Abstract
Quinones of Thermoplasma acidophilum HO-62 were analyzed by high-performance liquid chromatography, mass spectrometry, and nuclear magnetic resonance. Menaquinone, methionaquinone, and 2-trans and 2-cis forms of thermoplasmaquinone were identified. The relative amount of thermoplasmaquinone increased under anaerobic conditions, and those of menaquinone and methionaquinone increased under aerobic conditions.
Isoprenoid quinones are widely distributed in the bacterial plasma membrane and play important roles in the electron transport system (14). The bacterial quinone profile has often been used for microbial taxonomy (4–6, 15). The relationship between the quinone profile and growth conditions has also been discussed, since each quinone has specific redox potential and its content is expected to affect the respiratory system (7, 12, 13, 16).
Thermoplasma acidophilum is a facultative anaerobic and thermophilic archaeon. Two naphthoquinones, thermoplasmaquinone-7 (TPQ-7), which is a derivative of methylmenaquinones, and menaquinone-7 (MK-7) have been isolated from strain 122-1B2 (3, 11). A methyl group of methylmenaquinone isolated from Alteromonas putrefaciens was reported to be located at position 8 on the naphthoquinone ring (10). However, the exact position of the methyl group in TPQ-7 has not been reported yet. In this study, we determined the position of the methyl group. The other quinones in T. acidophilum HO-62 were also analyzed. We also investigated the influence of aeration on the relative amount of naphthoquinones in T. acidophilum.
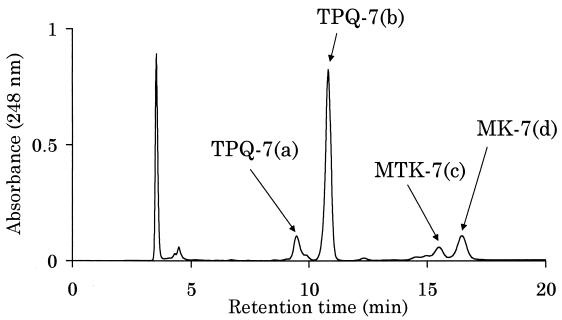
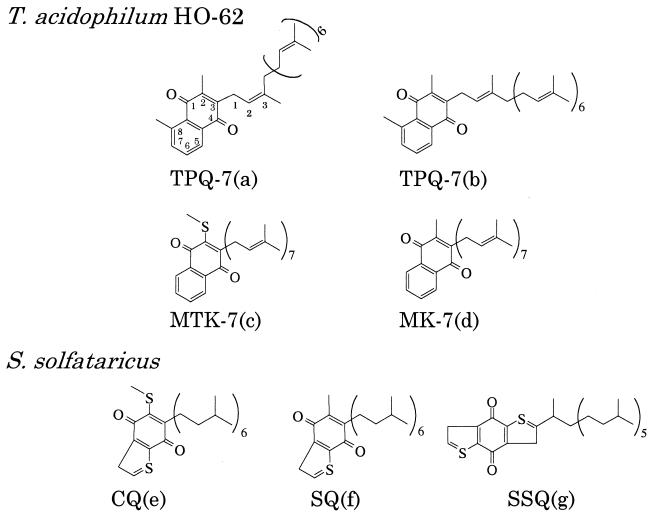
T. acidophilum HO-62 was statically grown in 10 liters of a medium described by Yasuda et al. (18). Total lipids were extracted by the Bligh-Dyer method (2) from the harvested cells (14.8 g [wet weight] in total) and then were passed through a Sephadex G-25 column (17). The total lipid was applied on to a silica gel column (30 by 250 mm) equilibrated with chloroform. Neutral lipid was eluted with 200 ml of chloroform and subjected to high-performance liquid chromatography (HPLC). Four peaks (a to d) were observed (Fig. 1). The structures of the compounds corresponding to these peaks obtained in this study are shown in Fig. 2.
FIG. 1.
HPLC chromatogram of a neutral lipid fraction from T. acidophilum HO-62. A Shiseido (Tokyo, Japan) Capcell pak silica UG80 column (4.6 by 250 mm) attached to an HPLC system (monitored at 248 nm) was used. The sample was separated using hexane-diethylether-trifluoroacetic acid (99:1:0.002, vol/vol/vol) at a flow rate of 0.86 ml · min−1.
FIG. 2.
Chemical structures of quinones elucidated from the results obtained in this report [TPQ-7(a) and -7(b) and MTK-7(c) and MK-7(d)] and the structures identified by Nicolaus et al. (13) [CQ(e), SQ(f), and SSQ(g), where SQ is sulfolobusquinone and SSQ is tricyclic quinone].
Electron impact mass spectrometry was used to analyze the compounds corresponding to the four peaks shown in Fig. 1. Both TPQ-7(a) and TPQ-7(b) showed a molecular ion [M]+ at m/z 662, and fragment ions at m/z 647, 594, 526, 457, 389, 321, and 253, which are similar to those reported for TPQ with seven isoprene units (3). TPQ-7(a) and TPQ-7(b) are considered to be cis-trans isomers. MTK-7(c) that showed [M]+ at m/z 680 seemed to be one of the methionaquinones (2-methylthio-3-multiprenyl-1, 4-naphthoquinone) (9), with seven isoprene units. The fragment ion observed at m/z 634 can be explained by the loss of S⩵CH2 from [M]+, caused by hydrogen transfer. This fragmentation was similar to that of MTK-7(H4) (9). The molecular ion derived from 34S ([M+2]+, m/z 682) was not distinct, because it overlapped with isotope peaks of 13C. MK-7(d) showed [M]+ at m/z 648. The fragmentation pattern was similar to that reported for MK-7 (10).
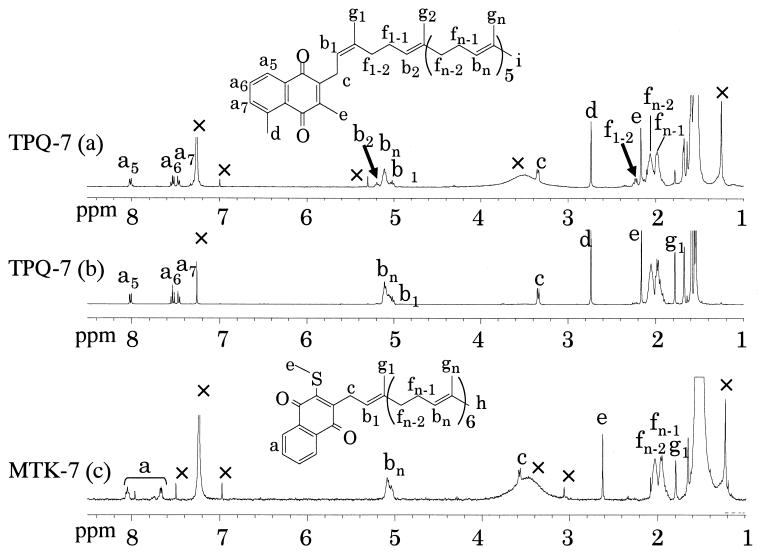
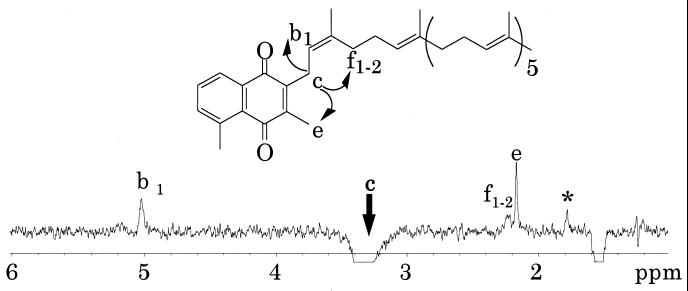
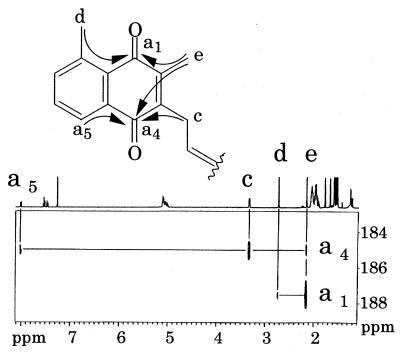
Nuclear magnetic resonance (NMR) spectra of these quinones were measured in deuterochloroform (CDCl3). The 1H NMR spectra of TPQ-7(a) and TPQ-7(b) were similar, except for the signals of b2 and f1–2 in TPQ-7(a) (Fig. 3). The 1H NMR spectrum of TPQ-7(b) agreed well with that of TPQ-7 (3). The signal of f1–2 in TPQ-7(a) was determined by the one-dimensional steady-state differential nuclear Overhauser effect (1D-NOE) presaturated at peak c. Because the correlation signals of f1–2 were observed, the double bond of an isoprene unit which was nearest to the naphthoquinone ring was thought to be a cis form (Fig. 4). TPQ-7(b) was found to be a trans form, because the 1D-NOE spectrum of TPQ-7(b) showed the correlation signal of g1 (data not shown). A fraction of TPQ-7(a) was isomerized to TPQ-7(b) in a storage solution and vice versa (data not shown).
FIG. 3.
1H NMR spectra (400 MHz). Shown are results of TPQ-7(a) in CDCl3, 128 scans; TPQ-7(b) in CDCl3, 16 scans; and MTK-7 (c) in CDCl3, 128 scans. All spectra were recorded at an ambient temperature. Signals of impurity, water, or solvent are denoted by a ×. In the TPQ-7(a) spectrum, signals different from those of TPQ-7(b) are denoted by arrows.
FIG. 4.
1D-NOE spectrum (400 MHz) of TPQ-7(a) presaturated at peak c (arrow) in CDCl3. A total of 32,000 scans were accumulated at an ambient temperature. The peak denoted by an asterisk probably corresponds to TPQ-7(b) contaminating the TPQ-7(a) sample.
To investigate the position of the CH3 group in the naphthoquinone ring of TPQ, the heteronuclear multiple-bond correlation (1) spectrum of TPQ-7(b) was measured (Fig. 5). The CH3(d) of TPQ was found to be present at position 8 (a8). The position of the CH3 group in TPQ-7 of T. acidophilum was the same as that in methylmenaquinone-7 (10) isolated from A. putrefaciens.
FIG. 5.
Heteronuclear multiple-bond correlation spectrum (500 MHz) of TPQ-7(b). The horizontal and vertical axes show the 1H chemical shift and 13C chemical shift, respectively (only for carbonyl carbons). A total of 1,024 scans were accumulated at an ambient temperature.
The 1H NMR spectrum of MTK-7(c) is shown in Fig. 3. The signal of peak e (2.62 ppm) assigned as the proton of SCH3 was similar to that of MTK-7(H4) (2.64 ppm) isolated from Hydrogenobacter thermophilus TK-6 (9). This signal is unique to MTKs and is significantly different from that of MK-7(d) (2.17 ppm) (data not shown). Based on mass spectrometry analysis, all the isoprene units of MTK-7(c) described in this report were found to be unsaturated. Although MTK-7(H4) is different from MTK-7 in that the former has two saturated isoprene units at the end of seven isoprene units, the structure of the naphthoquinone part is expected to be the same based on the NMR data. MTK-7, which was tentatively assumed to be unsaturated, was found in the microbial mat which existed in the hot spring reported by Hiraishi et al. (8). However, there have been no reports on MTK-7 obtained from any isolated bacteria.
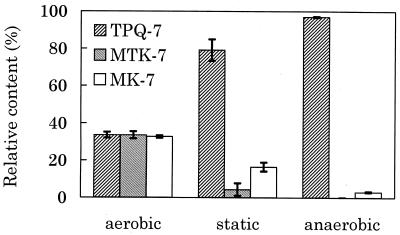
The samples used for the analysis of the quinone content under different growth conditions were extracted from cells of a 30-ml culture in the presence of sulfur (0.1 g · ml−1) at 57°C. Aerobic culture was performed with an aeration flux of 55 ml · min−1. Air was replaced by N2 gas for anaerobic culture. The extracted crude lipid was directly applied onto the HPLC column. The relative amount of quinones was estimated from the peak area monitored at 248 nm. Freshly presented samples tend to show a higher amount of 2-trans-TPQ-7 [TPQ-7(b)] than of 2-cis-TPQ-7 [TPQ-7(a)]. Because 2-cis-TPQ-7 [TPQ-7(a)] seemed to be formed by isomerization of 2-trans-TPQ-7 [TPQ-7(b)] during the purification process, the percentage of TPQ-7 was expressed as the sum of the amount of cis and trans forms. Under aerobic conditions, the relative amounts of TPQ-7, MTK-7, and MK-7 were 34, 34, and 33%, respectively (Fig. 6). While under anaerobic conditions, 97% of total quinone was TPQ-7 and only 3% was MK-7. TPQ-7 was also predominant in cells statically cultured. TPQ-7 was produced under anaerobic conditions. The relative amounts of MK-7 and MTK-7 were higher under aerobic conditions than those under anaerobic conditions.
FIG. 6.
Quinone composition in T. acidophilum cells cultured under different air supplies. Each value is a mean obtained from three independent experiments (error bars, standard deviations).
In the case of Escherichia coli, ubiquinone, a derivative of benzoquinones, is used for aerobic respiration, because it has a high redox potential, while for anaerobic respiration, MK is used due to its low redox potential (7, 12). Although the redox potential of quinones in Thermoplasma was not estimated, Fig. 6 shows that different naphthoquinones are used depending on the oxygen supply in Thermoplasma.
The relative amounts of CQ (caldariellaquinone) and SQ (sulfolobusquinone) in Solfolobus solfataricus and Desulfurolobus ambivalens were influenced by the oxygen supply. The amount of CQ increased under aerobic conditions (13, 16). The structure of CQ is similar to that of MTK: they both have a methylthio group (SCH3) attached to the second position in the quinone ring. The amount of MTK in T. acidophilum also increased under aerobic conditions. Accordingly, the methylthio group in the quinone ring appears to play an important role in growth under aerobic conditions.
The amount of MK-7 in T. acidophilum HO-62 was also increased under aerobic conditions. The only difference between MK-7 and TPQ-7 is a CH3 at position 8. The amount of MK-7 may be increased under aerobic conditions by suppressing methylation, which must occur at the last step of biosynthesis of TPQ-7. These results suggest that T. acidophilum HO-62 not only can grow in an anaerobic environment by producing an increased amount of TPQ-7 but also can adapt to an aerobic environment by producing MK-7 and MTK-7. It will be interesting to investigate the regulation of the biosynthesis process under different growth conditions.
Acknowledgments
We thank M. Iwashima for the valuable discussions of the NMR measurements.
REFERENCES
- 1.Bax A, Summers M F. 1H and 13C assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J Am Chem Soc. 1986;108:2093–2094. [Google Scholar]
- 2.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 3.Collins M D. Structure of thermoplasmaquinone from Thermoplasma acidophilum. FEMS Microbiol Lett. 1985;28:21–23. [Google Scholar]
- 4.Collins M D, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microb Rev. 1981;45:316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Rosa M, Gambacorta A. The lipids of archaebacteria. Prog Lipid Res. 1988;27:153–175. doi: 10.1016/0163-7827(88)90011-2. [DOI] [PubMed] [Google Scholar]
- 6.Gambacorta A, Trincone A, Nicolaus B, Lama L, de Rosa M. Unique features of lipids of archaea. Syst Appl Microbiol. 1994;16:518–527. [Google Scholar]
- 7.Hellemond J J V, Tielens A G M. Expression and functional properties of fumarate reductase. Biochem J. 1994;304:321–331. doi: 10.1042/bj3040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiraishi A, Umezawa T, Yamamoto H, Kato K, Maki Y. Changes in quinone profiles of hot spring microbial mats with a thermal gradient. Appl Environ Microbiol. 1999;65:198–205. doi: 10.1128/aem.65.1.198-205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii M, Kawasumi T, Igarashi Y, Kodama T, Minoda Y. 2-Methylthio-1, 4-naphthoquinone, a new quinone from an extremely thermophilic hydrogen bacterium. Agric Biol Chem. 1983;47:167–169. doi: 10.1128/jb.169.6.2380-2384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh T, Funabashi H, Katayama-Fujimura Y, Iwasaki S, Kuraishi H. Structure of methylmenaquinone-7 isolated from Alteromonas putrefaciens IAM12079. Biochem Biophys Acta. 1985;840:51–55. [Google Scholar]
- 11.Langworthy T A, Smith P F, Mayberry W R. Lipids of Thermoplasma acidophilum. J Bacteriol. 1972;112:1193–1200. doi: 10.1128/jb.112.3.1193-1200.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lester R L, Crane F L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959;234:2169–2175. [PubMed] [Google Scholar]
- 13.Nicolaus B, Trincone A, Palmieri G, Gambacorta A. Quinone composition in Sulfolobus solfataricus grown under different conditions. Syst Appl Microbiol. 1992;15:18–20. [Google Scholar]
- 14.Redfearn E R. Mode of action of ubiquinones in electron transport systems. Vitam Horm. 1966;24:465–488. doi: 10.1016/s0083-6729(08)60217-4. [DOI] [PubMed] [Google Scholar]
- 15.Thurl S, Witke W, Buhrow I, Schafer W. Different types of quinones from sulphur-dependent archaebacteria. Biol Chem Hoppe-Seyler. 1986;367:191–198. doi: 10.1515/bchm3.1986.367.1.191. [DOI] [PubMed] [Google Scholar]
- 16.Trincone A, Lanzotti V, Nicolaus B, Zillig W, de Rosa M, Gambacorta A. Comparative lipid composition of aerobically grown Desulfurolobus ambivalens, an autotrophic thermophilic archaebacterium. J Gen Microbiol. 1989;135:2751–2757. [Google Scholar]
- 17.Wells M A, Dittmer J C. The use of sephadex for the removal of nonlipid contaminants from lipid extracts. Biochemistry. 1963;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda M, Oyaizu H, Yamagishi A, Oshima T. Morphological variation of new Thermoplasma acidophilum isolates from Japanese hot springs. Appl Environ Microbiol. 1995;61:3482–3485. doi: 10.1128/aem.61.9.3482-3485.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]