Abstract
The outbreaks of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant in China have revealed a high rate of asymptomatic cases, making isolation and quarantine measures exceedingly difficult. Public health surveillance and intervention measures will require rapid and accurate testing preferably on-site using point-of-care tests (POCTs) technology for SARS-CoV-2 variants. However, delayed and/or inaccurate surveillance data is a major obstacle blocking the large-scale implementation of POCTs in curbing spread of infectious pathogens and reducing mortality during an outbreak. To determine levels of community transmission and timely strategies accordingly, highly sensitive and specific POCT embedded with the internet of things (IoT) technology could enable on-site screening and real-time data collection. A new Rapid Amplification with Sensitivity And Portability point-of-care test (RASAP-POCT) system based on thermal convection PCR is the first IoT-based isothermal nucleic acid amplification POCT, which can provide test results within 20–30 min using saliva and/or nasopharyngeal swab samples without nucleic acid extraction. With the IoT-imbedded feature, the RASAP-POCT system can be integrated easily and smoothly with China’s existing mobile-phone-based contact tracing system, which has previously proved to be highly effective in maintaining the dynamic zero-COVID policy. Current regulatory guidelines and rules should be modified to accelerate the adoption of new technologies under an emergency use authorization (EUA).
Keywords: POCT, Thermal convention PCT, Isothermal nucleic acid amplification, IoT
1. Effective point-of-care testing (POSTs) for on-site screening and real-time data collection is critical for COVID-19 control
More than two years into the coronavirus disease 2019 (COVID-19) pandemic, the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), classified as B.1.1.529 (now there are more Bs and B.2 is the dominant one) by World Health Organization (WHO) in late November 2021, has rapidly become a global dominant strain owing to its very high transmissibility [1]. Characterized by short incubation time before viral particles shedding and high prevalence of asymptomatic or mild symptoms, this variant can still disseminate amongst populations with high prior rates of SARS-COV-2 infection or cause breakthrough infections in COVID-19 vaccinated individuals [2]. The outbreak of the Omicron variant in Shanghai, China has revealed exceptionally high ratios of asymptomatic cases over infected individuals with symptoms. Under China's dynamic zero-COVID policy, which aims to keep virus transmission as close to zero as possible using a “find, test, trace, isolate, and support” protocol, one of the paramount challenges of stopping the Omicron outbreak is to identify asymptomatic individuals accurately before they relay the infectious agent to others. Thus, population-wide rapid and accurate surveillance for community transmission of any SARS-CoV-2 variants using new technologies is required.
The gold standard for SARS-CoV-2 testing uses reverse transcription-quantitative polymerase chain reaction (RT-qPCR). However, the RT-qPCR method has a long turnaround time (requiring at least 4 h of sample-to-result time) and could only be performed in a few testing facilities (requiring certified laboratories that have expensive equipment and skilled technicians) [3]. In employing conventional PCR devices, it is difficult to shorten the reaction time because of the need for nucleic acid extraction and the intrinsic large thermal inertia of the heating blocks for thermal cycling. Surveillance using large-scale accessible diagnostics is a foundation for developing public health measures to stop the spread of infectious pathogens and improve individual and population health outcomes during an outbreak of a pathogen such as Omicron. As numbers of asymptomatic are skyrocketing, preventing Omicron spreading poses a serious technical challenge. Because RT-qPCR testing resources cannot meet the timely requirement and real-time data collection for large-scale testing, delays in reporting test results further complicate the difficulties in controlling outbreaks of highly infectious agents. Therefore, rapid and sensitive point-of-care tests (POCTs) of SARS-CoV-2, which are cheaper, faster, and deployable in the field, are essential for on-site testing in controlling the Omicron outbreaks [4].
2. Internet of things (IoT) is required for on-site POCT in controlling COVID-19 outbreak
All POCTs are designed for portable use and easy operation, which can be performed on-site by individuals without extensive training or even be used for self-testing at home. However, for various reasons not in line with the disease control, persons who perform on-site testing might purposely delay POCT reporting and/or misreport test results. Inaccurate surveillance data is a major obstacle blocking the large-scale implementation of POCTs in curbing spread of infectious pathogens and reducing mortality during an outbreak. Before implementing public health intervention measures to control community transmission, it is crucial for decision-makers to obtain real-time epidemiologic statistics based on mass testing and surveillance [5]. To this end, Internet of Things (IoT) technologies offer great promises to enhance timely testing, computation, and dissemination of epidemiologic assessment of the outbreaks that are standard functions in surveillance systems [6], [7]. IoT refers to electronic devices that are connected to the internet that supports easy sharing and collection of data required for appropriate decision‐making, without the need for significant human intervention. Although IoT has been employed in the medical domains and wearable devices, there is no IoT embedded POCT available currently. Embedded into a POCT device, an IoT system enables real-time data transfer without hands-on operations, while simultaneously block/impede attempts of unwanted or malicious use of POCTs. Moreover, IoT can also save precious time by eliminating the need for sample transportation and simplifying the data upload processes. Further, the internet-ready feature of an IoT embedded POCT would allow easy and smooth integration of the system with China’s existing mobile-phone-based contact tracing system, which has been proved to be highly effective in maintaining the dynamic zero-COVID policy. This will be particularly useful for decision-makers in managing mass testing and population surveillance in ending the COVID-19 pandemic and transiting society into a post-pandemic world.
3. Problems with current POCTs for SARS-CoV-2 screening
RT-qPCR testing uses nasopharyngeal swab samples whose collection is invasiveness, increasing the risk of infection to health workers. Moreover, implementation of sample collection and testing requires skilled technicians (as inappropriate handling may lead to false negative test results). Research has shown that saliva sampling can be an alternative to nasopharyngeal swab sampling to collect specimens for SARS-CoV-2 RT-qPCR and POCTs [8].
During the global pandemic, the WHO has ensured that apropriate POCT can receive not only an emergency use authorization (EUA) but also a Clinical Laboratory Improvement Amendment (CLIA) waived certification. Current SARS-CoV-2 POCTs are either based on antigen testing (Ag-POCT) or isothermal nucleic acid amplification techniques (NAAT-POCT). Antigen testing, which employs immune-based detection of the viral nucleocapsid protein from respiratory samples, constitutes the most common POCT products approved for SARS-CoV-2 screening, although it is considered inferior to RT-qPCR in terms of sensitivity and specificity [9]. Antigen-based test results can be further affected by stages of viral infection and disease prevalence in the local community, as well as cross- reactivity with other human coronaviruses, such as the Middle East Respiratory Syndrome (MERS) and other SARS viruses. Due to the poor sensitivity of antigen tests, confirmation tests using a nucleic acid detection technique is required to rule out potential false-negative antigen test results when clinical suspicion of infection remains high. In addition, the antigen-based test is usually designed as a self-testing kit without an associated IoT-imbedded portable instrument, which is not only difficult to report results but also incompatible with mass data collection and surveillance.
Aside from standard PCR, several alternative NAAT have been developed, including 1) loop-mediated isothermal amplification (LAMP), 2) nucleic acid sequence-based amplification (NASBA), 3) recombinase polymerase amplification (RPA), and 4) rolling circle amplification (RCA). These techniques are isothermal NAAT and thus employ simple or no instrument, suitable for mobile testing or POCTs for nucleic acid detection. However, these NAAT-POCTs are often limited by their costs and unreliable results due to their complex reagent systems and poor sensitivity, especially when unpurified samples are used. A meta-analysis revealed that the performance of direct detection of viral RNA unpurified from patient samples is substantially poorer than that of purified RNA [10].
4. Applying thermal convection PCR technology in designing NAAT-POCT
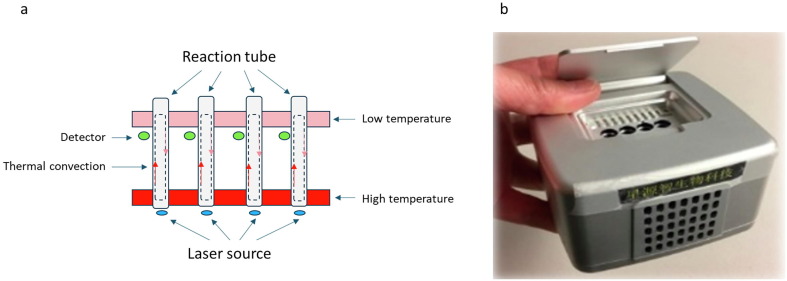
Thermal convection has been applied to PCR ever since it was originally invented by Hwang et al. in 2001. Unlike a conventional thermal cycler that has a heating block undergoing temperature cycling, the instrument for convection PCR is isothermal NAAT, in which two heating blocks with constant temperatures (the bottom one being set at a higher temperature) are used to maintain a temperature gradient in a columned reaction tube. Effective thermal cycling of the reaction mixture in a pseudo-isothermal manner can be created by imposing a steady-state vertical temperature gradient [11], [12]. This temperature gradient induces a continuous, sustainable circulation of reactants between the hot and cold zones in a reaction tube, thus spatially stratifying the reaction into stable melting, annealing, and extension zones (Fig. 1A). By eliminating the need for controlled time-domain temperature cycling, thermal convection PCR significantly shortens the cycling time which can be developed into POCT tool in testing for SARS-CoV-2 with reduced instrument size and cost. However, most previous attempts in applying the thermal convection PCR technology failed, because using conventional Taq DNA polymerase is not effective (low sensitivity and highly nonspecific products), particularly in on-site testing with complex or unpurified samples [13], [14]. To circumvent this problem, a new POCT system developed by HAVY Biotech ltd. (Changshu, Jiangsu, China) called RASAP – Rapid Amplification with Sensitivity And Portability - includes a palm-sized device with IoT components and a proprietary reagent system that can tolerate the less desirable temperature profile of the thermal convection (Fig. 1B). Compared with the conventional TaqMan reagents, the RASAP reagent contains a more robust DNA polymerase developed through genetic engineering and specifically designed primers and molecular probes. The system is insensitive to large temperature variations, has 2x faster extension rates, and, more importantly, is compatible with high ratios of unpurified samples. For example, DNA target molecules in a reaction containing 50% of human whole blood can be amplified by the reagent system.
Fig. 1.
Applying thermal convection PCR technology in designing NAAT-POCT. A) Schematic depiction of the real-time capillary convection polymerase chain reaction (PCR) system; B) A photograph of the Rapid Amplification with Sensitivity And Portability point-of-care test (RASAP-POCT) system.
5. RASAP-POCT system is a suitable IoT-imbedded NAAT-POCT for SARS-CoV-2 testing
The findings of saliva sample tests with a blind Proficiency Test Kit provided by the XPRIZE Rapid COVID Testing competition (a semi-finalist in the 6-month XPRIZE competition, September 2020) and the feasibility study conducted by the Shenzhen CDC, China (Oct., 2021), demonstrate that the RASAP-POCT system can provide test results within 20–30 min for saliva samples without nucleic acid extraction. For the early stages of SARS-CoV-2 infection, the detection sensitivity and accuracy of the RASAP-POCT system are higher than or equal to those of the conventional RT-qPCR technologies. In SARS-CoV-2 testing, the collection of saliva samples by patients themselves negates the need for direct interaction between healthcare workers and patients. This interaction is a major testing bottleneck and presents a risk of nosocomial infection. Collection of saliva samples by patients themselves also alleviates demands for supplies of swabs and personal protective equipment.
The RASAP-POCT system has advantages over other POCTs of isothermal NAAT techniques [15]. The reagent of the RASAP-POCT system is ∼ 50% less complex (fewer reactants per reaction) which offers low cost and better reliability. The RASAP-POCT system can detect the single-copy level of target molecules in a test containing a 15-μl saliva sample, which is equivalent to a detection limit of about 100–500 copies/ml.
There are four unique features in the innovative RASAP-POCT system. First, it is rapid and sensitive. Test results can be obtained in less than 25 min for a sample containing as low as a single copy of the target molecule. For this reason, the system can be used for on-site screening, eliminating the need for transporting hazardous biological samples. Second, it is accurate and specific. RASAP-POCT test reaction determines a target sequence using three nucleic acid sequences, which is similar to the gold-standard TaqMan method in RT-qPCR, resulting in an accuracy that is far better than other non-PCR-based rapid tests. Third, it is easy to use. Inactivated samples can be used directly without the need for nucleic acid extraction. The test process does not require laboratory equipment and does not have to be operated by trained professionals. Fourth, it is a simple portable device connected to the IoT. It is especially useful for mass testing and surveillance to be used in places, such as office buildings, schools, shopping centers, public places for exhibitions, sports events, cultural performances, tourist attraction sites, logistics centers, as well as toll booths; it is also applicable to various medical facilities, community centers, and medical observation stations.
6. Modification of existing regulatory guidelines is needed
With the IoT-imbedded feature, the RASAP-POCT system can be integrated into the existing contact tracing system that meets the standard requirements for public health surveillance. At this time, the RASAP-POCT system is the only IoT-imbedded NAAT POCT available, although a rapid advancement in this field is expected. Although the RASAP-POCT system has the promise to fill the gap of on-site viral nucleic acid testing with saliva samples in China, there are several regulatory gaps that need to be filled before this new technology could be adopted for practical use. Since the RASAP-POCT system is based on convection PCR that uses a different thermal control mechanism than the conventional qPCR, the current regulatory protocol for testing newly developed system for EUA is in a deadlock for further validation of RASAP-POCT system through the clinical trial, which is in urgent need for administrative and creative solutions, particularly during the outbreaks of COVID-19 in China.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Zhaoxi Wang: Conceptualization, Writing – Original Draft. Simin Liu: Conceptualization, Writing – Review & Editing.
References
- 1.Khandia R., Singhal S., Alqahtani T., Kamal M.A., El-Shall N.A., Nainu F., Desingu P.A., Dhama K. Emergence of SARS-CoV-2 Omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ. Res. 2022;209:112816. doi: 10.1016/j.envres.2022.112816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrett N., Tapley A., Andriesen J., Seocharan I., Fisher L.H., Bunts L., Espy N., Wallis C.L., Randhawa A.K., Ketter N., Yacovone M., Goga A., Bekker L.G., Gray G.E., Corey L. High rate of asymptomatic carriage associated with variant strain Omicron [Preprint] medRxiv. 2022 doi: 10.1101/2021.12.20.21268130. [DOI] [Google Scholar]
- 3.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92(9):1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustine R., Das S., Hasan A., A. S, S. Abdul Salam, P. Augustine, Y.B. Dalvi, R. Varghese, R. Primavera, H.M. Yassine, A.S. Thakor, et al. Rapid antibody-based COVID-19 mass surveillance: relevance, challenges, and prospects in a pandemic and post-pandemic world. J. Clin. Med. 2020;9(10) doi: 10.3390/jcm9103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Ridder D., Sandoval J., Vuilleumier N., Stringhini S., Spechbach H., Joost S., Kaiser L., Guessous I. Geospatial digital monitoring of COVID-19 cases at high spatiotemporal resolution. Lancet Digit. Health. 2020;2(8) doi: 10.1016/S2589-7500(20)30139-4. e393-e394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu P., Lamontagne P. Internet of things based contact tracing systems. Sensors. 2021;21(21):7124. doi: 10.3390/s21217124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahmunah V., Sudarshan V.K., Oh S.L., Gururajan R., Gururajan R., Zhou X., Tao X., Faust O., Ciaccio E.J., Ng K.H., Acharya U.R. Future IoT tools for COVID-19 contact tracing and prediction: A review of the state-of-the-science. Int. J. Imaging Syst. Technol. 2021:455–471. doi: 10.1002/ima.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakanashi D., Asai N., Nakamura A., Miyazaki N., Kawamoto Y., Muramatsu Y., Kishino T., Ohashi W., Yamagishi Y., Mikamo H., et al. Comparative evaluation of nasopharyngeal swab and saliva specimens for the molecular detection of SARS-CoV-2 RNA in Japanese patients with COVID-19. J. Infect Chemother. 2021;27(1):126–129. doi: 10.1016/j.jiac.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mak G.C., Cheng P.K., Lau S.S., Wong K.K., Lau C.S., Lam E.T., Chan R.C., Tsang D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subsoontorn P., Lohitnavy M., Kongkaew C. The diagnostic accuracy of isothermal nucleic acid point-of-care tests for human coronaviruses: A systematic review and meta-analysis. Sci. Rep. 2020;10(1):22349. doi: 10.1038/s41598-020-79237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao G., Zhang L., Zhang J., Ge S., Xia N., Qian S., Yu D., Qiu X. Free convective PCR: From principle study to commercial applications-A critical review. Anal. Chim. Acta. 2020;1108:177–197. doi: 10.1016/j.aca.2020.01.069. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S., Wang J., Zhuo Z., Su X., Chen M., Chen W., Li T., Zhang D., Min X., Ge S., Xia N. An efficient isothermal PCR method for on-site detection of nucleic acid. Biotechniques. 2019;67(2):63–69. doi: 10.2144/btn-2018-0190. [DOI] [PubMed] [Google Scholar]
- 13.Qiu X., Ge S., Gao P., Li K., Yang Y., Zhang S., Ye X., Xia N., Qian S. A Low-Cost and Fast Real-Time PCR System Based on Capillary Convection. SLAS Technol. 2017;22(1):13–17. doi: 10.1177/2211068216652847. [DOI] [PubMed] [Google Scholar]
- 14.Song K.Y., Hwang H.J., Kim J.H. Ultra-fast DNA-based multiplex convection PCR method for meat species identification with possible on-site applications. Food Chem. 2017;229:341–346. doi: 10.1016/j.foodchem.2017.02.085. [DOI] [PubMed] [Google Scholar]
- 15.Corman V.M., Haage V.C., Bleicker T., Schmidt M.L., Muhlemann B., Zuchowski M., Jo W.K., Tscheak P., Moncke-Buchner E., Muller M.A., Krumbholz A., Drexler J.F., Drosten C. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. Lancet Microbe. 2021;2(7) doi: 10.1016/S2666-5247(21)00056-2. e311-e319. [DOI] [PMC free article] [PubMed] [Google Scholar]