Abstract
During the coronavirus disease 2019 (COVID-19) pandemic, wastewater-based epidemiology (WBE) attracted attention as an objective and comprehensive indicator of community infection that does not require individual inspection. Although several severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection methods from wastewater have been developed, there are obstacles to their social implementation. In this study, we developed the COPMAN (Coagulation and Proteolysis method using Magnetic beads for detection of Nucleic acids in wastewater), an automatable method that can concentrate and detect multiple types of viruses from a limited volume (∼10 mL) of wastewater. The COPMAN consists of a high basicity polyaluminum chloride (PAC) coagulation process, magnetic bead-based RNA purification, and RT-preamplification, followed by qPCR. A series of enzymes exhibiting a high tolerance to PCR inhibitors derived from wastewater was identified and employed in the molecular detection steps in the COPMAN. We compared the detectability of viral RNA from 10-mL samples of virus-spiked (heat-inactivated SARS-CoV-2 and intact RSV) or unspiked wastewater by the COPMAN and other methods (PEG-qPCR, UF-qPCR, and EPISENS-S). The COPMAN was the most efficient for detecting spiked viruses from wastewater, detecting the highest level of pepper mild mottle virus (PMMoV), a typical intrinsic virus in human stool, from wastewater samples. The COPMAN also successfully detected indigenous SARS-CoV-2 RNA from 12 samples of wastewater at concentrations of 2.2 × 104 to 5.4 × 105 copies/L, during initial stages of an infection wave in the right and the left bank of the Sagami River in Japan (0.65 to 11.45 daily reported cases per 100,000 people). These results indicate that the COPMAN is suitable for detection of multiple pathogens from small volume of wastewater in automated stations.
Keywords: Wastewater-based epidemiology, COVID-19, Sewage, Environmental surveillance, Automation, PCR
Graphical abstract
Abbreviations
- COPMAN
Coagulation and Proteolysis method using Magnetic beads for detection of Nucleic acids in wastewater
- WBE
Wastewater-based epidemiology
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- HI-SARS-CoV-2
Heat-inactivated SARS-CoV-2
- RSV
Respiratory syncytial virus
- PMMoV
Pepper mild mottle virus
- PEG
Polyethylene glycol
- PAC
Polyaluminum chloride
- WWTP
Wastewater treatment plant
- UF
Ultrafiltration
1. Introduction
From an epidemiological point of view, comprehending disease prevalence in an area is crucial to predict outbreaks and make effective political decisions. WBE has recently attracted attention for the monitoring of infection trends in a community without individual inspection (Kitajima et al., 2020; Ahmed et al., 2020). Previous studies have revealed that viruses which cause respiratory illness, such as SARS-CoV-2 (Wölfel et al., 2020), RSV (von Linstow et al., 2006) and influenza A virus (Minodier et al., 2015), can be detected by RT-qPCR not only from the saliva but also from the stool of patients. Moreover, multiple studies have reported that SARS-CoV-2 RNA could be detected from stool even during the recovery period with mild symptoms (Ling et al., 2020; Wölfel et al., 2020). These findings suggest that respiratory viruses could be detected from the stool of even asymptomatic patients and that WBE could reveal the community-level disease prevalence including subclinical infections. Although several methods for SARS-CoV-2 RNA detection from wastewater have been developed, they analyze different fractions of wastewater: the liquid fraction (Fernandez-Cassi et al., 2021; Wu et al., 2020) or the solid fraction (Parra-Guardado et al., 2022; Wölfel et al., 2020; Ando et al., 2022). A recent study revealed that a substantial amount of SARS-CoV-2 RNA in wastewater is present in the solid fraction pelleted by low-speed centrifugation (Kitamura et al., 2021). On the other hand, for the detection of viruses which cause gastrointestinal illness, such as norovirus (Kazama et al., 2017), poliovirus (Brouwer et al., 2018), and sapovirus (McCall et al., 2020), the liquid fraction of wastewater has been used with polyethylene glycol (PEG) precipitation or ultrafiltration.
Although these methods have been optimized for the detection of viral RNA on the lab scale, there are several obstacles for social implementation via high-throughput sample processing. First, automated operation is needed to achieve high-throughput processing, good reproducibility, and safety of the operators. However, since almost all ready-made auto-pipetting stations are for small volumes (∼10 mL), methods handling the solid fraction (40 – 400 mL) or PEG precipitation (40 mL or above) are difficult to adopt. Also, previous methods do not analyze all fractions of wastewater (Kitamura et al., 2021). Thus, such methods are not suitable for the detection of multiple pathogens including non-enveloped and enveloped viruses from a single wastewater sample. Another issue is that wastewater samples include a considerable amount of PCR inhibitors derived from soil, such as humic acid or fumaric acid (Matheson et al., 2010), resulting in a poor sensitivity.
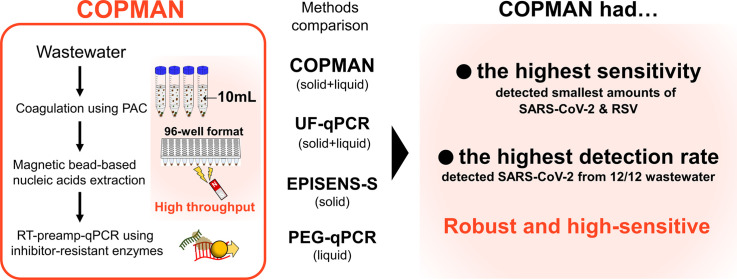
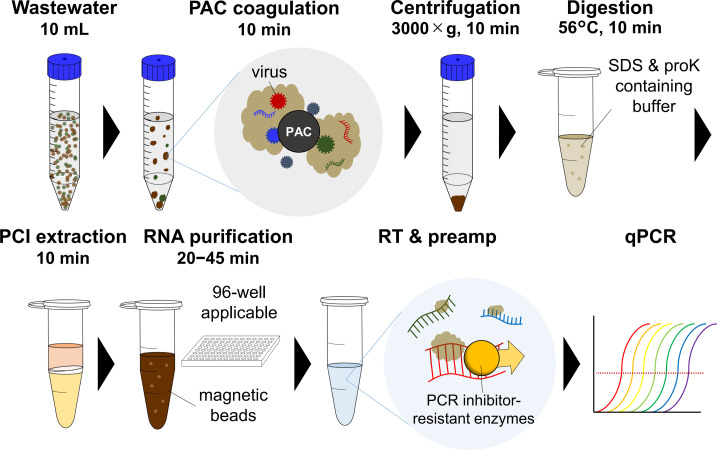
To solve these issues, we developed the COPMAN (Coagulation and Proteolysis method using Magnetic beads for detection of Nucleic acids in wastewater) (Fig. 1 ). To facilitate high-throughput processing, we employed magnetic bead-based RNA extraction that is directly transferable to the 96-well format, instead of column-based RNA purifications that need repeated centrifugations. To enable multiple pathogens detection from one vial, we targeted both the liquid and solid fractions of wastewater. We adopted polyaluminum chloride (PAC) as a coagulant which has been reported to be able to coagulate various types of viruses including SARS-CoV-2 more efficiently than PEG precipitation (Barril et al., 2021). To accurately estimate the copy number of viral RNA even in the presence of PCR inhibitors, we screened a set of enzymes that are highly tolerant to PCR inhibitors in wastewater. These improvements enabled COPMAN to achieve higher sensitivity than conventional methods (PEG-qPCR, UF-qPCR, and the solid fraction-based method EPISENS-S) even with samples of limited volume (10 mL).
Fig. 1.
Workflow of the COPMAN: coagulation and proteolysis method using magnetic beads for detection of nucleic acids in wastewater.
2. Materials and methods
2.1. Wastewater sampling
Between 21st July and 4th August 2021, 12 influent wastewater samples were collected from two wastewater treatment plants (WWTP) in the right and the left bank of the Sagami River in Kanagawa, Japan (WWTP R and L, respectively) with service populations of 0.54 and 1.26 million people, respectively. The daily reported numbers of newly cases ranged from 0.65 to 11.45 per 100,000 people in the area on the sampling days.
Influent wastewater samples were collected at the WWTP in Japan on 29th November 2021, when SARS-CoV-2 and RSV positive cases had not been reported for 2 weeks. These samples were used for the spike and recovery experiment using heat-inactivated SARS-CoV-2 (HI-SARS-CoV-2) and RSV described in a latter section. The collected samples were immediately transported to the laboratory on ice and kept at −30 °C or lower until use (3 – 6 months).
2.2. Viral stock preparation
An isolated SARS-CoV-2 strain (hCoV-19/Japan/TY-WK-521/2020, GISAID Accession ID: EPI_ISL_408667) was provided by the National Institute of Infectious Diseases, Japan. SARS-CoV-2 was propagated in VeroE6-TMPRSS2 cells (JCRB1819) (Matsuyama et al., 2020), and the virus was inactivated by heating at 65 °C for 30 min (Kim et al., 2020). RSV strain A2 was purchased from ATCC (VR-1540) and propagated in HEp-2 cells. To determine the viral RNA copy numbers, RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. cDNA was synthesized from 1-, 10-, and 100-fold diluted RNA using the Reliance Select cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA), which was quantified by qPCR using the TaqMan Environmental Master Mix 2.0 (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Comparison of four viral RNA quantification methods
2.3.1. Sample preparation and qPCR
The wastewater samples were slowly thawed at 4 °C. To assess the sensitivity and recovery rates of the methods, HI-SARS-CoV-2 and intact RSV with different amounts (500, 103, 104 and 105 genome copies) were spiked into 10-mL portions of wastewater. The detectability of indigenous SARS-CoV-2 RNA and PMMoV RNA from the untreated wastewater was evaluated using the 12 samples collected at two WWTPs (R and L) in Kanagawa on six occasions.
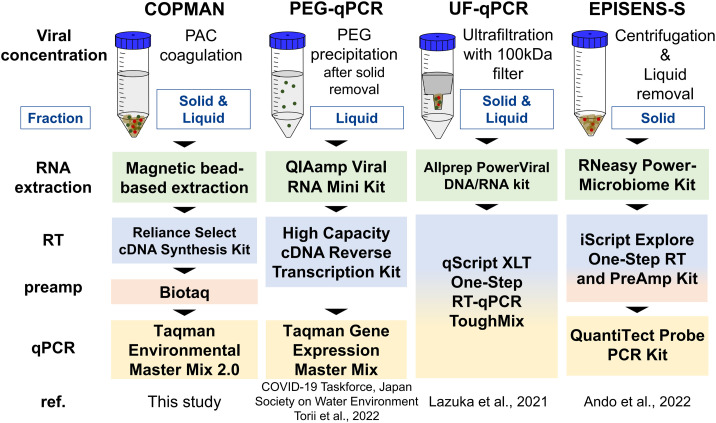
The COPMAN method was compared with three other methods, as described in Fig. 2 . In the present study, the pellet and supernatant fraction of wastewater obtained by low-speed centrifugation (3000 ×g, 10 min) were designated as the “solid fraction” and “liquid fraction”, respectively. With all methods, RNA was extracted from a concentrate derived from 10 mL of wastewater and eluted in 50-μL nuclease-free water. The concentrations of the RNA extracts were measured from 1-μL extracts by Nanodrop 2000c (Thermo Fisher Scientific). The viral RNA was quantified by RT-qPCR using TaqMan-based assays on an Applied Biosystems Fast 7500 Real-Time PCR System (Thermo Fisher Scientific). Plasmid DNA containing each target sequence was used as the standard (5, 101 to 105 copies/tube for SARS-CoV-2 and RSV: 50, 102 to 106 copies/tube for PMMoV). Nuclease-free water was used as a negative control in the qPCR reactions. Primers and probes used in the present study are listed in Table S1 in the Supplementary Material.
Fig. 2.
Comparative analysis of four methods.
The diagram shows four methods with different strategies for quantification of viral RNA from wastewater. Each method was used to detect viral RNA in 10 mL of wastewater. The COPMAN uses both liquid and solid fractions by employing a PAC coagulation procedure. PEG-qPCR uses the liquid fraction by removing the solid fraction before the assay. Ultrafiltration collects molecules larger than 100 kDa from both fractions. The EPISENS-S collects molecules in the solid fraction by removing the liquid fraction.
2.3.2. The COPMAN
The workflow of the COPMAN is presented in Fig. 1. The RNA extraction process of this method is described in the instructions of the COPMAN viral RNA kit (AdvanSentinel, Osaka, Japan), which was commercialized as a result of the present study. Briefly, viruses were coagulated with the addition of 1 μL of polyaluminum chloride (PAC) followed by vigorous shaking for 30 times, and subsequent gentle shaking at 80–120 rpm for 10 min at 4 °C. The samples were then centrifuged at 3000 ×g for 10 min, and the supernatant was discarded. The samples were centrifuged again at 3000 ×g for 3 min and the remaining liquids were removed by pipetting. The debris was then transferred to a 1.5-mL tube and lysed with 250-μL SDS-based lysis buffer and digested by 14.25-μL proteinase K solution at 56 °C for 10 min. Crude RNA of 200 μL was extracted from the samples with phenol/chloroform/isoamyl alcohol (25:24:1), which was then purified with carboxyl-modified magnetic beads, to obtain a final RNA extract volume of 50 μL. An aliquot (2 μg or 13 μL) of the magnetic bead-purified total RNA was subjected to cDNA synthesis using the Reliance Select cDNA synthesis kit (Bio-Rad Laboratories) under the following conditions: 50 °C for 60 min, 95 °C for 1 min in 20-μL reaction mix with 2 pmol each of reverse primers of SARS-CoV-2, RSV, and PMMoV. The resultant cDNAs of SARS-CoV-2 and RSV were pre-amplified for 10 cycles by the Biotaq HS (Bioline Reagents Ltd., London, UK) under the following conditions: 95 °C for 10 min, and 10 cycles of 95 °C for 15 s, 55 °C for 15 s, and 72 °C for 30 s, in 30-μL volume reaction mix containing 9 pmol each of forward and reverse primers. PMMoV cDNA was not preamplified because PMMoV RNA usually exists in wastewater with high amounts. Finally, viral RNA was quantified from 2.5 μL of the preamp product for SARS-CoV-2 and RSV, and 2.5 μL of cDNA for PMMoV by qPCR using the TaqMan Environmental Master Mix 2.0 (Thermo Fisher Scientific) under the following conditions: 95 °C for 10 min, and 45 cycles of 95 °C for 15 s and 60 °C for 30 s, in 20-μL singleplex reaction mix containing 10 pmol each of reverse and forward primers and 7.5 pmol of TaqMan probe.
2.3.3. The EPISENS-S
The EPISENS-S, a recently reported highly sensitive detection method for SARS-CoV-2 from the solid fraction of wastewater, was performed as previously described (Ando et al., 2022) with slight modifications. Briefly, 10 mL of wastewater samples were centrifuged at 3000 ×g for 10 min, and the supernatant was discarded. RNA was extracted from the resultant pellet with the RNeasy PowerMicrobiome Kit (Qiagen) using the QIAcube-based automated system, according to the manufacturer's instructions to obtain a final RNA extract volume of 50 μL. The 13-μL portion of the extracted RNA was subjected to 10-cycle preamplification following cDNA synthesis with the iScript Explore One-Step RT and PreAmp Kit (Bio-Rad) in 30-μL reaction volume according to the manufacturer's instructions. The RT-preamplification product (5 μL) was subjected to qPCR using the QuantiTect Probe PCR Kit (Qiagen) to quantify the viral RNA.
2.3.4. PEG-qPCR
PEG-qPCR is a RT-qPCR-based viral RNA detection method using PEG precipitation as a virus concentration procedure. The PEG precipitation was performed according to the Manual for Detection of SARS-CoV-2 RNA in Wastewater (Japan Society on Water Environment COVID-19 Taskforce, 2022) and a previous study (Torii et al., 2022) with slight modifications. RNA extraction kits and RT-qPCR kits were also selected based on the previous study (Torii et al., 2022). The method is referred to as “PEG-qPCR” in this study. Briefly, 10 mL of wastewater sample was centrifuged at 3000 ×g for 5 min and the supernatant was collected. The supernatant was supplemented with 1 g of PEG8000 and 0.59 g of NaCl, and incubated overnight at 4 °C with agitation at 100 rpm. Subsequently, the mixture was centrifuged at 11,000 ×g at 4 °C for 40 min and the supernatant was discarded. Additional flash centrifugation allowed for collection of the remaining liquid in the tube, which was removed by pipetting. The resultant precipitate was suspended by adding 200 μL of Trizol Reagent (Thermo Fisher Scientific) and RNA was extracted from 140 μL of the suspension by the QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer's instructions to obtain a final RNA extract volume of 50 μL. Carrier RNA (5.6 μg) was added to the sample during the RNA extraction procedure according to the manufacturer's instructions. cDNA was synthesized from the extracted RNA with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Finally, viral RNA was quantified from 5 μL of cDNA by qPCR using the TaqMan Gene Expression Master Mix (Thermo Fisher Scientific) according to the manufacturer's instructions.
2.3.5. UF-qPCR
UF-qPCR is a RT-qPCR-based viral RNA detection method using ultrafiltration as a virus concentration procedure. Ultrafiltration, RNA extraction and RT-qPCR were performed according to a previous report (Lazuka et al., 2021). The method is referred to as “UF-qPCR” in this study. In brief, 10 mL of wastewater sample was concentrated using the Amicon Ultra 15 mL centrifugal devices with a 100-kDa molecular weight cutoff by centrifugation at 2000 ×g for 15 min (Merck Millipore, MA, USA). The resulting retentate was transferred to 2-mL Lysing Matrix A tubes (MP Biomedicals) by carefully pipetting up and down with 600-μL solution I from the Allprep PowerViral DNA/RNA kit (QIAGEN) to obtain a final DNA/RNA extract volume of 50 μL. The samples were then disrupted using ShakeMaster NEO (Bio Medical Science, Tokyo, Japan) at 1500 rpm for 10 min. The following steps of RNA extraction with the PowerViral DNA/RNA kit (QIAGEN) were performed according to the manufacturer's instructions. Viral RNAs were quantified from 1 or 2 μL RNA extracts using the qScript XLT One-Step RT-qPCR ToughMix (Quantabio, MA, USA) according to the manufacturer's instructions.
3. Results
3.1. Development of the COPMAN
To develop a method that is suitable for automation, a small volume of wastewater (i.e., 10 mL) was used. Also, magnetic bead-based RNA extraction was adopted instead of widely used column-based RNA extraction protocols to minimize the number of centrifugations.
To achieve highly sensitive detection of viral RNA in wastewater, the processes for viral concentration, RNA extraction and RT-preamplification-qPCR were improved. Specifically, we found that PAC-based flocculation and enzymatic proteolysis processes enabled efficient recovery and extraction of viral nucleic acids from wastewater, respectively (data not shown), and thus these processes were included in the established protocol of the COPMAN (Fig. 1). However, proteolysis of the solids would inevitably promote the release of PCR inhibitors into the liquid phase, reducing the efficiency of the downstream molecular processes. To overcome this issue, we compared multiple RT-PCR enzymes using RNA from various wastewater samples in Japan, and successfully identified a set of inhibitor-resistant enzymes for the RT-preamp-qPCR step (i.e., the Reliance Select cDNA synthesis kit, the Biotaq HS, and the TaqMan Environmental Master Mix 2.0 for RT, preamplification, and qPCR, respectively) that enables efficient and sensitive detection of SARS-CoV-2 RNA in wastewater. As a result, the COPMAN achieved high detection sensitivity of viral RNA even in wastewater samples containing a significant amount of PCR inhibitors (data not shown).
3.2. Comparison of sensitivity of viral RNA detection among the COPMAN and three other methods
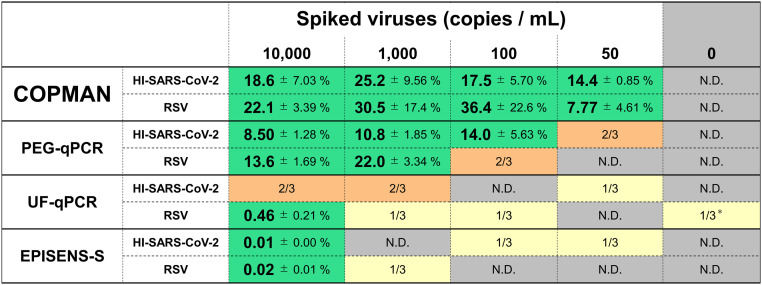
To evaluate the sensitivity of the COPMAN, we compared the method with other methods: PEG-qPCR (Japan Society on Water Environment COVID-19 Taskforce, 2022; Torii et al., 2022), UF-qPCR (Lazuka et al., 2021), and EPISENS-S (Ando et al., 2022) (Fig. 2). The comparison was performed using 10 mL of wastewater spiked with serial dilution of HI-SARS-CoV-2 and RSV to obtain final genome concentrations of 50, 102, 103, 104 GC/mL (Fig. 3 ). Among the four methods tested, the COPMAN was the most sensitive, detecting 50 GC/mL of HI-SARS-CoV-2 and RSV. PEG-qPCR detected 102 and 103 GC/mL of HI-SARS-CoV-2 and RSV, respectively. UF-qPCR detected 104 GC/mL of RSV, but it failed to detect HI-SARS-CoV-2 RNA. EPISENS-S detected 104 GC/mL of both viruses (Fig. 3).
Fig. 3.
Detection of viral RNA from wastewater spiked with various amounts of HI-SARS-CoV-2 and RSV.
Heat-inactivated (HI) SARS-CoV-2 and intact RSV were spiked and recovered from 10-mL wastewater. Recovery rates are shown in each cell with standard deviations when viral RNA was detected in 3/3 samples. 1/3 and 2/3 represents RNA detection in one and two samples out of three, respectively. N.D. represents no RNA detection in the samples (0/3). *One of three samples showed a Ct value of 44.94.
The recovery rates of the COPMAN (14.4–25.2 % for SARS-CoV-2; 7.77–36.4 % for RSV) and PEG-qPCR (8.50–14.0 % for SARS-CoV-2; 13.6–22.0 for RSV) were higher than those of UF-qPCR (not calculated for SARS-CoV-2; 0.46 % for RSV) and EPISENS-S (0.01 % for SARS-CoV-2; 0.02 % for RSV) under the experimental settings of the present study (Fig. 3). These results for the detectability of spiked viral RNA from 10–mL wastewater demonstrated the higher detection sensitivity of the COPMAN over the other three methods.
3.3. Comparison of indigenous viral RNA detection from wastewater by the COPMAN and the other three methods
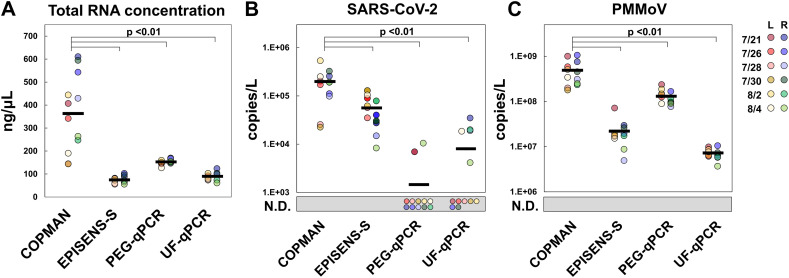
To demonstrate the applicability of the COPMAN to the quantification of indigenous SARS-CoV-2 in municipal wastewater and to evaluate its detection sensitivity by comparison with the other three methods, detection of the virus was tested from wastewater during the early stage of SARS-CoV-2 spread (0.65 to 11.45 newly reported cases per 100,000 people in a day) (Fig. 3). Total RNA yields from wastewater were significantly higher with the COPMAN than the other three methods (Tukey test, p < 0.01) (Fig. 3, A).
Indigenous SARS-CoV-2 RNA was detected with the COPMAN, PEG-qPCR, UF-qPCR, and EPISENS-S from 12 (100 %), 2 (17 %), 5 (42 %), and 12 (100 %) samples, respectively (Fig. 4, B). The average concentrations of SARS-CoV-2 RNA in the wastewater samples (n = 12, non-detects were substituted with 0 copies/L when calculating the averages) observed with the COPMAN were 137, 24.7, and 3.52 times higher than those observed with PEG-qPCR, UF-qPCR, and EPISENS-S, respectively (Fig. 4, B).
Fig. 4.
Comparison of detection abilities of viral RNA from wastewater.
Each method was used to quantify viral RNA from 10 mL of wastewater during the initial stage of a SARS-CoV-2 outbreak. The COPMAN was compared with the EPISENS-S, PEG-qPCR, and UF-qPCR. (A) Total RNA concentration of the purified RNA extracts. Note that for PEG-qPCR, 5.6 μg carrier RNA was added during the RNA extraction with the QIAamp Viral RNA Mini kit according to the manufacturer's instructions. (B, C) Detection of SARS-CoV-2 (B) and PMMoV (C). The black bar is the average of 12 samples (calculated by substituting N.D. to 0). L and R represent the WWTP in the left and the right bank of the Sagami river in Japan, respectively. N.D., not detected. Pairwise comparison was performed using the Tukey test (p < 0.01).
The observed amount of PMMoV RNA was also compared for the COPMAN and the three other methods (Fig. 4, C). All four methods detected PMMoV RNA from every sample, with the highest amount being observed by the COPMAN for all 12 samples (Fig. 4, C). The average concentrations of PMMoV RNA in the wastewater samples (n = 12) observed by the COPMAN were 3.8, 22, and 68 times higher than those observed by PEG-qPCR, UF-qPCR, and EPISENS-S, respectively.
4. Discussion
The presence of PCR inhibitory substances, low concentrations of target nucleic acids and burdensome processes are major challenges for the detection of human viral pathogens in wastewater samples using qPCR. Most previously developed methods for viral concentration and detection in wastewater have weaknesses such as inadequate detection sensitivity or low recovery capacity for viruses with different partitioning (Kitamura et al., 2021) and poor throughput. In the present study, we developed the COPMAN for highly sensitive and automatable detection of viral RNA in wastewater. This new method was then compared with PEG-qPCR, UF-qPCR, and EPISENS-S methods for the detection of SARS-CoV-2 RNA from 10 mL of untreated wastewater.
The COPMAN showed the highest sensitivity and/or recovery rates for the detection of spiked HI-SARS-CoV-2 and RSV as well as indigenous SARS-CoV-2 RNA and PMMoV RNA in the wastewater samples (Fig. 3, Fig. 4). The average recovery rates of COPMAN and PEG-qPCR were 25.2 % and 10.8 %, respectively, when 1.0 × 103 copies/mL of HI-SARS-CoV-2 was spiked into wastewater. Previous study showed that the recovery rate of EPISENS-S was 1.1 % when 2.11 × 103 copies/mL of HI-SARS-CoV-2 was spiked (Ando et al., 2022). Barril et al. (2021) showed that PAC flocculation or PEG precipitation adopted methods showed higher recovery of feline calicivirus among 11 methods, and these two methods showed the recovery rates of 9.2 % and 7.4 %, respectively, when 4.3 × 103 copies/mL of SARS-CoV-2 was spiked. It is difficult to directly compare the recovery rates of COPMAN with previously reported values because the experimental conditions and samples influence on recovery rates. However, it is interesting that both our study and the previous study by Barril et al. (2021) showed relatively high recovery rates of spiked SARS-CoV-2 in the methods adopting PAC flocculation (including COPMAN) and the methods adopting PEG precipitation in the spike and recovery experiments.
Previous studies have reported that viral partitioning between liquid and solid fractions in wastewater differs depending on the types of viruses. For example, enveloped viruses (murine hepatitis virus [MHV] and φ6 phage) showed higher tendencies to adsorb to the solid fraction of wastewater than nonenveloped viruses (MS2 and T3 phages) (Ye et al., 2016). More recent studies reported that high amounts of indigenous SARS-CoV-2, an enveloped virus, could be detected from the solid fraction (Kim et al., 2022; Kitamura et al., 2021; Ando et al., 2022), whereas PMMoV, a nonenveloped virus, was less associated with solids but abundantly present in the liquid fraction of wastewater (Kitamura et al., 2021). However, most of the previously reported methods were designed to collect either the liquid or the solid fraction, making it difficult to detect both enveloped and nonenveloped viruses simultaneously with high efficiency. For instance, the conventional PEG-qPCR method mainly uses the liquid fraction or eluates (Hewitt et al., 2007; Jones and Johns, 2009; Kevill et al., 2022; Lewis and Metcalf, 1988), while the EPISENS-S method uses only the solid fraction. Hence, we designed the COPMAN to recover viruses present in both the liquid and solid fractions of wastewater using a PAC-based coagulation-flocculation process. Previous studies have shown that the PAC flocculation method can concentrate poliovirus and norovirus (Shirasaki et al., 2010; Zhang et al., 2009). Thus, we hypothesized that the COPMAN would also be able to detect these viruses. Furthermore, the PAC coagulation process would enable the collection of viruses in environmental water or wastewater with a small amount of sediment. Although the UF-qPCR method could recover viruses from both liquid and solid fractions of wastewater, it detected fewer amounts of SARS-CoV-2 and PMMoV than the other methods (Fig. 4, B, C). This poorer performance of UF-qPCR could have been due to physical loss of viral RNA during the ultrafiltration step and/or inhibition in the molecular detection process.
For large-scale WBE implementation with less operational burden, automation is necessary. To automate the entire process of virus detection, the crucial factors were a simple coagulation process, reduction of centrifugation steps and minimalization of input wastewater volume. We employed the high-basicity PAC, which is a simple coagulation process, in the COPMAN for the following reasons: (i) precipitation time is short and pH adjustment is unnecessary and (ii) PAC coagulation occurs in 10 min, whereas PEG precipitation usually requires 1.5 h to overnight incubation prior to pelleting (Hewitt et al., 2007; Jones and Johns, 2009; Kevill et al., 2022; Lewis and Metcalf, 1988; Torii et al., 2022). The high-basicity PAC (Shirasaki et al., 2014) used in the COPMAN does not require the laborious pH adjustment necessary for normal-basicity PAC coagulation (Barril et al., 2021; Randazzo et al., 2020). Also, the COPMAN uses only 10 mL of wastewater, which can be handled by widely-used automated pipetting systems. The magnetic bead-based RNA extraction chosen for the COPMAN, which allowed for the use of 96-well format protocol and reduced the number of centrifugation steps compared to column-based RNA extraction protocols. The COPMAN was designed to be an automatable and less labor-intensive method by employing these protocols. Indeed, we have successfully implemented the COPMAN in a robot system in a pilot study, which will be reported in the future.
Further research is needed to validate the utility of the COPMAN for different types of wastewater with varying amounts of solids, enzyme inhibitors, and target nucleic acids. For more accurate prediction of infection in the community, effective normalization is necessary since inflow into a wastewater treatment plant substantially increases on rainy days due to intrusion of stormwater in combined sewer systems, which may dilute the viral RNA in wastewater. PMMoV may become a useful normalizer for correlation of the copy number of pathogens when using the COPMAN. As a potential problem with using 10 mL wastewater sample, very low concentration of viruses (theoretically under 0.1 copies/mL) in wastewater hardly be detected. To deal with the problem, we have been developing passive samplers to concentrate the wastewater which can be used in COPMAN.
5. Conclusions
-
•
We developed a sensitive and automatable viral RNA detection method, COPMAN, consisting of PAC coagulation process, magnetic bead-based RNA purification, and RT-preamplification-qPCR.
-
•
In comparison to the other three methods (PEG-qPCR, UF-qPCR, EPISENS-S), the COPMAN showed the highest sensitivity, recovering the smallest amounts of SARS-CoV-2 and RSV (50 GC/mL).
-
•
COPMAN detected SARS-CoV-2 RNA from only 10 mL of wastewater when 0.65 to 11.45 newly infected cases were reported per day per 100,000 people in the present study.
-
•
The COPMAN is expected to make wastewater-based epidemiological surveillance more useful and accessible.
The following is the supplementary data related to this article.
Primers and probes used in the present study.
CRediT authorship contribution statement
Yuka Adachi Katayama: Conceptualization, Methodology, Investigation, Writing – original draft, Visualization, Validation. Shin Hayase: Conceptualization, Methodology, Investigation, Writing – original draft, Visualization, Validation. Yoshinori Ando: Resources, Writing – review & editing. Tomohiro Kuroita: Methodology, Resources. Kazuya Okada: Resources. Ryo Iwamoto: Writing – review & editing. Toru Yanagimoto: Supervision. Masaaki Kitajima: Resources, Writing – review & editing. Yusaku Masago: Conceptualization, Methodology, Investigation, Writing – original draft, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Masaaki Kitajima reports financial support was provided by Shionogi & Co Ltd. Yuka Adachi Katayama reports a relationship with Shionogi & Co Ltd. that includes: employment. Shin Hayase reports a relationship with Shionogi & Co Ltd. that includes: employment. Yoshinori Ando reports a relationship with Shionogi & Co Ltd. that includes: employment. Tomohiro Kuroita reports a relationship with Shionogi & Co Ltd. that includes: employment. Kazuya Okada reports a relationship with Shionogi & Co Ltd. that includes: employment. Ryo Iwamoto reports a relationship with Shionogi & Co Ltd. that includes: employment. Toru Yanagimoto reports a relationship with Shionogi & Co Ltd. that includes: employment. Yusaku Masago reports a relationship with Shionogi & Co Ltd. that includes: employment. Yuka Adachi Katayama (Yuka Katayama) has patent Nucleic acid detection & quantification methods from environmental samples pending to Shionogi & Co Ltd., Hokkaido university. Shin Hayase has patent Nucleic acid detection & quantification methods from environmental samples pending to Shionogi & Co Ltd., Hokkaido university. Ryo Iwamoto has patent Nucleic acid detection and quantification methods from environmental samples pending to Shionogi & Co Ltd., Hokkaido University. Masaaki Kitajima has patent Nucleic acid detection and quantification methods from environmental samples pending to Shionogi & Co Ltd., Hokkaido University. Yusaku Masago has patent Nucleic acid detection and quantification methods from environmental samples pending to Shionogi & Co Ltd., Hokkaido university. Judy Noguchi had proofreaded this manuscript in English for a fee. Judy Noguchi works in Kobe Gakuin University.
Acknowledgments
Acknowledgements
The authors acknowledge Prof. Byung-Kwang Yoo and Prof. Ung-il Chung at Kanagawa University of Human Services for their support in obtaining permission for wastewater sampling. The authors thank the staff members of the WWTPs at Kanagawa Sewerage Works Foundation and crisis management office at Kanagawa prefecture for their support and permission to sample the wastewater. The authors would like to thank Yumi Sato for the technical support.
Funding
This study was funded by Shionogi & Co., Ltd. The employees of Shionogi & Co., Ltd. involved in the study design, data collection, analysis and interpretation, and the writing of the report made the decision to serve as authors.
Editor: Warish Ahmed
Data availability
The authors do not have permission to share data.
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H., Iwamoto R., Kobayashi H., Okabe S., Kitajima M. The efficient and practical virus identification system with ENhanced sensitivity for solids (EPISENS-S): a rapid and cost-effective SARS-CoV-2 RNA detection method for routine wastewater surveillance. Sci. Total Environ. 2022;843 doi: 10.1016/j.scitotenv.2022.157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barril P.A., Pianciola L.A., Mazzeo M., Ousset M.J., Jaureguiberry M.V., Alessandrello M., Sánchez G., Oteiza J.M. Evaluation of viral concentration methods for SARS-CoV-2 recovery from wastewaters. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A.F., Eisenberg J.N.S., Pomeroy C.D., Shulman L.M., Hindiyeh M., Manor Y., Grotto I., Koopman J.S., Eisenberg M.C. Epidemiology of the silent polio outbreak in Rahat, Israel, based on modeling of environmental surveillance data. Proc. Natl. Acad. Sci. 2018;115 doi: 10.1073/pnas.1808798115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Taskforce, Japan Society on Water Environment Manual for detection of SARS-CoV-2 RNA in wastewater. 2022. https://www.jswe.or.jp/aboutus/pdf/Manual%20for%20Detection%20of%20SARS-CoV-2%20RNA%20in%20Wastewater.pdf
- Fernandez-Cassi X., Scheidegger A., Bänziger C., Cariti F., Tuñas Corzon A., Ganesanandamoorthy P., Lemaitre J.C., Ort C., Julian T.R., Kohn T. Wastewater monitoring outperforms case numbers as a tool to track COVID-19 incidence dynamics when test positivity rates are high. Water Res. 2021;200 doi: 10.1016/j.watres.2021.117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Bell D., Simmons G.C., Rivera-Aban M., Wolf S., Greening G.E. Gastroenteritis outbreak caused by waterborne norovirus at a New Zealand ski resort. Appl. Environ. Microbiol. 2007;73:7853–7857. doi: 10.1128/AEM.00718-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.H., Johns M.W. Improved detection of F-specific RNA coliphages in fecal material by extraction and polyethylene glycol precipitation. Appl. Environ. Microbiol. 2009;75:6142–6146. doi: 10.1128/AEM.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama S., Miura T., Masago Y., Konta Y., Tohma K., Manaka T., Liu X., Nakayama D., Tanno T., Saito M., Oshitani H., Omura T. Environmental surveillance of norovirus genogroups I and II for sensitive detection of epidemic variants. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.03406-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevill J.L., Pellett C., Farkas K., Brown M.R., Bassano I., Denise H., McDonald J.E., Malham S.K., Porter J., Warren J., Evens N.P., Paterson S., Singer A.C., Jones D.L. A comparison of precipitation and filtration-based SARS-CoV-2 recovery methods and the influence of temperature, turbidity, and surfactant load in urban wastewater. Sci. Total Environ. 2022;808 doi: 10.1016/j.scitotenv.2021.151916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-I., Casel M.A.B., Kim S.-M., Kim S.-G., Park S.-J., Kim E.-H., Jeong H.W., Poo H., Choi Y.K. Development of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) thermal inactivation method with preservation of diagnostic sensitivity. J. Microbiol. 2020;58:886–891. doi: 10.1007/s12275-020-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kennedy L.C., Wolfe M.K., Criddle C.S., Duong D.H., Topol A., White B.J., Kantor R.S., Nelson K.L., Steele J.A., Langlois K., Griffith J.F., Zimmer-Faust A.G., McLellan S.L., Schussman M.K., Ammerman M., Wigginton K.R., Bakker K.M., Boehm A.B. SARS-CoV-2 RNA is enriched by orders of magnitude in primary settled solids relative to liquid wastewater at publicly owned treatment works. Environ. Sci. Water Res. Technol. 2022;8:757–770. doi: 10.1039/D1EW00826A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Sadamasu K., Muramatsu M., Yoshida H. Efficient detection of SARS-CoV-2 RNA in the solid fraction of wastewater. Sci. Total Environ. 2021;763 doi: 10.1016/j.scitotenv.2020.144587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazuka A., Arnal C., Soyeux E., Sampson M., Lepeuple A.-S., Deleuze Y., Pouradier Duteil S., Lacroix S. COVID-19 wastewater based epidemiology: long-term monitoring of 10 WWTP in France reveals the importance of the sampling context. Water Sci. Technol. 2021;84:1997–2013. doi: 10.2166/wst.2021.418. [DOI] [PubMed] [Google Scholar]
- Lewis G.D., Metcalf T.G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis a virus and human rotavirus, from oyster, water, and sediment samples. Appl. Environ. Microbiol. 1988;54:1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S.-B., Lin Y.-X., Tian D., Zhu Z.-Q., Dai F.-H., Wu F., Song Z.-G., Huang W., Chen J., Hu B.-J., Wang S., Mao E.-Q., Zhu L., Zhang W.-H., Lu H.-Z. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson C., Gurney C., Esau N., Lehto R. Assessing PCR inhibition from humic substances. Open Enzym. Inhib. J. 2010;3:38–45. [Google Scholar]
- Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C., Wu H., Miyani B., Xagoraraki I. Identification of multiple potential viral diseases in a large urban center using wastewater surveillance. Water Res. 2020;184 doi: 10.1016/j.watres.2020.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minodier L., Charrel R.N., Ceccaldi P.-E., van der Werf S., Blanchon T., Hanslik T., Falchi A. Prevalence of gastrointestinal symptoms in patients with influenza, clinical significance, and pathophysiology of human influenza viruses in faecal samples: what do we know? Virol. J. 2015;12:215. doi: 10.1186/s12985-015-0448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Guardado A.L., Sweeney C.L., Hayes E.K., Trueman B.F., Huang Y., Jamieson R.C., Rand J.L., Gagnon G.A., Stoddart A.K. Development of a rapid pre-concentration protocol and a magnetic bead-based RNA extraction method for SARS-CoV-2 detection in raw municipal wastewater. Environ. Sci. Water Res. Technol. 2022;8:47–61. doi: 10.1039/D1EW00539A. [DOI] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki N., Matsushita T., Matsui Y., Oshiba A., Marubayashi T., Sato S. Improved virus removal by high-basicity polyaluminum coagulants compared to commercially available aluminum-based coagulants. Water Res. 2014;48:375–386. doi: 10.1016/j.watres.2013.09.052. [DOI] [PubMed] [Google Scholar]
- Shirasaki N., Matsushita T., Matsui Y., Oshiba A., Ohno K. Estimation of norovirus removal performance in a coagulation–rapid sand filtration process by using recombinant norovirus VLPs. Water Res. 2010;44:1307–1316. doi: 10.1016/j.watres.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Torii S., Oishi W., Zhu Y., Thakali O., Malla B., Yu Z., Zhao B., Arakawa C., Kitajima M., Hata A., Ihara M., Kyuwa S., Sano D., Haramoto E., Katayama H. Comparison of five polyethylene glycol precipitation procedures for the RT-qPCR based recovery of murine hepatitis virus, bacteriophage phi6, and pepper mild mottle virus as a surrogate for SARS-CoV-2 from wastewater. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Linstow M.-L., Eugen-Olsen J., Koch A., Winther T.N., Westh H., Hogh B. Excretion patterns of human metapneumovirus and respiratory syncytial virus among young children. Eur. J. Med. Res. 2006;11:329–335. [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhao Z., Shen Z., Wang X.-W., Guo J., Jin M., Wang J., Qiu Z., Li J.-W. Polyaluminum chloride-enhanced concentration efficiency of poliovirus and f2 phage from sewage water. J. Appl. Microbiol. 2009;106:660–665. doi: 10.1111/j.1365-2672.2008.04041.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers and probes used in the present study.
Data Availability Statement
The authors do not have permission to share data.