Abstract
The global regulator LaeA plays crucial roles in morphological development and secondary metabolite biosynthesis in filamentous fungi. However, the functions of LaeA in basidiomycetes are less reported. The basidiomycete Pleurotus ostreatus is a well-known fungus used both in medicine and as food that produces polysaccharides and cellulolytic enzymes. In this study, we characterized three LaeA homologs (PoLaeA1, PoLaeA2, and PoLaeA3) in P. ostreatus. PoLaeA1 showed different expression patterns than PoLaeA2 and PoLaeA3 during different developmental stages. Silencing PoLaeA1 decreased the intracellular polysaccharide (IPS) content by approximately 28–30% and reduced intracellular ROS levels compared with those of the WT strain. However, silencing PoLaeA2 and PoLaeA3 decreased cellulase activity by 31–34% and 35–40%, respectively, and reduced the cytosolic Ca2+ content, compared with those of the WT strain. Further analysis showed that PoLaeA1 regulated IPS biosynthesis through intracellular ROS levels, whereas PoLaeA2 and PoLaeA3 regulated cellulase activity through intracellular Ca2+ signaling. Our results provide new insights into the regulation of polysaccharide biosynthesis and cellulase production in filamentous fungi.
Keywords: LaeA, fungal growth, cellulase, polysaccharides, Pleurotus ostreatus
1. Introduction
The basidiomycete Pleurotus ostreatus is a well-known fungus used both in medicine and as food that has been cultivated and consumed worldwide. In recent years, the commercial cultivation of P. ostreatus is believed to be one of the fastest-growing sectors in the edible mushroom market [1]. On the one hand, P. ostreatus is commonly used as food or as a food ingredient for its rich nutrient content, including essential unsaturated fatty acids, vitamins, and amino acids [2]. On the other hand, P. ostreatus produces multiple bioactive compounds that possess physiological and therapeutic effects on human chronic disorders. Aqueous extracts of P. ostreatus have been reported to exhibit antioxidant and antihypertensive properties [3]. Among the bioactive compounds in P. ostreatus, polysaccharides are mainly responsible for their significant anti-inflammatory, antitumor, antidiabetic, antioxidant, and immunomodulatory roles [4]. Therefore, the polysaccharide content is becoming a measure of the quality of this fungus, and improving polysaccharide content is an important goal. In addition, P. ostreatus is an important white-rot fungus that produces cellulases and laccases to degrade cellulose and lignin, and this fungus has potential applications in energy production, bioconversion of agricultural wastes, and biodegradation of organo-pollutants and industrial contaminants [5]. Recently, P. ostreatus has been proven to improve biomethane production from rice straw via its cellulase and xylanase activities [6]. Understanding the regulatory mechanisms of cellulases and laccases is an urgent need and is helpful in environmental protection and energy deficiency relief efforts.
In recent years, P. ostreatus has become a potentially good model system for elucidating physiological and chemical processes in basidiomycete fungi, such as the generation of various bioactive compounds and a series of cellulose-degrading enzymes in a short production cycle [7]. A relatively well-annotated genome database of P. ostreatus has been published [8] and is available via GenBank (AYUK00000000), drawing interest in exploring this fungus. Moreover, genetic tools including gene silencing and gene overexpression systems [9] and efficient genetic transformation systems [10], have been applied in P. ostreatus, providing more possibilities to explore the functions of genes of interest.
Global regulators have been found to play crucial roles in morphological development and secondary metabolite biosynthesis and in other biological processes in filamentous fungi [11]. LaeA (loss of aflR expression A) is a key global regulator that was first identified genetically in Aspergillus spp. [12] and is a nuclear protein that is highly conserved in filamentous fungi. Deletion and overexpression of LaeA suggested that LaeA regulates the biosynthesis of sterigmatocystin, penicillin, and lovastatin through their respective metabolic gene clusters [12]. Subsequently, homologs of LaeA were found in other fungi, and studies have confirmed the important roles of LaeA homologs in regulating secondary metabolism and morphological developmental processes. In Chaetomium globosum, CgLaeA is required for pigmentation, sporulation, and chaetoglobosin A production [13]. In Monascus purpureus, overexpression of LaeA not only causes higher monacolin K and pigment production but also results in altered mycelial morphology [14]. In addition to its significant role in secondary metabolism, LaeA also contributes to the fungal virulence of Aspergillus ochraceus [15] and the apple canker pathogen Valsa mali [16], among other fungi. In addition, LaeA was also found to regulate cellulolytic enzyme production in Penicillium oxalicum [17] and Trichoderma reesei [18]. However, studies of the function of LaeA mainly focus on ascomycetes and are relatively rare in basidiomycete fungi due to a lack of effective genetic tools.
Here, three LaeA homologs (PoLaeA1, PoLaeA2, and PoLaeA3) were characterized in P. ostreatus to explore their regulatory role in secondary metabolism and cellulase activities. Silencing any of these LaeA homologous genes caused a defect in the normal growth of P. ostreatus. Further analysis showed that the intracellular ROS level was involved in the regulation of polysaccharide biosynthesis by PoLaeA1. However, PoLaeA2 and PoLaeA3 regulated cellulase activities via the cytosolic Ca2+ content.
2. Materials and Methods
2.1. Microbial Strains and Culture Conditions
The P. ostreatus strain (ACCC50596) obtained from the Agricultural Culture Collection of China was also preserved in the China Center for Mushroom Spawn Standards and Control (CCMSSC00389), and was named P89. The P89 strain was used as a parental strain to construct PoLaeA-silenced strains. The wild-type (WT), empty vector control (Sicontrol) and LaeA-silenced strains (LaeA1i-16, LaeA1i-38, LaeA2i-12, LaeA2i-26, LaeA3i-15 and LaeA3i-32) were grown on complete yeast medium (CYM: 2% glucose, 1% maltose, 0.2% tryptone, 0.2% yeast extract, 0.46% KH2PO4, 0.05% MgSO4·7H2O, 2% agar) at 28 °C [10]. Seed cultures of the fungal strains were prepared in potato dextrose broth at 28 °C and 150 rpm for 5 days. The cultures were broken up with a mechanical crusher under aseptic conditions, after which the cultures were transferred to 100 mL of CYM liquid medium. The fermentation experiments were performed in liquid CYM at 28 °C and 150 rpm for 7 days [19]. The Escherichia coli DH5α strain (TaKaRa, Dalian, China) cultured in lysogeny broth (LB) containing kanamycin (50 μg/mL) was used for plasmid construction. Agrobacterium tumefaciens GV3101 (IMCAS, Beijing, China) used in the transformation of P. ostreatus was grown in LB containing kanamycin (50 μg/mL) and rifampicin (50 μg/mL).
2.2. Gene Cloning and Sequence Analysis
To identify the homologs of LaeA in P. ostreatus, the sequence of LaeA from Hypsizygus marmoreus (GenBank accession number: RDB28437, accessed on 16 May 2022) was used in a homology search of the genome database of P89 [9]. Three LaeA-like genes (PoLaeA1, PoLaeA2, and PoLaeA3 with the GenBank accession numbers ON804804, ON804805, and ON804806, respectively, accessed on 25 June 2022) were identified in the P89 genome database. The coding sequences of these genes were polymerase chain reaction (PCR) amplified using complementary DNA (cDNA) of P89 with the primers listed in Supplemental Table S1. The amplified fragments were cloned into the pMD19-T vector (TaKaRa, Dalian, China) for sequencing. The online tool ProtParam (http://web.expasy.org/protparam/, accessed on 16 May 2022) was used to predict the theoretical molecular weights and isoelectric points (pIs) of PoLaeA1, PoLaeA2, and PoLaeA3. The conserved domains present in the three LaeA-like proteins were analyzed using the NCBI Conserved Domains Database (http://www.ncbi.nlm.nih.gov/cdd, accessed on 16 May 2022). The phylogenetic tree was constructed with the MEGA 6.0 program [20] using the neighbor-joining method with 1000 bootstrap replicates.
2.3. Construction of PoLaeA-RNAi Plasmids and Strains
The original pBHG vector [21] was used to construct PoLaeA-silenced plasmids. Briefly, the sense and antisense fragments of the glyceraldehyde-3-phosphate dehydrogenase (gpd) promoter (GenBank: KY924471, accessed on 16 May 2022) of P89 were PCR amplified and digested and then inserted into pBHG at the corresponding restriction sites to generate the intermediate plasmid pHPG. The antisense fragments of PoLaeA1, PoLaeA2, and PoLaeA3 were PCR amplified and doubly digested with BglⅡ and ApaI, followed by insertion into pHPG to generate the PoLaeA-silenced vectors RNAi-PoLaeA1, RNAi-PoLaeA2, and RNAi-PoLaeA3, respectively (Figure S1). Then, these constructed plasmids were transferred into P. ostreatus using A. tumefaciens-mediated transformation [10]. The transformants were randomly selected on CYM plates containing 90 μg/mL hygromycin B (hyg). In addition, the SiControl was constructed with pHPG without PoLaeA fragments. The silencing efficiency of these randomly selected PoLaeA-silenced strains randomly selected was analyzed by quantitative real-time PCR (qRT–PCR). The PoLaeA-silenced strains (LaeA1i-16, LaeA1i-38, LaeA2i-12, LaeA2i-26, LaeA3i-15, and LaeA3i-32) with higher silencing efficiency were selected and used for subsequent studies. The primers used are listed in Supplemental Table S1.
2.4. Measurement of Mycelial Growth and Biomass
The WT, SiControl and PoLaeA-silenced strains were cultured on CYM plates at 28 °C for 7 days to detect the mycelial diameters of the resulting colonies. The relative mycelial diameters of the tested strains = (the mean diameter of the tested strains)/(the mean diameter of the WT strain) × 100%. Then, the dry weight (DW) of the fungal mycelia collected from CYM plates was measured gravimetrically after drying at 60 °C.
2.5. Measurement of the Intracellular Reactive Oxygen Species (ROS) Level
The fungal strains (WT, SiControl, and PoLaeA-silenced strains) were cultured on CYM plates. Sterilized cover slips were obliquely inserted into the CYM plates adjacent to the fungal mycelia. After the fungal mycelia had grown onto the cover slips, the cover slips were stained with 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and then incubated at 28 °C for 30 min. The green fluorescence of H2O2 in fungal hyphae was measured with an LSM780 confocal laser scanning microscope (Zeiss, Jena, Germany) [19]. Fluorescence intensity was analyzed by ZEN 3.3 (blue edition). In addition, the fungal hyphae collected from liquid CYM cultures were used to measure the intracellular H2O2 content with a commercial hydrogen peroxide assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions of the manufacturer [19]. The malondialdehyde (MDA) content of the fungal mycelia was determined as described previously [22]. Briefly, fungal mycelia were ground with liquid nitrogen, and then 0.5 mL of 5% trichloroacetic acid (TCA) was added. After centrifugation at 10,000× g for 10 min, the supernatant was collected and mixed with 0.5 mL of 0.67% β-thiobarbituric acid (TBA). The mixture was subsequently incubated at 95 °C for 10 min and then centrifuged at 10,000× g for 10 min. The absorbance of the supernatant was detected at 532 nm and corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. The MDA content was calculated using the MDA extinction coefficient of 155 mM−1 cm−1 at 532 nm.
2.6. Enzymatic Activity Determination
The WT, SiControl and PoLaeA-silenced strains were cultured in liquid CYM at 28 °C for 5 days, and the fungal hyphae were ground with liquid nitrogen, and suspended in 5 mL phosphate-buffered saline (PBS) buffer. After centrifugation at 12,000× g for 10 min at 4 °C, the supernatants were collected to measure the enzymatic activity of the antioxidant systems. The protein content was determined with the Bradford method with albumin from bovine serum as a standard. Ascorbate peroxidase (APX) activity was detected using a commercial ascorbate peroxidase assay kit (Nanjing jiancheng Bioengineering Institute, Nanjing, China). Catalase (CAT) activity was detected using a commercial catalase assay kit (Nanjing jiancheng Bioengineering Institute, Nanjing, China). Glutathione peroxidase (GPX) activity was measured by using a cellular glutathione peroxidase assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Superoxide dismutase (SOD) were determined by monitoring the inhibition of the photochemical reduction of nitro blue tetrazolium [22].
To measure the endoglucanase (CMCase) activities of the fungal mycelia, the fungal strains were cultured in liquid CYM at 28 °C for 5 days, and the cultures were washed with 0.85% (w/v) NaCl, followed by transfer to MCM medium (1% cellulose, 0.05% MgSO4•7H2O, 0.5% (NH4)2SO4, 0.46% KH2PO4 and 2 mL/L trace element) with or without CaCl2 (5 mM) at 28 °C for 2 days. The culture supernatants were collected to detect the CMCase activity. A 2.0 mL reaction mixture (containing 0.5 mL diluted culture supernatants and 1.5 mL of 1% carboxymethylcellulose sodium salt) was transferred into a 25 mL tube and mixed followed by incubation at 50 °C for 30 min. 3 mL of DNS reagent were added to stop the reaction. A blank tube (with boiled crude enzyme) was used as a control to correct for the presence of reducing sugars in the crude enzyme samples [23]. The absorbance of the reaction mixture was detected at 540 nm with a spectrophotometer UV-1800 (Shimadzu Corporation, Kyoto, Japan). One unit of enzyme activity was defined as the amount of enzyme required to release 1 μmol of glycoside bonds of substrate per minute under defined assay conditions.
2.7. Measurement of the Cytosolic Ca2+ Content
Fluo-3AM (Invitrogen) is a membrane-permeable compound that can be hydrolyzed into Fluo-3 (a Ca2+-binding form) by endogenous esterase and was used as a fluorescent calcium indicator dye to measure the cytosolic Ca2+ level in fungal cells. The WT, SiControl and PoLaeA-silenced strains were cultured on CYM plates. Sterilized cover slips were obliquely inserted into the CYM plates adjacent to the fungal mycelia. After the fungal mycelia had grown onto the cover slips, the cover slips were stained with 50 μM Fluo-3AM and then incubated for 30 min. The fungal hyphae were viewed microscopically with an LSM780 confocal laser scanning microscope (Zeiss, Jena, Germany) [23].
2.8. Determination of Intracellular Polysaccharide Content
The WT, SiControl and PoLaeA-silenced strains were cultured in liquid CYM at 28 °C for 7 days, and the fungal hyphae were collected. The intracellular polysaccharides (IPS) were extracted and analyzed as described previously [24]. Briefly, mycelia were washed with distilled water repeatedly and dried at 60 °C. Then the IPS of fungal mycelia was extracted with NaOH (1 M) and the supernatant was detected with the phenol-sulfuric acid method using glucose as a standard.
2.9. Analysis of Gene Expression with qRT–PCR
Synthesis of first-strand cDNA from the fungal strains (WT, SiControl, and PoLaeA-silenced strains) was performed with 5 × All-In-One RT MasterMix (ABM, Richmond, VA, Canada). qRT–PCR was performed with a Realplex2 System (Eppendorf, Hamburg, Germany) using EvaGreen 2 × qPCR MasterMix-S (ABM, Richmond, Canada) as described previously [25]. The glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) was used as a standard control. The 2−△△CT method [26] was used to analyze the relative gene-specific mRNA expression levels. The primers used here are listed in Supplemental Table S1.
2.10. Data Analysis
Statistical analysis was carried out using GraphPad Prism Version 7.00 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com, accessed on 16 May 2022). All experiments were conducted in three biological replications with similar results. The mean values are interpreted as the mean ± standard deviation (SD). The error bars indicate the SD from the means of triplicates. One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test was used to analyze the differences (p < 0.05) in the mean values between the analyzed samples.
3. Results
3.1. LaeA-like Genes in P. ostreatus
To identify LaeA homologs in P. ostreatus, LaeA of Hypsizygus marmoreus was used to BLAST against the genome database of P89. Using the criteria of E values of <1.0 × 10−50, three LaeA homologs were identified and termed PoLaeA1, PoLaeA2, and PoLaeA3. The open reading frames of PoLaeA1, PoLaeA2, and PoLaeA3 are 1212, 756, and 699 bp, respectively, and are interrupted by six, six, and five introns, respectively. The PoLaeA1 gene encodes a 45.75-kDa protein of 403 amino acids with a pI of 4.78; PoLaeA2 encodes a 28.29-kDa protein of 251 amino acids with a pI of 5.44; and PoLaeA3 encodes a 25.86 kDa protein of 232 amino acids with a pI of 4.32, respectively.
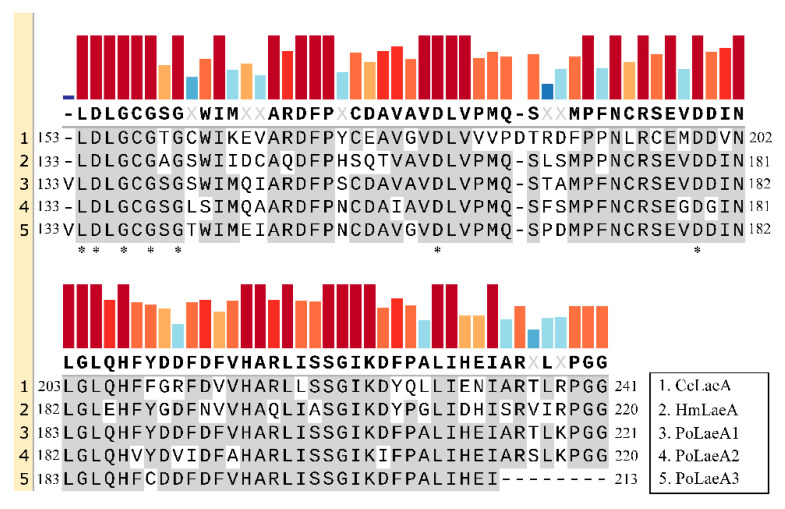
Domain analysis showed that all three LaeA-like proteins (PoLaeA1, PoLaeA2, and PoLaeA3) contain one Methyltransf_25 domain and belong to the AdoMet_Mtases superfamily, which uses S-adenosylmethionine (SAM) as a substrate for methyltransfer (Figure S2A–C). In addition, these LaeA-like proteins revealed a reciprocal best BLAST hit to LaeA of Hypsizygus marmoreus and Coprinopsis cinerea, and alignments of these proteins demonstrated that structural homology with the SAM binding motif [12] is highly conserved (Figure 1). In addition, we investigated the divergence of the three LaeA-like proteins in P. ostreatus from other known LaeA proteins in filamentous fungi. The phylogenetic tree could be divided into two major groups (ascomycetes and basidiomycetes). The three LaeA-like proteins in P. ostreatus are most homologous to LaeA in Hypsizygus marmoreus and distinct from those of the ascomycetes (Figure S2D).
Figure 1.
Amino acid sequence alignments of the conserved domain in LaeA-like proteins from basidiomycetes. The conserved domains were identified by Pfam (https://pfam.xfam.org/, accessed on 16 May 2022). The GenBank accession numbers for the LaeA-like proteins are Coprinopsis cinerea CcLaeA (XP_001829319, accessed on 16 May 2022) and Hypsizygus marmoreus HmLaeA (RDB28437, accessed on 16 May 2022). The results were constructed with SnapGene software (version 6.0.5) using the multiple sequence comparison function. Asterisks indicate the putative S-adenosylmethionine (SAM) binding sites in the conserved domain.
3.2. Expression of LaeA-like Genes during Different Developmental Stages of P. ostreatus
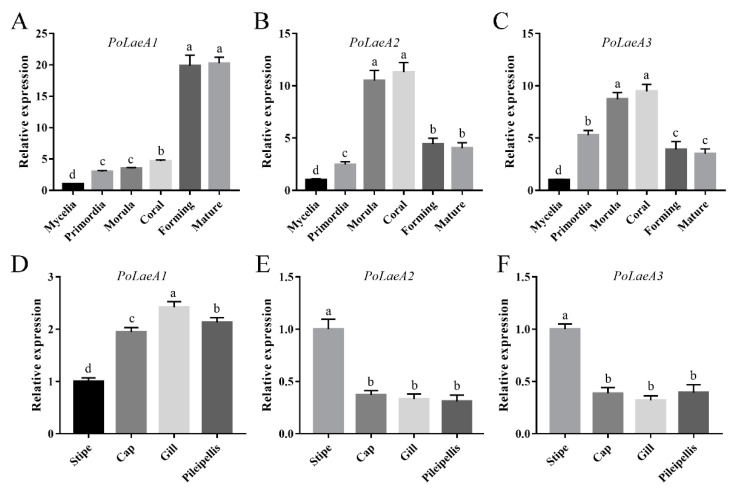
The developmental stages of P. ostreatus include mycelia, primordia, morula, coral, forming, and mature stages (Figure S3), and we first examined the expression patterns of the LaeA-like genes during P. ostreatus development. The results showed that the expression of the three LaeA-like genes was significantly lower in mycelia than in any other stage. The expression of PoLaeA1 was highest in the forming and mature stages and was 19.88- and 20.27-fold that of the mycelia (Figure 2A), respectively. However, the expression of PoLaeA2 and PoLaeA3 was highest in the morula and coral stages compared with expression in the mycelia (Figure 2B,C), respectively. In addition, we investigated the expression patterns of LaeA-like genes in different parts of the fruiting bodies of the WT strain. The expression of PoLaeA1 was upregulated in the cap (1.95-fold), gill (2.42-fold), and pileipellis (2.13-fold) than in the stipe (Figure 2D). In contrast, the highest expression of PoLaeA2 and PoLaeA3 was in the stipe (Figure 2E,F). These results indicate that these LaeA-like genes are required for the development of P. ostreatus but might play different roles in development.
Figure 2.
The expression of LaeA-like genes during different developmental stages and in different parts of fruiting bodies. (A–C) qRT–PCR analysis of LaeA-like gene expression in the WT strain during different developmental stages. The relative abundances of PoLaeA1 (A), PoLaeA2 (B) and PoLaeA3 (C) transcripts at different stages were normalized by comparison with those levels in the mycelial stage (relative transcript level = 1.0). (D–F) Expression of LaeA-like genes in different parts of fruiting bodies. The relative abundances of PoLaeA1 (D), PoLaeA2 (E) and PoLaeA3 (F) transcripts in different parts of fruiting bodies were normalized by comparison with those levels in the stipe (relative transcript level = 1.0). The values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the analyzed samples (p < 0.05, according to Dunnett’s multiple comparisons test).
3.3. Construction of P. ostreatus LaeA-Silenced Strains
To investigate the roles of the LaeA-like genes in physiological processes, PoLaeA-silencing plasmids (RNAi-PoLaeA1, RNAi-PoLaeA2, and RNAi-PoLaeA3) were constructed using the pBHG vector, which contains the Hyg resistance gene as a selectable marker (Figure S1) and used to transform P. ostreatus. qRT–PCR analysis was used to measure the expression of LaeA-like genes and confirm the silencing efficiency in the randomly selected transformants. The expression of PoLaeA1 in LaeA1i-16 and LaeA1i-38 was downregulated by 74% and 70%, and the expression of PoLaeA2 in LaeA2i-12 and LaeA2i-26 was downregulated by 82% and 79%, and the expression of PoLaeA3 in LaeA3i-15 and LaeA3i-32 was downregulated by 76% and 85%, respectively, compared with WT expression (Figure S1). Therefore, these PoLaeA-silenced strains were used for further study.
3.4. LaeA-like Genes Are Required for the Fungal Growth of P. ostreatus
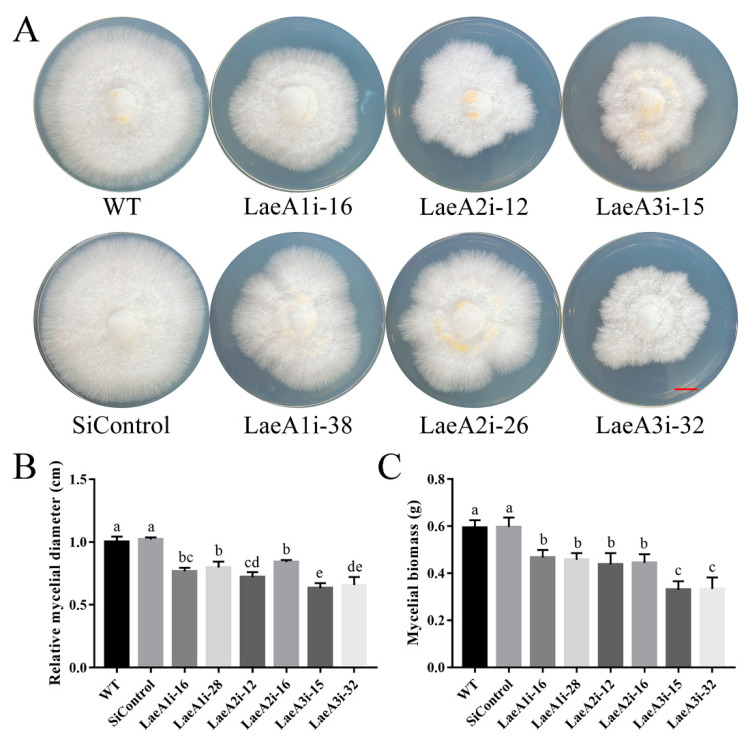
To determine whether LaeA-like genes impact the growth of P. ostreatus, the WT, SiControl and PoLaeA-silenced strains were cultured on CYM plates to measure the resulting mycelial diameters. All PoLaeA-silenced strains grew much more slowly than the WT strain (Figure 3A). The mycelial diameters of LaeA1i-16, LaeA1i-38, LaeA2i-12, LaeA2i-26, LaeA3i-15, and LaeA3i-32 were approximately 23%, 20%, 28%, 16%, 37%, and 34% smaller, respectively, than that of the WT strain (Figure 3B). In addition, we measured the biomass of PoLaeA-silenced strains cultured in liquid CYM. The dry weights (DW) of PoLaeA-silenced strains were significantly lower, by approximately 21–44% than that of the WT strain (Figure 3C). These results together reveal that silencing PoLaeA1, PoLaeA2 or PoLaeA3 causes a defect in the normal growth of P. ostreatus.
Figure 3.
Hyphal growth and biomass of PoLaeA-silenced strains. (A) Fungal growth of P. ostreatus strains grown on CYM plates at 28 °C for 7 days. (Red scale bar = 1 cm). (B) Mycelial diameters. (C) Mycelial biomass. The values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the strains (p < 0.05, according to Dunnett’s multiple comparisons test).
3.5. Silencing PoLaeA1 Decreased the Intracellular ROS Levels of P. ostreatus
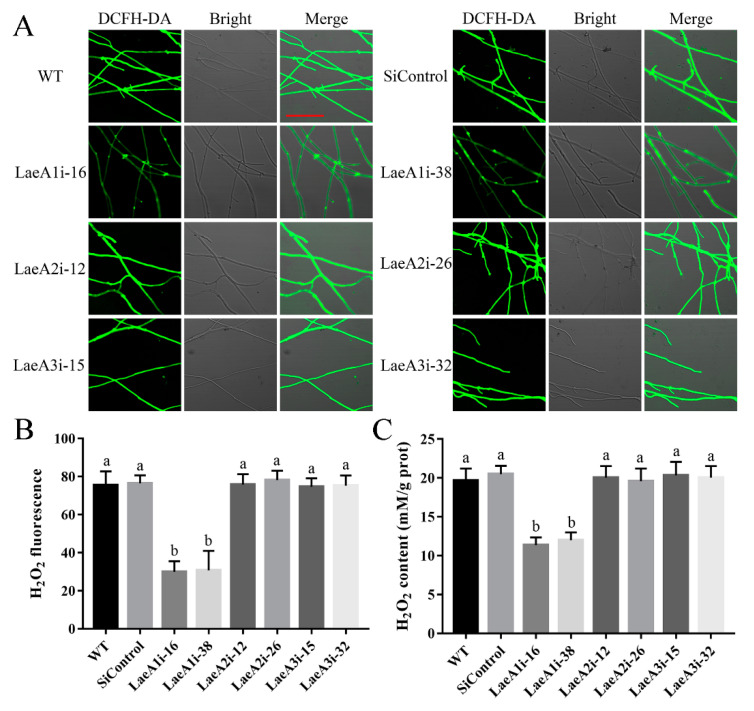
To decipher how LaeA-like genes affect fungal growth, we first examined intracellular ROS signaling in the PoLaeA-silenced strains using an H2O2 fluorescence probe (DCFH-DA). Figure 4A shows that the H2O2 fluorescence intensity of the PoLaeA1-silenced strains (LaeA1i-16 and LaeA1i-38) was significantly decreased, and the H2O2 fluorescence values in LaeAi-16 and LaeAi-38 were 40% and 41% that of the WT strain (Figure 4B), respectively. Furthermore, we tested the H2O2 levels of the PoLaeA-silenced strains. The intracellular H2O2 levels in LaeA1i-16 and LaeA1i-38 were 11.37 and 12.01 mM/g protein and were reduced by 42% and 39% (Figure 4C), respectively, compared with that of the WT strain. The MDA content can reflect the status of intracellular ROS [22]. In addition, the MDA content in LaeA1i-16 and LaeA1i-38 was reduced by approximately 45% and 49%, respectively, compared with that of the WT strain (Figure S4). However, the H2O2 fluorescence intensity, the intracellular H2O2 levels, and the MDA content in the PoLaeA2-silenced strains (LaeA2i-12 and LaeA2i-26) and PoLaeA3-silenced strains (LaeA3i-15 and LaeA3i-32) did not differ from those of the WT strain (Figure 4 and Figure S4). These results indicate that silencing PoLaeA1 reduces intracellular ROS levels in P. ostreatus.
Figure 4.
Silencing PoLaeA1 decreased the intracellular ROS levels. (A) DCFH-DA staining (scale bar = 100 μm). The tested strains cultured on CYM plates were stained with the fluorescent H2O2 probe DCFH-DA. (B) The H2O2 fluorescence values. The fluorescence was analyzed with ZEN 3.3 (blue edition). (C) Intracellular H2O2 levels. The tested strains were cultured in liquid CYM at 28 °C for 5 days. The grounded mycelia were used to determine the intracellular H2O2 levels with a commercial hydrogen peroxide assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the strains (p < 0.05, according to Dunnett’s multiple comparisons test).
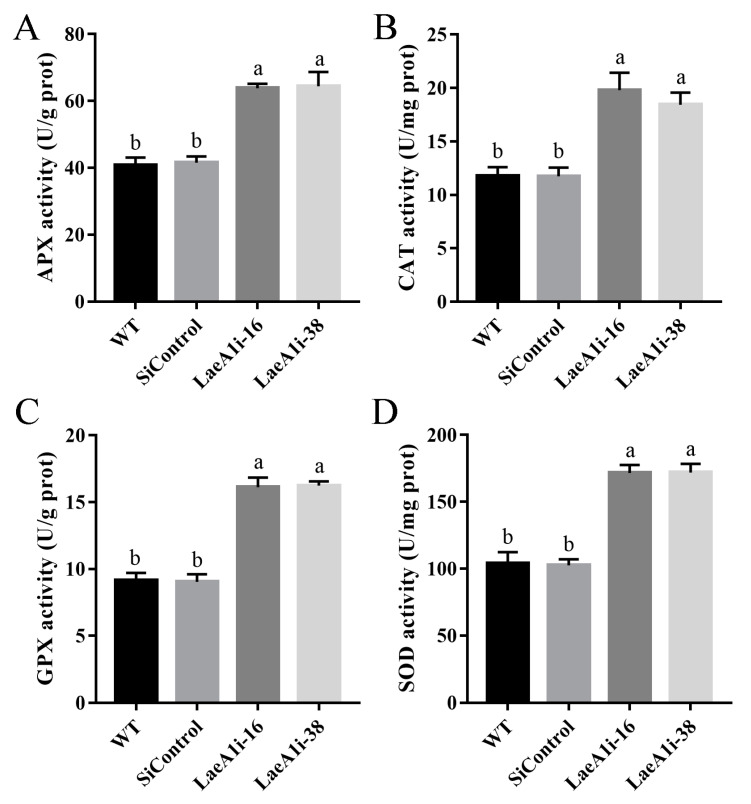
Next, we examined the major enzymes involved in the antioxidant system to understand how PoLaeA1 affects intracellular ROS levels. Figure 5 shows that the activities of APX (catalyzes the oxidation of ascorbic acid), CAT (decomposes H2O2), GPX (transfers glutathione to glutathione disulfide), and SOD (superoxide dismutase) in LaeA1i-16 and LaeA1i-38 were approximately 57–58%, 56–68%, 76–78%, and 64–65% higher, respectively, than those of the WT strain. Meanwhile, the expression of the genes APX, CAT1, CAT2, GPX, SOD1, and SOD2 in LaeA1i-16 and LaeA1i-38 was significantly upregulated compared with expression in the WT strain (Figure S5). These results imply that silencing PoLaeA1 increases the activity of enzymes involved in the antioxidant system.
Figure 5.
The activities of enzymes involved in the antioxidant system in PoLaeA1-silenced strains. The P. ostreatus strains (WT, SiControl, LaeA1i-16 and LaeA1i-38) were cultured in liquid CYM at 28 °C for 5 days. The grounded mycelia were suspended in phosphate-buffered saline buffer. After centrifuging, the supernatants were used to detect the enzyme activities involved in ROS scavenging. (A) APX activity. (B) CAT activity. (C) GPX activity. (D) SOD activity. The values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the strains (p < 0.05, according to Dunnett’s multiple comparisons test).
3.6. Silencing PoLaeA2 and PoLaeA3 Reduced the Cytosolic Ca2+ Content of P. ostreatus
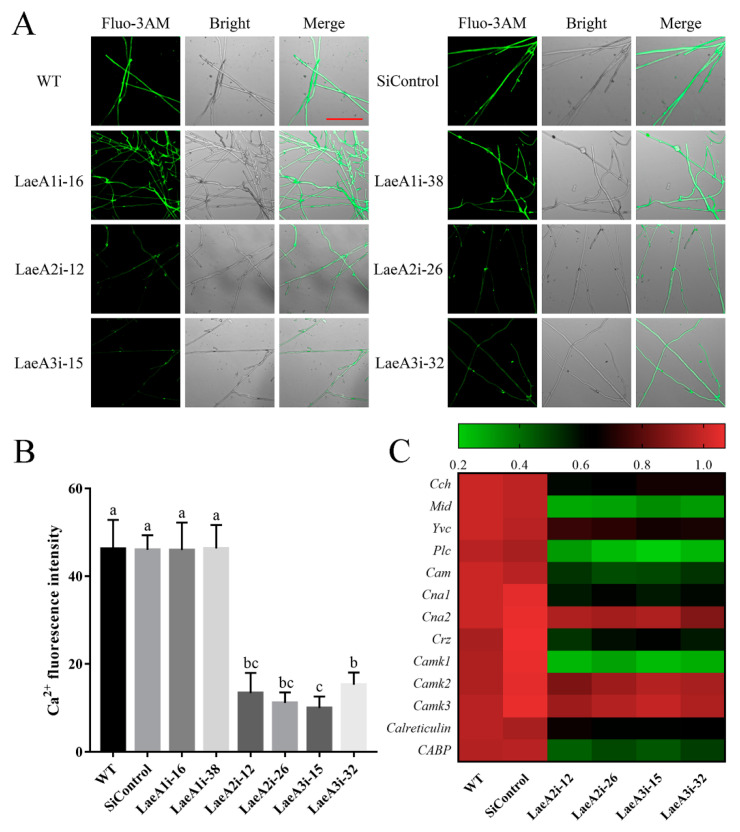
Ca2+ signaling plays a crucial role in fungal growth, and we measured the cytosolic Ca2+ content in PoLaeA-silenced strains using the Ca2+ fluorescence probe Fluo-3AM. As shown in Figure 6A, the cytosolic Ca2+ content was lower in the PoLaeA2- and PoLaeA3-silenced strains than in the WT strain. Fluorescence analysis showed that the Ca2+ fluorescence values in PoLaeA2- or PoLaeA3-silenced strains were approximately 67–78% lower than that of the WT strain (Figure 6B). However, the cytosolic Ca2+ content in the PoLaeA1-silenced strains was not different from that of the WT strain. Moreover, we examined the transcriptional regulation of Ca2+ signaling in the PoLaeA2- and PoLaeA3-silenced strains, including Ca2 + -permeable channel (Cch and Mid), vacuole Ca2+ channel (Yvc), phospholipase C (Plc), calmodulin (Cam), calcineurin (Cna1 and Cna2), calcineurin-responsive zinc finger transcription factor (Crz), Ca2+/Cam-dependent protein kinase (Camk1, Camk2 and Camk3), and calreticulin and Ca2+ binding protein (CABP). With the exceptions of Cna2, Camk2, and Camk3, the expression of Ca2+ signaling genes in PoLaeA2- and PoLaeA3-silenced strains was significantly downregulated compared with WT expression (Figure 6C). These results suggest that silencing PoLaeA2 and PoLaeA3 decreased the cytosolic Ca2+ content of P. ostreatus.
Figure 6.
The cytosolic Ca2+ content in the PoLaeA-silenced strains. The fungal strains were cultured on CYM plates at 28 °C for 4 days. (A) Fluo-3AM staining (scale bar = 100 μm). For detection, 50 μM Fluo-3AM, a Ca2+ fluorescent probe, was used to monitor the fluorescence intensity using an LSM780 confocal laser scanning microscope. Green fluorescence represents the free cytosolic Ca2+. (B) Ca2+ fluorescence values. The fluorescence was analyzed with ZEN 3.3 (blue edition). (C) Transcriptional analysis of Ca2+-related genes. The expression levels of the Ca2+-related genes in the WT strain were arbitrarily set to 1.0. The values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the strains (p < 0.05, according to Dunnett’s multiple comparisons test).
3.7. The IPS Contents Were Decreased in PoLaeA1-Silenced Strains and Could Be Recovered by H2O2
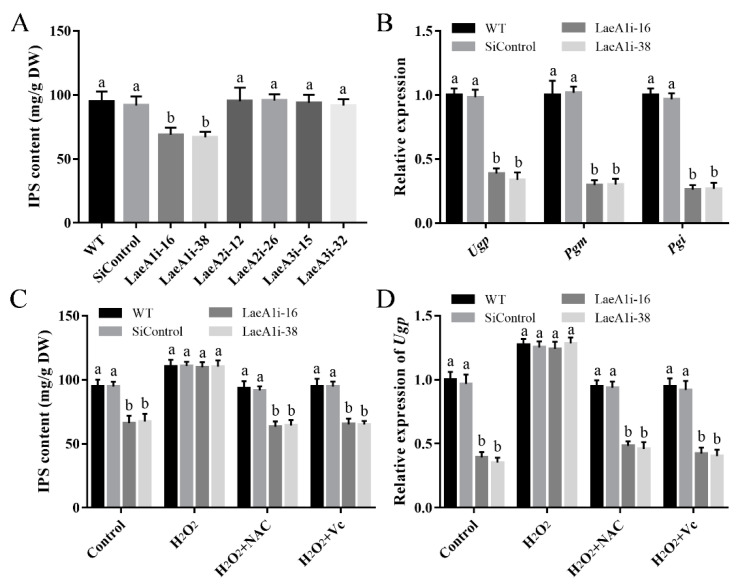
To test whether the LaeA-like genes affect the metabolites of P. ostreatus, the IPS contents of the PoLaeA-silenced strains were determined. The IPS contents in LaeA1i-16 and LaeA1i-38 were 68.95 and 66.94 mg/g DW and were approximately 28% and 30% lower than that of the WT strain (Figure 7A), respectively. However, the IPS contents of the PoLaeA2- and PoLaeA3-silenced strains were not different from that of the WT strain. Meanwhile, the transcripts of three key genes involved in polysaccharide biosynthesis, namely, Ugp (encodes UDP-glucose pyrophosphorylase), Pgm (encodes phosphoglucomutase), and Pgi (encodes glucose-6-phosphate isomerase), were all significantly lower in the PoLaeA1-silenced strains than in the WT strain (Figure 7B). The decreased IPS contents and reduced ROS content in the PoLaeA1-silenced strains might be related; to test this hypothesis, we added 1 mM H2O2 to the PoLaeA1-silenced strains. Figure 7C shows that adding H2O2 restored the decreased IPS contents in the PoLaeA1-silenced strains to the levels found in the WT strain. In addition, the ROS scavengers N-acetylcysteine (NAC) and ascorbate (Vc) were used to confirm the effect of H2O2 on the restored IPS contents in PoLaeA1-silenced strains. As expected, the IPS contents in the PoLaeA1-silenced strains decreased again after treatment with H2O2 plus 1 mM NAC or 1 mM Vc (Figure 7C). In addition, the downregulated expression of Ugp in PoLaeA1-silenced strains was restored to that of the WT strain when H2O2 was added and decreased again after treatment with H2O2 plus NAC or Vc (Figure 7D), presenting the same trend as the IPS contents. These results together indicate that the intracellular ROS level is involved in polysaccharide biosynthesis regulated by PoLaeA1.
Figure 7.
The intracellular ROS level was involved in the regulation of intracellular polysaccharide (IPS) content by PoLaeA1. The P. ostreatus strains were cultured in liquid CYM treatment with or without 1 mM H2O2, 1 mM NAC or 1 mM Vc at 28 °C for 7 days. (A) IPS content of the PoLaeA-silenced strains. (B) Transcriptional analysis of polysaccharide biosynthesis-related genes. (C) H2O2 recovered the decreased IPS content in the PoLaeA1-silenced strains. The IPS contents in the fungal mycelia were measured with the phenol-sulfuric acid method using glucose as a standard. (D) Transcriptional analysis of Ugp. The expression level of the Ugp gene in the WT strain of the control was arbitrarily set to 1.0. The values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the strains (p < 0.05, according to Dunnett’s multiple comparisons test).
3.8. The Cytosolic Ca2+ Content Affected the Regulation of Cellulase Activities by PoLaeA2 and PoLaeA3
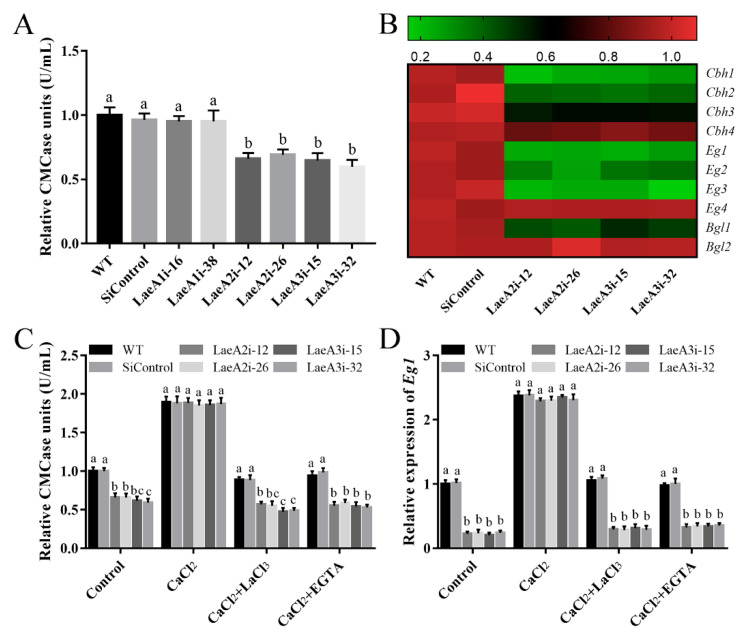
Cellulase activity is important for fungal growth; therefore, we measured endoglucanase (CMCase) activity to investigate the influence of LaeA-like genes on cellulase production in P. ostreatus. Figure 8A shows significantly lower CMCase activities in the PoLaeA2- and PoLaeA3-silenced strains, approximately 31–34% and 35–40% lower than that of the WT strain, respectively. Furthermore, the expression of genes related to cellulase (Cbh1, Cbh2, Cbh3, and Cbh4, encode cellobiohydrolases; Eg1, Eg2, Eg3, and Eg4, encode endoglucanases; Bgl1 and Bgl2, encode beta-glucosidases) was examined. The expression of three of the Cbh genes (Cbh1, Cbh2, and Cbh3), three of the Eg genes (Eg1, Eg2, and Eg3) and one of the Bgl genes (Bgl1) was significantly decreased in the PoLaeA2- or PoLaeA3-silenced strains compared with that in the WT strain (Figure 8B). These results indicate that silencing PoLaeA2 and PoLaeA3 causes a reduction in cellulase activity in P. ostreatus.
Figure 8.
The cytosolic Ca2+ content was involved in the regulation of cellulase activity by PoLaeA2 and PoLaeA3. The P. ostreatus strains cultured in liquid CYM at 28 °C for 5 days were transferred into MCM using cellulose as the sole carbon source, and treated with 5 mM CaCl2, LaCl3 or EGTA for 24 h. (A) CMCase (endoglucanase) activities of the PoLaeA-silenced strains. (B) Relative expression of the cellulase genes. The expression levels of the cellulase genes in the WT strain were arbitrarily set to 1.0. (C) CaCl2 recovered the decreased CMCase activities in the PoLaeA2- and PoLaeA3-silenced strains. (D) Transcriptional analysis of Eg1. The values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the strains (p < 0.05, according to Dunnett’s multiple comparisons test).
We further examined the relationship between the decreased Ca2+ content and the decreased cellulase activity in the PoLaeA2- and PoLaeA3-silenced strains. Figure 8C shows that adding 5 mM CaCl2 recovered the decreased CMCase activities in the PoLaeA2- and PoLaeA3-silenced strains to the levels found in the WT strain. Moreover, two Ca2+ inhibitors (LaCl3 and EGTA) were used to confirm the effect of Ca2+ content on the CMCase activities in the PoLaeA2- and PoLaeA3-silenced strains. As expected, the CMCase activities in the PoLaeA2- and PoLaeA3-silenced strains were decreased again after treatment with CaCl2 plus 5 mM EGTA or 5 mM LaCl3 (Figure 8C). In addition, the downregulated expression of EG1 in the PoLaeA2- and PoLaeA3-silenced strains was restored to that of the WT strain when CaCl2 was added and decreased again after treatment with CaCl2 plus EGTA or LaCl3 (Figure 8D). Together, these results imply that PoLaeA2 and PoLaeA3 improved cellulase activities through Ca2+ signaling.
4. Discussion
LaeA is a global regulator that plays a crucial role in secondary metabolite biosynthesis in filamentous fungi [27]. Since its initial identification in Aspergillus nidulans [12], numerous orthologs of LaeA have been identified and found to be highly conserved in filamentous fungi. LaeA and its orthologs contain a SAM binding domain and have some similarities to methyltransferases [28]. Numerous studies have revealed that LaeA orthologs are important for secondary metabolism biosynthesis and morphological development and for differentiation in filamentous fungi [11]. However, the functions of LaeA and its orthologs in edible fungi are relatively unknown. In the present study, we identified and characterized three LaeA homologs (PoLaeA1, PoLaeA2, and PoLaeA3) in P. ostreatus. Despite their similar roles in fungal growth, PoLaeA1 has different roles in secondary metabolism and cellulase production than PoLaeA2 and PoLaeA3 in P. ostreatus. Similarly, the LaeA-like genes (LaeA, LaeA2, and LaeA3) play different roles in citric acid accumulation in Aspergillus luchuensis mut. kawachii [29].
In ascomycetes, LaeA and its orthologs have a minor effect on fungal growth but are reported to participate in morphological and fungal development. The developmental regulatory role of LaeA has been well established in ascomycetes. In Aspergillus nidulans, LaeA is reported to control both sexual and asexual development [30] and is involved in light-responding developmental regulation [31]. In the present study, all three LaeA homologs were expressed at lower levels in mycelia than in any other developmental stage, implying that they are also required for fungal development. In addition, PoLaeA1 showed different expression patterns than PoLaeA2 and PoLaeA3 during the developmental stages of P. ostreatus (Figure 3), indicating that PoLaeA1 might play different roles than PoLaeA2 and PoLaeA3. Furthermore, we found that all three LaeA homologs were required for normal growth in P. ostreatus. Similarly, loss of LaeA decreases the colony diameters in Aspergillus ochraceus [15]. Nevertheless, given the few reports of LaeA on fungal growth in ascomycetes, its role in the regulation of fungal growth in basidiomycetes might differ from that of LaeA in ascomycetes.
As a global regulator, LaeA has been well characterized in secondary metabolism in numerous ascomycete fungi but in a few basidiomycetes. LaeA is reported to regulate secondary metabolism by forming velvet complexes to induce chromatin modification [32]. To date, the regulatory roles of LaeA on secondary metabolism-related genes, such as sterigmatocystin [12] and dothistromin [33] have been extensively studied. In addition, LaeA was also found to regulate the biosynthesis of organic acids. Knockout of LaeA eliminated kojic acid biosynthesis in Aspergillus oryzae [34] and inhibited citric acid production in Aspergillus luchuensis mut. kawachii [29]. Consistently, silencing PoLaeA1 decreased IPS production in P. ostreatus. In addition, silencing PoLaeA1 also decreased the intracellular ROS levels of P. ostreatus. Our further study showed that PoLaeA1 regulated IPS production through intracellular ROS signaling. Consistent with our results, intracellular ROS signaling is also involved in the regulation of secondary metabolism in Ganoderma lucidum [35]. However, the knockout of LaeA in Coprinopsis cinerea unexpectedly upregulates the biosynthesis of coprinoferrin [36]. These findings indicate that the role of LaeA in the regulation of secondary metabolism seems to be species specific in basidiomycetes.
Many filamentous fungi secrete cellulolytic enzymes, including cellulases, that are helpful in biofuel refining. Recently, LaeA was also found to regulate cellulase production in several ascomycetes. In Penicillium oxalicum, LaeA is reported to positively regulate prominent cellulase and hemicellulase but negatively regulate β-xylosidase formation [17]. In Trichoderma reesei, the deletion of LaeA completely blocks the expression of all seven cellulases, β-glucosidases, and xylanases [18]. As expected, we found that silencing PoLaeA2 and PoLaeA3 decreased cellulase production and downregulated the expression of cellulase-related genes. The signals involved in the regulation of cellulase production are limited thus far. The Ca2+-responsive signaling pathway is reported to play a crucial role in regulating cellulase production in Trichoderma reesei [37]. In addition, Ca2+ signaling is also involved in the regulation of cellulase by MAPK signaling [23]. These studies suggest that Ca2+ signaling is essential for cellulase production in filamentous fungi. However, the relationship between Ca2+ signaling and LaeA has not been reported. Our study showed that PoLaeA2 and PoLaeA3 positively regulated cellulase activities in Ca2+ signaling. Moreover, we found that PoLaeA2 and PoLaeA3 positively regulated the cytosolic Ca2+ content in P. ostreatus. In addition, silencing PoLaeA2 and PoLaeA3 downregulated the expression of Ca2+ signaling genes, demonstrating that PoLaeA2 and PoLaeA3 could positively regulate the influx of Ca2+. In addition, our pharmacological experiments indicated that the cytosolic Ca2+ content promotes the regulation of cellulase activities by PoLaeA2 and PoLaeA3. However, the specific regulatory mechanism of PoLaeA2 and PoLaeA3 on cellulase is still unclear and should be clarified in the future. Nevertheless, our study provides new insights into the regulation of cellulase production in filamentous fungi.
In summary, we characterized three LaeA homologs (PoLaeA1, PoLaeA2, and PoLaeA3) in P. ostreatus. These LaeA orthologs are all required for the normal growth of P. ostreatus. PoLaeA1 plays different roles than PoLaeA2 and PoLaeA3 in P. ostreatus. PoLaeA1 regulates IPS content through intracellular ROS levels, whereas PoLaeA2 and PoLaeA3 regulate cellulase activity via cytosolic Ca2+ signaling. Our findings provide new insights into the regulation of polysaccharide biosynthesis and cellulase production in filamentous fungi.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8090902/s1, Figure S1: Construction of PoLaeA-silenced plasmids and strains; Figure S2: Protein domain structures and phylogenetic analysis of PoLaeA1, PoLaeA2 and PoLaeA3; Figure S3: The diagram of the developmental phases and structures of Pleurotus ostreatus; Figure S4: The Malondialdehyde (MDA) content of the PoLaeA-silenced strains; Figure S5: Transcriptional analysis of ROS-related genes in the PoLaeA1-silenced strains; Table S1: Oligonucleotide primers used.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z. and P.Y.; software, D.L. and P.Y.; validation, G.Z., P.Y. and D.L.; formal analysis, G.Z. and P.Y.; investigation, G.Z.; resources, G.Z. and C.Z.; data curation, L.S.; writing—original draft preparation, G.Z. and P.Y.; writing—review and editing, G.Z. and P.Y.; visualization, L.S.; supervision, Z.W. (Zhongwei Wu); project administration, Z.W. (Zhenhe Wang); funding acquisition, G.Z. and C.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number: 31902090, Science and Technology Project of Henan Province, grant number: 222102110431 and 202102110019.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koutrotsios G., Danezis G., Georgiou C., Zervakis G.I. Elemental content in Pleurotus ostreatus and Cyclocybe cylindracea mushrooms: Correlations with concentrations in cultivation substrates and effects on the production process. Molecules. 2020;25:2179. doi: 10.3390/molecules25092179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez A., Nobre C., Simões L.S., Cruz M., Loredo A., Rodríguez-Jasso R.M., Contreras J., Texeria J., Belmares R. Evaluation of functional and nutritional potential of a protein concentrate from Pleurotus ostreatus mushroom. Food Chem. 2021;346:128884. doi: 10.1016/j.foodchem.2020.128884. [DOI] [PubMed] [Google Scholar]
- 3.Agunloye O.M. Effect of aqueous extracts of Pleurotus ostreatus and Lentinus subnudus on activity of adenosine deaminase, arginase, cholinergic enzyme, and angiotensin-1-converting enzyme. J. Food Biochem. 2021;45:e13490. doi: 10.1111/jfbc.13490. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A., Sharma A., Tripathi A. Biological activities of Pleurotus spp. polysaccharides: A review. J. Food Biochem. 2021;45:e13748. doi: 10.1111/jfbc.13748. [DOI] [PubMed] [Google Scholar]
- 5.Cohen R., Persky L., Hadar Y. Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl. Microbiol. Biotechnol. 2002;58:582–594. doi: 10.1007/s00253-002-0930-y. [DOI] [PubMed] [Google Scholar]
- 6.Huang W.B., Yuan H.R., Li X.J. Multi-perspective analyses of rice straw modification by Pleurotus ostreatus and effects on biomethane production. Bioresour. Technol. 2020;296:122365. doi: 10.1016/j.biortech.2019.122365. [DOI] [PubMed] [Google Scholar]
- 7.Ding Y., Liang S., Lei J.H., Chen L.G., Kothe E., Ma A.M. Agrobacterium tumefaciens mediated fused egfp-hph gene expression under the control of gpd promoter in Pleurotus ostreatus. Microbiol. Res. 2011;166:314–322. doi: 10.1016/j.micres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Riley R., Salamov A.A., Brown D.W., Nagy L.G., Floudas D., Held B.W., Levasseur A., Lombard V., Morin E., Otillar R., et al. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. USA. 2014;111:9923–9928. doi: 10.1073/pnas.1400592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou L.D., Wang L.N., Wu X.L., Gao W., Zhang J.X., Huang C.Y. Expression patterns of two pal genes of Pleurotus ostreatus across developmental stages and under heat stress. BMC Microbiol. 2019;19:231. doi: 10.1186/s12866-019-1594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei M., Wu X.L., Zhang J.X., Wang H.X., Huang C.Y. Establishment of an efficient transformation system for Pleurotus ostreatus. World J. Microbiol. Biotechnol. 2017;33:214. doi: 10.1007/s11274-017-2378-3. [DOI] [PubMed] [Google Scholar]
- 11.Martin J.F. Key role of LaeA and velvet complex proteins on expression of beta-lactam and PR-toxin genes in Penicillium chrysogenum: Cross-talk regulation of secondary metabolite pathways. J. Ind. Microbiol. Biotechnol. 2017;44:525–535. doi: 10.1007/s10295-016-1830-y. [DOI] [PubMed] [Google Scholar]
- 12.Bok J.W., Keller N.P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell. 2004;3:527–535. doi: 10.1128/EC.3.2.527-535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng M., Zhao S.S., Lin C.Y., Song J.Z., Yang Q. Requirement of LaeA for sporulation, pigmentation and secondary metabolism in Chaetomium globosum. Fungal Biol. 2021;125:305–315. doi: 10.1016/j.funbio.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C., Zhang H., Zhu Q.Q., Hao S., Chai S.Y., Li Y.H., Jiao Z., Shi J.C., Sun B.G., Wang C.T. Overexpression of global regulator LaeA increases secondary metabolite production in Monascus purpureus. Appl. Microbiol. Biotechnol. 2020;104:3049–3060. doi: 10.1007/s00253-020-10379-4. [DOI] [PubMed] [Google Scholar]
- 15.Wang G., Zhang H.Y., Wang Y.L., Liu F., Li E.F., Ma J.N., Yang B.L., Zhang C.X., Li L., Liu Y. Requirement of LaeA, VeA, and VelB on asexual development, ochratoxin A biosynthesis, and fungal virulence in Aspergillus ochraceus. Front. Microbiol. 2019;10:2759. doi: 10.3389/fmicb.2019.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y., Yin Z.Y., Wu Y.X., Xu L.S., Du H.X., Wang N.N., Huang L.L. LaeA controls virulence and secondary metabolism in apple canker pathogen Valsa mali. Front. Microbiol. 2020;11:581203. doi: 10.3389/fmicb.2020.581203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Zheng X.J., Zhang X.J., Bao L.F., Zhu Y.Y., Qu Y.B., Zhao J., Qin Y.Q. The different roles of Penicillium oxalicum LaeA in the production of extracellular cellulase and beta-xylosidase. Front. Microbiol. 2016;7:2091. doi: 10.3389/fmicb.2016.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiboth B., Karimi R.A., Phatale P.A., Linke R., Hartl L., Sauer D.G., Smith K.M., Baker S.E., Freitag M., Kubicek C.P. The putative protein methyltransferase LAE1 controls cellulase gene expression in Trichoderma reesei. Mol. Microbiol. 2012;84:1150–1164. doi: 10.1111/j.1365-2958.2012.08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G., Zhang C.H., Leng D.D., Yan P., Wang Z.H., Zhang M.X., Wu Z.W. The non-canonical functions of telomerase reverse transcriptase gene GlTert on regulating fungal growth, oxidative stress, and ganoderic acid biosynthesis in Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2021;105:7353–7365. doi: 10.1007/s00253-021-11564-9. [DOI] [PubMed] [Google Scholar]
- 20.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C.H., Zhang G., Wen Y.M., Li T., Gao Y.Q., Meng F.M., Qiu L.Y., Ai Y.C. Pseudomonas sp. UW4 acdS gene promotes primordium initiation and fruiting body development of Agaricus bisporus. World J. Microbiol. Biotechnol. 2019;35:163. doi: 10.1007/s11274-019-2741-7. [DOI] [PubMed] [Google Scholar]
- 22.Lei M., Wu X.L., Huang C.Y., Qiu Z.H., Wang L.N., Zhang R.Y., Zhang J.X. Trehalose induced by reactive oxygen species relieved the radial growth defects of Pleurotus ostreatus under heat stress. Appl. Microbiol. Biotechnol. 2019;103:5379–5390. doi: 10.1007/s00253-019-09834-8. [DOI] [PubMed] [Google Scholar]
- 23.Lian L.D., Shi L.Y., Zhu J., Liu R., Shi L., Ren A., Yu H.S., Zhao M.W. GlSwi6 positively regulates cellulase and xylanase activities through intracellular Ca2+ signaling in Ganoderma lucidum. J. Fungi. 2022;8:187. doi: 10.3390/jof8020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M.J., Chen T.X., Gao T., Miao Z.G., Jiang A.L., Shi L., Ren A., Zhao M.W. UDP-glucose pyrophosphorylase influences polysaccharide synthesis, cell wall components, and hyphal branching in Ganoderma lucidum via regulation of the balance between glucose-1-phosphate and UDP-glucose. Fungal Genet. Biol. 2015;82:251–263. doi: 10.1016/j.fgb.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Wang L.N., Wu X.L., Gao W., Zhao M.R., Zhang J.X., Huang C.Y. Differential expression patterns of Pleurotus ostreatus catalase genes during developmental stages and under heat stress. Genes. 2017;8:335. doi: 10.3390/genes8110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Linde T., Zoglowek M., Lübeck M., Frisvad J.C., Lübeck P.S. The global regulator LaeA controls production of citric acid and endoglucanases in Aspergillus carbonarius. J. Ind. Microbiol. Biotechnol. 2016;43:1139–1147. doi: 10.1007/s10295-016-1781-3. [DOI] [PubMed] [Google Scholar]
- 28.Yin S., Yang D.M., Zhu Y.Y., Huang B.Z. Methionine and S-adenosylmethionine regulate Monascus pigments biosynthesis in Monascus purpureus. Front. Microbiol. 2022;13:921540. doi: 10.3389/fmicb.2022.921540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadooka C., Nakamura E., Mori K., Okutsu K., Yoshizaki Y., Takamine K., Goto M., Tamaki H., Futagami T. LaeA Controls citric acid production through regulation of the citrate exporter-encoding cexA gene in Aspergillus luchuensis mut. kawachii. Appl. Environ. Microbiol. 2020;86:e01950-19. doi: 10.1128/AEM.01950-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayram O.S., Bayram O., Valerius O., Park H.S., Irniger S., Gerke J., Ni M., Han K.H., Yu J.H., Braus G.H. LaeA control of velvet family regulatory proteins for light-dependent development and fungal cell-type specificity. PLoS Genet. 2010;6:e1001226. doi: 10.1371/journal.pgen.1001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayram O., Krappmann S., Ni M., Bok J.W., Helmstaedt K., Valerius O., Braus-Stromeyer S., Kwon N.J., Keller N.P., Yu J.H., et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- 32.Bayram O., Braus G.H. Coordination of secondary metabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol. Rev. 2012;36:1–24. doi: 10.1111/j.1574-6976.2011.00285.x. [DOI] [PubMed] [Google Scholar]
- 33.Chettri P., Bradshaw R.E. LaeA negatively regulates dothistromin production in the pine needle pathogen Dothistroma septosporum. Fungal Genet. Biol. 2016;97:24–32. doi: 10.1016/j.fgb.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Oda K., Kobayashi A., Ohashi S., Sano M. Aspergillus oryzae laeA regulates kojic acid synthesis genes. Biosci. Biotechnol. Biochem. 2011;75:1832–1834. doi: 10.1271/bbb.110235. [DOI] [PubMed] [Google Scholar]
- 35.Shi D.K., Zhu J., Sun Z.H., Zhang G., Liu R., Zhang T.J., Wang S.L., Ren A., Zhao M.W. Alternative oxidase impacts ganoderic acid biosynthesis by regulating intracellular ROS levels in Ganoderma lucidum. Microbiology. 2017;163:1466–1476. doi: 10.1099/mic.0.000527. [DOI] [PubMed] [Google Scholar]
- 36.Tsunematsu Y., Takanshi S., Asai S., Masuya T., Nakazawa T., Watanabe K. Genomic mushroom hunting decrypts coprinoferrin, a siderophore secondary metabolite vital to fungal cell development. Org. Lett. 2019;21:7582–7586. doi: 10.1021/acs.orglett.9b02861. [DOI] [PubMed] [Google Scholar]
- 37.Chen L., Zou G., Wang J.Z., Wang J., Liu R., Jiang Y.P., Zhao G.P., Zhou Z.H. Characterization of the Ca2+-responsive signaling pathway in regulating the expression and secretion of cellulases in Trichoderma reesei Rut-C30. Mol. Microbiol. 2016;100:560–575. doi: 10.1111/mmi.13334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.