Abstract
Pericyclic processes such as [3,3]-sigmatropic rearrangements leading to the rapid generation of molecular complexity constitute highly valuable tools in organic synthesis. Herein, we report the formation of particularly hindered tertiary alcohols via rearrangement of Breslow intermediates formed in situ from readily available N-allyl thiazolium salts and benzaldehyde derivatives. Experimental mechanistic studies performed suggest that the reaction proceeds via a close radical pair which recombine in a regio- and diastereoselective manner, formally leading to [3,3]-rearranged products.
Graphical Abstract

Since the seminal reports by Breslow and co-workers on the formation of stabilized carbenes by deprotonation of thiazolium salts,1 N-heterocyclic carbenes (NHC) have become invaluable tools for the construction of carbon–carbon bonds in organic synthesis.2,3 In a typical mechanism, the NHC reacts with an aldehyde derivative to form a nucleophilic enaminol species denoted “Breslow intermediate”4 capable of reaction with a wide range of electrophiles, eventually leading to a functionalized ketone after NHC regeneration. While two-electron pathways are often operative and have been widely studied,2 radical processes have recently emerged,5 opening a new realm of reactivities to explore with these versatile enaminol intermediates. More specifically, Ohmiya5b,6 and others7 have shown that persistent α-hydroxy radicals can readily be formed from triazolidene- and thiazolidene-derived Breslow intermediates and react via radical–radical coupling to introduce sterically bulky substituents. Such radical coupling strategies are highly valuable to the synthetic community, since the extreme reactivity of the intermediates involved enables the possibility for C–C bond formation events difficult to achieve by two-electron pathways. In this regard, the elaboration of all-carbon quaternary centers remains a formidable challenge and is of great value to synthetic chemists,8 as these constitute ubiquitous units in natural products9 and are increasingly relevant to medicinal chemistry research.10 With this in mind, we reasoned that Breslow intermediates derived from N-allylated thiazolium salts would constitute ideal retrons for highly congested thiazole-containing tertiary alcohols via a [3,3] transform, where aromatization of the thiazole ring could exert an effective driving force. Notably, thiazole derivatives constitute important scaffolds found in synthetic drugs,11 natural products,12 or organic dyes for solar cells applications.13 While McIntosh and co-workers reported that benzothiazolium salts react to form the formal [1,3] (linear) products via radical pairs (Scheme 1a),14,15 initial experiments performed in our lab with thiazolium analogues instead led us to believe that a fully selective [3,3] disconnection, leading to branched products instead, might be possible.16
Scheme 1.

Divergent Reactivity of Breslow Intermediates Derived from (Benzo)thiazolium Salts
Herein, we report an expedient synthesis of highly congested tertiary homoallylic alcohols via the rearrangement of Breslow intermediates formed in situ from simple thiazolium salts and benzaldehyde derivatives (Scheme 1b). Mechanistic studies performed via radical trapping and EPR experiments revealed that the reaction likely proceeds via fragmentation of the Breslow intermediate into a close radical pair which can recombine in a regio- and diastereoselective manner, formally affording [3,3]-rearranged products. Divergent fragmentation of the congested tertiary alcohols obtained by this method via various C–C bond cleavage strategies is also documented.
Several N-allylated thiazolium salts 1a–1j with different substitution patterns were readily prepared by simple treatment of thiazole with substituted allyl bromide derivatives at high temperature, followed by recrystallization or purification by flash chromatography (eq 1).16
 |
(1) |
N-Prenylated thiazole 1a was elected as a model substrate for further optimization studies by reaction with benzaldehyde, leading to tertiary homoallylic alcohol 2a (Table 1). DBU was rapidly identified as an ideal base for this transformation, whereas trialkylamines such as DIPEA typically afforded substantial amounts of benzoin side-product 2ab (entries 1–2).1b,17 Ketone 2ac, presumably formed by radical decomposition of the Breslow intermediate, was also observed as a minor side-product in most cases. When the reaction was run in DMSO, complete conversion was observed in only 4 h at room temperature (entries 3–7), and decreasing the concentration to 0.02 M provided a substantially higher yield of 2a (entries 8–9). Notably, the corresponding linear product was not observed in any case with this substrate, in contrast to analogous reactions reported by McIntosh and co-workers involving benzothiazolium salts instead.14 In order to allow for isolation of the product by chromatography, the crude reaction mixture was treated with NaBH4 in methanol to reduce the excess benzaldehyde still present, which was found to coelute with 2a (entries 10–11). Under the optimized conditions, congested alcohol 2a was isolated in 73% yield, and the reaction could be performed on a 1 mmol scale of substrate with similar efficiency (Scheme 2). A range of functionalized benzaldehyde derivatives were found to be compatible in the reaction with thiazolium salt 1a (see 2a–2m), with electron-poor aldehydes often affording the highest yield of desired tertiary alcohol.18 Aliphatic aldehydes were found to be unsuitable in this transformation likely due to a decreased stabilization of the α-hydroxy radical intermediate (vide infra), with mostly the benzoin product being observed in those cases. Various other substituted thiazolium salts were evaluated as substrates with 4-chlorobenzaldehyde, leading to a range of different branched tertiary alcohols in good yields and moderate diastereoselectivity (3a–3i). X-ray crystal structures obtained for tertiary alcohol 3f and the corresponding minor isomer (3f’)16 revealed the identity of the major diastereomer formed in each case.19 Interestingly, while trans- and cis-propyl-substituted thiazolium salts 1d and 1e both reacted to afford similar yields, the major diastereomers observed for 3c and 3d were different, which might be indicative of a nonconcerted rearrangement proceeding via a close radical pair.
Table 1.
Optimization of the Formal [3,3] Deconstruction of Breslow Intermediates to Tertiary Homoallylic Alcohols

| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entry | solvent | temp (°C) | concn (M) | yield (%)a |
||
| 2a | 2ab | 2ac | ||||
| 1b | Dioxane | 80 | 0.15 | 36 | 0 | 0 |
| 2b,c | Dioxane | 80 | 0.15 | 38 | 21 | 5 |
| 3 | Dioxane | 80 | 0.15 | 40 | 0 | 4 |
| 4 | DMSO | 80 | 0.15 | 53 | 5 | 11 |
| 5 | DMSO | 70 | 0.15 | 56 | 8 | 8 |
| 6 | DMSO | 60 | 0.15 | 64 | 4 | 5 |
| 7 | DMSO | rt | 0.15 | 65 | 3 | 4 |
| 8 | DMSO | rt | 0.075 | 76 | 7 | 2 |
| 9 | DMSO | rt | 0.020 | 80 | 3 | 5 |
| 10d | DMSO | rt | 0.020 | 86 | 1 | 14 |
| 11d | DMSO | rt | 0.020 | 74 (73)e | – | – |
Yield on a 0.1 mmol scale 1a determined by 1H NMR using triphenylmethane as standard.
Reaction was stirred for 18 h.
N,N-Diisopropylethylamine (2.0 equiv) was used as base.
3.0 equiv of benzaldehyde were used.
NMR yield of 2a after treatment of the crude mixture with NaBH4 (isolated yield in parentheses).
Scheme 2.

Scope of Accessible Tertiary Homoallylic Alcoholsa
aIsolated yields from 1a–1i on a 1 mmol scale after treatment of the crude mixture with NaBH4 in methanol.
To gain further insight into the mechanism, the reaction with 1a was performed in the presence of various radical trapping reagents (Table 2). While the use of TEMPO20 as an additive did not significantly affect the yield of alcohol observed (entries 1–4), more reactive radical inhibitors such as molecular oxygen (O2)21 or 2-methyl-2-nitrosopropane dimer (MNP) led to an important reduction in the reaction efficiency (entries 5–6). The reactions performed in the presence of TEMPO also produced some 2,2,6,6-tetramethyl-piperidin-1-yl 4-chlorobenzoate (4) via oxidation of the Breslow intermediate,22 although the amounts isolated do not account for the inefficiency of TEMPO to inhibit the reaction. The fact that the reaction is only affected by highly reactive radical traps could be indicative of a mechanism where recombination of the radical pair is faster than diffusion from the solvent cage.23 Moreover, the diastereomeric mixtures observed when starting from a single thiazolium isomer (see 3b–3i) further point to the presence of radical intermediates, since concerted [3,3]-sigmatropic rearrangements are known to be stereospecific.24 Electron paramagnetic resonance (EPR) experiments were also performed and provided evidence of radical intermediates under the optimal conditions when MNP was employed as a radical trapping reagent.16 Based on these data and previous work involving thiazolidene-derived intermediates,5b we propose that the reaction proceeds via fragmentation and rearomatization of the corresponding Breslow intermediate B (or its deprotonated form), generating a close radical pair C in resonance with the more stable (substituted) D (Scheme 3). Rapid radical recombination of D leads to the formation of the congested branched product 2a–2m or 3a–3i. Notably, an oxidized radical form of the enaminol B as an intermediate is also plausible.17,25
Table 2.
Mechanistic Insights via Radical Inhibition Experiments
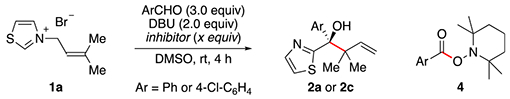
| ||||
|---|---|---|---|---|
|
| ||||
| entry | Ar | inhibitor (equiv) | product | yield (%)a |
| 1 | Ph | – | 2a | 72 |
| 2 | 4-Cl-C6H4 | – | 2c | 75 |
| 3 | 4-Cl-C6H4 | TEMPO (2.0) | 2c | 71b |
| 4 | 4-Cl-C6H4 | TEMPO (4.0) | 2c | 65c |
| 5 | 4-Cl-C6H4 | O2 (excess) | 2c | 13 |
| 6 | Ph | MNPd (1.0) | 2a | 17 |
Isolated yield from 1a after treatment of the crude mixture with NaBH4.
Product 4 (44%) was also isolated.
Product 4 (35%) was also isolated.
MNP: 2-methyl-2-nitrosopropane dimer.
Scheme 3.
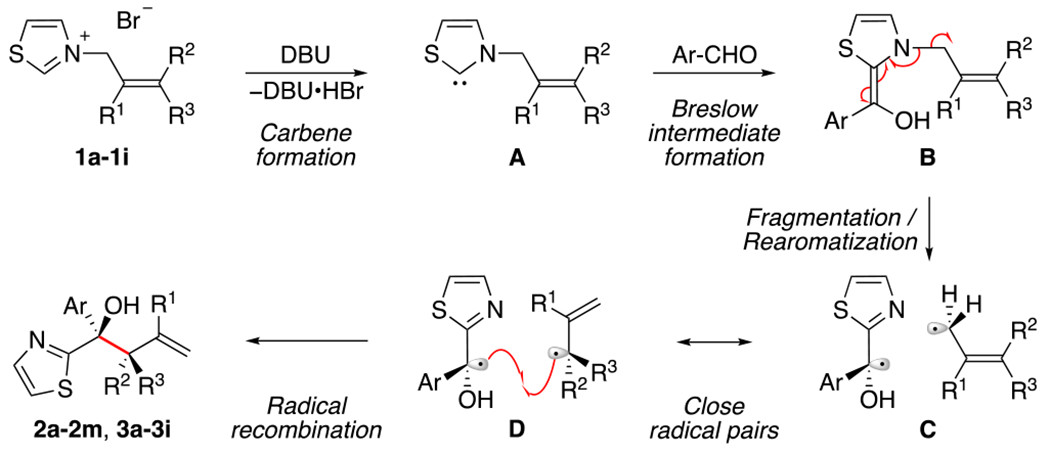
Proposed Mechanism of the Formal [3,3] Deconstruction of Breslow Intermediates to Tertiary Homoallylic Alcohols
When particularly hindered R2/R3 substituents are present on the thiazolium substrate, the selectivity can be reversed toward a formal [1,3] rearrangement,14,15 leading to the linear product 3j instead via recombination of radical pair C (Scheme 4a). Moreover, the tertiary alcohols obtained by this method can diverge into ketones via various C–C bond cleaving strategies, as demonstrated here starting from 2a (Scheme 4b). In the presence of methyl triflate, the thiazole ring is effectively eliminated as an NHC under basic conditions to afford α-quaternary ketone 5 (left). Alternatively, the (reverse) prenyl group can be selectively cleaved under acidic conditions via alkene protonation, producing diaryl ketone 6 in good yield (right).
Scheme 4.

Alternative [1,3] Deconstruction with Hindered Substrates (a) and Divergent Product Fragmentation to Ketones (b)
In summary, we report a simple and efficient synthesis of highly congested tertiary homoallylic alcohols via the rearrangement of Breslow intermediates formed in situ from simple N-allyl thiazolium salts and benzaldehyde derivatives.26 Experimental mechanistic studies suggest that the reaction likely proceeds via homolytic fragmentation of the Breslow intermediate into a close radical pair which can recombine in a regio- and diastereoselective manner, formally leading to [3,3]-rearranged products. Deconstruction of the congested tertiary alcohols obtained to various ketones is also shown to be possible via divergent C–C bond cleavage strategies. Considering the difficulty of constructing such congested alcohol motifs and their general prevalence in natural products and other relevant molecules, this method should find significant utility for the elaboration of complex and biologically active compounds.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIH (R35GM142965) and by North Carolina State University Faculty Research and Professional Development Program (FRPD) and startup funds. All X-ray, nuclear magnetic resonance (NMR) spectroscopy, and high-resolution mass spectrometry (HRMS) measurements were performed by the Molecular Education, Technology, and Research Innovation Center (METRIC) at NC State University, which is supported by the State of North Carolina. We are grateful to Dr. Roger D. Sommer (METRIC, NC State University) for X-ray analysis of 3f and 3f’, visualized here using CYLView.27 We are also grateful to Prof. David A. Shultz (Dept of Chemistry, NC State University), Dr. Patrick Hewitt (Dept of Chemistry, NC State University), and Remi Fayad (Dept of Chemistry, NC State University) for valuable insights with EPR experiments. R.M.R. is grateful to NC State University for Diversity Graduate Assistance grants, and for a Percy Lavon Julian Award in Organic Chemistry. N.R.B. is grateful to NC State University’s Office of Undergraduate Research for undergraduate fellowships.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c01627.
Experimental details and spectroscopic data; crystallographic data for compounds 3f (CCDC 2116375) and 3f’ (CCDC 2116376) (PDF)
Accession Codes
CCDC 2116375–2116376 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.orglett.2c01627
The authors declare no competing financial interest.
Contributor Information
Roger Machín Rivera, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States.
Nikolas R. Burton, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States.
Luke D. Call, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States
Marshall A. Tomat, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States
Vincent N. G. Lindsay, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States.
REFERENCES
- (1).(a) Breslow R Rapid Deuterium Exchange in Thiazolium Salts. J. Am. Chem. Soc 1957, 79, 1762–1763. [Google Scholar]; (b) Breslow R On the Mechanism of Thiamine Action. IV. Evidence from Studies on Model Systems. J. Am. Chem. Soc 1958, 80, 3719–3726. [Google Scholar]
- (2).For selected reviews on organocatalysis using N-heterocyclic carbenes, see:; (a) Enders D; Niemeier O; Henseler A Organocatalysis by N-Heterocyclic Carbenes. Chem. Rev 2007, 107, 5606–5655. [DOI] [PubMed] [Google Scholar]; (b) Bugaut X; Glorius F Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev 2012, 41, 3511–3522. [DOI] [PubMed] [Google Scholar]; (c) Grossmann A; Enders D N-Heterocyclic Carbene Catalyzed Domino Reactions. Angew. Chem., Int. Ed 2012, 51, 314–325. [DOI] [PubMed] [Google Scholar]; (d) Hopkinson MN; Richter C; Schedler M; Glorius F An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [DOI] [PubMed] [Google Scholar]; (e) Mahatthananchai J; Bode JW On the Mechanism of N-Heterocyclic Carbene-Catalyzed Reactions Involving Acyl Azoliums. Acc. Chem. Res 2014, 47, 696–707. [DOI] [PubMed] [Google Scholar]; (f) Flanigan DM; Romanov-Michailidis F; White NA; Rovis T Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev 2015, 115, 9307–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Menon RS; Biju AT; Nair V Recent advances in employing homoenolates generated by N-heterocyclic carbene (NHC) catalysis in carbon–carbon bond-forming reactions. Chem. Soc. Rev 2015, 44, 5040–5052. [DOI] [PubMed] [Google Scholar]; (h) Ohmiya H N-Heterocyclic Carbene-Based Catalysis Enabling Cross-Coupling Reactions. ACS Catal 2020, 10, 6862–6869. [Google Scholar]
- (3).For selected reviews on the use of NHC organocatalysis in medicinal chemistry and total synthesis, see:; (a) Izquierdo J; Hutson GE; Cohen DT; Scheidt KA A Continuum of Progress: Applications of N-Hetereocyclic Carbene Catalysis in Total Synthesis. Angew. Chem., Int. Ed 2012, 51, 11686–11698. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Todd Hovey M; Jaworski AA; Scheidt KA N-Heterocyclic Carbene Catalysis in Natural Product and Complex Target Synthesis. In N-Heterocyclic Carbenes in Organocatalysis; Biju AT, Ed.; Wiley: 2018; pp 345–404. [Google Scholar]; (c) Que Y; He H Advances in N-Heterocyclic Carbene Catalysis for Natural Product Synthesis. Eur. J. Org. Chem 2020, 2020, 5917–5925. [Google Scholar]; (d) Han B; He X-H; Liu Y-Q; He G; Peng C; Li J-L Asymmetric organocatalysis: an enabling technology for medicinal chemistry. Chem. Soc. Rev 2021, 50, 1522–1586. [DOI] [PubMed] [Google Scholar]
- (4).For studies on the identification and isolation of Breslow intermediates, see:; (a) DiRocco DA; Oberg KM; Rovis T Isolable Analogues of the Breslow Intermediate Derived from Chiral Triazolylidene Carbenes. J. Am. Chem. Soc 2012, 134, 6143–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Berkessel A; Elfert S; Yatham VR; Neudörfl J-M; Schlörer NE; Teles JH Umpolung by N-Heterocyclic Carbenes: Generation and Reactivity of the Elusive 2,2-Diamino Enols (Breslow Intermediates). Angew. Chem., Int. Ed 2012, 51, 12370–12374. [DOI] [PubMed] [Google Scholar]; (c) Paul M; Sudkaow P; Wessels A; Schlörer NE; Neudörfl J-M; Berkessel A Breslow Intermediates from Aromatic N-Heterocyclic Carbenes (Benzimidazolin-2-ylidenes, Thiazolin-2-ylidenes). Angew. Chem., Int. Ed 2018, 57, 8310–8315. [DOI] [PubMed] [Google Scholar]; (d) Paul M; Neudörfl J-M; Berkessel A Breslow Intermediates from a Thiazolin-2-ylidene and Fluorinated Aldehydes: XRD and Solution-Phase NMR Spectroscopic Characterization. Angew. Chem., Int. Ed 2019, 58, 10596–10600. [DOI] [PubMed] [Google Scholar]; (e) Paul M; Peckelsen K; Thomulka T; Martens J; Berden G; Oomens J; Neudörfl J-M; Breugst M; Meijer AJHM; Schäfer M; Berkessel A Breslow Intermediates (Amino Enols) and Their Keto Tautomers: First Gas-Phase Characterization by IR Ion Spectroscopy. Chem.—Eur. J 2021, 27, 2662–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).For reviews of single-electron pathways in NHC organocatalysis, see:; (a) Zhao K; Enders D Merging N-Heterocyclic Carbene Catalysis and Single Electron Transfer: A New Strategy for Asymmetric Transformations. Angew. Chem., Int. Ed 2017, 56, 3754–3756. [DOI] [PubMed] [Google Scholar]; (b) Ishii T; Nagao K; Ohmiya H Recent advances in N-heterocyclic carbene-based radical catalysis. Chem. Sci 2020, 11 , 5630–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li Q-Z; Zeng R; Han B; Li J-L Single-Electron Transfer Reactions Enabled by N-Heterocyclic Carbene Organocatalysis. Chem.—Eur. J 2021, 27, 3238–3250. [DOI] [PubMed] [Google Scholar]
- (6).(a) Ishii T; Kakeno Y; Nagao K; Ohmiya H N-Heterocyclic Carbene-Catalyzed Decarboxylative Alkylation of Aldehydes. J. Am. Chem. Soc 2019, 141, 3854–3858. [DOI] [PubMed] [Google Scholar]; (b) Ishii T; Ota K; Nagao K; Ohmiya H N-Heterocyclic Carbene-Catalyzed Radical Relay Enabling Vicinal Alkylacylation of Alkenes. J. Am. Chem. Soc 2019, 141, 14073–14077. [DOI] [PubMed] [Google Scholar]; (c) Ota K; Nagao K; Ohmiya H N-Heterocyclic Carbene-Catalyzed Radical Relay Enabling Synthesis of δ-Ketocarbonyls. Org. Lett 2020, 22, 3922–3925. [DOI] [PubMed] [Google Scholar]; (d) Kakeno Y; Kusakabe M; Nagao K; Ohmiya H Direct Synthesis of Dialkyl Ketones from Aliphatic Aldehydes through Radical N-Heterocyclic Carbene Catalysis. ACS Catal. 2020, 10, 8524–8529. [Google Scholar]; (e) Ishii T; Nagao K; Ohmiya H Radical N-heterocyclic carbene catalysis for β-ketocarbonyl synthesis. Tetrahedron 2021, 91, 132212. [Google Scholar]; (f) Matsuki Y; Ohnishi N; Kakeno Y; Takemoto S; Ishii T; Nagao K; Ohmiya H Aryl radical-mediated N-heterocyclic carbene catalysis. Nat. Commun 2021, 12, 3848. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Kusakabe M; Nagao K; Ohmiya H Radical Relay Trichloromethylacylation of Alkenes through N-Heterocyclic Carbene Catalysis. Org. Lett 2021, 23, 7242–7247. [DOI] [PubMed] [Google Scholar]; (h) Sato Y; Goto Y; Nakamura K; Miyamoto Y; Sumida Y; Ohmiya H Light-Driven N-Heterocyclic Carbene Catalysis Using Alkylborates. ACS Catal. 2021, 11, 12886–12892. [Google Scholar]
- (7).(a) Du Y; Wang Y; Li X; Shao Y; Li G; Webster RD; Chi YR N-Heterocyclic Carbene Organocatalytic Reductive β,β-Coupling Reactions of Nitroalkenes via Radical Intermediates. Org. Lett 2014, 16, 5678–5681. [DOI] [PubMed] [Google Scholar]; (b) White NA; Rovis T Enantioselective N-Heterocyclic Carbene-Catalyzed β-Hydroxylation of Enals Using Nitroarenes: An Atom Transfer Reaction That Proceeds via Single Electron Transfer. J. Am. Chem. Soc 2014, 136, 14674–14677. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang Y; Du Y; Huang Z; Xu J; Wu X; Wang Y; Wang M; Yang S; Webster RD; Chi YR N-Heterocyclic Carbene-Catalyzed Radical Reactions for Highly Enantioselective β-Hydroxylation of Enals. J. Am. Chem. Soc 2015, 137, 2416–2419. [DOI] [PubMed] [Google Scholar]; (d) White NA; Rovis T Oxidatively Initiated NHC-Catalyzed Enantioselective Synthesis of 3,4-Disubstituted Cyclopentanones from Enals. J. Am. Chem. Soc 2015, 137, 10112–10115. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chen X-Y; Chen K-Q; Sun D-Q; Ye S N-Heterocyclic carbene-catalyzed oxidative [3 + 2] annulation of dioxindoles and enals: cross coupling of homoenolate and enolate. Chem. Sci 2017, 8, 1936–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).For recent reviews on the elaboration of all-carbon quaternary centers, see:; (a) Das JP; Marek I Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem. Commun 2011, 47, 4593–4623. [DOI] [PubMed] [Google Scholar]; (b) Marek I; Minko Y; Pasco M; Mejuch T; Gilboa N; Chechik H; Das JP All-Carbon Quaternary Stereogenic Centers in Acyclic Systems through the Creation of Several C–C Bonds per Chemical Step. J. Am. Chem. Soc 2014, 136, 2682–2694. [DOI] [PubMed] [Google Scholar]; (c) Quasdorf KW; Overman LE Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 2014, 516, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liu Y; Han S-J; Liu W-B; Stoltz BM Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules. Acc. Chem. Res 2015, 48, 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zeng X-P; Cao Z-Y; Wang Y-H; Zhou F; Zhou J Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev 2016, 116, 7330–7396. [DOI] [PubMed] [Google Scholar]; (f) Feng J; Holmes M; Krische MJ Acyclic Quaternary Carbon Stereocenters via Enantioselective Transition Metal Catalysis. Chem. Rev 2017, 117, 12564–12580. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Wu G; Wu J-R; Huang Y; Yang Y-W Enantioselective Synthesis of Quaternary Carbon Stereocenters by Asymmetric Allylic Alkylation: A Review. Chem.—Asian J 2021, 16, 1864–1877. [DOI] [PubMed] [Google Scholar]; (h) Süsse L; Stoltz BM Enantioselective Formation of Quaternary Centers by Allylic Alkylation with First-Row Transition-Metal Catalysts. Chem. Rev 2021, 121, 4084–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Ling T; Rivas F All-carbon quaternary centers in natural products and medicinal chemistry: recent advances. Tetrahedron 2016, 72, 6729–6777. [Google Scholar]; (b) Hong AY; Stoltz BM The Construction of All-Carbon Quaternary Stereocenters by Use of Pd-Catalyzed Asymmetric Allylic Alkylation Reactions in Total Synthesis. Eur. J. Org. Chem 2013, 2013, 2745–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; (b) Feher M; Schmidt JM Property Distributions: Differences between Drugs, Natural Products, and Molecules from Combinatorial Chemistry. J. Chem. Inf. Comput. Sci 2003, 43, 218–227. [DOI] [PubMed] [Google Scholar]; (c) Allu TK; Oprea TI Rapid Evaluation of Synthetic and Molecular Complexity for in Silico Chemistry. J. Chem. Inf. Model 2005, 45, 1237–1243. [DOI] [PubMed] [Google Scholar]
- (11).(a) Chhabria TM; Patel S; Modi P; Brahmkshatriya SP Thiazole: A Review on Chemistry, Synthesis and Therapeutic Importance of its Derivatives. Curr. Top. Med. Chem 2016, 16, 2841–2862. [DOI] [PubMed] [Google Scholar]; (b) Liu Y; Li Z; Xie Y; He P; Qiao J; Fan X; Du Y Efficient One-Pot Synthesis of 2,4-Disubstituted Thiazoles and Dimeric Thiazoles Directly from Acyl Chlorides and β-Azido Disulfides. Synthesis 2017, 49, 4876–4886. [Google Scholar]; (c) Beno BR; Yeung K-S; Bartberger MD; Pennington LD; Meanwell NA A Survey of the Role of Noncovalent Sulfur Interactions in Drug Design. J. Med. Chem 2015, 58, 4383–4438. [DOI] [PubMed] [Google Scholar]; (d) Mjambili F; Njoroge M; Naran K; De Kock C; Smith PJ; Mizrahi V; Warner D; Chibale K Synthesis and biological evaluation of 2-aminothiazole derivatives as antimycobacterial and antiplasmodial agents. Bioorg. Med. Chem. Lett 2014, 24, 560–564. [DOI] [PubMed] [Google Scholar]; (e) Cascioferro S; Parrino B; Carbone D; Schillaci D; Giovannetti E; Cirrincione G; Diana P Thiazoles, Their Benzofused Systems, and Thiazolidinone Derivatives: Versatile and Promising Tools to Combat Antibiotic Resistance. J. Med. Chem 2020, 63, 7923–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Sinha S; Manju SL; Doble M Chalcone-Thiazole Hybrids: Rational Design, Synthesis, and Lead Identification against 5-Lipoxygenase. ACS Med. Chem. Lett 2019, 10, 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Sharma PC; Bansal KK; Sharma A; Sharma D; Deep A Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem 2020, 188, 112016. [DOI] [PubMed] [Google Scholar]
- (12).(a) Hughes R A.; Moody, C. J. From Amino Acids to Heteroaromatics—Thiopeptide Antibiotics, Nature’s Heterocyclic Peptides. Angew. Chem., Int. Ed 2007, 46, 7930–7954. [DOI] [PubMed] [Google Scholar]; (b) You S-L ; Kelly JW Highly Efficient Biomimetic Total Synthesis and Structural Verification of Bistratamides E and J from Lissoclinum bistratum. Chem.—Eur. J 2004, 10, 71–75. [DOI] [PubMed] [Google Scholar]; (c) Jin Z Muscarine, imidaozle, oxazole and thiazole alkaloids. Nat. Prod. Rep 2013, 30, 869–915. [DOI] [PubMed] [Google Scholar]
- (13).(a) Gao P; Tsao HN; Grätzel M; Nazeeruddin MK Fine-tuning the Electronic Structure of Organic Dyes for Dye-Sensitized Solar Cells. Org. Lett 2012, 14, 4330–4333. [DOI] [PubMed] [Google Scholar]; (b) Tao T; Ma B-B; Peng Y-X; Wang X-X; Huang W; You X-Z Asymmetrical/Symmetrical D–π–A/D–π-D Thiazole-Containing Aromatic Heterocyclic Fluorescent Compounds Having the Same Triphenylamino Chromophores. J. Org. Chem 2013, 78, 8669–8679. [DOI] [PubMed] [Google Scholar]; (c) Zhang L; Deng W; Wu B; Ye L; Sun X; Wang Z; Gao K; Wu H; Duan C; Huang F; Cao Y Reduced Energy Loss in Non-Fullerene Organic Solar Cells with Isomeric Donor Polymers Containing Thiazole π-Spacers. ACS Appl. Mater. Interfaces 2020, 12, 753–762. [DOI] [PubMed] [Google Scholar]; (d) Chen Z; Gao D; Huang J; Mao Z; Zhang W; Yu G Thiazole-Flanked Diketopyrrolopyrrole Polymeric Semiconductors for Ambipolar Field-Effect Transistors with Balanced Carrier Mobilities. ACS Appl. Mater. Interfaces 2016, 8, 34725–34734. [DOI] [PubMed] [Google Scholar]; (e) Radhakrishnan R; Sreejalekshmi KG Computational Design, Synthesis, and Structure Property Evaluation of 1,3-Thiazole-Based Color-Tunable Multi-heterocyclic Small Organic Fluorophores as Multifunctional Molecular Materials. J. Org. Chem 2018, 83, 3453–3466. [DOI] [PubMed] [Google Scholar]
- (14).(a) Alwarsh S; Ayinuola K; Dormi SS; McIntosh MC Intercepting the Breslow Intermediate via Claisen Rearrangement: Synthesis of Complex Tertiary Alcohols without Organometallic Reagents. Org. Lett 2013, 15, 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Alwarsh S; Xu Y; Qian SY; McIntosh MC Radical [1,3] Rearrangements of Breslow Intermediates. Angew. Chem., Int. Ed 2016, 55, 355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).For related studies and reviews on the formal [1,3] rearrangement of Breslow intermediates, see:; (a) Oka Y;Kishimoto S; Hirano H Studies on Vitamin B1 and Related Compounds. CIX. A Novel Cleavage of Thiamin and Its Homologues by the Reaction with Aromatic Aldehydes. Chem. Pharm. Bull 1970, 18, 527–533. [Google Scholar]; (b) Oka Y; Kishimoto S; Hirano H Studies on Vitamin B1 and Related Compounds. CX. Rearrangements of α-Hydroxybenzylthiamin and Its Homologues. Chem. Pharm. Bull 1970, 18, 534–541. [Google Scholar]; (c) Kluger R; Tittmann K Thiamin Diphosphate Catalysis: Enzymic and Nonenzymic Covalent Intermediates. Chem. Rev 2008, 108, 1797–1833. [DOI] [PubMed] [Google Scholar]; (d) Bielecki M; Kluger R The Need for an Alternative to Radicals as the Cause of Fragmentation of a Thiamin-Derived Breslow Intermediate. Angew. Chem., Int. Ed 2017, 56, 6321–6323. [DOI] [PubMed] [Google Scholar]
- (16).See the Supporting Information for details.
- (17).Rehbein J; Ruser S-M; Phan J NHC-catalysed benzoin condensation – is it all down to the Breslow intermediate? Chem. Sci 2015, 6, 6013–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).When 4-methoxybenzaldehyde was employed, less than 10% desired product was detected by 1H NMR.
- (19).The identity of the major diastereomer for other compounds (3b–3e and 3g–3i) was determined by NMR analysis in analogy to 3f and 3f’.
- (20).Bagryanskaya EG; Marque S R A. Scavenging of Organic C-Centered Radicals by Nitroxides. Chem. Rev 2014, 114, 5011–5056. [DOI] [PubMed] [Google Scholar]
- (21).Decker C; Jenkins AD Kinetic approach of oxygen inhibition in ultraviolet- and laser-induced polymerizations. Macromolecules 1985, 18, 1241–1244. [Google Scholar]
- (22).Guin J; De Sarkar S; Grimme S; Studer A Biomimetic Carbene-Catalyzed Oxidations of Aldehydes Using TEMPO. Angew. Chem., Int. Ed 2008, 47, 8727–8730. [DOI] [PubMed] [Google Scholar]
- (23).(a) Franck J; Rabinowitsch E Some remarks about free radicals and the photochemistry of solutions. Trans. Faraday Soc 1934, 30, 120–130. [Google Scholar]; (b) Rabinowitch E; Wood WC The collison mechanism and the primary photochemical process in solutions. Trans. Faraday Soc 1936, 32, 1381–1387. [Google Scholar]; (c) Herk L; Feld M; Szwarc M Studies of “Cage” Reactions. J. Am. Chem. Soc 1961, 83, 2998–3005. [Google Scholar]; (d) Braden DA; Parrack EE; Tyler DR Solvent cage effects. I. Effect of radical mass and size on radical cage pair recombination efficiency. II. Is geminate recombination of polar radicals sensitive to solvent polarity? Coord. Chem. Rev 2001, 211, 279–294. [Google Scholar]
- (24).For selected reviews on [3,3]-sigmatropic rearrangements, see:; (a) Wipf P Claisen Rearrangements. In Comprehensive Organic Synthesis; Trost BM, Fleming I., Eds.; Pergamon: Oxford, 1991; Vol. 5, pp 827–873. [Google Scholar]; (b) Enders D; Knopp M; Schiffers R Asymmetric [3.3]-sigmatropic rearrangements in organic synthesis. Tetrahedron: Asymmetry 1996, 7, 1847–1882. [Google Scholar]; (c) Martín Castro AM. Claisen Rearrangement over the Past Nine Decades. Chem. Rev 2004, 104, 2939–3002. [DOI] [PubMed] [Google Scholar]
- (25).(a) Delfau L; Nichilo S; Molton F; Broggi J; Tomás-Mendivil E; Martin D Critical Assessment of the Reducing Ability of Breslow-type Derivatives and Implications for Carbene-Catalyzed Radical Reactions. Angew. Chem., Int. Ed 2021, 60, 26783–26789. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Regnier V; Romero EA; Molton F; Jazzar R; Bertrand G; Martin D What Are the Radical Intermediates in Oxidative N-Heterocyclic Carbene Organocatalysis? J. Am. Chem. Soc 2019, 141, 1109–1117. [DOI] [PubMed] [Google Scholar]
- (26).A previous version of this manuscript was deposited on a preprint server:; Machín Rivera R; Burton NR; Call LD; Tomat MA; Lindsay VNG. Synthesis of Highly Congested Tertiary Alcohols via the [3,3] Radical Deconstruction of Breslow Intermediates. ChemRxiv 2022, DOI: 10.26434/chemndv-2022-160sc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).CYLview, 1.0b; Legault CY. Université de Sherbrooke, 2009. (https://www.cylview.org/). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


