Abstract
Escherichia coli acetyl coenzyme A carboxylase (ACC), the first enzyme of the fatty acid biosynthetic pathway, is inhibited by acylated derivatives of acyl carrier protein (ACP). ACP lacking an acyl moiety does not inhibit ACC. Acylated derivatives of ACP having chain lengths of 6 to 20 carbon atoms were similarly inhibitory at physiologically relevant concentrations. The observed feedback inhibition was specific to the protein moiety, as shown by the inability of the palmitoyl thioester of spinach ACP I to inhibit ACC.
Escherichia coli and other bacteria accurately regulate membrane lipid synthesis over a wide variety of growth rates. A well-studied aspect of this regulation is the close coupling of the rate of fatty acid synthesis to the rate of phospholipid synthesis (17, 19, 26, 27). Inhibition of phospholipid synthesis results in a rapid decrease in the rate of fatty acid synthesis and in the accumulation of acylated derivatives of the key lipid synthetic protein, acyl carrier protein (ACP) (17, 19, 26, 27). As shown by Jiang and Cronan (19) and subsequently by others (5, 6, 28, 32), the biosynthetic coupling between fatty acid and phospholipid syntheses could be disrupted by high-level expression of thioesterases, resulting in cleavage of the acylated derivatives of ACP (acyl-ACPs) to fatty acids plus ACP. A possible explanation for these results was that all of the ACP had been converted to acyl-ACPs such that the availability of ACP to initiate fatty acid synthesis was limiting in the absence of thioesterase action. However, this explanation has been ruled out by the finding that ACP levels were not limiting (17, 19). Therefore, another model is favored in which the accumulated acyl-ACPs inhibit fatty acid synthesis by feedback inhibition of one or more enzymes of the pathway. Heath and Rock (15–17) have reported in vitro data showing that acyl-ACPs inhibit both enoyl-ACP reductase and 3-ketoacyl-ACP synthase III of E. coli. These workers also have suggested that acetyl coenzyme A (acetyl-CoA) carboxylase (ACC), the first enzyme of the fatty acid biosynthetic pathway, could be an additional site of inhibition by acyl-ACPs (16). We have recently reported that the overproduction of ACC results in an increased rate of fatty acid synthesis in E. coli (8), thus strengthening the proposal of a regulatory role for this enzyme. We have now tested the proposal of Heath and Rock (16) and report that acyl-ACPs are potent inhibitors of ACC activity.
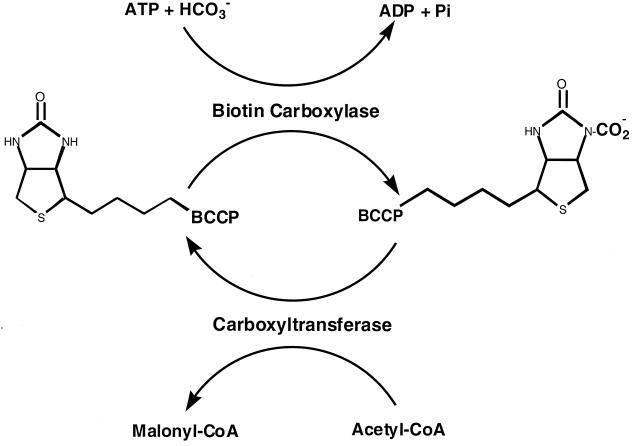
The ACC reaction consists of two readily assayed partial reactions (Fig. 1) (4, 10, 11, 24, 25). In the first partial reaction, biotin is carboxylated by bicarbonate in an ATP-dependent reaction to form carboxybiotin, whereas in the second partial reaction, the carboxyl group is transferred from carboxybiotin to acetyl-CoA to form malonyl coenzyme A (malonyl-CoA). In E. coli, these partial reactions are catalyzed by different components of a putative enzyme complex. The biotin carboxylase subunit, encoded by the accC gene (22, 23), is responsible for the first partial reaction, whereas carboxyl transfer is catalyzed by a complex of two different proteins (called α and β) encoded by the accA and accD genes, respectively (4, 24). Although in vitro free biotin functions with a very low efficiency in both partial reactions (4, 10, 23, 24), the function of ACC both in vitro (1, 10) and in vivo (23) requires that the biotin moiety be covalently attached to a fourth protein, biotin carboxyl carrier protein (BCCP), encoded by the accB gene. Extracts of wild-type strains of E. coli have no detectable ACC activity (1, 8–10), although both partial reactions are readily detected. The E. coli ACC complex is believed to dissociate at the low subunit concentrations of crude extracts, and the overall ACC reaction has been detected in vitro only when high concentrations of the four component proteins are present (8–10). We recently have obtained the necessary high protein concentrations by simultaneous overexpression of the four ACC subunits in a stoichiometric manner via induction of a synthetic acc operon (8). Extracts of strains overexpressing these four proteins contain high levels of ACC activity (8). In this report, we have used this system to examine the effects of acyl-ACPs on this fatty acid synthesis step.
FIG. 1.
Reaction mechanism of E. coli ACC. Biotin carboxylase is encoded by the accC gene, whereas BCCP is encoded by the accB gene. The two subunits involved in carboxyltransferase activity are encoded by the accA and accD genes. The covalently bound biotin of BCCP carries the carboxylate moiety.
Acyl-ACPs with different chain lengths were synthesized from E. coli ACP (21) and fatty acids by use of either the E. coli or the Vibrio harveyi acyl-ACP synthetase reactions (depending on the fatty acid) and purified as described previously (18, 30, 31). These acyl-ACP preparations were transferred into the same buffer as that used in the assays, and the mixtures were then added to extracts of the ACC overproduction strain, prepared as described previously (8). These mixtures were then incubated, and ACC activity was assayed after the addition of acetyl-CoA, ATP, and [14C]bicarbonate (8). We first examined the effects of palmitoyl-ACP, since palmitate is the most abundant fatty acid in E. coli. The addition of palmitoyl-ACP to these cell extracts resulted in a significant and concentration-dependent inhibition of ACC activity (Fig. 2). In contrast, ACP lacking an acyl group (ACP-SH) was not inhibitory. The specificity of inhibition was further examined by synthesis of an acyl-ACP having an ACP moiety which differed from that of E. coli. The ACP used was a plant chloroplast protein, ACP-I, of spinach. ACP-I was expressed in E. coli, purified to homogeneity as previously described (2, 12, 13), and then converted to its palmitoyl thioester as described above. The palmitoyl thioester of spinach ACP-I failed to inhibit E. coli ACC activity (Fig. 2), although this heterologous ACP has 44% sequence identity to E. coli ACP and a very similar overall structure (29). The concentrations of acyl-ACPs required to inhibit ACC activity are within the physiological range (15). An intracellular acyl-ACP concentration of 40 μM requires that only about one-third of the cellular ACP (the most abundant soluble protein of E. coli) be converted to acyl-ACP, whereas inhibition of phospholipid synthesis results in the conversion of over one-half of the cellular ACP to acyl-ACP (15).
FIG. 2.
Inhibition of the ACC reaction by palmitoyl-ACP. The reaction mixture contained 0.2 mg of extract protein prepared as described previously (8). E. coli ACP or the palmitoyl thioesters of ACP from either E. coli or spinach were added to the cell extract and incubated for 60 min at 37°C. The ACC reaction components were then added, and the reactions were carried out as previously described (8). Symbols: ○, E. coli ACP; ●, E. coli palmitoyl-ACP; ▪, spinach ACP-I.
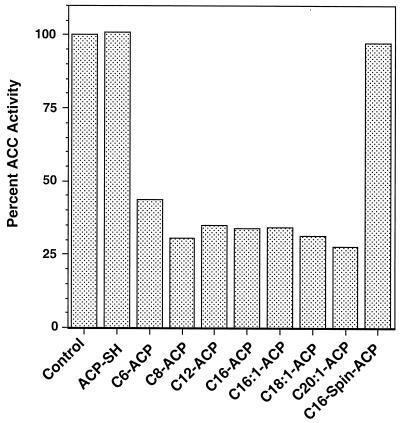
Acyl-ACPs having acyl chain lengths of from C6:0 to C20:1 gave essentially identical degrees of inhibition of ACC activity (Fig. 3), with 40 μM resulting in 60 to 70% inhibition. Complete inhibition was not obtained at any concentration of the acyl-ACP species tested. We tested the effects of acyl-ACPs on the partial reactions of ACC, biotin carboxylase, and carboxyltransferase for clues to the mechanism of ACC inhibition. No detectable inhibition of either activity was found at concentrations of up to 100 μM palmitoyl-ACP, either when all four ACC proteins were overexpressed or when overexpression was limited to only those subunits involved in a given partial reaction (AccC for biotin carboxylase and AccA plus AccD for carboxyltransferase) (Fig. 4). It should be noted that the ACC activity of extracts of strains overproducing only the AccBCD subunits (8) was inhibited by acyl-ACPs in a manner essentially identical to that shown in Fig. 3 (7).
FIG. 3.
Effects of different acyl chain lengths on ACC inhibition. ACP-SH and all acyl-ACPs were tested at 40 μM as described in the legend to Fig. 2. The control incubation lacked added ACP-SH or acyl-ACP. All of the acyl species were unbranched. The unsaturated fatty acyl chains were as follows: C16:1, cis-9-hexadecenoic acid (palmitoleic acid); C18:1, cis-11-octadecenoic acid (cis-vaccenic acid); and C20:1, cis-13-dodecenoic acid. Spin-ACP, spinach ACP-I.
FIG. 4.
Effects of acyl-ACPs on ACC partial reactions. Biotin carboxylase (A) was assayed by carboxylation of biotin with [14C]bicarbonate, whereas carboxyltransferase (B) was assayed in the reverse of the physiological reaction by decarboxylation of [2-14C]malonyl-CoA in the presence of d-biotin-ɛ-lysine (8). The extracts were incubated with palmitoyl-ACP as described in the legend to Fig. 2. Symbols: ▵, partial activities of an extract that contained all four overproduced ACC subunits; ▿, biotin carboxylase activity of an extract that contained overproduced AccC subunit; ○, carboxyltransferase activity of an extract that contained all overproduced AccA and AccD subunits (8). The 100% activity for biotin carboxylase was 4.6 nmol/min/mg of protein, whereas that for carboxyltransferase was 28.4 nmol/min/mg of protein.
The lack of acyl-ACP inhibition of the biotin carboxylase partial reaction indicated that acyl-ACPs did not inhibit the binding of ATP or bicarbonate to ACC. However, an effect on the binding of acetyl-CoA was not precluded by the partial-reaction assays, since the carboxyltransferase partial reaction was assayed in the reverse direction (4, 11, 24), in which acetyl-CoA is not a substrate. (Carboxybiotin, the substrate required for assay of the forward carboxyltransferase reaction, is a very unstable compound (11); thus, the standard assay for carboxyltransferase activity is the decarboxylation of malonyl-CoA in the presence of biotinyl-lysine). We therefore examined the kinetics of acetyl-CoA utilization in the overall ACC assay. The presence of palmitoyl-ACP increased the Km for acetyl-CoA, but the kinetics of inhibition indicated that the pattern of inhibition was mixed, that is, a combination of competitive inhibition and noncompetitive inhibition (Fig. 5).
FIG. 5.
Lineweaver-Burk plot of the effects of various acetyl-CoA concentrations on the inhibition of ACC by palmitoyl-ACP. The experiments were carried out as described in the legend to Fig. 2. Symbols: ▪, no inhibitor added; ▴, 20 μM palmitoyl-ACP added; ●, 40 μM palmitoyl-ACP added.
The lack of ACC inhibition by spinach palmitoyl-ACP provides strong evidence for the specificity of the observed inhibition. Spinach ACP-I and E. coli ACP show 44% sequence identity over 72 residues (essentially the full lengths of both proteins). Moreover, two-dimensional nuclear magnetic resonance analyses indicate that the overall protein structures of E. coli ACP and spinach ACP-I are very similar (29). Finally, both ACPs are substrates for E. coli acyl-ACP synthase, and each ACP will support in vitro fatty acid synthesis by the heterologous system (13).
It should be noted that the specificity that we observed is supported by published in vivo data. When spinach ACP-I is produced in E. coli, it can comprise as much as 90% of the total cellular ACP (10). A major fraction (50 to 70%) of spinach ACP-I is found as acyl-ACP, with the acyl group being cis-vaccenate (18:1) (12, 13). (The accumulation of this acylated protein species has been attributed to a failure of the acyltransferases of the E. coli phospholipid synthetic pathway to accept acyl–ACP-I species [12, 13].) From the data of Guerra and Browse (12), an intracellular concentration of >1 mM cis-vaccenoyl–ACP-I can be calculated. At this concentration, the cis-vaccenoyl thioester of E. coli ACP would be a potent inhibitor of ACC (Fig. 3), and inhibition of both cell growth and in vivo fatty acid synthesis would be expected. However, E. coli strains producing high levels of spinach cis-vaccenoyl–ACP-I have normal rates of growth and lipid synthesis (13). Therefore, spinach acyl-ACP is not inhibitory, providing in vivo results consistent with our in vitro data. It should be noted that Heath and Rock (16) have reported that the accumulation of acyl-ACPs in vivo fails to block malonyl-CoA synthesis. However, since these data were single time point measurements obtained under conditions where the utilization of malonyl-CoA was blocked, a reduced rate of malonyl-CoA synthesis could have been missed. Consistent with this premise, the amount of malonyl-CoA accumulated in the presence of acyl-ACPs was about 20% lower than that in cells lacking acyl-ACP accumulation. Indeed, later data obtained by these workers were consistent with the inhibition of ACC by acyl-ACPs (15) and led to their subsequent proposal that this enzyme could be a target of inhibition.
The accumulation of acyl-ACPs was found to decrease malonyl-ACP concentrations in vivo by about 60% (13). Since malonyl-CoA:ACP transacylase is not inhibited by acyl-ACP (16) and since this enzyme catalyzes a rapid interconversion of malonyl-ACP and malonyl-CoA (17), it seems very likely that the malonyl-CoA pool was similarly depleted in these experiments. The limited depletion of malonate pools observed in vivo was consistent with our in vivo results in which the complete inhibition of ACC activity was not attained.
The kinetics of ACC inhibition by acyl-ACP were neither purely competitive nor noncompetitive with respect to acetyl-CoA but showed a mixed form of inhibition having elements of both types of inhibition (Fig. 5). The competitive element suggests an interaction with the acetyl-CoA binding site. This notion seems reasonable, since the substrate, acetyl-CoA, and the inhibitor, acyl-ACP, share the structural elements of an acyl chain linked to 4′-phosphopanthetheine via a thioester bond. A straightforward mode of inhibition would be occupation of the acetyl-CoA binding site by the acylated 4′-phosphopanthetheine moiety of the acyl-ACP. However, this mode of inhibition seems precluded by the finding that spinach acyl-ACP failed to inhibit ACC. Moreover, since E. coli ACC is inactive with propionyl coenzyme A (1), the pocket that binds the acyl chain would seem too small to accommodate the much longer acyl chains of the inhibitory acyl-ACPs. Therefore, it seems likely that acyl-ACP binds to ACC (presumably to the AccA-AccD carboxyltransferase part of the complex) and indirectly alters the active site, a scenario consistent with a mixed form of inhibition. The proposed binding of acyl-ACP to ACC would require an acyl chain plus ACP amino acid residues distant in the primary sequence from the site of 4′-phosphopanthetheine attachment because the 10 residues bracketing the attachment site are strictly conserved between the E. coli and spinach ACPs.
It should be noted that this is the first example of an enzyme inhibited by acyl-ACPs that does not have an acyl-ACP substrate. Heath and Rock (15–17) have reported in vitro data showing that E. coli enoyl-ACP reductase and 3-ketoacyl-ACP synthase III are inhibited by acyl-ACPs. In the case of enoyl-ACP reductase, the observed inhibition might be the simple product inhibition characteristic of all enzymes, since the enoyl-ACP reductase produces finished (fully reduced) acyl-ACPs. However, in the case of 3-ketoacyl-ACP synthase III, the acyl-ACP species produced differ from those reported to inhibit the enzyme both in chain length (C4 versus C12 to C20) and in acyl chain oxidation state (3-keto versus fully reduced); thus, thus simple product inhibition seems unlikely. It should be noted that although genetic and inhibitor studies have demonstrated that enoyl-ACP reductase is an essential enzyme (3), such data are not yet available for 3-ketoacyl-ACP synthase III. Although all of the acyl-ACP acyl chain lengths that we tested had similar inhibitory potencies, it is possible that the inhibition of multiple enzymes results in a regulatory synergy. For example, the inhibition of enoyl-ACP reductase would result in the accumulation of 3-hydroxyacyl-ACPs and enoyl-ACPs (14), which might be more potent inhibitors of ACC than the fully reduced species. It is noteworthy that 50% inhibition of 3-ketoacyl-ACP synthase III (15) requires concentrations of acyl-ACP two- to threefold higher than those required to give comparable inhibition of ACC, whereas enoyl-ACP reductase (16) has a palmitoyl-ACP inhibition profile similar to that of ACC. It is interesting that, like that of ACC, the mode of acyl-ACP inhibition of 3-ketoacyl-ACP synthase III shows mixed kinetics when acetyl-CoA is the substrate varied (15).
The inhibition of acetyl-CoA carboxylase by acyl-ACPs seems physiologically reasonable. ACC is the first committed step of fatty acid synthesis and consumes ATP and acetyl-CoA. Therefore, regulation of ACC activity would allow conservation of both energy and a key metabolic intermediate. The use of acyl-ACPs as the inhibitory ligand would neatly tie the end of the fatty acid biosynthetic pathway to the beginning of the pathway. Therefore, if the rate of phospholipid synthesis fell below the rate of fatty acid synthesis, the resulting accumulation of acyl-ACPs would feedback inhibit ACC (and probably later steps in the pathway) in a coordinated manner until the excess acyl-ACP was consumed by incorporation of the acyl chains into phospholipid and lipid A.
Neither we nor Heath and Rock (15, 16) have obtained complete inhibition of a target enzyme by the addition of acyl-ACPs. One possible explanation for the partial nature of the observed inhibition profiles could be the need for the synthesis of nonphospholipid molecules, such as lipoic acid (20) and the acyl moieties of lipid A. Therefore, it is possible that E. coli must sustain a residual rate of fatty acid synthesis even in the presence of high levels of long-chain acyl-ACPs.
Acknowledgments
This work was supported by National Institutes of Health grant AI15650.
We thank John Ohlrogge for a plasmid encoding spinach ACP-I.
REFERENCES
- 1.Alberts A W, Vagelos P R. Acyl-CoA carboxylases. In: Boyer P D, editor. The enzymes. 3rd ed. Vol. 6. New York, N.Y: Academic Press, Inc.; 1972. pp. 37–82. [Google Scholar]
- 2.Beremand P D, Hannapel D J, Guerra D J, Kuhn D N, Ohlrogge J B. Synthesis, cloning, and expression in Escherichia coli of a spinach acyl carrier protein-I gene. Arch Biochem Biophys. 1987;256:90–100. doi: 10.1016/0003-9861(87)90428-0. [DOI] [PubMed] [Google Scholar]
- 3.Bergler H, Wallner P, Ebeling A, Leitinger B, Fuchsbichler S, Aschauer H, Kollenz G, Hogenauer G, Turnowsky F. Protein EnvM is the NADH-dependent enoyl-ACP reductase (FabI) of Escherichia coli. J Biol Chem. 1994;269:5493–5496. [PubMed] [Google Scholar]
- 4.Blanchard C Z, Waldrop G L. Overexpression and kinetic characterization of the carboxyltransferase component of acetyl-CoA carboxylase. J Biol Chem. 1998;273:19140–19145. doi: 10.1074/jbc.273.30.19140. [DOI] [PubMed] [Google Scholar]
- 5.Cho H, Cronan J E., Jr Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis. J Biol Chem. 1995;270:4216–4219. doi: 10.1074/jbc.270.9.4216. [DOI] [PubMed] [Google Scholar]
- 6.Cho H, Cronan J E., Jr “Protease I” of Escherichia coli functions as a thioesterase in vivo. J Bacteriol. 1994;176:1793–1795. doi: 10.1128/jb.176.6.1793-1795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis M S. Studies of acetyl-CoA carboxylase from Escherichia coli. Ph.D. thesis. University of Illinois, Urbana; 1998. [Google Scholar]
- 8.Davis M S, Solbiati J, Cronan J E., Jr Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem. 2000;275:28593–28598. doi: 10.1074/jbc.M004756200. [DOI] [PubMed] [Google Scholar]
- 9.Fall R R. Stabilization of an acetyl-coenzyme A carboxylase complex from Pseudomonas citronellolis. Biochim Biophys Acta. 1976;450:475–480. doi: 10.1016/0005-2760(76)90022-9. [DOI] [PubMed] [Google Scholar]
- 10.Guchhait R B, Polakis S E, Dimroth P, Stoll E, Moss J, Lane M D. Acetyl coenzyme A carboxylase system of Escherichia coli. Purification and properties of the biotin carboxylase, carboxyltransferase, and carboxyl carrier protein components. J Biol Chem. 1974;249:6633–6645. [PubMed] [Google Scholar]
- 11.Guchhait R B, Polakis S E, Hollis D, Fenselau C, Lane M D. Acetyl coenzyme A carboxylase system of Escherichia coli. Site of carboxylation of biotin and enzymatic reactivity of 1′-N-(ureido)-carboxybiotin derivatives. J Biol Chem. 1974;249:6646–6656. [PubMed] [Google Scholar]
- 12.Guerra D J, Browse J. The recombinant spinach acyl-acyl carrier protein-I expressed in Escherichia coli is the 18:1 delta 11(cis) thioester. Arch Biochem Biophys. 1989;271:246–253. doi: 10.1016/0003-9861(89)90275-0. [DOI] [PubMed] [Google Scholar]
- 13.Guerra D J, Dziewanowska K, Ohlrogge J B, Beremand P D. Purification and characterization of recombinant spinach acyl carrier protein I expressed in Escherichia coli. J Biol Chem. 1988;263:4386–4391. [PubMed] [Google Scholar]
- 14.Heath R J, Rock C O. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J Biol Chem. 1995;270:26538–26542. doi: 10.1074/jbc.270.44.26538. [DOI] [PubMed] [Google Scholar]
- 15.Heath R J, Rock C O. Inhibition of β-ketoacyl-acyl carrier protein synthase III (FabH) by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271:10996–11000. doi: 10.1074/jbc.271.18.10996. [DOI] [PubMed] [Google Scholar]
- 16.Heath R J, Rock C O. Regulation of fatty acid elongation and initiation by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271:1833–1836. doi: 10.1074/jbc.271.4.1833. [DOI] [PubMed] [Google Scholar]
- 17.Heath R J, Rock C O. Regulation of malonyl-CoA metabolism by acyl-acyl carrier protein and beta-ketoacyl-acyl carrier protein synthases in Escherichia coli. J Biol Chem. 1995;270:15531–15538. doi: 10.1074/jbc.270.26.15531. [DOI] [PubMed] [Google Scholar]
- 18.Jackowski S, Jackson P D, Rock C O. Sequence and function of the aas gene in Escherichia coli. J Biol Chem. 1994;269:2921–2928. [PubMed] [Google Scholar]
- 19.Jiang P, Cronan J E., Jr Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J Bacteriol. 1994;176:2814–2821. doi: 10.1128/jb.176.10.2814-2821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan S W, Cronan J E., Jr A new metabolic link. The acyl carrier protein of lipid synthesis donates lipoic acid to the pyruvate dehydrogenase complex in Escherichia coli and mitochondria. J Biol Chem. 1997;272:17903–17906. doi: 10.1074/jbc.272.29.17903. [DOI] [PubMed] [Google Scholar]
- 21.Keating D H, Carey M R, Cronan J E., Jr The unmodified (apo) form of Escherichia coli acyl carrier protein is a potent inhibitor of cell growth. J Biol Chem. 1995;270:22229–22235. doi: 10.1074/jbc.270.38.22229. [DOI] [PubMed] [Google Scholar]
- 22.Kondo H, Shiratsuchi K, Yoshimoto T, Masuda T, Kitazono A, Tsuru D, Anai M, Sekiguchi M, Tanabe T. Acetyl-CoA carboxylase from Escherichia coli: gene organization and nucleotide sequence of the biotin carboxylase subunit. Proc Natl Acad Sci USA. 1991;88:9730–9733. doi: 10.1073/pnas.88.21.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S J, Cronan J E., Jr The gene encoding the biotin carboxylase subunit of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992;267:855–863. [PubMed] [Google Scholar]
- 24.Li S J, Cronan J E., Jr The genes encoding the two carboxyltransferase subunits of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992;267:16841–16847. [PubMed] [Google Scholar]
- 25.Li S J, Rock C O, Cronan J E., Jr The dedB (usg) open reading frame of Escherichia coli encodes a subunit of acetyl coenzyme A carboxylase. J Bacteriol. 1992;174:5755–5757. doi: 10.1128/jb.174.17.5755-5757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mindich L. Control of fatty acid synthesis in bacteria. J Bacteriol. 1972;110:96–102. doi: 10.1128/jb.110.1.96-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunn W D, Kelly D L, Stumfall M Y. Regulation of fatty acid synthesis during the cessation of phospholipid biosynthesis in Escherichia coli. J Bacteriol. 1977;132:526–531. doi: 10.1128/jb.132.2.526-531.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlrogge J, Savage L, Jaworski J, Voelker T, Post-Beittenmiller D. Alteration of acyl-acyl carrier protein pools and acetyl-CoA carboxylase expression in Escherichia coli by a plant medium chain acyl-acyl carrier protein thioesterase. Arch Biochem Biophys. 1995;317:185–190. doi: 10.1006/abbi.1995.1152. [DOI] [PubMed] [Google Scholar]
- 29.Oswood C M, Kim Y, Ohlrogge J B, Prestegard J H. Structural homology of spinach acyl carrier protein and Escherichia coli acyl carrier protein based on NMR data. Proteins Struct Funct. 1997;27:131–143. [PubMed] [Google Scholar]
- 30.Rock C O, Garwin J L, Cronan J E., Jr Preparative enzymatic synthesis of acyl-acyl carrier protein. Methods Enzymol. 1981;72:397–403. doi: 10.1016/s0076-6879(81)72029-9. [DOI] [PubMed] [Google Scholar]
- 31.Shen Z, Fice D, Byers D M. Preparation of fatty-acylated derivatives of acyl carrier protein using Vibrio harveyi acyl-ACP synthetase. Anal Biochem. 1992;204:34–39. doi: 10.1016/0003-2697(92)90135-t. [DOI] [PubMed] [Google Scholar]
- 32.Voelker T A, Davies H M. Alteration of the specificity and regulation of fatty acid synthesis of Escherichia coli by expression of a plant medium-chain acyl-acyl carrier protein thioesterase. J Bacteriol. 1994;176:7320–7327. doi: 10.1128/jb.176.23.7320-7327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]