Highlights
-
•
The AIDS Drug Assistance Program (ADAP) is a $2.4 billion a year program.
-
•
Little formal economic analysis of the ADAP in the literature.
-
•
The few economic analyses of ADAP use 2008 or older data.
-
•
ADAP programs’ healthcare delivery have changed substantially since 2008.
-
•
Updated person-centered cost effectiveness models assessing ADAP are needed.
Keywords: HIV, AIDS Drug Assistance Program, Medication Access, Health Policy, Economic Evaluation, Cost Effectiveness
Abstract
As part of the Ryan White HIV/AIDs Program, the federally-funded, state-administered AIDS Drug Assistance Program (ADAP) provides prescription drug medications, including antiretroviral therapy, for people with HIV (PWH) who are uninsured/underinsured and have a low income. ADAP expenditures are ∼$2.4 billion annually, but there is a dearth of formal economic analysis supporting the societal perspective. We conducted a systematic review of economic analyses of the United States’ AIDS Drug Assistance Program to establish future research priorities based on gaps in knowledge. We searched six electronic databases for articles published before January 2022 that met inclusion criteria. We used the 2022 Consolidated Health Economic Evaluation Reporting Standards to assess the quality of reporting of the economic evaluations. We extracted data into categories to assess gaps and needs for future economic evaluation. Seven studies met inclusion criteria. Two used the same modeling approaches but were published with slightly different outcomes. The few economic analyses that focused solely on ADAP were conducted using 2008 or older data. The most recent study modeled the net cost per quality-adjusted life-year (QALY) secondary to reducing new HIV cases among those virally suppressed, but did not include the economic or health benefits for PWH. ADAP programs’ delivery of antiretroviral therapy has shifted from primarily direct provision to subsidizing insurance plans. None of the models take these shifts into account. Updated person-centered cost effectiveness models assessing ADAP are needed on a national and state-by-state level to guide policy decisions and coverage determinations.
1. Introduction
As part of the United States’ Ryan White HIV/AIDS Program, the AIDS Drug Assistance Program (ADAP) is a large federally-funded, state-administered program that provides prescription drug medications, including antiretroviral therapy (ART), for people with HIV (PWH) who lack adequate coverage from Medicaid or other forms of health insurance. PWH are eligible for ADAP support if they are uninsured/underinsured and have an income below 200–500 % federal poverty level, depending on their state’s eligibility criteria (Kaiser Family Foundation, 2012). While ADAP is a safety net, it is an important aspect of HIV care in the United States. Depending on the individual state program policy, ADAPs can provide financial assistance for client’s health insurance premiums and cost sharing (including deductibles, copayments, and coinsurance) (HIV/AIDS, 2016). In 2020, ADAPs provided ART and other essential medicines for treating comorbidities (e.g. opportunistic infections, Hepatitis C, mental health conditions) that can impact HIV care management for almost one out of every-four PWH in the United States (NASTAD et al., 2022). Through the federal government’s Ending the HIV Epidemic (EHE) initiative, the U.S. Department of Health and Human Services (HHS) aims to end HIV in the United States by scaling up evidence-based HIV prevention and treatment strategies in order to reduce new infections by 90 % by 2030 (Fauci et al., 2019), however, funding decisions need to be made about how to best direct resources. Currently, no additional funds from the Ending the HIV Epidemic Initiative are allocated to ADAP. As expenditures from ADAP are approximately $2.4 billion per year (NASTAD et al., 2022), economic analyses assessing the societal perspective of the federal program are warranted. The objective of this study was to conduct a systematic review of economic analyses of the United States’ ADAPs to establish future research and policy priorities based on gaps in knowledge.
2. Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and meta-analyses guideline (Page et al., 2020). This work is not human subjects research. A comprehensive review was initiated including articles up to January 15, 2022 using six electronic databases (PubMed/Medline, Web of Science, Google Scholar, CINHAL, the CEA Tufts Database, and EconLit). We also manually reviewed reference lists of included articles. The key words for the search strategies were built using three key concepts: (1) “cost effectiveness”, “cost utility”, “cost”, “cost analysis”, “cost consequence”, “cost benefit”, “pharmacoeconomic”, “economic”, “economic analysis”, “economic assessment”, “economic evaluation”; (2) “HIV”, “AIDS”; (3) “ADAP”, “drug assistance”, “antiretroviral drug assistance”, “ART drug assistance”, “ARV drug assistance”, “antiretroviral prescription assistance”, “Ryan White program”. Key words were combined using Boolean operators to broaden our results and identify relevant articles. Importantly, this review of economic evaluations focused on the coverage/program of antiretroviral therapy, not the cost effectiveness of antiretroviral therapies themselves. There were no restrictions of the years of articles included.
Studies were included if they were: (1) economic evaluations, meaning there was either a model-derived cost utility or cost effectiveness analysis, or clinical trials that incorporated full economic evaluations, (2) they specifically evaluated programs that provide assistance in obtaining or full coverage of antiretroviral medications for people living with HIV, (3) for policy translation, they also needed to be modeled on the context of the United States, and (4) written in English. Articles were identified and then 2 independent reviewers screened titles, abstracts, and full texts to ensure eligibility (Fig. 1). The Consolidated Health Economic Evaluation Reporting Standards checklist 2022 (Husereau et al., 2022) was used to assess the quality of reporting of the economic evaluations. Data were extracted into categories to assess gaps and needs for future economic evaluation.
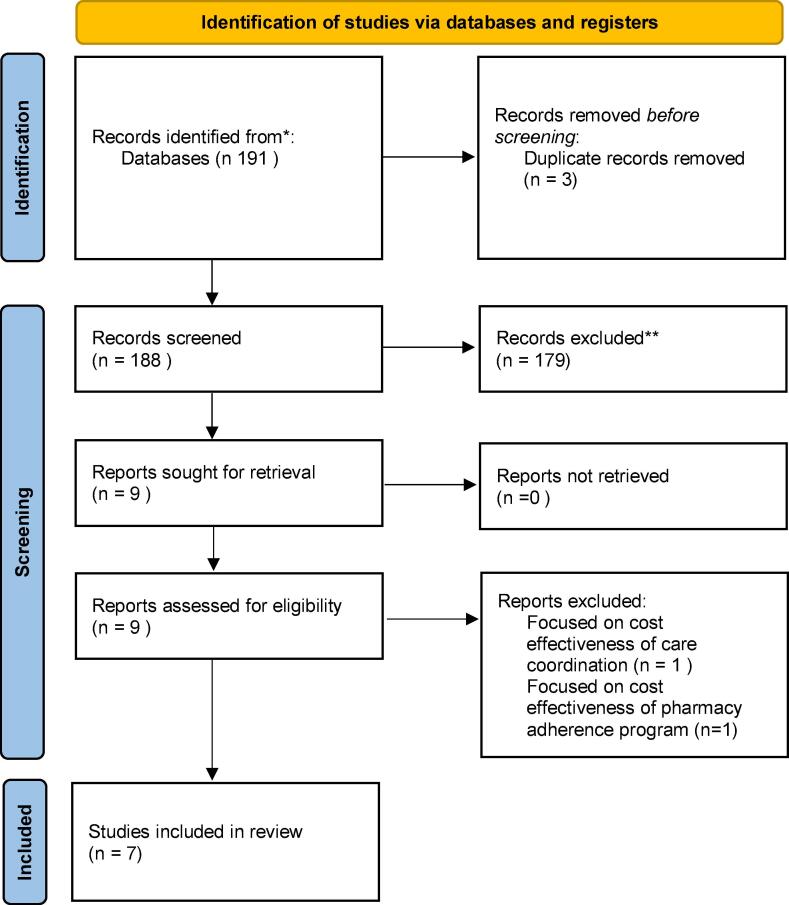
Fig. 1.
PRISMA flow diagram.
3. Results
After screening 188 articles following our identification, seven studies met our inclusion criteria (Table 1) (Goldman et al., 2001, Goyal et al., 2021, Goyal et al., 2021, Johri et al., 2002, Pinkerton et al., 2013, Schackman et al., 2001, Snider et al., 2016). Two of the studies used Markov or state transition models, two used agent-based stochastic models, two used econometrics methods (i.e. regression models to estimate effect size and extrapolate the results to the population of interest), and one study used differential equations to estimate the cases of HIV averted through viral suppression. The three economic analyses that focused solely on ADAP were conducted based on 2008 or older data for both costs and health outcomes, indicating that we are in dire need of economic analyses updated in terms of costs and health outcomes (Johri et al., 2002, Pinkerton et al., 2013, Schackman et al., 2001, Snider et al., 2016).
Table 1.
General characteristics of included studies.
| Author, year | Type of economic evaluation | Perspective | Sample | Comparison | Cost data | Model outcome | Horizon | Study funding Source |
|---|---|---|---|---|---|---|---|---|
| (Goldman et al., 2001) | Mutli-stage national probabilistic sample to assess state-level variation and simulation to estimate the effects across each state | Societal | Probabilstic sample of AIDS cases | Simulation based on Medicaid eligibility, ADAP eligibility, ADAP benefits | HCSUS sample and self-report data | Monthly expenditure on outpatient and inpatient care, ER visits, drug costs, full time labor force participation and earnings | Not specified | HRSA, NIH, AHRQ, RWJF |
| Goyal, Hu, et al., 2021 (Goyal et al., 2021)* | Agent-based stochastic model | US Health Care System | Overall HIV burden in US | Simulates the 5 types of Ryan White HIV/AIDS program | RWHAP and ADAP | HIV incidence for MSM, mortality rate, average life expectancy for low CD4, lifetime care costs | 50 years | HRSA |
| Goyal. Luca, et al., 2021 (Goyal et al., 2021)* | Agent-based stochastic model | US Health Care System | Overall HIV burden in US | Simulates the 5 types of Ryan White HIV/AIDs Program | RWHAP and ADAP | Proportion of people with HIV virally suppressed, deaths, cumulative health care costs, ICER, QALY | 50 years | HRSA |
| (Johri et al., 2002) | State transition model with Monte Carlo Simulations | State ADAPs | ADAP clients |
(1) “High efficacy”, (2) “Low efficacy” ADAP policy scenarios | AIDS Cost and Services Utilization Survey |
Projected life expectancy, cumulative healthcare costs, ICER, QALYs | Lifetime | CDC; NIAID; NIH; NIMH |
| (Pinkerton et al., 2013) | Transmission mathematical model | Societal | ADAP clients | ADAP clients | Kaiser Foundation ADAP Fact Sheet (2008 dollars) | Cost of reduction in new secondary cases each year for those who are ADAP clients (Net cost per QALY) |
Lifetime | NIH; RWJF |
| (Schackman et al., 2001) | Markov state-transition and microsimulation | Government/ payer and societal | People with HIV who present for medical care with CD4 counts of 500 | (1) Immediate ART, (2) ART initiated at CD4 count of 200 (3) no ART | AIDS Costs and Services Utilization Survey | Incidence of opportunistic infections, years of life, QALYs gained, lifetime costs | Lifetime | NIH |
| (Snider et al., 2016) | Ordinary least squares regression model and simulation |
State ADAPs | ADAP clients | ADAP Policies (income limit, medical requirements, enrollment cap, asset limits) | Literature Review (Staszewski et al., 1996, Freedberg et al., 2001) | ADAP clients served, survival benefits (QALYs and $), and cumulative healthcare costs | Lifetime | Bristol-Myers Squibb |
*Same modeling approach, reporting on different outcomes.
Abbreviations: ADAP – AIDS Drug Assistance Programs; RWHAP – Ryan White HIV-AIDS Program; QALY – Quality-adjusted life-year; ICER – Incremental cost-effectiveness ratio; CDC – Centers for Disease Control and Prevention; HRSA – Health Resources and Services Administration; NIH – National Institutes of Health; NIAID – National Institute of Allergy and Infectious Diseases; NIDA – National Institute on Drug Abuse, RWJF – Robert Wood Johnson Foundation; NIMH – National Institute on Mental Health. HCSUS - HIV Cost and Services Utilization Study.
Two of the studies (Goldman et al., 2001, Pinkerton et al., 2013) conducted the economic analysis from the societal perspective, which is considered to be the gold standard (Garrison et al., 2010, Sanders et al., 2016). Using 2008 data, Pinkerton and colleagues (Pinkerton et al., 2013) modeled the net cost per quality-adjusted life-year (QALY) secondary to reducing new HIV cases among those that are virally suppressed and found that ADAP prevented 3,191 secondary infections and saved 24,922 QALYs. Furthermore, they asserted ADAP as a cost-effective program based on the net cost per QALY saved at $11,955. Notably, this analysis did not include the economic or health benefits for PWH (Pinkerton et al., 2013). Goldman and colleagues (Goldman et al., 2001) used data from the 1998 HIV Cost and Services Utilization Study (Bozzette et al., 1998) to evaluate the effects of state Medicaid and ADAP policies on the direct and indirect costs of treating PWH. They found that if states expanded ADAP by improving ART access, there would be reduction in societal medical expenditures mainly due to the reduction in medical care expenses for comorbid conditions resulting from inadequate HIV treatment. Furthermore, they found that the expanded ADAP policies could result in indirect gains of employment and income for PWH.
Six of the studies (Goyal et al., 2021, Goyal et al., 2021, Johri et al., 2002, Pinkerton et al., 2013, Schackman et al., 2001, Snider et al., 2016) reported economic outcomes in terms of quality-adjusted life-years (QALY) and incremental cost-effectiveness analysis (ICER). While not solely focusing on ADAP, Goyal and colleagues (Goyal et al., 2021) model demonstrated that the Ryan White HIV AIDS Program (RWHAP) led to increased care engagement, viral suppression, QALYs, and reduction in deaths for PWH. Furthermore, they found that RWHAP led to an ICER of $29,573 per QALY gained, and included sensitivity analyses using the ICER estimates and found the program to be cost-effective in all scenarios. Compared to the previous study, Snider and colleagues (Snider et al., 2016) focused specifically on state-specific ADAP policies (e.g. income-based eligibility, enrollment caps, etc.) related to ART access and its impact on QALYs, healthcare spending, and population health. They simulated a hypothetical reduction in the ADAP income ceiling by 50 percentage points in each state, and found that while the policy could save $274 million in health care expenditures, the resulting 12,352 QALYs lost for the 4,626 ineligible PWH would result in a net societal loss of $962 million (Snider et al., 2016).
There were several gaps identified during our review. Currently, no studies expanded the indirect costs from the societal perspective to include caregiver burden and Goldman and colleagues (Goldman et al., 2001) were the only ones that included ability to remain employed in the workforce while Pinkerton and colleagues (Pinkerton et al., 2013) were the only group to include the costs per case averted while virally suppressed. None of the models included person-centered health adverse effects into the model (costs in terms of outpatient, emergency department, or hospital utilization), health consequences of opportunistic infections, or early death.
4. Discussion
In this study, we conducted a systematic review of economic analyses of United States’ ADAP in order to summarize what is currently understood and establish future research and policy priorities based on knowledge gaps. Through our review, we included seven studies that were screened from 188 initially identified from our key word search criteria. Of the seven, only two were assessed from a societal perspective. Although limited, the studies focusing on ADAP found the program to be cost-effective and policies limiting access were a significant net negative for society (Goldman et al., 2001, Snider et al., 2016). Furthermore, the most recent study conducted by Goyal and colleagues (Goyal et al., 2021) found the RWHAP as a whole was cost-effective and contributed to increases in care engagement, viral suppression, QALYs, and an ICER of $29,573 per QALY gained. Albeit limited, the economic literature supports the notion that ADAP is a cost-effective program for society and limiting access would be an economic and quality of life detriment for those who depend on its services. Important to consider, the majority of these analyses were conducted during an era where ADAP’s roles had not yet expanded to their current functionality and these limiting policy changes may have an even greater detriment now. Analyses with more recent data could provide more accurate estimates of the current impact of ADAP.
Through our review we found key limitations among the published economic models which would make any evaluations of the contemporary program incongruous. Before 2008, ADAP’s main method of providing medications was direct provision of prescription drugs, but following changes to the US health insurance landscape their roles expanded to include providing financial assistance for ADAP-subsidized insurance plans (HIV/AIDS, 2016). For example, 83 % of ADAP clients received direct provision of medications in 2008 (Kaiser Family Foundation et al., 2009), but by 2020, 60 % of ADAP clients received treatment through ADAP-subsidized insurance plans.(NASTAD et al., 2022) Studying the impact of incorporating this type of medication delivery is essential because the two types of ART coverage can result in differences in viral suppression rates (2015: ADAP-subsidized insurance plan: 86.0 %, ADAP-provision of ART only: 80.2 %) (McManus et al., 2020) and costs to a state health department (2015: ADAP-subsidized insurance plan: $5,399/client/year, ADAP-provision of ART only: $10,224/client/year) (McManus et al., 2016). Moreover, the analyses that addressed ADAP’s cost effectiveness assumed a uniform ADAP structure across jurisdictions. This is an oversimplification. While every state has an ADAP, they vary widely in their service delivery due to different state funding allocations and legislative requirements, eligibility policies, pharmacy structures, and insurance subsidization benefits. Extending and updating Goldman et al. (Goldman et al., 2001) by modeling the state level impact would help individual states assess the cost effectiveness of their particular ADAP structure and guide policy decisions and coverage determinations.
Using the Consolidated Health Economic Evaluation Reporting Standards checklist 2022 (Husereau et al., 2022), we identified specific gaps in the economic models and their reporting. It is essential that updated models take into account the fact the often transitional nature of care engagement, different types of health care coverage, and viral suppression. This requires person-level, longitudinal data that has been lacking in previous models. There is also a need for models that can estimate indirect costs from a societal perspective such as cost/cases averted while suppressed, employment engagement, and caregiver burden. Additionally, we should aim to incorporate health adverse effects (e.g. emergency care, hospitalization, etc.) that occur when PWH are not virally suppressed or unable to access essential medicines for opportunistic infections and comorbidities. Lastly, future studies should also incorporate an assessment of the impact of ADAP programs on secondary outcomes, such as improvements resulting in improved safety-net engagement (i.e. improved levels of housing security, improved behavioral-health engagement, and increased financial stability).
Updated models could help to determine the ADAP investments that are necessary to help achieve HIV-related public health goals, such as the US federal government’s Ending the HIV Epidemic’s goal of 90 % reduction in new HIV infections by 2030 (Fauci et al., 2019). A recent CDC modeling study demonstrated that helping PWH achieve viral suppression is one of the most cost-effective ways to reduce new HIV infections and end the HIV epidemic (Sansom et al., 2021). Using contemporary data to demonstrate that ADAPs are cost effective could lead to them being formally and independently incorporated into the Ending the HIV Epidemic Initiative strategy.
Our review has some limitations including that we restricted the search to six databases. This could have missed some potentially relevant studies or “grey literature”, particularly if they were presented at conferences but then not formally published. Nevertheless, this is an attempt to catalog the knowledge about the economic impact of ADAP.
Despite an annual $2.4 billion investment, there is a paucity of published economic analyses of the United States’ ADAPs that address their ever-expanding role in HIV care for PWH with low incomes. There is a need for more nuanced assessment that accounts for changes in healthcare delivery options, variability in program structures, and person-level data. Future research and policy priorities include updating economic evaluations of ADAP using current data and person-centered outcomes while accounting for different ways that ADAPs provide medication access. Updated economic models of the ADAP program are warranted on a national and state-by-state level to guide policy decisions and coverage determinations for our most vulnerable populations.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (Grant No K08AI136644 to K.A.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CRediT authorship contribution statement
Kathleen A. McManus: Conceptualization, Funding acquisition, Visualization, Project administration, Supervision, Formal analysis, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. Andrew Strumpf: Data curation, Investigation, Software, Visualization, Formal analysis, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. Amy Killelea: Writing – original draft, Writing – review & editing. Tim Horn: Writing – original draft, Writing – review & editing. Auntré Hamp: Writing – original draft, Writing – review & editing. Jessica Keim-Malpass: Conceptualization, Project administration, Supervision, Formal analysis, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Kathleen A. McManus reports stock ownership in Gilead Sciences, Inc. Tim Horn and Auntré Hamp report that NASTAD receives grant funding from Gilead Sciences Inc, ViiV Healthcare, and Janssen Pharmaceuticals for organizational support; all funding to the institution was separate from this project. Amy Killelea reports being a paid consultant for the University of Virginia related to this work; outside this work, she reports being a paid consultant for NASTAD and JSI, working on RWHAP Part B/ADAP technical assistance, and a paid consultant for the HRSA HIV/AIDS Bureau for their Division of State HIV/AIDS Programs.
The remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Bozzette S.A., Berry S.H., Duan N., et al. The care of HIV-infected adults in the United States. HIV cost and services utilization study consortium. N. Engl. J. Med. 1998;339(26):1897–1904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- Fauci A.S., Redfield R.R., Sigounas G., Weahkee M.D., Giroir B.P. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321(9):844–845. doi: 10.1001/jama.2019.1343. [DOI] [PubMed] [Google Scholar]
- Garrison L.P., Mansley E.C., Abbott T.A., Bresnahan B.W., Hay J.W., Smeeding J. Good research practices for measuring drug costs in cost-effectiveness analyses: a societal perspective: the ISPOR Drug Cost Task Force report–Part II. Value Health. 2010;13(1):8–13. doi: 10.1111/j.1524-4733.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- Goldman D.P., Bhattacharya J., Leibowitz A.A., Joyce G.F., Shapiro M.F., Bozzette S.A. The impact of state policy on the costs of HIV infection. Med. Care Res. Rev. 2001;58(1):31–53. doi: 10.1177/107755870105800102. [DOI] [PubMed] [Google Scholar]
- Goyal R., Hu C., Klein P.W., Hotchkiss J., Morris E., Mandsager P., Cohen S.M., Luca D., Gao J., Jones A., Addison W., O'Brien-Strain M., Cheever L.W., Gilman B. Development of a mathematical model to estimate the cost-effectiveness of HRSA’s Ryan White HIV/AIDS program. J. Acquir. Immune Defic. Syndr. 2021;86(2):164–173. doi: 10.1097/QAI.0000000000002546. [DOI] [PubMed] [Google Scholar]
- Goyal R., Luca D., Klein P.W., Morris E., Mandsager P., Cohen S.M., Hu C., Hotchkiss J., Gao J., Jones A., Addison W., O'Brien-Strain M., Cheever L.W., Gilman B. Cost-Effectiveness of HRSA’s Ryan white HIV/AIDS program? J. Acquir. Immune Defic. Syndr. 2021;86(2):174–181. doi: 10.1097/QAI.0000000000002547. [DOI] [PubMed] [Google Scholar]
- HIV/AIDS Bureau Division of State HIV/AIDS Programs. AIDS Drug Assistant Program (ADAP) Manual. Published 2016. Accessed April 10, 202 https://ryanwhite.hrsa.gov/sites/default/files/ryanwhite/resources/adap-manual.pdf.
- Husereau D., Drummond M., Augustovski F., et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975. doi: 10.1136/bmj-2021-067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri M., David Paltiel A., Goldie S.J., Freedberg K.A. State AIDS Drug Assistance Programs: equity and efficiency in an era of rapidly changing treatment standards. Med. Care. 2002;40(5):429–441. doi: 10.1097/00005650-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation, NASTAD. National ADAP Monitoring Project Annual Report. Published 2009. Accessed July 12, 2021. https://www.kff.org/wp-content/uploads/2013/01/7861.pdf.
- Kaiser Family Foundation. HIV/AIDS Policy Fact Sheet: AIDS Drug Assistance Program. Published 2012. Accessed March 24, 2013. http://www.kff.org/hivaids/upload/1584-11.pdf.
- McManus K.A., Christensen B., Nagraj V.P., et al. Evidence from a multistate cohort: enrollment in affordable care act qualified health plans’ association with viral suppression. Clin. Infect. Dis. 2020;71(10):2572–2580. doi: 10.1093/cid/ciz1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus K.A., Rodney R.C., Rhodes A., Bailey S., Dillingham R. Affordable care act qualified health plan enrollment for AIDS drug assistance program clients: Virginia’s experience and best practices. AIDS Res. Hum. Retroviruses. 2016;32(9):885–891. doi: 10.1089/aid.2016.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASTAD, Horn T, McManus K, Steen A. 2021-2022 National Ryan White HIV/AIDS Program Part B and AIDS Drug Assistance Program Monitoring Project Report. Published 2022. Accessed January 25, 2022. https://nastad.org/partb-adap-2021-2022-report.
- Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton S.D., Kibicho J., Galletly C.L. Is the US AIDS drug assistance program cost-effective? AIDS Behav. 2013;17(1):1–4. doi: 10.1007/s10461-012-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders G.D., Neumann P.J., Basu A., Brock D.W., Feeny D., Krahn M., Kuntz K.M., Meltzer D.O., Owens D.K., Prosser L.A., Salomon J.A., Sculpher M.J., Trikalinos T.A., Russell L.B., Siegel J.E., Ganiats T.G. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- Sansom S.L., Hicks K.A., Carrico J., Jacobson E.U., Shrestha R.K., Green T.A., Purcell D.W. Optimal allocation of societal HIV prevention resources to reduce HIV incidence in the United States. Am. J. Public Health. 2021;111(1):150–158. doi: 10.2105/AJPH.2020.305965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schackman B.R., Goldie S.J., Weinstein M.C., Losina E., Zhang H., Freedberg K.A. Cost-effectiveness of earlier initiation of antiretroviral therapy for uninsured HIV-infected adults. Am. J. Public Health. 2001;91(9):1456–1463. doi: 10.2105/ajph.91.9.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider J.T., Goldman D.P., Rosenblatt L., Seekins D., Juday T., Sanchez Y., Wu Y., Peneva D., Romley J.A. The impact of State AIDS drug assistance policies on clinical and economic outcomes of people with HIV. Med Care Res Rev. 2016;73(3):329–348. doi: 10.1177/1077558715614479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.