Abstract
Background
This longitudinal study of autism symptom trajectories provides unique information that can characterize autism features and diagnostic patterns from childhood to adulthood.
Methods
Participants (n = 155) were part of a longitudinal cohort referred for possible autism where in‐person assessments were completed at ages 2, 3, 5, 9, 19, and 25. Assessors were blinded to previous diagnoses. Based on adult best estimate diagnoses, participants were categorized into one of the four groups: Retained ASD, Lost ASD, Never Had ASD, or Gained ASD Diagnosis. To examine developmental changes in autism symptoms, mixed models indicated the rate of change in ADOS CSS and ADI‐R scores in each diagnostic group.
Results
A subset of participants with VIQ> and <70 were assigned a diagnosis in adulthood that differed from diagnoses earlier in development. Across cognitive levels, the majority of novel diagnoses emerged in adulthood. For those with VIQ > 70, improvements in ADOS CSS over time for the Lost Diagnosis group and worsening in CSS in the Gained Diagnosis group were gradual. Individuals with VIQ > 70 who lost a diagnosis even in adulthood could be distinguished on CSS and ADI‐R scores by age 5 from those who retained their ASD diagnosis. Although most participants with VIQ < 70 saw decreases in autistic symptoms as a whole, changes in autism diagnoses were confounded by disentangling profound intellectual disability as a differential diagnosis or co‐occurrence. Only the Never Had Diagnosis group revealed significant changes in ADOS scores over time, with autism symptoms increasing.
Conclusions
Associated with gradual changes in core features of autism beginning in childhood, diagnoses of autism can shift across development.
Keywords: Autism, adult, outcome, longitudinal
Introduction
Autism Spectrum Disorder (ASD) is a heterogeneous condition where core features are expressed differently within a person across the lifespan and between individuals with the same diagnosis. Though studies of core symptom trajectories are few, most autistic individuals show characteristics of ASD across the lifespan (Giserman‐Kiss & Carter, 2019; Guthrie, Swineford, Nottke, & Wetherby, 2013; Seltzer, Shattuck, Abbeduto, & Greenberg, 2004; Wiggins et al., 2012). In contrast, symptom presentations may change between childhood and adolescence (Baghdadli et al., 2018; Georgiades, Pickles, & Lord, 2021; King & Bearman, 2009). Methodologies vary, including assessment tools, sample sizes, cognitive abilities, and the amount of time elapsed between diagnosis and follow‐up (Lord, Bishop, & Anderson, 2015; Simonoff et al., 2019; Woodman, Smith, Greenberg, & Mailick, 2014). Here, we examine the phenotypic trajectories of a longitudinal cohort between the ages of 2 and 25 (Lord, McCauley, Pepa, Huerta, & Pickles, 2020), stratified by best estimate diagnoses.
Bal, Kim, Fok, and Lord (2019) explored change in parent‐reported social‐communication from ages 2 to 19 in the same sample described in this paper. Participants showed decreases in social‐communication difficulties on the Autism Diagnostic Interview‐Revised (ADI‐R), with language development and maturation contributing to trajectories of skills. Modest decreases of ADI‐R social‐communication impairments between childhood and adulthood have similarly been reported in other longitudinal samples (Gillespie‐Lynch et al., 2012; McGovern & Sigman, 2005; Shattuck et al., 2007; Woodman et al., 2014). Relatedly, in a longitudinal sample spanning ages 5–15, low language and high autism symptoms were risk factors for low growth trajectories of socialization and communication on the Vineland (Baghdadli et al., 2012).
In a sample overlapping with this study, using direct measures of social‐communication and RRBs in the Autism Diagnostic Observation Schedule (ADOS), symptom change between 2 and 15 years of age occurred in about 7% of participants who were assigned to an improving class and 9% in a worsening class (Gotham et al., 2012). Using a less cognitively impaired cohort, Georgiades et al. (2021) identified a larger subset of participants (27% of sample) with ADOS trajectories that continuously improved between initial preschool diagnosis and age 10. For the remainder of the sample, a “turning point” emerged when improvements plateaued around 6 years old.
Several studies have gone beyond these findings to examine individuals who change diagnostic status from clearly defined autism in childhood to an adult presentation of no symptoms of ASD (Billstedt, Gillberg, & Gillberg, 2005; Fein et al., 2013; Zachor & Ben‐Itzchak, 2020). In a previous report of the present sample, 9% of study participants no longer met diagnostic criteria for ASD (not including history) by age 19 (Anderson, Liang, & Lord, 2014). Other longitudinal follow‐up studies have reported similar proportions of previously diagnosed individuals, including those with lower cognitive abilities, who no longer show autistic core features in adulthood (Baghdadli et al., 2018; Billstedt et al., 2005; Howlin, Goode, Hutton, & Rutter, 2004; Mawhood, Howlin, & Rutter, 2000). The alternative, symptom worsening such that individuals gain a diagnosis later in development, is also possible (Ozonoff et al., 2018). Often, studies of first diagnoses in adulthood assume that an earlier childhood diagnosis was missed, but, often standardized assessments of ASD were never received.
Fein et al. (2013) retrospectively characterized a group of 34 individuals with prior early childhood community diagnoses of ASD, who no longer met diagnostic criteria. Participants had more early, intensive applied behavioral analysis relative to people who retained a diagnosis of ASD (Orinstein et al., 2014). We did not find this relationship between outcomes at 19 and intensive treatment services among our longitudinal, but quite different sample (Anderson et al., 2014). Rather, the only treatment variable associated with very positive outcomes was minimal but regular amounts of treatment of any type in the year after diagnosis at 2 (Anderson et al., 2014).
Given that there are few longitudinal studies examining diagnostic stability into adulthood, this study seeks to create trajectories of the core features of ASD between 2 and 25 years of age among individuals with different cognitive levels. We also set out to establish the diagnostic stability of individuals who received repeated, in‐person, and diagnostic evaluations between early childhood and young adulthood. We expected that a majority of participants diagnosed with ASD in childhood would continue to meet current diagnostic criteria in adulthood (Anderson et al., 2014).
Methods
Participants
Participants were part of the Early Diagnosis Study (EDX; Lord et al., 2020), a longitudinal cohort initially recruited in 1990 as consecutive community‐referrals for a diagnostic autism evaluation (Figure S1 for CONSORT). Initial aims of the study were to determine if diagnoses of autism or developmental delay made under age 3 were stable across time. Participants were drawn from 3 locales in the United States. In early childhood (M age = 2.5 years, SD = 0.43, Range = 1.25–3.33), 124 participants enrolled in North Carolina (n = 74) and Chicago (n = 50). An additional 31 participants (M age = 9.14 years, SD = 2.49, Range = 7.75–15.33) with prior early diagnostic evaluations (<3 years old) were recruited in Michigan and entered the study at age 9 (see Pickles, McCauley, Pepa, Huerta, & Lord, 2020). They were then followed concurrently with the same frequency as the rest of the sample into adulthood. All 155 individuals included in this paper (80.65% male) were seen at entry and participated in at least one assessment battery in young adulthood (M age = 23.46 years; SD = 3.40; Range = 17.58–30.08).
Since baseline, attrition occurred due to unreachable status and refusals (Table S1). Between study entry (n = 253, inclusive of Michigan recruits) and adulthood (n = 155), attrition constituted 38.74% of the sample, and was not significantly associated with gender, diagnosis, IQ at baseline, or rural/urban status (25% rural). It was significantly higher for Black families (79% White, 19% Black, 2% Other) and those with lower levels of education (54% of caregivers had college degrees). Most participants (78.71%) had a diagnosis of ASD at some point during the study; 21.29% had a history of developmental delay but no ASD.
Over half (55.48%) of the sample was less cognitively able as measured at age 19 (i.e., Verbal Intelligence Quotient [VIQ] < 70). As adults, less cognitively able participants (n = 86) had significantly lower VIQ scores (M VIQ = 24.58, SD = 16.51), Non‐Verbal Intelligence Quotient [NVIQ] scores (M NVIQ = 31.45, SD = 21.77), and adaptive skills (M VABC = 40.36, SD = 18.30) than more cognitively abled participants (n = 69; M VIQ = 102.56, SD = 16.97; M NVIQ = 101.73, SD = 17.74; M VABC = 80.19, SD = 18.30), with a bimodal distribution. Mean adult ADOS CSS scores met autism spectrum clinical thresholds (CSS = 4) for less cognitively able (M = 5.57; SD = 2.14, Range = 1–10) and more cognitively able participants (M = 4.45, SD = 2.46, Range = 1–10).
Procedure
Face‐to‐face diagnostic assessments were conducted between 1990 and 2018 with timepoints corresponding to mean participant ages (2, 3, 5, 9, 19, 25). Assessments (Table S1) were administered by clinicians who were aware that many, but not all, participants had an autism diagnosis. Clinicians were research‐reliable and administered diagnostic batteries without knowledge of previous test results, histories, or diagnoses, making the assessment blinded. Research was IRB‐approved and consent/assent forms were obtained for participants.
Measures
Diagnostic assessments
The ADOS‐2 (Lord et al., 2012), is a clinician‐administered measure which distinguishes behaviors of autism from those in the typical population. Calibrated severity scores from ADOS modules, determined by age and language level are comparable across modules (CSS; Gotham, Pickles, & Lord, 2009), including the Adapted‐ADOS administered to minimally verbal adults (Bal et al., 2020), which uses calibrated scores from Modules 1 and 2. The ADI‐R (Lord, Rutter, le Couteur, & Free Hospital, 1994) is a standardized parent‐report interview of social behaviors, communication, and repetitive interests. Current ADI‐R social‐communication scores were generated from the nonverbal algorithm (14‐items administered at all ages between 2 and 19; see Bal et al., 2019). Parent‐reported treatment hours prior to age 3 were aggregated using procedures from Anderson et al., 2014.
Cognitive and adaptive assessments
NVIQ and VIQ were derived depending on the age and skills of the participant. Measures included the Wechsler Adult Intelligence Scale‐IV (Wechsler, 2008), Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), Differential Ability Scales (Elliott, 2007), or the Mullen Scale of Early Learning (Mullen, 1995). Adaptive skills were assessed via the Vineland Adaptive Behavior Composite (VABC; Sparrow, Cicchetti, & Balla, 2005).
Social and behavioral assessments
The Aberrant Behavior Checklist‐Community (ABC; Aman, Singh, Stewart & Field, 1985) measures behavior problems in individuals with developmental delay. The Social and Emotional Functioning Interview (Rutter et al., 1988) was clinician‐administered independently to parents and more cognitively able participants. Information on aspects of adult functioning, including education, living status, driving status, and finances were gathered. The Well‐Being Questionnaire (Ryff, 1989) was a self‐report measure of personal growth, purpose in life, and self‐acceptance. Adult outcomes were characterized by Pickles et al. (2020), who generated four empirically derived latent classes from a range of adult functioning measures for this sample (1‐ “Best Outcome Class”; 2‐“High‐IQ ASD”; 3‐“Low‐IQ ASD without Behavioral Problems”; 4‐“Low‐IQ ASD with Behavioral Problems”) and by McCauley and Sigman (2020) who conceptualized outcomes a priori as a count variable encompassing autonomy, social relationships, and purpose (range = 0–3).
Diagnosis
After each in‐person assessment, a best estimate autism diagnosis was assigned by research‐reliable clinicians who conducted in‐person testing with multiple informants and reviewed all available testing information (including cognitive functioning, ADOS‐2, ADI‐R, adaptive functioning, and mood/behavior screens). If the final diagnosis conflicted with the ADOS‐2 and ADI‐R (current), senior researchers reviewed all records, watched the ADOS‐2 recording and discussed the case with the examiners to reach a consensus diagnosis. Importantly, these were not DSM‐5 diagnoses, where historical information can be included; diagnoses were solely based on current functioning using DSM‐IV criteria at early ages and DSM‐5 from age 19 on.
Data analysis
Participants were divided into four groups based on diagnostic impressions in adulthood. Of those who received an ASD diagnosis (autism or Pervasive Developmental Disorder‐Not Otherwise Specified [PDD‐NOS]) in early childhood, two groups were identified: Retained Diagnosis and Lost Diagnosis. Of those who had never received an ASD diagnosis in early childhood, an additional two groups emerged: Never Had Diagnosis and Gained Diagnosis. Descriptive statistics characterized individuals in each diagnostic category, separating those with VIQs above 70 or VIQs below 70. The two cognitive groups have a bimodal distribution and differing ability to self‐report. Concerns most relevant to families and outcomes among those with average IQ and those with moderate–severe intellectual disability vary greatly. Group level differences were assessed via one‐way ANOVAs or Chi‐Square Tests.
Mixed models (MIXED in STATA 16) indicated the rate of change over time in ADOS CSS (total, social‐communication, and RRB), ADI‐R Social‐Communication current, and VABC scores among individuals in the four diagnostic groupings. The marginal mean estimates of diagnosis group were compared at each timepoint. The linear mixed model flexibly handled participant missing data using maximum likelihood estimation. Diagnostic groupings were compared at initial starting point (intercept) and rate of change (linear slopes). Time was centered at zero (i.e., the start of the study when children were approximately 2‐years old). All models included a subject level random intercept to account for the nonindependence of the data caused by repeated measures of the same individuals over time. Race and maternal education were included as covariates.
The beginning and endpoints of the trajectories over time were set by our best estimate diagnoses. Group differences on measures from the start and end of our study are to be expected because they contributed to the diagnoses that formed the categories. Therefore, of greatest empirical interest are the trajectories of autism symptoms between early childhood and adulthood.
Results
More cognitively able group composition
Most participants (71.01%) with VIQ > 70 had stable diagnostic impressions from early childhood into young adulthood (Retained Diagnosis, n = 31 and Never Had Diagnosis, n = 18). For a minority of the sample (28.99%), diagnostic perceptions differed from what was assigned earlier in childhood (Lost Diagnosis, n = 13 and Gained Diagnosis, n = 7). Early in the study, PDD‐NOS diagnoses for those with VIQ>70 was common (72%) and associated with the four diagnostic groups. It should be noted, however, that for the purposes of this paper, PDD‐NOS and autism classifications were collapsed into a greater ASD diagnosis per DSM‐5.
Thirteen individuals with VIQ > 70 comprised the Lost Diagnosis group, which was exclusively male and mostly White (84.62%). All 13 participants were diagnosed prior to age 3 (M age = 2.51years; SD = 0.39). We can estimate when autistic symptom severity dropped below the clinical threshold by the first face‐to‐face visit when the diagnosis was not autism, recognizing that we did not do face‐to‐face assessments between 9 and 19 and that there were missing data in some cases. Given these limitations, our estimates are that two participants moved from autism to nonspectrum at the 5‐year‐old assessment, one participant was identified as nonspectrum at the 9‐year‐old assessment, four participants moved out of ASD between 9 and 19, and six participants were identified as nonspectrum between the 19 and 25‐year‐old assessments. Thus, nearly half (46.15%) of the individuals who moved out of ASD did so after age 18. We recognize that between the ages of 9 and 18, classification switched from DSM‐IV‐TR to DSM‐5. Even though this period of time represented a change in DSM diagnostic criteria, the four individuals who were identified as nonspectrum (current) between 9 and 19 did not meet DSM‐IV criteria for autism or PDD‐NOS or DSM‐5 criteria for ASD.
The Gained Diagnosis group (n = 7) was entirely male with the majority (85.71%) White. All received ASD diagnoses in adulthood, with five assigned this categorization for the first time at the 19‐year‐old assessment and the remaining two given the diagnosis at age 21. For all individuals in both the Lost and Gained diagnosis groups, once a changed classification was made, diagnoses were sustained in subsequent assessments.
See Table 1 for adult more cognitively able demographic characteristics. Residence in adulthood differed by diagnosis group, χ2 (1, 54) = 19.109, p < .001 such that those who retained their diagnosis lived with their parents significantly more (ps < .001) than those who never had a diagnosis or acquired a diagnosis in adulthood. 32.14% of the sample was still enrolled in college during their most recent adult assessment. When diagnostic groups were compared to Pickles et al. (2020) outcome groups, significance emerged (p = .007). However, individuals belonging to Class 1 (i.e., least ASD symptomology, high IQs, and good functional/behavioral outcomes) were found in each diagnostic group (Retained Diagnosis = 37.5%, Never Had Diagnosis = 54.54%, Lost Diagnosis = 100%, Gained Diagnosis = 50%). Using McCauley et al.s' (2020) definition, outcomes were also related to diagnostic group (p = .003). All of the Lost Diagnosis group achieved at least 2 of the three outcomes (i.e., independent living, employment, and friendships), whereas 36% of the Retained, 54.54% of the Never Had, and 50% of the Gained Diagnosis groups had. Thus, while good outcomes were more frequent in those who no longer had autism symptoms, some participants who retained or even gained autism diagnoses also were doing well.
Table 1.
Adult demographic variables (VIQ > 70; n = 69)
| Sample characteristics | Retained diagnosis (n = 31) | Lost diagnosis (n = 13) | Never had diagnosis (n = 18) | Gained diagnosis (n = 7) | Unadj. p‐value |
|---|---|---|---|---|---|
| Male (%) | 93.55 | 100 | 61.11 | 100 | .002 |
| Site (%) | |||||
| North Carolina | 48.39 | 46.15 | 38.89 | 14.29 | <.001 |
| Chicago | 32.26 | 53.85 | 5.56 | 0 | |
| Michigan | 19.35 | 0 | 55.56 | 85.71 | |
| Urban | 74.19 | 80 | 66.67 | 100 | .363 |
| Rural | 25.81 | 20 | 33.33 | 0 | |
| Race (%) | |||||
| White | 74.19 | 92.31 | 88.89 | 85.71 | .105 |
| Black | 25.80 | 7.69 | 5.56 | 0 | |
| Other | 0 | 0 | 5.56 | 14.29 | |
| Cognitive ability (M, SD) | |||||
| VIQ | 101.36 (17.37) | 113.33 (12.44) | 94.59 (16.00) | 108.29 (15.61) | .019 |
| NVIQ | 100.11 (15.97) | 114.09 (11.80) | 98.94 (20.50) | 95.29 (19.50) | .071 |
| Autism features (M, SD) | |||||
| CSS Total | 6.04 (2.01) | 1.92 (0.76) | 3.06 (1.95) | 6.14 (0.90) | <.001 |
| CSS Soc. Affect | 6.07 (2.09) | 2.31 (1.32) | 3.71 (2.34) | 6.43 (1.40) | <.001 |
| CSS RRB | 7.03 (1.64) | 3.54 (2.50) | 3.47 (2.45) | 6.14 (2.54) | <.001 |
| ADI‐R Social a | 17.63 (8.13) | 11.17 (7.47) | 5.50 (3.72) | 10.14 (6.47) | <.001 |
| ADI‐R Comm. a | 14.48 (5.91) | 8.92 (7.28) | 5.64 (3.99) | 9.57 (6.71) | <.001 |
| ADI‐R RRB a | 6.52 (2.82) | 3.67 (1.50) | 2.29 (3.34) | 2.29 (1.80) | <.001 |
| Adaptive skills (M, SD) | |||||
| Vineland ABC | 73.93 (14.56) | 90.92 (10.56) | 83.07 (17.36) | 78.29 (20.20) | .014 |
| Independent living (%) | |||||
| Drives | 59.26 | 84.62 | 71.43 | 71.43 | .261 |
| Lives on own | 14.81 | 84.62 | 28.57 | 57.14 | <.001 |
| Paid Job | 65.38 | 92.31 | 53.33 | 71.43 | .08 |
| ABC (M, SD) | |||||
| Irritability | 6.41 (7.27) | 0.14 (0.38) | 5.53 (4.88) | 1.00 (1.73) | .067 |
| Social withdrawal | 10.36 (8.64) | 0.71 (1.25) | 3.60 (3.68) | 1.33 (2.31) | .001 |
| Stereotypy | 3.77 (4.42) | 0.43 (1.13) | 0.87 (1.41) | 0.33 (0.58) | .020 |
| Hyperactivity/noncompliance | 7.50 (7.37) | 0.57 (1.13) | 5.13 (4.21) | 0.33 (0.58) | .024 |
| Inap. speech | 3.05 (2.89) | 0 | 0.67 (1.13) | 0 | .001 |
| Well‐being (M, SD) | 187.73 (27.49) | 202.33 (22.47) | 154.50 (63.59) | 144.67 (69.79) | .095 |
ADI‐R scores were from the 19‐year‐old assessment only.
Although diagnosed early, participants did not receive high amounts of intervention prior to 3 years (Anderson et al., 2014). Individuals seen in early childhood in the Lost Diagnosis group received more cumulative hours of one‐to‐one structured teaching prior to 36 months than individuals in the Retained Diagnosis group, χ2 (1, 44) = 4.60, p = .032. Between ages 2 and 3, those in the Lost Diagnosis group received on average 45‐min of structured teaching per week, whereas those in the Retained Diagnosis group received a weekly average less than 10‐min. Nobody in the Gained Diagnosis group received one‐to‐one structured teaching before age 3.
Trajectories of autism features across time (VIQ > 70)
ASD features as measured by the ADOS were examined across time for members in each diagnostic group. Growth mixture modeling revealed a significant time by group interaction for individuals in the Lost and Gained diagnosis groups only (Table 2, Figure 1). Race and education were included in the model as predictors to account for attrition but were not significant. There were steady, incremental improvements in CSS scores over time for those in the Lost Diagnosis group and gradual worsening in the Gained Diagnosis group.
Table 2.
Change in autistic symptoms from 2–25 (VIQ > 70; n = 69)
| Predictors | Social affect CSS coefficient (SE) | RRB CSS coefficient (SE) | Total CSS coefficient (SE) | ADI social‐communication coefficient (SE) b |
|---|---|---|---|---|
| Intercept | 6.29 (0.34)** | 6.25 (0.36)** | 6.10 (0.35)** | 14.10 (0.92)** |
| Group | ||||
| Retained | – | – | – | |
| Never had | −3.49 (0.66)** | −3.09 (0.69)** | −3.62 (0.66)** | −9.75 (1.79)** |
| Lost | −0.86 (0.57) | −0.75 (0.59) | −0.96 (0.57) | −2.40 (1.43) |
| Gained | −2.95 (1.11)** | −3.33 (1.19)** | −3.46 (1.09)** | −7.55 (4.76) |
| Time | −0.03 (0.02) | 0.05 (0.02)* | −0.01 (0.02) | −0.53 (0.09)** |
| Interaction | ||||
| Time*Retained | – | – | – | |
| Time*Never Had | 0.07 (0.04) | −0.03 (0.04) | 0.04 (0.04) | 0.36 (0.16)* |
| Time*Lost | −0.11 (0.03)** | −0.12 (0.04)** | −0.14 (0.03)** | −0.12 (0.15) |
| Time*Gained | 0.16 (0.06)* | 0.14 (0.07)* | 0.17 (0.06)** | 0.53 (0.34) |
| White | – | – | – | |
| Minority | 0.59 (0.51) | −0.86 (0.52) | 0.22 (0.52) | −0.94 (1.24) |
| Parent college educated | – | – | – | |
| Parent not college educated | 0.03 (0.51) | 0.23 (0.52) | 0.22 (0.52) | 0.88 (1.26) |
| Variance | ||||
| Random effects | ||||
| Intercept | 1.18 (0.37) | 1.04 (0.40) | 1.35 (0.39) | 3.79 (2.21) |
| Residual a | 3.78 (0.37) | 4.72 (0.46) | 3.45 (0.34) | 30.49 (3.33) |
*p < .05, **p < .01.
LR test versus linear model suggested support for random effect.
ADI‐R only administered until 19.
Figure 1.
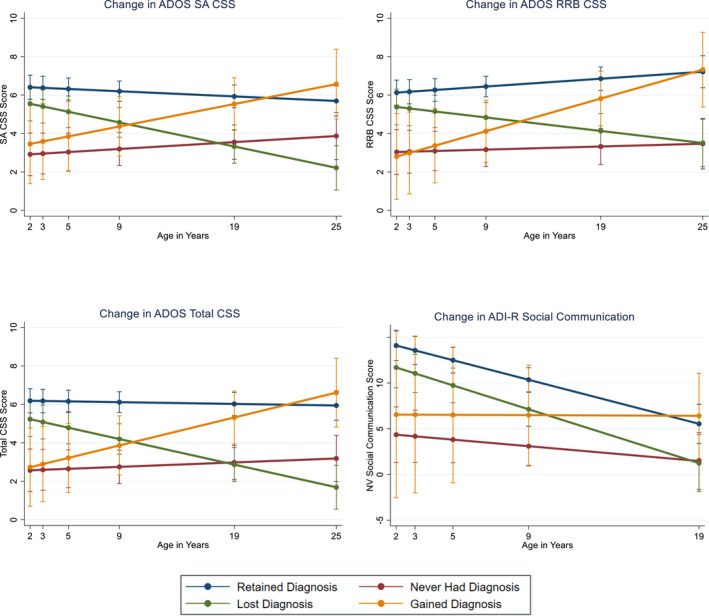
Autism symptom change (VIQ > 70; n = 69). ADI‐R, Autism Diagnostic Interview‐Revised; ADOS, Autism Diagnostic Observation Schedule; CSS, Calibrated Severity Score; NV, non‐verbal; RRB, restricted and repetitive behavior; SA, social affect
Individuals who were assigned to the Retained Diagnosis and Lost Diagnosis group in adulthood could first be distinguished from one another both on social affect CSS scores (p = .02) and RRB CSS scores (p = .04) as early as age 5 (but not at age 2 or 3), with significant group differences continuing at each assessment thereafter (Figure 1). Thus, for those diagnosed with autism in early childhood, both social affect and RRB trajectories may begin diverging prior to school age. See Figure S2a,b for individual trajectories.
Social communication scores on the ADI‐R replicated the divergence in autistic behaviors beginning at age 5 for those in the Retained and Lost diagnosis groups (p = .02). Social‐communication improvements over time occurred across the sample (Table 2, Figure 1) with only one significant diagnosis by time interaction (Never Had Diagnosis group), whose social communication scores worsened relative to the Retained group. With respect to adaptive skills, a significant diagnosis by time trajectory emerged for the Lost Diagnosis group (p = .003; Table S2) with adaptive skill differences between the Lost and Retained Diagnosis groups emerging beginning at age 9 (p = .007).
Less cognitively able group composition
Fewer participants with lower cognitive abilities had changing diagnostic categorizations in adulthood, resulting in very small group sizes. Four participants with VIQ < 70 were characterized by “blinded” clinicians as not autistic in adulthood, even though they had repeated diagnoses of autism in childhood (M IQ = 11.75, SD = 9.57). All of them received non‐ASD diagnoses of Profound Intellectual Disability and had lower mean VIQ and adaptive scores than the Retained Diagnosis group (Table 3). The majority (75%) of this group were female and Black. An additional four individuals were identified as gaining a diagnosis of ASD in adulthood (M IQ = 31.50, SD = 8.19), with 50% male and 100% White. Unlike the more cognitively able participants, individuals in the Lost Diagnosis group continued to have mean ADOS CSS scores above the clinical threshold (Table 3). There were no differences in treatment among groups.
Table 3.
Adult demographic variables (IQ < 70; n = 86)
| Sample characteristics | Retained diagnosis (n = 62) | Lost diagnosis (n = 4) | Never had diagnosis (n = 16) | Gained diagnosis (n = 4) | Unadj. p‐value |
|---|---|---|---|---|---|
| Male (%) | 87.10 | 25 | 50 | 50 | .001 |
| Site (%) | |||||
| North Carolina | 48.39 | 75.00 | 62.50 | 50 | <.001 |
| Chicago | 50.00 | 25.00 | 0 | 0 | |
| Michigan | 1.61 | 0 | 37.50 | 50 | |
| Urban | 73.77 | 25.00 | 73.33 | 100 | .106 |
| Rural | 26.23 | 75.00 | 26.67 | 0 | |
| Race (%) | |||||
| White | 74.19 | 25 | 87.5 | 100 | .081 |
| Black | 24.19 | 75 | 6.25 | 0 | |
| Other | 1.61 | 0 | 6.25 | 0 | |
| Cognitive ability (M, SD) | |||||
| VIQ | 21.69 (15.51) | 11.75 (9.57) | 37.06 (16.61) | 31.50 (8.19) | .002 |
| NVIQ | 32.19 (23.17) | 9.75 (7.27) | 34.47 (18.49) | 30.25 (4.03) | .228 |
| Autism features (M, SD) | |||||
| CSS Total | 5.98 (1.97) | 6.00 (0.00) | 4.21 (2.29) | 4.75 (2.50) | .047 |
| CSS Social Affect | 6.52 (1.98) | 7.00 (1.00) | 5.00 (2.25) | 5.00 (1.83) | .052 |
| CSS RRB | 5.92 (2.31) | 6.33 (0.58) | 4.21 (2.19) | 5.00 (4.61) | .119 |
| ADI‐R Social a | 21.61 (9.23) | 11.00 (14/73) | 9.88 (6.55) | 16.67 (12.86) | <.001 |
| ADI‐R Comm. a | 14.61 (6.70) | 4.67 (8.03) | 8.31 (5.33) | 14.00 (8.72) | .002 |
| ADI‐R RRB a | 5.83 (2.73) | 2.67 (3.05) | 3.06 (2.49) | 6.33 (2.89) | .002 |
| Adaptive skills (M, SD) | |||||
| Vineland ABC | 37.13 (16.67) | 28.75 (10.56) | 56.56 (17.40) | 37.25 (20.17) | .001 |
ADI‐R scores were from the 19‐year‐old assessment only.
Trajectories of autism features across time (VIQ < 70)
As a whole, participants with VIQ < 70 (n = 86) saw significant gradual decreases in autistic symptoms as they aged (Table 4, Figure 2). However, a Diagnosis by Time interaction occurred in the Never Had Diagnosis group for ADOS and VABC scores suggesting subtle increases in autistic presentation yet gains in adaptive skills across time (Table 4, Table S2) relative to those who retained their diagnosis.
Table 4.
Change in autistic symptoms from 2–25 (VIQ < 70; n = 86)
| Predictors | Social affect CSS coefficient (SE) | RRB CSS coefficient (SE) | Total CSS coefficient (SE) | ADI social‐communication coefficient (SE) b |
|---|---|---|---|---|
| Intercept | 8.26 (0.21)** | 7.65 (0.23)** | 8.29 (0.21)** | 20.68 (0.75)** |
| Group | ||||
| Retained | – | – | – | |
| Never had | −5.06 (0.56)** | −3.55 (0.59)** | −5.33 (0.55)** | −14.14 (1.86)** |
| Lost | −0.21 (0.91) | 0.45 (0.98) | −0.09 (0.91) | 2.49 (3.14) |
| Gained | −2.40 (1.07)* | −1.81 (1.14) | −2.36 (1.07)* | −11.79 (3.74)** |
| Time | −0.10 (0.02)** | −0.06 (0.02)** | −0.11 (0.02)** | −0.28 (0.04)** |
| Interaction | ||||
| Time*Retained | – | – | – | |
| Time*Never Had | 0.23 (0.04)** | 0.11 (0.04)** | 0.21 (0.04)** | 0.20 (0.12) |
| Time*Lost | 0.01 (0.10) | −0.04 (0.11) | −0.03 (0.10) | −0.15 (0.24) |
| Time*Gained | 0.08 (0.07) | 0.06 (0.07) | 0.08 (0.07) | 0.32 (0.29) |
| White | – | – | – | |
| Minority | 0.03 (0.37) | −0.35 (0.40) | −0.19 (0.38) | −0.04 (1.47) |
| Parent college educated | – | – | – | |
| Parent not college educated | −0.75 (0.41) | −0.16 (0.43) | −0.51 (0.40) | 2.05 (1.56) |
| Variance | ||||
| Random effects | ||||
| Intercept | 0.66 (0.25) | 0.73 (0.29) | 0.59 (0.24) | 17.17 (3.58) |
| Residual a | 3.39 (0.31) | 3.94 (0.35) | 3.52 (0.32) | 17.65 (1.65) |
*p < .05, **p < .01.
LR test versus linear model suggested support for random effect.
ADI‐R only administered until 19.
Figure 2.
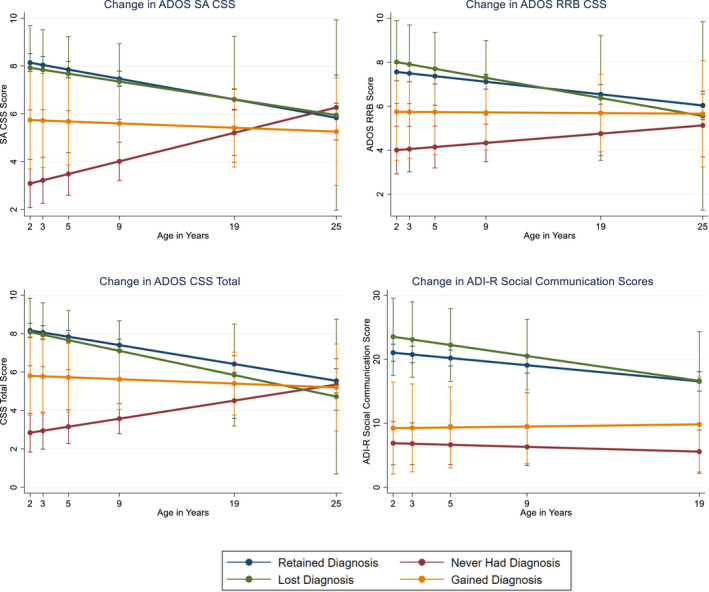
ADOS symptom change (VIQ < 70; n = 86). ADI‐R, Autism Diagnostic Interview‐Revised; ADOS, Autism Diagnostic Observation Schedule; CSS, Calibrated Severity Score; NV, non‐verbal; RRB, restricted and repetitive behavior; SA, social affect
Those who never had a diagnosis of ASD were statistically different (ps < .05) from those who: (1) retained a diagnosis up until age 25 on social affect CSS and RRB CSS, (2) lost a diagnosis until age 19 (social affect and RRB CSS), and (3) gained a diagnosis until age 19 on social affect CSS only. For ADI‐R Social‐Communication scores, significant differences emerged between those who never had a diagnosis and those who retained and lost diagnoses throughout the lifespan. Taken together, results indicate convergence of observable social difficulties in young adulthood for individuals with severe to profound intellectual disability with or without autism diagnoses.
Discussion
This paper highlights that diagnoses of autism can shift across development and are associated with gradual changes in core features of autism beginning in childhood. The present sample is unique due to the repeated “blinded” comprehensive assessments and best estimate diagnoses across over 20 years. For most participants, assessment started under age 3. A critical part of the design was that not all participants had ASD, and though these individuals represent a heterogeneous group defined only by having early developmental disorders and not ASD, their inclusion means that the examiners carrying out the assessments were not expecting autism in every participant.
Repeated measurement meant that we could assess incremental changes and trajectories not only in the dimensional measures but also in the diagnostic characterizations that clinicians use as they formulate plans for the children and adults with whom they work. We do not propose that diagnostic conceptualizations are more important than dimensional measures. However, given that best estimate diagnoses are considered the gold standard for diagnosis of autism by many professional and governmental agencies (NICE, DSM‐5, ICD‐11, AAP), with these data we have an opportunity to address how overall formulations change over time, as well as specific measurements.
Over the years of this study, diagnostic criteria as specified in the DSM for an ASD have slightly shifted. Due to the timing of initial assessment (prior to age 3), no individuals in our study were given an early diagnosis of Asperger’s Disorder. However, some were given diagnoses of PDD‐NOS between ages 2 and 9. In this paper, we collapsed Autistic Disorder and PDD‐NOS into a single classification of ASD as in DSM‐5. It is possible that the change from DSM‐IV and DSM‐5, between ages 9 and 19, resulted in reclassifications but reviewing the data on core features of autism and considering the linear changes in the diagnostic and assessment measures (direct observation and parent interview) throughout the study, the criteria changes do not account for shifts in diagnoses.
A crucial component of the study was the need to differentiate participants by language level and intellectual ability. Doing so has statistical consequences, but, by age 5, children who are becoming fluent speakers and those who have little functional speech and marked delays in nonverbal problem solving begin to diverge in ways that require separate consideration. Pooling across huge differences in cognitive functioning, as we have shown here through the different trajectories, can be misleading.
Because recruitment was many years ago at young ages, it has a higher proportion of participants with limited language and cognitive abilities than current populations.
The majority of the children who received ASD diagnoses at early ages continued to meet formal diagnostic criteria for ASD as young adults. However, 19% of the higher IQ participants “lost” their ASD diagnosis by age 25. Again, adult diagnostic judgments were based on current functioning and not historical information as is recommended by the DSM‐5. Addressing the personal perspectives (such as camouflaging) of these people is beyond the scope of this paper. But, it is worth noting that none currently identified as autistic, though many acknowledged that autism was a condition they had when younger.
Most striking is the gradual nature of differential changes in social communication, repetitive behaviors, and adaptive skills associated with overall shifts in ASD diagnoses based on current functioning. While the “turning points” in these analyses were not as distinct as those found in Georgiades et al. (2021), we also found changes in autism symptoms, based not only on the ADOS but also the ADI‐R, that were apparent by age 5, but importantly not in very early years (2 and 3). Trajectories for adaptive skills were also gradual but associated with even later differentiation in mid‐school years.
Over half of the changes in best estimate ASD diagnosis occurred at age 19 or later. One could argue that this is because diagnoses of adults are more difficult and liable to variability. However, follow‐up assessments yielded stable results. Moreover, not only were there participants who “moved out of” current ASD diagnoses but also several participants (24% of the developmental delay group) who gained (and then retained) diagnoses of ASD as young adults. Of note, the majority of the Gained Diagnosis group belonged to the site which had the oldest recruitment age. In part, this region also had the highest number of participants without ASD diagnoses at the earlier time points, despite referrals for possible autism. Though this was a small group, changes in their clinician‐observed autism symptoms also turned out to be gradual, beginning in childhood. These findings suggest that changing expectations/demands or behaviors from individuals may occur as youth with longstanding developmental disorders move into adulthood.
Although less cognitively able participants were identified in each of the four groups, reasons for switching diagnostic categorization were less straightforward. Differential diagnoses were primarily between ASD plus intellectual disability and profound intellectual disability without ASD suggesting that for those with marked cognitive deficits, clinical diagnostic decision‐making may be more difficult. With the small sample, we can only speculate about possible sex or race effects when the ASD diagnosis was not retained or gained, perhaps in part because of caregivers’ perspectives. Some of the measures, (i.e., Adaptive‐ADOS), are relatively new so conclusions need to be replicated both across samples and methods. Given the variability in outcomes even within minimally verbal adults (McCauley et al., 2020), more research is needed.
Limitations include missing data and attrition, which was higher in less well‐educated and Black participants. Convergence across samples from different countries using different measures and samples is encouraging and will continue to be a critical factor in interpretation. Similarly, the sample was approximately 80% male, which makes it difficult to postulate if sex‐based differences exist with respect to diagnostic classification or stability over time. Further analyses in different samples will yield important contributions about those who still self‐report symptoms but successfully camouflage them.
Small sample sizes, particularly in each subgroup, should temper interpretation of statistical significance. Further, although assessors were not aware which participants had autism and which had not, unavoidable factors (e.g., caregiver reports) may have unintentionally introduced bias into the blinded assessment. It was also beyond the scope of this paper to address other important issues in outcome, including co‐occurring disorders, behavior and emotional issues, nor the perspectives of the participants we studied, which are important.
This study demonstrates that when comparing diagnostic groups in cognitively able adults to previously published outcome variables, “good” outcomes are achieved not just in the Lost Diagnosis group, but also in the three other groups as well (McCauley et al., 2020; Pickles et al., 2020). Thus, independent living, employment, and friendships are achieved by those who retained an autism diagnosis over more than 20 years, but also those who gained or lost it. For the less cognitively able group, there is also variation in these factors, including well‐being and happiness (McCauley et al., 2020), which are in many ways more important than a formal diagnosis. Further, these outcomes occurred in individuals who received far fewer services early in development in contrast to other published research examining diagnostic outcomes (e.g. Orinstein et al., 2014).
These findings are from longitudinal data that followed individuals across time. Cross‐sectional studies have much to offer but are likely affected by changes over time in awareness of autism, reduced stigma, and differential opportunities for services. We hope the effort and commitment of the participants in this study over a very long time can move us forward to better understand and support individuals with autism and their families and to support the gradual changes that accompany development and outcomes possible for all autistic people.
Supporting information
Figure S1. CONSORT diagram for the EDX study.
Figure S2. (a) Overall CSS trajectories for the Lost Diagnosis (VIQ > 70) group by individual ID. (b) Overall CSS trajectories for the Gained Diagnosis (VIQ > 70) group by individual ID.
Table S1. Schedule of Assessments used in study analyses.
Table S2. Change in adaptive skills from 2 to 25.
Acknowledgments
The authors are grateful for the time and commitment of participants, families, and staff. This study was funded by R01HD081199(PI:CL) and R01MH081873(PI:CL). C.L. acknowledges royalties from the sale of the ADOS‐2 and the ADI‐R. Royalties generated from this study were donated to a not‐for‐profit agency, Have Dreams. R.E. has no conflicts to declare.
Key points.
Regarded as a fixed disorder, most autistic individuals show clinical impairments across the lifespan. Studies of core symptom trajectories of ASD across the lifespan, however, are relatively few.
The majority of children who received ASD diagnoses at early ages continue to meet formal diagnostic criteria for ASD as young adults. However, for a minority of the sample, current diagnostic impressions differed and were coupled with gradual changes in autism symptoms first apparent beginning in childhood.
Conflict of interest statement: See Acknowledgments for full disclosures.
References
- Aman, M.G. , Singh, N.N. , Stewart, A.W. , & Field, C.J. (1985). The aberrant behavior checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89, 485–491. [PubMed] [Google Scholar]
- Anderson, D.K. , Liang, J.W. , & Lord, C. (2014). Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. Journal of Child Psychology and Psychiatry, 55, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdadli, A. , Assouline, B. , Sonié, S. , Pernon, E. , Darrou, C. , Michelon, C. , … & Pry, R. (2012). Developmental trajectories of adaptive behaviors from early childhood to adolescence in a cohort of 152 children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42, 1314–1325. [DOI] [PubMed] [Google Scholar]
- Baghdadli, A. , Michelon, C. , Pernon, E. , Picot, M.‐C. , Miot, S. , Sonié, S. , … & Mottron, L. (2018). Adaptive trajectories and early risk factors in the autism spectrum: A 15‐year prospective study. Autism Research, 11), 1455–1467. [DOI] [PubMed] [Google Scholar]
- Bal, V.H. , Kim, S.H. , Fok, M. , & Lord, C. (2019). Autism spectrum disorder symptoms from ages 2 to 19 years: Implications for diagnosing adolescents and young adults. Autism Research, 12, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal, V.H. , Maye, M. , Salzman, E. , Huerta, M. , Pepa, L. , Risi, S. , & Lord, C. (2020). The adapted ADOS: A new module set for the assessment of minimally verbal adolescents and adults. Journal of Autism and Developmental Disorders, 50, 719–729. 10.1007/s10803-019-04302-8/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstedt, E. , Gillberg, C. , & Gillberg, C. (2005). Autism after adolescence: Population‐based 13‐ to 22‐year follow‐up study of 120 individuals with autism diagnosed in childhood. Journal of Autism and Developmental Disorders, 35, 351–360. [DOI] [PubMed] [Google Scholar]
- Elliott, C.D. (2007). Differential ability scales (2nd edn). San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Fein, D. , Barton, M. , Eigsti, I.‐M. , Kelley, E. , Naigles, L. , Schultz, R.T. , … & Tyson, K. (2013). Optimal outcome in individuals with a history of autism. Journal of Child Psychology and Psychiatry, 54, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades, S. , Tait, P.A. , McNicholas, P.D. , Duku, E. , Zwaigenbaum, L. , Smith, I.M. , … & Szatmari, P. (2021). Trajectories of symptom severity in children with autism: Variability and turning points through the transition to school. Journal of Autism and Developmental Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie‐Lynch, K. , Sepeta, L. , Wang, Y. , Marshall, S. , Gomez, L. , Sigman, M. , & Hutman, T. (2012). Early childhood predictors of the social competence of adults with autism. Journal of Autism and Developmental Disorders, 42(2), 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giserman‐Kiss, I. , & Carter, A.S. (2019). Stability of autism spectrum disorder in young children with diverse backgrounds. Journal of Autism and Developmental Disorders, 50, 3263–3275. 10.1007/s10803-019-04138-2/ [DOI] [PubMed] [Google Scholar]
- Gotham, K. , Pickles, A. , & Lord, C. (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham, K. , Pickles, A. , & Lord, C. (2012). Trajectories of autism severity in children using standardized ADOS scores. Pediatrics, 130, e1278–e1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, W. , Swineford, L.B. , Nottke, C. , & Wetherby, A.M. (2013). Early diagnosis of autism spectrum disorder: Stability and change in clinical diagnosis and symptom presentation. Journal of Child Psychology and Psychiatry, 54, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin, P. , Goode, S. , Hutton, J. , & Rutter, M. (2004). Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, 45, 212–229. [DOI] [PubMed] [Google Scholar]
- King, M. , & Bearman, P. (2009). Diagnostic change and the increased prevalence of autism. International Journal of Epidemiology, 38, 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C. , Bishop, S. , & Anderson, D. (2015). Developmental trajectories as autism phenotypes. American Journal of Medical Genetics, 169, 198–208. 10.1002/ajmg.c.31440/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C. , McCauley, J.B. , Pepa, L.A. , Huerta, M. , & Pickles, A. (2020). Work, living, and the pursuit of happiness: Vocational and psychosocial outcomes for young adults with autism. Autism, 24(7), 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , le Couteur, A. , & Free Hospital, R. (1994). Autism Diagnostic Interview‐Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , DiLavore, P.C. , Risi, S. , Gotham, K. , & Bishop, S.L. (2012). Autism diagnostic observation schedule (2nd edn). Torrance, CA: Western Psychological Services. [Google Scholar]
- Mawhood, L. , Howlin, P. , & Rutter, M. (2000). Autism and developmental receptive language disorder – A comparative follow‐up in early adult life. Journal of Child Psychology and Psychiatry, 41, 547–559. [DOI] [PubMed] [Google Scholar]
- McCauley, J.B. , Pickles, A. , Huerta, M. , & Lord, C. (2020). Defining positive outcomes in more and less cognitively able autistic adults. Autism Research, 13, 1548–1560. [DOI] [PubMed] [Google Scholar]
- McGovern, C.W. , & Sigman, M. (2005). Continuity and change from early childhood to adolescence in autism. Journal of Child Psychology and Psychiatry, 46, 401–408. [DOI] [PubMed] [Google Scholar]
- Mullen, E.M. (1995). Mullen scales of early learning. San Antonio, TX: Pearson. [Google Scholar]
- Orinstein, A.J. , Helt, M. , Troyb, E. , Tyson, K.E. , Barton, M.L. , Eigsti, I.M. , … & Fein, D.A. (2014). Intervention for optimal outcome in children and adolescents with a history of autism. Advances in Nursing Science, 37, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff, S. , Young, G.S. , Brian, J. , Charman, T. , Shephard, E. , Solish, A. , & Zwaigenbaum, L. (2018). Diagnosis of autism spectrum disorder after age 5 in children evaluated longitudinally since infancy. Journal of the American Academy of Child and Adolescent Psychiatry, 57), 849–857.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles, A. , McCauley, J.B. , Pepa, L.A. , Huerta, M. , & Lord, C. (2020). The adult outcome of children referred for autism: typology and prediction from childhood. Journal of Child Psychology and Psychiatry, 61, 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter, M. , LeCouteur, A. , Lord, C. , Macdonald, H. , Rios, P. , & Folstein, S. (1988). Diagnosis and subclassification of autism: Concepts and instrument development. In Schopler E. & Mesibov G. (Eds.), Diagnosis and subclassification of autism: Concepts and instrument development (pp. 239–259). Plenum. [Google Scholar]
- Ryff, C.D. (1989). Happiness is everything, or is it? Explorations on the meaning of psychological well‐being. Journal of Personality and Social Psychology, 57, 1069–1081. [Google Scholar]
- Seltzer, M.M. , Shattuck, P. , Abbeduto, L. , & Greenberg, J.S. (2004). Trajectory of development in adolescents and adults with autism. Mental Retardation and Developmental Disabilities Research Reviews, 10, 234–247. [DOI] [PubMed] [Google Scholar]
- Shattuck, P.T. , Seltzer, M.M. , Greenberg, J.S. , Orsmond, G.I. , Bolt, D. , Kring, S. , … & Lord, C. (2007). Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. Journal of Autism and Developmental Disorders, 37, 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff, E. , Kent, R. , Stringer, D. , Lord, C. , Briskman, J. , Lukito, S. , … & Baird, G. (2019). Trajectories in symptoms of autism and cognitive ability in autism from childhood to adult life: Findings From a longitudinal epidemiological cohort. Journal of the American Academy of Child & Adolescent Psychiatry, 59, 1342–1352. [DOI] [PubMed] [Google Scholar]
- Sparrow, S.S. , Cicchetti, D.V. , & Balla, D.A. (2005). Vineland adaptive behavior scales (2nd edn). Circle Pines, MN: American Guidance Service. [Google Scholar]
- Wechsler, D. (1999). Manual for the Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler, D. (2008). Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX: Pearson. [Google Scholar]
- Wiggins, L.D. , Baio, J. , Schieve, L.A. , Lee, L.C. , Nicholas, J. , & Rice, C.E. (2012). Retention of autism spectrum diagnoses by community professionals: Findings from the autism and developmental disabilities monitoring network, 2000 and 2006. Journal of Developmental & Behavioral Pediatrics, 33, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman, A.C. , Smith, L.E. , Greenberg, J.S. , & Mailick, M.R. (2014). Change in autism symptoms and maladaptive behaviors in adolescence and adulthood: The role of positive family processes. Journal of Autism and Developmental Disorders, 45, 111–126. 10.1007/s10803-014-2199-2/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachor, D.A. , & Ben‐Itzchak, E. (2020). From toddlerhood to adolescence, trajectories and predictors of outcome: Long‐Term follow‐up study in autism spectrum disorder. Autism Research, 13, 1130–1143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CONSORT diagram for the EDX study.
Figure S2. (a) Overall CSS trajectories for the Lost Diagnosis (VIQ > 70) group by individual ID. (b) Overall CSS trajectories for the Gained Diagnosis (VIQ > 70) group by individual ID.
Table S1. Schedule of Assessments used in study analyses.
Table S2. Change in adaptive skills from 2 to 25.


