Abstract
The Enterococcus faecalis general stress protein Gsp65 has been purified from two-dimensional gel electrophoresis. Determination of its N-terminal sequence and characterization of the corresponding gene revealed that the gsp65 product is a 133-amino-acid protein sharing homologies with organic hydroperoxide resistance (Ohr) proteins. Transcriptional analysis of gsp65 gave evidence for a monocistronic mRNA initiated 52 nucleotides upstream of the ATG start codon and for an induction in response to hydrogen peroxide, heat shock, acid pH, detergents, ethanol, sodium chloride, and tert-butylhydroperoxide (tBOOH). A gsp65 mutant showed increased sensitivity to the organic hydroperoxide tBOOH and to ethanol.
The gram-positive bacterium Enterococcus faecalis is a natural member of the human and animal flora. This ubiquitous microorganism, which may be responsible for serious diseases (i.e., endocarditis, meningitis, intra-abdominal and urinary tract wounds, and other infections [23, 27]), is able to grow in hostile conditions that are usually detrimental to the development of most mesophilic microorganisms, and it can survive in many harsh environments (i.e., extreme temperatures, pHs, and salt concentrations). The ability of E. faecalis to resist many kinds of stresses is of particular importance (i) for its selective isolation and characterization (selective media with 6.5% NaCl, 40% bile salts, 0.1% methylene blue milk, or pH 9.6 are commonly used [22, 28]), (ii) for the detection of fecal contamination (E. faecalis is considered a good indicator of fecal contamination in water and foods, and its detection can be used to indicate the presence of pathogenic bacteria [3, 24]), and (iii) because it copes with stresses frequently used to prevent the proliferation of foodborne pathogens in food processes (4, 9). Numerous reports reflect the ability of exponentially growing E. faecalis cells to resist stresses such as heating, high osmolarity, and the presence of ethanol, detergents, hydrogen peroxide, sodium hypochlorite, and heavy metals (2, 4–7, 10, 18, 25). Moreover, starvation promoted by exhaustion of the carbon and energy source glucose, and after incubation in an oligotrophic microcosm, strongly enhances the resistance of E. faecalis to environmental stresses and can be correlated with an increased synthesis of many proteins (11, 12, 15). The adaptation phenomenon has also been observed when exponentially growing cultures of E. faecalis are subjected to sublethal conditions, e.g., 30 min at 50 or 37°C in the presence of hydrogen peroxide (2.4 mM), sodium dodecyl sulfate (SDS) (0.01%), bile salts (0.08%), cadmium chloride (50 μg/ml), or pH 4.8 or 10.5 (2, 4–10, 17). Under these conditions, E. faecalis developed phenotypic resistances towards usually lethal stresses (heating at 62°C, pH 3.2 or 11.9, 45 mM H2O2, CdCl2 [50 mg/ml], SDS [0.017%], or bile salts [0.3%]). Analysis of protein synthesis during preincubation of exponentially growing cells of E. faecalis with sublethal stresses led to the detection of overexpression of 167 stress proteins. While most of these stress proteins were shown to be specifically induced by one or two treatments, six proteins, called general stress proteins (Gsp62 to Gsp67), were induced by at least six stress conditions (26). A previous study carried out by Western blot analysis allowed the identification of Gsp66 and Gsp67 as being DnaK and GroEL chaperonins, respectively (8). A recent study revealed that the E. faecalis glucose starvation protein Gls24 was not induced exclusively during carbohydrate and complete starvation but also after exposure to several stresses and consequently could also be qualified as a general stress protein (13).
In order to identify the general stress protein Gsp65, we purified this protein from two-dimensional (2-D) electrophoresis, and after sequencing of its N-terminal extremity, we identified the corresponding gene. Sequence analysis and mutation experiments revealed that this gene encodes a protein involved in organic hydroperoxide resistance.
Identification of gsp65.
Among the different stress proteins identified on 2-D electropherograms (26) of proteins extracted from E. faecalis ATCC 19433 (16), Gsp65 was purified after electroblotting from a 2-D electrophoresis of proteins extracted after a 30-min sublethal treatment at 50°C onto a polyvinylidene difluoride membrane as described by Giard et al. (12). The purified protein was then subjected to N-terminal sequencing. This allowed the identification of the N-terminal 39-amino-acid sequence MKKIYETTIINTGGRAGEVHSPDKSFXYAVASPGVKKEN. Homology searches carried out with the BLAST program (1) gave no significant homologies with sequences from databases. The corresponding open reading frame (ORF) was obtained within the genomic sequence of E. faecalis (V583) available at http://www.tigr.org. The analysis of the deduced N-terminal amino acid sequence revealed that the unidentified amino acid (X) in position 27 of our sequence was a serine. Observation of the nucleotide sequence immediately upstream of this ORF gave evidence for the presence of a ribosome binding site sequence (RBS) (AGAGGA) located 7 bp upstream of the initiation codon (ATG). Translation of the entire ORF revealed that it encodes a 133-amino-acid protein with a calculated molecular mass of 14.4 kDa, in concordance with the location of the protein on 2-D electropherograms.
From this entire deduced amino acid sequence, homologies were found with organic hydroperoxide resistance (Ohr) proteins which are similar to the Escherichia coli OsmC protein. The higher homology scores were observed with proteins encoded by the organic hydroperoxide resistance gene (ohr) of Xanthomonas campestris (44% homology and 64% similarity) (21) and by hypothetical ohr genes of Bacillus subtilis (YklA, 51% homology and 66% similarity; YkzA, 48% homology and 62% similarity) (30) and Deinococcus radiodurans (46% homology and 61% similarity) (31). Alignments with these proteins and with the OsmC protein from E. coli (Fig. 1) showed that the sequence corresponding to the first 40 amino acids is less conserved than the other parts of the proteins and that the central domain harbors a 15-amino-acid segment that is highly conserved within all sequences. The hydrophobicity profiles of Gsp65 and of proteins belonging to the OsmC family are well conserved and showed that the 15-amino-acid conserved region is part of a hydrophobic domain located between positions 43 and 63 (data not shown). From a previous alignment of Ohr sequences with OsmC proteins of E. coli and Mycoplasma genitalium, Mongkolsuk et al. (21) suggested that two highly conserved redox-sensitive cysteine residues could be important in the structure and function of Ohr. These two cysteines are also present in Gsp65 (amino acids at positions 62 and 129 in Fig. 1).
FIG. 1.
Amino acid sequence comparison of Gsp65 with Ohr proteins from B. subtilis (YkzA Bs and YklA Bs) (accession numbers F69870 and D69857) (30), X. campestris (Ohr Xc) (accession number AF036166) (21), D. radiodurans (Ohr Dr) (accession number AE002025.1) (31), and E. coli OsmC (OsmC Ec) (accession number P23929) (14). Complete amino acid sequence identity in three out of the six proteins is indicated in boldface. Asterisks and dots indicate positions where, respectively, identical and functionally related (H, K, and R; F, Y, and W; L, I, M, and V; G and A; S and T; D and E; N and Q; C and P) amino acid are found in the six proteins. The consensus sequence is given with lowercase and capital letters, which represent identity in all and in five out of the six proteins, respectively.
Genetic organization of gsp65.
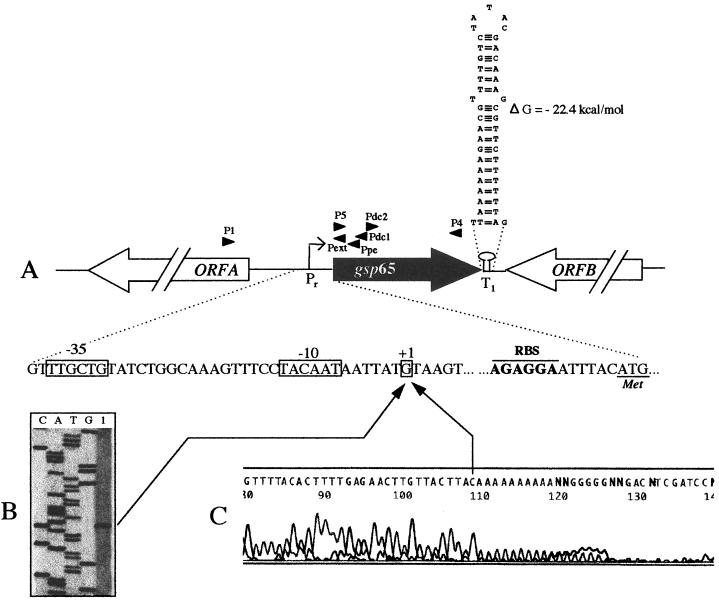
Analysis of the genomic sequence of B. subtilis revealed two ohr genes (yklA and ykzA), while Mongkolsuk et al. (21) gave evidence for a single ohr gene in X. campestris. In order to search for the presence of another gene in E. faecalis, a Southern blot analysis was carried out with E. faecalis JH2-2 (32) chromosomal DNA digested by ClaI or EcoRV and hybridized with a 32P-labeled PCR DNA fragment obtained with primers P4 and P5 (Fig. 2). A unique band was observed with each digest (data not shown), suggesting a unique ohr gene in E. faecalis. Moreover, searches for homology between the almost-complete genomic sequence of E. faecalis V583 (http://www.tigr.org) and the entire amino acid sequence of Gsp65 or the 15-amino-acid domain that is highly conserved within Ohr proteins suggested that there is no paralog in the E. faecalis chromosome. Analysis of the nucleotide sequence indicated that gsp65 is surrounded by two ORFs located on the other strand of the DNA (Fig. 2A). The amino acid sequences of these ORFs (named orfA and orfB) corresponded to proteins of 57.4 and 28.0 kDa with pIs of 4.81 and 9.22, respectively, which did not share significant homology with known proteins. An inverted repeat (ΔG = −22.4 kcal/mol) (Fig. 2A) which could act as a Rho-independent terminator was observed 2 nucleotides (nt) downstream from the gsp65 termination codon TAA.
FIG. 2.
(A) Schematic representation of the genetic organization of the gsp65 chromosomal region. Large arrows represent the ORFs, and their orientation shows the transcriptional direction. The nucleotide sequences of the gsp65 promoter region (Pr) and of a putative Rho-independent terminator (T1) located 2 nt downstream of the gsp65 stop codon are shown. The transcriptional initiation nucleotide (+1) and the putative −35 and −10 motifs are boxed. Primer positions are indicated by small arrows. (B) Primer extension experiment. Lane 1, primer extension signal obtained with the primer Pext and RNA extracted from E. faecalis JH2-2 cells incubated for 10 min with 0.08% bile salts. Lanes C, A, T, and G, products of the sequencing reactions performed with the transcribed DNA strand used for standardization. The arrowhead indicates the primer extension signal which corresponds to the transcriptional start site. (C) Electropherogram obtained from 5′ RACE PCR experiment. The sequence in the electropherogram was obtained using the primer Pext and cDNA from 5′ A-tailed RNA, using the 3′/5′ RACE kit (Roche Molecular Biochemicals). The last base (C) upstream from the 16-n A tail corresponded to the first nucleotide transcribed. The corresponding G on the reverse complement strand is indicated (+1).
Transcriptional analysis of gsp65.
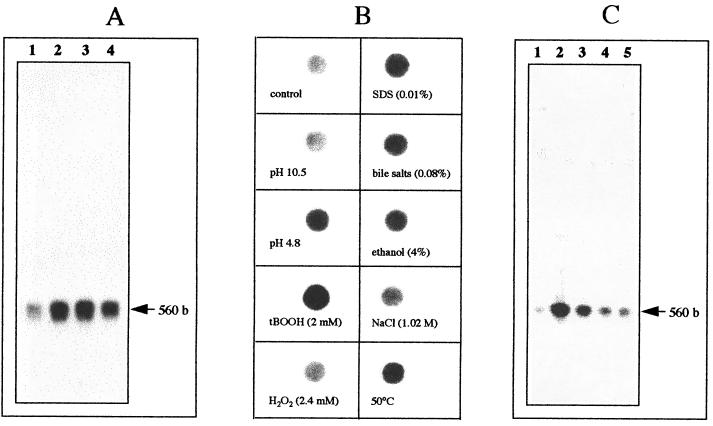
A Northern blot of 10 μg of RNA extracted with the Rneasy Midi Kit (Qiagen, Santa Clara, Calif.) from an exponentially growing culture of E. faecalis JH2-2 or from cells subjected to a sublethal treatment with 0.08% bile salts was carried out as described by Giard et al. (13). The RNA was then hybridized with a gsp65 single-stranded probe synthesized by elongation of the oligonucleotide P4 using Taq DNA polymerase, deoxynucleoside triphosphates (including 2 μCi of [α-32P]dATP), and 10 ng of template DNA corresponding to an amplimer previously obtained from a PCR with primers P4 and P5 (Fig. 2A; Table 1). The results confirmed the presence of a monocistronic mRNA of 560 bp. The transcription of gsp65 was obviously induced in the presence of bile salts, and the maximum expression was observed after 10 min of stress exposure (Fig. 3A). A dot blot obtained with 1 μg of total RNA extracted from E. faecalis JH2-2 cells growing in M17 medium containing 0.5% glucose (29) or from cells pretreated for 10 min with sublethal doses of several agents was hybridized with the same probe. It revealed that the transcription of the gsp65 gene is also induced after incubation at 50°C, at acid pH (pH 4.8), or in the presence of 0.01% SDS, 4% ethanol, 2 mM tert-butylhydroperoxide (tBOOH), or, at a lower level, H2O2 (Fig. 3B). The major induction observed with the dot blot experiments was obtained with tBOOH. While gsp65 is homologous to osmotically induced genes of the osmC family, only a weak induction was observed when cells were exposed for 10 min to 6% (1.02 M) sodium chloride. Northern blot experiments were then carried out with RNA extracted from cells treated for 10 min with 0.3, 0.6, 0.9, and 1.2 M NaCl (Fig. 3C). The results clearly demonstrated that gsp65 is induced by salt, with a maximum induction of transcription at a concentration of 0.3 M NaCl. These transcriptional analyses lead to an unambiguous demonstration that Gsp65 belongs to the group of general stress proteins. However, it has to be noted that the amount of stress used for the dot blot analysis corresponded to that allowing the maximum tolerance of cells towards a lethal treatment, and this does not necessarily correlate with the optimum concentration allowing the maximum induction of gsp65 transcription. Such an absence of a correlation been demonstrated at least for the NaCl concentration: 1.02 M allowed the maximum tolerance, while the concentration allowing the maximum induction of transcription was 0.3 M.
TABLE 1.
Primers used for PCR, mutagenesis, sequencing, primer extension, and 5′ RACE PCR experiments
| Primer | Sequencea | Orientationb |
|---|---|---|
| P1 | 5′-AAGTGCAGGAAAAGCAGG-3′ | + |
| P4 | 5′-GTTGCTTTAGAATATGGGC-3′ | − |
| P5 | 5′-CTATCATTAATACGGGTGG-3′ | + |
| P6 | 5′-TTTAGCTTGATCTACCCCA-3′ | − |
| Ppe | 5′-GCGAAGCAACCGCATAAG-3′ | − |
| Pext | 5′-CCGGTGAGTGAACTTCTCCTGCAC-3′ | − |
| Pdc1 | 5′-ttgcCCGggGCtcATAATTGTTCTGGATT-3′ | − |
| Pdc2 | 5′-acaccCGGgTTAaAGTGCTTGCTTTAACG-3′ | + |
Bases in lowercase letters are not complementary to the target sequence. Bases in boldface represent the two translational stop codons TGA and TAA introduced in the gsp65 gene during the mutagenesis experiment. Underlined nucleotides corresponded to SmaI sites.
+, primer directed towards the 3′ end of gsp65; −, primer oriented in the opposite direction.
FIG. 3.
(A) Northern blot hybridization of E. faecalis JH2-2 RNA extracted from exponentially growing cells (lane 1) and from cells incubated for 10, 20, and 30 min (lanes 2 to 4, respectively) with 0.08% bile salts. Hybridization was performed with a single-stranded DNA probe corresponding to the DNA region located between primers P4 and P5. The size of the transcript determined with RNA molecular size markers (Amersham) is indicated on the right. (B) Dot blot hybridization of total RNA (1 μg) from E. faecalis JH2-2 cells harvested in exponential growth phase (control) and from exponentially growing cells incubated for 10 min under the indicated conditions with the single-stranded DNA probe corresponding to the DNA region located between primers P4 and P5. (C) Northern blot hybridization of E. faecalis JH2-2 RNA extracted from exponentially growing cells (lane 1) and from cells incubated for 10 min with 0.3, 0.6, 0.9, and 1.2 M NaCl (lanes 2 to 5, respectively) with the single-stranded DNA probe corresponding to the DNA region located between primers P4 and P5. The size of the transcript determined with RNA molecular size markers (0.56- to 0.94-kb RNA ladder; Amersham) is indicated on the right.
Mapping of the transcriptional start site of gsp65.
In order to locate the region involved in the initiation of the transcription of gsp65, primer extension and 5′ rapid amplification of cDNA ends (RACE) PCR experiments were carried out with RNA extracted from E. faecalis JH2-2 cells treated for 10 min with 2 mM tBOOH. Primer extension performed as described by Giard et al. (13) with the primer Pext (Table 1) revealed a unique band corresponding to a cDNA whose size positioned the gsp65 mRNA 5′ end 40 nt upstream of the RBS (Fig. 2B). Electrophoresis on a 6% acrylamide gel of the 5′ RACE PCR product obtained with the 3′/5′ RACE kit (Roche Molecular Biochemicals) using primer P6 for the reverse transcriptase reaction and primer Ppe for poly(A) tailing and PCR amplification revealed a unique 250-bp fragment (data not shown). The nucleotide sequence of this 5′ RACE PCR product was determined after purification with the QIAquick kit (Qiagen) by the dideoxy chain termination method with the ABI prism sequencing system (PE Biosystems) and Pext primer. It confirmed the sole transcriptional start on the G located 40 nt upstream of the gsp65 RBS (Fig. 2C). At 6 bp upstream of this transcriptional start site can be found a putative −10 sequence (TACAAT) separated by 17 bp from a putative −35 sequence (TTGCTG), each of which partially resembled the −10 and −35 consensus sequences (TATAAT and TTGACA, respectively). Upstream of this gsp65 promoter, two direct repeats, DR1 (TGTACAANTGTACAA) and DR2 [ACAACGT(N)27ACAACGTT] and an inverted repeat (AAAAATAcAACgTTcAAtGTTATTTTT) can be identified. Because our results demonstrate that the gsp65 promoter is stress inducible, such a structure may have an important role in the initiation of transcription.
Construction of a gsp65 mutant by double crossing over.
A gsp65 mutant was constructed by introducing two translational stop codons within the gsp65 gene via a double-crossover event using a method based on the conditional replication pORI19/ pG+host3 system first described for the single-crossover interruption of chromosomal genes in Lactococcus lactis by Law et al. (19). First, a 705-bp DNA fragment was amplified by PCR using chromosomal DNA of E. faecalis JH2-2 and oligonucleotides P1 and P4 (Fig. 2A; Table 1). This PCR fragment was then cloned into the SmaI site of the vector pORI19-1 using E. coli Ec101 (19). The resulting plasmid (p65) was used as a DNA template to perform a second PCR using oligonucleotides Pdc1 and Pdc2 (Fig. 2A; Table 1). The 2.9-kb PCR product obtained was digested by SmaI, purified, and ligated to obtain a circular plasmid (p65m) which was then used to transform E. coli Ec101. The insert of plasmid p65m isolated from transformant clones and containing a SmaI site was sequenced to confirm the mutation in the gsp65 ORF by insertion of two translational stop codons. This plasmid was then used to transform E. faecalis JH2-2 in which plasmid pG+host3 (pVE6007) (20), encoding a thermosensitive RepA protein, had previously been introduced. After electroporation, cells were plated on GM17 agar medium containing erythromycin and chloramphenicol and incubated at 30°C. Clones resistant to both antibiotics were shown to harbor the two plasmids pG+host3 and p65m. One of these clones was then grown for 1 h at 30°C in GM17 broth without antibiotics and transferred for 3 h at 42°C before being plated on GM17 agar medium containing erythromycin. After a 48-h incubation at 42°C, clones resistant to erythromycin were analyzed by PCR and were shown to be integrants containing both the wild-type and the mutated alleles of the gsp65 gene. Southern blot experiments confirmed that the p65m integration event occurred within the gsp65 gene (data not shown). To obtain the second crossing over, integrant cells were transformed with plasmid pG+host3. After electroporation, transformants were selected at a permissive temperature (30°C) on GM17 plates with chloramphenicol. They were grown for 100 generations at 30°C on GM17 broth containing chloramphenicol and then grown for 1 h at 30°C on GM17 without antibiotics and transferred at 42°C for 3 h before being plated in the same medium and incubated at 42°C. Twelve erythromycin-sensitive clones were isolated out of 600 clones analyzed. Total DNA was extracted from these erythromycin-sensitive clones, digested by ClaI, and hybridized with a gsp65 probe. This revealed that all 12 clones were generated by a second crossing over which resulted in the excision of the pORI19-1 vector plus one copy of gsp65. Results of a SmaI digest of PCR fragments amplified with primers P1 and P4 showed that half of the clones kept the wild-type copy of gsp65 while the six others harbored a SmaI site within the gsp65 gene and were considered mutants. The genotype of one of these mutant (called the gsp65 mutant) was confirmed by nucleotide sequencing and by the disappearance of Gsp65 on 2-D gel electrophoresis. To our knowledge, this is the first report on chromosomal inactivation in E. faecalis via double crossing over. This mutagenesis system, which limits the polar effect of mutation on transcription of downstream genes, was successfully used in our laboratory for the inactivation of other stress genes and should probably be usable with a large number of bacterial species, as it derives from the large-host-range pWV01 replicon.
Characterization of the gsp65 mutant.
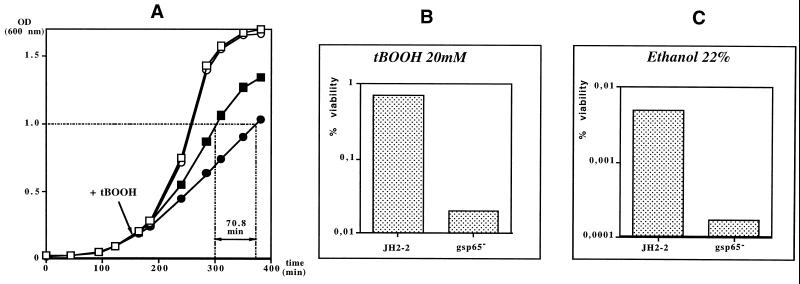
Growth studies of the gsp65 mutant strain did not reveal any significant difference compared to the wild-type JH2-2 strain when cultured at 37°C in brain heart infusion (BHI) broth, indicating that gsp65 is dispensable for optimal growth. The generation time for these two strains was 37.5 min (data not shown). To determine the potential role of the general stress protein Gsp65 in the resistance of E. faecalis to environmental stresses, sensitivity to sublethal stress conditions and survival with different individual challenges were tested. To analyze the sensitivity of the gsp65 mutant, cells were taken at the begining of exponential growth (optical density [OD] of 0.2), harvested, and resuspended in BHI medium containing 5% ethanol, 0.002% SDS, 0.06% bile salts, 5.5% NaCl, 2 mM H2O2, or 0.15 mM tBOOH; resuspend in BHi medium adjusted to pH 9.8 or pH 5.8; or incubated at 48°C. Each of these stresses did reduce the growth rates of both the mutant and wild-type strains without inducing a complete arrest of cell proliferation. A significant difference in sensitivity between the two strains was observed only with the tBOOH treatment (Fig. 4A). Indeed, the gsp65 culture reached an OD of 1 with a 70.8-min delay compared to the JH2-2 strain.
FIG. 4.
(A) Effect of tBOOH on wild-type E. faecalis JH2-2 and gsp65 mutant growth. Cultures grown in BHI medium to an OD at 600 nm of 0.2 were divided and either treated with 0.15 mM tBOOH or not. Squares, JH2-2; circles, gsp65 mutant; closed symbols, treated; open symbol; untreated. The values are the averages of results obtained in three independent experiments. (B and C) Effect of tBOOH (B) and ethanol (C) on wild-type E. faecalis JH2-2 and gsp65 mutant survival. Bacteria grown in BHI medium to an OD at 600 nm of 0.5 were harvested and resuspended in BHI medium (control) or BHI medium containing 20 mM tBOOH or 22% ethanol (challenges). The percent viability represents the ratio between the number of cells surviving after 30 min of challenge and the number surviving prior challenge.
The survival of wild-type strain JH2-2 and the gsp65 mutant after different individual lethal treatments was analyzed as follows. Bacteria from the mid-exponential growth phase were harvested, resuspended in 10 ml of fresh BHI medium, and incubated at 37°C (control), at 62°C (thermal challenge), or at 37°C in medium supplied with (i) 22% ethanol, (ii) 0.3% bile salts, (iii) 0.017% SDS, (iv) 28.5% NaCl, (v) 45 mM H2O2, (vi) lactic acid to adjust the pH to 3.2, (vii) NaOH to adjust the pH to 11.9, or (viii) 20 mM tBOOH. Challenges were performed for 30 min except for the detergents bile salts and SDS (30 s). Samples (0.5 ml) were removed, diluted in 0.9% NaCl, and poured in GM17 agar for determination of CFU. Plates were incubated at 37°C for 48 h. These individual lethal treatments induced no significant reduction of the percent survival when heat shock, NaCl, pH, detergent, and H2O2 challenges were applied. However, the gsp65 mutant was shown to be less resistant to the oxidative stress generated by 20 mM tBOOH and to the 22% ethanol challenge; 35- and 30-fold reductions of the percent survival were observed when cells were incubated for 30 min with tBOOH and ethanol, respectively (Fig. 4B and C). When cells were preincubated for 30 minutes with 2 mM tBOOH or 4% ethanol before the homologous challenge, the gsp65 mutant was partially adapted, and, in comparison with the wild-type strain, 10- and 6-fold reductions of the percent survival were observed with 20 mM tBOOH and 22% ethanol, respectively. Sublethal heterologous pretreatments with stresses inducing gsp65 were also applied prior the tBOOH challenge in order to search for treatments which can increase resistance against tBOOH. When a sublethal treatment with heat, bile salts, NaCl, or acid pH was applied as described by Flahaut et al. (10) prior to the tBOOH challenge, partial adaptations were observed for both the wild-type and mutant strains (data not shown). However, this led to a reduction of the survival difference between the strains, indicating that these heterologous tolerances are the result of other inducible stress resistance mechanisms. A 30-min pretreatment with 0.01% SDS, which was previously shown to induce tolerance towards SDS and bile salts challenges (5), did not induce tolerance against tBOOH.
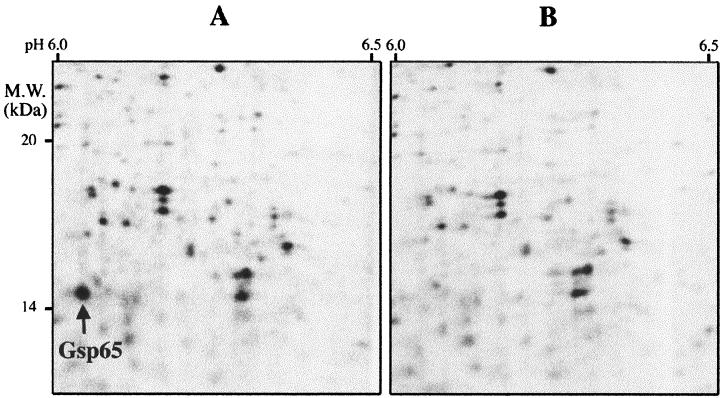
A 2-D protein gel electrophoresis approach was used for the identification of modifications in the protein pattern due to the gsp65 mutation. 2-D polyacrylamide gel electrophoresis (PAGE) of proteins extracted as previously described by Giard et al. (12) from exponentially growing JH2-2 cells exposed or not to 2 mM tBOOH for 30 min revealed the induction of numerous proteins by the tBOOH treatment. Gsp65 belongs to these overexpressed proteins, with an induction factor of 9 (data not shown). 2-D PAGE revealed no significant difference between the gsp65 and wild-type strains, except for the expected absence of Gsp65 in the mutant pattern (Fig. 5). The fact that 2D-PAGE of the mutant did not revealed a pleiotropic effect of the mutation on the synthesis of other proteins, while the gsp65 strain was shown to be sensitive to the organic hydroperoxide tBOOH, argues for a direct role of Gsp65 in tBOOH resistance. Such a phenotype was also obtained with an ohr mutant of X. campestris by Mongkolsuk et al. (21), who suggested that Ohr might function directly in detoxification of organic hydroperoxide. However, those authors did not exclude the possibility that Ohr might be involved in the transport of organic molecules. The latter hypothesis can be argued by the fact that the most conserved region within Ohr proteins corresponded to unique hydrophobic segment of Ohr that probably crosses the membrane. Interestingly, the gsp65 mutant was also shown to be more sensitive to ethanol challenge. To our knowledge, this is the first report of an ohr mutant phenotype towards a stress different from oxidative stress.
FIG. 5.
Autoradiogram of 2-D-labeled protein gels of E. faecalis JH2-2 (A) and the gsp65 mutant (B). The pictures represent parts of the autoradiograms obtained after electrophoresis of labeled proteins during the 30-min treatment with 2 mM tBOOH. The arrow shows the position of the spot corresponding to Gsp65.
Acknowledgments
We are grateful to C. J. Leenhouts (Department of Genetics, University of Groningen, Groningen, The Netherlands) and E. Maguin (INRA, Jouy-en-Josas, France) for providing us with plasmid pORI19-1 and the E. coli Ec101 strain and pG+host3 plasmid, respectively. The expert technical assistance of Annick Blandin and Béatrice Gillot was greatly appreciated.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Boutibonnes P, Giard J C, Hartke A, Thammavongs B, Auffray Y. Characterization of the heat shock response in Enterococcus faecalis. Antonie Leeuwenhoek. 1993;64:47–55. doi: 10.1007/BF00870921. [DOI] [PubMed] [Google Scholar]
- 3.Burton M C. Comparison of coliform and Enterococcus organisms as indices of pollution in frozen foods. Food Res. 1949;14:434–448. doi: 10.1111/j.1365-2621.1949.tb16253.x. [DOI] [PubMed] [Google Scholar]
- 4.Flahaut S, Hartke A, Giard J C, Benachour A, Boutibonnes P, Auffray Y. Relationship between stress response toward bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiol Lett. 1996;138:49–54. doi: 10.1111/j.1574-6968.1996.tb08133.x. [DOI] [PubMed] [Google Scholar]
- 5.Flahaut S, Frere J, Boutibonnes P, Auffray Y. Comparison of the bile salts and sodium dodecyl sulfate stress responses in Enterococcus faecalis. Appl Environ Microbiol. 1996;62:2416–2420. doi: 10.1128/aem.62.7.2416-2420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flahaut S, Benachour A, Giard J C, Boutibonnes P, Auffray Y. Defense against lethal treatments and de novo protein synthesis induced by NaCl in Enterococcus faecalis ATCC 19433. Arch Microbiol. 1996;165:317–324. doi: 10.1007/s002030050333. [DOI] [PubMed] [Google Scholar]
- 7.Flahaut S, Hartke A, Giard J C, Auffray Y. Alkaline stress response in Enterococcus faecalis: adaptation, cross-protection, and changes in protein synthesis. Appl Environ Microbiol. 1997;63:812–814. doi: 10.1128/aem.63.2.812-814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flahaut S, Frere J, Boutibonnes P, Auffray Y. Relationship between the thermotolerance and the increase of DnaK and GroEL synthesis in Enterococcus faecalis ATCC19433. J Basic Microbiol. 1997;37:251–258. doi: 10.1002/jobm.3620370404. [DOI] [PubMed] [Google Scholar]
- 9.Flahaut S, Boutibonnes P, Auffray Y. Les Enterocoques dans l'environnement proche de l'homme. Can J Microbiol. 1997;43:699–708. [PubMed] [Google Scholar]
- 10.Flahaut S, Laplace J M, Frere J, Auffray Y. The oxidative stress response in Enterococcus faecalis: relationship between H2O2 tolerance and H2O2 stress proteins. Lett Appl Microbiol. 1998;26:259–264. doi: 10.1046/j.1472-765x.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 11.Giard J C, Hartke A, Flahaut S, Benachour A, Boutibonnes P, Auffray Y. Starvation-induced multiresistance in Enterococcus faecalis JH2–2. Curr Microbiol. 1996;32:264–271. doi: 10.1007/s002849900048. [DOI] [PubMed] [Google Scholar]
- 12.Giard J C, Hartke A, Flahaut S, Benachour A, Boutibonnes P, Auffray Y. Glucose starvation response in Enterococcus faecalis JH2–2: survival and proteins analysis. Res Microbiol. 1997;148:27–35. doi: 10.1016/S0923-2508(97)81897-9. [DOI] [PubMed] [Google Scholar]
- 13.Giard J C, Rincé A, Capiaux H, Auffray Y, Hartke A. Inactivation of the stress- and starvation-inducible gls24 operon has a pleiotropic effect on cell morphology, stress sensitivity and gene expression in Enterococcus faecalis. J Bacteriol. 2000;182:4512–4520. doi: 10.1128/jb.182.16.4512-4520.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez C, Devedjian J C. Osmotic induction of gene osmC expression in Escherichia coli K12. J Mol Biol. 1991;220:959–973. doi: 10.1016/0022-2836(91)90366-e. [DOI] [PubMed] [Google Scholar]
- 15.Hartke A, Giard J C, Laplace J M, Auffray Y. Survival of Enterococcus faecalis in an oligotrophic microcosm: changes in morphology, development of general stress resistance, and analysis of protein synthesis. Appl Environ Microbiol. 1998;64:4238–4245. doi: 10.1128/aem.64.11.4238-4245.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones D, Shattock P M. The location of the group antigen of group D Streptococcus. J Gen Microbiol. 1960;23:335–343. doi: 10.1099/00221287-23-2-335. [DOI] [PubMed] [Google Scholar]
- 17.Laplace J M, Boutibonnes P, Auffray Y. Unusual resistance and acquired tolerance to cadmium chloride in Enterococcus faecalis. J Basic Microbiol. 1996;36:311–317. doi: 10.1002/jobm.3620360504. [DOI] [PubMed] [Google Scholar]
- 18.Laplace J M, Thuault M, Hartke A, Boutibonnes P, Auffray Y. Sodium hypochlorite stress in Enterococcus faecalis: influence of antecedent growth conditions and induced proteins. Curr Microbiol. 1997;34:284–289. doi: 10.1007/s002849900183. [DOI] [PubMed] [Google Scholar]
- 19.Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol. 1995;177:7011–7018. doi: 10.1128/jb.177.24.7011-7018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maguin E, Duwat T, Hege T, Ehrlich D, Gruss A. New thermosensitive plasmid for gram-positive bacteria. J Bacteriol. 1992;174:5633–5638. doi: 10.1128/jb.174.17.5633-5638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J Bacteriol. 1998;180:2636–2643. doi: 10.1128/jb.180.10.2636-2643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundt J O. Enterococci. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1063–1065. [Google Scholar]
- 23.Nosking G A, Patterson T M, Clarke J Y, Warren J R. High-level gentamicin resistance in Enterococcus faecalis bacteremia. J Infect Dis. 1991;164:1212–1215. doi: 10.1093/infdis/164.6.1212. [DOI] [PubMed] [Google Scholar]
- 24.Ostrolenk M, Kramer N, Clerverdon R C. Comparative studies of enterococci and Escherichia coli as indices of pollution. J Bacteriol. 1947;53:197–203. doi: 10.1128/jb.53.2.197-203.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichereau V, Bourot S, Flahaut S, Blanco C, Auffray Y, Bernard T. The osmoprotectant glycine betaine inhibits salt-induced cross-tolerance towards lethal treatment in Enterococcus faecalis. Microbiology. 1999;145:427–435. doi: 10.1099/13500872-145-2-427. [DOI] [PubMed] [Google Scholar]
- 26.Rincé A, Flahaut S, Auffray Y. Identification of general stress genes in Enterococcus faecalis. Int J Food Microbiol. 2000;55:87–91. doi: 10.1016/s0168-1605(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 27.Schaberg D R, Culver D H, Gaynes R P. Major trends in microbial etiology of nosocomial infection. Am J Med Suppl. 1991;3B:725–758. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 28.Sherman J M. The streptococci. Bacteriol Rev. 1937;1:3–97. doi: 10.1128/br.1.1.3-97.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Environ Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volker U, Andersen K K, Antelmann H, Devine K M, Hecker M. One of two osmC homologs in Bacillus subtilis is part of the sigmaB-dependent general stress regulon. J Bacteriol. 1998;180:4212–4218. doi: 10.1128/jb.180.16.4212-4218.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, Dodson R J, Haft D H, Gwinn M L, Nelson W C, Richardson D L, Moffat K S, et al. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagi Y, Clewell D B. Recombination-deficient mutant of Streptococcus faecalis. J Bacteriol. 1980;143:966–970. doi: 10.1128/jb.143.2.966-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]