Abstract
Approximately 5% of all human cancers are attributable to human papillomavirus (HPV) infections. HPV-associated diseases and cancers remain a substantial public health and economic burden worldwide despite the availability of prophylactic HPV vaccines. Current diagnosis and treatments for HPV-associated diseases and cancers are predominantly based on cell/tissue morphological examination and/or testing for the presence of high-risk HPV types. There is a lack of robust targets/markers to improve the accuracy of diagnosis and treatments. Several naturally occurring animal papillomavirus models have been established as surrogates to study HPV pathogenesis. Among them, the Cottontail rabbit papillomavirus (CRPV) model has become known as the gold standard. This model has played a pivotal role in the successful development of vaccines now available to prevent HPV infections. Over the past eighty years, the CRPV model has been widely applied to study HPV carcinogenesis. Taking advantage of a large panel of functional mutant CRPV genomes with distinct, reproducible, and predictable phenotypes, we have gained a deeper understanding of viral–host interaction during tumor progression. In recent years, the application of genome-wide RNA-seq analysis to the CRPV model has allowed us to learn and validate changes that parallel those reported in HPV-associated cancers. In addition, we have established a selection of gene-modified rabbit lines to facilitate mechanistic studies and the development of novel therapeutic strategies. In the current review, we summarize some significant findings that have advanced our understanding of HPV pathogenesis and highlight the implication of the development of novel gene-modified rabbits to future mechanistic studies.
Keywords: rabbit, papillomavirus, CRPV, HPV, tumor regression, disease progression, cancer, gene modified rabbits, RNAseq, codon optimization, wound healing, immune responses
1. Introduction
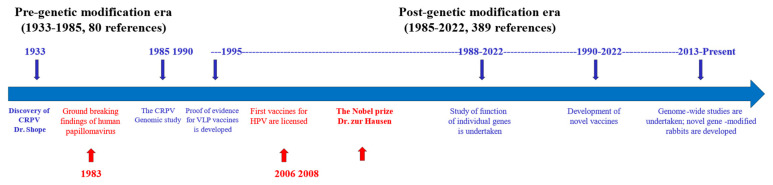
Human papillomavirus (HPV)-associated diseases and cancers remain a significant public health problem worldwide [1]. Due to the species-specific properties of HPV, several naturally occurring animal papillomavirus models have been critical in studying HPV pathogenesis [2,3]. Among these preclinical models, the Cottontail rabbit papillomavirus (CRPV) was the first identified papillomavirus and the CRPV model has been widely used to study viral–host interactions for HPV-associated diseases and cancers since the first report by Shope in 1933 [4,5,6,7,8]. The extensive genetic and functional homology of CRPV with high-risk HPVs has made this model system a gold standard for testing novel anti-viral and anti-tumor treatments leading to clinical applications and providing the first proof-of-evidence for the current HPV vaccines [9,10,11,12,13,14,15,16,17]. Over the past eighty years since the discovery of this tumor virus [4], especially after the CRPV genome sequence was reported in 1985 [18], we have gained a significant understanding about viral pathogenesis by using tools such as genetic modification to alter this virus genome (mutations /insertions /deletions) without destroying its ability to induce tumors. Several key milestones in CRPV studies correlating to breakthroughs in HPV cancer research are updated in Figure 1 from previous reviews [8,19,20,21,22,23,24,25,26,27,28].
Figure 1.
Several milestones of the CRPV rabbit model (blue) and HPV study (red). The rabbit model has played a pivotal role in HPV vaccine development and better understanding of HPV pathogenesis. The research using the rabbit model can be divided into two periods based on the first report on the genomic sequence of CRPV: pre-genetic modification era and post-genetic modification era. The notable research activities on the rabbit model have continued to reduce over the last two decades.
CRPV has significant biosafety advantages relative to HPVs in preclinical experiments because its species specificity ensures that it does not pose harm to humans and other animals. Therefore, the CRPV model is ideal to test many novel anti-viral and anti-tumor compounds, as well as novel vaccines [9]. To facilitate vaccine development for both prophylactic and therapeutic purposes, many vaccine strategies have been developed including peptide, protein, and DNA vaccines targeting both viral early and late genes (E1, E2, E6, E7, E8, L1, L2) [29,30,31,32,33,34,35,36,37,38,39,40,41]. Some of these strategies have moved on to clinical trials (see review paper [8]). We also synthesized the HPV/CRPV pseudovirus to test novel vaccines, including a broadly protective minor capsid protein L2 vaccine in the CRPV rabbit model [42,43,44,45].
In addition to different mutant viral genomes, rabbits with different genetic backgrounds (inbred, outbred, transgenic, and gene knockout) have been used to advance our understanding of the interaction of viral pathogenesis and host immunogenicity [7,33,46,47,48,49,50,51,52,53,54]. In the current review, which is not inclusive of all of the research performed in the rabbit papillomavirus field, we focused on some of our recent findings relating to viral pathogenesis in the post-genetic modification era (Figure 1) and highlight recent advances in gene-modified rabbits [55] that can be used for future studies.
2. Cottontail Rabbit Papillomavirus (CRPV)-Associated Pathogenesis
The CRPV genome exhibits a genetic structure and biology similar to those of high-risk HPVs [3,8,27,56]. Three oncogenes, E6, E7, and E8 (an equivalent for E5 of HPV, which is now also called E10), corresponding to those of HPVs have been identified [33,57,58,59,60]. To investigate the oncogenicity and immunogenicity of viral genes, a large panel of mutant CRPV genomes have been generated by different groups over the years, including 300 plus mutant genomes generated in our laboratory [20,23,25,26,50,53,58,61,62,63,64]. Some unique features of our mutant CRPV genomes are summarized in Table 1.
Table 1.
Published mutant CRPV genomes with unique phenotypes.
| Constructs (>300) | Tumor Phenotype | Cancer |
|---|---|---|
| Wild type (>3) | Latent, persistent, cancer | Yes, >12 months (Hu et al., 2002, 2005, 2009; Cladel et al., 2009, 2013) |
| Regressive strain (>5) | Regressive | No (Hu et al., 2002, 2005, 2009) |
| Hybrid, epitope etc., mutants (>200) | Varies | Maybe (Hu et al., 2002, 2005, 2009; Cladel et al., 2009, 2013; Bounds, 2010) and unpublished |
| E8 and SE6 mutants (>10) | Persistent, benign, and small | Maybe, >12 months (Hu et al., 2002, 2005, 2009; Cladel et al., 2009, 2013) |
| E7 mutant genomes (>5) | Persistent and benign | No (unpublished observations) |
| E6 and E7 codon optimized genomes (>20) | Regressive or Cancer | Yes, >3 months (Cladel et al., 2009, 2013) |
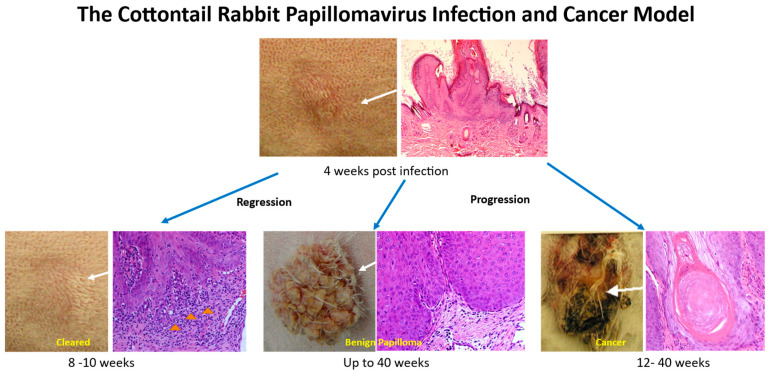
CRPV-infected tissues can either progress to cancer, maintain persistent and benign, or regress completely depending on the viral and host genetic background (Figure 2) [8,50,53,65]. The CRPV rabbit model is an excellent model to assess the role of both early and late genes in vivo because infection can be initiated with the viral DNA cloned into a plasmid [20,23,27,50,66,67,68]. Intriguingly, some of these mutant CRPV genomes display unique phenotypes in disease outcomes at predictable time frames [8,27,49]. Using an improved pre-wounding strategy established in our laboratory, we were able to achieve consistent and reproducible results among different experiments [69].
Figure 2.
The CRPV rabbit model mimics HPV-associated infections and diseases with predictable disease outcomes at different time frames. Significant immune cell infiltrates (yellow arrows) were found in the tumors undergoing regression.
2.1. Increased Viral Infection and Tumor Growth Using a Pre-Wounding Strategy
In the original study, Shope used a scarification strategy to successfully inoculate wart suspensions and to induce tumor growth on the skin of both wild and domestic rabbits [4]. This method has been adopted in most published studies for CRPV viral infections. Since the development of genetic modification technology, mutant CRPV genomes have been generated to further understand the function of individual genes in the viral life cycle and tumor progression. The best strategies to effectively induce infections with viral DNA have been a road block for researchers until the pre-wounding method was tested and validated [20,22,23,24,25,27,33,50,53,56,58,62,64,65,66,67,69,70,71,72]. The pre-wounding technique greatly improved the efficiency of infections initiated by plasmid DNA. Using this technique, plasmid loads as low as 40 ng yielded infection [69]. Interestingly, this new method also significantly increased viral infectivity by a thousand-fold, and increased induction of tumors from a dilution of 10−2 of viral stock to as low as 10–5 from the same viral stock [69]. In addition to improvements in both reproducibility and consistency, the pre-wounding technique is cost-effective considering the limited resources of viral stock and the cost of making large quantities of highly purified viral DNA plasmid [50]. It was especially helpful in increasing the sensitivity of some viral mutants, such as the E8ATGko mutant [33,58], that are less viable than the wild type. Using the pre-wounding method, we demonstrated that an E8ATGko mutant genome induced significantly smaller tumors than those of the wild type [64], whereas no lesions were found using a gene-gun delivery method by another group [58]. Therefore, this improved pre-wounding technique for viral inoculation played a significant role to gain a more accurate and deeper understanding of the in vivo oncogenicity of the individual oncogenes.
The mechanisms underlying the improved viral infection by pre-wounding in our inoculation protocol need further investigation [69]. Skin wounding triggers innate immune responses including inflammatory reactions via recruiting immune cells to counteract local infections [73,74]. We postulate that the wounding strategy plus CRPV infections further promote the local chronic inflammation that has been associated with cancer development [75,76,77]. Coincidently, we have identified a panel of wound healing-related molecules in CRPV tumors using the genome-wide transcriptome assay for which a high homology is shared between rabbits and humans [73,74,78,79,80,81]. Some of these molecules are significantly dysregulated in CRPV tumors [82]. Recent studies confirmed the important role of wound healing-related molecules including Arginase1 and Cox-2 in cutaneous wound repair; interestingly, these molecules were found to be dysregulated in CRPV-induced lesions [81,82]. Therefore, this model holds the promise of further understanding the role of these inflammation-associated molecules in HPV-associated viral infection, persistence, and tumor progression, which would improve our ability to identify interventions to treat and prevent HPV-associated diseases and cancers.
In addition to local infections, we also demonstrated that productive infections could be established by delivery of virions or viral DNA intravenously [82]. The intravenous infection was first reported in the original study by Shope, using wart suspensions [4]. Using careful controls and different viral doses, our study provided solid and new proof-of-evidence to show that papillomavirus especially viral DNA can be transmitted through the bloodstream and induce local infections at pre-wounded sites of domestic rabbits [82]. These findings suggest the possibility that the same could pertain in humans [83].
2.2. The Use of Mutant CRPV Genomes to Understand the Viral Life Cycle In Vivo
We have made modifications in both the early and late genes of the CRPV genome [27,33,35,36,50,71]. Among many mutant genomes that induce visible skin tumors on rabbits, the regions that tolerate insertions, deletions, and mutations cluster in the two late capsid genes, L1 and L2. As expected, most mutants with changes in the L1 and L2 genes did not significantly reduce the capacity to induce tumor growth in vivo [35,36,62,84].
2.2.1. Early Genes Play a Crucial Role in Viral Life Cycle and Tumor Growth
The early genes E1, E2, E6, and E7 are essential for tumor growth in vivo [20,21,27,60]. However, we were able to insert small fragments at the end of the E6 and E7 genes of some of the mutant genomes without losing the capacity for induction of tumor growth [51,84]. Many of these early gene-modified constructs became less vigorous in promoting tumor growth even with the pre-wounding method [27]. It should be noted that viral DNA can be readily detected in the lesions of wild cottontail rabbits after infection with the wild-type CRPV genome cloned as a plasmid by in situ hybridization [8]. However, the tumors induced by the same genome in New Zealand White (NZW) rabbits show much lower copies of viral DNA despite similar levels of tumor growth and antibody detection in these animals [8,35,56,82]. These findings suggest that the plasmid may interfere in some way in the domestic laboratory rabbits, while the Cottontail rabbits could overcome this interference (unpublished observations). Plasmid interference in the laboratory rabbits is supported by our findings that the release of DNA from the vector prior to infection resulted in increased L1 signals in the resulting lesions [56]. To increase the viability of the mutant constructs, we leveraged the knowledge of naturally identified tandem repeat papillomavirus sequences [82] and generated tandem repeat CRPV constructs as described in our previous publications [26,84]. These tandem repeat mutant constructs with duplicate numbers of early (E6, E7, E1, E2) and/or late (L1 and L2) genes that showed increased infectivity resulting in tumor outgrowth in vivo [26,84] display additive pathological functions and could be also complemental [26]. The tandem repeat strategy was not only used to explore the impact of different early and late genes in the viral life cycle in vivo [27,84] but also to generate hybrid constructs with HPV genes/epitopes inserted into different regions for vaccine development against HPV-associated diseases and cancers [51,84].
2.2.2. Synonymous Codon Optimization Increases Oncogenicity and Immunogenicity of the Virus
As in the case of HPVs, the CRPV genome contains many rare codons, presumably to escape host immune and miRNA surveillance by inhibiting the expression of its early and late genes [72,85,86,87]. To release the brake on this suppression, we introduced synonymous codons (without changes in the protein sequences) into the oncogenes E6 and E7 of the wild-type CRPV to match mammalian codons [25,72]. The codon-optimized E6 (CoE6) and E7 (CoE7) proteins promote cell proliferation in vitro [25,72]. CoE7 also induces primary centrosome duplication errors leading to abnormal centrosome numbers (>2) in CoE7 transfected cells (our unpublished observations), as shown in HPV16E7 [87,88]. Therefore, aneuploidy associated with codon-optimized CRPV (designated as CoCRPV) E6 and E7 may have played a role in accelerated cancer development observed for some CoCRPV genomes [25,72]. We identified one particular CoCRPV containing 15 and 18 synonymous codon changes in E6 and E7, respectively, that could induce cancers within 16 weeks post infection [25]. The accelerated cancer development by the CoCRPV genomes is characterized by a disruption of the basement membrane and invasion into the dermis as early as week 10 post infection, and contrasts significantly with the one-year average time scale to cancer for wild-type CRPV [25]. The lesions generated from the CoCRPV genomes contain higher viral copies, suggesting an increased viral replication in the codon-optimized CRPV-infected cells [25]. Comparable levels of E6 and E7 transcripts between the CoCRPV and wtCRPV lesions suggest that the levels of these two viral transcripts may not be critical in triggering a malignant transition [89,90,91], even though they might be important for tumor initiation, given that the UV light reactivation of latent CRPV infections significantly increased the E6/E7 transcripts [92]. In contrast, a third of the CoCRPV papillomas showed a greater tendency for regression or reduced growth [72]. This outcome may be due to larger amounts of oncoproteins being produced in codon-modified papillomas and subsequently targeted by the immune system, as we observed increased immune cell infiltration in these lesions [41,72]. We look forward to utilizing these unique constructs for further elucidating the functions of these oncoproteins in the viral life cycle and pathogenesis.
2.2.3. Early Gene E6 Is Important for Tumor Regression
HPV E6 has been shown to play a key role in disease progression by binding and degrading tumor suppressor protein p53 [93,94,95]. Similarly, CRPV E6 has been shown to bind tumor suppressors [59]. Based on the phenotype, two CRPV viral strains have been isolated: the progressive strain and the regressive strain that mimics high- and low-risk HPV types, respectively [53,65]. By swapping the E6 genes between these two unique CRPV strains, we observed that the E6 of the progressive strain is the key oncogene for viral persistence, a prerequisite for cancer development [50,59]. To further understand E6 function in vivo, we generated several CRPV genomes with hybrid progressive and regressive E6 [27,96]. We determined that the carboxyl terminus of regressive E6 is crucial for the regressive phenotype [50]. Interestingly, the same construct could display the opposite phenotype based on the host’s genetic background [50,53], or when the host T cells were depleted, which parallels the increased HPV disease and cancers in organ transplant patients [57]. The contribution of the host immune control of papillomavirus infections has benefited from studies on tumors that regressed [97,98,99]. Tumor regression correlated with infiltration of the CD4 and CD8 T cells that target early genes, such as E2 and E6 [100,101,102,103,104,105,106,107,108]. The CRPV model will be a useful tool to gain deeper understanding of the roles of infiltrating cells in the regression, using novel technology such as single-cell omics.
The constructs with hybrid E6 between the progressive CRPV E6 and E6 of the rabbit oral papillomavirus (ROPV), a mucosotropic papillomavirus, have been used to further understand tissue tropism and the underlying mechanisms [36,96,109,110,111]. Interestingly, all of these latter hybrid constructs failed to promote tumor growth in the skin sites of rabbits [27]. Despite this lack of viability in vivo, some of these hybrid E6 constructs show oncogenicity in vitro [109], suggesting that in vivo failure may be related to the tissue specificity of CRPV versus ROPV. Further studies will be needed to better understand the role of E6 in the pathogenesis and regression of different tissues.
3. Genetic Analyses of Changes during CRPV Infections
By taking advantage of recent genome-wide transcriptome analyses, we have begun to gain a deeper understanding of the changes occurring at the molecular level during infection [8,82,90]. Unbiased whole-genome RNA-seq analysis has been utilized and host gene transcript profiling of tumor tissues has been reported in our recent study [82].
3.1. Host Changes during Viral Infection
Immune cell infiltration is correlated with CRPV-induced tumor regression, as demonstrated in other PV models and HPV [41,103,104,105,106,107,108]. The changes at the transcription level of the host genes that were identified and correlated with CRPV infection have been reported [82,90,112,113], including common signal transduction pathways/ molecules in HPV specimens. Using two representative host genes that play critical roles in DNA repair (Apobec2) and inflammation (IL36r) that were identified in the wild-type CRPV-infected tissues as examples [82], we also observed a similar expression pattern in persistent and benign tumors induced by a CRPVE8ATGko (E8m) mutant genome suggesting that both the wild type and E8 mutant interfere with these pathways [33,58]. As we have a large selection of mutant CRPV genomes with different phenotypes that can be used for comparative studies, we may determine whether the differences we observed in some of the genes/pathways among different mutant genome--induced lesions are predictive for tumor growth and disease trajectory. These can be measured by in vitro and in vivo T cell function assays [51], neutralization assays, ELISA, Western blot, immuno-precipitation, and cytokine profiling assays [8,24,82].
3.2. In Situ Analysis of Tissues at Different Disease Stages
To study the virus-induced expression changes in host genes during disease progression, a panel of in situ assays have been developed over the years by different groups [3,24,25,36,56,70,71,92,114]. Newly improved assays, including the in situ hybridization for detection of CRPV DNA in CRPV-infected tumor tissues and improved RNA-ISH analysis to detect CRPV E4 transcripts have been applied to recent studies [82]. To validate host gene expression in the infected tissues, we have identified a panel of cancer-related genes that are upregulated in advanced CRPV lesions (Table 2, based on secondary analyses of our published RNAseq dataset) [24,25,82]. These include the biomarkers PCNA, Cyclin E, and MCM7 [25] which are also upregulated in HPV-associated cancer tissues (Table 2) [82,115,116,117,118,119,120,121,122]. A good example is pro-inflammatory molecule calcium-binding protein A9 or S100A9 which is highly dysregulated in both CRPV-infected tissues [82] and HPV-associated cancers [123,124,125,126]. These striking parallels between the rabbit model and the HPV cancers further enhance the value of this preclinical model for new targeted therapies. To facilitate study in different immune cell populations, we have also developed antibodies to rabbit T cell surface markers including a CD4 T cell antibody that has been used successfully for in vivo depletion studies [25,127]. These antibodies are useful for the validation of our earlier observations that fewer T cells (CD4 and CD8) infiltrate in tumors relative to those undergoing regression [41].
Table 2.
Representative molecules related to cancers and T cell functions that are significantly changed in both the CRPV-induced tumor tissues AND cervical cancer.
| Genes | Changes in CRPV-Infected Tumors | Pathways |
|---|---|---|
| Krt1, 2, 3, 4, 7, 10, 14, 16, 78; Krt13, 75 | UP/Down | Cytokeratin |
| KLF3, 10; KLF 1, 9, 11, 15 | UP/Down | Keratinocyte proliferation |
| BRCA1, BRCA2, FANCD2, PCNA; DDR2 | UP/Down | DNA damage |
| MAPK6, 13; MAPK12 | UP/Down | p38 MAPKs |
| PCNA, CDK2, CASP8, ERBB3, PDCD5,6; TGFBR2, PDCD4 | UP/Down | Cell growth and death |
| TP53I3, CDKN2A | UP | Tumor suppressor |
| CTLA-4, RNF149, Cblb, Rel, PD-L1; Gata3, NFATC1, 4, CD34, NR4A1, Foxp1, CD8b | UP/Down | T cell function |
| CXCL8, IFNgR1, STAT4; Cox-2, CX3CL1 | UP/Down | Cytokines, chemokines, and ligands |
| IL1A, IL4R, IL10RA, IL13, IL17F, IL23A, IL36A, IL36g; IL6R, 11RA, IL13, IL16 | UP/Down | Interleukins |
4. Rabbits for Studying Viral–Host Interactions during Tumor Progression
Rabbits have been used for studying a number of human diseases, including papillomavirus infections [128]. The host genetic background, including HLA class II alleles, plays an important role in HPV-associated disease progression and cancer development [129,130,131,132,133]. Similarly, rabbit MHCII has been linked to CRPV-induced tumor regression [53,54]. In agreement with these findings, we and others have demonstrated, using a variety of rabbit strains, that the host genetic constitution plays a role in disease outcome of CRPV infections [7,8,33,50,54,65,134]. Different responses to the same CRPV genomic construct have been reported in outbred and inbred rabbits in our studies [33,50]. We generated transgenic rabbits, including EJ-ras and HLA-A2.1 rabbits, to facilitate determination of the role of host oncogene and immune responses in the CRPV infection [33,46,50,134]. In recent years, novel gene modification technologies, especially CRISPR editing, have enabled rapid production of gene-modified rabbits [135,136,137].
4.1. Inbred and Outbred Rabbits
Most studies have used outbred rabbits that are supplied by several vendors including Charles River, Robinson, and Covance [3,50,61]. During the early years of our studies, we used rabbits from each of these suppliers [36,39,110,138]. While rabbits from different suppliers are all susceptible to CRPV infections, we did observe different natural regression rates following infections [39]. To maintain consistency from study to study, we have used the same supplier for most of our studies in the past two decades [8]. The inbred rabbit strain (EIII/JC) has been maintained in our facility for over thirty years and was originally acquired from NIH [33,50]. These inbred rabbits appear to be normal except for a heightened sensitivity to noise. These rabbits showed higher regression rates after CRPV infection [33,49]. In our previous studies, we have tested our CRPV mutant constructs on both outbred and inbred rabbits; the results are summarized in Table 3 [50]. It would be interesting to compare the host gene expression profiles after CRPV infections in these different rabbit strains.
Table 3.
Rabbit strains used in our studies.
| Rabbit Strain | Phenotype after Infection | References |
|---|---|---|
| Outbred | Persistent and cancer (wild-type CRPV) Regressive (regressive CRPV) |
Hu et al., 2002, 2005, 2009; Cladel et al., 2009, 2013, 2019 |
| EIII/JC inbred | Higher regression rate for wild-type CRPV | Hu et al., 2002, 2005, 2006, 2007, 2009 |
| HLA-A2.1 outbred | Persistent and cancer (wild-type CRPV) with higher regression rates Regressive (regressive CRPV) |
Hu et al., 2006, 2007, Bounds et al., 2009, Cladel et al., 2019 |
4.2. Transgenic Rabbits
To understand the pathogenesis and tissue-tropism of CRPV in rabbits, we generated a CRPV/EJ-ras transgenic rabbit strain [48]. We observed that the tissue specificity of CRPV DNA expression in these rabbits was the same as in the virion-infected wild-type animals. It appears that the strict tissue-tropism of CRPV is controlled by the URR of the CRPV genome [46,47,48,139].
To facilitate vaccine development for HPV-associated diseases and cancers, we also developed an HLA-A2.1 transgenic rabbit model to test HPV vaccines in the context of a human MHCI background (HLA-A2.1) [134,140]. We have tested the immunogenicity of several known and unknown HLA-A2.1 restricted epitopes delivered by either DNA or peptides and have demonstrated both the prophylactic and therapeutic effects of these candidates [35,51,134,141]. The HLA-A2.1 transgenic rabbit model will continue contributing to future studies that lead to novel prophylactic and therapeutic strategies against HPV-associated diseases and cancers.
4.3. Novel Genetically Modified Rabbits
The recent development of gene-editing technologies has brought new tools to the development of animal models, especially for species for which germ-line embryonic stem cells (ESCs) are not available [55]. The attempts to produce gene-targeted rabbits date back two decades, after Chesne et al. reported the successful cloning of rabbits by somatic cell nuclear transfer [142]. The idea was to generate targeted mutations, for example, a gene knockout in the somatic cells (bypassing the need for ESCs), and to use these cells for animal cloning. Knockout pigs and cows had been produced via this strategy [143]. Unfortunately, despite large numbers of embryo transfers, no gene-targeted rabbits were cloned and produced using this approach.
The first gene knockout rabbit was produced shortly after zinc finger nuclease (ZFN) was introduced to researchers [144]. This first-generation gene editing nuclease (GEN) was quickly replaced by TALEN and then CRISPR/Cas9. To date, CRISPR/Cas9 represents the most commonly used GEN in the production of rabbit models [55]. Our group reported the first success in producing gene knockout rabbits by CRISPR/Cas9 in 2014 [135]. More than ten animal lines were efficiently produced, highlighting the power of CRISPR/Cas9 in the gene editing of rabbits. Later, in 2016, we reported that the efficiency of gene knock-in in rabbits by Cas9 or TALEN can be improved two–five-fold when a small molecular compound RS-1 is used [145].
In 2017, we reported the production of multiple lines of immunodeficient rabbits [138]. The targeted knockout genes include Foxn1, Il2rg, and Rag2. Foxn1 is essential for thymus and hair follicle epithelial cell development. The knockout of Foxn1 leads to the hairless “nude” phenotype and an impaired T cell development, as shown in athymic nude mice [146]. Il2rg is a gene that codes for the common gamma chain (γc), which is a cytokine receptor sub-unit that is common to the receptor complexes for different interleukin receptors. These include IL-2, IL-4, IL-7, IL-9, and IL-15. The loss-of-function mutation of Il2rg leads to defective B and T cell development and subsequently to severe combined immunodeficiency (SCID) disease [147]. Rag2 is involved in the V(D)J recombination process for B and T cells and is essential for the generation of mature B and T lymphocytes. Individuals with defective Rag2 therefore also often suffer from SCID. These immunodeficient rabbit lines will be useful for delineating the contributions of the B and T cells in viral pathogenesis, and for developing therapeutic strategies in the CRPV rabbit model.
5. Summary and Conclusions
HPV infection causes approximately 5% of human cancers and 30% of all cancers caused by infectious agents [148]. Most of the HPV infections (>90%) are cleared within two years because the host immune system is effective in eliminating HPV infections in most situations [148]. The CRPV rabbit model has provided opportunities to study the fine balance between viral oncogenicity and immunogenicity in deciding disease outcomes over the past several decades [1,3,8,149,150]. In addition to the key role of adaptive immune responses, we and others have also demonstrated that innate immune modulators, such as select cytokines, play a role in viral persistence and tumor progression [34,141]. However, an in-depth understanding of viral pathogenesis in the rabbit model has been delayed due to the slow advancement in whole genome sequencing and annotation of the rabbit genome [8]. Only recently were we able to obtain the genome-wide transcriptome profile of CRPV-infected lesions [83]. These datasets identified many parallel changes in different signal transduction pathways/genes that have been reported in HPV studies [150], which further confirmed the applicability of the CRPV rabbit model to HPV pathogenesis. The noticeable limitation for most human studies is that they have focused on HPV disease at a single time point assuring that the dynamics of tumor progression are difficult to follow [151]. This limitation can be overcome by using the rabbit model with predictable disease outcomes within a reasonable time frame. We can monitor dynamic changes at different disease stages and determine how the balance of oncogenicity and immunogenicity is associated with cancer development. Equipped with the availability of novel gene-modified rabbit lines, we expect to conduct more mechanistic studies leading to significant contributions to the deeper understanding of HPV pathogenesis.
The CRPV model continues to hold great promise for mechanistic studies of papillomavirus-associated disease progression or regression, especially with recent technological advances such as single-cell omics which provide unprecedented opportunities to analyze the complexities of biological systems at the single cell level. Novel hypotheses, including the dynamic changes in the balance of oncogenicity and immunogenicity during cancer development can be tested in this model system in future studies based on newly acquired knowledge as well as unique resources and reagents that will continue to be established for rabbit researchers.
Acknowledgments
We acknowledge our colleagues Lynn Budgeon, Jingwei Li, Karla Balogh, Vonn Walter, Sarah Brendle, Debra Shearer, Yuka Imamura Kawasawa, Anna Salzberg, Timothy Cooper, Ricai Han, Timothy Culp, Ann Benko, Syndi Reed, and Callie Bounds who have contributed to our rabbit projects over the past two decades.
Author Contributions
Conceptualization, N.M.C., J.X., N.D.C. and J.H.; writing—original draft preparation, N.M.C., J.X. and J.H.; writing—review and editing, N.M.C., J.X., X.P., P.J., N.D.C., Z.-M.Z. and J.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors declare no conflict of interest.
Funding Statement
This work was supported by the National Cancer Institute under Award Number R01 CA47622 (Christensen), NIH contract HHSN272201000020I (Christensen), the Jake Gittlen Memorial Golf Tournament, and the Department of Pathology and Laboratory Medicine, Penn State College of Medicine.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roden R.B.S., Stern P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer. 2018;18:240–254. doi: 10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doorbar J. Host control of human papillomavirus infection and disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;47:27–41. doi: 10.1016/j.bpobgyn.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Christensen N.D., Budgeon L.R., Cladel N.M., Hu J. Recent advances in preclinical model systems for papillomaviruses. Virus Res. 2017;231:108–118. doi: 10.1016/j.virusres.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shope R.E., Hurst E.W. Infectious papillomatosis of rabbits; with a note on the histopathology. J. Exp. Med. 1933;58:607–624. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escudero Duch C., Williams R.A., Timm R.M., Perez-Tris J., Benitez L. A Century of Shope Papillomavirus in Museum Rabbit Specimens. PLoS ONE. 2015;10:e0132172. doi: 10.1371/journal.pone.0132172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandsma J.L. The cottontail rabbit papillomavirus model of high-risk HPV-induced disease. Methods Mol. Med. 2005;119:217–235. doi: 10.1385/1-59259-982-6:217. [DOI] [PubMed] [Google Scholar]
- 7.Breitburd F., Salmon J., Orth G. The rabbit viral skin papillomas and carcinomas: A model for the immunogenetics of HPV-associated carcinogenesis. Clin. Dermatol. 1997;15:237–247. doi: 10.1016/S0738-081X(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 8.Cladel N.M., Peng X., Christensen N., Hu J. The rabbit papillomavirus model: A valuable tool to study viral-host interactions. Philos. Trans. R. Soc. B. 2019;374:20180294. doi: 10.1098/rstb.2018.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen N.D., Pickel M.D., Budgeon L.R., Kreider J.W. In vivo anti-papillomavirus activity of nucleoside analogues including cidofovir on CRPV-induced rabbit papillomas. Antivir. Res. 2000;48:131–142. doi: 10.1016/S0166-3542(00)00124-8. [DOI] [PubMed] [Google Scholar]
- 10.Morse M.A., Balogh K.K., Brendle S.A., Campbell C.A., Chen M.X., Furze R.C., Harada I.L., Holyer I.D., Kumar U., Lee K., et al. BET bromodomain inhibitors show anti-papillomavirus activity in vitro and block CRPV wart growth in vivo. Antivir. Res. 2018;154:158–165. doi: 10.1016/j.antiviral.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolhassani A., Mohit E., Rafati S. Different spectra of therapeutic vaccine development against HPV infections. Hum. Vaccin. 2009;5:671–689. doi: 10.4161/hv.5.10.9370. [DOI] [PubMed] [Google Scholar]
- 12.Fausch S.C., Da Silva D.M., Eiben G.L., Le Poole I.C., Kast W.M. HPV protein/peptide vaccines: From animal models to clinical trials. Front. Biosci. 2003;8:s81–s91. doi: 10.2741/1009. [DOI] [PubMed] [Google Scholar]
- 13.Amella C.A., Lofgren L.A., Ronn A.M., Nouri M., Shikowitz M.J., Steinberg B.M. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency. Am. J. Pathol. 1994;144:1167–1171. [PMC free article] [PubMed] [Google Scholar]
- 14.Duan J., Paris W., De Marte J., Roopchand D., Fleet T., Cordingley M.G. Topical effects of cidofovir on cutaneous rabbit warts: Treatment regimen and inoculum dependence. Antivir. Res. 2000;46:135–144. doi: 10.1016/S0166-3542(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 15.Christensen N.D., Cladel N.M., Hu J., Balogh K.K. Formulation of cidofovir improves the anti-papillomaviral activity of topical treatments in the CRPV/rabbit model. Antivir. Res. 2014;108:148–155. doi: 10.1016/j.antiviral.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breitburd F., Kirnbauer R., Hubbert N.L., Nonnenmacher B., Trin-Dinh-Desmarquet C., Orth G., Schiller J.T., Lowy D.R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen N.D., Reed C.A., Cladel N.M., Han R., Kreider J.W. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 1996;70:960–965. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giri I., Danos O., Yaniv M. Genomic structure of the cottontail rabbit (Shope) papillomavirus. Proc. Natl. Acad. Sci. USA. 1985;82:1580–1584. doi: 10.1073/pnas.82.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandsma J.L., Xiao W. Some human papillomavirus (HPV) E7 DNA sequences can replace wild-type E7 sequences in cottontail rabbit papillomavirus (CRPV) to induce papillomas in rabbits; Proceedings of the 14th International Papillomavirus Conference; Quebec City, QC, Canada. 23–28 July 1995; p. 92. Abstract book. Abstract book. [Google Scholar]
- 20.Brandsma J.L., Yang Z.-H., Barthold S.W., Johnson E.A. Use of a rapid, efficient inoculation method to induce papillomas by cottontail rabbit papillomavirus DNA shows that the E7 gene is required. Proc. Natl. Acad. Sci. USA. 1991;88:4816–4820. doi: 10.1073/pnas.88.11.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X., Xiao W., Brandsma J. Papilloma formation by cottontail rabbit papillomavirus requires E1 and E2 regulatory genes in addition to E6 and E7 transforming genes. J. Virol. 1994;68:6097–6102. doi: 10.1128/jvi.68.9.6097-6102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeckel S., Loetzsch E., Huber E., Stubenrauch F., Iftner T. Identification of the E9/E2C cDNA and functional characterization of the gene product reveal a new repressor of transcription and replication in cottontail rabbit papillomavirus. J. Virol. 2003;77:8736–8744. doi: 10.1128/JVI.77.16.8736-8744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeckel S., Huber E., Stubenrauch F., Iftner T. A transactivator function of cottontail rabbit papillomavirus e2 is essential for tumor induction in rabbits. J. Virol. 2002;76:11209–11215. doi: 10.1128/JVI.76.22.11209-11215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Probst S., Notz E., Wolff M., Buehlmann J., Stubenrauch F., Iftner T. A recombinant cottontail rabbit papillomavirus genome for ectopic expression of genes in cells infected with virus in vivo. J. Virol. Methods. 2013;187:110–113. doi: 10.1016/j.jviromet.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Cladel N.M., Hu J., Balogh K.K., Christensen N.D. CRPV genomes with synonymous codon optimizations in the CRPV E7 gene show phenotypic differences in growth and altered immunity upon E7 vaccination. PLoS ONE. 2008;3:e2947. doi: 10.1371/journal.pone.0002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu J., Cladel N.M., Budgeon L., Balogh K.K., Christensen N.D. Papillomavirus DNA complementation in vivo. Virus Res. 2009;144:117–122. doi: 10.1016/j.virusres.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J., Cladel N.M., Balogh K., Budgeon L., Christensen N.D. Impact of genetic changes to the CRPV genome and their application to the study of pathogenesis in vivo. Virology. 2007;358:384–390. doi: 10.1016/j.virol.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egawa N., Egawa K., Griffin H., Doorbar J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses. 2015;7:3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandsma J.L., Shylankevich M., Su Y., Roberts A., Rose J.K., Zelterman D., Buonocore L. Vesicular stomatitis virus-based therapeutic vaccination targeted to the E1, E2, E6, and E7 proteins of cottontail rabbit papillomavirus. J. Virol. 2007;81:5749–5758. doi: 10.1128/JVI.02835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han R., Reed C.A., Cladel N.M., Christensen N.D. Immunization of rabbits with cottontail rabbit papillomavirus E1 and E2 genes: Protective immunity induced by gene gun-mediated intracutaneous delivery but not by intramuscular injection. Vaccine. 2000;18:2937–2944. doi: 10.1016/S0264-410X(00)00110-9. [DOI] [PubMed] [Google Scholar]
- 31.Schneider M., Yigitliler A., Stubenrauch F., Iftner T. Cottontail Rabbit Papillomavirus E1 and E2 Proteins Mutually Influence Their Subcellular Localizations. J. Virol. 2018;92:E00704–E00718. doi: 10.1128/JVI.00704-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen N.D., Han R., Cladel N.M., Pickel M.D. Combination treatment with intralesional cidofovir and viral-DNA vaccination cures large cottontail rabbit papillomavirus-induced papillomas and reduces recurrences. Antimicrob. Agents Chemother. 2001;45:1201–1209. doi: 10.1128/AAC.45.4.1201-1209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J., Han R., Cladel N.M., Pickel M.D., Christensen N.D. Intracutaneous DNA vaccination with the E8 gene of cottontail rabbit papillomavirus induces protective immunity against virus challenge in rabbits. J. Virol. 2002;76:6453–6459. doi: 10.1128/JVI.76.13.6453-6459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J., Cladel N.M., Wang Z., Han R., Pickel M.D., Christensen N.D. GM-CSF enhances protective immunity to cottontail rabbit papillomavirus E8 genetic vaccination in rabbits. Vaccine. 2004;22:1124–1130. doi: 10.1016/j.vaccine.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 35.Hu J., Cladel N.M., Budgeon L.R., Reed C.A., Pickel M.D., Christensen N.D. Protective cell-mediated immunity by DNA vaccination against Papillomavirus L1 capsid protein in the Cottontail Rabbit Papillomavirus model. Viral Immunol. 2006;19:492–507. doi: 10.1089/vim.2006.19.492. [DOI] [PubMed] [Google Scholar]
- 36.Embers M.E., Budgeon L.R., Pickel M., Christensen N.D. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of l2, the minor capsid protein. J. Virol. 2002;76:9798–9805. doi: 10.1128/JVI.76.19.9798-9805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer K.E., Benko A., Doucette S.A., Cameron T.I., Foster T., Hanley K.M., McCormick A.A., McCulloch M., Pogue G.P., Smith M.L., et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine. 2006;24:5516–5525. doi: 10.1016/j.vaccine.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y.L., Borenstein L.A., Selvakumar R., Ahmed R., Wettstein F.O. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992;187:612–619. doi: 10.1016/0042-6822(92)90463-Y. [DOI] [PubMed] [Google Scholar]
- 39.Kalnin K., Tibbitts T., Yan Y., Stegalkina S., Shen L., Costa V., Sabharwal R., Anderson S.F., Day P.M., Christensen N., et al. Low doses of flagellin-L2 multimer vaccines protect against challenge with diverse papillomavirus genotypes. Vaccine. 2014;32:3540–3547. doi: 10.1016/j.vaccine.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vambutas A., DeVoti J., Nouri M., Drijfhout J.W., Lipford G.B., Bonagura V.R., Van der Burg S.H., Melief C.J. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine. 2005;23:5271–5280. doi: 10.1016/j.vaccine.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 41.Hu J., Budgeon L.R., Balogh K.K., Peng X., Cladel N.M., Christensen N.D. Long-peptide therapeutic vaccination against CRPV-induced papillomas in HLA-A2.1 transgenic rabbits. Trials Vaccinol. 2014;3:134–142. doi: 10.1016/j.trivac.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mejia A.F., Culp T.D., Cladel N.M., Balogh K.K., Budgeon L.R., Buck C.B., Christensen N.D. Preclinical Model To Test Human Papillomavirus Virus (HPV) Capsid Vaccines In Vivo Using Infectious HPV/Cottontail Rabbit Papillomavirus Chimeric Papillomavirus Particles. J. Virol. 2006;80:12393–12397. doi: 10.1128/JVI.01583-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gambhira R., Jagu S., Karanam B., Gravitt P.E., Culp T.D., Christensen N.D., Roden R.B. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J. Virol. 2007;81:11585–11592. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jagu S., Karanam B., Wang J.W., Zayed H., Weghofer M., Brendle S.A., Balogh K.K., Tossi K.P., Roden R.B., Christensen N.D. Durable immunity to oncogenic human papillomaviruses elicited by adjuvanted recombinant Adeno-associated virus-like particle immunogen displaying L2 17–36 epitopes. Vaccine. 2015;33:5553–5563. doi: 10.1016/j.vaccine.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olczak P., Matsui K., Wong M., Alvarez J., Lambert P., Christensen N.D., Hu J., Huber B., Kirnbauer R., Wang J.W., et al. RG2-VLP: A Vaccine Designed to Broadly Protect against Anogenital and Skin Human Papillomaviruses Causing Human Cancer. J. Virol. 2022;96:e00566-22. doi: 10.1128/jvi.00566-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng X., Griffith J.W., Han R., Lang C.M., Kreider J.W. Development of keratoacanthomas and squamous cell carcinomas in transgenic rabbits with targeted expression of EJras oncogene in epidermis. Am. J. Pathol. 1999;155:315–324. doi: 10.1016/S0002-9440(10)65125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng X., Lang C.M., Kreider J.W. Immortalization of inbred rabbit keratinocytes from a Shope papilloma and tumorigenic transformation of the cells by EJ-ras. Cancer Lett. 1996;108:101–109. doi: 10.1016/S0304-3835(96)04415-1. [DOI] [PubMed] [Google Scholar]
- 48.Peng X., Olson R.O., Christian C.B., Lang C.M., Kreider J.W. Papillomas and carcinomas in transgenic rabbits carrying EJ-ras DNA and cottontail rabbit papillomavirus DNA. J. Virol. 1993;67:1698–1701. doi: 10.1128/jvi.67.3.1698-1701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu J., Cladel N.M., Christensen N.D. Increased immunity to cottontail rabbit papillomavirus infection in EIII/JC inbred rabbits after vaccination with a mutant E6 that correlates with spontaneous regression. Viral Immunol. 2007;20:320–325. doi: 10.1089/vim.2006.0104. [DOI] [PubMed] [Google Scholar]
- 50.Hu J., Cladel N.M., Pickel M.D., Christensen N.D. Amino acid residues in the carboxy-terminal region of cottontail rabbit papillomavirus E6 influence spontaneous regression of cutaneous papillomas. J. Virol. 2002;76:11801–11808. doi: 10.1128/JVI.76.23.11801-11808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu J., Peng X., Schell T.D., Budgeon L.R., Cladel N.M., Christensen N.D. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J. Immunol. 2006;177:8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 52.Hu J., Schell T.D., Peng X., Cladel N.M., Balogh K.K., Christensen N.D. Using HLA-A2.1 Transgenic Rabbit Model to Screen and Characterize New HLA-A2.1 Restricted Epitope DNA Vaccines. J. Vaccines Vaccin. 2010;1 doi: 10.4172/2157-7560.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salmon J., Nonnenmacher M., Caze S., Flamant P., Croissant O., Orth G., Breitburd F. Variation in the nucleotide sequence of cottontail rabbit papillomavirus a and b subtypes affects wart regression and malignant transformation and level of viral replication in domestic rabbits. J. Virol. 2000;74:10766–10777. doi: 10.1128/JVI.74.22.10766-10777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han R., Breitburd F., Marche P.N., Orth G. Linkage of Regression and Malignant Conversion of Rabbit Viral Papillomas to MHC Class II Genes. Nature. 1992;356:66–68. doi: 10.1038/356066a0. [DOI] [PubMed] [Google Scholar]
- 55.Xu J., Zhang J., Yang D., Song J., Pallas B., Zhang C., Hu J., Peng X., Christensen N.D., Han R., et al. Gene Editing in Rabbits: Unique Opportunities for Translational Biomedical Research. Front. Genet. 2021;12:642444. doi: 10.3389/fgene.2021.642444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu J., Budgeon L.R., Cladel N.M., Culp T.D., Balogh K.K., Christensen N.D. Detection of L1, infectious virions and anti-L1 antibody in domestic rabbits infected with cottontail rabbit papillomavirus. J. Gen. Virol. 2007;88:3286–3293. doi: 10.1099/vir.0.82879-0. [DOI] [PubMed] [Google Scholar]
- 57.Han R., Cladel N.M., Reed C.A., Christensen N.D. Characterization of transformation function of cottontail rabbit papillomavirus E5 and E8 genes. Virology. 1998;251:253–263. doi: 10.1006/viro.1998.9416. [DOI] [PubMed] [Google Scholar]
- 58.Nonnenmacher M., Salmon J., Jacob Y., Orth G., Breitburd F. Cottontail rabbit papillomavirus E8 protein is essential for wart formation and provides new insights into viral pathogenesis. J. Virol. 2006;80:4890–4900. doi: 10.1128/JVI.80.10.4890-4900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du M., Fan X., Hanada T., Gao H., Lutchman M., Brandsma J.L., Chishti A.H., Chen J.J. Association of cottontail rabbit papillomavirus E6 oncoproteins with the hDlg/SAP97 tumor suppressor. J. Cell. Biochem. 2005;94:1038–1045. doi: 10.1002/jcb.20383. [DOI] [PubMed] [Google Scholar]
- 60.Meyers C., Harry J., Lin Y.-L., Wettstein F.O. Identification of three transforming proteins encoded by cottontail rabbit papillomavirus. J. Virol. 1992;66:1655–1664. doi: 10.1128/jvi.66.3.1655-1664.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu J., Peng X., Cladel N.M., Pickel M.D., Christensen N.D. Large cutaneous rabbit papillomas that persist during cyclosporin A treatment can regress spontaneously after cessation of immunosuppression. J. Gen. Virol. 2005;86:55–63. doi: 10.1099/vir.0.80448-0. [DOI] [PubMed] [Google Scholar]
- 62.Nasseri M., Meyers C., Wettstein F.O. Genetic analysis of CRPV pathogenesis: The L1 open reading frame is dispensable for cellular transformation but is required for papilloma formation. Virology. 1989;170:321–325. doi: 10.1016/0042-6822(89)90388-7. [DOI] [PubMed] [Google Scholar]
- 63.Defeo-Jones D., Vuocolo G.A., Haskell K.M., Hanobik M.G., Kiefer D.M., McAvoy E.M., Ivey-Hoyle M., Brandsma J.L., Oliff A., Jones R.E. Papillomavirus E7 protein binding to the retinoblastoma protein is not required for viral induction of warts. J. Virol. 1993;67:716–725. doi: 10.1128/jvi.67.2.716-725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cladel N.M., Hu J., Balogh K.K., Christensen N.D. Differences in methodology, but not differences in viral strain, account for variable experimental outcomes in laboratories utilizing the cottontail rabbit papillomavirus model. J. Virol. Methods. 2010;165:36–41. doi: 10.1016/j.jviromet.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salmon J., Ramoz N., Cassonnet P., Orth G., Breitburd F. A cottontail rabbit papillomavirus strain (CRPVb) with strikingly divergent E6 and E7 oncoproteins: An insight in the evolution of papillomaviruses. Virology. 1997;235:228–234. doi: 10.1006/viro.1997.8680. [DOI] [PubMed] [Google Scholar]
- 66.Reuter J.D., Gomez D., Brandsma J.L., Rose J.K., Roberts A. Optimization of cottontail rabbit papilloma virus challenge technique. J. Virol. Methods. 2001;98:127–134. doi: 10.1016/S0166-0934(01)00370-6. [DOI] [PubMed] [Google Scholar]
- 67.Kreider J.W., Cladel N.M., Patrick S.D., Welsh P.A., DiAngelo S.L., Bower J.M., Christensen N.D. High efficiency induction of papillomas in vivo using recombinant cottontail rabbit papillomavirus DNA. J. Virol. Methods. 1995;55:233–244. doi: 10.1016/0166-0934(95)00062-Y. [DOI] [PubMed] [Google Scholar]
- 68.Brandsma J.L., Xiao W. Infectious virus replication in papillomas induced by molecularly cloned cottontail rabbit papillomavirus DNA. J. Virol. 1993;67:567–571. doi: 10.1128/jvi.67.1.567-571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cladel N.M., Hu J., Balogh K., Mejia A., Christensen N.D. Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection. J. Virol. Methods. 2008;148:34–39. doi: 10.1016/j.jviromet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peh W.L., Middleton K., Christensen N., Nicholls P., Egawa K., Sotlar K., Brandsma J., Percival A., Lewis J., Liu W.J., et al. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J. Virol. 2002;76:10401–10416. doi: 10.1128/JVI.76.20.10401-10416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peh W.L., Brandsma J.L., Christensen N.D., Cladel N.M., Wu X., Doorbar J. The viral E4 protein is required for the completion of the cottontail rabbit papillomavirus productive cycle in vivo. J. Virol. 2004;78:2142–2151. doi: 10.1128/JVI.78.4.2142-2151.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cladel N.M., Budgeon L.R., Hu J., Balogh K.K., Christensen N.D. Synonymous codon changes in the oncogenes of the cottontail rabbit papillomavirus lead to increased oncogenicity and immunogenicity of the virus. Virology. 2013;438:70–83. doi: 10.1016/j.virol.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abd El-Aleem S.A., Abdelwahab S., Am-Sherief H., Sayed A. Cellular and physiological upregulation of inducible nitric oxide synthase, arginase, and inducible cyclooxygenase in wound healing. J. Cell. Physiol. 2019;234:23618–23632. doi: 10.1002/jcp.28930. [DOI] [PubMed] [Google Scholar]
- 74.Theilgaard-Monch K., Knudsen S., Follin P., Borregaard N. The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J. Immunol. 2004;172:7684–7693. doi: 10.4049/jimmunol.172.12.7684. [DOI] [PubMed] [Google Scholar]
- 75.Sistigu A., Di Modugno F., Manic G., Nistico P. Deciphering the loop of epithelial-mesenchymal transition, inflammatory cytokines and cancer immunoediting. Cytokine Growth Factor Rev. 2017;36:67–77. doi: 10.1016/j.cytogfr.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 76.Woodworth C.D. HPV innate immunity. Front. Biosci. 2002;7:d2058–d2071. doi: 10.2741/woodworth. [DOI] [PubMed] [Google Scholar]
- 77.Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Yang T.H., Porta-Pardo E., Gao G.F., Plaisier C.L., Eddy J.A., et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830.e14. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cerqueira C., Samperio Ventayol P., Vogeley C., Schelhaas M. Kallikrein-8 Proteolytically Processes Human Papillomaviruses in the Extracellular Space To Facilitate Entry into Host Cells. J. Virol. 2015;89:7038–7052. doi: 10.1128/JVI.00234-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hung V.C., Lee J.Y., Zitelli J.A., Hebda P.A. Topical tretinoin and epithelial wound healing. Arch. Dermatol. 1989;125:65–69. doi: 10.1001/archderm.1989.01670130067008. [DOI] [PubMed] [Google Scholar]
- 80.Szondi D.C., Wong J.K., Vardy L.A., Cruickshank S.M. Arginase Signalling as a Key Player in Chronic Wound Pathophysiology and Healing. Front. Mol. Biosci. 2021;8:773866. doi: 10.3389/fmolb.2021.773866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crompton R.A., Williams H., Campbell L., Hui Kheng L., Saville C., Ansell D.M., Reid A., Wong J., Vardy L.A., Hardman M.J., et al. An Epidermal-Specific Role for Arginase1 during Cutaneous Wound Repair. J. Investig. Dermatol. 2022;142:1206–1216.e8. doi: 10.1016/j.jid.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 82.Cladel N.M., Jiang P., Li J.J., Peng X., Cooper T.K., Majerciak V., Balogh K.K., Meyer T.J., Brendle S.A., Budgeon L.R., et al. Papillomavirus can be transmitted through the blood and produce infections in blood recipients: Evidence from two animal models. Emerg. Microbes. Infect. 2019;8:1108–1121. doi: 10.1080/22221751.2019.1637072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bodaghi S., Wood L.V., Roby G., Ryder C., Steinberg S.M., Zheng Z.M. Could human papillomaviruses be spread through blood? J. Clin. Microbiol. 2005;43:5428–5434. doi: 10.1128/JCM.43.11.5428-5434.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bounds C.E., Hu J., Cladel N.M., Balogh K., Christensen N.D. Vaccine generated immunity targets an HPV16 E7 HLA-A2.1-restricted CD8(+) T cell epitope relocated to an early gene or a late gene of the cottontail rabbit papillomavirus (CRPV) genome in HLA-A2.1 transgenic rabbits. Vaccine. 2011;29:1194–1200. doi: 10.1016/j.vaccine.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leder C., Kleinschmidt J.A., Wiethe C., Muller M. Enhancement of capsid gene expression: Preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. J. Virol. 2001;75:9201–9209. doi: 10.1128/JVI.75.19.9201-9209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu W.J., Gao F., Zhao K.N., Zhao W., Fernando G.J., Thomas R., Frazer I.H. Codon modified human papillomavirus type 16 E7 DNA vaccine enhances cytotoxic T-lymphocyte induction and anti-tumour activity. Virology. 2002;301:43–52. doi: 10.1006/viro.2002.1584. [DOI] [PubMed] [Google Scholar]
- 87.Schaeffer A.J., Nguyen M., Liem A., Lee D., Montagna C., Lambert P.F., Ried T., Difilippantonio M.J. E6 and E7 oncoproteins induce distinct patterns of chromosomal aneuploidy in skin tumors from transgenic mice. Cancer Res. 2004;64:538–546. doi: 10.1158/0008-5472.CAN-03-0124. [DOI] [PubMed] [Google Scholar]
- 88.Duensing S., Duensing A., Crum C.P., Munger K. Human papillomavirus type 16 E7 oncoprotein-induced abnormal centrosome synthesis is an early event in the evolving malignant phenotype. Cancer Res. 2001;61:2356–2360. [PubMed] [Google Scholar]
- 89.Nasseri M., Wettstein F.O. Differences exist between viral transcripts in cottontail rabbit papillomavirus-induced benign and malignant tumors as well as non-virus-producing and virus-producing tumors. J. Virol. 1984;51:706–712. doi: 10.1128/jvi.51.3.706-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huber E., Vlasny D., Jeckel S., Stubenrauch F., Iftner T. Gene profiling of cottontail rabbit papillomavirus-induced carcinomas identifies upregulated genes directly Involved in stroma invasion as shown by small interfering RNA-mediated gene silencing. J. Virol. 2004;78:7478–7489. doi: 10.1128/JVI.78.14.7478-7489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Probst-Hunczek S., Jager G., Schneider M., Notz E., Stubenrauch F., Iftner T. RNA sequencing analysis identifies novel spliced transcripts but does not indicate quantitative or qualitative changes of viral transcripts during progression of cottontail rabbit papillomavirus-induced tumours. J. Gen. Virol. 2015;96:3083–3089. doi: 10.1099/jgv.0.000239. [DOI] [PubMed] [Google Scholar]
- 92.Zhang P., Nouri M., Brandsma J.L., Iftner T., Steinberg B.M. Induction of E6/E7 expression in cottontail rabbit papillomavirus latency following UV activation. Virology. 1999;263:388–394. doi: 10.1006/viro.1999.9950. [DOI] [PubMed] [Google Scholar]
- 93.zur Hausen H. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 94.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 2006;110:525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 95.Tewari K.S., Monk B.J. New strategies in advanced cervical cancer: From angiogenesis blockade to immunotherapy. Clin. Cancer Res. 2014;20:5349–5358. doi: 10.1158/1078-0432.CCR-14-1099. [DOI] [PubMed] [Google Scholar]
- 96.Christensen N.D., Cladel N.M., Reed C.A., Han R. Rabbit oral papillomavirus complete genome sequence and immunity following genital infection. Virology. 2000;269:451–461. doi: 10.1006/viro.2000.0237. [DOI] [PubMed] [Google Scholar]
- 97.Evans C.A., Gorman L.R., Ito Y., Weiser R.S. Antitumor immunity in the Shope papilloma-carcinoma complex of rabbits. I. Papilloma regression induced by homologous and autologous tissue vaccines. JNCI. 1962;29:277–285. [PubMed] [Google Scholar]
- 98.Tagami H. Regression phenomenon of numerous flat wart—An experiment on the nature of tumor immunity in man. Int. J. Dermatol. 1983;22:570–571. doi: 10.1111/j.1365-4362.1983.tb02126.x. [DOI] [PubMed] [Google Scholar]
- 99.Nicholls P.K., Stanley M.A. The immunology of animal papillomaviruses. Vet. Immunol. Immunopathol. 2000;73:101–127. doi: 10.1016/S0165-2427(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 100.Selvakumar R., Ahmed R., Wettstein F.O. Tumor regression is associated with a specific immune response to the E2 protein of cottontail rabbit papillomavirus. Virology. 1995;208:298–302. doi: 10.1006/viro.1995.1152. [DOI] [PubMed] [Google Scholar]
- 101.Okabayashi M., Angell M.G., Christensen N.D., Kreider J.W. Morphometric analysis and identification of infiltrating leucocytes in regressing and progressing Shope rabbit papillomas. Int. J. Cancer. 1991;49:919–923. doi: 10.1002/ijc.2910490620. [DOI] [PubMed] [Google Scholar]
- 102.Okabayashi M., Pickel M.D., Budgeon L.R., Cladel N.M., Kreider J.W. Podofilox-induced regression of Shope papillomas may be independent of host immunity. J. Investig. Dermatol. 1993;101:852–857. doi: 10.1111/1523-1747.ep12371706. [DOI] [PubMed] [Google Scholar]
- 103.Selvakumar R., Schmitt A., Iftner T., Ahmed R., Wettstein F.O. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and persistence of viral DNA after regression. J. Virol. 1997;71:5540–5548. doi: 10.1128/jvi.71.7.5540-5548.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Höpfl R.M., Christensen N.D., Angell M.G., Kreider J.W. Skin test to assess immunity against cottontail rabbit papillomavirus antigens in rabbits with progressing papillomas or after papilloma regression. J. Investig. Dermatol. 1993;101:227–231. doi: 10.1111/1523-1747.ep12364825. [DOI] [PubMed] [Google Scholar]
- 105.Hopfl R.M., Christensen N.D., Heim K., Kreider J.W. Skin test reactivity to papilloma cells is long lasting in domestic rabbits after regression of cottontail rabbit papillomavirus induced papillomas. In: Stanley M.A., editor. Immunology of Human Papillomavirus. Plenum Press; New York, NY, USA: London, UK: 1994. p. 259. [Google Scholar]
- 106.Hopfl R., Christensen N.D., Angell M.G., Kreider J.W. Leukocyte proliferation in vitro against cottontail rabbit papillomavirus in rabbits with persisting papillomas/cancer or after regression. Arch. Dermatol. Res. 1995;287:652–658. doi: 10.1007/BF00371738. [DOI] [PubMed] [Google Scholar]
- 107.Hibma M.H. The immune response to papillomavirus during infection persistence and regression. Open Virol. J. 2012;6:241–248. doi: 10.2174/1874357901206010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coleman N., Birley H.D., Renton A.M., Hanna N.F., Ryait B.K., Byrne M., Taylor-Robinson D., Stanley M.A. Immunological events in regressing genital warts. Am. J. Clin. Pathol. 1994;102:768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 109.Hu J., Cladel N.M., Budgeon L.R., Christensen N.D. Characterization of three rabbit oral papillomavirus oncogenes. Virology. 2004;325:48–55. doi: 10.1016/j.virol.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 110.Wilgenburg B.J., Budgeon L.R., Lang C.M., Griffith J.W., Christensen N.D. Characterization of immune responses during regression of rabbit oral papillomavirus infections. Comp. Med. 2005;55:431–439. [PubMed] [Google Scholar]
- 111.Maglennon G.A., McIntosh P., Doorbar J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology. 2011;414:153–163. doi: 10.1016/j.virol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muench P., Probst S., Schuetz J., Leiprecht N., Busch M., Wesselborg S., Stubenrauch F., Iftner T. Cutaneous papillomavirus E6 proteins must interact with p300 and block p53-mediated apoptosis for cellular immortalization and tumorigenesis. Cancer Res. 2010;70:6913–6924. doi: 10.1158/0008-5472.CAN-10-1307. [DOI] [PubMed] [Google Scholar]
- 113.Wurdak M., Schneider M., Iftner T., Stubenrauch F. The contribution of SP100 to cottontail rabbit papillomavirus transcription and replication. J. Gen. Virol. 2018;99:344–354. doi: 10.1099/jgv.0.001012. [DOI] [PubMed] [Google Scholar]
- 114.Zeltner R., Borenstein L.A., Wettstein F.O., Iftner T. Changes in RNA expression pattern during the malignant progression of cottontail rabbit papillomavirus-induced tumors in rabbits. J. Virol. 1994;68:3620–3630. doi: 10.1128/jvi.68.6.3620-3630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Biesaga B., Mucha-Malecka A., Janecka-Widla A., Kolodziej-Rzepa M., Szostek S., Slonina D., Kowalczyk A., Halaszka K., Przewoznik M. Differences in the prognosis of HPV16-positive patients with squamous cell carcinoma of head and neck according to viral load and expression of P16. J. Cancer Res. Clin. Oncol. 2018;144:63–73. doi: 10.1007/s00432-017-2531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jelen M.M., Chen Z., Kocjan B.J., Burt F.J., Chan P.K., Chouhy D., Combrinck C.E., Coutlee F., Estrade C., Ferenczy A., et al. Global genomic diversity of human papillomavirus 6 based on 724 isolates and 190 complete genome sequences. J. Virol. 2014;88:7307–7316. doi: 10.1128/JVI.00621-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jelen M.M., Chen Z., Kocjan B.J., Hosnjak L., Burt F.J., Chan P.K., Chouhy D., Combrinck C.E., Estrade C., Fiander A., et al. Global Genomic Diversity of Human Papillomavirus 11 Based on 433 Isolates and 78 Complete Genome Sequences. J. Virol. 2016;90:5503–5513. doi: 10.1128/JVI.03149-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen L., Qiu X., Zhang N., Wang Y., Wang M., Li D., Wang L., Du Y. APOBEC-mediated genomic alterations link immunity and viral infection during human papillomavirus-driven cervical carcinogenesis. Biosci. Trends. 2017;11:383–388. doi: 10.5582/bst.2017.01103. [DOI] [PubMed] [Google Scholar]
- 119.Yang-Chun F., Sen-Yu W., Yuan Z., Yan-Chun H. Genome-Wide Profiling of Human Papillomavirus DNA Integration into Human Genome and Its Influence on PD-L1 Expression in Chinese Uygur Cervical Cancer Women. J. Immunol. Res. 2020;2020:6284960. doi: 10.1155/2020/6284960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.von Knebel D.M. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur. J. Cancer. 2002;38:2229–2242. doi: 10.1016/S0959-8049(02)00462-8. [DOI] [PubMed] [Google Scholar]
- 121.Ojesina A.I., Lichtenstein L., Freeman S.S., Pedamallu C.S., Imaz-Rosshandler I., Pugh T.J., Cherniack A.D., Ambrogio L., Cibulskis K., Bertelsen B., et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu L., Zhang X.L., Zhao Z.K., Wang L., Li B., Li G.B., Dean M., Yu Q.C., Wang Y.H., Lin X.X., et al. Full-length single-cell RNA-seq applied to a viral human cancer: Applications to HPV expression and splicing analysis in HeLa S3 cells. Gigascience. 2015;4:s13742-015. doi: 10.1186/s13742-015-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leemans C.R., Snijders P.J.F., Brakenhoff R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer. 2018;18:269–282. doi: 10.1038/nrc.2018.11. [DOI] [PubMed] [Google Scholar]
- 124.The Cancer Genome Atlas Research Network Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–384. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Argyris P.P., Slama Z.M., Ross K.F., Khammanivong A., Herzberg M.C. Calprotectin and the Initiation and Progression of Head and Neck Cancer. J. Dent. Res. 2018;97:674–682. doi: 10.1177/0022034518756330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khammanivong A., Sorenson B.S., Ross K.F., Dickerson E.B., Hasina R., Lingen M.W., Herzberg M.C. Involvement of calprotectin (S100A8/A9) in molecular pathways associated with HNSCC. Oncotarget. 2016;7:14029–14047. doi: 10.18632/oncotarget.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Christensen N.D., Kreider J.W. Neutralization of CRPV infectivity by monoclonal antibodies that identify conformational epitopes on intact virions. Virus Res. 1991;21:169–179. doi: 10.1016/0168-1702(91)90031-P. [DOI] [PubMed] [Google Scholar]
- 128.Esteves P.J., Abrantes J., Baldauf H.M., BenMohamed L., Chen Y., Christensen N., Gonzalez-Gallego J., Giacani L., Hu J., Kaplan G., et al. The wide utility of rabbits as models of human diseases. Exp. Mol. Med. 2018;50:66. doi: 10.1038/s12276-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mehta A.M., Mooij M., Brankovic I., Ouburg S., Morre S.A., Jordanova E.S. Cervical Carcinogenesis and Immune Response Gene Polymorphisms: A Review. J. Immunol. Res. 2017;2017:8913860. doi: 10.1155/2017/8913860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Breitburd F., Ramoz N., Salmon J., Orth G. HLA control in the progression of human papillomavirus infections. Sem. Cancer Biol. 1997;7:359. doi: 10.1006/scbi.1996.0045. [DOI] [PubMed] [Google Scholar]
- 131.Allen M., Kalantari M., Ylitalo N., Pettersson B., Hagmar B., Scheibenpflug L., Johansson B., Petterson U., Gyllensten U. HLA DQ-DR haplotype and susceptibility to cervical carcinoma: Indications of increased risk for development of cervical carcinoma in individuals infected with HPV 18. Tissue Antigens. 1996;48:32–37. doi: 10.1111/j.1399-0039.1996.tb02602.x. [DOI] [PubMed] [Google Scholar]
- 132.Beskow A.H., Josefsson A.M., Gyllensten U.B. HLA class II alleles associated with infection by HPV16 in cervical cancer in situ. Int. J. Cancer. 2001;93:817–822. doi: 10.1002/ijc.1412. [DOI] [PubMed] [Google Scholar]
- 133.Lin P., Koutsky L.A., Critchlow C.W., Apple R.J., Hawes S.E., Hughes J.P., Toure P., Dembele A., Kiviat N.B. HLA class II DR-DQ and increased risk of cervical cancer among Senegalese women. Cancer Epidemiol. Biomarkers Prev. 2001;10:1037–1045. [PubMed] [Google Scholar]
- 134.Hu J., Peng X., Budgeon L.R., Cladel N.M., Balogh K.K., Christensen N.D. Establishment of a Cottontail Rabbit Papillomavirus/HLA-A2.1 Transgenic Rabbit Model. J. Virol. 2007;81:7171–7177. doi: 10.1128/JVI.00200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yan Q., Zhang Q., Yang H., Zou Q., Tang C., Fan N., Lai L. Generation of multi-gene knockout rabbits using the Cas9/gRNA system. Cell Regen. 2014;3:12. doi: 10.1186/2045-9769-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang D., Xu J., Chen Y.E. Generation of Rabbit Models by Gene Editing Nucleases. Methods Mol. Biol. 2019;1874:327–345. doi: 10.1007/978-1-4939-8831-0_19. [DOI] [PubMed] [Google Scholar]
- 137.Song J., Yang D., Ruan J., Zhang J., Chen Y.E., Xu J. Production of immunodeficient rabbits by multiplex embryo transfer and multiplex gene targeting. Sci. Rep. 2017;7:12202. doi: 10.1038/s41598-017-12201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Christensen N.D., Kreider J.W., Kan N.C., DiAngelo S.L. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181:572–579. doi: 10.1016/0042-6822(91)90890-N. [DOI] [PubMed] [Google Scholar]
- 139.Peng X., Griffith J.W., Lang C.M. Reinitiated expression of EJras transgene in targeted epidermal cells of transgenic rabbits by cottontail rabbit papillomavirus infection. Cancer Lett. 2001;171:193–200. doi: 10.1016/S0304-3835(01)00576-6. [DOI] [PubMed] [Google Scholar]
- 140.Zhao K.N., Liu W.J., Frazer I.H. Codon usage bias and A+T content variation in human papillomavirus genomes. Virus Res. 2003;98:95–104. doi: 10.1016/j.virusres.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 141.Hu J., Cladel N., Balogh K., Christensen N. Mucosally delivered peptides prime strong immunity in HLA-A2.1 transgenic rabbits. Vaccine. 2010;28:3706–3713. doi: 10.1016/j.vaccine.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chesne P., Adenot P.G., Viglietta C., Baratte M., Boulanger L., Renard J.P. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat. Biotechnol. 2002;20:366–369. doi: 10.1038/nbt0402-366. [DOI] [PubMed] [Google Scholar]
- 143.Wolf D.P., Mitalipov S., Norgren R.B., Jr. Nuclear transfer technology in mammalian cloning. Arch. Med. Res. 2001;32:609–613. doi: 10.1016/S0188-4409(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 144.Flisikowska T., Thorey I.S., Offner S., Ros F., Lifke V., Zeitler B., Rottmann O., Vincent A., Zhang L., Jenkins S., et al. Efficient immunoglobulin gene disruption and targeted replacement in rabbit using zinc finger nucleases. PLoS ONE. 2011;6:e21045. doi: 10.1371/journal.pone.0021045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Song J., Yang D., Xu J., Zhu T., Chen Y.E., Zhang J. RS-1 enhances CRISPR/Cas9- and TALEN-mediated knock-in efficiency. Nat. Commun. 2016;7:10548. doi: 10.1038/ncomms10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bosticardo M., Yamazaki Y., Cowan J., Giardino G., Corsino C., Scalia G., Prencipe R., Ruffner M., Hill D.A., Sakovich I., et al. Heterozygous FOXN1 Variants Cause Low TRECs and Severe T Cell Lymphopenia, Revealing a Crucial Role of FOXN1 in Supporting Early Thymopoiesis. Am. J. Hum. Genet. 2019;105:549–561. doi: 10.1016/j.ajhg.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Song J., Wang G., Hoenerhoff M.J., Ruan J., Yang D., Zhang J., Yang J., Lester P.A., Sigler R., Bradley M., et al. Bacterial and Pneumocystis Infections in the Lungs of Gene-Knockout Rabbits with Severe Combined Immunodeficiency. Front. Immunol. 2018;9:429. doi: 10.3389/fimmu.2018.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vonsky M., Shabaeva M., Runov A., Lebedeva N., Chowdhury S., Palefsky J.M., Isaguliants M. Carcinogenesis Associated with Human Papillomavirus Infection. Mechanisms and Potential for Immunotherapy. Biochemistry. 2019;84:782–799. doi: 10.1134/S0006297919070095. [DOI] [PubMed] [Google Scholar]
- 149.Nunes R.A.L., Morale M.G., Silva G.A.F., Villa L.L., Termini L. Innate immunity and HPV: Friends or foes. Clinics. 2018;73:e549s. doi: 10.6061/clinics/2018/e549s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang X., Cheng Y., Li C. The role of TLRs in cervical cancer with HPV infection: A review. Signal Transduct. Target. Ther. 2017;2:17055. doi: 10.1038/sigtrans.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cattani P., Zannoni G.F., Ricci C., D’Onghia S., Trivellizzi I.N., Di Franco A., Vellone V.G., Durante M., Fadda G., Scambia G., et al. Clinical performance of human papillomavirus E6 and E7 mRNA testing for high-grade lesions of the cervix. J. Clin. Microbiol. 2009;47:3895–3901. doi: 10.1128/JCM.01275-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.