Abstract
A Bacillus subtilis disruption mutant with a mutation in sigI (formerly ykoZ) shows a temperature-sensitive growth on agar plates. The transcription of the sigI gene is heat shock induced in rich medium but not in minimal medium. Proteome studies revealed a reduced amount of GsiB protein in the sigI mutant under heat shock conditions.
The soil bacterium Bacillus subtilis has to adapt to a huge variety of environmental conditions like high-salt conditions, nutrition starvation, and cold and heat stress. During the last few years, much progress has been made in unraveling the heat shock response of B. subtilis. The heat shock genes can be classified into several groups according to their regulation: class I is controlled by HrcA binding to CIRCE (15, 26, 27), class II is controlled by the alternative sigma factor ςB (1, 7, 8, 18, 24, 25), and class III is controlled by CtsR, the negative regulator of clpP, clpE, and the clpC operon I (3, 12). There are other heat shock genes like htpG (22) and ykdA (17), which do not belong to classes I to III. In Escherichia coli two alternative sigma factors (ς32 and ςE) are involved in heat shock regulation and show a complex type of interplay (16, 19, 21). Under heat stress conditions there is a need for additional capacity of chaperones and proteases, due to the heat denaturation of proteins. In B. subtilis the nature of heat-inducible regulation of the DnaK and GroE chaperone machines, as well as the ClpCP proteases, is already known (3, 15). However, the details of the regulation of several heat shock genes like clpX (U. Gerth, personal communication), htpG (S. Versteeg, personal communication), and ykdA, coding for a HtrA-like protease (17), are still unclear. Therefore, additional regulators seem to be involved in the regulation of the heat shock stimulon in B. subtilis.
Several new sigma factors have been described in the genome sequence of B. subtilis (9–11, 13). For our understanding of the global regulatory network (the “regulome”), analysis of sigma factor mutants is very promising. If the conditions under which a sigma factor is active are known, a comparison between the behavior of wild-type and mutant strains under those conditions will lead to the detection of genes which are controlled by that specific sigma factor, using global methods like two-dimensional polyacrylamide gel electrophoresis (2-D PAGE) or DNA array technologies. In this type of analysis, we initially looked for conditions under which the transcription of ykoZ coding for a sigma factor with unknown function is induced, even if an induced transcription does not necessarily mean that the sigma factor is also active under these conditions.
A sigI mutant cannot grow at high temperature.
BFA 251, a sigI mutant was constructed by Mathieu Simon and Patrick Stragier. Briefly, a PCR was performed using chromosomal DNA of B. subtilis 168 and a primer pair; this resulted in an amplicon consisting of sigI internal bases 1411388 to 1411627 according to the BSUB genome annotation (SubtiList database) and BamHI (HindIII) cleavable ends. The PCR product was purified and digested with BamHI and HindIII and ligated into the vector pMUTIN (23), which was also digested with BamHI and HindIII, resulting in plasmid pDG1773. B. subtilis 168 was transformed with pDG1773 and selected for Ermr on Luria-Bertani LB plates containing 0.3 μg of erythromycin per ml to favor single integration events. Colonies were subsequently transferred to LB plates containing 150 μg of erythromycin per ml to select for true integrants. The correct integration was then checked by PCR.
During the systematic function analysis project, we made the initial observation that strain BFA 251 (sigI::pMUTIN4) did not grow at very high temperatures (55°C) after replica plating on LB plates. Also, the strain did not form colonies at 54°C on SMM plates (1 liter of SMM consists of 200 ml of 5× minimal salt solution [0.057 M K2SO4, 0.31 M K2HPO4, 0.22 M KH2PO4, 0.017 N sodium citrate, 0.004 M MgSO4; pH 7.0], 20 ml of glucose [20%, wt/vol]), 5 ml of l-tryptophane [1%, wt/vol], 50 ml of l-glutamine [4%, wt/vol], 2 ml of FeCl3 [2 mg/ml], 2 ml of MnSO4 [0.1 mg/ml], 10 ml of 100× trace element solution [0.55 g of CaCl2, 0.17 g of ZnCl2, 0.043 g of CuCl2, 0.06 g of CoCl2, and 0.06 g of Na2MoO4 dissolved in H2O to 1 liter]; for plates, 15 g of agar per liter was added).
To be sure that the temperature-sensitive (TS) phenotype is indeed linked to the ykoZ::pMUTIN4 insertion and does not result from putative second-site mutations, we transformed chromosomal DNA from BFA 251 into B. subtilis 168 and checked the transformants for growth on LB plates at 55°C. All the 16 tested clones showed the TS phenotype. The survival rate of that mutant was determined by using serial dilutions after plating and incubation at 55°C for 18 h. As described in Table 1, the survival rate of the sigI mutant was about 1,000-fold reduced compared to that of the wild type. Nevertheless, some colonies grew on plates at high temperature. Several clones were streaked out and cultivated at 55°C, and some of these clones showed wild-type-like growth at high temperature. These clones were considered to be TS suppressors.
TABLE 1.
Heat shock survival at 55°Ca
| Strain | CFU after incubation at:
|
Survival rate (%) | |
|---|---|---|---|
| 37°C | 55°C | ||
| 168 | 4.6 × 108 | 2.6 × 108 | 57 |
| BFA 251 | 0.9 × 108 | 1.6 × 105 | 0.17 |
The titers of different strains after incubation on LB plates at 55 and 37°C for 20 h and the survival rate under heat stress are given.
Transcription of sigI is heat shock induced in rich medium.
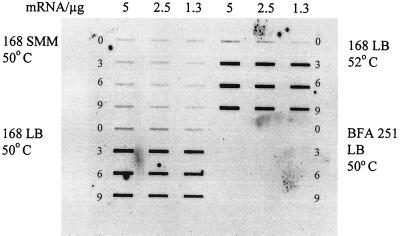
To study the transcriptional profile of sigI, a digoxigenin-labeled riboprobe of sigI was created by using the primers Ykoz7F1 (CTAATCGACTCACTATAGGGAGCACCCGTAATGATAA) and Ykoz7R1 (AAACCAGTGCTTAGCTTTT) in a PCR. The amplicon was purified with the QIAquick gel extraction kit (Qiagen) after the right sized fragment was cut out of an agarose gel. For the digoxigenin labeling reaction, the T7 transcription kit from Roche and primer Ykoz7F1 were used as specified in the manufacturer's protocol. This riboprobe was used for slot blot hybridization against total RNA prepared from B. subtilis cells grown in LB medium under different stress and starvation conditions as described earlier (12). The sigI gene was clearly induced under heat shock conditions, leading to the idea that it is an additional heat shock-specific sigma factor. Total RNA was prepared from cells grown in minimal medium and in rich medium before and after the heat shock and was used for additional slot blot experiments probed with the sigI riboprobe. As presented in Fig. 1, sigI is induced by heat shock only in rich medium (DSM [6] or LB medium), not in minimal medium (SMM). As expected, the sigI mutant BFA 251 did not show any signal, due to the pMutin insertion. No CIRCE sequence, CtsR binding site, or SigB-dependent promoter could be detected within the upstream region of sigI. Therefore, the sigI gene should be classified into group IV of the B. subtilis heat shock genes.
FIG. 1.
Concentration of sigI/ykoZ mRNA in B. subtilis 168 and BFA 251 grown in LB medium and SMM. A slot blot analysis of total RNA, which was isolated before (0 min) and 3, 6, and 9 min after a heat shock from 37 to 50°C (or 52°C, as indicated) is shown. The amount of total RNA used was 5, 2.5, or 1.3 μg as indicated.
To characterize the heat induction kinetics, additional slot blot experiments with RNA prepared after different time points (3, 6, 9, 30, and 60 min [data not shown]) were used for quantification. As calculated from several experiments, the maximal induction rate was about 15-fold 3 to 6 minutes after heat shock and declined to about 4-fold after 60 min. Like other heat shock genes, sigI is also transiently induced.
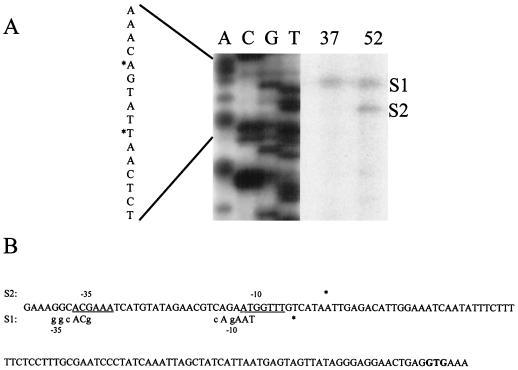
To define putative transcriptional start sites, a primer extension experiment was performed as described elsewhere (12), using YkozPE1 (TGCAGATCTTTATTGCCTTTTTG) as the primer and RNA prepared from B. subtilis 168 before and 5 min after a heat shock from 37 to 52°C. Two putative transcriptional start sites (S1 and S2) were detected, as shown in Fig. 2. The intensity of the signal of S1 remained unchanged before and after the heat shock, and the putative promoter resembles a weak ςA-dependent recognition sequence (Fig. 2B). After the heat shock an additional signal of a putative transcriptional start site was visible (S2). The deduced recognition sequence does not have homology to any known promoter sequences (5, 9–11). Consistent with the observations mentioned in this paper, the sigI gene could be autoregulated, and this sequence could resemble a ςI-dependent promoter.
FIG. 2.
(A) Autoradiograph from a primer extension experiment mapping the 5′ end of sigI mRNA. Equal amounts of RNA isolated before (lane 37) and 5 min after (lane 52) heat shock were used as templates for reverse transcription. The corresponding sequence was obtained with the appropriate primer. The DNA sequence illustrated in the central part is shown at the left side. Putative transcriptional start sites are indicated by asterisks and by S1 and S2. (B) Sequence of the upstream region of sigI. Putative transcriptional start sites are marked by asterisks. The start codon is given in bold letters. The putative promoter sequence (−35 and −10 regions) belonging to S1 is shown below the sequence line. Bases representing the ςA consensus sequence are labeled in capital letters. Above the sequence the potential −10 and −35 regions (underlined bases) belonging to the heat-inducible putative transcriptional start site are shown.
The sigI mutant shows a reduced level of GsiB under heat shock conditions.
If SigI is active under heat shock conditions, 2-D PAGE analyses of [35S]methionine-pulse-labeled protein extracts of wild-type and sigI mutant strains after heat shock should lead to detection of the proteins which were controlled by SigI and which could be responsible for the TS phenotype. Protein extract preparation, 2-D PAGE conditions, and gel staining were performed as described previously (12). To obtain an efficient uptake and incorporation of the labeled methionine in pulse-labeling experiments, cells have to be grown in minimal medium. Ironically, we found a heat shock induction of the sigI gene only during growth in rich medium, which could not be used for pulse-labeling experiments with [35S]methionine. Comparisons of gels derived from minimal medium extracts showed no relevant changes between wild-type and sigI mutant strains. In addition, 2-D PAGE was performed with cultures grown in DSM to an optical density at 540 nm of 0.5 with subsequent transfer from 37 to 50°C for 15 or 30 min. Compared to the pulse-labeling experiments, which visualize changes in the protein synthesis rate, these gels show the accumulated amount of proteins. As a consequence, a putative SigI dependence will not be seen as clearly as in pulse-labeling experiments.
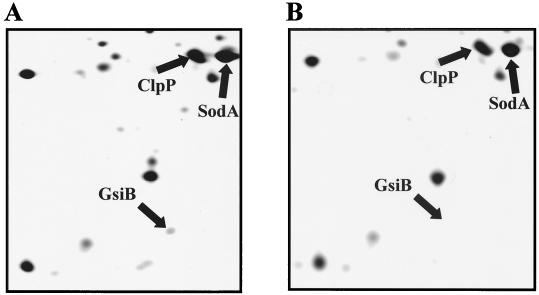
Figure 3 presents a comparison of a part of the silver-stained protein pattern of wild-type and BFA 251 strains. Only the GsiB protein spot showed a significant size reduction in the mutant in all repeated experiments, whereas the other sigB-dependent proteins showed the same heat-inducible pattern as in the wild type. Similar results were obtained with Coomassie blue-stained gels (data not shown).
FIG. 3.
2-D PAGE analysis of proteins present in the wild-type B. subtilis 168 (A) and in BFA 251 (B) grown in LB medium for 30 min after the heat shock. The GsiB spot was identified by comparison with a B. subtilis master gel (1), and the GsiB spot-containing sections of the gels are presented. For generating the images, 80-μg portions of protein extracts were fractionated by 2-D PAGE and the gels were stained with silver (12).
It was suggested that GsiB is exclusively transcribed by SigB (14). In contrast to the sigI mutant BFA 251, sigB null mutants can grow at high temperature; in these mutants transcription of gsiB does not occur and GsiB protein is not visible in 2-D PAGE under conditions where GsiB is expressed in the B. subtilis wild-type strain. This may indicate that GsiB is not essential for survival at high temperature. Therefore it is more likely that another factor controlling the amount of GsiB (e.g., a protease) is the reason for the observed size reduction of the GsiB spot and the TS phenotype in the sigI mutant.
To clarify whether gsiB is transcribed in a SigI-dependent manner, we used a gsiB probe to hybridize a similar slot blot to that presented in Fig. 1. A similar heat shock induction pattern was observed in the wild-type and sigI mutant strains (data not shown). This also argues against a SigI-dependent transcription of gsiB.
The limitations of the 2-D PAGE system will be overcome by using the DNA array technology as tool for monitoring global gene regulation (2, 4, 20). Experiments are in progress to define the SigI-dependent regulon by hybridizing commercially available genomic DNA arrays on nylon filters with radiolabeled cDNA, which was prepared from the wild type and from the sigI mutant under heat shock conditions. Comparison of these images should reveal directly or indirectly sigI-controlled genes, which could be responsible for the TS phenotype of the sigI mutant strain BFA251.
Acknowledgments
This work was supported by the EU-funded project Systematic Function Analysis of the Bacillus subtilis genes (BIO4-CT95-0278). As part of this project, the mutant BFA 251 was constructed and verified by Mathieu Simon and Patrick Stragier.
We are grateful to Mathieu Simon and Patrick Stragier for constructing the mutant BFA 251, making the β-galactosidase measurements, and providing fruitful comments. We thank Elke Krüger, Susanne Engelmann and Britta Jürgen for useful discussions. We are especially grateful to Karin Binder, Renate Gloger, Anita Harang, and Annette Tschirner for excellent technical assistance.
REFERENCES
- 1.Bernhard J, Völker U, Völker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two-dimensional protein electrophoresis study. Microbiology. 1997;143:999–1017. doi: 10.1099/00221287-143-3-999. [DOI] [PubMed] [Google Scholar]
- 2.DeRisi J L, Iyer V R, Brown P O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 3.Derré I, Rapoport G, Msadek T. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in Gram-positive bacteria. Mol Microbiol. 1999;31:117–131. doi: 10.1046/j.1365-2958.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 4.Fawcett P, Eichenberger P, Losick R, Youngman P. The transcriptional profile of early to middle sporulation in Bacillus subtilis. Proc Natl Acad Sci USA. 2000;97:8063–8068. doi: 10.1073/pnas.140209597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons Ltd.; 1990. [Google Scholar]
- 7.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 8.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the ςB regulon. Mol Microbiol. 1998;29:1129–1136. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 9.Horsburgh M J, Moir A. ςM, an ECF RNA polymerase sigma factor of Bacillus subtilis 168, is essential for growth and survival in high concentrations of salt. Mol Microbiol. 1999;32:41–50. doi: 10.1046/j.1365-2958.1999.01323.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang X, Helmann J D. Identification of target promoters for the Bacillus subtilis ςX factor using a consensus-directed search. J Mol Biol. 1998;279:165–173. doi: 10.1006/jmbi.1998.1765. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Gaballa A, Cao M, Helmann J D. Identification of target promoters for the Bacillus subtilis extracytoplasmic function ς factor, ςW. Mol Microbiol. 1999;31:361–371. doi: 10.1046/j.1365-2958.1999.01180.x. [DOI] [PubMed] [Google Scholar]
- 12.Krüger E, Hecker M. The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes. J Bacteriol. 1998;180:6681–6688. doi: 10.1128/jb.180.24.6681-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunst F, Ogasawara N, Moszer I, Albertini A M, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1998;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 14.Maul B, Völker U, Riethdorf S, Engelmann S, Hecker M. ςB-dependent induction of gsiB by multiple stimuli in Bacillus subtilis. Mol Gen Genet. 1995;248:114–120. doi: 10.1007/BF02456620. [DOI] [PubMed] [Google Scholar]
- 15.Mogk A, Homuth G, Scholz C, Kim L, Schmid F X, Schumann W. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 1997;16:4579–4590. doi: 10.1093/emboj/16.15.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita M T, Tanaka Y, Kodama T S, Kyogoku Y, Yanagi H, Yura T. Translational induction of heat shock transcription factor sigma32: evidence for a built-in RNA thermosensor. Genes Dev. 1999;13:655–665. doi: 10.1101/gad.13.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noone D, Howell A, Devine K M. Expression of ykdA, encoding a Bacillus subtilis homologue of HtrA, is heat shock inducible and negatively autoregulated. J Bacteriol. 2000;182:1592–1599. doi: 10.1128/jb.182.6.1592-1599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersohn A, Bernhardt J, Gerth U, Höper D, Koburger T, Völker U, Hecker M. Identification of ςB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J Bacteriol. 1999;181:5718–5724. doi: 10.1128/jb.181.18.5718-5724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raina S, Missiakas D, Georgopoulos C. The rpoE gene encoding the ςE (ς24) heat shock sigma factor of Escherichia coli. EMBO. 1995;14:1043–1055. doi: 10.1002/j.1460-2075.1995.tb07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richmond C R, Glasner J D, Mau R, Jin H, Blattner F R. Genome-wide expression profiling in Escherichia coli K-12. Nucleic Acids Res. 1999;27:3821–3835. doi: 10.1093/nar/27.19.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouviere P E, De Las Penas A, Mecsas J, Lu C Z, Rudd K E, Gross C A. rpoE, the gene encoding the second heat-shock sigma factor, ςE, in Escherichia coli. EMBO. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz A, Schwab S, Homuth G, Versteeg S, Schumann W. The htpG gene of Bacillus subtilis belongs to class III heat shock genes and is under negative control. J Bacteriol. 1997;179:3103–3109. doi: 10.1128/jb.179.10.3103-3109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vagner V, Dervyn E, Ehrlich S D. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
- 24.Vijay K, Brody M S, Fredlund E, Price C W. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the sigmaB transcription factor of Bacillus subtilis. Mol Microbiol. 2000;35:180–188. doi: 10.1046/j.1365-2958.2000.01697.x. [DOI] [PubMed] [Google Scholar]
- 25.Völker U, Maul B, Hecker M. Expression of the ςB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J Bacteriol. 1999;181:3942–3948. doi: 10.1128/jb.181.13.3942-3948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan G, Wong S L. Isolation and characterization of Bacillus subtilis groE regulatory mutants: evidence for orf39 in the dnaK operon as a repressor gene in regulating the expression of both groE and dnaK. J Bacteriol. 1995;177:6462–6468. doi: 10.1128/jb.177.22.6462-6468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuber U, Schumann W. CIRCE, a novel heat shock element involved in regulation of the heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]