Abstract
This study was conducted to examine the chemical constituents of Origanum majorana L. essential oils (EOs) that originate in Nepal, as well as their biological activities, antioxidant properties, and enantiomeric compositions. The EOs were extracted by the hydro-distillation method using a Clevenger-type apparatus and their chemical compositions were determined through gas chromatography and mass spectrometry (GC-MS). Chiral GC-MS was used to evaluate the enantiomeric compositions of EOs. The minimum inhibitory concentrations (MICs) of the essential oils were determined by the micro-broth dilution method, and the antioxidant activity was evaluated by the 2,2-diphenyl-1-picrylhydrazyl scavenging assay and ferric-reducing antioxidant power (FRAP). GC-MS analysis showed the presence of 50 and 41 compounds in the EO samples, (S1) and (S2), respectively, representing the Kathmandu and Bhaktapur districts. The oxygenated monoterpenoids, along with terpinen-4-ol, were predominant constituents in both EO samples. However, the EOs from two locations showed some variations in their major components. The chiral terpenoids for two EO samples of marjoram have also been reported in this study in an elaborative way for the first time in accordance with the literature review. A hierarchical cluster analysis based on the compositions of EOs with 50 compositions reported in the literature revealed at least 5 different chemotypes of marjoram oil. The antioxidant activity for the sample (S2) was found to be relatively moderate, with an IC50 value of 225.61 ± 0.05 μg/mL and an EC50 value of 372.72 ± 0.84 µg/mL, as compared to the standard used. Furthermore, with an MIC value of 78.1 µg/mL, the EO from sample (S2) demonstrated effective antifungal activity against Aspergillus niger and Candida albicans. Moreover, both samples displayed considerable antimicrobial activity. The results suggest that EOs of Origanum majorana possess some noteworthy antimicrobial properties as well as antioxidant activity, and hence can be used as a natural preservative ingredient in the food and pharmaceutical industries.
Keywords: Origanum majorana L., essential oil, bio-active component, chiral GC-MS analysis, hierarchical cluster analysis, antimicrobial activity, DPPH assay, FRAP assay
1. Introduction
The use of plants as traditional health remedies has become very important and popular all over the world in recent years, because more than 80% of the world’s population directly or indirectly rely on herbal drugs for their primary healthcare [1]. They have gained significant attention in recent years in fields such as medicine, nutraceuticals, dietary supplements, pharmaceutical intermediates, and chemical entities for synthetic pharmaceuticals [2,3]. The screening of such local plants for their constituents and antimicrobial properties has always been of great interest to researchers who are looking for new compounds to treat various microbial diseases [4,5].
Essential oils (EOs) are natural complex mixtures of volatile organic compounds with a strong fragrance that can be obtained from several medicinal and aromatic plants. EOs have a predominance of terpenes in which monoterpenes and sesquiterpenes are the two major classes of constituents, with isoprene as a building block of terpenes [6]. Nowadays, essential oils have a wide application in the food industry, e.g., flavors, fragrances, preservatives; in the cosmetic industry, e.g., perfumes, skin products; in feed additives, e.g., antioxidants, growth promoters, and in the pharmaceutical industry, e.g., medicines [7]. This is because they possess strong antimicrobial, antioxidant, antiparasitic, antiprotozoal, antifungal, and anti-inflammatory properties. The use of synthetic flavoring, fragrance, preservatives, and other antimicrobial compounds is growing rapidly. However, such synthetic chemicals are highly hazardous, and detrimental to health upon exceeding the permissible level of intake [8]. Therefore, essential oils may be a reasonable alternative for ensuring food safety, maintaining the nutritional content and quality of food, and removing risks to human health. Since many EOs have a predominance of oxygenated monoterpenes, which have a potential antibacterial effect, they are being used legally as flavorings [9,10,11].
Origanum majorana L. (syn. Majorana hortensis Moench), which belongs to the Lamiaceae family (Labiatae) and has medicinal values, is often known as ‘sweet marjoram’. It is a bushy perennial herb of the Origanum genus [12]. This herb is native to the Mediterranean region and is grown in many Asian, North African, and European nations [13]. It grows up to a height of 30 to 60 cm. It features an oblique rhizome, hairy shrub-like stalks, opposite dark green oval leaves, and clustered bracts with white or red flowers. O. majorana is an important culinary herb and is widely distributed in the central zone of Nepal at an altitude of about 1300 m to 3000 m, mostly in moist places. It is locally known as ‘Mu-swan’ in Newari (or maruwa phool) and ‘Raam tulsi’ in Nepali. O. majorana has been reported to possess very good anti-bacterial and antifungal activity against different pathogenic bacteria [14,15] and fungi [16,17]. It has also been reported to have antispasmodic, digestive, expectorant, and diuretic properties. It is effective for curing asthma and coughs and is widely used in gastronomy and natural medicine. Moreover, EOs of O. majorana have great potential in the cosmetic, pharmaceutical, perfume, food, and flavor industries [17,18]. The essential oil of this plant has also been used for pain, gastrointestinal problems, and respiratory tract disorders [19,20]. The antiparasitic and larvicidal activities of marjoram have also been reported in several studies [21]. Moreover, majorana was reported to show antidiabetic activity [22], nephrotoxicity protective effects [23], anti-inflammatory, analgesic, and anti-pyretic activities [24].
The gas chromatography and mass spectrometry (GC-MS) method has been used to investigate the mixture of bioactive volatile compounds produced by aromatic plants [25]. Similarly, an improved approach known as chiral GC-MS has been utilized in conjunction with GC-MS in order to determine the chirality of secondary metabolites. This method is critical for ensuring the integrity of the essential oil constituents, as well as assisting in the detection of impurities.
Even though there are numerous reports on the EOs of O. majorana from different geographical and climatic conditions of the world, there is a lack of extensive information about the chemical composition and biological activities of O. majorana of Nepalese origin. Because the Nepal Himalaya possesses a wide range of unique and valuable medicinal and aromatic plants (MAPs) on its land, due to its biologically diverse ecosystems, this study has set out to investigate the antimicrobial activity, chemical profiles, hierarchical cluster analysis, and antioxidant activities of EOs from two locations in Nepal and has attempted to identify the leading active constituents, along with the enantiomeric distribution of chiral terpenoids.
2. Results and Discussion
2.1. Isolation and Yields of Essential Oils
The yields of O. majorana essential oils from Kathmandu (sample S1) and Bhaktapur (sample S2) were found to be 0.5% (v/w) and 0.8% (v/w), respectively. The variation in the yields of EOs is attributed to various factors, such as geographical origin, harvesting period, extraction techniques, temperature, and time of extraction [26]. The essential oils of O. majorana were characterized by the senses, including taste, sight, smell, and touch. Both EOs of O. majorana were slightly viscous liquids and had a colorless to pale yellow color. They had a strong sweet and spicy odor.
2.2. Comparision of Chemical Composition of Two Essential Oils
The analysis of the chemical composition of O. majorana essential oil revealed that a total of 50 and 41 compounds were characterized in the marjoram EO for samples S1 and S2, respectively, representing 91.19% to 98.80% of total volatile oil. The relative percentages of all individual components present in the O. majorana EOs are shown in Table 1.
Table 1.
Chemical composition of essential oils of Origanum majorana L. from Nepal.
| RI | Compound Name (S1) | % | RI | Compound Name (S2) | % |
|---|---|---|---|---|---|
| 849 | (3Z)-Hexenol e | tr | - | - | - |
| 920 | Tricyclene a | tr | - | - | - |
| 923 | α-Thujene a | 0.2 | 924 | α –Thujene a | 0.05 |
| 930 | α-Pinene a | 0.5 | 931 | α –Pinene a | 0.15 |
| 947 | Camphene a | 0.3 | 948 | Camphene a | 0.09 |
| 971 | Sabinene a | 3.6 | 972 | Sabinene a | 1.29 |
| 975 | β-Pinene a | 0.3 | 978 | β –Pinene a | 0.12 |
| 987 | Myrcene a | 1.3 | 989 | Myrcene a | 0.16 |
| 1003 | p-Mentha-1(7),8-diene b | tr | - | - | - |
| 1005 | α-Phellandrene a | 0.1 | - | - | - |
| 1016 | α-Terpinene a | 5.0 | - | - | - |
| 1023 | p-Cymene a | 1.8 | 1024 | p-Cymene a | 6.90 |
| 1027 | Limonene a | 1.4 | 1028 | Limonene a | 0.52 |
| 1029 | β-Phellandrene a | 1.1 | 1029 | β -Phellandrene a | 0.06 |
| 1030 | 1,8-Cineole b | 0.1 | 1031 | 1,8-Cineole b | 0.12 |
| 1033 | (Z)-β-Ocimene a | 0.1 | - | - | - |
| 1042 | Benzene acetaldehyde f | tr | - | - | - |
| 1043 | (E)-β-Ocimene a | 0.1 | - | - | - |
| 1058 | γ-Terpinene a | 9.5 | - | - | - |
| 1070 | cis-Sabinene hydrate b | 4.4 | 1071 | cis-Sabinene hydrate b | 3.48 |
| - | - | - | 1087 | trans-Linalool oxide b | 0.52 |
| 1084 | Terpinolene a | 2.5 | - | - | - |
| 1088 | p-Cymenene a | tr | - | - | - |
| 1102 | Linalool b | 13.8 | 1099 | Linalool b | 15.37 |
| 1103 | trans-Sabinene hydrate b | 1.6 | 1102 | trans-Sabinene hydrate b | 0.71 |
| 1124 | cis-p-Menth-2-en-1-ol b | 1.9 | 1124 | cis-p-Menth-2-en-1-ol b | 1.35 |
| - | - | - | 1124 | Cyclooctanone | 0.47 |
| 1142 | trans-p-Menth-2-en-1-ol b | 1.0 | 1142 | trans-p-Menth-2-en-1-ol b | 0.78 |
| 1154 | Menthone b | 0.1 | - | - | - |
| 1174 | Borneol b | 0.2 | - | - | - |
| 1186 | Terpinen-4-olb | 32.1 | 1180 | Terpinen-4-ol b | 33.35 |
| - | - | - | 1187 | p-Cymen-8-ol b | 0.53 |
| - | - | - | 1188 | 3-cis-Hexenyl butyrate b | 0.13 |
| - | - | - | 1190 | 1,4-Hydroxy cineole b | 3.35 |
| 1196 | α-Terpineol b | 3.7 | 1195 | α –Terpineol b | 2.63 |
| 1197 | cis-Piperitol b | 0.4 | 1197 | cis-Piperitol b | 0.12 |
| 1203 | p-Cumenol g | tr | - | - | - |
| 1208 | trans-Piperitol b | 0.6 | 1208 | trans-Piperitol b | 0.26 |
| - | - | - | 1224 | Isoascaridole b | 0.15 |
| 1214 | cis-Sabinene hydrateacetate b | tr | - | - | - |
| 1222 | Nerol b | 0.2 | - | - | - |
| 1236 | Pulegone b | tr | - | - | - |
| 1248 | Linalyl acetate b | 5.9 | 1252 | Linalyl acetate b | 6.67 |
| - | - | - | 1255 | p-menthane-1,2,3-triol b | 0.69 |
| 1273 | trans-Ascaridol glycol b | 0.1 | 1276 | trans-Ascaridol glycol b | 1.15 |
| 1282 | Bornyl acetate b | 2.4 | 1282 | Bornyl acetate b | 2.83 |
| - | - | - | 1291 | Terpinen-4-ol acetate b | 0.31 |
| - | - | - | 1297 | Carvacrol b | 0.08 |
| - | - | - | 1345 | 2-Methyl-2-(para-tolyl) propionaldehyde | 0.24 |
| - | - | - | 1354 | Terpen-diol | 0.46 |
| 1293 | Terpin-1-en-4-yl acetate b | 0.2 | - | - | - |
| 1329 | δ-Elemene c | tr | - | - | - |
| 1355 | Neryl acetate b | 0.2 | 1361 | Neryl acetate b | 0.14 |
| 1375 | Geranyl acetate b | 0.3 | 1377 | Geranyl acetate b | 0.48 |
| 1417 | β-Caryophyllene c | 2.4 | 1418 | β –Caryophyllene c | 0.13 |
| - | - | - | 1486 | Hydroxy linalyl acetate b | 1.12 |
| - | - | - | 1488 | p-Menthane-1,2,4-triol | 1.44 |
| 1452 | α-Humulene c | 0.1 | - | - | - |
| 1492 | Bicyclogermacrene c | 0.4 | - | - | - |
| 1500 | (E,E)-α-Farnesene c | tr | - | - | - |
| 1573 | Spathulenol d | tr | 1579 | Spathulenol d | 0.14 |
| 1578 | Caryophyllene oxide d | 0.1 | 1580 | Caryophyllene oxide d | 2.54 |
| - | - | - | 1612 | Humulene epoxide II d | 0.13 |
| 2047 | Abietatriene h | tr | - | - | - |
| Total identified | 99.8 | 91.19 |
Note: RI = retention index determined in reference to a series of n-alkanes (C8–C40) on a ZB-5ms column; compounds are listed in order of elution (increasing RI). tr = trace (˂0.05%), ‘-‘ = not detected;% = percent composition. Symbols: ‘a’= monoterpene hydrocarbon; ‘b’ = oxygenated monoterpene; ‘c’ = sesquiterpene hydrocarbon; ‘d’ = oxygenated sesquiterpene; ‘e’ = alcohol; ‘f’ = aldehyde; ‘g’ = phenol carrying an isopropyl group at position 4; and ‘h’ = diterpenoid. “Sample S1”= Kathmandu district; “Sample S2”= Bhaktapur district.
For our O. majorana oil (S1), terpinen-4-ol (32.1%), linalool (13.8%) and γ-terpinene (9.5%) were the most prominent compounds, followed by linalyl acetate (5.9%), α-terpinene (5%), cis-sabinene hydrate (4.4%), α-terpineol (3.7%), terpinolene (2.5%), bornyl acetate (2.4%), β-caryophyllene (2.4%), cis-p-menth-2-en-1-ol (1.8%) and p-cymene (1.8%) in smaller amounts. Similarly, terpinen-4-ol (33.35%), linalool (15.37%), p-cymene (6.90%) and linalyl acetate (6.67%) were the most major compounds, followed by cis-sabinene hydrate (3.48%), 1,4-hydroxy cineole (3.35%), bornyl acetate (2.83%), α-terpineol (2.63%), and caryophyllene oxide (2.54%) in smaller amounts in the oil of marjoram (S2). The oxygenated monoterpenoids, monoterpene hydrocarbons and sesquiterpene hydrocarbons were the major classes of terpenes in these O. majorana essential oils. Figure 1. shows a typical GC-MS chromatogram of O. majorana essential oil, displaying the separation of chemical components. Similarly, Figure 2. depicts the chemical structure of the prominent constituents in the essential oil of samples (S1) and (S2). The obtained results are in accordance with the reports of several studies carried out previously because they also found terpinen-4-ol as the most predominant compound, along with other major compounds in the marjoram EOs [15,27,28,29,30,31,32]. Table 2. shows the major components of Origanum majorana essential oil from different countries, which makes the comparative study easier. In a similar way, a study carried out on the EO of majorana from Nepal found terpinen-4-ol (22.42%), γ-terpinene (14.69%) and linalool (11.61%) as the main components [33]. Some other studies reported that terpinen-4-ol, either alone or in combination with cis-sabinene hydrate, linalyl acetate, γ-terpinene, etc., was found as the predominant constituent, along with some others, such as α- terpineol, α-terpinene, linalyl acetate and linalool in the majorana oil [23,34,35,36], which are also mentioned in Table 2. However, some other studies reported that thymol or carvacrol were the predominant compounds in marjoram EO [37]. Likewise, O. majorana oil from Turkey exhibited carvacrol (78.27–79.46%) as the major component [38]. In general, O. majorana EO is rich in terpinen-4-ol, cis-sabinene hydrate, γ-terpinene, α-terpinene, α-terpineol, p-cymene and linalool, which can be clearly observed in Table 2.
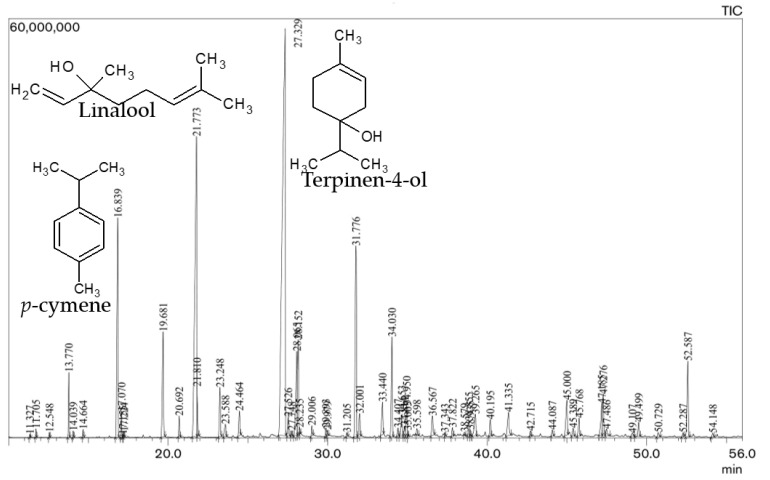
Figure 1.
Typical GC-MS chromatogram of O. majorana L. essential oil.
Figure 2.
Major chemical constituents identified in the essential oil of Origanum majorana L. (Sample S1 and Sample S2) and the chiral compounds present, such as terpinen-4-ol, cis-sabinene hydrate, linalool, linalyl acetate, bornyl acetate, α-terpineol and β-caryophyllene in both EOs.
Table 2.
The major chemical compounds in the essential oil of O. majorana in the different countries.
| Country | Major compounds | References |
|---|---|---|
| Morocco | Terpinen-4-ol (34.1%), α-Terpinene (19.2%), Terpineol (8.9%) | [15] |
| Iran | Terpinen-4-ol (32.69%), γ -Terpinene (12.88%), trans-Sabinene hydrate (8.47%), α -Terpinene (7.98%) | [27] |
| Iran | Terpinene-4-ol (22.15%-25.65%), γ-Terpinene (13.94%-16.22%), α-Terpinene (8.11%-10.39%), α-Terpineol (4.53%-6.39%) | [28] |
| India | Terpinen-4-ol (31.15%), cis-Sabinene hydrate (15.76%), p-Cymene (6.83%), Sabinene (6.91%), trans-Sabinene hydrate (3.86%), α-Terpineol (3.71%) | [29] |
| Tunisia | Terpinene-4-ol, γ-Terpinene, cis-Sabinene-hydrate, α-Terpineol | [30] |
| China | Terpinen-4-ol (33.0 %), Caryophyllene oxide (11.9 %), p-Cymene (6.8 %), α-Terpineol (6.7 %), Spathulenol (6.0 %) | [31] |
| Egypt | Terpinen-4-ol (30.4%), γ-Terpinene, cis-Sabinene hydrate, α-Terpinene, Sabinene, α-Terpineol | [32] |
| Reunion Island | Terpinen-4-ol (38.4%), cis-Sabinene hydrate (15.0%), p-Cymene (7.0%), γ-Terpinene (6.9%). | [40] |
| Albania | Terpinen-4-ol (21.3%), trans-Sabinene hydrate (15.5%), γ-Terpinene (14.0%), α-Terpinene (8.9%) | [41] |
| Tunisia | Terpinen-4-ol | [4] |
| Hungary | Terpinen-4-ol | [11] |
| Tunisia | Terpinen-4-ol (23.2%), cis-Sabinene hydrate (17.5%), γ-Terpinene (10.5%), p-Cymene (9%), α–Terpineol (5.6%), α-Terpinene (4.7%), trans-Sabinene hydrate (4.0%) | [14] |
| Nepal | Terpinen-4-ol (22.42%), Linalool (11.61%), γ-Terpinene (14.69%), α-Terpineol (7.02%), α-Phellandrene (9.8%), p-Cymene (8.91%) | [33] |
| Egypt | cis-Sabinene hydrate, Linalyl acetate, 𝛾-Terpinene | [23] |
| Cyprus | cis-Sabinene hydrate (7.4–33.3%), Terpinen- 4-01 (16.6–21.6%), α-Terpineol (7.3%), trans-Sabinene hydrate, (4.7%), γ-Terpinene (8.3%), α-Terpinene(4.7%) | [34] |
| Turkey | Trace amounts of Carvacrol. cis-Sabinene hydrate (30–44%), Terpinen-4-ol (8–14%) | [36] |
| Greece | 4-Terpineol (37%), p-Cymene (12%), α-Terpineol (7%) | [42] |
| Iran | Linalyl acetate (26.1%), Sabinene (12%) | [43] |
| Brazil | 𝛾-Terpinene (25.73%), 𝛼-Terpinene (17.35%), Terpinen-4-ol (17.24%), Sabinene (10.8%) | [44] |
| Venezuela | cis-Sabinene hydrate (30.2%), Terpinen-4-ol (28.8%), γ-Terpinene (7.2%), α-Terpineol (6.9%), trans-Sabinene hydrate (4.4%), Linalyl acetate (3.8%), α-Terpinene (3.6%) | [45] |
Here, the variations in the chemical content and compositions of O. majorana essential oil across the world might be attributed to several factors, such as the varied agro-climatic (climatical, seasonal, geographical) conditions of the regions, isolation regimes, plant species, adaptive metabolism of plants, and the plant part being analyzed [39]. Indeed, two O. majorana oils from Nepal (S1 and S2) showed some minor variations in volatile constituents. However, both EOs were found to be consistent with the reports presented previously, except for some slight variations due to the environmental and climatic conditions. The high content of terpinen-4-ol may be attributed to the rearrangements of components during the distillation processes, which were mentioned in the studies carried out previously, and cis-sabinene hydrate (responsible for the intense spicy marjoram aroma) is also present in our sample [29]. This study will provide information regarding O. majorana EO from Nepalese land, for those who wish to carry out further research on it.
2.3. Chiral GC-MS Analysis for Enantiomeric Distribution
The chiral GC-MS analysis was performed for the identification of enantiomeric compounds that are present in the EOs of O. majorana. The relative percentages of the dextrorotatory and laevorotatory compounds identified in the O. majorana EO are presented in Table 3. It also shows the enantiomeric compositions of majorana oil and identified the presence of twelve chiral compounds in total for both EOs. Figure 2 shows the structure of some of the chiral compounds present in both EO samples. The determination of enantiomeric composition is a powerful tool in order to authenticate the EOs because EOs obtained from different plants may be adulterated, due to the addition of several foreign components. In simple terms, geographical location and distillation time do not affect the enantiomeric distribution of chiral compounds present in the EOs. This analysis is the first attempt to capture the marjoram EO in more detail, which belong to Nepalese origin. Terpinen-4-ol is the major oxygenated monoterpene found in the EO of our two samples, in which the dextrorotatory (+) enantiomer is the most predominant chiral compound. In our study, the essential oil of sample (S2) has nearly the racemic mixture of terpinen-4-ol, (+) 52.6% and (–) 47.4%, whereas terpinen-4-ol, (+) 58.31%, is dominant over terpinen-4-ol, (–) 47.69%, for the EO for sample (S1). In both EO samples, (–) β-caryophyllene, (–) bornyl acetate, and (–) linalyl acetate were detected as enantiomerically pure with 100% in levorotatory form. Camphene is the enantiomerically most dominant component that exists in levorotatory form with slight variations, including (–) 96.8% to (–) 97.87% in our both EO samples, which is followed by (+) sabinene and (+) cis-sabinene hydrate, with the variation in the enantiomeric distribution in this study. Terpinen-4-ol in the EO of majorana from Israel was reported as not optically pure and the enantiomeric composition was about (+)-terpinen-4-ol (73.0%) and (–)-terpinen-4-ol (27.0%) [46]. The enantiomeric distribution of linalool was reported only in a sample of marjoram oil with (–)-linalool (82.0%) and (+)-linalool (18.0%) [47]. These previous results were found to be in close agreement with the chiral distribution of linalool and terpinen-4-ol as compared to our EOs, although we did not obtain information from the previous study on the enantiomeric distribution for other components in detail. Finally, we can conclude that the (+)/(−) ratios of each of the terpenoids remain relatively constant in our samples, regardless of the geographical location of the oil source.
Table 3.
Enantiomeric distributions of chiral compounds in EOs of Origanum majorana L. from Nepal.
| Compounds | O. majorana (Sample S1) | O. majorana (Sample S2) | ||
|---|---|---|---|---|
| + (D) | – (L) | + (D) | – (L) | |
| α-Pinene | 55.81 | 44.19 | 50.81 | 49.2 |
| Camphene | 2.31 | 97.87 | 3.18 | 96.8 |
| Sabinene | 95.63 | 4.37 | 91.6 | 8.4 |
| β-Pinene | 24.31 | 75.69 | 29.3 | 70.7 |
| Limonene | 66.48 | 33.52 | 66.82 | 33.2 |
| cis-Sabinene hydrate | 88.52 | 11.48 | 86.8 | 13.2 |
| Linalool | 31.43 | 68.57 | 29.6 | 70.4 |
| Terpinen-4-ol | 58.31 | 41.69 | 52.6 | 47.4 |
| Linalyl acetate | 0.0 | 100.0 | 0.0 | 100.0 |
| Bornyl acetate | 0.0 | 100.0 | 0.0 | 100.0 |
| α-Terpineol | 75.3 | 24.7 | 72.4 | 27.6 |
| β-Caryophyllene | 0.0 | 100.0 | 0.0 | 100.0 |
2.4. Chemotypes of O. majorana EOs
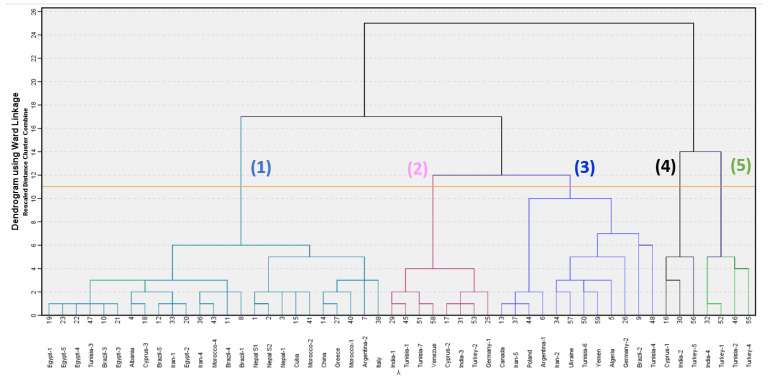
In order to highlight the chemotypes of O. majorana EOs, we performed an agglomerative hierarchical cluster (AHC) analysis based on the chemical compositions of the two EOs (S1 and S2) under this study, along with fifty additional marjoram oil chemical compositions from the literature review. The dendrogram of this analysis is shown in Figure 3. From the analysis of AHC, there are five different chemotypes, which are classified as follows: (1) terpinen-4-ol/γ-terpinene/α-terpinene/sabinen/α-terpineol, (2) cis-sabinene hydrate/terpinen-4-ol/γ-terpinene, (3) trans-sabinene hydrate/cis-sabinene hydrate/terpinen-4-ol/α-terpineol, (4) linalool/p-cymene/estragole and (5) carvacrol/linalool/p-cymene.
Figure 3.
A dendrogram obtained from the agglomerative hierarchical cluster analysis of 52 Origanum majorana essential oil compositions. Numbers, (1), (2), (3), (4) and (5) represent different chemotypes dominated by terpinen-4-ol, cis-sabinene hydrate, trans-sabinene hydrate, linalool and carvacrol, respectively.
Chemotype-1, dominated by terpinen-4-ol [16,28,31,32,33,37,48,49,50], is a cluster made up of twenty-five samples, including both samples (S1 and S2) from Nepal in our study, along with the previous marjoram sample from Nepal-1. Chemotype-2, dominated by cis-sabinene hydrate [29,36,39,45,51,52], has eight samples. Chemotype-3, dominated by trans-sabinene hydrate [53,54,55,56,57,58], has twelve samples in this analysis. Chemotype-4 is dominated by linalool [59,60] and is comprised of three samples. The chemotype-5 is dominated by carvacrol, which is comprised of four samples [38,61,62,63].
2.5. Antibacterial and Antifungal Activity
The antimicrobial activities of O. majorana essential oils were examined against several microorganisms. Both EOs were noted to be active against all microbial strains, but their efficacies were found to be variable to a different degree. The results obtained for the antimicrobial activities are given in Table 4. In the EO of marjoram for sample (S1), terpinen-4-ol, linalool and γ-terpinene were the major components. The essential oil sample (S2) had terpinen-4-ol, linalool and p-Cymene as the most dominant components. The EO for sample (S2) showed moderate antibacterial activity against Staphylococcus aureus, with an MIC value of 156 µg/mL. Both EOs had weaker activity against Bacillus cereus and Staphylococcus epidermidis, with an MIC value of 312.5 µg/mL as compared to the positive control, gentamicin (MIC = 19.5 µg/mL). The EO for sample (S2) exhibited good antifungal activity against Aspergillus niger and Candida albicans, with an MIC value of 78.1 µg/mL. Similarly, it showed moderate antifungal activity against Trichophyton mentagrophytes and Aspergillus fumigatus, with an MIC value of 156 µg/mL. The EO for sample (S1) had moderate antifungal activity against Aspergillus niger, Candida albicans and Trichophyton mentagrophytes. However, when compared to the positive control, amphotericin B (MIC = 19.5 µg/mL), both EOs were found to have weaker activity against the remaining fungal strains, with an MIC value of 312.5 µg/mL.
Table 4.
Minimum inhibitory concentrations (MICs) of O. majorana essential oils against tested bacterial and fungal strains.
| Name of Micro-Organism | MICs (µg/mL) | |
|---|---|---|
| EO Sample (S1) | EO Sample (S2) | |
| Bacillus cereus (ATCC 14579) | 312.5 | 312.5 |
| Staphylococcus aureus (ATCC 29213) | 312.5 | 156.3 |
| Staphylococcus epidermidis (ATCC 14990) | 312.5 | 312.5 |
| Aspergillusniger (ATCC 16888) | 156.3 | 78.1 |
| Candida albicans (ATCC 18804) | 156.3 | 78.1 |
| Trichophytonmentagrophytes (ATCC 18748) | 156.3 | 156.3 |
| Aspergillusfumigatus (ATCC 96918) | 312.5 | 156.3 |
| Cryptococcus neoformans (ATCC32045) | 312.5 | 312.5 |
| Microsporumcanis (ATCC11621) | 312.5 | 312.5 |
| Microsporumgypseum (ATCC24102) | 312.5 | 312.5 |
| Trichophytonrubrum (ATCC28188) | 312.5 | 312.5 |
Note: Gentamicin was used as the standard for bacteria (MIC = 19.5 µg/mL) and amphotericin B as the standard for fungi (MIC = 19.5 µg/mL). MICs = minimum inhibitory concentrations (in µg/mL).
According to several investigations, the EO of majoram showed a moderately varied antibacterial effect on Staphylococcus aureus, with an MIC value of 782 µg/mL [32], 192 μg/mL [14], 50 μg/mL [64], from 150.0 to 250.0 μg/mL [65], 50 μg/mL [64], and from 2.5 μL/mL (for S. aureus BH3), 5 μL/mL(for S. aureus BH01) to 10 μL/mL(for S. aureus BH02), respectively [66]. Similarly, this oil had a good antibacterial effect on Bacillus cereus, with an MIC value of 75.0 to 150.0 μg/mL [65] and 97 μg/mL [14]. It had an antibacterial effect on Staphylococcus epidermidis, with an MIC value of 390 µg/mL [14]. The marjoram EOs also exhibited strong effects against different fungal species in terms of MICs or growth inhibition doses. Likewise, strong inhibitory effects were observed with 10 μg/mL at 90% concentration [67], 2000 ppm concentration [16] and 22 mg/mL concentration [68] against Aspergillus niger. The majorana EO showed very good antifungal activity against Candida albicans, with MIC values of 58 µg/mL and 468 µg/mL [14]. Similarly, against Microsporum canis and Trichophyton rubrum, marjoram EO had mild activity with MIC values of 234 µg/mL, while it had moderate activity against Trichophyton mentagrophytes, with an MIC value of 117 µg/mL [14]. On the basis of the literature reviews, we can conclude that these results obtained for antimicrobial activities of EOs are in close agreement with some of the previous reports in the literature.
The antimicrobial activities of the EOs are mostly associated with their high content of oxygenated monoterpenes, particularly major components, such as terpinen-4-ol [69], α-terpineol, α-pinene, p-cymene etc. γ-terpinene, β-caryophyllene and sabinene are also known for their strong antimicrobial activities [70]. Indeed, the observed activity of EOs against bacterial and fungal species may be attributed to the synergistic effect among them and other constituents, rather than a single constituent. Our finding is in agreement with the previous studies that the essential oils exhibit more antifungal substances from aromatic plants than antibacterial substances, especially in the Lamiaceae [15,16]. This result may indicate that the essential oil of O. majorana can be used as natural preservatives in food against foodborne diseases and food spoilage, such as Bacillus sp., Staphylococcus aureus, Candida sp., Fusarium sp., Aspergillus sp., etc.
2.6. Antioxidant Activity
2.6.1. DPPH free Radical Scavenging Activity
In the present study, the antioxidant activity of O. majorana EO was determined by using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method and was compared to ascorbic acid activity. The antioxidant activity of EO samples is summarized in Table 5 in terms of IC50 values.
Table 5.
Antioxidant activity of O. majorna EOs (S1 and S2) and ascorbic acid (standard).
| Samples and Standard | DPPH Radical Scavenging IC50 Value (µg/mL) |
FRAP EC50 Value (µg/mL) |
|---|---|---|
| O. majorana (S1) | 503.08 ± 0.06 | 511.43 ± 0.61 |
| O. majorana (S2) | 225.61 ± 0.05 | 372.72 ± 0.84 |
| Ascorbic acid | 9.74 ± 0.07 | 217.23 ± 0.34 |
Note: Values are mean ± standard deviations from three experiments (n = 3).
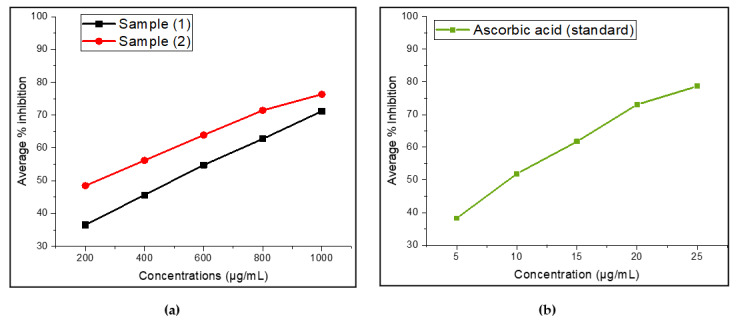
The DPPH is a stable free radical, which accepts the hydrogen radical or an electron, forming a stable diamagnetic molecule [71]. Indeed, DPPH solution is initially purple in color, which fades into the yellow color of diphenylpicryl hydrazine when bioactive components (antioxidants) from essential oils donate hydrogen radicals [72]. A standard criterion for measuring the antioxidant activity of EO samples is the IC50 values, which represents the concentration of antioxidant needed to minimize the initial DPPH concentration by 50% [72]. Our study revealed that O. majorana EO from two locations exhibited moderate DPPH free radical-scavenging activity, with IC50 values of 503.08 ± 0.06 μg/mL (S1) and 225.61 ± 0.05 μg/mL (S2), as compared to the results reported previously. Here, marjoram EO (S2) showed slightly stronger antioxidant activity than that of sample (S1). However, the antioxidant activities of both EO samples were lower than that of the positive control, ascorbic acid (9.74 ± 0.07 μg/mL). The average percentage of free-radical scavenging activity of essential oils and the reference standard (ascorbic acid) by DPPH assay is shown in Figure 4a,b. According to a literature review, O. majorana EOs were found to exhibit stronger antioxidant activity in terms of smaller IC50 values, smaller than 200 μg/mL [14,21,63,73,74,75]. In other studies, antioxidant activity of marjoram EO was reported to be weaker, with IC50 values greater than 200 μg/mL [41,68,76,77]. Based on these previous reports, both our EO samples may be considered moderate antioxidant sources.
Figure 4.
Average percentage of free-radical scavenging activity by DPPH assay (n = 3). (a) Essential oils from majorana species (S1 and S2); (b) standard reference (ascorbic acid).
Our EO observed radical-scavenging activity could be entirely attributed to the higher activity of the major component terpinen-4-ol. Several other EO components have also exhibited DPPH radical-scavenging action, despite the fact that phenolic compounds are typically recognized as being the source of the strongest antioxidant activity. However, it is hard to attribute the radical scavenging activity to one or a few active volatile compounds of the total EO, because EO is a complex mixture of different volatile compounds. Generally, the antioxidant activity of the whole essential oil showed better radical scavenging capacity than the individual components, indicating the possible synergistic interaction between different components of essential oils [75].
2.6.2. Ferric-Reducing Antioxidant Power (FRAP)
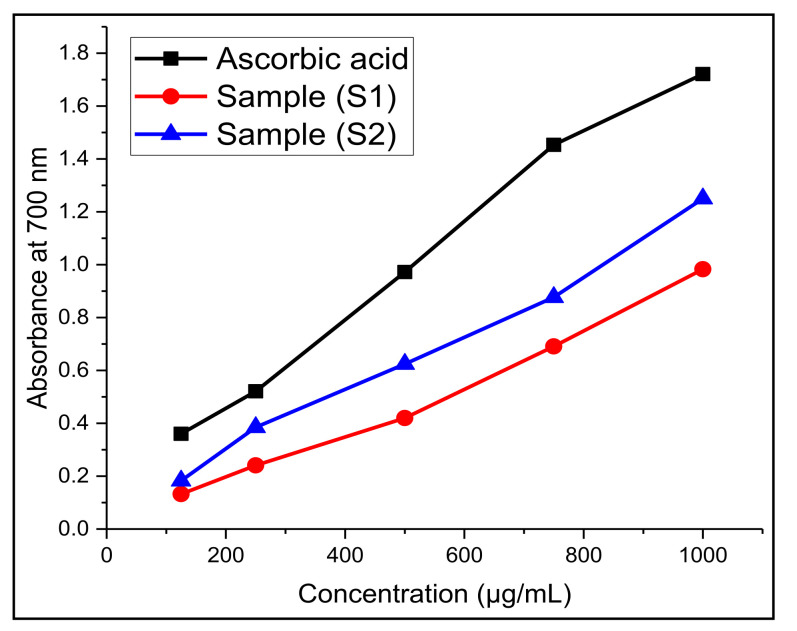
For further confirmation of the antioxidant activity of EO samples, we investigated the total reducing power of EOs using the FRAP assay. The results showed that there is an increase in absorbance at 700 nm with an increase in the concentration of EO samples. This is because the assay involves the transformation of Fe3+/ferricyanide complexes to the ferrous (Fe2+) form in the presence of reducers (i.e., antioxidants) in EO extracts. The results are expressed in terms of effective concentration (EC50), which are listed in Table 5 and Figure 5. In general, the lower the EC50 values, the higher the reducing ability of the EO extract to convert ferric to the ferrous ion form.
Figure 5.
Ferric-reducing antioxidant power of O. majorana EO (S1 and S2) and standard reference (ascorbic acid) (n = 3).
From the analysis, it was observed that the EO of sample (S2) has higher reducing ability, with an EC50 value of 372.72 ± 0.84 µg/mL, than that of sample (S1), with an EC50 value of 511.4 3 ± 0.61 µg/mL. On the other hand, both EO samples showed lower EC50 values as compared to the standard positive control, ascorbic acid, at 217.23 ± 0.34 µg/mL. However, the EO sample (S1) showed considerable reducing power. This reducing capacity could presumably be due to their hydrogen donating ability from the phenolic compounds [78]. In addition to this, the number and position of the hydroxyl groups of phenolic compounds also lead to their antioxidant activity [79].
3. Materials and Methods
3.1. Plant Material Collection
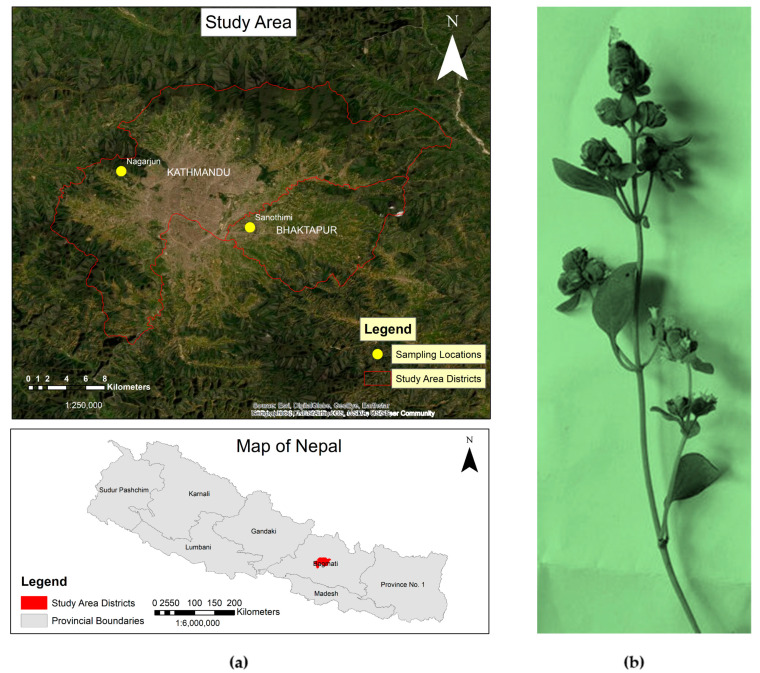
The fresh plant samples of O. majorana during the flowering stage were collected in May/June 2019, from the following two locations: Nagarjun, Kathmandu with latitude 27°43′58.857″ N and longitude 85°15′26.5572″ E at an elevation of 1537 m, (S1) and Sanothimi, Bhaktapur with latitude 27°40′48.8856″ N and longitude 85°22′42.3654″ E at an elevation of 1336 m (S2) in Nepal. Figure 6a,b show the geographical locations for plant sample collection and a photograph of the plant sample. The taxonomic identification of plant materials was confirmed by Ms. Rita Chhetri (senior research officer), at the National Herbarium and Plant Laboratories, Godawari, Lalitpur, Nepal.
Figure 6.
(a) The geographical location of majorana species collection sites; (b) a photograph of a twig of O. majorana L.
3.2. Extraction of Essential Oils
The shade-dried and chopped aerial parts of plant material (ca. 100 g) were submitted to hydro-distillation for 3 hours with 500 mL of distilled water, using a Clevenger-type apparatus (Jain Scientific Glass Works, JSGW, India) following standard protocol [80]. The obtained essential oil was dried over anhydrous sodium sulfate and, after filtration, stored at 4 °C until further testing and analysis. The yields were calculated based on the volume to weight ratio with the percentages 0.5% (S1) and 0.8% (S2) of EO samples of O. majorana.
3.3. Chemical Composition Analysis by Gas Chromatography-Mass Spectrometry
The essential oil of O. majorana was analyzed by GC-MS using a Shimadzu GC-MS-QP2010 Ultra apparatus (Shimadzu Scientific Instruments, Columbia, MD, USA) operated in the electron impact (EI) mode (electron energy = 70 eV), with a scan range of 40–400 amu, scan rate of 3.0 scans/s, and GC-MS solution software version 4.5 (Shimadzu Scientific Instruments, Columbia, MD, USA). The GC column was a ZB-5 MS fused silica capillary column with a (5%phenyl)–polymethyl siloxane stationary phase and a film thickness of 0.25 μm. The carrier gas was helium, with a column head pressure of 552 kPa and a flow rate of 1.37 mL/min. The injector temperature was 250 °C and the ion source temperature was 200 °C. The GC oven temperature program was programmed with 50 °C initial temperature and the temperature increased at a rate of 2 °C/min to 260 °C. A 5% w/v solution of the sample in CH2Cl2 was prepared and 0.1 μL was injected with a splitting mode (30:1) [81,82].
Identification of the oil components was based on their retention indices, determined by reference to a homologous series of n-alkanes (C8–C40), and by comparison of their mass spectral fragmentation patterns with those found in the MS databases using LabSolution GC-MS solution software and those reported in the literature [83]. The relative percentages of the individual components are listed in Table 1.
3.4. Chiral GC-MS analysis for Enantiomeric Components
Enantiomeric analysis for O. majorana oil was carried out by using a Shimadzu GC–MS–QP2010S (Shimadzu Scientific Instruments, Columbia, MD, USA) with EI mode (70 eV) and a B-Dex 325 chiral capillary GC column. It allows scanning at a rate of 3.0 scans/s in the range of 40–400 m/z. The column temperature was set at 50 °C, then increased by 1.5 °C/min until it reached 120 °C, and then by 2 °C/min until it reached 200 °C. The final temperature of the column was 200 °C and it was kept constant. Helium was used as the carrier gas, with a constant flow rate of 1.8 mL/min. A 3% w/v solution in CH2Cl2 was prepared for the essential oil sample, and 0.1 µL was injected at a split ratio of 1:45 [44,45,47]. The percentage composition of enantiomers was calculated from the peak area. The enantiomers were identified by comparing retention times and mass spectral fragmentation patterns with authentic samples acquired from Sigma-Aldrich (Milwaukee, WI, USA). The enantiomeric distribution of components for EO is presented in Table 3.
3.5. Hierarchical Cluster Analysis for Chemical Composition of EOs
A total of 50 O. majorana essential oil compositions from the different published literatures, besides the 2 EO samples (S1 and S2) from this study, were taken as the operational taxonomic units (OTUs). The percentage composition of major essential oil components (such as terpinen-4-ol, γ-terpinene, cis-sabinene hydrate, linalool, trans-sabinene hydrate, carvacrol, α-terpinene, α-terpineol, sabinene, p-cymene, linalyl acetate, β-caryophyllene, α-terpinolene, myecene, pulegone, α-pinene, p-menth-1-en-4-ol, thymol, cis-sabinene hydrate acetate, β-phellandrene, limone, cis-p-Menth-2-en-1-ol, bicyclogermacrene, camphene, α- phellandrene, 2-carene, β –pinene, estragole, α-thujene, caryophyllene oxide, trans-4 thujanol, trans-p-Menth-2-en-1-ol, and bornyl acetate) was used to determine the chemical relationship between the various O. majorana essential oil samples by agglomerative hierarchical cluster (AHC) analysis, using the IBM SPSS STATISTICS VERSION 8.5.5, IBM: Armonk, NY, USA.
3.6. Antimicrobial Activity
The in-vitro antimicrobial activities of essential oils were evaluated in terms of minimum inhibitory concentration (MIC) using the micro-broth dilution technique. The bacterial strains used were Bacillus cereus (ATCC 14579), Staphylococcus aureus (ATCC 29213), and Staphylococcus epidermidis (ATCC 14990). All bacteria were cultured on tryptic soy agar (Sigma-Aldrich, St. Louis, MO, USA). The solution of each essential oil was prepared at a concentration of 5000-µg/mL, using dimethyl sulfoxide (DMSO). Then, 50 µL of this solution was diluted with 50 μL of cation-adjusted Mueller Hinton broth (CAMHB) (Sigma-Aldrich, St. Louis, MO, USA), which was transferred to the top well of a 96-well micro-dilution plate. The solution of the EO sample thus prepared was serially two-fold diluted in fresh CAMHB, resulting in the final concentrations of 2500, 1250, 625, 312.5, 156.3, 78.1, 39.1 and 19.5 µg/mL. The bacterial strains were obtained from a fresh culture and added to each well of 96-well micro-dilution plates (~ 0.1 μL) at a concentration of approximately 1.5 × 108 CFUs/mL (determined using Mcfarland standard). Then, they were incubated at 37 °C for 24 hours. Gentamicin (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive antibiotic control, and DMSO was used as a negative control (50 µL DMSO diluted in 50 µL broth medium and then serially diluted as mentioned above). This experiment was carried out using the standard protocols [84,85,86].
The fungal strains were Aspergillus niger (ATCC 16888), Candida albicans (ATCC 18804), Trichophyton mentagrophytes (ATCC 18748), Aspergillus fumigatus (ATCC 96918), Cryptococcus neoformans (ATCC 32045), Microsporum canis (ATCC 11621), Microsporum gypseum (ATCC 24102), and Trichophyton rubrum (ATCC 28188). All fungi were cultured on yeast-nitrogen base growth medium (Sigma-Aldrich, St. Louis, MO, USA). All the stock solutions of EOs were prepared in accordance with the method discussed above. The freshly cultivated fungi strains, with approximately 7.5 × 107 CFUs/mL final concentrations, were added to each well of 96-well micro-dilution plates, which were then incubated at 35 °C for 24 hours. Here, DMSO was used as a negative control, and amphotericin B (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control [45,47]. The antibacterial and antifungal activities (MICs) of EOs are listed in Table 4. The microorganisms were purchased from ATCC (Lines 199–203), and cells were harvested from freshly cultured plates for the further assay.
3.7. Antioxidant Activity
3.7.1. DPPH Radical Scavenging Activity
The antioxidant activity of the EO was evaluated based on its ability to scavenge 2,2-diphenyl-1-picrylhydrazylstable free radicals (DPPH) (Sigma-Aldrich, St. Louis, MO, USA). The assay was carried out by using the colorimetric method with some modifications using the standard method [87]. Briefly, 2 mL of different concentrations of the EO were mixed with 2mL of DPPH solutions (100 µM). The mixture was then allowed to stand at room temperature in the dark for 30 minutes to complete the reaction and the absorbance was measured at 517 nm using a UV spectrophotometer (UV-1800 Shimadzu, Japan). Ascorbic acid was used as the reference standard and methanol (2mL each) was used as a negative control. The radical scavenging activity was calculated as a percentage of DPPH discoloration using the following equation:
| DPPH scavenging effect (%) = [(A0 − As)/A0] ∗ 100 | (1) |
A0 is the absorbance of the control reaction (containing all reagents without the test sample) and AS is the absorbance of the test sample. The antiradical activity was evaluated in terms of IC50 values (µg/mL), indicating the EO extract doses required to cause 50% inhibition. A lower IC50 value corresponds to higher antioxidant activity of the EO extract. A similar process was carried out for the reference standard, ascorbic acid. The experiments were conducted in triplicate. The IC50 values calculated for EOs and standards are listed in Table 5.
3.7.2. Ferric-Reducing Antioxidant Power (FRAP)
The ferric-reducing antioxidant power (FRAP) of EO samples was analyzed following the standard method [88]. All the chemicals used in this assay were purchased from the Fisher Scientific Co., Ltd., Bengalaru, India. First, 1 mL of each of the sample concentrations was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide, K3[Fe(CN)6]. The mixture was incubated at 50° C for 20 min. After incubation, 2.5 mL of 10% trichloroacetic acid (TCA) was added to the solution, which was then centrifuged for 10 min at 1000 rpm. The supernatant was collected, and 2.5 mL of the supernatant was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min, and the absorbance was measured at 700 nm on a UV–visible spectrophotometer, where higher absorbance indicates higher reducing power. The above assays were carried out in triplicate, and the results were expressed as mean values ± standard deviation. Finally, the mean of absorbance values was plotted against the concentration values. Increased absorbance of the reaction mixture indicated the increased reducing power. The results were expressed as effective concentrations (EC50 value in μg/mL) when the absorbance was 0.5 at 700 nm and compared with standard ascorbic acid, which was used as a positive control. The EC50 values calculated for EOs and standards are listed in Table 5.
3.8. Data Analysis
The data obtained from the overall experiment were processed and analyzed using various software, including Microsoft Excel OriginPro 2016 64Bit (Origin version 9.3, OriginLab Corporation, ORIGIN: Northampton, MA, USA) and IBM SPSS STATISTICS VERSION 8.5.5, IBM: Armonk, NY, USA, and GIS software (ArcGIS software, ESRI, Redlands, CA, USA), which was used to create a GIS map of the sampling sites. Finally, the antioxidant activity of EOs was expressed as the mean ± standard deviation (SD) of the three replications.
4. Conclusions
In summary, the chemical composition, biological activities, and enantiomeric distribution of O. majorana EOs from Nepal have been broadly determined for the first time. Our results demonstrated that EOs of O. majorana from two locations in Nepal were found to contain oxygenated monoterpenoids as their predominant bioactive constituents, in which terpinen-4-ol was the most dominant compound, with a higher percentage compared to previous results and with a slight variation in the major components. The O. majorana EO possessed moderate activity against the tested bacteria, while it showed prominent activity against the fungal strains. Likewise, the EO also showed relatively moderate antioxidant activity as compared to the studies carried out previously, although their radical scavenging activity was lower than the control. This study also reported the chiral compounds found in the EOs of marjoram, which are very useful for identifying the adulteration and authentication of samples. On the basis of the relative concentration of major components in marjoram EOs, this study showed at least five different chemotypes. Furthermore, the O. majorana EOs could be a promising natural ingredient in flavoring, perfumery, aromatherapy, and pharmaceuticals, due to their possession of biological properties with some synergistic and antagonistic effects that are associated with minor and/or major bioactive volatile compounds. Some in-vivo and clinical tests are needed to further justify the potency of marjoram EOs, along with the mode of action of the bioactive components present.
Acknowledgments
The authors are thankful to the Aromatic Plant Research Center, Lehi, UT, USA, for providing GC-MS and chiral GC-MS data and other financial support to publish this article. The authors would like to express their gratitude to the Department of Chemical Science and Engineering, and the Department of Pharmacy at Kathmandu University, Nepal, for the technical assistance. We would also like to thank Balram Pokhrel, Ajaya Acharya and Milan Phuyal for their support during the analysis.
Author Contributions
Conceptualization, R.G., P.N.P. and P.S.; methodology, P.N.P., R.G., P.S. and W.N.S.; validation, P.S.; formal analysis, P.N.P., R.G., P.S. and R.S.; investigation, P.N.P., P.S. and R.S.; data curation, R.G. and P.S.; writing—original draft preparation, P.N.P. and R.S.; writing—review and editing, P.N.P., R.G., P.S., W.N.S. and R.S.; supervision, R.G., P.S. and W.N.S.; antimicrobial activities, P.N.P. and P.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. World Health Organization; Geneva, Switzerland: 2000. pp. 1–73. [Google Scholar]
- 2.Hammer K.A., Carson C.F., Riley T.V. Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 3.Balunas M.J., Kinghorn A.D. Drug Discovery from Medicinal Plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Wayland C. The Failure of Pharmaceuticals and the Power of Plants: Medicinal Discourse as a Critique of Modernity in the Amazon. Soc. Sci. Med. 2004;58:2409–2419. doi: 10.1016/j.socscimed.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Gyawali R., Bhandari J., Amatya S., Piya E., Pradhan U.L., Paudyal R., Shrestha R., Shrestha T.M. Antibacterial and Cytotoxic Activities of High Altitude Essential Oils from Nepalese Himalaya. J. Med. Plants Res. 2013;7:738–743. doi: 10.5897/JMPR11.1713. [DOI] [Google Scholar]
- 6.Baser K.H.C., Buchbauer G. Handbook of Essential Oils: Science, Technology, and Application. CRC Press; Boca Raton, FL, USA: London, UK: New York, NY, USA: 2010. [Google Scholar]
- 7.Lubbe A., Verpoorte R. Cultivation of Medicinal and Aromatic Plants for Specialty Industrial Materials. Ind. Crops Prod. 2011;34:785–801. doi: 10.1016/j.indcrop.2011.01.019. [DOI] [Google Scholar]
- 8.Bhavaniramya S., Vishnupriya S., Al-Aboody M.S., Vijayakumar R., Baskaran D. Role of Essential Oils in Food Safety: Antimicrobial and Antioxidant Applications. Grain Oil Sci. Technol. 2019;2:49–55. doi: 10.1016/j.gaost.2019.03.001. [DOI] [Google Scholar]
- 9.Benali T., Habbadi K., Khabbach A., Marmouzi I., Zengin G., Bouyahya A., Chamkhi I., Chtibi H., Aanniz T., Achbani E.H., et al. GC-MS Analysis, Antioxidant and Antimicrobial Activities of Achillea odorata subsp. pectinata and Ruta montana Essential Oils and Their Potential Use as Food Preservatives. Foods. 2020;9:668. doi: 10.3390/foods9050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burt S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prerna P., Vasudeva N. Origanum majorana, L. Phyto-Pharmacological Review. Indian J. Nat. Prod. Resour. 2015;6:261–267. [Google Scholar]
- 13.Ietswaart J.H. A Taxonomic Revision of the Genus Origanum (Labiatae) Leiden University Press; Hague, The Netherlands: Borton, UK: 1980. [Google Scholar]
- 14.Hajlaoui H., Mighri H., Aouni M., Gharsallah N., Kadri A. Chemical Composition and in-vitro Evaluation of Antioxidant, Antimicrobial, Cytotoxicity and Anti-Acetylcholinesterase Properties of Tunisian Origanum majorana, L. Essential Oil. Microb. Pathog. 2016;95:86–94. doi: 10.1016/j.micpath.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Amor G., Caputo L., Storia A., De La Feo V., Mauriello G., Fechtali T. Artemisia Herba-Alba and Origanum majorana Essential Oils from Morocco. Molecules. 2019;24:4021. doi: 10.3390/molecules24224021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della Pepa T., Elshafie H.S., Capasso R., De Feo V., Camele I., Nazzaro F., Scognamiglio M.R., Caputo L. Antimicrobial and Phytotoxic Activity of Origanum heracleoticum and O. majorana Essential Oils Growing in Cilento (Southern Italy) Molecules. 2019;24:2576. doi: 10.3390/molecules24142576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thanh V.M., Bui L.M., Bach L.G., Nguyen N.T., Thi H. Le Origanum majorana L. Essential Oil-Associated Polymeric Nano Dendrimer for Antifungal Activity against Phytophthora infestans. Materials. 2019;12:1446. doi: 10.3390/ma12091446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vági E., Simándi B., Suhajda Á., Héthelyi É. Essential Oil Composition and Antimicrobial Activity of Origanum majorana L. Extracts Obtained with Ethyl Alcohol and Supercritical Carbon Dioxide. Food Res. Int. 2005;38:51–57. doi: 10.1016/j.foodres.2004.07.006. [DOI] [Google Scholar]
- 19.Charles D.J. Antioxidant Properties of Spices, Herbs and Other Sources. Springer; London, UK: 2013. [Google Scholar]
- 20.Erenler R., Sen O., Aksit H., Demirtas I., Yaglioglu A.S., Elmastas M., Telci I. Isolation and Identification of Chemical Constituents from Origanum majorana and Investigation of Antiproliferative and Antioxidant Activities. J. Sci. Food Agric. 2016;96:822–836. doi: 10.1002/jsfa.7155. [DOI] [PubMed] [Google Scholar]
- 21.do Chaves R.S.B., Martins R.L., Rodrigues A.B.L., de Menezes Rabelo É., Farias A.L.F., da Conceicao Vieira Araújo C.M., Sobral T.F., Galardo A.K.R., da Silva de Almeida S.S.M. Larvicidal Evaluation of the Origanum majorana L. Essential Oil against the Larvae of the Aedes aegypti Mosquito. Cold Spring Harbor. 2019 doi: 10.1101/595900. [DOI] [Google Scholar]
- 22.Pimple B.P., Kadam P.V., Patil M.J. Ulcer Healing Properties of Different Extracts of Origanum majorana in Streptozotocin-Nicotinamide Induced Diabetic Rats. Asian Pac. J. Trop. Dis. 2012;2:312–318. doi: 10.1016/S2222-1808(12)60068-1. [DOI] [Google Scholar]
- 23.Soliman A.M., Desouky S., Marzouk M., Sayed A.A. Origanum majorana Attenuates Nephrotoxicity of Cisplatin Anticancer Drug through Ameliorating Oxidative Stress. Nutrients. 2016;8:264. doi: 10.3390/nu8050264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seoudi D.M., Medhat A.M., Hewedi I.H., Osman S.A., Mohamed M.K., Arbid M. Evaluation of the Anti-Inflammatory, Analgesic, and Anti-Pyretic Effects of Origanum majorana Ethanolic Extract in Experimental Animals. J. Radiat. Res. Appl. Sci. 2009;2:513–534. [Google Scholar]
- 25.Silva E.M., Souza J.N.S., Rogez H., Rees J.F., Larondelle Y. Antioxidant Activities and Polyphenolic Contents of Fifteen Selected Plant Species from the Amazonian Region. Food Chem. 2007;101:1012–1018. doi: 10.1016/j.foodchem.2006.02.055. [DOI] [Google Scholar]
- 26.Tran T.H., Cam Quyen N.T., Kieu Linh H.T., Le Ngoc T.T., Quan P.M., Toan T.Q. Essential Oil from Vietnamese Mandarin (Citrus reticulata Blanco) Using Hydro-distillation Extraction Process and Identification of It’s Components. Solid State Phenom. 2019;298:100–105. doi: 10.4028/www.scientific.net/SSP.298.100. [DOI] [Google Scholar]
- 27.Abbasi-Maleki S., Kadkhoda Z., Taghizad-Farid R. The Antidepressant-like Effects of Origanum majorana Essential Oil on Mice through Monoaminergic Modulation Using the Forced Swimming Test. J. Tradit. Complement. Med. 2020;10:327–335. doi: 10.1016/j.jtcme.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahmasebi S., Majd A., Mehrafarin A., Jonoubi P. Comparative Ontogenetic Survey of the Essential Oil Composition in Origanum vulgare L., and Origanum majorana L. Acta Biol. Szeged. 2016;60:105–111. [Google Scholar]
- 29.Raina A.P., Negi K.S. Essential Oil Composition of Origanum majorana and Origanum vulgare ssp. Hirtum Growing in India. Chem. Nat. Compd. 2012;47:1015–1017. doi: 10.1007/s10600-012-0133-4. [DOI] [Google Scholar]
- 30.Jelali N., Dhifi W., Chahed T., Marzouk B. Salinity Effects on Growth, Essential Oil Yield and Composition and Phenolic Compounds Content of Marjoram (Origanum majorana L.) Leaves. J. Food Biochem. 2011;35:1443–1450. doi: 10.1111/j.1745-4514.2010.00465.x. [DOI] [Google Scholar]
- 31.Jiang Z.T., Li R., Wang Y., Chen S.H., Guan W.Q. Volatile Oil Composition of Natural Spice, Origanum majorana L. Grown in China. J. Essent. Oil-Bear. Plants. 2011;14:458–462. doi: 10.1080/0972060X.2011.10643601. [DOI] [Google Scholar]
- 32.Busatta C., Vidal R.S., Popiolski A.S., Mossi A.J., Dariva C., Rodrigues M.R.A., Corazza F.C., Corazza M.L., Vladimir Oliveira J., Cansian R.L. Application of Origanum majorana L. Essential Oil as an Antimicrobial Agent in Sausage. Food Microbiol. 2008;25:207–211. doi: 10.1016/j.fm.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Shrestha S., Nyaupane D.R., Yahara S., Rajbhandari M., Gewali M.B. Quality Assessment of the Essential Oils From Artemisia Gmelinii and Orifanum Majorana of Nepali Origin. Sci. World. 2013;11:77–80. doi: 10.3126/sw.v11i11.8557. [DOI] [Google Scholar]
- 34.Arnold N., Bellomaria B., Valentini G., Arnold H.J. Comparative Study of the Essential Oils from Three Species of Origanum Growing Wild in the Eastern Mediterranean Region. J. Essent. Oil Res. 1993;5:71–77. doi: 10.1080/10412905.1993.9698172. [DOI] [Google Scholar]
- 35.Calín-Sánchez Á., Figiel A., Lech K., Szumny A., Martínez-Tomé J., Carbonell-Barrachina Á.A. Dying Methods Affect the Aroma of Origanum majorana L. Analyzed by GC-MS and Descriptive Sensory Analysis. Ind. Crops Prod. 2015;74:218–227. doi: 10.1016/j.indcrop.2015.04.067. [DOI] [Google Scholar]
- 36.Tabanca N., Özek T., Baser K.H.C., Tümen G. Comparison of the Essential Oils of Origanum majorana L. and Origanum x majoricum Cambess. J. Essent. Oil Res. 2004;16:248–252. doi: 10.1080/10412905.2004.9698713. [DOI] [Google Scholar]
- 37.Banchio E., Bogino P.C., Zygadlo J., Giordano W. Plant Growth Promoting Rhizobacteria Improve Growth and Essential Oil Yield in Origanum majorana L. Biochem. Syst. Ecol. 2008;36:766–771. doi: 10.1016/j.bse.2008.08.006. [DOI] [Google Scholar]
- 38.Baser K.H.C., Kirimer N., Tümen G. Composition of the Essential Oil of Origanum majorana L. from Turkey. J. Essent. Oil Res. 1993;5:577–579. doi: 10.1080/10412905.1993.9698283. [DOI] [Google Scholar]
- 39.Sellami I.H., Maamouri E., Chahed T., Wannes W.A., Kchouk M.E., Marzouk B. Effect of Growth Stage on the Content and Composition of the Essential Oil and Phenolic Fraction of Sweet Marjoram (Origanum majorana L.) Ind. Crops Prod. 2009;30:395–402. doi: 10.1016/j.indcrop.2009.07.010. [DOI] [Google Scholar]
- 40.Vera R.R., Chane-Ming J. Chemical Composition of the Essential Oil of Marjoram (Origanum majorana L.) from Reunion Island. Food Chem. 1999;66:143–145. doi: 10.1016/S0308-8146(98)00018-1. [DOI] [Google Scholar]
- 41.Schmidt E., Bail S., Buchbauer G., Stoilova I., Krastanov A., Stoyanova A., Jirovetz L. Chemical Composition, Olfactory Evaluation and Antioxidant Effects of the Essential Oil of Origanum majorana L. from Albania. Nat. Prod. Commun. 2008;3:1051–1056. doi: 10.1177/1934578X0800300704. [DOI] [Google Scholar]
- 42.Komaitis M.E., Ifanti-Papatragianni N., Melissari-Panagiotou E. Composition of the Essential Oil of Marjoram (Origanum majorana L.) Food Chem. 1992;45:117–118. doi: 10.1016/0308-8146(92)90020-3. [DOI] [Google Scholar]
- 43.Barazandeh M.M. Essential Oil Composition of Origanum majorana L. from Iran. J. Essent. Oil Res. 2001;13:76–77. doi: 10.1080/10412905.2001.9699616. [DOI] [Google Scholar]
- 44.Dantas A.D.S., Klein-Júnior L.C., Machado M.S., Guecheva T.N., Santos L.D.D., Zanette R.A., Mello F.B.D., Pêgas Henriques J.A., De Mello J.R.B. Origanum majorana Essential Oil Lacks Mutagenic Activity in the Salmonella/Microsome and Micronucleus Assays. Sci. World J. 2016;2016:1–8. doi: 10.1155/2016/3694901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos S., Rojas L.B., Lucena M.E., Meccia G., Usubillaga A. Chemical Composition and Antibacterial Activity of Origanum majorana L. Essential Oil from the Venezuelan Andes. J. Essent. Oil Res. 2011;23:45–49. doi: 10.1080/10412905.2011.9700481. [DOI] [Google Scholar]
- 46.Ravid U., Bassat M., Putievsky E., Ikan R., Weinstein V. Determination of the Enantiomeric Composition of (+)-terpinen- 4-ol from Sweet Marjoram Origanum majorana L. Using a Chiral Lanthanide Shift Reagent. Flavour Fragr. J. 1987;2:17–19. doi: 10.1002/ffj.2730020104. [DOI] [Google Scholar]
- 47.Casabianca H., Graff J.B. Chiral Analysis of Linalool and Linalyl Acetate in Various Plants. Riv. Ital. EPPOS Numero Spec. 1996:227–243. [Google Scholar]
- 48.El-Akhal F., Abdelhakim E.O.L., Ez Zoubi Y.E., Greche H., Guemmouh R. Chemical Composition and Larvicidal Activity of Essential Oil of Origanum majorana (Lamiaceae) Cultivated in Morocco against Culex pipiens (Diptera: Culicidae) Asian Pac. J. Trop. Biomed. 2014;4:746–750. doi: 10.12980/APJTB.4.2014APJTB-2014-0392. [DOI] [Google Scholar]
- 49.Ben Hamida-Ben Ezzeddine N., Abdelkéfi M.M., Ben Aissa R., Chaabouni M.M. Antibacterial Screening of Origanum majorana L. Oil from Tunisia. J. Essent. Oil Res. 2001;13:295–297. doi: 10.1080/10412905.2001.9699698. [DOI] [Google Scholar]
- 50.Waller S.B., Cleff M.B., de Mattos C.B., da Silva C.C., Giordani C., Dalla Lana D.F., Fuentefria A.M., Freitag R.A., Viegas Sallis E.S., de Mello J.R.B., et al. In Vivo Protection of the Marjoram (Origanum majorana Linn.) Essential Oil in the Cutaneous Sporotrichosis by Sporothrix Brasiliensis. Nat. Prod. Res. 2021;35:2977–2981. doi: 10.1080/14786419.2019.1678617. [DOI] [PubMed] [Google Scholar]
- 51.Verma R.S., Padalia R.C., Chauhan A., Verma R.K., ur Rahman L., Singh A. Changes in the Essential Oil Composition of Origanum majorana L. During Post Harvest Drying. J. Essent. Oil-Bear. Plants. 2016;19:1547–1552. doi: 10.1080/0972060X.2014.935039. [DOI] [Google Scholar]
- 52.Novak J., Pank F., Langbehn J., Blüthner W.D., Vender C., Van Niekerk L., Junghanns W., Franz C. Determination of Growing Location of Marjoram (Origanum majorana L.) Samples by Comparison of Essential Oil Profiles. Flavour Fragr. J. 2004;19:263–267. doi: 10.1002/ffj.1303. [DOI] [Google Scholar]
- 53.Baatour O., Kaddour R., Wannes W.A., Lachaâl M., Marzouk B. Salt Effects on the Growth, Mineral Nutrition, Essential Oil Yield and Composition of Marjoram (Origanum majorana) Acta Physiol. Plant. 2010;32:45–51. doi: 10.1007/s11738-009-0374-4. [DOI] [Google Scholar]
- 54.Alizadeh A., Khosh-Khui M., Javidnia K., Firuzi O., Jokar S.M. Chemical Composition of the Essential Oil, Total Phenolic Content and Antioxidant Activity in Origanum majorana L. (Lamiaceae) Cultivated in Iran. Adv. Environ. Biol. 2011;5:2326–2331. [Google Scholar]
- 55.Nurzyñska-Wierdak R., Zawislak G., Kowalski R. The Content and Composition of Essential Oil of Origanum majorana L. Grown in Poland Depending on Harvest Tme and Method of Raw Material Preparation. J. Essent. Oil-Bear. Plants. 2015;18:1482–1489. doi: 10.1080/0972060X.2013.831569. [DOI] [Google Scholar]
- 56.Dambolena J.S., Zunino M.P., Lucini E.I., Olmedo R., Banchio E., Bima P.J., Zygadlo J.A. Total Phenolic Content, Radical Scavenging Properties, and Essential Oil Composition of Origanum Species from Different Populations. J. Agric. Food Chem. 2010;58:1115–1120. doi: 10.1021/jf903203n. [DOI] [PubMed] [Google Scholar]
- 57.Baj T., Baryluk A., Sieniawska E. Application of Mixture Design for Optimum Antioxidant Activity of Mixtures of Essential Oils from Ocimum basilicum L., Origanum majorana L. and Rosmarinus officinalis L. Ind. Crops Prod. 2018;115:52–61. doi: 10.1016/j.indcrop.2018.02.006. [DOI] [Google Scholar]
- 58.Al-Fatimi M. Volatile Constituents, Antimicrobial and Antioxidant Activities of the Aerial Parts of Origanum majorana L. from Yemen. J. Pharm. Res. Int. 2018;23:1–10. doi: 10.9734/JPRI/2018/35932. [DOI] [Google Scholar]
- 59.Novak J., Lukas B., Franz C.M. The Essential Oil Composition of Wild Growing Sweet Marjoram (Origanum majorana L., Lamiaceae) from Cyprus—Three Chemotypes. J. Essent. Oil Res. 2008;20:339–341. doi: 10.1080/10412905.2008.9700026. [DOI] [Google Scholar]
- 60.Goel P., Vasudeva N. Comparative Study of Volatile Oil of Stem and Aerial Parts of Origanum majorana Linn. J. Essent. Oil-Bear. Plants. 2016;19:2091–2099. doi: 10.1080/0972060X.2016.1264276. [DOI] [Google Scholar]
- 61.Jan S., Mir J.I., Shafi W., Faktoo S.Z., Singh D.B., Wijaya L., Alyemeni M.N., Ahmad P. Divergence in Tissue-Specific Expression Patterns of Genes Associated with the Terpeniod Biosynthesis in Two Oregano Species Origanum vulgare L., and Origanum majorana. Ind. Crops Prod. 2018;123:546–555. doi: 10.1016/j.indcrop.2018.07.006. [DOI] [Google Scholar]
- 62.Abidi A., Sebai E., Dhibi M., Darghouth M.A., Akkari H. Chemical Analyses and Evaluation of the Anthelmintic Effects of Origanum majorana Essential Oil, in-vitro and in-vivo Studies. Vet. Med. (Praha). 2020;65:495–505. doi: 10.17221/115/2019-VETMED. [DOI] [Google Scholar]
- 63.Erdogan A., Ozkan A. Investigatıon of Antioxıdative, Cytotoxic, Membrane-Damaging and Membrane-Protective Effects of the Essentıal Oil of Origanum majorana and Its Oxygenated Monoterpene Component Linalool in Human-Derived Hep G2 Cell Line. Iran. J. Pharm. Res. 2017;16:24–34. [PMC free article] [PubMed] [Google Scholar]
- 64.de Marques J.L., Volcão L.M., Funck G.D., Kroning I.S., da Silva W.P., Fiorentini Â.M., Ribeiro G.A. Antimicrobial Activity of Essential Oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus Isolated from Poultry Meat. Ind. Crops Prod. 2015;77:444–450. doi: 10.1016/j.indcrop.2015.09.013. [DOI] [Google Scholar]
- 65.Selim S., Abdel M., Abdel H., Nuclear E., Authority R.R., Warrad M.F. Antibacterial Activities, Chemical Constitutes and Acute Toxicity of Egyptian Origanum majorana L., Peganum harmala, L. and Salvia officinalis, L. Essential Oils. African J. Pharm. Pharmacol. 2013;7:725–735. doi: 10.5897/AJPP2013.3518. [DOI] [Google Scholar]
- 66.de Oliveira J.L.T.M., de Fátima Melo Diniz M., de Oliveira Lima E., de Souza E.L., Trajano V.N., Santos B.H.C. Effectiveness of Origanum vulgare L. and Origanum majorana L. Essential Oils in Inhibiting the Growth of Bacterial Strains Isolated from the Patients with Conjunctivitis. Brazilian Arch. Biol. Technol. 2009;52:45–50. doi: 10.1590/S1516-89132009000100006. [DOI] [Google Scholar]
- 67.Deans S.G., Svoboda K.P. The Antimicrobial Properties of Marjoram (Origanum majorana L.) Volatile Oil. Flavour Fragr. J. 1990;5:187–190. doi: 10.1002/ffj.2730050311. [DOI] [Google Scholar]
- 68.Ben Salha G., Herrera Díaz R., Labidi J., Abderrabba M. Deterpenation of Origanum majorana L. Essential Oil by Reduced Pressure Steam Distillation. Ind. Crops Prod. 2017;109:116–122. doi: 10.1016/j.indcrop.2017.08.016. [DOI] [Google Scholar]
- 69.Tabanca N., Kirimer N., Demirci B., Demirci F., Can Başer K.H. Composition and Antimicrobial Activity of the Essential Oils of Micromeria cristata Subsp. Phrygia and the Enantiomeric Distribution of Borneol. J. Agric. Food Chem. 2001;49:4300–4303. doi: 10.1021/jf0105034. [DOI] [PubMed] [Google Scholar]
- 70.Cimanga K., Apers S., De Bruyne T., Van Miert S., Hermans N., Totté J., Pieters L., Vlietinck A.J., Kambu K., Tona L. Correlation between Chemical Composition and Antifungal Activity of Essential Oils of Some Aromatic Medicinal Plants Growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002;79:213–220. doi: 10.1016/S0378-8741(01)00384-1. [DOI] [PubMed] [Google Scholar]
- 71.Soares J.R., Dinis T.C.P., Cunha A.P., Almeida L.M. Antioxidant Activities of Some Extracts of Thymus zygis. Free Radic. Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- 72.Szabo M.R., Idiţoiu C., Chambre D., Lupea A.X. Improved DPPH Determination for Antioxidant Activity Spectrophotometric Assay. Chem. Pap. 2007;61:214–216. doi: 10.2478/s11696-007-0022-7. [DOI] [Google Scholar]
- 73.Olfa B., Mariem A., Salah A.M., Mouhiba B.N.A. Chemical Content, Antibacterial and Antioxidant Properties of Essential Oil Extract from Tunisian Origanum majorana L. Cultivated under Saline Condition. Pak. J. Pharm. Sci. 2016;29:1951–1958. [PubMed] [Google Scholar]
- 74.Mossa A.T.H., Nawwar G.A.M. Free Radical Scavenging and Antiacetylcholinesterase Activities of Origanum majorana L. Essential Oil. Hum. Exp. Toxicol. 2011;30:1501–1513. doi: 10.1177/0960327110391686. [DOI] [PubMed] [Google Scholar]
- 75.Hussain A.I., Anwar F., Rasheed S., Nigam P.S., Janneh O., Sarker S.D. Composition, Antioxidant and Chemotherapeutic Properties of the Essential Oils from Two Origanum Species Growing in Pakistan. Rev. Bras. Farmacogn. 2011;21:943–952. doi: 10.1590/S0102-695X2011005000165. [DOI] [Google Scholar]
- 76.Guerra-Boone L., Alvarez-Román R., Salazar-Aranda R., Torres-Cirio A., Rivas-Galindo V.M., de-Torres N.W., González G., Pérez-López L.A. Antimicrobial and Antioxidant Activities and Chemical Characterization of Essential Oils of Thymus vulgaris, Rosmarinus officinalis, and Origanum majorana from Northeastern México. Pak. J. Pharm. Sci. 2015;28:363–369. [PubMed] [Google Scholar]
- 77.Khadhri A., Bouali I., Aouadhi C., Lagel M.C., Masson E., Pizzi A. Determination of Phenolic Compounds by MALDI–TOF and Essential Oil Composition by GC–MS during Three Development Stages of Origanum majorana L. Biomed. Chromatogr. 2019;33:e4665. doi: 10.1002/bmc.4665. [DOI] [PubMed] [Google Scholar]
- 78.Ruberto G., Baratta M.T. Antioxidant Activity of Selected Essential Oil Components in Two Lipid Model Systems. Food Chem. 2000;69:167–174. doi: 10.1016/S0308-8146(99)00247-2. [DOI] [Google Scholar]
- 79.Lee K.G., Shibamoto T. Antioxidant Activities of Volatile Components Isolated from Eucalyptus Species. J. Sci. Food Agric. 2001;81:1573–1579. doi: 10.1002/jsfa.980. [DOI] [Google Scholar]
- 80.Chu S.S., Jiang G.H., Liu Z.L. Insecticidal Compounds from the Essential Oil of Chinese Medicinal Herb Atractylodes chinensis. Pest Manag. Sci. 2011;67:1253–1257. doi: 10.1002/ps.2180. [DOI] [PubMed] [Google Scholar]
- 81.Satyal P., Jones T.H., Lopez E.M., McFeeters R.L., Ali N.A.A., Mansi I., Al-Kaf A.G., Setzer W.N. Chemotypic Characterization and Biological Activity of Rosmarinus officinalis. Foods. 2017;6:20. doi: 10.3390/foods6030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poudel D.K., Rokaya A., Ojha P.K., Timsina S., Satyal R., Dosoky N.S., Satyal P., Setzer W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum camphora L. and Their Antimicrobial Activities. Molecules. 2021;26:5132. doi: 10.3390/molecules26175132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 84.Decarlo A., Zeng T., Dosoky N.S., Satyal P., Setzer W.N. The Essential Oil Composition and Antimicrobial Activity of Liquidambar formosana Oleoresin. Plants. 2020;9:822. doi: 10.3390/plants9070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahm D.F. Antibacterial Susceptibility Tests: Dilution Methods. In: Wersalovic J., Carroll K.C., Funke G., Jorgensen J.H., Landry M.L., Warnock D.W., editors. Manual of Clinical Microbiology. ASM Press; Washington, DC, USA: 1995. [Google Scholar]
- 86.Rodríguez-Tudela J.L., Barchiesi F., Bille J., Chryssanthou E., Cuenca-Estrella M., Denning D., Donnelly J.P., Dupont B., Fegeler W., Moore C., et al. Method for the Determination of Minimum Inhibitory Concentration (MIC) by Broth Dilution of Fermentative Yeasts. Clin. Microbiol. Infect. 2003;9:i–viii. doi: 10.1046/j.1469-0691.2003.00789.x. [DOI] [Google Scholar]
- 87.Kim M.K., Lee H.S., Kim E.J., Won N.H., Chi Y.M., Kim B.C., Lee K.W. Protective Effect of Aqueous Extract of Perilla frutescens on Tert-Butyl Hydroperoxide-Induced Oxidative Hepatotoxicity in Rats. Food Chem. Toxicol. 2007;45:1738–1744. doi: 10.1016/j.fct.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Park Y.S., Jung S.T., Kang S.G., Heo B.G., Arancibia-Avila P., Toledo F., Drzewiecki J., Namiesnik J., Gorinstein S. Antioxidants and Proteins in Ethylene-Treated Kiwifruits. Food Chem. 2008;107:640–648. doi: 10.1016/j.foodchem.2007.08.070. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the manuscript.