Abstract
Introduction
Insulin resistance can be assessed by the Triglyceride-Glucose Index (TyG), a simple, low-cost, and easy-to-apply method.
Objective
To assess the predictive capacity of the TyG index about cardiovascular risk and identify its cutoff point in a population at cardiometabolic risk.
Methods
Cross-sectional study with 264 individuals at cardiometabolic risk (54.9% women, age: 43.1 ± 16.3 years). Demographic, anthropometric, clinical-laboratory, and lifestyle data were collected. The TyG index was determined using the formula Ln [fasting triglycerides (mg/dL) × fasting plasma glucose (mg (dL)/2]. The ten-year cardiovascular risk was assessed by the Framingham risk score (FRS). The receiver operating characteristic curve (ROC) was used to define the cutoff point for the TyG index, and the associations were tested by Poisson regression.
Results
ROC curve analysis indicated an area under the curve of 0.678 (95% CI = 0.618–0.734; p < 0.001), with a cutoff of 9.04 (sensitivity = 62.5%, specificity = 66.7%, positive predictive value = 29.4% and negative predictive value = 88.9%). Elevated TyG values (≥9.04) were positively associated with cardiometabolic risk factors (total cholesterol, LDL, VLDL, uric acid, alanine aminotransferase, aspartate aminotransferase, waist-hip ratio, systolic blood pressure, HOMA-IR, smoking, metabolic syndrome, diabetes, and hepatic steatosis). After adjustment for confounding factors, individuals with high TyG showed an increase of 69% (RP = 1.69; 95%CI = 1.03–2.78) in the prevalence of intermediate/high risk by FRS, compared to those with low TyG.
Conclusion
The TyG index showed a good predictive capacity for cardiovascular risk in ten years assessed by the FRS.
Keywords: TyG index, Cardiovascular disease, Framingham score, Insulin resistance, Cardiovascular risk
Abbreviations: CVD, Cardiovascular diseases; IR, Insulin resistance; TyG, triglyceride-glucose index; FRS, Framingham risk score; ALT, Alanine aminotransferase; AST, Apartate aminotransferase; TC, Total cholesterol; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; VLDL, Very-low-density lipoprotein; ROC, Receiver operating characteristic
1. Introduction
Cardiovascular diseases (CVD) are the main causes of morbidity and mortality worldwide, while insulin resistance (IR) is an important risk factor for these diseases (Ormazabal et al., 2018). Previous studies have shown an independent association between IR and CVD, assessed by hyperinsulinemic-euglycemic clamping, a gold standard method in the assessment of IR (De Fronzo et al., 1979).
In fact, IR is the pathophysiological basis of cardiovascular events (Aronis and Mantzoros, 2012) that involves multiple mechanisms, including changes in classic cardiovascular risk factors and downregulation of insulin signaling pathways in different tissues (Laakso, 2015). In this sense, the TyG index, biomarker of has been proposed to indirectly identify IR in various ethnic groups (Zhang et al., 2017, Won et al., 2020, Sánches-Iñigo et al., 2016, Simental-Mendía et al., 2008), including in the Brazilian population (Vasques et al., 2011).
The triglyceride-glucose (TyG) index indirectly assesses IR through a mathematical model that uses only laboratory data on fasting plasma triglyceride and glucose concentrations (Simental-Mendía et al., 2008). This is a simple, low-cost indicator that has been shown to determine IR in a more appropriate way than other surrogate indices, such as the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), when both are compared to hyperinsulinemic euglycemic clamping (Irace et al., 2013, Vasques et al., 2011, Nam et al., 2020). In this sense, the TyG index has been considered a surrogate marker of IR (Guerrero-Romero et al., 2010). Furthermore, this index has shown good accuracy in predicting cardiovascular events, with sensitivity and specificity values between 67 and 96% and 32.5–85%, respectively (Sánchez-García et al., 2020).
The Framingham risk score (FRS), in turn, is an instrument capable of predicting the risk of future cardiovascular events according to the presence or absence of certain risk factors (Dawber et al., 1951). However, to date, no study has evaluated the predictive capacity of the TyG index about the recognized risk stratification.
Overall, the present study aimed to evaluate the predictive capacity of the TyG index about cardiovascular risk in ten years according to the FRS and to identify its cutoff point in a population at cardiometabolic risk.
2. Methods
2.1. Subjects
This is a cross-sectional study, with data collection carried out between March 2012 and December 2017, with participants of the Cardiovascular Health Care Program at the Universidade Federal de Viçosa - PROCARDIO-UFV (ReBEC, id: RBR-5n4y2g), which aims to improve the clinical-metabolic status and quality of life of individuals at cardiovascular risk. The inclusion and exclusion criteria for this program are described in Table 1 (de Almeida et al., 2020). Of the total number of participants in the baseline (n = 320), referring to the first consultation in the program, 264 individuals were selected, as they had complete data on the main variables of the study.
Table 1.
The inclusion and exclusion criteria for participants of the study.
| Inclusion | Exclusion |
|---|---|
| Both sexes | Children, adolescents and pregnant women |
| Age > 20 years | No CVD |
| Be a server/dependent or student of Brazilian university | No occurrence of cardiometabolic risk factors |
| Present CVD diagnosed or occurrence of cardiometabolic risk factors such: - excess weight (body mass index (BMI) ≥ 25 kg/m2) - hypertriglyceridemia (triglycerides ≥ 150 mg/dL) - hypercholesterolemia (total cholesterol ≥ 200 mg/dL) - high-density lipoprotein (HDL) at low concentrations (men < 40 mg/dl and women < 50 mg/dl) - systolic and diastolic blood pressure ≥ 130/≥ 85 mmHg or arterial hypertension (AH) diagnosed - glucose fasting ≥ 100 mg/DL or Diabetes Mellitus (DM) diagnosed and/or medical record |
This work was approved by the Ethics Committee on Research with Human Beings at UFV (Of. Ref. 066/2012/CEPH), by Resolution 466/2012 of the National Health Council on research involving human beings. All participants read and signed the Informed Consent Form.
2.2. TyG index and cardiometabolic risk markers
The TyG index was calculated according to the formula: Ln [fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2] (Simental-Mendía et al., 2008), and the HOMA-IR by the formula: [(fasting glucose (mg/dL)) × (fasting insulin (μU/mL))]/405 (Matthews et al., 1985).
Weight, height, and waist and hip circumferences were measured according to the protocol established in PROCARDIO-UFV (de Almeida et al., 2020). For clinical and laboratory evaluation, data on triglycerides, fasting glucose, total cholesterol (TC), HDL, LDL, VLDL, non-HDL cholesterol, insulin, uric acid, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were used. Such analyzes were carried out at the Clinical Analysis Laboratory of the Health Division of the UFV, according to a standardized protocol.
Diabetes, hypertension, dyslipidemia, and hepatic steatosis were considered through medical referral or patient self-report. Systolic and diastolic blood pressure values (SBP and DBP, respectively) were measured according to the technique described in the VI Brazilian Guidelines on Hypertension (SBC, 2010). Metabolic syndrome was classified according to the Joint Interim Statement of the Task Force on Epidemiology and Prevention of the International Diabetes Federation; National Heart, Lung and Blood Institute; American Heart Association; World Heart Federation; International Society of Atherosclerosis; and International Association for the Study of Obesity (Alberti et al., 2009).
2.3. Framingham risk score (FRS)
Previous cardiovascular diseases were determined by self-reported questionnaires. Framingham risk score was calculated to evaluate cardiovascular risk (Agostino et al., 2008).
FRS points are estimated according to the Brazilian Society of Cardiology (Sposito et al., 2007). The evaluation of the score is different for men and women, and the evaluation in TH three steps. In the first stage, they were scored as age (-9 to + 13 and −7 to + 16 points, respectively, for men and women), TC (zero to + 11 and zero to + 13 points, respectively, for men and women), smoking (zero to + 8 and zero to + 9 points, respectively, for men and women), HDL (-1 to 2 points for men and women) and untreated SBP (zero to 2 and zero to + 4 points, for men and women, respectively) and treated SBP (zero to + 3 and zero to + 6 points, for men and women, respectively). In the second stage, the sum of the points was performed, at the absolute risk in 10 years. The third step, finally, consists of comparing the sum of points in relation to the probability of the individual developing cardiovascular disease 10 years old, as alerted by the score. Men who scored < 0 to 12 points were classified as low risk (<10%); intermediate risk (≥10% ≤-20%) those who scored 13 to 15 points; and high risk (>20%) those who scored 16 or more points. Women, on the other hand, were classified as low risk (<10%) when they scored < 9 to 19 points; intermediate risk (≥10% ≤20%) those who scored 20 to 22 points; and high risk (>20%) those who scored 23 or more points.
Due to the small number of individuals who were classified as high risk (n = 6), we chose to combine the intermediate risk and high-risk categories. Thus, the FRS in this study has two classifications: low risk and intermediate/high risk.
2.4. Statistical analysis
Categorical data were presented as frequency measures and quantitative data as mean (standard deviation) or median (25th and 75th percentile). To compare variables according to TyG index categories, Student's t-test or Mann-Whitney test was used, depending on the normality of quantitative variables, and Pearson's chi-square test was used for categorical variables. Associations of the TyG index with the FRS were tested by Poisson regression, with robust variance. A crude model was estimated and another adjusted for possible confounding factors, defined from the literature (gender, age, diabetes, hypertension, and physical activity). Analyzes were performed using the Statistical Package for Social Science (SPSS, ® 22, Chicago, IL, USA) and STATA ® 13.0.
Data normality was assessed using the Kolmogorov-Smirnov test. The receiver operating characteristic curve (ROC) was developed in the MedCalc ® program to assess the ability of the TyG index to predict cardiovascular risk by FRS. The ROC curve was summarized by the area under the curve, with its respective 95% confidence interval. In addition, sensitivity, specificity, positive predictive value and negative predictive value were estimated. The cutoff point for the TyG index was identified at the point equivalent to the best balance between sensitivity and specificity values. In all analyses, a significance level of 5% was adopted.
3. Results
The sample consisted of 264 individuals, with a mean age of 43.1 ± 16.3 years and a mean BMI of 28.8 ± 5.8 kg/m2, of which 32.4% were smokers and the majority (54.9%) female.
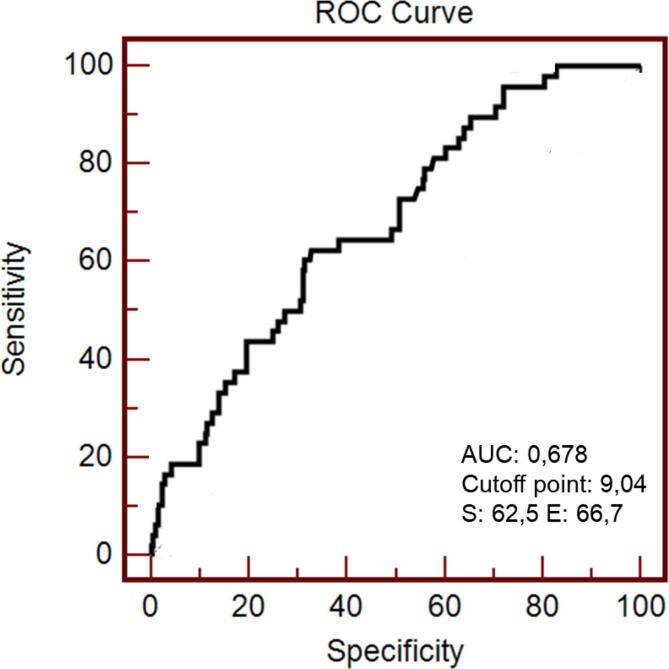
ROC curve analysis indicated an area under the curve (AUC) of 0.678 (95% CI = 0.618 – 0.734; p < 0.001). The cutoff point of the TyG index to identify intermediate/high risk FRS was 9.04, with a sensitivity of 62.5%, specificity of 66.7%, a positive predictive value of 29.4%, and an equal negative predictive value of 88.9% (Fig. 1).
Fig. 1.
Operational characteristic curve of the receptor (ROC) of the TyG index to predict cardiovascular risk in ten years assessed by the Framingham score in individuals at cardiometabolic risk (n = 264).
Individuals with high TyG (TyG ≥ 9.04) had significantly higher waist-hip ratio, TC, LDL, VLDL, uric acid, ALT, AST, SBP, HOMA-IR values, as well as a higher prevalence of smoking, metabolic syndrome, diabetes, and hepatic steatosis compared to those with low TyG (TyG < 9.04). Furthermore, we noticed lower HDL in the high TyG group compared to the group with lower index values. Finally, a higher frequency of participants with intermediate/high risk of developing cardiovascular events in ten years (FRS) was observed in those with high values of the TyG index (Table 2).
Table 2.
Demographic, anthropometric, clinical-laboratory, and lifestyle characteristics of the sample according to TyG index categories (n = 264).
| Características | TyG Index |
||
|---|---|---|---|
| Lowest (<9.04) (n = 161) |
Highest (≥9.04) (n = 103) |
p-values | |
| TyG index | 8.48 (8.25–8.68) | 9.35 (9.15–9.65) | <0.001 |
| Triglycerides (mg/dl) | 104 (86–135) | 242.5 (196.75–306.50) | <0.001 |
| Glucose (mg/dl) | 88 (82–98) | 96 (87.75–121.25) | <0.001 |
| Age (years) | 41 (25 – 55) | 48 (29 – 60) | 0.010 |
| BMI (kg/m2) | 28.06 (25.01–32.41) | 30.29 (25.65–32.96) | 0.424 |
| Waist circumference (cm) | 95.54 ± 15.28 | 98.83 ± 12.38 | 0.051 |
| Waist-to-height ratio | 0.91 ± 0.09 | 0.96 ± 0.08 | <0.001 |
| Total cholesterol (mg/dl) | 201.04 ± 41.94 | 212.43 ± 46.19 | 0.047 |
| Non-HDL cholesterol (mg/dl) | 150.21 ± 39.11 | 169.91 ± 43.20 | 0.060 |
| HDL (mg/dl) | 48 (41–56) | 40 (33.5–44.9) | <0.001 |
| LDL (mg/dl) | 127.83 (±38.38) | 117.97 ± 39.59 | <0.001 |
| VLDL (mg/dl) | 20.8 (17.2–27) | 48.5 (39.35–61.3) | <0.001 |
| Uric acid (mg/dl) | 4 (3.3–4.8) | 4.75 (6.65–6.02) | <0.001 |
| ALT (mg/dl) | 21 (15–26) | 22.5 (19.25–29) | 0.007 |
| AST (mg/dl) | 24 (21–31) | 28.5 (21.75–34.25) | 0.016 |
| SBP (mmHg) | 120 (110–130) | 130 (120–150.25) | 0.003 |
| DBP (mmHg) | 80 (70–80) | 80 (80–90) | 0.131 |
| HOMA-IR | 2.03 (1.42–3.03) | 2.84 (1.49–4.41) | 0.003 |
| Metabolic Syndrome [%] | 51 [41.1] | 73 [58.9] | <0.001 |
| Smoking [%] | 37 [44.6] | 46 [55.4] | <0.001 |
| Physical Actitivity practice [%] | 94 [64.8] | 51 [35.2] | 0.249 |
| Diabetes [%] | 16 [30] | 35 [70] | <0.001 |
| Dyslipidemia [%] | 121 [58.5] | 86 [41.5] | 0.119 |
| Hypertension [%] | 66 [62.9] | 39 [37.1] | 0.698 |
| Hepatic steatosis [%] | 8 [44.4] | 10 [55.6] | 0.421 |
| Framingham Risk Score | |||
| Low risk [%] | 143 [66.5] | 72 [33.5] | <0.001 |
| Intermediate/high risk [%] | 18 [37.5] | 30 [62.5] | |
Data are mean ± SD, median (25th and 75th percentile), or absolute and relative frequencies [%]. P values are based on the Mann-Whitney test or Student's t-test, depending on the normality of quantitative variables, and Pearson's chi-square for categorical variables. TyG: Triglyceride-glucose index; SBP: Systolic blood pressure; DBP: diastolic blood pressure; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very-low-density lipoprotein; HOMA-IR: Homeostasis Model Assessment of Insulin Resistance.
When comparing the average of the FRS groups, we observed a score of 6.36 (±7.04) and 8.92 (±6.61) for the low and intermediate/high TyG index, respectively. In the regression model adjusted for confounding factors, individuals with high TyG showed a 69% increase (PR = 1.69; 95%CI = 1.03–2.78) in the prevalence of intermediate/high risk by FRS, compared to those with low TyG (Table 3).
Table 3.
Association between the TyG index (independent variable) and the risk of developing cardiovascular disease in ten years assessed by the Framingham score (dependent variable) in individuals at cardiometabolic risk (n = 264).
| TyG index tertiles | Framingham risk score classification |
||
|---|---|---|---|
| Lowest risk |
Intermediate/Highest Risk |
||
| RP (IC95%) |
|||
| Model 1 | Model 2 | ||
| Lowest TyG | 1 (Ref) | 1 (Ref) | |
| Highest TyG | 2.63 (1.54–4.46) | 1.69 (1.03 – 2.78) | |
Prevalence ratio data (95%CI) based on Poisson regression with robust variance.
Model 1: crude.
Model 2: adjusted by sex, age, diabetes, high blood pressure and physical activity.
4. Discussion
In our study, the increase in TyG index values was significantly associated with an increased risk of CVD over ten years, and the cutoff point established was 9.04, with a sensitivity of 62.5% and a specificity of 66 0.7%. To date, this is the first study to investigate the cutoff point of the TyG index and its predictive capacity about cardiovascular risk in ten years assessed by the FRS in adults and elderly individuals at cardiometabolic risk.
The TyG index also has been positively associated with CVD, both in individuals at cardiovascular risk (Nam et al., 2020, Da Silva et al., 2019) and in healthy individuals (Park et al., 2020). Besides, a higher TyG index was associated with an increased risk of coronary artery stenosis in asymptomatic individuals with type 2 diabetes (Lee et al., 2016), which is considered an independent risk factor for incident CVD (Luo et al., 2019).
One previous study included the TyG index as one of the variables for calculating the FRS and compared the new model (TyG index plus FRS model) with the traditional model (FRS only) in predicting cardiovascular events from a cohort study (Sánchez-Íñigo et al., 2016). The authors identified that the accuracy of the risk for CVD was improved with the inclusion of the TyG index, reaching a higher AUC value (0.719) than that observed with the traditional model (0.708; p = 0.014) (Sánchez-Íñigo et al., 2016). We emphasize, however, that our proposal is different, because we analyzed the TyG index as a predictor of cardiovascular risk in ten years, using FRS as reference.
As for the TyG index cutoff point for predicting cardiovascular risk, previous studies also found TyG index values >9.0 for predicting cardiovascular events (Luo et al., 2019, Wang et al., 2020). Wang et al (Sánchez-Íñigo et al., 2016) identified a value of 9.323 (AUC = 0.560, sensitivity = 46.0%, and specificity = 63.6%) for the predictive capacity of the TyG index about major adverse cardiovascular events (MACE) in people with diabetes and acute coronary syndrome (Wang et al., 2020). In a study with patients with ST-segment elevation myocardial infarction (STEMI) after percutaneous coronary intervention, the AUC of the TyG index to predict the occurrence of adverse cardiovascular events was 0.685. When considering the presence of diabetes (DM), the AUC of the TyG index in predicting adverse cardiovascular events was higher (0.699) in patients with DM compared to those without DM (0.678) (Luo et al., 2019). Furthermore, our cutoff point (TyG = 9.04) corroborates a previous study, in which the TyG index was an independent predictor of the progression of coronary artery calcification (CAC) in individuals with cardiovascular risk factors (Lee and Kang, 2019).
In the present study, high values of the TyG index also were associated with cardiometabolic risk factors such as TC, LDL, VLDL, uric acid, AST, ALT, SBP, smoking, metabolic syndrome, and diabetes. Recent cross-sectional data from the Brazilian Cardioprotective Nutritional Program Trial also showed a positive association of the TyG index with metabolic and behavioral risk factors (Da Silva et al., 2019).
As a limitation, this study had a cross-sectional design, which does not ensure the temporality of the observed associations. Thus, longitudinal studies in different populations and age groups are needed to confirm our findings regarding the accuracy of the TyG index in predicting cardiovascular risk in ten years assessed by the FRS. Besides, the fact of merging the groups between intermediate and high-risk participants would be a limitation. As strengths of this study, to our knowledge, this is the first to assess the predictive ability of the TyG index according to the FRS in a population at cardiovascular risk, and the proposed cutoff point had fair sensitivity and specificity, which reinforces the applicability of this index in tracking individuals at high cardiovascular risk.
5. Conclusion
Our results indicate a fair predictive ability of the TyG index to assess cardiovascular risk in ten years (according to the FRS), considering the cutoff value of 9.04 for the TyG index. Thus, the use of the TyG index for cardiovascular risk screening seems promising to early identification for high risk for cardiovascular events in clinical and public health practice.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank to the PROCARDIO-UFV’s patients for their participation in this study, and the UFV’s professionals for the excellent technical support. The CAPES Foundation (Ministry of Education, Brazil, code 001), National Council for Scientific, Technological Development (CNPq/MCT/Brazil), and Foundation for Research Support of the State of Minas Gerais (FAPEMIG) provided financial support. J Bressan and HHM Hermsdorff are CNPq Research Productivity Fellowship.
Data availability
The data that has been used is confidential.
References
- Agostino R.B.D., Vasan R.S., Pencina M.J., Wolf P.A., Cobain M., Massaro J.M., et al. General Cardiovascular Risk Profile for Use in Primary Care The Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., et al. Harmonizing the Metabolic Syndrome International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Aronis K.N., Mantzoros C.S. A brief history of insulin resistance: From the first insulin radioimmunoassay to selectively targeting protein kinase C pathways. Metabolism. 2012;61(4):445–449. doi: 10.1016/j.metabol.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Da Silva A., Caldas A.P.S., Hermsdorff H.H.M., Bersch-Ferreira Â.C., Torreglosa C.R., Weber B., et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc. Diabetol. 2019;18(1):89. doi: 10.1186/s12933-019-0893-2. https://www2.scopus.com/inward/record.uri?eid=2-s2.0-85069461836&doi=10.1186%2Fs12933-019-0893-2&partnerID=40&md5=b9e7a0d4985abb37fde4d35cddae54ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawber T.R., Meadors G.F., Moore F.E. Epidemiological Disease: The Approaches to Heart Framingham Study. Am. J. Public Health. 1951;41(3):279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida A.P., Rocha D.M.U.P., Moreira A.V.B., Moraes e Lima H.C.F., Hermsdorff H.H.M. Personalized Nutrition Using PROCARDIO to Reduce Cardiometabolic Risk in the Academic Community: A Study Protocol with Preliminary Results. J. Am. Coll. Nutr. 2020;39(7):591–600. doi: 10.1080/07315724.2019.1706663. [DOI] [PubMed] [Google Scholar]
- De Fronzo R.A., Tobin J.D., Andres R. Glucose clamp technique: a method insulin secretion and resistance for quantifying. Am. J. Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Guerrero-Romero F., Simental-Mendía L.E., González-Ortiz M., Martínez-Abundis E., Ramos-Zavala M.G., Hernández-González S.O., et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- Irace C., Carallo C., Scavelli F.B., De Franceschi M.S., Esposito T., Tripolino C., et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int. J. Clin. Pract. 2013;67(7):665–672. doi: 10.1111/ijcp.12124. [DOI] [PubMed] [Google Scholar]
- Laakso M. Is Insulin Resistance a Feature of or a Primary Risk Factor for Cardiovascular Disease? Curr. Diab. Rep. 2015;15(12):105. doi: 10.1007/s11892-015-0684-4. [DOI] [PubMed] [Google Scholar]
- Lee E.Y., Yang H.K., Lee J., Kang B., Yang Y., Lee S.H., et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15:155. doi: 10.1186/s12944-016-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo E., Wang D., Yan G., Qiao Y., Liu B., Hou J., et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc. Diabetol. 2019;18(1):150. doi: 10.1186/s12933-019-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.R., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C., et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Nam K.W., Kwon H.M., Jeong H.Y., Park J.H., Kwon H., Jeong S.M. High triglyceride-glucose index is associated with subclinical cerebral small vessel disease in a healthy population: A cross-sectional study. Cardiovasc. Diabetol. 2020;19(1):53. doi: 10.1186/s12933-020-01031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G.M., Cho Y.R., Won K.B., Yang Y.J., Park S., Ann S.H., et al. Triglyceride glucose index is a useful marker for predicting subclinical coronary artery disease in the absence of traditional risk factors. Lipids Health Dis. 2020;19(1):7. doi: 10.1186/s12944-020-1187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánches-Iñigo L., Navarro-gonza D., Sa L., Martı J.A. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J. Hypertens. 2016;34(7):1257–1265. doi: 10.1097/HJH.0000000000000941. [DOI] [PubMed] [Google Scholar]
- Sánchez-García A., Rodríguez-Gutiérrez R., Mancillas-Adame L., González-Nava V., Díaz González-Colmenero A., Solis R.C., et al. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int. J. Endocrinol. 2020;2020:4678526. doi: 10.1155/2020/4678526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Íñigo L., Navarro-González D., Fernández-Montero A., Pastrana-Delgado J., Martínez J.A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Invest. 2016;46(2):189–197. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- SBC Sociedade Brasileira de Cardiologia/Sociedade Brasileira de Hipertensão/Sociedade Brasileira de Nefrologia. VI Diretrizes Brasileiras de hipertensão [VI Brazilian Guidelines on Hypertension] Arq. Bras. Cardiol. 2010;95(Suppl 1):1–51. [PubMed] [Google Scholar]
- Simental-Mendía L.E., Rodríguez-Morán M., Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- Sposito A.C., Caramelli B., Fonseca F.A., et al. Sociedade Brasileira de Cardiologia IV Diretriz Brasileira sobre Dislipidemias e Prevenção da Aterosclerose: Departamento de Aterosclerose da Sociedade Brasileira de Cardiologia [IV Brazilian Guideline for Dyslipidemia and Atherosclerosis prevention: Department of Atherosclerosis of Brazilian Society of Cardiology] Arq Bras Cardiol. 2007;88(Suppl 1):2–19. doi: 10.1590/s0066-782x2007000700002. [DOI] [PubMed] [Google Scholar]
- Vasques A.C.J., Novaes F.S., de Oliveira M.D.S., Matos Souza J.R., Yamanaka A., Pareja J.C., et al. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes. Res. Clin. Pract. 2011;93(3):e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Wang L., Cong H.L., Zhang J.X., Hu Y.C., Wei A., Zhang Y.Y., et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc. Diabetol. 2020;19(1):80. doi: 10.1186/s12933-020-01054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won K.B., Han D., Lee J.H., Choi S.Y., Chun E.J., et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19(1):34. doi: 10.1186/s12933-020-01008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Du T., Zhang J., Lu H., Lin X., Xie J., et al. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16(1):15. doi: 10.1186/s12944-017-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.B., Kang S. Elevated TyG Index Predicts Progression of Coronary Artery Calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.