Abstract
Background: General pathophysiological mechanisms regarding associations between fluid administration and intra-abdominal hypertension (IAH) are evident, but specific effects of type, amount, and timing of fluids are less clear. Objectives: This review aims to summarize current knowledge on associations between fluid administration and intra-abdominal pressure (IAP) and fluid management in patients at risk of intra-abdominal hypertension and abdominal compartment syndrome (ACS). Methods: We performed a structured literature search from 1950 until May 2021 to identify evidence of associations between fluid management and intra-abdominal pressure not limited to any specific study or patient population. Findings were summarized based on the following information: general concepts of fluid management, physiology of fluid movement in patients with intra-abdominal hypertension, and data on associations between fluid administration and IAH. Results: We identified three randomized controlled trials (RCTs), 38 prospective observational studies, 29 retrospective studies, 18 case reports in adults, two observational studies and 10 case reports in children, and three animal studies that addressed associations between fluid administration and IAH. Associations between fluid resuscitation and IAH were confirmed in most studies. Fluid resuscitation contributes to the development of IAH. However, patients with IAH receive more fluids to manage the effect of IAH on other organ systems, thereby causing a vicious cycle. Timing and approach to de-resuscitation are of utmost importance, but clear indicators to guide this decision-making process are lacking. In selected cases, only surgical decompression of the abdomen can stop deterioration and prevent further morbidity and mortality. Conclusions: Current evidence confirms an association between fluid resuscitation and secondary IAH, but optimal fluid management strategies for patients with IAH remain controversial.
Keywords: fluid therapy, abdominal hypertension, abdominal compartment syndrome, open abdomen, crystalloids, colloids, hypertonic, resuscitation, maintenance, sepsis
1. Background
Over the past two decades, the pathophysiological consequences of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) have received more research and awareness. The Abdominal Compartment Society (WSACS, www.wsacs.org (accessed on: 26 May 2022)) has set out clear definitions for intra-abdominal hypertension (subclassified into primary and secondary) and abdominal compartment syndrome, including the importance of concepts such as abdominal perfusion pressure (APP) [1,2,3,4].
Elevation of IAP leads to compromise in several organ systems, including cardiovascular (decreasing preload, increasing afterload, and decreasing cardiac output), respiratory (elevated diaphragm, decreased compliance, decreased functional residual capacity), central nervous system (intracranial hypertension due to functional obstruction of cerebral venous outflow), renal (compression of both the renal veins and arteries), and the gastrointestinal system (GIT) [5,6,7,8,9,10,11,12,13,14]. The effect on the GIT is multiple and includes mesenteric vein compression, decreased perfusion, intestinal edema, bacterial translocation, and disruption of the gut microbiome and immune system [3].
The pathophysiological impact of elevated IAP on the various organ systems mimics a state like sepsis, with loss of vasomotor tone and dysfunction of the intercellular junctions of the endothelium. Fluid resuscitation is often the first choice to restore hemodynamic stability. However, administration of large volumes of intravenous fluids may paradoxically result in ACS. The increased abdominal pressure stimulates anti-diuretic hormone (ADH) release, further promoting fluid retention [4]. Dabrowski et al., documented a significant correlation between IAP and extravascular water content in critically ill patients and patients undergoing prolonged elective surgery [5]. Reintam et al., showed that mortality among patients with secondary IAH was significantly higher than among patients with primary IAH [6], whereas a meta-analysis looking at various risk factors for IAH identified fluid balance as an independent predictor for IAH [7].
The effect of intravenous fluid administration on IAP has been studied, however, the effects of fluids on IAP in different patient populations and conditions remain largely unexplored.
The effect of pressures on neighboring anatomical compartments highlights the importance of appropriate management of patients with IAH or elevated pressures in adjacent compartments (abdomen, thorax, skull) [10,11,12,13]. In 2007, Thomas Scalea was the first to suggest the complex and constant interplay of elevated pressure between different compartments [8]. The poly-compartment syndrome (PCS) as “terminus technicus” coined by Malbrain has been well described in the medical literature [10,11,12,13]. Genuine PCS is a rare, but life-threatening condition, when two or more compartments have simultaneously elevated pressures. Releasing the pressure of one of the affected compartments usually improves the clinical scenario [15,16].
The goals of treatment for PCS are:
To reduce the pressure in the compartment by improving compliance (e.g., muscle relaxation) and, or opening different compartments (e.g., through escharotomy or decompressive surgery).
Individualized fluid management strategies and supportive therapy.
Apply the concepts of the four stages of fluid resuscitation (ROSE model, Figure 1) [17].
Avoid the adverse effects of ischemia-reperfusion after surgical decompression [11,12,13].
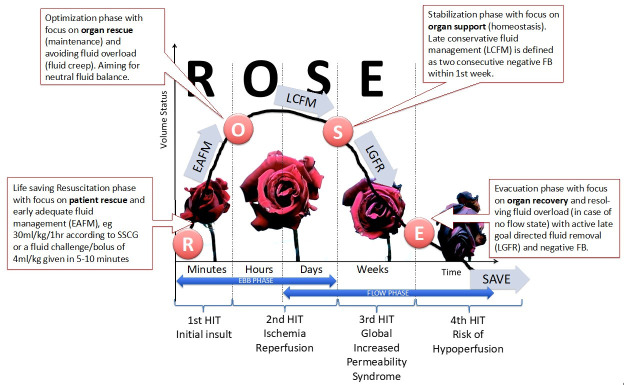
Figure 1.
The four hits of shock. Graph showing the four-hit model of shock with evolution of patients’ cumulative fluid volume status over time during the five distinct phases of resuscitation: Resuscitation (R), Optimization (O), Stabilization (S), and Evacuation (E) (ROSE), followed by a possible risk of Hypoperfusion in case of too aggressive de-resuscitation. On admission patients are hypovolemic, followed by normovolemia after fluid resuscitation (EAFM, early adequate fluid management), and possible fluid overload, again followed by a phase going to normovolemia with late conservative fluid management (LCFM) and late goal directed fluid removal (LGFR) or de-resuscitation. In the case of hypovolemia, O2 cannot get into the tissue because of convective problems; in the case of hypervolemia, O2 cannot get into the tissue because of diffusion problems related to interstitial and pulmonary edema, gut edema (ileus and abdominal hypertension). Adapted according to the Open Access CC BY License 4.0 from Malbrain et al., with permission [17].
As the available data is scarce, this scoping review aims to describe the impact of fluid resuscitation on the development of intra-abdominal hypertension. Firstly, current knowledge on the pathophysiology of fluid administration is summarized, focusing on specific aspects related to increased IAP. Secondly, studies addressing fluid management in subjects with IAH are summarized and discussed.
2. Methods
Methods for inclusion, analysis and reporting of results were according to recommendations from the preferred reporting items for systematic reviews and meta-analyses (PRISMA).
Search Strategy
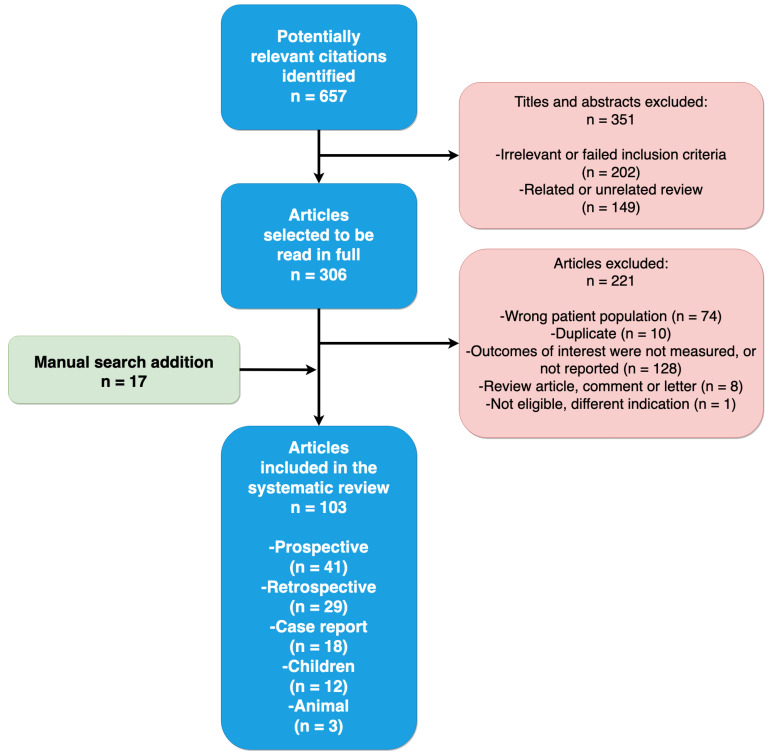
Two investigators performed a literature search for articles between 1950 and May 2021 using Scopus and PubMed electronic databases. We used the following search terms: (“abdominal hypertension” OR “abdominal compartment syndrome” OR “abdominal pressure”) AND (“fluid therapy” OR “fluid resuscitation” OR “risk factor”). The search was limited to English-language articles. PubMed search formula included (“intra-abdominal hypertension” [All Fields] OR “intra-abdominal pressure” [All Fields]) OR “abdominal compartment syndrome” [All Fields]) AND (“fluid” [All Fields] OR “resuscitation” [All Fields]). Identified citations had their titles and abstracts independently screened for the relationship between fluid therapy and IAH (Figure 2). We used the following inclusion criteria: (1) No age limitation and animal studies included; (2) studies that examined the association between fluid resuscitation and IAH or ACS; (3) IAH diagnosed using trans-bladder pressure measurements; and (4) no limitation to the type of study design. Disagreements between investigators regarding study inclusion were resolved by consensus. Reference lists of these papers, and related articles featured in PubMed, were screened to identify additional studies not identified through the initial literature search.
Figure 2.
Flowchart of literature review and selection of included publications.
The same two authors extracted the following data independently (as tabulated in the manuscript): (1) design and setting; (2) study participant diagnosis (for example, trauma, burn, severe acute pancreatitis); (3) type and amount of fluid administered; (4) IAP measurement in mmHg; (5) patient outcomes; (6) management/intervention of IAH or ACS.
All relevant studies underwent a full-text assessment, and data were extracted into tables according to the study type/design. Studies and hypotheses on pathophysiological mechanisms are summarized as narrative text. One hundred and three of the 657 potentially relevant publications identified during the literature search were included (Figure 2).
3. Results
3.1. Data on Associations between Fluid Administration and IAH
3.1.1. Study selection and characteristics
Among 764 unique citations, 103 studies enrolling 12015 critically ill adults, 107 critically ill children, and 104 animals met the inclusion criteria (Figure 2) [5,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119]. Among the 103 included studies, 3 were randomized controlled trials (RCTs) (Table 1), 39 prospective observational studies (Table 1), 29 retrospective studies (Table 2), 17 case reports in adult patients (Table 3), two observational studies and 10 case reports in children (Table 4), and three animal studies (Table 5).
Table 1.
Summary of findings of prospective studies on fluid administration and IAH.
| Author | Year | Type of Study | Patients | Resuscitation Fluids | IAP (mmHg) | Results |
|---|---|---|---|---|---|---|
| Severe burn patients | ||||||
| Ivy et al. [19] | 2000 | Prospective | n = 10 (7 IAH; 2 ACS) | IAH: volume of fluid 9 to 35 L 579 ACS: volume of fluid 33 to 48 L |
IAP: 9 to 44 | 2 DL; 2 patients died |
| O’Mara et al. [23] | 2005 | Observational | Crystalloid (n = 15) vs. Plasma (n = 16) | 561 mL/kg crystalloid 360 mL/kg plasma |
Crystalloid: 32.5 Plasma: 16.4 |
Crystalloid group:
|
| Oda et al. [21] | 2006 | Observational | HLS (n = 14) LR (n = 22) (≥40% TBSA) |
Needed to maintain UO: HLS 3.1 ± 0.9 mL/24 h/kg/% TBSA vs. LR 5.2 ± 1.2 mL24 h/kg/% TBSA |
HLS 14% vs. LR 50% developed IAH |
HLS resuscitation can reduce risk of secondary ACS with lower fluid load than LR solution |
| Oda et al. [22] | 2006 | Observational | n = 48 | ACS patients received 398.7 ± 105.5 mL/kg fluid the first 24 h after injury | IAP (49 ± 12 cm H2O) ACS: n = 8 |
>300 mL/kg/24 h fluid resuscitation → ACS |
| Ennis et al. [18] | 2008 | Prospective | n = 56 BRG group n = 62 control group |
>250 mL/kg volume in the first 24 h | Not reported | ACS and mortality significantly lower in BRG group (p = 0.03) |
| Ruiz-Castilla et al. [24] | 2014 | Observational | n = 25 (>20% TBSA) |
10473 mL in pts with IAH vs. 4100 mL in no IAH (p = 0.03) | 13 vs. 10 | IAH pts:
|
| Wise et al. [26] | 2016 | Observational | n = 56 | ACS 13.6 ± 16 L vs. No ACS 7.6 ± 4.1 L |
IAH: n = 44 ACS: n = 16 |
Non-survivors:
|
| Mbiine et al. [20] | 2017 | Observational | n = 64 (adults and children) | IAH in fluid overloaded patients: 16 vs. 13 IAH in patients not fluid overloaded: 10 vs. 9 |
Prevalence IAH: 57.8% 3.3 times increased risk of mortality with IAH Mortality with IAH: 82.6% |
More IAH among the fluid overloaded patients, but difference not significant, probably due to small sample size |
| Talizin et al. [25] | 2018 | Prospective | n = 46 (38 IAH; 8 no IAH) | 48 h fluid balance: With IAH: 5370 (3857–8828) mL Without IAH: 3894 (2411–5946) mL (p = 0.091) |
Not applicable | IAH was associated with↑ mortality rate: 24 IAH + vs. 1 IAH – p = 0.016 |
| Severe acute pancreatitis | ||||||
| Mao et al. [43] | 2009 | RCT | n = 76 | Amount of crystalloid and colloid on admission day (mL):
|
Incidence of ACS 72.2% in group I vs. 32.5% in group II | Total amount of fluid sequestration, rate of mechanical ventilation, incidence of ACS and mortality were significantly higher in group I |
| Du et al. [41] | 2011 | RCT | HES = 20 RL = 21 |
Total infusion volumes not significantly different between 2 groups | HES: 11.25 ± 2.35 RL: 17.08 ± 4.98 |
HES group (p < 0.05): IAP lower; more urine production, earlier negative fluid balance and fewer patients received mechanical ventilation |
| Ke et al. [42] | 2012 | Observational | n = 58 | 24 h fluid balance: IAH: 503 (373–1431) mL No IAH 74 (−31–409) mL |
Median max IAP 13.1 mmHg 36 patients developed IAH 7 patients developed ACS |
Risk factors for IAH include 24 h positive fluid balance (first day), number of fluid collections, and serum calcium level |
| Zhao et al. [40] | 2013 | RCT | n = 120 | NS: 61.79 ± 7.61 mL/kg/day SH (NS + HES): 46.93 ± 12.38 mL/kg/day SHG (SH + glutamine) 44.75 ± 8.53) mL/kg/day (p < 0.05) |
IAP in NS significant higher | Compared to the NS group: patients in the SH and SHG groups:
|
| Trauma | ||||||
| Raeburn et al. [51] | 2001 | Observational | n = 77 | 28 patients with ACS vs. 49 patients no ACS |
Mortality ACS 43% vs. no ACS 22% (p = 0.002) |
|
| Balogh et al. [47] | 2002 | Prospective | n = 128 total n = 11 ACS |
26 ± 2 U RCC 38 ± 3 L crystalloid |
Mortality ACS: 54% |
|
| Balogh et al. [48] | 2003 | Observational | n = 188 | Amount of crystalloid (L) received in:
|
ACS: Primary 11 patients vs. Secondary 15 patients Mortality ACS (prim 64% vs. sec 53% vs. no ACS 17% |
|
| Cotton et al. [74] | 2009 | Prospective | Pre-TEP: n = 141 TEP: n = 125 |
Blood products intraoperative:
|
Higher 30-day survival in TEP 56.8% vs. 37.6% pre-TEP (p = 0.001) |
|
| Neal et al. [50] | 2012 | Multi-centre, Prospective | n = 452 |
|
Overall mortality 22.6% | Patients with a ratio > 1.5:1 Crystalloid: RCC have 70% higher risk of MOF and 2-fold higher risk of ARDS and ACS |
| Mahmood et al. [49] | 2014 | Observational | n = 117 DL = 102 No DL = 15 |
Crystalloid (L):
|
16.7% developed IAP > 20 mmHg in DL Mortality: 6% in DL vs. 20% in no DL p = 0.05 |
Blood transfusion and IV fluids significant correlation with IAP >20 mmHg and more metabolic acidosis |
| Vatankhah et al. [52] | 2018 | Observational | n = 100 28 ACS vs. 72 no ACS |
Crystalloid:
|
21% mortality in ACS | Mean volume of fluids significantly higher in pts. with ACS |
| Medical | ||||||
| Daugherty et al. [86] | 2007 | Observational | n = 40 | Positive fluid balance > 5 L/24 h | n = 34 IAP > 12 mmHg n = 13 IAP > 20 mmHg n = 10 ACS |
25% of patients with 5 L or > positive fluid balance in 24 h developed ACS |
| Cordemans et al. [87] | 2012 | Observational | n = 123 | Cumulative fluid balance:
|
20% IAH | Not achieving CLFM & being non-responder: strong independent predictors of mortality |
| Dorigatti et al. [85] | 2019 | Observational | n = 25 | Accumulated fluid balance (mL): n = 13 (death): 15165.4 ± 12719.2 vs. n = 12 (survival): 6194.5 ± 6517.1 |
IAP 14.1 ± 4.2 vs. 9.4 ± 2.0 |
Higher admission and consecutive SOFA score of > 7 associated with higher ACS incidence and higher mortality rate. |
| Surgical | ||||||
| Biancofiore et al. [92] | 2003 | Observational | n = 34 IAH n = 74 no IAH |
IAH:
|
Not Reported | High IAP pressure:
|
| Šerpytis et al. [97] | 2008 | Observational | n = 77 | Not reported | POD 1: 45.5% IAH POD 2: 41.7% IAH POD 3: 35.6% IAH |
Positive correlation between 24-h fluid balance and IAP |
| Makar et al. [95] | 2009 | Prospective | n = 14 eEVR n = 16 eOR |
Units RCC: (p ≤ 0.001)
|
1 ACS in eEVR, 1 ACS eOR | Correlation between IAP and the following:
|
| Dalfino et al. [93] | 2013 | Observational | n = 22 IAH n = 47 no IAH |
Positive fluid balance: independent risk factor for IAH | Not Reported | Mortality IAH 53% vs. 27% (p = 0.02) |
| Muturi et al. [96] | 2017 | Observational | n = 113 | IV fluid over 24 h (mL): IAH: 3946.6 vs. No IAH: 2931.1 (p = 0.003) |
n = 76 IAH n = 37 no IAH n = 5 ACS |
Of those who had IAH; age, amount of iv fluids over 24 h, fluid balance & ventilator mode were significant determinants of risk of progression to ACS |
| Kotlińska-Hasiec et al. [94] | 2017 | Observational | Liberal: n = 32 vs. Restrictive: n = 31) |
Liberal = 2822 ± 606 mL Restrictive = 823 ± 223 mL (p < 0.001) |
Significant higher IAP in pts receiving liberal crystalloid therapy | Correlation between IAP and ECW |
| Medical-surgical | ||||||
| Biffl et al. [104] | 2001 | Prospective | 14 ACS: 8 trauma 6 medical |
Averages administered: 16.7 ± 3.0 L crystalloid 13.3 ± 2.9 RBC |
10 patients underwent DL |
|
| Malbrain et al. [110] | 2004 | Observational | n = 97 | Patients with IAH:
|
IAH 50.5% ACS 8.2% |
|
| Malbrain et al. [109] | 2005 | Observational | n = 265 | Not reported | IAH: 32.1% on admission Mortality 27.5% |
Fluid resuscitation was independent predictor for IAH (OR, 1.88; 95% CI, 1.04– 3.42; p = 0.04) |
| Dalfino et al. [107] | 2008 | Observational | n = 123 | Cumulative fluid balance in ml
|
Primary IAH: 27.1% Secondary IAH: 67.5% ACS: 5.4% |
Acute renal failure: 19.7% in IAH vs. 8.1% in no IAH Age, cumulative fluid balance and shock are all independent predictive factors of IAH |
| Vidal et al. [113] | 2008 | Prospective | n = 83 | Intense fluid resuscitation, was significantly greater in patients with IAH and in non-survivors |
53 patients with IAH 30 patients with no IAH |
IAH associated with organ failure and mortality |
| Reintam Blaser et al. [116] | 2011 | Observational | n = 563 | >5 L fluid resuscitation/24 h:
|
No IAH: 381 patients IAH: 182 patients 33% mortality in ACS patients |
|
| Kim et al. [114] | 2012 | Observational | n = 100 52 medical, 37 surgical, 11 trauma |
No correlation with 24-h fluid balance | 42% IAH, 4% ACS |
|
| Iyer et al. [111] | 2014 | Observational | n = 403 | IAH: 4.24 (2.54–5.56) L No IAH: 2.75 (1.75–4.05) L (p < 0.001) |
39% IAH 2% ACS |
IV fluid > 2.3 L is an independent predictor of IAH |
| Malbrain et al. [115] | 2014 | Systematic review | n = 1669 | Not reported | Overall mean IAP:
|
|
| Dąbrowski et al. [5] | 2015 | Observational | n = 120 48 surgical 72 medical |
Cut-off points for development of IAH:
|
Not Reported | IAP strongly correlates with ECW |
| Murphy et al. [108] | 2018 | Observational | n = 285 | No IAH: 1135 (145–2685) mL IAH: 2019 (716–4.000) mL (p < 0.001) |
45% IAH, 3% ACS Mortality: 30% IAH vs. 11% no IAH |
24-h fluid balance > 3 L is an independent predictor for IAH |
| Reintam Blaser et al. [112] | 2019 | Observational | n = 491 | 48.9% IAH (46.3% primary vs. 53.7% secondary). IAH vs. no IAH: 5 L fluid resuscitation before ICU (p = 0.036) | 6.3% ACS | Positive fluid balance and BMI and PEEP ≥ 7 cmH2O associated with development of IAH |
DL: damage control laparotomy; UO: urine output; IV: intravenous; HLS: hypertonic lactated saline; TBSA: total body surface area; OF: organ failure; SAPS II: Simplified Acute Physiology Score II; SOFA: Sequential Organ Failure Assessment Score; ICU: intensive care unit; PEEP: positive end expiratory pressure; BRG: burn resuscitation guidelines, TEP: Trauma Exsanguination Protocol; RF: risk factor; CLFM: conservative late fluid management; 0.9% saline (NS group), combination of 0.9% saline and hydroxyethyl starch (HES) (SH group), combination of 0.9% saline, hydroxyethyl starch and glutamine (SHG group); L: liters; P: platelets; LOS: length of stay; MV: mechanical ventilation; OF: organ failure; ECW: extracellular body water; eEVR: emergency endovascular repair; eOR: emergency open repair; POD: post-operative day.
Table 2.
Summary of findings of retrospective studies on fluid administration and IAH.
| Author | Year | Population | Patients | Resuscitation Fluids | IAP (mmHg) | Intervention | Results |
|---|---|---|---|---|---|---|---|
| Boehm et al. [27] | 2019 | Burn | 38 ACS+ vs. control | Average FB/day: ACS vs. control 13.3 L ± 7.7 L vs. control 7.9 L ± 7.9 L (NS) |
Not reported | Not reported | ↑ mortality rate of 84% in ACS+ vs. 32% in control (p = 0.00008) |
| Hershberger et al. [28] | 2007 | Burn | 25 ACS+ | Mean fluid infused 2102 mL/h before DL | Mean IAP 57 ± 4.2 | DL | 22 patients (88%) died |
| Hobsen et al. [29] | 2002 | Burn | n = 1014 10 ACS |
3.1 mL/kg/% TBSA for the first 12 h | Mean 40 ± 10 | DL | 40% of ACS patients survived |
| Markell et al. [30] | 2009 | Burn | n = 1825 ACS: 32 |
6.02 mL/kg/% TBSA | >30 | DL | 90% mortality for ACS |
| McBeth et al. [31] | 2014 | Burn | 110 | 48-h FB: 25.6 (± 11.1) L exceeding predicted Parkland formula estimates by 86% (± 32) |
12.1 (± 4.2) | 3 patients DL | 39 patients died |
| Park et al. [32] | 2012 | Burn | 159 | Pre-protocol 4.6 ± 2.3 mL/kg/% TBS. Post-protocol: 4.2 ± 1.7 mL/kg/% TBS, mean ± SD; p not significant |
Pre-protocol:
|
DL, n (%) Pre-protocol: 6 Post-protocol: 0 (p < 0.05) |
Mortality, n (%)
|
| Britt et al. [38] | 2005 | Burn, trauma | 10 ACS | Mean volume in the first 24 h: 33 L (12.4–69) | Mean 44.6 | DL |
|
| Reed et al. [39] | 2006 | Trauma, burn, solid organ injury | 12 | 12 L of fluids or >500 mL/h for 4 consecutive hours | Average before and after catheter insertion 44.8 and 58.7 | 2 patients DL, 8 patients intra-abdominal catheters |
7 patients survived |
| Gracias et al. [54] | 2002 | Trauma | 5 ACS vs. 15 control | ACS: 37 L crystalloid vs. Control: 16.1 L crystalloid |
>25 | Decompression | 60% in ACS vs. 7% in control |
| Balogh et al. [53] | 2003 | Trauma | 71 N vs. 85 SN | SN vs. LR infusion:
|
SN vs. LR:
|
Not reported | Mortality SN vs. LR: 27% vs. 11% (p < 0.05) |
| He et al. [55] | 2019 | Trauma | 455 pts (44 IAH; 5 ACS) | Volume of IV fluids over 24 h: 3.965 ± 739 mL | Mean IAP 24.4 ± 8.5 | DL |
|
| Hwabejire et al. [56] | 2016 | Trauma | n = 1976 of which 122 ACS |
Total fluid/kg:
|
Not reported | 98.4% DL | ACS+: 37.7% vs. ACS-: 14.6% (p < 0.001) Rise in ACS risk after total volume + 1302 mL/kg |
| Joseph et al. [57] | 2014 | Trauma | 799 |
|
18 patients ACS | DL in 18.9% |
|
| Macedo et al. [58] | 2016 | Trauma | 10 |
|
Not reported | DL | 60% overall mortality |
| Shaheen et al. [62] | 2016 | Trauma | 28 | >10 U of RCC in 24 h | 60.7% developed ACS | Not reported | - 30-day mortality was 32.1% |
| Madigan et al. [59] | 2008 | Trauma | ACS (n = 48) vs. control (n = 48) |
Net fluid for DC until 48 h post-admission was 18.2 L vs. 5.1 L (p < 0.0001) | Not reported | DL | Mortality 60% ACS vs. 2% controls (p < 0.0001) |
| Maxwell et al. [60] | 1999 | Trauma | 46 | Mean 19 ± 5 L crystalloid 29 ± 10 U RCC |
Mean: 33± 3 | DL | 67% mortality |
| Rodas et al. [61] | 2005 | Trauma | 5 | Crystalloid: 15 ± 1.7 L Blood: 11 ± 0.4 U |
NR | DL | No mortality |
| Strang et al. [75] | 2015 | Trauma | 567 509 no IAH 58 IAH |
No IAH: 4.2 L Crystalloid vs. IAH: 6 L crystalloid; no IAH: 1.5 L colloids vs. IAH: 2.5 L colloids; no IAH: 2 U RCC vs. IAH: 17 U | 30 patients ACS | NR | IAH: 25.9% vs. 12.2% no IAH; p = 0.012). |
| Zaydfudim et al. [69] | 2010 | Trauma | 39 pre-TEP vs. 36 TEP | Pre-TEP: 12 U RCC vs. TEP: 12.5 U RCC Pre-TEP: 4 U FFP, vs. TEP: 8 U FFP; p < 0.01 Pre-TEP: 1 U platelets vs. TEP: 2 U platelets; p < 0.01 Pre-TEP: 6 L of crystalloids vs. TEP: 4 L crystalloids; p < 0.01 |
20% ACS in pre-TEP vs. 0% ACS in TEP | NR | pre-TEP cohort: 31% 30-day survival TEP cohort: 53% 30-day survival |
| Cothren et al. [106] | 2007 | Surgical & Medical patients | 54 patients | Total fluid resuscitation before DL:
|
Medical: 33.5 ± 1.1 vs.
|
DL | MOF:
|
| Cordemans et al. [78] | 2012 | ALI | 57 PAL vs. 57 control |
Cumulative FB after 1 week 8.027 ± 5.254 mL/day vs. −1.451 ± 7.761 (p < 0.001) |
IAP at baseline: PAL: 10 ± 4.2 Control: 8 ± 3.7 (p = 0.013) |
PAL treatment |
|
| Pupelis et al. [44] | 2012 | Pancreatitis | 130 patients 75 CVVH 55 control |
Not reported | CVVH: 19.6 ± 7.1 Control: 16.3 ± 5.5 p = 0.05 |
DL n = 36 | 11.7% CVVH and 13.8% no CVVH NS |
| Struck et al. [79] | 2012 | TEN | 29 patients 5 ACS |
+ FB 4.6 ± 1.2 L | 33 ± 7 | DL | Mortality: ACS+ 100% vs. ACS- 0% |
| Aik-Yong et al. [105] | 2014 | Surgical & medical patients | 17 patients: 14 primary ACS 3 secondary ACS |
>3.5 L in 24 h | DL | Overall mortality 47.1% | |
| McNelis et al. [99] | 2002 | Surgery | 22 ACS vs. 22 control | 24-h FB: ACS: 15.9 ± 10.3 L vs. Control: 7 ± 3.5 L (p < 0.05) |
Not reported | Not reported | Mortality: 66.7% in ACS vs. none in control |
| Rubenstein et al. [89] | 2015 | rAAA open repair. 44 pts (60%) EVAR: 29 pts (40%) |
73 | Intraoperative fluid higher in EVAR patients ACS+ vs. ACS-
|
ACS% 34% in open21% in EVARp not significant | DL | Overall mortality 42%:
|
| Leclerc et al. [98] | 2017 | rAAA | 47 | ACS+: 5.250 (4.625; 9.375) L ACS-: 4.125 (2.925; 5.500) L (p = 0.053) |
8 patients developed ACS | 30-day mortality in ACS+ higher (p = 0.108) | |
| Miranda et al. [88] | 2018 | rAAA | 25 |
|
12% (n = 3) developed ACS |
|
FB: fluid balance; pts: patients; ACS+: with abdominal compartment syndrome; ACS-: without abdominal compartment syndrome; TBSA: total body surface area; DL: decompressive laparotomy; EVAR: endovascular aortic repair, NS: not significant; rAAA: ruptured abdominal aortic aneurysms; U: units; RCC: Red cell concentrate; PAL: peep-albumin-Lasix; CVVH: continuous veno-venous hemofiltration; S: surgical; M: medical; SN: supranormal resuscitation group; LR: lactated ringer infusion; d: day.
Table 3.
Summary of findings of case reports on fluid administration and IAH.
| Author | Year | Population | Resuscitation Fluids/Fluid Balance | IAP (mmHg) | Intervention | Results |
|---|---|---|---|---|---|---|
| Fietsam et al. [101] | 1989 | Surgery | >25 L of fluid | NR | DL | NR |
| Burrows et al. [63] | 1995 | Surgery | 21 L of crystalloid; 4 U RCC | NR | DL | Alive |
| Burrows et al. [63] | 1995 | Trauma | Pre-op: 7.3 mL/kg/h vs. Postop: 14.2 mL/kg/h | 39 | DL | NR |
| Burrows et al. [63] | 1995 | Trauma | Pre-op: 9.2 mL/kg/h vs. Postop: 5.5 mL/kg/h | 40 | DL | Died |
| Burrows et al. [63] | 1995 | Trauma | Pre-op: 14.7 mL/kg/h vs. Postop: 3.2 mL/kg/h | NR | DL | Alive |
| Ivy et al. [33] | 1999 | Burn | 32 L | 49 | DL | Died |
| Ivy et al. [33] | 1999 | Burn | 24 L | 50 | Escharotomy | Died |
| Ivy et al. [33] | 1999 | Burn | 32 L | 36 | None | Died |
| Kopelman et al. [65] | 2000 | Trauma | + FB: 25 L | 34 | DL | Died |
| Kopelman et al. [65] | 2000 | Trauma | 26 L of crystalloid | 25 | DL | Died |
| Kopelman et al. [65] | 2000 | Trauma | + FB: 29.5 L | 22 | DL | Died |
| Kopelman et al. [65] | 2000 | Trauma | + FB: 10 L | 26 | DL | Alive |
| Kopelman et al. [65] | 2000 | Trauma | + FB: 5 L | 46 | DL | Alive |
| Macalino et al. [77] | 2002 | Sepsis | 14 L crystalloids | 27 | NMB | Died |
| Kula et al. [72] | 2004 | Sepsis | 10 L + FB first 96 h. 4:1 (crystalloid: colloid) |
>25 | DL CVVH |
Died |
| Kula et al. [72] | 2004 | Sepsis | 12.5 L + FB first 96 h (crystalloids) | 29 | CVVH | Died |
| Shiiya et al. [103] | 2005 | Surgery | 34.1 L crystalloids vs. 13.7 L blood products | NR | DL | Alive |
| Parra et al. [34] | 2006 | Burn/Trauma | 25.55 L of crystalloid 12 U RCC |
34 | DL | Alive |
| De Wolf et al. [100] | 2008 | Surgery | Massive fluid resuscitation | 24 in 1st patient 27 in 2nd patient |
DL | Alive |
| Tsuang et al. [76] | 2007 | Sepsis | 17 L fluid during first 20 h | 54 | DL | Alive |
| Chamisa et al. [64] | 2008 | Trauma | Not reported | >35 | DL | Died |
| Kula et al. [73] | 2008 | Trauma | 7.5 L + FB first 48 h. 4:1 (crystalloid: colloid) | 26 | CVVH | NR |
| Kula et al. [73] | 2008 | Trauma | 17 L + FB first 96 h. 3:1 (crystalloid: colloid) | 28 | CVVH | NR |
| Augustin et al. [90] | 2010 | Surgery | 16 L + FB | 19 | DL | Died |
| Augustin et al. [90] | 2010 | Surgery | 23 L + FB | 35 | None | Died |
| Rabbi et al. [102] | 2012 | Surgery | Not reported | 50 | DL | Alive |
| Park et al. [46] | 2014 | SAP | Not reported | 31 | PCD | Alive |
| Bressan et al. [91] | 2016 | Surgery | 4 L crystalloids 2 RCC during first 24 h |
21 | DL | Alive |
| Michel et al. [66] | 2016 | Trauma | 10.5 L (crystalloids, colloids & blood products) | NR | DL | Alive |
| Lee et al. [45] | 2019 | SAP | 6 L | 28 | DL | Alive |
+ FB: positive fluid balance; NR: not reported; CVVH: continuous veno-venous hemofiltration; NMB: neuromuscular blocker; SAP: severe acute pancreatitis; PCD: Percutaneous Catheter Drainage; DL: decompressive laparotomy; RCC: red cell concentrate.
Table 4.
Summary of findings of pediatric studies on fluid administration and IAH.
| Author | Year | Type of Study | Population | Resuscitation Fluids | IAP (mmHg) | Intervention | Results |
|---|---|---|---|---|---|---|---|
| Divarci et al. [81] | 2016 | Prospective | Sepsis | NR | 14 patients with IAH (13–15) 6 patients ACS (17–24) |
Decompressive measures DL |
1 Dead |
| Ranjit et al. [84] | 2018 | Prospective | Sepsis | ST group (n = 30): 17.8 (10.8–25.2) L TI group (n = 38): 10.02 (5.7–18.2) L (p = 0.009) |
NR | Percutaneous drainage of ACS, n (%) ST group: 9 (30) TI group: 3 (7.9) (p = 0.01) |
Mortality: ST: 8 (26%) TI: 1 (2.6%) p = 0.008 |
| DeCou et al. [70] | 2000 | Case report | Trauma | Crystalloids and 16 U RCC and 4 U FFP |
NR | Silo decompression | Alive |
| DeCou et al. [70] | 2000 | Case report | Trauma | Replacement of 2 x blood volume | NR | Silo decompression | Alive |
| DeCou et al. [70] | 2000 | Case report | Sepsis | NR | 26 | Silo decompression | Alive |
| Perks et al. [68] | 2005 | Case report | Trauma | NR | NR | Surgical decompression | Alive |
| Jensen et al. [37] | 2006 | Case report | Burn | 5990 mL crystalloids | >22 | DL | Dead |
| Jensen et al. [37] | 2006 | Case report | Burn | 8580 mL crystalloids + 990 mL blood products + 805 mL albumin |
NR | Abdominal wall escharotomy and NMB and peritoneal dialysis catheter | Alive |
| Jensen et al. [37] | 2006 | Case report | Burn | 10300 mL crystalloids | 44 | Surgical decompression | Dead |
| Jensen et al. [37] | 2006 | Case report | Trauma | 1950 mL crystalloids | 26 | Silo decompression | Alive |
| Morell et al. [67] | 2007 | Case report | Trauma | 10000 mL crystalloids and 10 U RCC | NR | Laparotomy | Alive |
| Lam et al. [83] | 2008 | Case report | Sepsis | 272 mL/kg | 35 | Paracentesis | Died |
| Lam et al. [83] | 2008 | Case report | Sepsis | 220 mL/kg | NR | DL | Died |
| Lam et al. [83] | 2008 | Case report | Reanimated after drowning | 334 mL/kg | NR | DL | Died |
| Lam et al. [83] | 2008 | Case report | Sepsis | 500 mL/kg | 120 | None | Died |
| Lam et al. [83] | 2008 | Case report | Sepsis | NR | NR | Peritoneal catheter | Alive |
| Dauplaise et al. [80] | 2010 | Case report | Sepsis | 70 mL/kg in first h and 330 mL/kg in first 24 h | 43 | DL | Alive |
| Gala et al. [82] | 2012 | Case report | Sepsis | NR | NR | Paracentesis | Alive |
| Streit et al. [35] | 2013 | Case report | Burn | NR | 27 | Decompression | Alive |
| Sun et al. [36] | 2015 | Case report | Burn | 5600 mL LR during first 24 h | 22 | NMB, diuresis; percutaneous drain | Alive |
| Kobayashi et al. [71] | 2016 | Case report | Trauma | 560 mL RCC. 960 mL FFP. 400 mL platelets and fluids |
NR | Laparotomy | Alive |
NR: not reported; RCC: red cell concentrate; FFP: fresh frozen plasma; ST group: standard therapy; TI group: targeted intervention; DL: decompressive laparotomy; NMB: neuromuscular blockers.
Table 5.
Summary of findings of animal studies on fluid administration and IAH.
| Author | Year | Population | Intervention | Results |
|---|---|---|---|---|
| Schachtrupp et al. [119] | 2005 | 12 Pigs:
|
Fluid intake: Intervention group vs. control (p < 0.01) 10570 ± 1928 mL vs. 3918 ± 1042 mL |
Acidosis, liver, bowel, kidney and lung damage higher in intervention group (p < 0.01) |
| Moore-Olufemi et al. [117] | 2005 | 44 Rats Experiment 1: 20 mL/kg saline Experiment 2: 80 mL/kg saline In each experiment 4 groups
|
A mesenteric venous hypertension/gut edema model was created to evaluate whether gut edema caused by acute mesenteric venous hypertension and/or crystalloid resuscitation is associated with impaired intestinal transit, mucosal barrier dysfunction, and/or injury | Delayed intestinal transit, increased permeability, and decreased epithelial resistance are associated with gut edema |
| Chang et al. [118] | 2016 | 48 rats:
|
Induced portal hypertension, hemorrhage to a MAP of 40 mmHg for 2 h (except for sham group) Collected blood reinfused and treatment with:
|
Melatonin use associated with less inflammatory and oxidative injury, less intestinal permeability and injury, lower incidence of secondary IAH |
LR: Ringer’s lactate solution, HES: hydroxyethyl starch, IAH: intra-abdominal hypertension.
From the analyzed and discussed studies, twenty included burn patients [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37], seven included severe acute pancreatitis (SAP) patients [40,41,42,43,44,45,46], thirty included trauma patients [38,39,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,73,74,75], fourteen included medical patients [70,72,76,77,78,79,80,81,82,83,84,85,86,87], seventeen included surgical patients [63,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103], fourteen included medical-surgical (and critically ill) patients [5,104,105,106,107,108,109,110,111,112,113,114,115,116].
Pooled analysis was not possible because of the heterogeneity in study populations and data, and the lack of details on IAP measurement techniques.
Several above-cited studies have investigated the relationship between the volume of intravenous fluids administered and their effect on IAP. Most of these trials reported an association between the volume of intravenous fluids administered and the rise in IAP or the development of IAH.
3.1.2. Severe burn patients
There are nine prospective studies in 434 burn patients [18,19,20,21,22,23,24,25,26], six retrospective studies in 3171 burn patients [27,28,29,30,31,32], two case reports in adults [33,34], and three case reports in children [35,36,37] investigating the relationship between fluid resuscitation and ACS (Supplementary Table S1).
Prevalence
The reported prevalence of IAH ranged from 57.8% to 82.6% among patients with ≥20% total body surface area (TBSA) burned. Six of the nine studies reported ACS rates between 5.5 and 28.6% [18,19,20,21,22,23,24,25,26].
Resuscitation Fluids & Risk Factors
A capillary leak is common in critically ill patients and leads to interstitial edema. This can be a particular problem in burn patients who require large volumes of intravenous fluid resuscitation. There is a significant correlation between IAP and resuscitation volume [22,33,34]. A volume administration of > 250 mL/kg in the first 24 h is a risk factor for ACS, and this amount is known as the Ivy index [19]. Hypertonic lactated saline (HLS) resuscitation may reduce the risk of developing secondary ACS and is associated with a lower fluid volume when compared to Ringer’s lactate (LR) solution [21,120]. Compared to colloid resuscitation, crystalloid resuscitation resulted in higher volumes of fluid per kilogram body weight, both in the first 24 h and during resuscitation with a significantly greater increase in IAP [23]. The implementation of 5% albumin in the first 24 h of resuscitation showed a trend towards less intravenous fluid. However, this did not translate into differences in the overall incidence of ACS, but it did improve outcomes [32]. Risk factors identified for acute kidney injury were IAH and the use of glycopeptides, vasopressors, and mechanical ventilation. Acute kidney injury was associated with increased 30-day mortality [25].
Management
Bladder pressure measurements should be performed after infusion of more than 25 mL during the acute resuscitation phase [18]. While IAH usually responds to medical therapy, the presence of ACS warrants escharotomy or surgical decompression of the abdominal cavity [18]. Non-resolution of IAH is related to a worse outcome [26,29,38].
Outcome
Mortality rates in the prospective studies varied from 18% to 82.6% [18,19,20,21,22,23,24,25,26]. The implementation of burn resuscitation guidelines can significantly lower mortality rates [18].
3.1.3. Severe acute pancreatitis
Severe acute pancreatitis (SAP) is a disease with a 30% mortality rate and is characterized by a systemic inflammatory response, pancreatic necrosis, and multiple organ failure [40]. Appropriate early fluid resuscitation is essential to prevent complications. Three RCTs [40,41,43], one observational study [42] (total of 295 patients), one retrospective study [44], and two case reports [45,46], investigated the relationship between intravenous fluids and IAH in SAP (Supplementary Table S2).
Prevalence
The incidence of ACS is lower when controlled fluid resuscitation is applied [40]. This was shown in an RCT where the incidence of ACS was 72.2% in the rapid fluid expansion group versus 32.5% in the controlled fluid expansion group [43].
Resuscitation Fluid and Risk Factors
The type of intravenous fluid used is important in the prevention of IAH. Resuscitation with colloids resulted in less IAH compared with crystalloids [41]. Using a combination of 0.9% saline, colloids, and glutamine is possibly a more efficient resuscitation strategy for SAP (by relieving inflammation and maintaining the intestinal barrier) than 0.9% saline [40]. Significant risk factors for the development of IAH in patients with SAP include the first 24-h fluid balance, number of fluid collections (which is included in the definitive Balthazar’s CT score for severity stratification in acute pancreatitis), and serum calcium level [42].
Management
Early management of patients with SAP includes the initiation of CVVH to facilitate achieving a negative fluid balance and a subsequent reduction in IAH [44]. Abdominal decompression in patients with ACS may lead to a reversal of MOF [45,46].
Outcome
IAH is associated with a poor prognosis and an increased need for surgical interventions with associated morbidity and mortality. The reported mortality rate varied between 7.3% to 31.6% [41].
3.1.4. Trauma patients
Trauma patients frequently pose a fluid resuscitation challenge since they often require rapid intravenous fluid administration to treat hypovolemia. This may include red cell concentrate (RCC) and platelets. Rapid fluid administration, together with reperfusion injury and activation of inflammatory mediators, leads to increased capillary permeability and an increased risk of developing IAH and ACS [120,121]. There are seven prospective studies investigating the relationship between intravenous fluids and IAH in 1329 trauma patients [47,48,49,50,51,52,74], fourteen retrospective studies in 4233 trauma patients [38,39,53,54,55,56,57,58,59,60,61,62,69,75], five case reports [63,64,65,66,73], and five case reports in children [37,67,68,70,71] (Supplementary Table S3).
Prevalence
The reported prevalence of ACS in the prospective studies varied between 8% and 36% (with a mean Injury Severity Score (ISS) range of 13–35) [47,48,49,50,51,52]. In a retrospective study by Zaydfudim et al., the implementation of a trauma exsanguination protocol significantly reduced ACS from 20% to zero [69]. Balogh found that the implementation of a standard resuscitation compared to a supranormal resuscitation reduced the incidence of IAH (20 vs. 42%) and ACS (8 vs. 16%) [53].
Resuscitation Fluid and Risk Factors
Trauma patients that develop ACS, as a complication of massive volume loading, receive significantly more crystalloids and blood products [48]. Aggressive crystalloid resuscitation should be minimized in severely injured patients. Neal et al., found that patients requiring massive transfusions (crystalloid resuscitation in a ratio greater than 1.5:1 per unit of RCC) were associated with a higher risk of MOF, ARDS, and ACS [50]. Although massive transfusion is associated with more complications, when blood products are delivered in a 3:2 ratio of RCC: FFP (red blood cells: fresh frozen plasma) and 5:1 for RCC: platelets, it is associated with a reduction in MOF and infectious complications, as well as an increase in ventilator-free days [63].
Management
Bladder pressures should be checked routinely when resuscitation volumes approach 10 L of crystalloid or ten units of packed red cells [60]. Following the resuscitation phase, fluid removal with diuretics or CVVH may restore euvolemia and may reduce IAP leading to improvement of organ failure [5,72,76].
Outcome
Trauma patients with ACS have more complications, mechanical ventilation, organ failure, and a longer length of stay. Mortality for this group varies between 6% and 54% [47,48,49,50,51,52,120]. Limiting crystalloids during resuscitation in trauma patients was associated with better outcomes and almost eliminated ACS [57,58].
3.1.5. Medical patients
Three prospective studies (188 patients) [85,86,87], two retrospective studies (143 patients) [78,79], three case reports in adult patients [72,76,77], two prospective trials (88 patients) in children [81,84], and four case reports in children [70,80,82,83] discuss fluid resuscitation in patients with sepsis (Supplementary Table S4).
Incidence
The observed incidence of IAH varied between 20 and 85%, and ACS developed in 25–28% of cases [78,85]. The incidence of IAH and ACS in a group of 40 medical ICU patients with a positive fluid balance of more than 5 L/24 h was high, with 85% developing IAH and 25% developing ACS [86].
Resuscitation Fluid and Management
In a prospective trial of 68 children, the replacement of crystalloid fluid resuscitation with albumin for refractory shock resulted in a smaller positive fluid balance, decreased morbidity, and improved outcomes [84]. Treatment (PAL therapy) that combined high levels of positive end-expiratory pressure (PEEP), small volume resuscitation with hyperoncotic 20% albumin (up to serum albumin levels of 30 g/L), and fluid removal using furosemide (a bolus of 1 mg/kg followed by continuous infusion at 10 mg/hour and titrated according to urine output) or renal replacement therapy with net ultrafiltration was associated with a reduction of extravascular lung water index (EVLWI) and IAP, was associated with improved clinical outcomes (better survival and faster weaning from mechanical ventilation) [87].
Decompressive laparotomy (open abdomen with silo bag) has been previously successful in medical patients [5,76]. Fluid removal with diuretics or CVVH may restore fluid balance and may reduce IAP, leading to improvement of organ failure [72].
Outcome
ACS is associated with a high mortality rate (52.8–77.4%) [78]. Moreover, Cordemans et al., concluded that there is a correlation between poor outcomes and a high capillary leak index (CLI), a positive fluid balance, high IAPs, high extravascular lung water indices (EVLWI), and low abdominal perfusion pressures (APP) [78]. The ACS-associated mortality rate in children was 16% [81].
3.1.6. Surgical patients
Six prospective studies (460 surgical patients) [92,93,94,95,96,97], four retrospective studies (189 patients) [88,89,98,99] and seven case reports (see Table 3) in adults [63,90,91,100,101,102,103], describe the association between fluid and ACS (Supplementary Table S4).
Incidence
Dalfino et al., showed how a positive fluid balance comprised one of three independent predictors for developing IAH (31.8%), together with baseline IAP and central venous pressure [104].
Resuscitation Fluid and Risk Factors
There is a significant positive correlation between increased IAP with a positive fluid balance and decreased IAP with a negative fluid balance [97]. A liberal fluid strategy, compared to a restrictive fluid strategy, is associated with a significantly higher rise in IAP after surgery [94]. Furthermore, there was a strong correlation between IAP and extracellular water content in the liberal subgroup, which is in keeping with the hypothesis of fluid extravasation being one of the critical mechanisms in the development of IAH.
Makar et al., conducted an observational study in patients following open and endovascular repair of ruptured abdominal aortic aneurysms (rAAA). The results suggested that endovascular repair is associated with less intra-abdominal hypertension and host inflammatory response, less blood loss, blood transfusion, and total intraoperative intravenous fluid infusion compared to open repair [95]. In 25 patients with rAAA who underwent emergency EVAR [88], hypotension on arrival, transfusion of three or more units of red cell concentrate, and postoperative anemia were all significantly associated with the development of postoperative ACS.
Outcome
Patients with high IAP have more frequent renal failure, delayed postsurgical weaning from mechanical ventilation, and worse outcomes [92]. Reported mortality among patients with IAH was 53% [93]. The development of ACS after the repair of ruptured abdominal aortic aneurysms (rAAAs) results in increased mortality, especially in patients treated by endovascular aortic repair (EVAR) [89]. Intraoperative fluid requirements were significantly higher in EVAR patients who developed ACS than those without ACS. Furthermore, Leclerc et al., showed that in patients who underwent rAAA repair, patients with ACS appeared to have higher mortality [98]. For a positive prediction, they required three of the following eight factors: anemia, prolonged shock, preoperative cardiac arrest, body mass index >30 kg/m2, massive fluid resuscitation and transfusions, severe hypothermia, and acidosis.
3.1.7. Mixed ICU patients
Twelve prospective studies (see Table 1) [5,104,107,108,109,110,111,112,113,114,115,116] (4213 patients) and 2 retrospective studies (71 patients) [105,106] describe fluid resuscitation in medical-surgical patients.
Incidence
The incidence of ACS varied between 2% and 12.9% [105,106]. The incidence of IAH is 25–30% on admission and 50% after the first week of ICU stay [115].
Independent Predictors for IAH
Fluid resuscitation and positive fluid balance are independent predictors for IAH [108]. Body mass index is significantly associated with the development of IAH [109]. Elevated vascular permeability due to a stress-related inflammatory response is associated with a positive fluid balance. It leads to extravascular fluid accumulation, which is likely to result in gastrointestinal tract edema and increased IAP [5].
Outcome
Mortality rates for IAH vary from 3 to 80% [110]. The grade of IAH is inversely related to outcome [111]. Biffl et al., showed that medical patients with ACS have a 100% mortality vs. 38% in trauma patients [104]. Similar results were seen in a retrospective (see Table 2) study that showed no significant differences in fluid resuscitation and bladder pressures between groups. However, there was a significantly higher incidence of MOF and a trend towards higher mortality in medical ACS [106]. Finally, the summary of findings of pediatric studies is presented in Table 4.
3.2. Animal data
We found eleven animal studies, of which three were suitable, reporting on resuscitation and secondary IAH (Table 5). Fluid resuscitation leads to IAH and venous congestion (or venous hypertension), resulting in gut edema and diminished gut contractility [117]. Melatonin may prevent deleterious effects related to fluid overload [118]. Extensive fluid resuscitation preserves cardiac output, urine output, and serum parameters (e.g., ALT, lipase, AP, lactate, creatinine) in pigs with ACS, but organ damage occurs (vicious cycle) [119]. Previous animal studies showed that IAH provokes the release of pro-inflammatory cytokines which may serve as a second insult for the induction of MOF [121]. This is illustrated in Figure 3.
Figure 3.
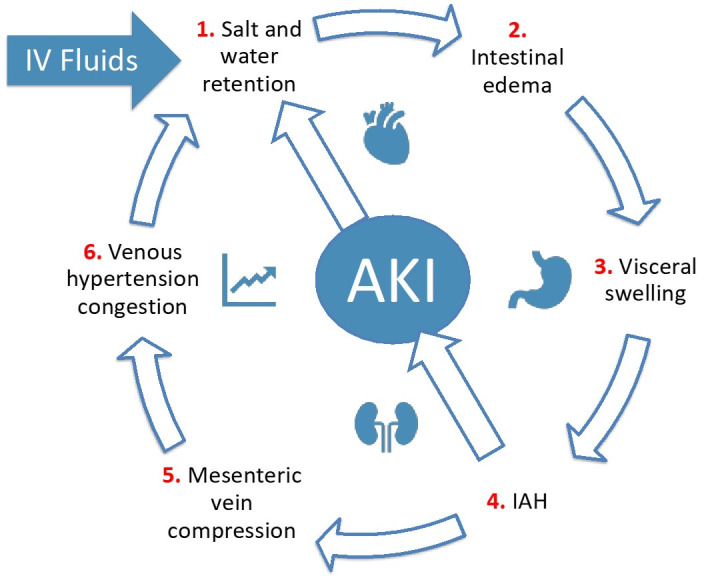
The vicious cycle of fluid resuscitation, abdominal hypertension and kidney injury. Adapted according to the Open Access CC BY License 4.0 from Malbrain et al., with permission [17]. AKI: acute kidney injury; IAH: intra-abdominal hypertension.
4. Discussion
Existing studies and pathophysiological rationale support the association between fluid administration and IAH. However, current evidence does not allow clinicians to accurately identify specific fluid management strategies for patients with IAH. IAH often occurs in patients with sepsis, trauma, burns, and severe acute pancreatitis [122,123,124]. These conditions are united by an accompanying inflammatory response that often progresses to shock and requires ongoing intravenous fluid therapy. Addressing the underlying cause of the pathophysiological process is essential; however, in all these patients, fluid management remains a challenge. Avoiding hypovolemia as well as unnecessary excessive intravenous fluids and subsequent interstitial edema, with progression to IAH and ACS, is a difficult balance to achieve [125,126].
The origin of intravenous fluid therapy [127] dates back to the cholera outbreak in the 1830s. Resuscitation fluids are administered to restore intravascular volume and maintain tissue perfusion [17]. However, determining the volume status of a critically ill patient remains a diagnostic challenge [123]. Furthermore, the ideal synthetic intravenous resuscitation fluid does not exist. Both crystalloid and colloid solutions offer therapeutic options. Albumin is considered safe for use as a resuscitation fluid in most critically ill patients; however, in patients with traumatic brain injury, its use is associated with increased mortality [128]. The use of hydroxyethyl starch (HES) solutions is associated with increased rates of renal-replacement therapy and blood transfusion in patients with sepsis and surgery. The use of 0.9% saline has been associated with the development of metabolic acidosis and acute kidney injury.
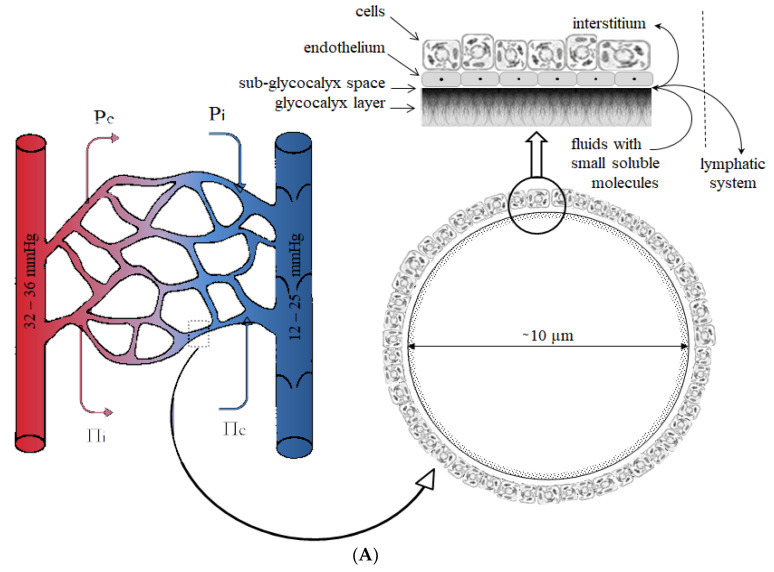
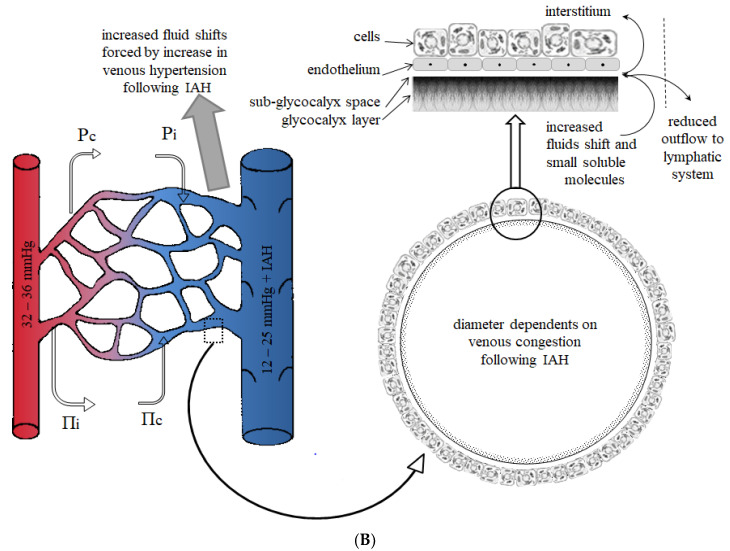
Fluid movement through the microcirculation is partly determined by the imbalance between colloid osmotic and hydrostatic forces (Starling equation). Following this theory in IAH, an increase in microvenule blood pressure following venous compression reduces the difference in hydrostatic pressure, resulting in disturbance of microcirculatory fluid movement. The entire vascular endothelium is covered by the endothelial glycocalyx which consists of various proteoglycans, glycoproteins, and glycolipids. It which plays a vital role in the movement of fluids. The endothelial glycocalyx is semi-permeable to small molecules and ions and impermeable to molecules greater than 70 kDa [129,130]. The Starling equation has been revised to account for the sub-glycocalyx layer that contributes to a reflectance coefficient responsible for larger molecules staying intravascular. According to this revised Starling equation, the differences in plasma-sub-glycocalyx colloid osmotic pressure play a crucial role in trans-endothelial fluid movement [131]. The revised Starling equation has the sub-glycocalyx oncotic pressure replacing the interstitial oncotic pressure as a primary factor in transvascular fluid movement (Figure 4). The rule states that colloids such as albumin may delay transvascular fluid escape under selected conditions but will not pull fluids from the interstitium back into the vascular compartment; rather, albumin only returns to the intravascular compartment by the lymphatics [132,133]. A decreased arterial pressure in conjunction with an increased venous pressure is frequently observed in patients with IAH. Increased pressure in venules may increase hydrostatic capillary pressure and augment transcapillary fluid extravasation causing loss of plasma volume. This is because of the dependence on differences in transendothelial pressure for the movement of fluid. Thus, the administration of colloid solutions to restore mean arterial pressure may maintain colloid osmotic pressure but increase hydrostatic capillary pressure, which may intensify fluid filtration. Crystalloid solutions decrease colloid osmotic pressure and increase hydrostatic capillary pressure, theoretically leading to higher fluid filtration than colloids [131]. However, IAH is often the result of several pathologies coinciding, damaging the glycocalyx and causing increased vascular permeability. As a result, both crystalloid and colloid solutions leak from the intravascular compartment into the interstitial space.
Figure 4.
Fluid movement in normal conditions (A) and abdominal hypertension (B). The physiological movement of fluid is determined by the imbalance between hydrostatic and colloid osmotic pressures. It is best described by the revised Starling equation: Jv = LpA[(Pc − Pi) − σ(IIc − IIi)], where Jv is net fluid filtration, Lp the capillary hydraulic permeability, A the capillary surface area (which is available for fluids and small molecule filtration), σ the capillary reflection coefficient, Pc the capillary hydrostatic pressure, Pi the interstitial hydrostatic pressure, IIc and IIi the capillary and interstitial colloid osmotic pressures, respectively. Generally, Pc dependent on the differences between the arteriole hydrostatic pressure (PA) and the venule hydrostatic pressure (PV). This difference strongly corresponds to the hydraulic resistances in arterioles and venule (RA and RV, respectively), which was described by the Pappenheimer Soto-Riviera Equation: Pc = (Pv [RA/RV] + PA)/(1 + [RA/RV]). According to this equation, every increase in PA or PV, as well as an increase in RA/RV (e.g., following intra-abdominal hypertension leading to venous congestion) or increase Pc. Under normal physiological conditions, the sub-glycocalyx colloid osmotic pressure strongly corresponds to interstitial pressure and its value ranges between 70% and 90% of the interstitial colloid pressure. Adapted from Levick et al. [133].
Experimental models have confirmed that when maintaining a normal MAP of approximately 65 mmHg (using vasopressors), fluid movement and reduction of plasma volume are more pronounced when the capillary permeability is disrupted versus normal conditions [134]. Interestingly, the plasma-reducing effect was lower in hypovolemic conditions compared to normovolemic subjects. These findings may suggest that the decrease in hydrostatic capillary pressure following hypovolemia leads to higher fluid retention in the intravascular space [135]. This effect may be disrupted by IAH; however, this hypothesis is yet to be confirmed.
4.1. Type of Patients
The incidence of ACS and IAH differs across various patient populations, but with a high mortality rate, regardless of the population.
In severe burns, the systemic release of inflammatory and vasoactive mediators is responsible for a systemic capillary leak, intravascular fluid loss, and significant fluid shifts that should be managed with aggressive intravenous fluid resuscitation [136]. The implementation of the Parkland formula, developed by Baxter and Shires, reduced inadequate resuscitation in acute burn patients, which in turn significantly decreased burn mortality [137]. However, excessive intravenous fluid administration during resuscitation can also be detrimental and lead to an IAH prevalence as high as 82.6% in patients with more than 20% TBSA burned. Fluid creep is applied to a burn resuscitation, during which more fluid than predicted by standard formulas is administered. Increased fluid requirements may be necessary, but dangerous fluid creep is also caused by overly permissive fluid infusion and the lack of colloid supplementation [138]. Fluid creep is reported in 30% to 90% of patients with major burns [139,140]. Complications of fluid overload include extremity and abdominal compartment syndromes, respiratory failure, and ocular hypertension [138]. Factors that predispose to increased fluid requirements are inhalation injury, delay in resuscitation, and polytrauma or high voltage electrical injury [120]. The use of hypertonic saline, 5% albumin, and routine use of a burn resuscitation guideline are all measures to help limit unnecessary fluid resuscitation.
Severe acute pancreatitis is associated with high mortality rates [141], and the local and systemic inflammatory response in SAP leads to intravascular fluid depletion and extravascular fluid accumulation, leading to IAH and ACS. Generally, in patients with IAH, volume status is probably best monitored with volumetric preload indicators instead of barometric ones (such as central venous pressure and pulmonary capillary wedge pressure) [142]. The primary aim of fluid replacement is to improve circulatory dysfunction, which leads to tissue hypoperfusion, ischemia, and self-sustaining disease with persistent pancreatic injury, extra-pancreatic tissue damage, and organ failure [143]. Although many controversies exist about the ideal fluid strategy, an RCT performed on 76 patients with SAP showed that controlled, more conservative, fluid resuscitation offers a better prognosis in patients with severe volume deficit within 72 h of SAP onset [43,144]. Initiation of renal replacement therapy should be considered to help manage fluid accumulation and ACS.
In patients with SAP, sepsis, septic shock, or severe trauma, shock-induced endotheliopathy (SHINE) is responsible for endothelial cell and glycocalyx damage [145]. Disruption of the endothelial glycocalyx layer (EGL) can also be induced by rapid infusion of intravenous fluids (partly due to the release of atrial natriuretic peptide) and acute hyperglycemia [131]. In septic patients, interstitial oncotic pressure increases due to the capillary leak, leading to a reduction of the plasma-expanding efficacy of any infused fluid [131] and aggravating the development of tissue edema. More recently, it has been suggested that non-resuscitation fluids in critically ill patients may even have a more considerable absolute impact on cumulative positive fluid balance than resuscitation fluids. In contrast, unintentional fluid administration in the form of IV medications and concentrated electrolytes contributes to the phenomenon of ‘fluid creep’ [146].
Understanding the different phases of intravenous fluid management (Figure 1 represents the ROSE concept) is key to planning optimal fluid management. Hypovolemia should generally be treated with fluids and vasoplegia with vasopressors, but this balance is difficult to find in septic patients. Early vasopressors, in addition to fluid resuscitation, instead of fluids alone, may be necessary to avoid fluid overload [17,74,75,119,122,147,148]. The recent results of the CLASSIC trial have shed more light on this topic and showed that giving less fluids is not harmful [149]. On average IAH is observed in up to 43.5% of patients with severe sepsis [150].
4.2. Type of Resuscitation Fluids
Crystalloid fluids are the mainstay of fluid resuscitation; however, the findings of this review suggest alternative strategies require further investigation. A randomized controlled trial (RCT) compared HES with Ringer’s lactate resuscitation in 41 patients with SAP. Resuscitation using colloids resulted in a lower IAP and reduced need for mechanical ventilation compared to those in which Ringer’s lactate was used [41]. However, there is no evidence from RCTs that resuscitation with colloids in patients with trauma, burns, or following surgery, reduces the risk of death compared to resuscitation with crystalloids [151]. There is evidence of harm from synthetic colloids, especially synthetic starch solutions [152].
Balanced crystalloids may have advantages over 0.9% saline, possibly reducing inflammation, but no apparent effect on mortality or morbidity was demonstrated in patients with SAP [153,154]. The recently conducted pragmatic SMART study (involving 15802 critically ill adults) showed that using balanced crystalloids for intravenous fluid administration resulted in a lower rate of composite outcomes, including death from any cause, new renal-replacement therapy, or persistent renal dysfunction than the use of saline [155]. Accordingly, several current guidelines suggest using balanced rather than unbalanced crystalloids in extensive volume replacements, surgical patients, and in SAP [142,154,156].
Several studies (SAFE [157], FEAST [158], ALBIOS [159]), evaluated the use of albumin as a resuscitation fluid. Except for patients with traumatic brain injury, evidence suggests that albumin is well tolerated as a resuscitation fluid. However, there is no evidence to suggest that albumin offers substantial outcome benefits over crystalloid solutions, albeit that their use may result in a less positive fluid balance [160,161,162]. This was demonstrated in an RCT by Martensson et al., where resuscitation with 20% albumin decreased resuscitation fluid requirements, minimized positive early fluid balance, and was not associated with any harm compared with 4–5% albumin. The use of 5% albumin in severe burn patients requires further research [161].
Only one retrospective study involving 114 patients incorporated IAP into the respiratory and fluid management concept. This study showed that using PAL treatment (PEEP set at the level of IAP, albumin 20%, followed by Lasix®) was able to keep the cumulative fluid balance in check with a significant drop in IAP, EVLWI, and rise in P/F ratio. This also resulted in faster weaning from the ventilator and improved survival compared to the matched control group [87].
Wang et al., conducted an RCT in 132 patients with SAP using fresh frozen plasma as a resuscitation fluid. Fresh frozen plasma shortens the duration of positive fluid balance, decreases the overall fluid balance within 72 h, reduces the duration of mechanical ventilation and admissions to ICU, and improves PaO2/FiO2 and mortality in severe acute pancreatitis [163].
Several animal studies proved that hypertonic saline (HTS) resuscitation improves hemodynamics [164,165,166,167]. HTS treatment allows smaller fluid volume resuscitation in the burn shock period and reduces the risk of low abdominal perfusion and secondary ACS 21]. The American Burn Association evaluated the efficacy of HTS in burn patients, however, the evidence in favor is equivocal. Additional studies are required to define the correct dosage and timing [168].
4.3. Fluid Resuscitation Strategies
The 4 D’s of fluid therapy (drug, dosing, duration, and de-escalation) should be considered during the administration of resuscitation fluids [17,148]. Fluid requirements of critically ill patients tend to change throughout their illness, and fluid therapy should be adjusted to account for these changes. Therefore, we distinguish four phases of fluid administration (ROSE) (Figure 1): the Resuscitation phase, the Optimization phase, the Stabilization phase, and the Evacuation phase [17]. The ROSE concept may help to guide therapeutic decision-making [17].
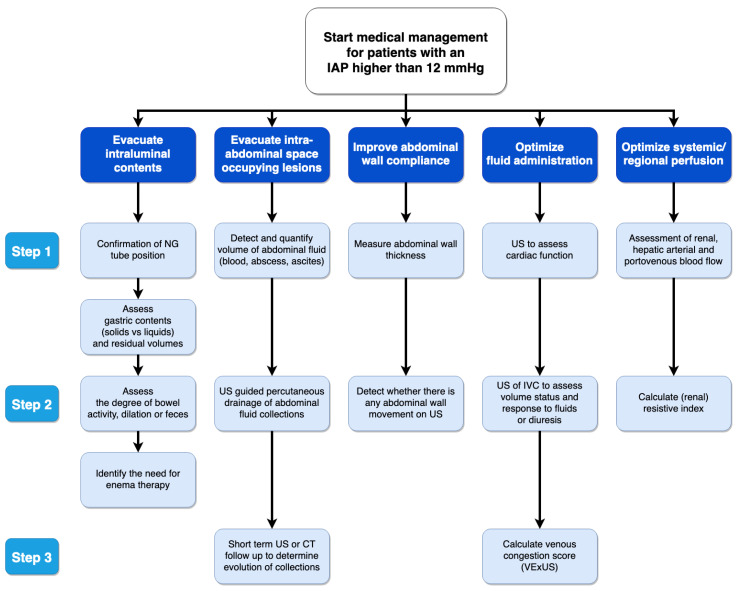
Decisions regarding the administration of intravenous fluids should be guided by functional hemodynamic measurements, such as pulse pressure or stroke volume variation. They should not be solely based on increased lactate, low MAP, or oliguria (<0.5 mL/kg/hour) [169,170]. With the increased use of ultrasound as a bedside modality in both emergency and critical care patients, it is important to consider point-of-care ultrasound (POCUS) as an adjuvant tool for IAH and management of fluid strategies (Figure 5). POCUS during the first three days of admission improved clinical performance in IAH scenarios and fluid management [171].
Figure 5.
Potential use of POCUS according to WSACS medical management algorithm.
All of these factors should be carefully considered, to avoid the dangerous complications and vicious cycle of fluid accumulation, as illustrated in Figure 3 [172]. Fluid overload was identified as an independent risk factor for developing intra-abdominal hypertension [7,173].
The ideal rate at which fluid is administered appears to depend on how much it takes to maintain perfusion, and thus there is no clear guidance from the available literature. This would largely depend on the systemic inflammation, rate of fluid extravasation out of the intravascular compartment, and effects on cardiac function. An RCT involving 60 patients with acute pancreatitis, but without organ failure, that received either aggressive (20 mL/kg bolus followed by 3 mL/kg/h) or standard (10 mL/kg bolus followed by 1.5 mg/kg/h) resuscitation with Ringer’s lactate solution. The rate of clinical improvement was more significant with aggressive hydration, and no patients developed signs of fluid overload [156]. Another RCT in 76 patients with SAP showed that rapid, uncontrolled fluid resuscitation (10–15 mL/kg/h or until a hematocrit <35% within 48 h) significantly worsened the rates of infections, ACS, the need for mechanical ventilation, and mortality [43]. Although these studies are relatively small, they suggest an optimum therapeutic range for fluid therapy. Further research in this field is required to help determine appropriate fluid resuscitation strategies in this group, particularly whether targeting a hematocrit is helpful [43].
4.4. Interventions with Potential Beneficial Effects That Need Further Investigation
Fluid requirements may be reduced by ascorbic acid, which has an apparent (osmotic) diuretic effect that may lead to hypovolemia and reduced inflammatory response [174]. This was shown in a prospective, randomized study where the use of high-dose ascorbic acid led to a significantly reduced amount of resuscitation volume [175].
Peritoneal resuscitation (PR) corrected many of the physiologic derangements that lead to eventual organ dysfunction, including endothelial cell dysfunction, tissue ischemia, reduction in capillary blood flow, derangements in fluid exchange, and electrolyte handling, and increased inflammatory mediators. Studies in trauma patients have shown that PR was associated with accelerated abdominal closure, reduced abdominal complications, and reduced mortality [176]. Further research in this field is required.
4.5. Limitations
Although the literature search was broad, it was limited to those studies published in English. There were potential sampling errors in the search terms, and the search was limited to Scopus and PubMed. Negative studies are less likely to be published and hence would not have come to our attention during the literature search. The studies included were also heterogeneous in their sampled populations and data, making pooled analysis impossible. Future studies should broaden the search to include other languages.
Final take-home messages on the relation between fluid resuscitation and IAH:
There is a relationship between fluid resuscitation, fluid accumulation, and secondary IAH. This signal, from the limited number of RCTs, needs further confirmation.
Crystalloids are associated with a more positive fluid balance and a greater likelihood of developing IAH compared to colloids or hypertonic solutions.
Fluid resuscitation in IAH may preserve cardiac output, however, it does not prevent organ damage.
Delivery of blood products in a 3:2 ratio of RCC: FFP (red blood cells: fresh frozen plasma) and 5:1 for RCC: platelets, may reduce MOF and infectious complications, and increase ventilator-free days [63].
Fluid resuscitation leads to IAH and venous congestion (or venous hypertension), contributing to gut edema and diminished gut contractility.
The relationship between fluid resuscitation, fluid accumulation, and secondary IAH holds in the setting of sepsis (capillary leak), severe burn injury, emergency surgery, and trauma with the presence of the deadly triad (coagulopathy, acidosis, hypothermia).
Fluid removal with diuretics or CVVH may restore cumulative fluid balance and may reduce IAP. The time to initiate RRT in this setting remains unclear.
Bladder pressure measurements should be performed after infusion of more than 25 mL during the acute resuscitation phase, and one should check for peak inspiratory pressures greater than 40 cm H2O.
The presence of IAH is associated with a poor prognosis. The presence of ACS warrants escharotomy or surgical decompression of the abdominal cavity, while IAH usually responds to medical therapy [48].
5. Conclusions
Intravenous fluid administration plays an essential role in developing IAH and ACS. Multiple pathophysiological mechanisms have been described, notably damaging the endothelial glycocalyx. Fluid balance has been identified as an independent risk factor in several clinical studies and can contribute to the development of IAH, venous congestion, gut edema, and diminished gut contractility. Evidence identifying the best resuscitation targets and management strategies regarding type, timing, and volume of fluids in patients with IAH is scarce. It is striking how there has been little advancement of new studies or data in recent years, as the bulk of the literature is more than five years old. Therefore, further research is required to improve insights into this topic.
Acknowledgments
This open access article is endorsed by the WSACS and IFA. The mission statement of the IFA is to foster education, promote research on fluid management and hemodynamic monitoring, and thereby improve survival of critically ill by bringing together physicians, nurses, and others throughout the world and from a variety of clinical disciplines. The IFA is integrated within the not-for-profit charitable organization iMERiT (International Medical Education and Research Initiative) under Belgian law.
Abbreviations
| ACS | abdominal compartment syndrome |
| ADH | anti-diuretic hormone |
| ALI | acute lung injury |
| APP | abdominal perfusion pressure |
| ARDS | acute respiratory distress syndrome |
| BMI | body mass index |
| BMT | bone marrow transplantation |
| BRG | burn resuscitation guidelines |
| CLFM | conservative late fluid management |
| CLI | capillary leak index |
| CO | cardiac output |
| CPB | cardiopulmonary bypass |
| CR | case report |
| CVVH | continuous veno-venous hemofiltration |
| CVP | central venous pressure |
| d | day |
| DL | damage control laparotomy |
| ECMO | extra-corporeal membrane oxygenation |
| ECW | extracellular body water |
| EGL | endothelial glycocalyx layer |
| eOR | emergency open repair |
| EVAR | endovascular aortic repair |
| EVLWI | extravascular lung water index |
| eEVR | emergency endovascular repair |
| FOAM | free open access medical education |
| FB | fluid balance |
| FFP | fresh frozen plasma |
| HES | hydroxyethyl starch |
| HLS | hypertonic lactated saline |
| IAP | intra-abdominal pressure |
| IAH | intra-abdominal hypertension |
| ICP | intra-cranial pressure |
| ICU | intensive care unit |
| ITP | intra-thoracic pressure |
| IV | intra-venous |
| L | liters |
| LR | ringer’s lactate solution |
| LOS | length of stay |
| M | medical |
| MAP | mean arterial pressure |
| MOF | multiple organ failure |
| MV | mechanical ventilation |
| NMB | neuromuscular blocker |
| NGT | nasogastric tube |
| NR | not reported |
| NS | 0.9% saline |
| OF | organ failure |
| PAL | positive end-expiratory pressure, albumin, and Lasix® (furosemide) |
| PCD | percutaneous catheter drainage |
| PCS | poly-compartment syndrome |
| PEEP | positive end-expiratory pressure |
| POCUS | point-of-care ultrasound |
| pts | patients |
| rAAAs | ruptured abdominal aortic aneurysms |
| RCC | red cell concentrate |
| RF | risk factor |
| S | surgical |
| SAP | severe acute pancreatitis |
| SAPS II | Simplified Acute Physiology Score II |
| SH group | combination of 0.9% saline and hydroxyethyl starch (HES) |
| SHG group | combination of 0.9% saline, hydroxyethyl starch and glutamine |
| SHINE | shock induced endotheliopathy |
| SN | supranormal resuscitation group |
| SOFA | Sequential Organ Failure Assessment Score |
| ST group | standard therapy |
| TBSA | total body surface area |
| TEP | trauma exsanguination protocol |
| TI group | targeted intervention |
| U | units |
| UO | urine output |
| WSACS | The Abdominal Compartment Society |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/life12091390/s1, Table S1: Overview of included studies on burn patients, Table S2: Overview of included studies on SAP patients, Table S3: Overview of included studies on trauma patients, Table S4: Overview of included studies on medical and surgical patients.
Author Contributions
All authors participated in the literature search, data collection and analysis, and manuscript preparation. All authors read and approved the final version of the manuscript.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
ARB received speaker’s fees from Fresenius Kabi and Nestlé, and her institution (University of Tartu) received a study grant from Fresenius Kabi. AWK is the Principal Investigator of the COOL Trial (https://clinicaltrials.gov/ct2/show/NCT03163095 (accessed on: 26 May 2022)), which has received unrestricted funding from the Abdominal Compartment Society and the Acelity Corp. AWK has also consulted for Zoll Medical, the Innovative Trauma Care, and the SAM Medical Corporations. MLNGM is a member of the medical advisory Board of Pulsion Medical Systems (now fully integrated in Getinge, Solna, Sweden) and Serenno Medical (Tel Aviv, Israel), consults for Baxter, Maltron, ConvaTec, Acelity, Spiegelberg and Holtech Medical. He is co-founder and President of the International Fluid Academy (IFA). He is co-founder, past-president and current treasurer of the Abdominal Compartment Society, formerly known as the World Society of Abdominal Compartment Syndrome (https://www.wsacs.org/ (accessed on: 26 May 2022)). XM is a member of the Medical Advisory Board of Pulsion Medical Systems. He made paid lectures for Cheetah Medical. MS had undertaken consulting with Smith and Nephew, Acelity and Novus Scientific All other authors declare that they have no competing interests in relation to the content published in this manuscript.
Ethics Approval and Consent to Participate
Not applicable.
Funding Statement
No funding was provided except for support by WSACS (www.wsacs.org (accessed on: 26 May 2022)) and University Hospital Antwerp to cover the Open Access fee.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Keulenaer B.L., Regli A., Dabrowski W., Kaloiani V., Bodnar Z., Cea J.I., Litvin A.A., Davis W.A., Palermo A.M., De Waele J.J., et al. Does femoral venous pressure measurement correlate well with intrabladder pressure measurement? A multicenter observational trial. Intensive Care Med. 2011;37:1620–1627. doi: 10.1007/s00134-011-2298-x. [DOI] [PubMed] [Google Scholar]
- 2.Kotlinska-Hasiec E., Dabrowski W., Rzecki Z., Rybojad B., Pilat J., De Keulenaer B., Lng Malbrain M. Association between intra-abdominal pressure and jugular bulb saturation in critically ill pa-tients. Minerva Anestesiol. 2014;80:785–795. [PubMed] [Google Scholar]
- 3.Druml W. [Intestinal cross-talk: The gut as motor of multiple organ failure] Med. Klin. Intensivmed. Notfmed. 2018;113:470–477. doi: 10.1007/s00063-018-0475-1. [DOI] [PubMed] [Google Scholar]
- 4.Holte K., Sharrock N.E., Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br. J. Anaesth. 2002;89:622–632. doi: 10.1093/bja/aef220. [DOI] [PubMed] [Google Scholar]
- 5.Dabrowski W., Kotlinska-Hasiec E., Jaroszynski A., Zadora P., Pilat J., Rzecki Z., Zaluska W., Schneditz D. Intra-abdominal pressure correlates with extracellular water content. PLoS ONE. 2015;10:e0122193. doi: 10.1371/journal.pone.0122193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reintam A., Parm P., Kitus R., Kern H., Starkopf J. Primary and secondary intra-abdominal hypertension--different impact on ICU outcome. Intensive Care Med. 2008;34:1624–1631. doi: 10.1007/s00134-008-1134-4. [DOI] [PubMed] [Google Scholar]
- 7.Holodinsky J.K., Roberts D.J., Ball C.G., Blaser A.R., Starkopf J., Zygun D.A., Stelfox H.T., Malbrain M.L., Jaeschke R.C., Kirkpatrick A.W. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: A systematic review and meta-analysis. Crit. Care. 2013;17:R249. doi: 10.1186/cc13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scalea T.M., Bochicchio G.V., Habashi N., McCunn M., Shih D., McQuillan K., Aarabi B. Increased intra-abdominal, intrathoracic, and intracranial pressure after severe brain injury: Multiple compartment syndrome. J. Trauma. 2007;62:647–656; discussion 656. doi: 10.1097/TA.0b013e31802ee542. [DOI] [PubMed] [Google Scholar]
- 9.De Waele J.J., Malbrain M.L., Kirkpatrick A.W. The abdominal compartment syndrome: Evolving concepts and future directions. Crit Care. 2015;19:211. doi: 10.1186/s13054-015-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malbrain M., De Laet I., De Waele J. The polycompartment syndrome: What’s all the fuss about? Yearbook of Intensive Care and Emergency Medicine. 1st ed. Springer; Berlin/Heidelberg, Germany: 2010. pp. 465–484. [Google Scholar]
- 11.Bodnar Z. Polycompartment syndrome—intra-abdominal pressure measurement. Anaesthesiol. Intensive Ther. 2019;51:316–322. doi: 10.5114/ait.2019.87474. [DOI] [PubMed] [Google Scholar]
- 12.Malbrain M.L., Roberts D.J., Sugrue M., De Keulenaer B.L., Ivatury R., Pelosi P., Verbrugge F., Wise R., Mullens W. The polycompartment syndrome: A concise state-of-the-art review. Anaesthesiol. Intensive Ther. 2014;46:433–450. doi: 10.5603/AIT.2014.0064. [DOI] [PubMed] [Google Scholar]
- 13.Malbrain M.L., Wilmer A. The polycompartment syndrome: Towards an understanding of the interactions between different compartments! Intensive Care Med. 2007;33:1869–1872. doi: 10.1007/s00134-007-0843-4. [DOI] [PubMed] [Google Scholar]
- 14.Malbrain M. Respiratory effects of increased intra-abdominal pressure. Réanimation. 2007;16:49–60. doi: 10.1016/j.reaurg.2006.12.001. [DOI] [Google Scholar]
- 15.Armanious M., Bacon L.N., Harris J., George S., Goulbourne K.K., Danner O., Matthews L.R., Wilson K.L. Decompressive laparotomy for reduction of incessant increased intracranial pressure in the absence of abdominal compartment syndrome: A case report. Int. J. Case Rep. Images. 2013;4:419–422. doi: 10.5348/ijcri-2013-08-346-CR-5. [DOI] [Google Scholar]
- 16.Mackay E.J., Nunn A.M., Cannon J.W., Martin N.D. Secondary extremity compartment syndrome after traumatic cardiac arrest. Trauma. 2016;2016:4. doi: 10.1177/1460408616646588. [DOI] [Google Scholar]
- 17.Malbrain M., Van Regenmortel N., Saugel B., De Tavernier B., Van Gaal P.J., Joannes-Boyau O., Teboul J.L., Rice T.W., Mythen M., Monnet X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care. 2018;8:66. doi: 10.1186/s13613-018-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ennis J.L., Chung K.K., Renz E.M., Barillo D.J., Albrecht M.C., Jones J.A., Blackbourne L.H., Cancio L.C., Eastridge B.J., Flaherty S.F., et al. Joint Theater Trauma System implementation of burn resuscitation guidelines improves outcomes in severely burned military casualties. J. Trauma. 2008;64:S146–S151. discussion S142–S151. doi: 10.1097/TA.0b013e318160b44c. [DOI] [PubMed] [Google Scholar]
- 19.Ivy M.E., Atweh N.A., Palmer J., Possenti P.P., Pineau M., D’Aiuto M. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J. Trauma. 2000;49:387–391. doi: 10.1097/00005373-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Mbiine R., Alenyo R., Kobusingye O., Kuteesa J., Nakanwagi C., Lekuya H.M., Kituuka O., Galukande M. Intra-abdominal hypertension in severe burns: Prevalence, incidence and mortality in a sub-Saharan African hospital. Int. J. Burns Trauma. 2017;7:80–87. [PMC free article] [PubMed] [Google Scholar]
- 21.Oda J., Ueyama M., Yamashita K., Inoue T., Noborio M., Ode Y., Aoki Y., Sugimoto H. Hypertonic lactated saline resuscitation reduces the risk of abdominal compartment syndrome in severely burned patients. J. Trauma. 2006;60:64–71. doi: 10.1097/01.ta.0000199431.66938.99. [DOI] [PubMed] [Google Scholar]
- 22.Oda J., Yamashita K., Inoue T., Harunari N., Ode Y., Mega K., Aoki Y., Noborio M., Ueyama M. Resuscitation fluid volume and abdominal compartment syndrome in patients with major burns. Burns. 2006;32:151–154. doi: 10.1016/j.burns.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 23.O’Mara M.S., Slater H., Goldfarb I.W., Caushaj P.F. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J. Trauma. 2005;58:1011–1018. doi: 10.1097/01.TA.0000162732.39083.15. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Castilla M., Barret J.P., Sanz D., Aguilera J., Serracanta J., Garcia V., Collado J.M. Analysis of intra-abdominal hypertension in severe burned patients: The Vall d’Hebron experience. Burns. 2014;40:719–724. doi: 10.1016/j.burns.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Talizin T.B., Tsuda M.S., Tanita M.T., Kauss I.A.M., Festti J., Carrilho C., Grion C.M.C., Cardoso L.T.Q. Acute kidney injury and intra-abdominal hypertension in burn patients in intensive care. Rev. Bras. Ter. Intensiva. 2018;30:15–20. doi: 10.5935/0103-507X.20180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise R., Jacobs J., Pilate S., Jacobs A., Peeters Y., Vandervelden S., Van Regenmortel N., De Laet I., Schoonheydt K., Dits H., et al. Incidence and prognosis of intra-abdominal hypertension and abdominal compartment syndrome in severely burned patients: Pilot study and review of the literature. Anaesthesiol. Intensive Ther. 2016;48:95–109. doi: 10.5603/AIT.a2015.0083. [DOI] [PubMed] [Google Scholar]
- 27.Boehm D., Schroder C., Arras D., Siemers F., Siafliakis A., Lehnhardt M., Dadras M., Hartmann B., Kuepper S., Czaja K.U., et al. Fluid Management as a Risk Factor for Intra-abdominal Compartment Syndrome in Burn Patients: A Total Body Surface Area-Independent Multicenter Trial Part I. J. Burn Care Res. 2019;40:500–506. doi: 10.1093/jbcr/irz053. [DOI] [PubMed] [Google Scholar]
- 28.Hershberger R.C., Hunt J.L., Arnoldo B.D., Purdue G.F. Abdominal compartment syndrome in the severely burned patient. J. Burn Care Res. 2007;28:708–714. doi: 10.1097/BCR.0b013E318148C988. [DOI] [PubMed] [Google Scholar]
- 29.Hobson K.G., Young K.M., Ciraulo A., Palmieri T.L., Greenhalgh D.G. Release of abdominal compartment syndrome improves survival in patients with burn injury. J. Trauma. 2002;53:1129–1133; discussion 1133–1124. doi: 10.1097/00005373-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Markell K.W., Renz E.M., White C.E., Albrecht M.E., Blackbourne L.H., Park M.S., Barillo D.A., Chung K.K., Kozar R.A., Minei J.P., et al. Abdominal complications after severe burns. J. Am. Coll. Surg. 2009;208:940–947; discussion 947–949. doi: 10.1016/j.jamcollsurg.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 31.McBeth P.B., Sass K., Nickerson D., Ball C.G., Kirkpatrick A.W. A necessary evil? Intra-abdominal hypertension complicating burn patient resuscitation. J. Trauma Manag. Outcomes. 2014;8:12. doi: 10.1186/1752-2897-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park S.H., Hemmila M.R., Wahl W.L. Early albumin use improves mortality in difficult to resuscitate burn patients. J. Trauma Acute Care Surg. 2012;73:1294–1297. doi: 10.1097/TA.0b013e31827019b1. [DOI] [PubMed] [Google Scholar]
- 33.Ivy M.E., Possenti P.P., Kepros J., Atweh N.A., D’Aiuto M., Palmer J., Pineau M., Burns G.A., Caushaj P.F. Abdominal compartment syndrome in patients with burns. J. Burn. Care Rehabil. 1999;20:351–353. doi: 10.1097/00004630-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Parra M.W., Al-Khayat H., Smith H.G., Cheatham M.L. Paracentesis for resuscitation-induced abdominal compartment syndrome: An alternative to decompressive laparotomy in the burn patient. J. Trauma. 2006;60:1119–1121. doi: 10.1097/01.ta.0000217274.48792.4d. [DOI] [PubMed] [Google Scholar]
- 35.Streit S., Hebra A. Abdominal compartment syndrome in a three year old child following a severe burn injury. J. Pediatric Surg. Case Rep. 2013;1:177–179. doi: 10.1016/j.epsc.2013.04.008. [DOI] [Google Scholar]
- 36.Sun K., Hancock B.J., Logsetty S. Ischemic bowel as a late sequela of abdominal compartment syndrome secondary to severe burn injury. Plast. Surg. 2015;23:218–220. doi: 10.1177/229255031502300407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen A.R., Hughes W.B., Grewal H. Secondary abdominal compartment syndrome in children with burns and trauma: A potentially lethal complication. J. Burn Care Res. 2006;27:242–246. doi: 10.1097/01.BCR.0000202643.16576.75. [DOI] [PubMed] [Google Scholar]
- 38.Britt R.C., Gannon T., Collins J.N., Cole F.J., Weireter L.J., Britt L.D. Secondary abdominal compartment syndrome: Risk factors and outcomes. Am. Surg. 2005;71:982–985. doi: 10.1177/000313480507101114. [DOI] [PubMed] [Google Scholar]
- 39.Reed S.F., Britt R.C., Collins J., Weireter L., Cole F., Britt L.D. Aggressive surveillance and early catheter-directed therapy in the management of intra-abdominal hypertension. J. Trauma. 2006;61:1359–1363; discussion 1363–1355. doi: 10.1097/01.ta.0000245975.68317.5a. [DOI] [PubMed] [Google Scholar]
- 40.Zhao G., Zhang J.G., Wu H.S., Tao J., Qin Q., Deng S.C., Liu Y., Liu L., Wang B., Tian K., et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J. Gastroenterol. 2013;19:2044–2052. doi: 10.3748/wjg.v19.i13.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du X.J., Hu W.M., Xia Q., Huang Z.W., Chen G.Y., Jin X.D., Xue P., Lu H.M., Ke N.W., Zhang Z.D., et al. Hydroxyethyl starch resuscitation reduces the risk of intra-abdominal hypertension in severe acute pancreatitis. Pancreas. 2011;40:1220–1225. doi: 10.1097/MPA.0b013e3182217f17. [DOI] [PubMed] [Google Scholar]
- 42.Ke L., Ni H.B., Sun J.K., Tong Z.H., Li W.Q., Li N., Li J.S. Risk factors and outcome of intra-abdominal hypertension in patients with severe acute pancreatitis. World J. Surg. 2012;36:171–178. doi: 10.1007/s00268-011-1295-0. [DOI] [PubMed] [Google Scholar]
- 43.Mao E.Q., Tang Y.Q., Fei J., Qin S., Wu J., Li L., Min D., Zhang S.D. Fluid therapy for severe acute pancreatitis in acute response stage. Chin. Med. J. 2009;122:169–173. [PubMed] [Google Scholar]
- 44.Pupelis G., Plaudis H., Zeiza K., Drozdova N., Mukans M., Kazaka I. Early continuous veno-venous haemofiltration in the management of severe acute pancreatitis complicated with intra-abdominal hypertension: Retrospective review of 10 years’ experience. Ann. Intensive Care. 2012;2((Suppl. S1)):S21. doi: 10.1186/2110-5820-2-S1-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee A.H.H., Lee W.S., Anderson D. Severe pancreatitis complicated by abdominal compartment syndrome managed with decompressive laparotomy: A case report. BMC Surg. 2019;19:113. doi: 10.1186/s12893-019-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park S., Lee S., Lee H.D., Kim M., Kim K., Jeong Y., Park S.M. Abdominal compartment syndrome in severe acute pancreatitis treated with percutaneous catheter drainage. Clin. Endosc. 2014;47:469–472. doi: 10.5946/ce.2014.47.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balogh Z., McKinley B.A., Cocanour C.S., Kozar R.A., Holcomb J.B., Ware D.N., Moore F.A. Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am. J. Surg. 2002;184:538–543; discussion 543–534. doi: 10.1016/S0002-9610(02)01050-4. [DOI] [PubMed] [Google Scholar]
- 48.Balogh Z., McKinley B.A., Holcomb J.B., Miller C.C., Cocanour C.S., Kozar R.A., Valdivia A., Ware D.N., Moore F.A. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J. Trauma. 2003;54:848–859; discussion 859–861. doi: 10.1097/01.TA.0000070166.29649.F3. [DOI] [PubMed] [Google Scholar]
- 49.Mahmood I., Mahmood S., Parchani A., Kumar S., El-Menyar A., Zarour A., Al-Thani H., Latifi R. Intra-abdominal hypertension in the current era of modern trauma resuscitation. ANZ J. Surg. 2014;84:166–171. doi: 10.1111/ans.12169. [DOI] [PubMed] [Google Scholar]
- 50.Neal M.D., Hoffman M.K., Cuschieri J., Minei J.P., Maier R.V., Harbrecht B.G., Billiar T.R., Peitzman A.B., Moore E.E., Cohen M.J., et al. Crystalloid to packed red blood cell transfusion ratio in the massively transfused patient: When a little goes a long way. J. Trauma Acute Care Surg. 2012;72:892–898. doi: 10.1097/TA.0b013e31823d84a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raeburn C.D., Moore E.E., Biffl W.L., Johnson J.L., Meldrum D.R., Offner P.J., Franciose R.J., Burch J.M. The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery. Am. J. Surg. 2001;182:542–546. doi: 10.1016/S0002-9610(01)00821-2. [DOI] [PubMed] [Google Scholar]
- 52.Vatankhah S., Sheikhi R.A., Heidari M., Moradimajd P. The relationship between fluid resuscitation and intra-abdominal hypertension in patients with blunt abdominal trauma. Int. J. Crit. Illn. Inj. Sci. 2018;8:149–153. doi: 10.4103/IJCIIS.IJCIIS_17_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balogh Z., McKinley B.A., Cocanour C.S., Kozar R.A., Valdivia A., Sailors R.M., Moore F.A. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch. Surg. 2003;138:637–642; discussion 642–633. doi: 10.1001/archsurg.138.6.637. [DOI] [PubMed] [Google Scholar]
- 54.Gracias V.H., Braslow B., Johnson J., Pryor J., Gupta R., Reilly P., Schwab C.W. Abdominal compartment syndrome in the open abdomen. Arch. Surg. 2002;137:1298–1300. doi: 10.1001/archsurg.137.11.1298. [DOI] [PubMed] [Google Scholar]
- 55.He L., Yi C., Hou Z., Hak D.J. Intraabdominal hypertension/abdominal compartment syndrome after pelvic fractures: How they occur and what can be done? Injury. 2019;50:919–925. doi: 10.1016/j.injury.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 56.Hwabejire J.O., Nembhard C.E., Oyetunji T.A., Seyoum T., Siram S.M., Cornwell E.E., 3rd, Greene W.R. Abdominal compartment syndrome in traumatic hemorrhagic shock: Is there a fluid resuscitation inflection point associated with increased risk? Am. J. Surg. 2016;211:733–738. doi: 10.1016/j.amjsurg.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 57.Joseph B., Zangbar B., Pandit V., Vercruysse G., Aziz H., Kulvatunyou N., Wynne J., O’Keeffe T., Tang A., Friese R.S., et al. The conjoint effect of reduced crystalloid administration and decreased damage-control laparotomy use in the development of abdominal compartment syndrome. J. Trauma Acute Care Surg. 2014;76:457–461. doi: 10.1097/TA.0b013e3182a9ea44. [DOI] [PubMed] [Google Scholar]
- 58.Macedo F.I., Sciarretta J.D., Otero C.A., Ruiz G., Ebler D.J., Pizano L.R., Namias N. Secondary abdominal compartment syndrome after complicated traumatic lower extremity vascular injuries. Eur. J. Trauma Emerg. Surg. 2016;42:207–211. doi: 10.1007/s00068-015-0524-x. [DOI] [PubMed] [Google Scholar]
- 59.Madigan M.C., Kemp C.D., Johnson J.C., Cotton B.A. Secondary abdominal compartment syndrome after severe extremity injury: Are early, aggressive fluid resuscitation strategies to blame? J. Trauma. 2008;64:280–285. doi: 10.1097/TA.0b013e3181622bb6. [DOI] [PubMed] [Google Scholar]
- 60.Maxwell R.A., Fabian T.C., Croce M.A., Davis K.A. Secondary abdominal compartment syndrome: An underappreciated manifestation of severe hemorrhagic shock. J. Trauma. 1999;47:995–999. doi: 10.1097/00005373-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Rodas E.B., Malhotra A.K., Chhitwal R., Aboutanos M.B., Duane T.M., Ivatury R.R. Hyperacute abdominal compartment syndrome: An unrecognized complication of massive intraoperative resuscitation for extra-abdominal injuries. Am. Surg. 2005;71:977–981. doi: 10.1177/000313480507101113. [DOI] [PubMed] [Google Scholar]
- 62.Shaheen A.W., Crandall M.L., Nicolson N.G., Smith-Singares E., Merlotti G.J., Jalundhwala Y., Issa N.M. Abdominal compartment syndrome in trauma patients: New insights for predicting outcomes. J. Emerg. Trauma Shock. 2016;9:53–57. doi: 10.4103/0974-2700.179452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burrows R., Edington J., Robbs J.V. A wolf in wolf’s clothing--the abdominal compartment syndrome. S. Afr. Med. J. 1995;85:46–48. [PubMed] [Google Scholar]
- 64.Chamisa I. Secondary Abdominal Compartment Syndrome in a Patientwith Isolated Extraperitoneal Injuries. Eur. J. Trauma Emerg. Surg. 2008;34:313–314. doi: 10.1007/s00068-008-7092-2. [DOI] [PubMed] [Google Scholar]
- 65.Kopelman T., Harris C., Miller R., Arrillaga A. Abdominal compartment syndrome in patients with isolated extraperitoneal injuries. J. Trauma. 2000;49:744–747; discussion 747–749. doi: 10.1097/00005373-200010000-00025. [DOI] [PubMed] [Google Scholar]
- 66.Michel P., Wahnert D., Freistuhler M., Laukoetter M.G., Rehberg S., Raschke M.J., Garcia P. Acute transfusion-related abdominal injury in trauma patients: A case report. J. Med. Case Rep. 2016;10:294. doi: 10.1186/s13256-016-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrell B.J., Vinden C., Singh R.N., Kornecki A., Fraser D.D. Secondary abdominal compartment syndrome in a case of pediatric trauma shock resuscitation. Pediatr. Crit. Care Med. 2007;8:67–70. doi: 10.1097/01.pcc.0000256615.32641.ab. [DOI] [PubMed] [Google Scholar]
- 68.Perks D.H., Grewal H. Abdominal compartment syndrome in the pediatric patient with blunt trauma. J. Trauma Nurs. 2005;12:50–54. doi: 10.1097/00043860-200512020-00005. [DOI] [PubMed] [Google Scholar]
- 69.Zaydfudim V., Dutton W.D., Feurer I.D., Au B.K., Pinson C.W., Cotton B.A. Exsanguination protocol improves survival after major hepatic trauma. Injury. 2010;41:30–34. doi: 10.1016/j.injury.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 70.DeCou J.M., Abrams R.S., Miller R.S., Gauderer M.W. Abdominal compartment syndrome in children: Experience with three cases. J. Pediatric Surg. 2000;35:840–842. doi: 10.1053/jpsu.2000.6857. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi T., Kubota M., Arai Y., Ohyama T., Yokota N., Miura K., Ishikawa H., Soma D., Takizawa K., Sakata J., et al. Staged laparotomies based on the damage control principle to treat hemodynamically unstable grade IV blunt hepatic injury in an eight-year-old girl. Surg. Case Rep. 2016;2:134. doi: 10.1186/s40792-016-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kula R., Szturz P., Sklienka P., Neiser J., Jahoda J. A role for negative fluid balance in septic patients with abdominal compartment syndrome? Intensive Care Med. 2004;30:2138–2139. doi: 10.1007/s00134-004-2423-1. [DOI] [PubMed] [Google Scholar]
- 73.Kula R., Szturz P., Sklienka P., Neiser J. Negative fluid balance in patients with abdominal compartment syndrome--case reports. Acta Chir. Belg. 2008;108:346–349. doi: 10.1080/00015458.2008.11680236. [DOI] [PubMed] [Google Scholar]
- 74.Cotton B.A., Au B.K., Nunez T.C., Gunter O.L., Robertson A.M., Young P.P. Predefined massive trans-fusion protocols are associated with a reduction in organ failure and postinjury complications. J. Trauma. 2009;66:41–48; discussion 48–49. doi: 10.1097/TA.0b013e31819313bb. [DOI] [PubMed] [Google Scholar]
- 75.Strang S., Van Imhoff D., Van Lieshout E., D’Amours S., Van Waes O. Identifying patients at risk for high-grade intra-abdominal hypertension following trauma laparotomy. Injury. 2015;46:843–848. doi: 10.1016/j.injury.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 76.Tsuang W., Pohlman M., Hall J. A 25-year-old woman with acute pancreatitis and hypotension refractory to aggressive fluid resuscitation. Diagnosis: Abdominal compartment syndrome. Chest. 2007;132:1702–1705. doi: 10.1378/chest.07-0717. [DOI] [PubMed] [Google Scholar]
- 77.Macalino J.U., Goldman R.K., Mayberry J.C. Medical management of abdominal compartment syndrome: Case report and a caution. Asian J. Surg. 2002;25:244–246. doi: 10.1016/S1015-9584(09)60184-6. [DOI] [PubMed] [Google Scholar]
- 78.Cordemans C., De Laet I., Van Regenmortel N., Schoonheydt K., Dits H., Huber W., Malbrain M.L. Fluid management in critically ill patients: The role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann. Intensive Care. 2012;2((Suppl. S1)):S1. doi: 10.1186/2110-5820-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Struck M.F., Illert T., Schmidt T., Reichelt B., Steen M. Secondary abdominal compartment syndrome in patients with toxic epidermal necrolysis. Burns. 2012;38:562–567. doi: 10.1016/j.burns.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 80.Dauplaise D.J., Barnett S.J., Frischer J.S., Wong H.R. Decompressive abdominal laparotomy for abdominal compartment syndrome in an unengrafted bone marrow recipient with septic shock. Crit. Care Res. Pract. 2010;2010:102910. doi: 10.1155/2010/102910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Divarci E., Karapinar B., Yalaz M., Ergun O., Celik A. Incidence and prognosis of intraabdominal hypertension and abdominal compartment syndrome in children. J. Pediatric Surg. 2016;51:503–507. doi: 10.1016/j.jpedsurg.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 82.Gala H.C., Avasthi B.S., Lokeshwar M.R. Dengue shock syndrome with two atypical complications. Indian J. Pediatr. 2012;79:386–388. doi: 10.1007/s12098-011-0551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lam M.C., Yang P.T., Skippen P.W., Kissoon N., Skarsgard E.D. Abdominal compartment syndrome complicating paediatric extracorporeal life support: Diagnostic and therapeutic challenges. Anaesth Intensive Care. 2008;36:726–731. doi: 10.1177/0310057X0803600517. [DOI] [PubMed] [Google Scholar]
- 84.Ranjit S., Ramanathan G., Ramakrishnan B., Kissoon N. Targeted Interventions in Critically Ill Children with Severe Dengue. Indian J. Crit. Care Med. 2018;22:154–161. doi: 10.4103/ijccm.IJCCM_413_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dorigatti A., Pereira B., Melek M., Dos Santos J., Teramoto F., Fraga G. Clinical warning signs for intra-abdominal hypertension in septic shock patients. Anaesthesiol. Intensive Ther. 2019;51:200–204. doi: 10.5114/ait.2019.87330. [DOI] [PubMed] [Google Scholar]
- 86.Daugherty E.L., Hongyan L., Taichman D., Hansen-Flaschen J., Fuchs B.D. Abdominal compartment syndrome is common in medical intensive care unit patients receiving large-volume resuscitation. J. Intensive Care Med. 2007;22:294–299. doi: 10.1177/0885066607305247. [DOI] [PubMed] [Google Scholar]
- 87.Cordemans C., De Laet I., Van Regenmortel N., Schoonheydt K., Dits H., Martin G., Huber W., Malbrain M.L. Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: A pilot study looking at the effects of PAL-treatment. Ann. Intensive Care. 2012;2((Suppl. S1)):S15. doi: 10.1186/2110-5820-2-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miranda E., Manzur M., Han S., Ham S.W., Weaver F.A., Rowe V.L. Postoperative Development of Abdominal Compartment Syndrome among Patients Undergoing Endovascular Aortic Repair for Ruptured Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2018;49:289–294. doi: 10.1016/j.avsg.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 89.Rubenstein C., Bietz G., Davenport D.L., Winkler M., Endean E.D. Abdominal compartment syndrome associated with endovascular and open repair of ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2015;61:648–654. doi: 10.1016/j.jvs.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 90.Augustin P., Lasocki S., Dufour G., Rode J., Karsenti A., Al-Attar N., Bazeli R., Montravers P. Abdominal compartment syndrome due to extracorporeal membrane oxygenation in adults. Ann. Thorac. Surg. 2010;90:e40–e41. doi: 10.1016/j.athoracsur.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 91.Bressan A.K., Kirkpatrick A.W., Ball C.G. Abdominal intra-compartment syndrome—A non-hydraulic model of abdominal compartment syndrome due to post-hepatectomy hemorrhage in a man with a localized frozen abdomen due to extensive adhesions: A case report. J. Med. Case Rep. 2016;10:251. doi: 10.1186/s13256-016-1045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biancofiore G., Bindi M.L., Romanelli A.M., Boldrini A., Consani G., Bisa M., Filipponi F., Vagelli A., Mosca F. Intra-abdominal pressure monitoring in liver transplant recipients: A prospective study. Intensive Care Med. 2003;29:30–36. doi: 10.1007/s00134-002-1552-7. [DOI] [PubMed] [Google Scholar]
- 93.Dalfino L., Sicolo A., Paparella D., Mongelli M., Rubino G., Brienza N. Intra-abdominal hypertension in cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2013;17:644–651. doi: 10.1093/icvts/ivt272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kotlinska-Hasiec E., Rutyna R.R., Rzecki Z., Czarko-Wicha K., Gagala J., Pawlik P., Zaluska A., Jaroszynski A., Zaluska W., Dabrowski W. The effect of crystalloid infusion on body water content and intra-abdominal pressure in patients undergoing orthopedic surgery under spinal anesthesia. Adv. Clin. Exp. Med. 2017;26:1189–1196. doi: 10.17219/acem/63140. [DOI] [PubMed] [Google Scholar]
- 95.Makar R.R., Badger S.A., O’Donnell M.E., Loan W., Lau L.L., Soong C.V. The effects of abdominal compartment hypertension after open and endovascular repair of a ruptured abdominal aortic aneurysm. J. Vasc. Surg. 2009;49:866–872. doi: 10.1016/j.jvs.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 96.Muturi A., Ndaguatha P., Ojuka D., Kibet A. Prevalence and predictors of intra-abdominal hypertension and compartment syndrome in surgical patients in critical care units at Kenyatta National Hospital. BMC Emerg. Med. 2017;17:10. doi: 10.1186/s12873-017-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Serpytis M., Ivaskevicius J. The influence of fluid balance on intra-abdominal pressure after major abdominal surgery. Medicina. 2008;44:421–427. doi: 10.3390/medicina44060055. [DOI] [PubMed] [Google Scholar]
- 98.Leclerc B., Salomon Du Mont L., Besch G., Rinckenbach S. How to identify patients at risk of abdominal compartment syndrome after surgical repair of ruptured abdominal aortic aneurysms in the operating room: A pilot study. Vascular. 2017;25:472–478. doi: 10.1177/1708538116689005. [DOI] [PubMed] [Google Scholar]
- 99.McNelis J., Marini C.P., Jurkiewicz A., Fields S., Caplin D., Stein D., Ritter G., Nathan I., Simms H.H. Predictive factors associated with the development of abdominal compartment syndrome in the surgical intensive care unit. Arch. Surg. 2002;137:133–136. doi: 10.1001/archsurg.137.2.133. [DOI] [PubMed] [Google Scholar]
- 100.De Wolf A., Poelaert J., Herck I., De Waele J.J. Surgical decompression for abdominal compartment syndrome after emergency cardiac surgery. Ann. Thorac. Surg. 2008;85:2133–2135. doi: 10.1016/j.athoracsur.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 101.Fietsam R., Jr., Villalba M., Glover J.L., Clark K. Intra-abdominal compartment syndrome as a complication of ruptured abdominal aortic aneurysm repair. Am. Surg. 1989;55:396–402. [PubMed] [Google Scholar]
- 102.Rabbi J.F., Valaulikar G., Appling N.A., Bee T.K., Ostrow B.F., Weiman D.S. Secondary abdominal compartment syndrome causing failure to wean from cardiopulmonary bypass. Ann. Thorac. Surg. 2012;93:e99–e100. doi: 10.1016/j.athoracsur.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 103.Shiiya N., Matsuzaki K., Miyatake T., Yoshimoto K., Yasuda K. Abdominal compartment syndrome causing respiratory failure during surgery for a ruptured descending thoracic aneurysm: Report of a case. Surg. Today. 2005;35:320–322. doi: 10.1007/s00595-004-2923-1. [DOI] [PubMed] [Google Scholar]
- 104.Biffl W.L., Moore E.E., Burch J.M., Offner P.J., Franciose R.J., Johnson J.L. Secondary abdominal compartment syndrome is a highly lethal event. Am. J. Surg. 2001;182:645–648. doi: 10.1016/S0002-9610(01)00814-5. [DOI] [PubMed] [Google Scholar]
- 105.Aik-Yong C., Ye-Xin K., Yi N.S., Hway W.T. Abdominal compartment syndrome: Incidence and prognostic factors influencing survival in Singapore. Indian J. Crit. Care. Med. 2014;18:648–652. doi: 10.4103/0972-5229.142173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cothren C.C., Moore E.E., Johnson J.L., Moore J.B. Outcomes in surgical versus medical patients with the secondary abdominal compartment syndrome. Am. J. Surg. 2007;194:804–807; discussion 807–808. doi: 10.1016/j.amjsurg.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 107.Dalfino L., Tullo L., Donadio I., Malcangi V., Brienza N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. 2008;34:707–713. doi: 10.1007/s00134-007-0969-4. [DOI] [PubMed] [Google Scholar]
- 108.Murphy P.B., Parry N.G., Sela N., Leslie K., Vogt K., Ball I. Intra-Abdominal Hypertension Is More Common Than Previously Thought: A Prospective Study in a Mixed Medical-Surgical ICU. Crit. Care Med. 2018;46:958–964. doi: 10.1097/CCM.0000000000003122. [DOI] [PubMed] [Google Scholar]
- 109.Malbrain M.L.N.G., Chiumello D., Pelosi P., Bihari D., Innes R., Ranieri V.M., Del Turco M., Wilmer A., Brienza N., Malcangi V., et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: A multiple-center epidemiological study. Crit. Care Med. 2005;33:315–322. doi: 10.1097/01.CCM.0000153408.09806.1B. [DOI] [PubMed] [Google Scholar]
- 110.Malbrain M.L., Chiumello D., Pelosi P., Wilmer A., Brienza N., Malcangi V., Bihari D., Innes R., Cohen J., Singer P., et al. Prevalence of intra-abdominal hypertension in critically ill patients: A multicentre epidemiological study. Intensive Care Med. 2004;30:822–829. doi: 10.1007/s00134-004-2169-9. [DOI] [PubMed] [Google Scholar]
- 111.Iyer D., Rastogi P., Aneman A., D’Amours S. Early screening to identify patients at risk of developing intra-abdominal hypertension and abdominal compartment syndrome. Acta Anaesthesiol. Scand. 2014;58:1267–1275. doi: 10.1111/aas.12409. [DOI] [PubMed] [Google Scholar]
- 112.Reintam Blaser A., Regli A., De Keulenaer B., Kimball E.J., Starkopf L., Davis W.A., Greiffenstein P., Starkopf J., Incidence R.F., Outcomes of Intra-Abdominal Study I. Incidence, Risk Factors, and Outcomes of Intra-Abdominal Hypertension in Critically Ill Patients-A Prospective Multicenter Study (IROI Study) Crit. Care Med. 2019;47:535–542. doi: 10.1097/CCM.0000000000003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vidal M.G., Ruiz Weisser J., Gonzalez F., Toro M.A., Loudet C., Balasini C., Canales H., Reina R., Es-tenssoro E. Incidence and clinical effects of intra-abdominal hypertension in critically ill pa-tients. Crit. Care Med. 2008;36:1823–1831. doi: 10.1097/CCM.0b013e31817c7a4d. [DOI] [PubMed] [Google Scholar]
- 114.Kim I.B., Prowle J., Baldwin I., Bellomo R. Incidence, risk factors and outcome associations of in-tra-abdominal hypertension in critically ill patients. Anaesth Intensive Care. 2012;40:79–89. doi: 10.1177/0310057X1204000107. [DOI] [PubMed] [Google Scholar]
- 115.Malbrain M.L., Chiumello D., Cesana B.M., Reintam Blaser A., Starkopf J., Sugrue M., Pelosi P., Sev-ergnini P., Hernandez G., Brienza N., et al. A systematic review and individual patient data me-ta-analysis on intra-abdominal hypertension in critically ill patients: The wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!) Minerva Anestesiol. 2014;80:293–306. [Google Scholar]
- 116.Reintam Blaser A., Parm P., Kitus R., Starkopf J. Risk factors for intra-abdominal hypertension in mechanically ventilated patients. Acta Anaesthesiol. Scand. 2011;55:607–614. doi: 10.1111/j.1399-6576.2011.02415.x. [DOI] [PubMed] [Google Scholar]
- 117.Moore-Olufemi S.D., Xue H., Attuwaybi B.O., Fischer U., Harari Y., Oliver D.H., Weisbrodt N., Allen S.J., Moore F.A., Stewart R., et al. Resuscitation-induced gut edema and intestinal dysfunction. J. Trauma. 2005;58:264–270. doi: 10.1097/01.TA.0000133571.64393.D2. [DOI] [PubMed] [Google Scholar]
- 118.Chang M., Tang H., Liu D., Li Y., Zhang L. Comparison of Melatonin, Hypertonic Saline, and Hydroxyethyl Starch for Resuscitation of Secondary Intra-Abdominal Hypertension in an Animal Model. PLoS ONE. 2016;11:e0161688. doi: 10.1371/journal.pone.0161688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schachtrupp A., Lawong G., Afify M., Graf J., Toens C., Schumpelick V. Fluid resuscitation preserves cardiac output but cannot prevent organ damage in a porcine model during 24 h of intraabdominal hypertension. Shock. 2005;24:153–158. doi: 10.1097/01.shk.0000172094.73918.c2. [DOI] [PubMed] [Google Scholar]
- 120.Regan A., Hotwagner D.T. Burn Fluid Management. StatPearls; Treasure Island, FL, USA: 2020. [PubMed] [Google Scholar]
- 121.Rezende-Neto J.B., Moore E.E., Melo de Andrade M.V., Teixeira M.M., Lisboa F.A., Arantes R.M., de Souza D.G., da Cunha-Melo J.R. Systemic inflammatory response secondary to abdominal compartment syndrome: Stage for multiple organ failure. J. Trauma. 2002;53:1121–1128. doi: 10.1097/00005373-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 122.Silversides J.A., Perner A., Malbrain M. Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med. 2019;45:1440–1442. doi: 10.1007/s00134-019-05713-y. [DOI] [PubMed] [Google Scholar]
- 123.Van der Mullen J., Wise R., Vermeulen G., Moonen P.J., Malbrain M. Assessment of hypovolaemia in the critically ill. Anaesthesiol. Intensive Ther. 2018;50:141–149. doi: 10.5603/AIT.a2017.0077. [DOI] [PubMed] [Google Scholar]
- 124.Harrell B.R., Miller S. Abdominal Compartment Syndrome as a Complication of Fluid Resuscitation. Nurs. Clin. North Am. 2017;52:331–338. doi: 10.1016/j.cnur.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 125.De Waele J.J., Ejike J.C., Leppaniemi A., De Keulenaer B.L., De Laet I., Kirkpatrick A.W., Roberts D.J., Kimball E., Ivatury R., Malbrain M.L. Intra-abdominal hypertension and abdominal compartment syndrome in pancreatitis, paediatrics, and trauma. Anaesthesiol. Intensive Ther. 2015;47:219–227. doi: 10.5603/AIT.a2015.0027. [DOI] [PubMed] [Google Scholar]
- 126.Peeters Y., Bernards J., Mekeirele M., Hoffmann B., De Raes M., Malbrain M.L. Hemodynamic monitoring: To calibrate or not to calibrate? Part 1--Calibrated techniques. Anaesthesiol. Intensive Ther. 2015;47:487–500. doi: 10.5603/AIT.a2015.0073. [DOI] [PubMed] [Google Scholar]
- 127.Cosnett J.E. The origins of intravenous fluid therapy. Lancet. 1989;1:768–771. doi: 10.1016/S0140-6736(89)92583-X. [DOI] [PubMed] [Google Scholar]
- 128.Myburgh J.A., Mythen M.G. Resuscitation fluids. N. Engl. J. Med. 2013;369:1243–1251. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 129.Reitsma S., Slaaf D.W., Vink H., van Zandvoort M.A., oude Egbrink M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Henry C.B., Duling B.R. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2815–H2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- 131.Woodcock T.E., Woodcock T.M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 2012;108:384–394. doi: 10.1093/bja/aer515. [DOI] [PubMed] [Google Scholar]
- 132.Erstad B.L. The Revised Starling Equation: The Debate of Albumin Versus Crystalloids Continues. Ann. Pharmacother. 2020;54:921–927. doi: 10.1177/1060028020907084. [DOI] [PubMed] [Google Scholar]
- 133.Levick J.R., Michel C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 134.Dubniks M., Persson J., Grande P.O. Effect of blood pressure on plasma volume loss in the rat under increased permeability. Intensive Care Med. 2007;33:2192–2198. doi: 10.1007/s00134-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 135.Jacobs R., Lochy S., Malbrain M. Phenylephrine-induced recruitable preload from the venous side. J. Clin. Monit. Comput. 2019;33:373–376. doi: 10.1007/s10877-018-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schaefer T., Nunez Lopez O. Burn Resuscitation And Management. [(accessed on 26 May 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK430795/
- 137.Baxter C.R., Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann. New York Acad. Sci. 1968;150:874–894. doi: 10.1111/j.1749-6632.1968.tb14738.x. [DOI] [PubMed] [Google Scholar]
- 138.Saffle J.R. Fluid Creep and Over-resuscitation. Crit. Care Clin. 2016;32:587–598. doi: 10.1016/j.ccc.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 139.Saffle J.I. The phenomenon of “fluid creep” in acute burn resuscitation. J. Burn Care Res. 2007;28:382–395. doi: 10.1097/BCR.0B013E318053D3A1. [DOI] [PubMed] [Google Scholar]
- 140.Pruitt B.A., Jr. Protection from excessive resuscitation: “pushing the pendulum back”. J. Trauma. 2000;49:567–568. doi: 10.1097/00005373-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 141.Working Group IAPAPAAPG IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–e15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 142.Huber W., Malbrain M.L. Goal-directed fluid resuscitation in acute pancreatitis: Shedding light on the penumbra by dynamic markers of preload? Intensive Care Med. 2013;39:784–786. doi: 10.1007/s00134-012-2783-x. [DOI] [PubMed] [Google Scholar]
- 143.Trikudanathan G., Navaneethan U., Vege S.S. Current controversies in fluid resuscitation in acute pancreatitis: A systematic review. Pancreas. 2012;41:827–834. doi: 10.1097/MPA.0b013e31824c1598. [DOI] [PubMed] [Google Scholar]
- 144.Mao E.Q., Fei J., Peng Y.B., Huang J., Tang Y.Q., Zhang S.D. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin. Med. J. 2010;123:1639–1644. [PubMed] [Google Scholar]
- 145.Johansson P.I., Stensballe J., Ostrowski S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care. 2017;21:25. doi: 10.1186/s13054-017-1605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Van Regenmortel N., Verbrugghe W., Roelant E., Van den Wyngaert T., Jorens P.G. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: A retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44:409–417. doi: 10.1007/s00134-018-5147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Myles P.S., Bellomo R., Corcoran T., Forbes A., Peyton P., Story D., Christophi C., Leslie K., McGuinness S., Parke R., et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N. Engl. J. Med. 2018;378:2263–2274. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 148.Malbrain M.L., Van Regenmortel N., Owczuk R. It is time to consider the four D’s of fluid management. Anaesthesiol. Intensive Ther. 2015;47:s1–s5. doi: 10.5603/AIT.a2015.0070. [DOI] [PubMed] [Google Scholar]
- 149.Meyhoff T.S., Hjortrup P.B., Moller M.H., Wetterslev J., Lange T., Kjaer M.N., Jonsson A.B., Hjortso C.J.S., Cronhjort M., Laake J.H., et al. Conservative vs liberal fluid therapy in septic shock (CLASSIC) trial-Protocol and statistical analysis plan. Acta Anaesthesiol. Scand. 2019;63:1262–1271. doi: 10.1111/aas.13434. [DOI] [PubMed] [Google Scholar]
- 150.Pereira B., Dorigatti A., Melek M., Dos Santos J., Ferreira M., Calderan T., Carmona C., Fraga G. Septic shock patients admitted to the intensive care unit with higher SOFA score tend to have higher incidence of abdominal compartment syndrome—A preliminary analysis. Anaesthesiol. Intensive Ther. 2019;51:370–372. doi: 10.5114/ait.2019.88184. [DOI] [PubMed] [Google Scholar]
- 151.Perel P., Roberts I., Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst. Rev. 2013;2:1465–1858. doi: 10.1002/14651858.CD000567.pub6. [DOI] [PubMed] [Google Scholar]
- 152.Hahn R.G. Adverse effects of crystalloid and colloid fluids. Anaesthesiol. Intensive Ther. 2017;49:303–308. doi: 10.5603/AIT.a2017.0045. [DOI] [PubMed] [Google Scholar]
- 153.Iqbal U., Anwar H., Scribani M. Ringer’s lactate versus normal saline in acute pancreatitis: A systematic review and meta-analysis. J. Dig. Dis. 2018;19:335–341. doi: 10.1111/1751-2980.12606. [DOI] [PubMed] [Google Scholar]
- 154.Wu B.U., Hwang J.Q., Gardner T.H., Repas K., Delee R., Yu S., Smith B., Banks P.A., Conwell D.L. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin. Gastroenterol. Hepatol. 2011;9:710–717.e711. doi: 10.1016/j.cgh.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 155.Semler M.W., Self W.H., Wanderer J.P., Ehrenfeld J.M., Wang L., Byrne D.W., Stollings J.L., Kumar A.B., Hughes C.G., Hernandez A., et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Buxbaum J.L., Quezada M., Da B., Jani N., Lane C., Mwengela D., Kelly T., Jhun P., Dhanireddy K., Laine L. Early Aggressive Hydration Hastens Clinical Improvement in Mild Acute Pancreatitis. Am. J. Gastroenterol. 2017;112:797–803. doi: 10.1038/ajg.2017.40. [DOI] [PubMed] [Google Scholar]
- 157.Finfer S., Bellomo R., Boyce N., French J., Myburgh J., Norton R., Investigators S.S. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 158.Maitland K., Kiguli S., Opoka R.O., Engoru C., Olupot-Olupot P., Akech S.O., Nyeko R., Mtove G., Reyburn H., Lang T., et al. Mortality after fluid bolus in African children with severe infection. N. Engl. J. Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 159.Caironi P., Tognoni G., Masson S., Fumagalli R., Pesenti A., Romero M., Fanizza C., Caspani L., Faenza S., Grasselli G., et al. Albumin replacement in patients with severe sepsis or septic shock. N. Engl. J. Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 160.Eljaiek R., Heylbroeck C., Dubois M.J. Albumin administration for fluid resuscitation in burn patients: A systematic review and meta-analysis. Burns. 2017;43:17–24. doi: 10.1016/j.burns.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 161.Martensson J., Bihari S., Bannard-Smith J., Glassford N.J., Lloyd-Donald P., Cioccari L., Luethi N., Tanaka A., Crisman M., Rey de Castro N., et al. Small volume resuscitation with 20% albumin in intensive care: Physiological effects: The SWIPE randomised clinical trial. Intensive Care Med. 2018;44:1797–1806. doi: 10.1007/s00134-018-5253-2. [DOI] [PubMed] [Google Scholar]
- 162.Finfer S. Reappraising the role of albumin for resuscitation. Curr. Opin. Crit. Care. 2013;19:315–320. doi: 10.1097/MCC.0b013e3283632e42. [DOI] [PubMed] [Google Scholar]
- 163.Wang M.D., Ji Y., Xu J., Jiang D.H., Luo L., Huang S.W. Early goal-directed fluid therapy with fresh frozen plasma reduces severe acute pancreatitis mortality in the intensive care unit. Chin. Med. J. 2013;126:1987–1988. [PubMed] [Google Scholar]
- 164.Horton J.W., Dunn C.W., Burnweit C.A., Walker P.B. Hypertonic saline-dextran resuscitation of acute canine bile-induced pancreatitis. Am. J. Surg. 1989;158:48–56. doi: 10.1016/0002-9610(89)90315-2. [DOI] [PubMed] [Google Scholar]
- 165.Machado M.C., Coelho A.M., Pontieri V., Sampietre S.N., Molan N.A., Soriano F., Matheus A.S., Patzina R.A., Cunha J.E., Velasco I.T. Local and systemic effects of hypertonic solution (NaCl 7.5%) in experimental acute pancreatitis. Pancreas. 2006;32:80–86. doi: 10.1097/01.mpa.0000191645.01926.8f. [DOI] [PubMed] [Google Scholar]
- 166.Ni H.B., Ke L., Sun J.K., Tong Z.H., Ding W.W., Li W.Q., Li N., Li J.S. Beneficial effect of hypertonic saline resuscitation in a porcine model of severe acute pancreatitis. Pancreas. 2012;41:310–316. doi: 10.1097/MPA.0b013e3182297fec. [DOI] [PubMed] [Google Scholar]
- 167.Shields C.J., Winter D.C., Sookhai S., Ryan L., Kirwan W.O., Redmond H.P. Hypertonic saline attenuates end-organ damage in an experimental model of acute pancreatitis. Br. J. Surg. 2000;87:1336–1340. doi: 10.1046/j.1365-2168.2000.01626.x. [DOI] [PubMed] [Google Scholar]
- 168.Pham T.N., Cancio L.C., Gibran N.S., American Burn A. American Burn Association practice guidelines burn shock resuscitation. J. Burn Care Res. 2008;29:257–266. doi: 10.1097/BCR.0b013e31815f3876. [DOI] [PubMed] [Google Scholar]
- 169.Saugel B., Malbrain M.L., Perel A. Hemodynamic monitoring in the era of evidence-based medicine. Crit. Care. 2016;20:401. doi: 10.1186/s13054-016-1534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vandervelden S., Malbrain M.L. Initial resuscitation from severe sepsis: One size does not fit all. Anaesthesiol. Intensive Ther. 2015;47:s44–s55. doi: 10.5603/AIT.a2015.0075. [DOI] [PubMed] [Google Scholar]
- 171.Pereira B.M., Pereira R.G., Wise R., Sugrue G., Zakrison T.L., Dorigatti A.E., Fiorelli R.K., Malbrain M. The role of point-of-care ultrasound in intra-abdominal hypertension management. Anaesthesiol. Intensive Ther. 2017;49:373–381. doi: 10.5603/AIT.a2017.0074. [DOI] [PubMed] [Google Scholar]
- 172.Marik P.E., Malbrain M. The SEP-1 quality mandate may be harmful: How to drown a patient with 30 mL per kg fluid! Anaesthesiol. Intensive Ther. 2017;49:323–328. doi: 10.5603/AIT.a2017.0056. [DOI] [PubMed] [Google Scholar]
- 173.O’Connor M.E., Prowle J.R. Fluid Overload. Crit. Care Clin. 2015;31:803–821. doi: 10.1016/j.ccc.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 174.Rizzo J.A., Rowan M.P., Driscoll I.R., Chung K.K., Friedman B.C. Vitamin C in Burn Resuscitation. Crit. Care Clin. 2016;32:539–546. doi: 10.1016/j.ccc.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 175.Tanaka H., Matsuda T., Miyagantani Y., Yukioka T., Matsuda H., Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Arch. Surg. 2000;135:326–331. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 176.Smith J.W., Garrison R.N., Matheson P.J., Franklin G.A., Harbrecht B.G., Richardson J.D. Direct peritoneal resuscitation accelerates primary abdominal wall closure after damage control surgery. J. Am. Coll Surg. 2010;210:658–664, 664–657. doi: 10.1016/j.jamcollsurg.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.