Abstract
A Mediterranean diet and intentional weight loss each positively affect cognitive functioning. Combining both could produce synergistic effects on cognition. The purpose of this study is to compare a Mediterranean diet lifestyle intervention with and without caloric restriction versus control on cognition, lifestyle, and cardiometabolic disease. In a three-arm trial conducted between 2017 and 2020 in Chicago, one hundred and eight-five, 55–85-year-old, predominately non-Hispanic black females with obesity were randomized (2:2:1) to an 8-month Mediterranean diet plus caloric restriction intervention, Mediterranean diet alone, or control. The primary outcome was change from baseline to post-intervention in cognitive composite scores: attention, information & processing; executive function; and learning, memory, & recognition. Secondary outcomes were weight, lifestyle and cardiometabolic markers. The 8-month Mediterranean diet interventions did not significantly affect cognition. Adherence to a Mediterranean diet improved more in the Mediterranean diet plus caloric restriction arm (mean [SE] score change, +6.3 [0.7] points) and Mediterranean diet alone arm (+4.8 [0.7] points) relative to controls (+0.6 [0.9] points). Mean weight loss was greater among the Mediterranean diet plus caloric restriction arm (−4.6 [0.6] kg) compared to the Mediterranean diet alone (−2.6 [0.6] kg) and control arms (−0.6 [0.7] kg). The interventions did not affect activity or cardiometabolic risk markers; although, fasting insulin did decline in the Mediterranean diet plus caloric restriction arm relative to the Mediterranean diet alone and control arms. A Mediterranean diet lifestyle intervention with and without caloric restriction did not significantly affect cognitive function compared to controls. The Mediterranean diet interventions, however, significantly affected diet quality and body weight.
Keywords: Mediterranean diet, Weight loss, Cognition, Dietary quality, Older adults
1. Introduction
Excess body fat, lifestyle factors including diet and physical activity, and cardiometabolic disorders are risk factors for cognitive decline and dementia (van den Brink et al., 2019, Veronese et al., 2017, Norton et al., 2014, Tönnies and Trushina, 2017). There are racial inequities in cognitive health in the U.S. with non-Hispanic blacks more likely than non-Hispanic whites to suffer from cognitive impairment, Alzheimer’s, and related dementias (Shadlen et al., 2006, Mehta et al., 2004, Rovner et al., 2013, Steenland et al., 2016). Non-Hispanic blacks also have disproportionate exposure to risk factors for cognitive decline and dementia including poor diet quality and a higher prevalence of obesity and cardiometabolic diseases (Ogden et al., 2020, Saab et al., 2015, Lackland, 2014, Neeland et al., 2013). There are currently no efficacious pharmacological treatments to prevent, delay, or treat cognitive decline, but lifestyle interventions may show promise in at-risk populations.
The Mediterranean diet (MedDiet) includes high consumption of fruits, vegetables, and olive oil, low intake of animal products, and moderate alcohol intake with food (Trichopoulou et al., 2015, Martínez-Lapiscina et al., 2013, Buckland et al., 2008). Observational studies have examined the relationship between MedDiet adherence and cognitive functioning (Gu et al., 2010, Feart and Barberger-Gateau, 2015, Kesse-Guyot et al., 2013, Cherbuin and Anstey, 2012, Scarmeas et al., 2006, Tangney et al., 2014, Wengreen et al., 2013, Samieri et al., 2013, Titova et al., 2013). Some studies show a positive association between adherence to a MedDiet and cognitive function (Gu et al., 2010, Feart and Barberger-Gateau, 2015, Tangney et al., 2014, Titova et al., 2013), whereas others do not (Kesse-Guyot et al., 2013, Cherbuin and Anstey, 2012, Samieri et al., 2013). Randomized clinical trials (RCTs) also show mixed results, with some reporting no difference in cognition post-intervention between groups randomized to a MedDiet versus control treatment (e.g., (Knight et al., 2016) and others reporting significant cognitive differences between groups (e.g., (Mehta et al., 2004, Rovner et al., 2013, Steenland et al., 2016, Ogden et al., 2020, Saab et al., 2015, Lackland, 2014, Neeland et al., 2013, Trichopoulou et al., 2015, Martínez-Lapiscina et al., 2013, Buckland et al., 2008, Gu et al., 2010, Feart and Barberger-Gateau, 2015, Kesse-Guyot et al., 2013, Cherbuin and Anstey, 2012, Scarmeas et al., 2006, Tangney et al., 2014, Wengreen et al., 2013, Samieri et al., 2013, Titova et al., 2013, Knight et al., 2016, Valls-Pedret et al., 2015, Wade et al., 2018, Wade et al., 2019)).
In a meta-analysis of trials focused on intentional weight loss through calorie restriction, weight loss among cognitively intact obese, primarily older adults, was linked to improved cognitive functioning across several domains with similar effects observed in a RCT among obese older adults with mild cognitive impairment (Horie et al., 2016). However, the effect of intentional weight loss on cognition remains equivocal. In a large decade-long RCT of older U.S. adults with type 2 diabetes, intentional weight loss did not affect the prevalence of cognitive impairment at 10-year follow-up (Espeland et al., 2017). Moreover, in a sub-study from the same trial, engagement in the behavioral weight loss intervention was associated with deficits in processing speed and long-term memory relative to controls at 10-year follow-up (Espeland et al., 2018). However, these results should be interpreted with caution given the cognitive assessments occurred after the interventions commenced.
Adherence to a MedDiet and intentional weight loss through caloric restriction each show important positive effects on risk indicators of cardiometabolic disease, including insulin resistance, blood lipids, blood pressure, and systemic inflammation (Schwingshackl and Hoffmann, 2014, Abd El-Kader and Al-Dahr, 2016, Esposito et al., 2003, Ard et al., 2018, Pedersen et al., 2019, Papadaki et al., 2020, Wu et al., 2021, Filippou et al., 2021). These physiological adaptations likely account for the cognitive benefit observed in some of the studies highlighted Therefore, combining a MedDiet with caloric restriction for intentional weight loss could have a synergistic effect on cardiometabolic health leading to greater cognitive improvement than either intervention alone. To our knowledge, no RCT has evaluated and reported on the efficacy of this combined approach among primarily non-Hispanic black older adults with obesity while also assessing related biological underpinnings.
We conducted a RCT to compare the effect of a MedDiet alone lifestyle intervention and a MedDiet plus caloric restriction lifestyle intervention compared to control on cognition, lifestyle, and risk indicators for cardiometabolic disease among older adult, predominantly non-Hispanic black females with obesity. We hypothesized that participants in the MedDiet alone and MedDiet plus caloric restriction lifestyle interventions would show improved cognition as well as improvements in diet quality and risk indicators for cardiometabolic disease compared to the control group. We also hypothesized that the greatest effects on cognition would be observed in the MedDiet plus caloric restriction lifestyle intervention group, given the benefit of intentional weight loss.
2. Methods
The trial design, including methodology, inclusion and exclusion criteria and detailed baseline characteristics of the study cohort, have been published elsewhere and are summarized below (Tussing-Humphreys et al., 2017, Sanchez-Flack et al., 2021). The trial is registered at clinicaltrials.gov (NCT03129048). Participants were followed for 14 months; this article presents findings following the completion of the 8-month active intervention phase.
2.1. Study population
We conducted the Building Research in Diet and Cognition study between January 2017 and October 2020 at several community sites in Chicago, Illinois. Participants were recruited using passive and active strategies including, advertising in local neighborhoods and presentations in senior facilities. Participants from a previous study who had agreed to be contacted for future research were also recruited (Hughes et al., 2020). Potential participants were screened by phone and in-person. Individuals included were 55–85 years old, body mass index (BMI) 30–50 kg/m2, Montreal Cognitive Assessment (MoCA) score ≥ 19, MedDiet adherence screener score ≤ 6 out of 13 indicating that they were less than 50 % adherent to a MedDiet pattern (Martínez-González et al., 2012), and English-speaking. Exclusion criteria has been discussed elsewhere (Tussing-Humphreys et al., 2017) and included inability to exercise based on the EASY screener (Ory et al., 2005), hemoglobin A1c (HbA1c) > 9 % from a blood sample obtained during a screening visit, significant health conditions including autoimmune diseases, and severe pulmonary, cardiovascular, hepatic or renal diseases, Warfarin use, severe neurological or psychiatric condition, bariatric surgery, concurrent enrollment in a formal weight loss program or participation in cognitive research in the past 12 months. The protocol was approved by the University of Illinois Chicago Institutional Review Board (#2016–0258), and written informed consent was obtained from all participants.
2.2. Randomization and intervention groups
The study was conducted in three cohorts of approximately 60 participants each. Participants were randomized by a statistician in a 2:2:1 ratio to Mediterranean diet alone lifestyle intervention, Mediterranean diet plus caloric restriction lifestyle intervention or control. Randomization was stratified by cohort, age (55–69 and 70–85 years), and baseline MoCA score (19–25 and 26–30) to ensure that the groups were approximately balanced with respect to the stratification variables. The stratified block randomization sequence, with a block size of 5, was created in SAS and imported into the Research Electronic Data Capture (REDCap) randomization module. The data manager, who had no contact with participants, used the randomization module to assign participants to the intervention groups. Research staff conducting cognitive interviews were blinded to allocation.
2.3. Interventions
The MedDiet alone lifestyle intervention and MedDiet plus caloric restriction lifestyle intervention participants met for 26 sessions (only 25 sessions were offered in the third cohort due to timing of the fall/winter holidays) including 1 individual in-person session with a nutrition professional that was assigned to the specific intervention arm and 25 in-person group sessions that were held weekly at a community location over an 8-month period. The Social Cognitive Theory (Bandura, 1982, Bandura, 1989) and Social Determination Theory (Ryan and Deci, 2000) were used to guide the development of the manualized interventions. Participants in both MedDiet groups received almonds (enough to consume 1 oz daily) and extra virgin olive oil (enough to consume 3 tablespoons daily) that were donated to the study (Almond Board of California & John Healy Columbus Vegetable Oil Company). Intervention instructor training and session content are described elsewhere (Tussing-Humphreys et al., 2017).
2.3.1. Mediterranean diet alone lifestyle intervention
Participants were instructed to adhere to an isocaloric MedDiet using an adapted exchange list during the initial one-on-one session (Djuric et al., 2008). During group sessions (60 min), participants engaged in didactic and hands-on (e.g., meal prep) training to accommodate adoption, adherence, and maintenance of a MedDiet pattern. Participants in this group were asked to maintain their baseline body weight and physical activity pattern.
2.3.2. Mediterranean diet plus caloric restriction lifestyle intervention
Participants were instructed to adhere to a calorie restricted (∼25 % kcal restricted to achieve ∼5-7 % weight loss) MedDiet during the initial one-on-one session. Like the MedDiet alone lifestyle intervention arm, an exchange list approach was used to facilitate adoption and adherence to the diet pattern. Participants were also instructed to gradually increase moderate to vigorous physical activity to a goal of 150 min weekly. Group sessions (90 min) were like the MedDiet alone intervention but included additional session content focused on weight loss through caloric restriction and increasing physical activity to meet prescribed recommendations. MedDiet plus caloric restriction group sessions also offered 30 min of supervised physical activity including stretching, flexibility and moderate cardiovascular exercise led by a certified exercise instructor.
2.3.3. Control
Participants met one-on-one with the control nutrition professional for 60 min to review procedures for the study. No dietary or physical activity recommendations were offered. Participants were provided with weekly general health education materials (newsletters). Upon completion of the study, control participants were offered a one-on-one session with the MedDiet plus caloric restriction dietitian and provided the curriculum in a self-directed format.
2.4. Outcome measures
2.4.1. Primary outcome
Cognition. The primary study outcome was change in cognition across three domains from baseline to the end of the 8-month active interventions. Cognitive interviews were performed by a trained interviewer blinded to the participant’s randomization assignment with the participant in a post-prandial state. Separate domain-specific composite scores were calculated for attention, information, & processing; executive function; and learning, memory, & recognition.
Attention, information & processing (AIP). The AIP cognitive domain was assessed via raw scores from the Digit Span Forward subtest and the Digit Symbol subtest of the Wechsler Adult Intelligence Scale – IV (Wechsler, 1955) time to completion on Part A of the Trail Making Test (TMT), (Reitan et al., 1988) and raw scores from the Stroop word and color subtests (Stroop, 1935).
Executive function (EF). The EF cognitive domain was assessed using the raw scores from the Digit Span Backward and Sequencing subtests of the WAIS-IV, TMT Part B time to completion, total correct words produced on letter fluency (Lezak et al., 2004) and the Stroop Color-Word Interference score (Stroop, 1935).
Learning, memory & recognition (LMR). The LMR cognitive domain was assessed using three measures from the California Verbal Learning Test – II (CVLT-II): total recall across the 5 learning trials, total delay free recall, and recognition discriminability scores (Delis, 2000).
Cognitive composite scores. A composite score was created for each of the three domains outlined above (AIP, EF, and LMR) by converting the relevant raw scores to z-scores, then taking the mean of the z-scores. The z-scores for the TMT Parts A and B were multiplied by −1 so that a higher z-score indicate better performance to be consistent with all other individual z-scores. The 8-month z-scores were created using the baseline means and standard deviations.
2.5. Secondary outcomes
Participants completed surveys to determine socio-demographics characteristics including race and ethnicity and underwent assessments and provided biospecimen data to assess secondary outcomes related to body weight/composition, lifestyle, and cardiometabolic risk markers.
Height, weight, BMI and body composition. Research staff measured participant height using a stadiometer and weight using a calibrated electronic scale. BMI was calculated from these measurements. Whole body composition was assessed using the Lunar iDXA scanner (GE Healthcare) with central adiposity estimated by the scanner.
Habitual diet and Mediterranean diet adherence. Habitual dietary intake was estimated using the Harvard Food Frequency Questionnaire (HFFQ) (Willett et al., 1985). The HFFQ is a semi-quantitative questionnaire querying consumption of 131 foods and beverages during the previous 12 months. Completed HFFQ surveys were processed by the Channing Lab at Harvard University. Adherence to a MedDiet-like pattern. Data from the HFFQ were used to calculate a MedDiet adherence score. The MedDiet score, first developed by Panagiotakos et al. (2007) and later modified for a Chicago-based population by Tangney et al. (2011) was adapted further for applicability to the HFFQ variables and data. Briefly, food and beverage items from the HFFQ used to create adherence scores (0–5) for 11 components included: non-refined grains (summary variable included in the HFFQ output file), potatoes (1 item), fruit (19 items), vegetables (24 items), legumes and nuts (8 items), fish (4 items), red meat and processed meat (12 items), poultry (3 items), full-fat dairy products (8 items), olive oil (3 items), alcohol (milliliters per week based on 5 items). The score ranges from 0 to 55 points, with 5 points maximum for each component awarded for full compliance and scores scaled proportionately based on intake. For example, for non-refined grains, those consuming 33 or more servings weekly received 5 points, 19–32 servings 4 points, 13–18 servings 3 points, 7–12 servings 2 points, 1–6 servings 1 point and consuming 0 servings 0 points.
Physical activity. Participants were asked to wear a wrist triaxial accelerometer for 7 days at each assessment point to determine moderate to vigorous physical activity (Kamada et al., 2016). Participants were asked to wear the accelerometer on their non-dominant wrist for 7 days. Data were included if the participant wore the accelerometer for ≥ 4 days and ≥ 10 h/day.
Cardiometabolic risk markers. Systolic and diastolic blood pressure were assessed in duplicate using an automated blood pressure monitor (Omron HEM-907, Lake Forest, IL) with the participant in a seated position after sitting quietly for five minutes.
Blood samples were obtained following a minimum 8 h fast for the assessment of total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, glucose, insulin, HbA1c and high sensitivity C-reactive protein (hs-CRP) by a local commercial lab (Quest Diagnostics, Wood Dale, IL). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as insulin mIU/ml X glucose mg/dl/405 (Matthews et al., 1985).
2.6. Statistical analysis
Power and sample size estimates were based on data results presented in the ENCORE trial (Blumenthal et al., 2010) with an intent to generate separate linear models for the three domain-specific cognitive composite scores. We calculated that at least 180 subjects (72 Mediterranean diet lifestyle intervention, 72 Mediterranean diet plus caloric restriction lifestyle intervention, 36 control) assuming 20 % attrition using the intention to treat analysis and alpha = 0.05 would yield power of about 0.80 to detect treatment effects of 0.32 standard deviations between Mediterranean diet groups and control and 0.38 standard deviations between Mediterranean diet groups for both executive function and attention, information, & processing (Tussing-Humphreys et al., 2017). Similarly, we estimated for learning, memory, & recognition our sample would yield power of 0.80 to detect a treatment effect of about 0.35 standard deviations between both Mediterranean diet groups and control and 0.44 standard deviations between the Mediterranean diet groups.
We tested for differences in retention between groups using a chi-square test and for differences in attendance between Mediterranean diet plus caloric restriction lifestyle intervention and Mediterranean diet lifestyle intervention using a Wilcoxon rank sum test. To test for intervention effects on cognition and the secondary outcomes, we used repeated-measures linear models in SAS Proc Mixed with a fully specified (unstructured) covariance matrix and the baseline value included in the outcome vector. All models included intervention group, visit, and a group*visit interaction term. Cohort, baseline age (continuous), and baseline Montreal Cognitive Assessment score (continuous) were included in all models as covariates since they were the stratification variables for randomization. In models where the overall group*visit term was statistically significant (p <.05), we used SAS LSMESTIMATE statements to test for pairwise differences in change between groups.
Three of the secondary outcome variables (insulin, HOMA-IR, and triglycerides) were log-transformed to improve normality. The adjusted baseline and 8-month means were back-transformed, and their standard errors were estimated using the delta method. The adjusted change from baseline to post-intervention and the corresponding 95 % confidence interval were back-transformed using the method suggested by Laursen et al. (2014).
A repeated-measures analysis using restricted maximum likelihood and unstructured covariance has been found to give unbiased estimates in cases with missing data, if the data are missing at random (Mallinckrodt, 2013). Therefore, our analysis is an intention-to-treat analysis. All statistical analyses were performed using SAS (SAS Institute, Cary, NC), v9.4.
3. Results
3.1. Participant characteristics and attrition
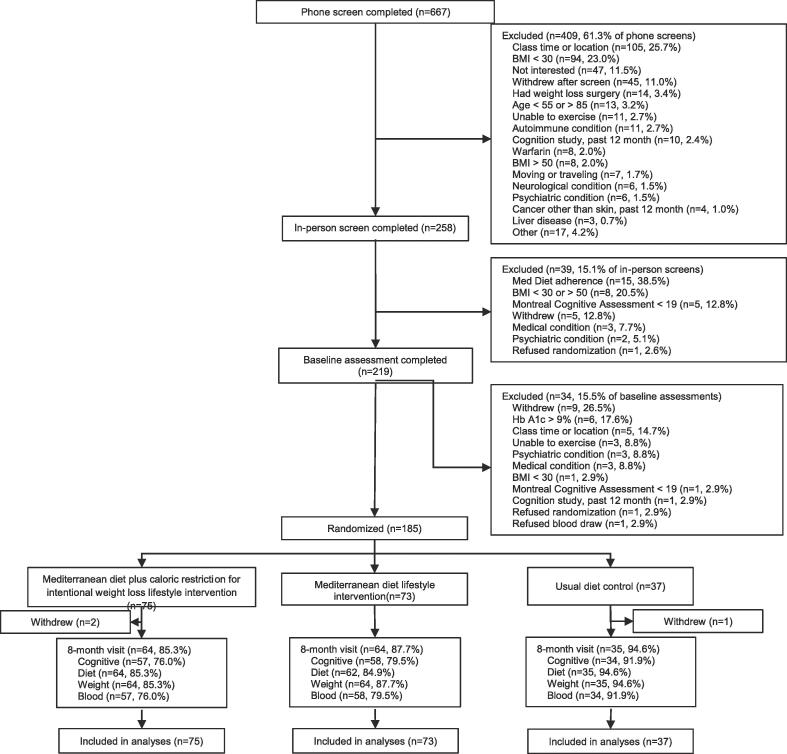
Of the 258 individuals screened in person, 185 were randomized as shown in the CONSORT diagram (Fig. 1) and 163 (88 %) completed some and 149 (81 %) completed all the cognitive assessments following the 8-month intervention. There was no statistically significant difference in attrition between the groups at follow-up (p = 0.36). All baseline characteristics had comparable distributions between the MedDiet arms and control arm (Table 1). Participants were primarily non-Hispanic black (91 %) based on self-report, female (86 %), with mean hs-CRP indicative of elevated cardiovascular risk (Malik et al., 2005) and 67 % reported a diagnosis of hypertension.
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Table 1.
Participant characteristics at baseline.
| Mediterranean diet plus caloric restriction lifestyle intervention n = 75 |
Mediterranean diet lifestyle intervention n = 73 |
Control n = 37 |
All n = 185a |
No cognitive data at 8 months n = 36 |
Cognitive data at 8 months n = 149 |
|
|---|---|---|---|---|---|---|
| Age at randomization, yr, mean (SD) | 65.7 (6.0) | 66.2 (6.0) | 67.6 (6.5) | 66.3 (6.1) | 65.8 (6.6) | 66.4 (6.0) |
| Gender, n (%) | ||||||
| Female | 61 (81.3) | 66 (90.4) | 32 (86.5) | 159 (85.9) | 28 (77.8) | 131 (87.9) |
| Male | 14 (18.7) | 7 (9.6) | 5 (13.5) | 26 (14.1) | 8 (22.2) | 18 (12.1) |
| Race, n (%) | ||||||
| Black or African-American, not Hispanic | 69 (92.0) | 69 (94.5) | 31 (83.8) | 169 (91.4) | 35 (97.2) | 134 (89.9) |
| Hispanic | 1 (1.3) | 1 (1.4) | 0 (0.0) | 2 (1.1) | 0 (0.0) | 2 (1.3) |
| White, not Hispanic | 0 (0.0) | 0 (0.0) | 2 (5.4) | 2 (1.1) | 0 (0.0) | 2 (1.3) |
| Native American | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (2.8) | 0 (0.0) |
| Multiracial | 4 (5.3) | 3 (4.1) | 4 (10.8) | 11 (5.9) | 0 (0.0) | 11 (7.4) |
| Education, yr, mean (SD) | 14.8 (2.3) | 15.2 (2.5) | 15.3 (2.4) | 15.1 (2.4) | 14.7 (2.6) | 15.1 (2.3) |
| Highest degree earned, n (%) | ||||||
| Not HS graduate | 1 (1.3) | 3 (4.1) | 0 (0.0) | 4 (2.2) | 2 (5.6) | 2 (1.4) |
| HS graduate | 31 (41.3) | 24 (32.9) | 14 (38.9) | 69 (37.5) | 14 (38.9) | 55 (37.2) |
| Associate’s degree | 6 (8.0) | 9 (12.3) | 3 (8.3) | 18 (9.8) | 4 (11.1) | 14 (9.5) |
| College graduate | 20 (26.7) | 11 (15.1) | 6 16.7) | 37 (20.1) | 6 (16.7) | 31 (20.9) |
| Graduate or professional degree | 17 (22.7) | 26 (35.6) | 13 (36.1) | 56 (30.4) | 10 (27.8) | 46 (31.1) |
| Employed full or part-time, n (%) | 20 (26.7) | 24 (32.9) | 8 (21.6) | 52 (28.1) | 11 (30.6) | 41 (27.5) |
| Marital status | ||||||
| Single | 17 (22.7) | 20 (27.4) | 10 (27.0) | 47 (25.4) | 7 (19.4) | 40 (26.8) |
| Married | 28 (37.3) | 14 (19.2) | 9 (24.3) | 51 (27.6) | 14 (38.9) | 37 (24.8) |
| Widowed | 8 (10.7) | 14 (19.2) | 7 (18.9) | 29 (15.7) | 9 (25.0) | 20 (13.4) |
| Divorced | 22 (29.3) | 25 (34.2) | 11 (29.7) | 58 (31.4) | 6 (16.7) | 52 (34.9) |
| Income, $, (median, quartile range) | 50,000 (40,000) | 50,000 (40,000) | 50,000 (20,000) | 50,000 (40,000) | 50,000 (50,000) | 50,000 (20,000) |
| Income category, n (%) | ||||||
| <$20,000 | 16 (22.2) | 16 (22.2) | 8 (22.2) | 40 (22.2) | 7 (20.0) | 33 (22.8) |
| $20,000–$40,000 | 18 (25.0) | 17 (23.6) | 7 (19.4) | 42 (23.3) | 6 (17.1) | 36 (24.8) |
| ≥$40,000 | 38 (52.8) | 39 (54.2) | 21 (58.3) | 98 (54.4) | 22 (62.9) | 76 (52.4) |
| Has health insuranceb, n (%) | 74 (98.7) | 72 (98.6) | 37 (100.0) | 183 (98.9) | 36 (100.0) | 147 (98.7) |
| Medical conditionsc, n (%) | ||||||
| High blood pressure | 47 (62.7) | 50 (68.5) | 27 (73.0) | 124 (67.0) | 24 (66.7) | 100 (67.1) |
| High cholesterol | 28 (37.3) | 24 (32.9) | 20 (54.1) | 72 (38.9) | 17 (47.2) | 55 (36.9) |
| Type 2 diabetes | 18 (24.0) | 8 (11.0) | 4 (10.8) | 30 (16.2) | 7 (19.4) | 23 (15.4) |
| Sleep apnea | 18 (24.0) | 17 (23.3) | 11 (29.7) | 46 (24.9) | 11 (30.6) | 35 (23.5) |
| Total prescription medications, mean (SD) | 2.5 (2.1) | 2.7 (2.1) | 2.4 (2.0) | 2.5 (2.1) | 2.8 (2.1) | 2.5 (2.1) |
| Mediterranean diet screener score (0–13)d, mean (SD) | 4.1 (1.3) | 4.3 (1.6) | 4.3 (1.3) | 4.2 (1.4) | 3.8 (1.5) | 4.3 (1.4) |
| Weight, kg, mean (SD) | 103.3 (15.9) | 99.2 (13.5) | 97.5 (13.0) | 100.5 (14.5) | 104.7 (10.7) | 99.5 (15.2) |
| Height, cm, mean (SD) | 165.0 (8.0) | 164.5 (6.3) | 164.2 (8.0) | 164.6 (7.4) | 166.7 (7.8) | 164.1 (7.2) |
| BMI, kg/m2, mean (SD) | 37.9 (5.1) | 36.7 (4.6) | 36.1 (4.2) | 37.1 (4.8) | 37.8 (4.7) | 36.9 (4.8) |
| Percent body fat, mean (SD) | 48.0 (6.4) | 47.2 (5.9) | 47.8 (5.1) | 47.7 (6.0) | 46.4 (6.9) | 48.0 (5.7) |
| VAT mass, g, mean (SD) | 1689 (803) | 1583 (670) | 1565 (905) | 1621 (774) | 1881 (828) | 1562 (752) |
| Percentage of classes attendede, (median, quartile range) | 75.0 (56.0) | 70.8 (51.5) | na | 72.0 (53.3) | ||
| Attendance categories, n (%) | na | |||||
| < 25% | 18 (24.0) | 16 (21.9) | 34 (23.0) | |||
| 25%-<50% | 5 (6.7) | 6 (8.2) | 11 (7.4) | |||
| 50%-<75% | 14 (18.7) | 16 (21.9) | 30 (20.3) | |||
| ≥ 75% | 38 (50.7) | 35 (47.9) | 73 (49.3) |
N=184 for education, prescription medications, and percent body fat,180 for income, and 174 for VAT mass. For attendance, N=148 (Mediterranean diet plus caloric restriction lifestyle intervention+ Mediterranean diet lifestyle intervention).
Medicare, Medicaid, or private insurance.
Self-reported, current or past conditions.
Screener scores can range from 0 to 13, with higher scores indicating greater adherence. Only those with scores < 7 were eligible for the study.
Number of classes used to calculate attendance: 25 for cohorts 1 and 2 and 24 for cohort 3. Wilcoxon rank sum test for difference between groups: p=.98.
3.2. Intervention participation
Attendance at group sessions for the MedDiet arms is presented in Table 1. There was no difference in group session attendance by intervention arm. For both groups, almost 70 % of participants attended 50 % or more of the sessions, and almost 50 % of participants in both MedDiet arms attended 75 % or more of intervention group sessions.
3.3. Cognitive outcomes
Changes in standardized cognitive composite scores are presented in Table 2 (Supplementary Table 1 reports on the unadjusted results). There was no significant group*time interaction for change in AIP, EF, or LMR composite scores following the 8-month interventions. We explored if response differed in participants with high and low baseline MoCA scores by running the repeated measure model separately for each grouping and second by using a 3-way interaction term (MoCA category*group*visit) to the repeated-measures model. We found no significant results by baseline MoCA category for any of the cognitive outcomes (data not shown).
Table 2.
Adjusted mean change in cognitive composites from baseline to end of the 8-month interventions.
| Mediterranean diet plus caloric restriction lifestyle intervention |
Mediterranean diet lifestyle intervention |
Control |
|||||
|---|---|---|---|---|---|---|---|
| Adjusteda Mean (SE) | Adjusteda change from baseline to 8 months | Adjusteda Mean (SE) | Adjusteda change from baseline to 8 months | Adjusteda Mean (SE) | Adjusteda change from baseline to 8 months | Group * Time | |
| Outcome Measure | Baseline | Meanb (95 % CI) | Baseline | Meanb (95 % CI) | Baseline | Meanb (95 % CI) | pc |
| Attention, Information & Processing Composite | −0.02 (0.07) | 0.04 (−0.05 to 0.13) | −0.01 (0.07) | 0.06 (−0.03 to 0.15) | 0.07 (0.10) | 0.14 (0.03 to 0.26) | 0.36 |
| Executive Function Composite | 0.08 (0.06) | 0.10 (−0.00 to 0.20) | −0.09 (0.06) | 0.10 (0.00 to 0.20) | 0.04 (0.09) | 0.18 (0.04 to 0.31) | 0.61 |
| Learning, Memory & Recognition Composite | −0.07 (0.09) | 0.04 (−0.15 to 0.23) | 0.04 (0.09) | 0.03 (−0.16 to 0.22) | 0.09 (0.13) | 0.06 (−0.19 to 0.31) | 0.98 |
From repeated-measures linear models with a fully specified (unstructured) covariance matrix and the baseline value included in the outcome vector. Covariates included in models: cohort, baseline age and Montreal Cognitive Assessment score. For Ns and unadjusted means, see Electronic Supplementary Table 1.
Estimated mean change is the difference between the follow-up and baseline adjusted means.
Test for group*time interaction.
3.4. Secondary outcomes
3.4.1. Weight loss and body composition
Change in body weight, BMI, and body composition following the 8-month interventions is detailed in Table 3 (Supplementary Table 2 reports on the unadjusted results). There was a significant group*time interaction for change in body weight, BMI, % body fat, and estimated visceral adipose tissue mass. The MedDiet plus caloric restriction group lost significantly more body weight and significantly reduced their BMI and % body fat relative to the MedDiet alone and control groups. Both MedDiet arms experienced a significant reduction in both body weight and BMI compared to the control group. Both MedDiet groups experienced similar and statistically significant reductions in estimated visceral adipose tissue mass compared to the control group. However, % total body fat was not statistically different among the study arms at the post-intervention assessment.
Table 3.
Adjusted mean change in secondary outcomes from baseline to end of the 8-month interventions.
| Mediterranean diet plus caloric restriction lifestyle intervention |
Mediterranean diet lifestyle intervention |
Control |
|||||
|---|---|---|---|---|---|---|---|
| Adjusteda Mean (SE) | Adjusteda change from baseline to 8 months | Adjusteda Mean (SE) | Adjusteda change from baseline to 8 months | Adjusteda Mean (SE) | Adjusteda change from baseline to 8 months | Group*Time | |
| Outcome Measure | Baseline | Meanb (95 % CI) | Baseline | Meanb (95 % CI) | Baseline | Meanb (95 % CI) | pc |
| Mediterranean Diet Score (0–55)g | 31.9 (0.6) | 6.3 (4.9 to 7.6) | 33.4 (0.6) | 4.8 (3.4 to 6.2) | 33.4 (0.9) | 0.6 (−1.3 to 2.4) | <.001d,e |
| Weight, kg | 103.0 (1.6) | −4.6 (−5.6 to −3.5) | 99.1 (1.7) | −2.6 (−3.7 to −1.5) | 98.1 (2.3) | −0.6 (−2.1 to 0.8) | <.001d,e,f |
| BMI, kg/m2 | 37.9 (0.5) | −1.7 (−2.1 to −1.3) | 36.6 (0.6) | −1.0 (−1.4 to −0.6) | 36.2 (0.8) | −0.2 (−0.8 to 0.3) | <.001d,e,f |
| Percent body fat | 48.1 (0.7) | −1.6 (−2.1 to −1.1) | 47.3 (0.7) | −0.8 (−1.2 to −0.3) | 47.7 (1.0) | −0.2 (−0.8 to 0.4) | .002d,f |
| Visceral adipose mass, g | 1747 (94) | −147 (−235 to −59) | 1598 (93) | −162 (−248 to −76) | 1560 (131) | 91 (−18 to 200) | <.001d,e |
| Systolic BP, mm Hg | 135.0 (2.1) | 1.5 (−3.2 to 6.2) | 132.7 (2.1) | 3.2 (−1.5 to 7.9) | 132.7 (3.0) | 2.6 (−3.7 to 8.8) | 0.88 |
| Diastolic BP, mm Hg | 80.2 (1.3) | 0.2 (−2.8 to 3.1) | 79.7 (1.3) | −0.6 (−3.5 to 2.3) | 78.8 (1.9) | 1.4 (−2.6 to 5.3) | 0.73 |
| Moderate to vigorous physical activity min/dayh | 9.1 (0.8) | 0.5 (−1.0 to 2.0) | 9.4 (0.8) | 1.0 (−0.5 to 2.5) | 10.1 (1.1) | −0.0 (−2.1 to 2.0) | 0.73 |
| HbA1c, % | 6.2 (0.1) | −0.1 (−0.2 to −0.0) | 6.0 (0.1) | −0.1 (−0.2 to 0.0) | 6.0 (0.1) | −0.0 (−0.2 to 0.1) | 0.72 |
| Glucose, mg/dL | 106.1 (2.8) | −0.5 (−6.3 to 5.2) | 98.6 (2.8) | −0.9 (−6.7 to 4.8) | 103.5 (3.9) | −5.9 (−13.6 to 1.8) | 0.51 |
| Insulin, uIU/mLi | 9.6 (0.6) | −2.2 (−3.2 to −1.2) | 10.0 (0.7) | −0.6 (−1.6 to 0.4) | 9.9 (0.9) | −0.5 (−1.8 to 0.8) | .046d,f |
| HOMA-IRi | 2.5 (0.2) | −0.6 (−0.9 to −0.3) | 2.4 (0.2) | −0.2 (−0.4 to 0.1) | 2.5 (0.3) | −0.2 (−0.6 to 0.1) | 0.08 |
| Total cholesterol, mg/dL | 188.2 (4.3) | −3.3 (−11.9 to 5.4) | 190.3 (4.4) | 1.2 (−7.4 to 9.7) | 187.2 (6.1) | 6.2 (−5.1 to 17.5) | 0.42 |
| HDL, mg/dL | 60.0 (1.8) | 0.4 (−1.9 to 2.6) | 57.6 (1.8) | 0.4 (−1.8 to 2.6) | 60.8 (2.5) | 3.9 (1.0 to 6.9) | 0.11 |
| LDL, mg/dL | 109.0 (3.8) | −3.4 (−10.5 to 3.8) | 112.0 (3.8) | 1.5 (−5.6 to 8.5) | 107.0 (5.4) | 2.8 (−6.6 to 12.1) | 0.51 |
| Triglycerides, mg/dLi | 89.2 (4.1) | −3.8 (−9.9 to 2.3) | 96.1 (4.4) | −3.5 (−9.6 to 2.5) | 88.8 (5.8) | −1.7 (−9.8 to 6.4) | 0.91 |
| hs-CRP mg/Lj | 4.7 (0.3) | −0.5 (−1.1 to −0.0) | 3.7 (0.3) | 0.2 (−0.3 to 0.7) | 3.8 (0.5) | −0.5 (−1.2 to 0.2) | 0.12 |
From repeated-measures linear models with a fully specified (unstructured) covariance matrix and the baseline value included in the outcome vector. Covariates included in models: cohort, baseline age and Montreal Cognitive Assessment score. For Ns and unadjusted means, see Electronic Supplementary Table 1.
Estimated mean change is the difference between the follow-up and baseline adjusted means, except in the case of the log-transformed variablesi.
Test for group*time interaction.
p <.05 for difference between Mediterranean diet plus caloric restriction for intentional weight loss lifestyle intervention and usual diet control.
p <.05 for difference between Mediterranean diet lifestyle intervention and usual diet control.
p <.05 for difference between Mediterranean diet plus caloric restriction for intentional weight loss lifestyle intervention and Mediterranean diet lifestyle intervention.
A higher score indicates greater adherence to the Mediterranean diet.
Calculated using the ActiLife program; moderate to vigorous physical activity was defined as ≥ 7500 counts per minute (Lezak et al., 2004).
Insulin, HOMA-IR, and triglycerides were log-transformed to improve normality. Baseline means shown are estimates of the geometric means (back-transformed adjusted means from the model), with SEs estimated using the delta method. Adjusted mean change from baseline to 8 months and the 95 % CI were estimated using the method suggested by Laursen et al. (2014).
hs-CRP values > 10 mg/L excluded: 29 at baseline and 23 at follow-up.
3.4.2. Mediterranean diet adherence
MedDiet adherence score changes following the 8-month interventions are presented in Table 3 (Supplementary Table 2 reports the unadjusted results). There was a significant group*time interaction for change in MedDiet adherence score. Both MedDiet groups increased their adherence scores similarly and with statistical and clinical significance from baseline compared to the control group (Anastasiou et al., 2017). Their baseline scores were aligned with medium adherence to a MedDiet (e.g., (Espeland et al., 2018, Schwingshackl and Hoffmann, 2014, Esposito et al., 2003, Ard et al., 2018, Pedersen et al., 2019, Papadaki et al., 2020, Anastasiou et al., 2017, Psaltopoulou et al., 2013)), but after the intervention their scores categorized them into the highly adherent, cognitively beneficial range and above (i.e., (Wu et al., 2021, Anastasiou et al., 2017, Psaltopoulou et al., 2013)).
3.4.3. Physical activity
Change in moderate to vigorous physical activity is presented in Table 3 (Supplementary Table 3 reports the unadjusted results). There was no significant group*time interaction for moderate to vigorous physical activity following the interventions.
3.4.4. Cardiometabolic risk markers
Cardiometabolic risk marker changes are reported in Table 3 (Supplementary Tables 3 & 4 report the unadjusted results). There was a significant group*time interaction for change in fasting insulin post-intervention with a significant reduction observed in the MedDiet plus caloric restriction group relative to the MedDiet alone and control groups. There were no significant group*time interactions observed for the other cardiometabolic risk markers.
4. Discussion
Findings from observational MedDiet, intentional weight loss, and cognitive functioning studies need to be confirmed through RCTs to establish a causal link between these modifiable factors for successful aging. We are unaware of any RCTs that have examined the combined effect of MedDiet and caloric restriction on cognition, although several have examined the effects of each in isolation. To our knowledge, this study is the first RCT to evaluate a MedDiet lifestyle intervention with and without caloric restriction on cognitive functioning, lifestyle and cardiometabolic health risk among a sample of older, predominately non-Hispanic black females with obesity. The results showed no statistical differences in three domains of cognitive functioning despite use of a comprehensive neuropsychological battery. Both MedDiet interventions did result, however, in decreased body weight and central adiposity and improved diet quality.
There are several potential explanations for our null cognitive findings. It may be that the benefit of a MedDiet and potential further benefit of a combination of a MedDiet and caloric restriction lies in slowing or preventing pathological cognitive aging, rather than maintaining normal cognitive aging (Feart and Barberger-Gateau, 2015, Chen et al., 2019). Indeed, several of the RCTs showing a positive effect of a MedDiet on cognition were conducted in persons with mild cognitive impairment or cardiometabolic disease (Valls-Pedret et al., 2015, Wade et al., 2019, Wade et al., 2020). Alternatively, our signal strength may have been reduced by using distinct cognitive domain scores rather than a single composite score. Several RCTs using a composite score reported positive effects of a MedDiet on cognition (Valls-Pedret et al., 2015, Wade et al., 2019, Wade et al., 2020). However, Knight et al. reported null effects after a 6-month MedDiet RCT using cognitive outcomes similar to ours (Knight et al., 2016). Specifically, we clearly delineated between AIP and higher-order executive control whereas other RCTs combine tasks from separate domains into one score. Thus, what we gained in distinct cognitive domain scores may have limited our signal strength. There is also the possibility that the relatively small study sample and limited duration of the active interventions were insufficient to detect an effect of a MedDiet and caloric restriction on cognitive function. Two papers from the PREvencion con DIeta MEDiterranea (PREDIMED) study with larger samples, longer duration, and the similar inclusion of supplements of extra virgin olive oil and/or nuts did show cognitive benefit in participants at high cardiometabolic risk (Martínez-Lapiscina et al., 2013, Valls-Pedret et al., 2015).
In a related RCT among overweight racially diverse adults with cardiovascular disease risk, performance on an executive function-memory-learning composite score significantly improved compared to controls following four months of aerobic exercise combined with a calorie restricted Dietary Approaches to Stop Hypertension (DASH) diet (Smith et al., 2010). Importantly, a calorie restricted DASH diet alone did not affect cognition. In fact, the significant effect of the combined intervention was mediated through improvement in cardiorespiratory fitness and not change in body weight or diet pattern. In a more recent trial, among adults with cognitive impairment and cardiometabolic risk, a 6-month aerobic exercise intervention with and without a DASH dietary pattern change had significant effects on a global measure of executive function compared to DASH alone or health education control (Blumenthal et al., 2019). The benefits of improving cardiorespiratory fitness on cognition are supported by several other RCTs reporting that inactive adults beginning an exercise program experienced cognitive improvement (Northey et al., 2018, Stern et al., 2019, Peven et al., 2020). Although our MedDiet plus caloric restriction lifestyle intervention included recommendations to achieve 150 min per week of moderate to vigorous physical activity and included 30 min per week supervised physical activity, moderate to vigorous physical activity increased minimally.
5. Strengths and limitations
There are limitations to our study including a dietary assessment based on self-report which is a limitation that is well documented and is prone to recall bias and social desirability. We did not assess objective biomarkers to determine dietary adherence. Second, the MedDiet adherence score used in our study does not account for minimally vs highly processed foods. Food processing greatly affects the nutrient composition and nutritional value of MedDiet specific foods that may provide health benefits (e.g., vegetables, olive oil) (Hoffman and Gerber, 2015). The generalizability of our findings is also limited by the enrollment of individuals self-selecting to participate in a RCT. Despite these limitations, our findings contribute to the field in several important ways. First, we recruited and retained an obese, a predominantly non-Hispanic black female sample, which is a unique and underrepresented group in prevention trials. Second, our two diet intervention approaches were designed to be similar in content and intensity, to rigorously test the addition of intentional weight loss. Third, we only randomized participants who had a suboptimal MedDiet score at baseline. Fourth, the cognitive test battery chosen had significant methodological rigor, assessing several cognitive domains, rather than assessing only global cognition or relying on a single task screener for our primary outcome metric.
In conclusion, we did not observe positive changes in cognitive function among older obese adults randomized to an 8-month MedDiet alone or MedDiet plus caloric restriction lifestyle intervention. Longer trials with larger samples, particularly among individuals representing a wider range of cognitive functioning, are warranted and will further confirm and explain our outcomes.
6. Compliance with Ethical Standards
Authors’ statement of conflict of interest and adherence to ethical standards: Drs. Fitzgibbon, Tussing-Humphreys, Lamar, McLeod, Sanchez-Flack and Berbaum reported receiving grant funding from the National Institutes of Health during the conduct of the study. The authors declare that they have no conflict of interest.
Authors’ contributions: Dr. Fitzgibbon had full access to all the data in the study and takes responsibility for the integrity of the data and the overall conduct of the trial as Principal Investigator, and for the preparation of this manuscript as its corresponding author. All coauthors received a copy of this manuscript and provided Dr. Fitzgibbon with their comments on earlier version of the draft.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Trial registration.clinicaltrials.gov Identifier: NCT03129048.
CRediT authorship contribution statement
Lisa Tussing-Humphreys: Conceptualization, Funding acquisition, Writing – original draft. Melissa Lamar: Conceptualization, Funding acquisition, Writing – review & editing. Andrew McLeod: Data curation, Writing – review & editing. Linda Schiffer: Data curation, Formal analysis, Writing – review & editing. Lara Blumstein: Project administration, Writing – review & editing. Roxanne Dakers: Data curation, Project administration, Writing – review & editing. Aimee Karstens: Data curation, Writing – review & editing. Nefertiti Oji Njideka Hemphill: Investigation, Project administration, Writing – review & editing. Desmona Strahan: Investigation, Writing – review & editing. Leilah Siegel: Investigation, Writing – review & editing. Jennifer Sanchez Flack: Data curation, Formal analysis, Methodology, Writing – review & editing. Mirjana Antonic: Project administration, Writing – review & editing. Leo Restrepo: Data curation. Michael Berbaum: Conceptualization, Formal analysis, Writing – review & editing. Marian Fitzgibbon: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by a grant from the National Heart, Lung, and Blood Institute to Dr. Fitzgibbon (R01HL129153). Drs. Sanchez-Flack and McLeod’s efforts were supported by T32CA057699. The statements in the article are solely the responsibility of the authors and do not necessarily represent the views of the sponsor.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.101955.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abd El-Kader S.M., Al-Dahr M.H.S. Weight loss improves biomarkers endothelial function and systemic inflammation in obese postmenopausal Saudi women. Afr. Health Sci. 2016;16(2):533–541. doi: 10.4314/ahs.v16i2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou C.A., Yannakoulia M., Kosmidis M.H., Dardiotis E., Hadjigeorgiou G.M., Sakka P., et al. Mediterranean diet and cognitive health: Initial results from the Hellenic Longitudinal Investigation of Ageing and Diet. PloS One. 2017;12(8) doi: 10.1371/journal.pone.0182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ard J.D., Gower B., Hunter G., Ritchie C.S., Roth D.L., Goss A., et al. Effects of calorie restriction in obese older adults: the CROSSROADS randomized controlled trial. J. Gerontol.: Series A. 2018;73(1):73–80. doi: 10.1093/gerona/glw237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy mechanism in human agency. Am. Psychol. 1982;37(2):122. [Google Scholar]
- Bandura A. Human agency in social cognitive theory. Am. Psychol. 1989;44(9):1175. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- Blumenthal J.A., Babyak M.A., Hinderliter A., Watkins L.L., Craighead L., Lin P.H., et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch. Intern. Med. 2010;170(2):126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal J.A., Smith P.J., Mabe S., Hinderliter A., Lin P.H., Liao L., et al. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology. 2019;92(3):e212–e223. doi: 10.1212/WNL.0000000000006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland G., Bach A., Serra-Majem L. Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes. Rev. 2008;9(6):582–593. doi: 10.1111/j.1467-789X.2008.00503.x. [DOI] [PubMed] [Google Scholar]
- Chen X., Maguire B., Brodaty H., O’Leary F. Dietary patterns and cognitive health in older adults: a systematic review. J. Alzheimers Dis. 2019;67(2):583–619. doi: 10.3233/JAD-180468. [DOI] [PubMed] [Google Scholar]
- Cherbuin N., Anstey K.J. The Mediterranean diet is not related to cognitive change in a large prospective investigation: the PATH Through Life study. Am. J. Geriatr. Psychiatry. 2012;20(7):635–639. doi: 10.1097/JGP.0b013e31823032a9. [DOI] [PubMed] [Google Scholar]
- Delis D.C. Adult Version Manual Psychological Corporation; 2000. California verbal learning test. [Google Scholar]
- Djuric Z., Vanloon G., Radakovich K., Dilaura N.M., Heilbrun L.K., Sen A. Design of a Mediterranean exchange list diet implemented by telephone counseling. J. Am. Diet. Assoc. 2008;108(12):2059–2065. doi: 10.1016/j.jada.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland M.A., Luchsinger J.A., Baker L.D., Neiberg R., Kahn S.E., Arnold S.E., et al. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology. 2017;88(21):2026–2035. doi: 10.1212/WNL.0000000000003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland M.A., Luchsinger J.A., Neiberg R.H., Carmichael O., Laurienti P.J., Pi-Sunyer X., et al. Long term effect of intensive lifestyle intervention on cerebral blood flow. J. Am. Geriatr. Soc. 2018;66(1):120–126. doi: 10.1111/jgs.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K., Pontillo A., Di Palo C., Giugliano G., Masella M., Marfella R., et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289(14):1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- Feart C., Barberger-Gateau P. Mediterranean diet and cognitive health. Diet Nutr. Dementia Cogn. Decline. 2015:265–283. [Google Scholar]
- Filippou C.D., Thomopoulos C.G., Kouremeti M.M., Sotiropoulou L.I., Nihoyannopoulos P.I., Tousoulis D.M., et al. Mediterranean diet and blood pressure reduction in adults with and without hypertension: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021 doi: 10.1016/j.clnu.2021.01.030. [DOI] [PubMed] [Google Scholar]
- Gu Y., Nieves J.W., Stern Y., Luchsinger J.A., Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch. Neurol. 2010;67(6):699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R., Gerber M. Food processing and the Mediterranean diet. Nutrients. 2015;7(9):7925–7964. doi: 10.3390/nu7095371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie N.C., Serrao V.T., Simon S.S., Gascon M.R.P., Dos Santos A.X., Zambone M.A., et al. Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment. J. Clin. Endocrinol. Metab. 2016;101(3):1104–1112. doi: 10.1210/jc.2015-2315. [DOI] [PubMed] [Google Scholar]
- Hughes S.L., Tussing-Humphreys L., Schiffer L., Smith-Ray R., Marquez D.X., DeMott A.D., et al. Fit & strong! plus trial outcomes for obese older adults with osteoarthritis. The Gerontologist. 2020;60(3):558–570. doi: 10.1093/geront/gny146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada M., Shiroma E.J., Harris T.B., Lee I.M. Comparison of physical activity assessed using hip- and wrist-worn accelerometers. Gait Posture. 2016;44:23–28. doi: 10.1016/j.gaitpost.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesse-Guyot E., Ahluwalia N., Lassale C., Hercberg S., Fezeu L., Lairon D. Adherence to Mediterranean diet reduces the risk of metabolic syndrome: a 6-year prospective study. Nutr. Metab. Cardiovasc. Dis. 2013;23(7):677–683. doi: 10.1016/j.numecd.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Knight A., Bryan J., Wilson C., Hodgson J.M., Davis C.R., Murphy K.J. The Mediterranean diet and cognitive function among healthy older adults in a 6-month randomised controlled trial: the MedLey Study. Nutrients. 2016;8(9):579. doi: 10.3390/nu8090579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackland D.T. Racial differences in hypertension: implications for high blood pressure management. Am. J. Med. Sci. 2014;348(2):135–138. doi: 10.1097/MAJ.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen R.P., Dalskov S.M., Damsgaard C.T., Ritz C. Back-transformation of treatment differences–an approximate method. Eur. J. Clin. Nutr. 2014;68(2):277–280. doi: 10.1038/ejcn.2013.259. [DOI] [PubMed] [Google Scholar]
- Lezak M.D., Howieson D.B., Loring D.W., Fischer J.S. Oxford University Press; USA: 2004. Neuropsychological Assessment. [Google Scholar]
- Malik S., Wong N.D., Franklin S., Pio J., Fairchild C., Chen R. Cardiovascular disease in US patients with metabolic syndrome, diabetes, and elevated C-reactive protein. Diab. Care. 2005;28(3):690–693. doi: 10.2337/diacare.28.3.690. [DOI] [PubMed] [Google Scholar]
- Mallinckrodt C.H. Cambridge University Press; 2013. Preventing and Treating Missing Data in Longitudinal Clinical Trials: A Practical Guide. [Google Scholar]
- Martínez-González M.A., García-Arellano A., Toledo E., Salas-Salvadó J., Buil-Cosiales P., Corella D., et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS ONE. 2012;7(8):e43134. doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Lapiscina E.H., Clavero P., Toledo E., Estruch R., Salas-Salvadó J., San Julián B., et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry. 2013;84(12):1318–1325. doi: 10.1136/jnnp-2012-304792. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J., Rudenski A., Naylor B., Treacher D., Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mehta K.M., Simonsick E.M., Rooks R., Newman A.B., Pope S.K., Rubin S.M., et al. Black and white differences in cognitive function test scores: what explains the difference? J. Am. Geriatr. Soc. 2004;52(12):2120–2127. doi: 10.1111/j.1532-5415.2004.52575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeland I.J., Ayers C.R., Rohatgi A.K., Turer A.T., Berry J.D., Das S.R., et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity. 2013;21(9):E439–E447. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northey J.M., Cherbuin N., Pumpa K.L., Smee D.J., Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br. J. Sports Med. 2018;52(3):154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- Ogden C.L., Fryar C.D., Martin C.B., Freedman D.S., Carroll M.D., Gu Q., et al. Trends in obesity prevalence by race and hispanic origin—1999-2000 to 2017–2018. JAMA. 2020;324(12):1208–1210. doi: 10.1001/jama.2020.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory M., Resnick B., Jordan P.J., Coday M., Riebe D., Ewing Garber C., et al. Screening, safety, and adverse events in physical activity interventions: collaborative experiences from the behavior change consortium. Ann. Behav. Med. 2005;(29 Suppl):20–28. doi: 10.1207/s15324796abm2902s_5. [DOI] [PubMed] [Google Scholar]
- Panagiotakos D.B., Pitsavos C., Arvaniti F., Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007;44(4):335–340. doi: 10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Papadaki A., Nolen-Doerr E., Mantzoros C.S. The effect of the Mediterranean diet on metabolic health: a systematic review and meta-analysis of controlled trials in adults. Nutrients. 2020;12(11):3342. doi: 10.3390/nu12113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen L.R., Olsen R.H., Anholm C., Astrup A., Eugen-Olsen J., Fenger M., et al. Effects of 1 year of exercise training versus combined exercise training and weight loss on body composition, low-grade inflammation and lipids in overweight patients with coronary artery disease: a randomized trial. Cardiovasc. Diabetol. 2019;18(1):1–13. doi: 10.1186/s12933-019-0934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peven J.C., Jakicic J.M., Rogers R.J., Lesnovskaya A., Erickson K.I., Kang C., et al. The effects of a 12-month weight loss intervention on cognitive outcomes in adults with overweight and obesity. Nutrients. 2020;12(10) doi: 10.3390/nu12102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaltopoulou T., Sergentanis T.N., Panagiotakos D.B., Sergentanis I.N., Kosti R., Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013;74(4):580–591. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- Reitan R.M., Hom J., Wolfson D. Verbal processing by the brain. J. Clin. Exp. Neuropsychol. 1988;10(4):400–408. doi: 10.1080/01688638808408248. [DOI] [PubMed] [Google Scholar]
- Rovner B.W., Casten R.J., Harris L.F. Cultural diversity and views on Alzheimer’s disease in older African Americans. Alzheimer Dis. Assoc. Disord. 2013;27(2):133. doi: 10.1097/WAD.0b013e3182654794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R.M., Deci E.L. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am. Psychol. 2000;55(1):68. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Saab K.R., Kendrick J., Yracheta J.M., Lanaspa M.A., Pollard M., Johnson R.J. New insights on the risk for cardiovascular disease in African Americans: the role of added sugars. J. Am. Soc. Nephrol. 2015;26(2):247–257. doi: 10.1681/ASN.2014040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samieri C., Grodstein F., Rosner B.A., Kang J.H., Cook N.R., Manson J.E., et al. Mediterranean diet and cognitive function in older age: results from the Women’s Health Study. Epidemiology (Cambridge, Mass.) 2013;24(4):490. doi: 10.1097/EDE.0b013e318294a065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Flack J.C., Tussing-Humphreys L., Lamar M., Fantuzzi G., Schiffer L., Blumstein L., et al. Building research in diet and cognition (BRIDGE): baseline characteristics of older obese African American adults in a randomized controlled trial to examine the effect of the Mediterranean diet with and without weight loss on cognitive functioning. Prev. Med. Rep. 2021;22 doi: 10.1016/j.pmedr.2020.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Stern Y., Tang M.X., Mayeux R., Luchsinger J.A. Mediterranean diet and risk for Alzheimer's disease. Ann. Neurol. 2006;59(6):912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl L., Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014;24(9):929–939. doi: 10.1016/j.numecd.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Shadlen M.F., Siscovick D., Fitzpatrick A.L., Dulberg C., Kuller L.H., Jackson S. Education, cognitive test scores, and black-white differences in dementia risk. J. Am. Geriatr. Soc. 2006;54(6):898–905. doi: 10.1111/j.1532-5415.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- Smith P.J., Blumenthal J.A., Babyak M.A., Craighead L., Welsh-Bohmer K.A., Browndyke J.N., et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331–1338. doi: 10.1161/HYPERTENSIONAHA.109.146795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K., Goldstein F.C., Levey A., Wharton W. A meta-analysis of Alzheimer’s disease incidence and prevalence comparing African-Americans and Caucasians. J. Alzheimers Dis. 2016;50(1):71–76. doi: 10.3233/JAD-150778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., MacKay-Brandt A., Lee S., McKinley P., McIntyre K., Razlighi Q., et al. Effect of aerobic exercise on cognition in younger adults: A randomized clinical trial. Neurology. 2019;92(9):e905–e916. doi: 10.1212/WNL.0000000000007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18(6):643. [Google Scholar]
- Tangney C.C., Kwasny M.J., Li H., Wilson R.S., Evans D.A., Morris M.C. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am. J. Clin. Nutr. 2011;93(3):601–607. doi: 10.3945/ajcn.110.007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney C.C., Li H., Wang Y., Barnes L., Schneider J.A., Bennett D.A., et al. Relation of DASH-and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410–1416. doi: 10.1212/WNL.0000000000000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titova O.E., Ax E., Brooks S.J., Sjögren P., Cederholm T., Kilander L., et al. Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp. Gerontol. 2013;48(12):1443–1448. doi: 10.1016/j.exger.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Tönnies E., Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimers Dis. 2017;57(4):1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichopoulou A., Kyrozis A., Rossi M., Katsoulis M., Trichopoulos D., La Vecchia C., et al. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur. J. Nutr. 2015;54(8):1311–1321. doi: 10.1007/s00394-014-0811-z. [DOI] [PubMed] [Google Scholar]
- Tussing-Humphreys L., Lamar M., Blumenthal J.A., Babyak M., Fantuzzi G., Blumstein L., et al. Building research in diet and cognition: The BRIDGE randomized controlled trial. Contemp. Clin. Trials. 2017;59:87–97. doi: 10.1016/j.cct.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Pedret C., Sala-Vila A., Serra-Mir M., Corella D., De la Torre R., Martínez-González M.Á., et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern. Med. 2015;175(7):1094–1103. doi: 10.1001/jamainternmed.2015.1668. [DOI] [PubMed] [Google Scholar]
- van den Brink A.C., Brouwer-Brolsma E.M., Berendsen A.A., van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer's disease—a review. Adv. Nutr. 2019;10(6):1040–1065. doi: 10.1093/advances/nmz054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese N., Facchini S., Stubbs B., Luchini C., Solmi M., Manzato E., et al. Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017;72:87–94. doi: 10.1016/j.neubiorev.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Wade A.T., Davis C.R., Dyer K.A., Hodgson J.M., Woodman R.J., Murphy K.J. A Mediterranean diet supplemented with dairy foods improves markers of cardiovascular risk: results from the MedDairy randomized controlled trial. Am. J. Clin. Nutr. 2018;108(6):1166–1182. doi: 10.1093/ajcn/nqy207. [DOI] [PubMed] [Google Scholar]
- Wade A.T., Davis C.R., Dyer K.A., Hodgson J.M., Woodman R.J., Keage H.A., et al. A Mediterranean Diet with fresh, lean pork improves processing speed and mood: Cognitive findings from the MedPork randomised controlled trial. Nutrients. 2019;11(7):1521. doi: 10.3390/nu11071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade A.T., Davis C.R., Dyer K.A., Hodgson J.M., Woodman R.J., Keage H.A., et al. A Mediterranean diet supplemented with dairy foods improves mood and processing speed in an Australian sample: Results from the MedDairy randomized controlled trial. Nutr. Neurosci. 2020;23(8):646–658. doi: 10.1080/1028415X.2018.1543148. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. Arch. Clin. Neuropsychol. 1955 [Google Scholar]
- Wengreen H., Munger R.G., Cutler A., Quach A., Bowles A., Corcoran C., et al. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am. J. Clin. Nutr. 2013;98(5):1263–1271. doi: 10.3945/ajcn.112.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W.C., Sampson L., Stampfer M.J., Rosner B., Bain C., Witschi J., et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- Wu P.-Y., Chen K.-M., Tsai W.-C. The Mediterranean dietary pattern and inflammation in older adults: a systematic review and meta-analysis. Adv. Nutr. 2021;12(2):363–373. doi: 10.1093/advances/nmaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.