determined by using an ADP-coupled continuous spectrophotometric assay at pH 7.0 and 25°C with 2 mM glycerol and 2.5 mM ATP (8). Protein concentrations were determined by using the Bio-Rad assay with bovine serum albumin as the standard. The glycerol kinase activity of strain KH11 is much higher than that of strains MC4100, MG1655, and MM130. High expression of glycerol kinase after growth in rich medium was reported for Lin strain 43 (11) and is verified as shown in Table 1. The phenotype has been termed constitutive and is associated with an undetermined defect in the glpR2 allele (5). Table 1 shows also that the same specific activity is obtained for strains KH11, MC4100, and MG1655 after growth under normal induction conditions in minimal glycerol medium. The absence of further induction in strain KH11 under these conditions indicates that the expression is constitutive rather than an elevation of the basal level. However, the specific activity after the growth of strain KH11 in LB medium is about twofold higher, indicating that additional induction is possible in rich medium.
TABLE 1.
Glycerol kinase specific activities in E. coli strains
| Strain | Genotypea | Source and/or reference | Growth mediumb | Mean glycerol kinase sp act (U/mg) ± SD |
|---|---|---|---|---|
| JM83 | F−ara glpR208 Δ(lac-proAB) rpsL(Strr) [φ80dlacΔ(lacZ)M15] thi | Ry Young (7) | LB | 1.9 ± 0.1 |
| JM101 | glpR208 supE thi Δ(lac-proAB) [F′ traD36 proAB lacIqZΔM15] | CGSC (7) | LB | 1.8 ± 0.1 |
| TB1 | JM83 glpR208 hsdR(rK− mK+) | Ry Young (1) | LB | 1.9 ± 0.1 |
| DG1 | TB1 ΔglpK202 glpR208 | 6 | LB | 0.3 ± 0.02 |
| KH11 | DG1 glpK+glpR208 pro+ | DG1 × Hfr PK3 | LB | 2.5 ± 0.6 |
| mG | 1.3 ± 0.5 | |||
| KH62 | KH11 glpR+malT::Tn10 | KH11 × P1.MM130 | LB | 0.3 ± 0.02 |
| KH20 | JM83 glpR+malT::Tn10 | JM83 × P1.MM130 | LB | 0.3 ± 0.02 |
| KH21 | JM83 glpR208 malT::Tn10 | JM83 × P1.MM130 | LB | 1.7 ± 0.6 |
| Lin43 | HfrC fadL701 glpK22 glpR2 phoA8 pit-10 relA1 sopT1 tonA22 T2R | CGSC (11) | LB | 2.4 ± 0.9 |
| MC4100 | F−araD139 Δ(argF-lac)U169 deoC1 flbB5301 ptsF25 rbsR relA1 rpsL150(Strr) | CGSC | LB | 0.4 ± 0.2 |
| mG | 1.6 ± 0.7 | |||
| TS100 | MC4100 glpR2 | Tim Larson | LB | 2.5 ± 0.5 |
| KH44 | MC4100 ΔglpEGR::kan | MC4100 × P1.NZ42 | LB | 1.7 ± 0.2 |
| NZ42 | MC4100 φ(glpK::lacZ)(Hyb) ΔglpEGR::kan | Tim Larson | NDc | |
| MG1655 | λ−rph-1 | CGSC | LB | 0.2 ± 0.1 |
| mG | 1.5 ± 0.2 | |||
| MM130 | malT::Tn10 | Mike Manson | LB | 0.4 ± 0.02 |
The allele number glpR208 was assigned by the E. coli Genetic Stock Center (CGSC) after the DNA sequence was determined and is added here to the genotypes of the relevant strains.
LB, LB medium; mG, minimal M9 salts with 1% (vol/vol) glycerol.
ND, not determined.
None of the Escherichia coli strains that were used in construction of KH11 were known to contain defective glpR alleles. Strain DG1, from which strain KH11 was derived, was constructed to provide a GlpK− background (6). DG1 is a derivative of TB1, which was chosen for efficient transformation and α-complementation (1). Table 1 shows that both TB1 and its parent, JM83 (7), display the constitutive phenotype. These results suggest that parent strain JM83 and its derivatives lack glycerol 3-phosphate repressor function. Strain JM83 was generated from strains CSH51 and 71.18 during the construction of strain JM101 (7). Table 1 also shows that JM101 displays the constitutive phenotype.
The role of the glpR locus in the constitutive phenotype of strain JM83 was investigated by using P1 transduction. The entry for strain KH20 in Table 1 shows that repair of the constitutive phenotype cotransduces with the malT::Tn10 marker from strain MM130. The cotransduction frequencies were 70 and 72% in two separate experiments, a finding which agrees well with the frequency that is expected (73% [9]) from the proximity of the marker, located at 76.5 min, to the glpEGR locus at 76.7 min (2). Strain KH21, isolated as Tetr from the same transduction, retains the constitutive phenotype, showing that the restored repression is not associated with the marker per se. Table 1 shows also that P1 transduction of strain KH11 restores the GlpR+ phenotype (KH62). These results indicate that the constitutive phenotype is not associated with defects at the glpFKX locus at 88.8 min, i.e., the altered operator.
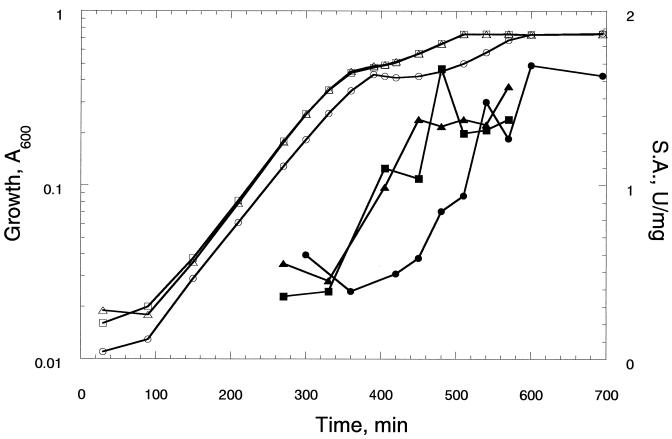
Diauxic growth in glucose-glycerol was used to evaluate the role of the repressor in glucose control of glycerol utilization and the expression of glycerol kinase. Figure 1 shows growth and glycerol kinase specific activity in cultures incubated at 37°C in minimal M9 medium with 2.5 mM glucose and 5 mM glycerol for strains MC4100, TS100, and KH44. The medium was inoculated with cells from overnight cultures in minimal glucose that were washed with minimal M9 salts. The constitutive phenotype is associated with shortening of the plateau of growth that occurs following glucose depletion and preceding glycerol utilization. However, repression of glycerol kinase by glucose does not depend on the glpR phenotype. This contrasts with results reported for glucose repression of the lactose operon, for which β-galactosidase increases in lacI strains (4). Glycerol kinase activity is repressed by glucose in the absence of glycerol in the glpR2 strains, Lin7 and Lin43 (11). Results from diauxic growth curves and glycerol kinase expression for strains KH62 and KH11 (not shown) are the same as those shown for MC4100 and TS100, respectively. Strain KH44 (ΔglpR) was constructed to assess the extent of the repressor defect. Diauxic growth and glycerol kinase expression are indistinguishable for strains TS100 and KH44, indicating that the constitutive phenotype is associated with complete loss of repressor function.
FIG. 1.
Effect of glpR mutations on diauxic growth and the expression of glycerol kinase. Cultures were prepared as described in the text. The open symbols show the change in the A600 following inoculation, and the filled symbols show the glycerol kinase specific activity (S.A.). Symbols: circles, MC4100; squares, TS100; triangles, KH44.
Loss of repressor function could be associated with glpEGR promoter defects and/or the expression of a defective repressor. To determine whether these alleles encode variant repressors, the glpR locus from strains MC4100, MM130, Lin43, JM83, and DG1 was amplified from chromosomal DNA by PCR using Taq DNA polymerase and a Perkin-Elmer 2400 thermal cycler. Thermocycling conditions were as follows: 92°C for 4 min, followed by 30 cycles of 92°C for 30 s, 45°C for 1 min, and 72°C for 2 min, and finally, 72°C for 6 min. Both strands of the amplification products were sequenced using ABI Prism Dye Terminator Cycle Sequencing Core Kit with Big Dye premix from Perkin-Elmer, and thermocycling conditions were as recommended by ABI. Sequence products were purified using spin columns of P-30 polyacrylamide gel from Bio-Rad. The purified product was loaded onto an ABI 373 or 377 DNA Sequencing System in the Gene Technologies Laboratory of Texas A&M University. Sequence analysis was performed using Sequencher software.
The sequences of the alleles from strains MC4100 and MM130 are identical to the sequence reported for the allele from strain MC4100 (10). The sequence of the glpR2 allele from strain 43 contains only one mutation relative to the sequence from MC4100, a G-to-C transversion at nucleotide 164 resulting in the amino acid change of glycine-55 to alanine. The sequences of the alleles from strains JM83 and DG1 are identical and contain only one mutation relative to the sequence from MC4100, i.e., a C-to-T transition at nucleotide 245 resulting in the amino acid change of alanine-82 to valine. This allele is designated glpR208. Thus, glpR2 and glpR208 are different alleles that are associated with the same phenotype. The same amino acid substitution found in glpR2 has been identified independently in the glpR200 allele from strain C600 (3) (Ann Flower, University of North Dakota, personal communication).
The E. coli glycerol 3-phosphate repressor is related to several other repressors in both gram-negative and gram-positive bacteria, as revealed by protein sequence alignments reported by Zeng et al. (10). It contains a helix-turn-helix DNA-binding motif which is located at amino acid residues 22 to 41. Thus, the substitutions that result from the glpR2 and glpR208 alleles do not occur in the DNA-binding motif, and direct effects on the recognition helix do not account for the loss of repressor function for these alleles. The sites of the substitutions are, however, highly conserved in the members of this repressor family. The glycine at position 55 is found in all but one member of the family, and the valine at position 82 is conserved absolutely. The extent of conservation at these two positions indicates their importance for repressor structure and function, and the complete loss of function that is associated with the variant alleles is consistent with the effects of substitutions of such highly conserved amino acids. Defective repressors associated with the substitutions found here could have effects on DNA-binding amino acids not in the helix-turn-helix motif, altered conformations that affect DNA binding, or decreased stability that prevents proper protein folding and expression.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM-49992) and by the Texas Agricultural Experiment Station (H-6559).
We thank Donna Barker, Audra Boettcher, and Geneva Sampson for expert technical assistance and Ry Young, Mike Manson, and Tim Larson for generously providing strains.
REFERENCES
- 1.Baldwin T O, Berends T, Bunch T A, Holzman T F, Rausch S K, Shamansky L, Treat M L, Ziegler M M. Cloning of the luciferase structural genes from Vibrio harveyi and expression of bioluminescence in Escherichia coli. Biochemistry. 1984;23:3663–3667. doi: 10.1021/bi00311a014. [DOI] [PubMed] [Google Scholar]
- 2.Berlyn M K B, Low K B, Rudd K E. Linkage map of Escherichia coli K-12, 9th ed. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: ASM Press; 1996. pp. 1715–1902. [Google Scholar]
- 3.Elvin C M, Hardy C M, Rosenberg H. Pi exchange mediated by the GlpT-dependent sn-glycerol-3-phosphate transport system in Escherichia coli. J Bacteriol. 1985;161:1054–1058. doi: 10.1128/jb.161.3.1054-1058.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inada T, Kimata K, Aiba H. Mechanism responsible for glucose-lactose diauxie in Escherichia coli: challenge to the cAMP model. Genes Cells. 1996;1:293–301. doi: 10.1046/j.1365-2443.1996.24025.x. [DOI] [PubMed] [Google Scholar]
- 5.Koch J P, Hayashi S-I, Lin E C C. The control of dissimilation of glycerol and l-α-glycerophosphate in Escherichia coli. J Biol Chem. 1964;239:3106–3108. [PubMed] [Google Scholar]
- 6.Liu W Z, Faber R, Feese M, Remington S J, Pettigrew D W. Escherichia coli glycerol kinase: role of a tetramer interface in regulation by fructose 1,6-bisphosphate and phosphotransferase system regulatory protein IIIglc. Biochemistry. 1994;33:10120–10126. doi: 10.1021/bi00199a040. [DOI] [PubMed] [Google Scholar]
- 7.Messing J. A multipurpose cloning system based on the single-stranded DNA bacteriophage M13. NIH publication no. 79–99. Recombinant DNA Tech Bull. 1979;2:43–48. [Google Scholar]
- 8.Pettigrew D W, Smith G B, Thomas K P, Dodds D C. Conserved active site aspartates and domain-domain interactions in regulatory properties of the sugar kinase superfamily. Arch Biochem Biophys. 1998;349:236–245. doi: 10.1006/abbi.1997.0444. [DOI] [PubMed] [Google Scholar]
- 9.Wu T T. A model for three point analysis of random general transduction. Genetics. 1966;54:405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng G, Ye S, Larson T J. Repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K-12: primary structure and identification of the DNA-binding domain. J Bacteriol. 1996;178:7080–7089. doi: 10.1128/jb.178.24.7080-7089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwaig N, Kistler W S, Lin E C C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970;102:753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]