Abstract
Introduction
Duodenal Mucosal Resurfacing (DMR) is an endoscopic ablation technique aimed at improving glycaemia and metabolic health in patients with type 2 diabetes mellitus (T2DM). DMR has an insulin sensitizing effect in patients with T2DM. Reducing hyperinsulinemia can improve cardiovascular health. In the INSPIRE trial, we combined a single DMR with a glucagon-like-peptide-1 receptor agonist (GLP-1RA) and demonstrated elimination of insulin treatment in 69% of patients at 6 months and 53% of patients at 18 months while improving glycaemic control and metabolic health. We hypothesized that this treatment approach is associated with improved cardiovascular health, by reducing hyperinsulinemia.
Methods
Before and 6 months after starting the combination treatment to replace insulin, the following assessments were performed to evaluate cardiovascular health: magnetic resonance imaging (MRI) to measure abdominal visceral adipose tissue volume, ambulatory 24 h blood pressure (ABPM) analysis, postprandial insulin and triglycerides, fasting lipid panel and urine microalbumin. The Atherosclerotic Cardiovascular Disease (ASCVD) score was calculated to estimate 10-year risk of cardiovascular disease or stroke and the diabetes lifetime-perspective prediction (DIAL) score was calculated to estimate years free of cardiovascular disease.
Results
Six months after replacing exogenous insulin by DMR and GLP-1RA, visceral adipose tissue decreased significantly by 24%. Postprandial triglyceride and insulin concentrations decreased significantly (p < 0.001), as did total cholesterol (from median 3.64 (IQR 3.34–4.89) to 3.48 (3.18–3.97) mmol/l, p = 0.008), LDL (from median 1.92 (IQR 1.49–2.30) to 1.79 (1.49–2.08 mmol/l, p = 0.044), and urine microalbumin (from median 7 (IQR 3–27) to 4 (3–8) mg/l, p = 0.018). All daytime blood pressure values decreased significantly. The ASCVD 10-year risk score decreased (from median 13.6 (IQR 5.7–26.0) to 11.5 (4.2–22.5) %, p = 0.030)) and the DIAL score increased (from median 82 (IQR 81–83) to 83 (81–84) years, (p = 0.039)).
Discussion
The combination of DMR and GLP-1RA to replace insulin therapy in patients with T2DM is associated with a positive effect on multiple parameters of cardiovascular health. Taken together, they show a pattern of overall improvement in cardiovascular health, as evidenced by decreased risk scores for cardiovascular complications. However, it is not yet clear whether these improvements will translate into a true reduction in cardiovascular events.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01628-z.
Keywords: Diabetes type 2, Duodenal ablation, DMR, Endoscopy, GLP-1 and duodenum
Introduction
Diabetes mellitus is an important public health challenge. Currently about 1 in 11 adults worldwide has type 2 diabetes mellitus (T2DM) and its prevalence is still rising [1]. Treatment of hyperglycaemia is paramount and multiple glucose lowering treatment options are established. Atherosclerotic cardiovascular disease (ASCVD), defined as coronary heart disease, cerebrovascular disease, and peripheral arterial disease, is the leading cause of morbidity and mortality for individuals with T2DM [2]. Consequently, hospitalization due to heart failure is twofold higher in patients with T2DM compared to the general population [3, 4]. Numerous studies demonstrated that controlling individual cardiovascular risk factors including hypertension, hyperlipidemia, and abdominal adiposity can prevent or slow down the progression of ASCVD in people with T2DM [5].
Although there is a variety of effective oral glucose lowering drugs, eventually treatment with exogenous insulin is necessary in many patients with T2DM. Unfortunately, hyperinsulinemia can elicit weight gain and further deterioration of metabolic health [6] and it does not treat insulin resistance, the root cause of T2DM. Therefore, finding alternatives for insulin therapy while maintaining glycaemic control in patients with T2DM is necessary.
Evidence is accumulating that the duodenum is an important regulator of glucose homeostasis and therefore a new target for the treatment of T2DM [7, 8]. Roux-en-Y gastric bypass (RYGB) surgery, which includes the bypassing of the duodenum, is a well-established treatment for T2DM and greatly improves insulin resistance. Patients undergoing RYGB surgery also demonstrate significant decreases in weight, body fat content, and plasma lipids [9–11].
Duodenal Mucosal Resurfacing (DMR) is a minimally invasive endoscopic procedure that ablates the duodenal mucosa with subsequent regeneration [12]. Data from animal model and human studies suggest that this is followed by an insulin-sensitizing effect that is similar to the metabolic improvements seen after bariatric surgery, but to a lesser extent [12–14]. In the INSPIRE pilot study we found that DMR combined with GLP-1RA successfully replaced insulin therapy in 69% (11/16) of patients with type 2 diabetes at 6 months and in 53% of patients at 18 months [14]. Moreover, improvements in glycaemic (HOMA-IR, fasting plasma glucose (FPG)) and metabolic parameters (weight, body fat and liver fat content) were seen in these patients.
In this sub study we investigated whether replacing exogenous insulin by the combination of DMR and GLP-1RA in these 16 patients also resulted in changes in cardiovascular health parameters and the 10-year risk score for cardiovascular disease.
Materials and methods
Study design and intervention
The INSPIRE study was a single-center, single-arm, prospective, open-label clinical study that evaluated the effect of a single DMR procedure combined with GLP-1RA (liraglutide), in patients with T2DM, treated with insulin therapy. The study protocol was approved by the medical ethics committee of the Amsterdam University Medical Center. The study was conducted in accordance with ICH Good Clinical Practice Guidelines and the Declaration of Helsinki. The study is registered under EudraCT number 2017-00,349-30 at Clinicaltrialsregister.eu. The primary endpoints of this study have been reported elsewhere [14]. This report presents the results of a pre-defined sub-study of the INSPIRE study investigating the changes in parameters of cardiovascular health after replacing insulin by DMR with GLP-1RA.
Clinical study summary
We included 16 patients with T2DM using basal insulin, aged 28–75 years, with a body mass index of 24–40 kg/m2, a hemoglobin A1c (HbA1c) ≤ 8.0% (64 mmol/mol), and an adequate β-cell reserve (defined as fasting C-peptide > 0.5 nmol/l) [14]. Baseline characteristics can be found in Additional file 1: Table S1. The endoscopic DMR procedure was performed under deep sedation with propofol by a single endoscopist (JB) with experience in endoscopic DMR procedures. The DMR procedure involved circumferential hydrothermal ablation of the duodenal mucosa using an over-the-guidewire catheter, as described previously [12, 13]. Exogenous insulin administration was discontinued immediately after the DMR procedure. Patients were instructed to adhere to a 2 week post-procedural diet (i.e. gradual transition from liquid to solid food to allow adequate regeneration of the duodenal mucosa). After finishing the 2 week post-procedural diet, patients began with self-administration of subcutaneous GLP-1RA, liraglutide (Victoza®, Novo Nordisk A/S). Standard mild nutritional counselling and lifestyle education were provided before DMR and during follow-up [14]. All 16 enrolled patients underwent a successful DMR procedure defined as ≥ 5 sequential ablations of 2 axial centimeters each.
Cardiovascular assessments
At baseline and 6 months after DMR multiple measurements were conducted to assess cardiovascular health and the risk of cardiovascular events. These assessments are listed below.
Visceral and subcutaneous fat volume measurements
During the clinical study, MRI images (MRI; model clinical 3 Tesla scanner, Achieva, Philips) were made to measure liver fat content at baseline and 6 months after DMR. We decided to use these available MRI images to measure abdominal visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT). Body composition measures are usually estimated at the level of lumbar vertebra 3 (L3) or 4 (L4) [15]. As these levels were not consistently available on the MRI examinations of the upper abdomen, measurements at one transversal slide closest to mid L2 were used. VAT and SAT segmentation was performed using manual outlining (mDixon fat images) and semi-automated thresholding using Radiant DICOM viewer (Medixant, Poznan, Poland) by an experienced radiologist. This method is considered the reference standard for the quantitative assessment of intra-abdominal adipose tissue.
Ambulatory blood pressure monitoring
Systolic and diastolic blood pressure, and heart rate were measured during 24 h using an ambulatory blood pressure monitoring (ABPM) device (IEM, Mobil-O-Graph NG ABPM Monitor) at baseline and at 6 months follow-up. The ABPM cuff was placed on the non-dominant arm unless there was a 20/10 mmHg difference between arms, in which case the arm with the higher reading was used. Patients were instructed to maintain normal activity during ABPM and to hold the arm still and at heart level during recording. Recordings were programmed for every 30 min during the day (07.00 to 22.00) and every 60 min during the night (22.00 to 07.00). After 24 h, the monitor was detached and returned to the hospital.
Lipid panel
At baseline and 6 months, blood was drawn to measure fasting total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL) and triglycerides. Blood for total cholesterol, HDL, and triglyceride quantification was collected in a heparin tube at room temperature. Samples were brought to the laboratory for processing and analysis. Quantification was done using enzymatic colorimetric test performed on a Cobas c502 machine.
Postprandial insulin and triglycerides
All patients underwent a mixed meal tolerance test (MMTT) to measure postprandial plasma insulin and triglyceride concentrations at baseline and 6 months. All patients were using exogenous insulin at baseline and none were using exogenous insulin at the 6 month follow-up MMTT. If insulin was restarted in patients with an HbA1c > 7.5%, it was restarted after the 6 months follow-up visit. Patients were asked to ingest a liquid meal (Fresubin 200 ml, 2.0 kcal/ml) within 10 min. During the MMTT, blood samples were drawn at 0 min (fasting) and at 15, 30, 45, 60, 90, 120, 180, and 240 min following the start of the meal. Triglyceride quantification was performed with serum heparin tubes at room temperature with use of the enzymatic colorimetric test on a Cobas c702 machine. Insulin quantification was performed on SST tubes with gel and clot activator with use of the ILMA method on an Atellica (Siemens) machine.
Urine microalbumin
All patients delivered a urine sample at baseline and 6 months. Microalbumin quantification was performed with urine monovette tubes at room temperature with use of the immunoturbidimetry on a Cobas c502 machine.
10-years cardiovascular risk score
Ten year risk of cardiovascular events was estimated with use of an online calculator of the ASCVD risk score (ACC/AHA ASCVD Risk Calculator (cvriskcalculator.com). The calculator has been validated and is based on the algorithm published by Goff et al. 2013 in the ACC/AHA Cardiovascular Risk Assessment Guidelines [16]. Calculation of the 10-year risk estimate for ASCVD risk can best be described as a series of steps, in which multiple calculated risks variables, based on pooled data, result in a combined risk estimate. The risk variables include age, gender, race, plasma cholesterol levels, blood pressure values, diabetes and smoking status, and the use of blood pressure-lowering medications.
Estimated life-years free of cardiovascular disease
Life-years free of cardiovascular disease were estimated by using the diabetes lifetime-perspective prediction (DIAL) model, consisting of two complementary competing risk adjusted Cox proportional hazards functions using data from people with T2DM registered in the Swedish National Diabetes Registry (n = 389,366) [17]. The risk variables include age, gender, geographic region, smoking status, history of ASCVD, duration of diabetes, insulin use, systolic blood pressure, body mass index (BMI), plasma cholesterol levels, HbA1c level, glomerular filtration rate (eGFR) and albuminuria values, cholesterol-lowering drug and anticoagulant use.
Statistical analysis
Data are expressed as medians (interquartile ranges). The Wilcoxon paired signed-rank test was used to detect differences between baseline and 6 months follow-up of all parameters derived from the MRI (surface area VAT and SAT), fasting lipid panel (total cholesterol, HDL, LDL and triglycerides), urine microalbumin, ASCVD risk and DIAL score. Mixed effect models were used to detect differences between the calculations of 24 h ABPM assessment (24 h, per daytime, per nighttime, systolic and diastolic blood pressure, and heart rate), postprandial triglycerides and insulin concentrations at baseline and 6 months. The intervention was set as fixed effect whereas the time points and patient number were set as random effects. For the mixed effect model analyses, 12 measurements (evenly distributed) were used to assess 24 h ABPM, 12 measurements to assess daytime (07:00–22:00) and 10 measurements to assess nighttime (22:00–07:00) to compare systolic and diastolic blood pressure and MAP before and 6 months after DMR. Data was analyzed using SPSS IBM SPSS Statistics 25 (IBM, Armonk, New York, United States). Graphs were created with Graphpad Prism 8 (GraphPad Software Inc., La Jolla, California, United States). Statistical test were done two-sided with p-values ≤ 0.05 considered as statistically significant.
Results
Patient characteristics
All 16 enrolled patients underwent a successful DMR procedure. Patients were on average 61 years old, their T2DM duration was 11 years, and used 31 units of insulin per day prior to DMR. All baseline characteristics can be found in Additional file 1: Table S1. As mentioned in the introduction, 69% of patients (11/16) at 6 months and 53% (8/15) of patients at 18 months remained off insulin therapy with improved glycaemic control and improved parameters of metabolic health. Details on these clinical outcomes have been published previously [14].
Abdominal visceral and subcutaneous fat volume decreased significantly
Cross-sectional area measurements of VAT were performed in 14/16 patients and for SAT in 13/16 patients, since one patient suffered from claustrophobia and did not undergo a second MRI and MRI slides of two patients did not include the L2 level. We observed a significant relative reduction in VAT (24.2%) and SAT (20.4%) at 6 months compared to baseline (Table 1).
Table 1.
Abdominal VAT and SAT at baseline and 6 months follow-up, measured using MRI
| Baseline | 6 months post DMR | p-value | |
|---|---|---|---|
| VAT (cm2) | 248 (184–294) | 188 (156–244) | 0.002 |
| SAT (cm2) | 152 (136–190) | 121 (93–158) | 0.002 |
Data are expressed as median (Q1-Q3)
Paired Wilcoxon signed-rank tests were used to compare measurements between baseline and 6 months
DMR duodenal mucosal resurfacing, VAT visceral adipose tissue, SAT subcutaneous adipose tissue, MRI magnetic resonance imaging
P-values ≤ 0.05 are displayed in bold
Decrease in daytime blood pressure
ABPM was performed in all 16 patients. During the study period, one patient started with amlodipine 5 mg 3 months after DMR and one patient stopped hydrochlorothiazide 3 months after DMR. All other patients had no changes in their blood pressure lowering medication. Daytime systolic, diastolic and MAP decreased. Heart rate increased. The other values did not show significant changes. (Table 2).
Table 2.
Overview of variables of 24 h ambulatory blood pressure monitoring. Data are expressed as median (Q1-Q3)
| Baseline | 6 months post DMR | p-value | |
|---|---|---|---|
| 24 h systole (mmHg) | 127 (114–141) | 126 (114–136) | 0.194 |
| 24 h diastole (mmHg) | 79 (69–86) | 77 (69–85) | 0.325 |
| 24 h MAP (mmHg) | 101 (91–111) | 99 (92–108) | 0.195 |
| Daytime systole (mmHg) | 132 (119–148) | 127 (115–137) | 0.001 |
| Daytime diastole (mmHg) | 83 (73–89) | 79 (72–86) | 0.037 |
| Daytime MAP (mmHg) | 104 (95–115) | 100 (93–109) | < 0.001 |
| Nighttime systole (mmHg) | 121 (110–133) | 121 (112–132) | 0.667 |
| Nighttime diastole (mmHg) | 75 (65–81) | 73 (64–83) | 0.812 |
| NIghttime MAP (mmHg) | 97 (86–106) | 96 (86–105) | 0.554 |
| 24 h heart rate (beats/min) | 78 (67–84) | 81 (73–89) | < 0.001 |
Mixed effect models were used to compare measurements between baseline and 6 months post DMR
DMR Duodenal Mucosal Resurfacing, 24 h 24 h, mmHg millimeter of mercury, MAP mean arterial pressure, min minute
P-values ≤ 0.05 are displayed in bold
Fasting lipid panel improved
Fasting total cholesterol, LDL, and triglyceride concentrations significantly decreased at 6 months post DMR compared to baseline, whereas HDL concentrations did not change (Table 3).
Table 3.
Fasting total cholesterol, HDL, LDL and triglyceride plasma concentrations at baseline and 6 months follow-up
| Baseline | 6 months post DMR | p-value | |
|---|---|---|---|
| Total cholesterol (mmol/l) | 3.64 (3.34–4.89) | 3.48 (3.18–3.97) | 0.008 |
| HDL (mmol/l) | 1.21 (0.95–1.32) | 1.15 (1.05–1.47) | 0.103 |
| LDL (mmol/l) | 1.92 (1.49–2.30) | 1.79 (1.49–2.08) | 0.044 |
| Triglycerides (mmol/l) | 1.79 (1.15–2.66) | 1.09 (0.91–1.89) | 0.023 |
Data are expressed as median (Q1-Q3). Paired Wilcoxon signed-rank tests were used to compare measurements between baseline and 6 months
HDL high-density lipoprotein, LDL0 low-density lipoprotein
P-values ≤ 0.05 are displayed in bold
Postprandial triglycerides and insulin decreased
Postprandial triglyceride concentrations decreased significantly at 6 months post DMR compared to baseline, (p < 0.001, using mixed effect models) (Fig. 1). Postprandial insulin concentrations also dtecreased significantly, (p < 0.001)(Fig. 2).
Fig. 1.
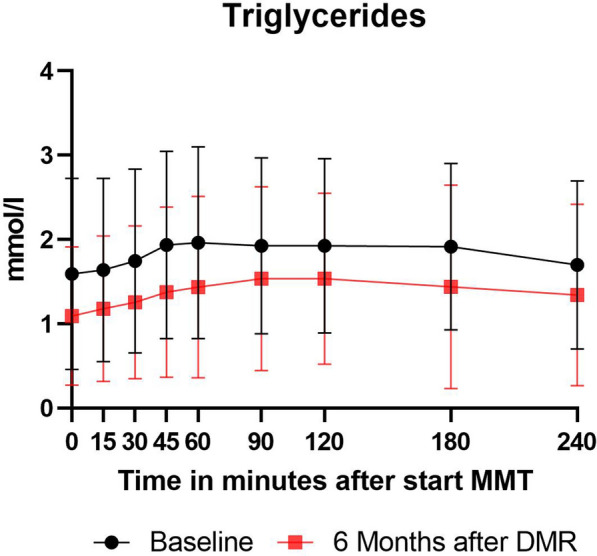
Postprandial triglyceride concentration curve during MMTT at baseline and 6 months in all 16 patients. Values are expressed as median (Q1-Q3). DMR: Duodenal Mucosal resurfacing, MMTT: mixed meal tolerance test
Fig. 2.
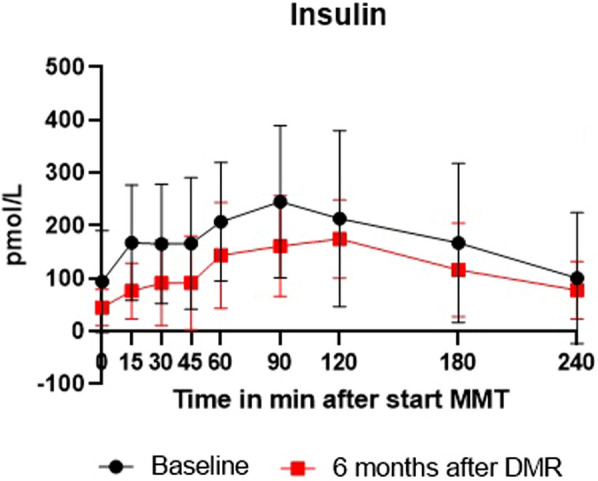
Postprandial insulin concentration curve during MMTT at baseline and 6 months in all 16 patients. Values are expressed as median (Q1-Q3). DMR: Duodenal Mucosal resurfacing, MMTT: mixed meal test
Urine microalbumin decreased
Urine microalbumin decreased significantly from 7 (3–27) mg/l at baseline to 4 (3–8) mg/l at 6 months post DMR, (p = 0.018).
Cardiovascular risk score decreased and ASCVD-free years increased
The ASCVD risk score decreased significantly at 6 months after DMR. At baseline 6/16 (37.5%) patients had a ≥ 20.0% estimated 10-year risk of heart disease or stroke, this decreased to 4/16 (25%) at 6 months post DMR. (Table 4) The median ASCVD risk score also decreased significantly from 13.6 (5.7–26.0)% to 11.5 (4.2–22.5)%, (p = 0.030). In five patients the risk score lowered by a category. None of the patients increased in risk category. (Additional file 1: figure S1) The estimated ASCVD-free years, according to the DIAL model, increased significantly with one year, (p = 0.039). (Table 4).
Table 4.
ASCVD risk score for estimated 10-year risk of heart disease or stroke
| ASCVD Score | Baseline | 6 months post DMR |
|---|---|---|
| Number of patients (%) | Number of patients (%) | |
| ≥ 20.0% (High risk) | 6 (37.5%) | 4 (25%) |
| 7.5–19.9% (Intermediate risk) | 5 (31.25%) | 7 (6.25%) |
| 5.0–7.4% (Borderline risk) | 3 (18.75%) | 0 |
| < 5.0% (Low risk) | 2 (12.5%) | 5 (18.75%) |
| Median risk score | 13.6 (5.7–26.0)% | 11.5 (4.2–22.5) % |
| Median ASCVD-free years (DIAL) | 82 (81–83) | 83 (81–84) |
ASCVD risk score was assessed by the ASCVD algorithm using the following variables: gender, age, systolic blood pressure, total cholesterol and smoking status in our patient population at baseline and 6 months post DMR [16]. Data are expressed as number of patients (% of population). ASCVD Risk score Calculator can be found on ACC/AHA ASCVD Risk Calculator (cvriskcalculator.com) and DIAL score calculator on https://u-prevent.com/calculators/dialModel
DMR Duodenal Mucosal Resurfacing, ASCVD atherosclerotic cardiovascular disease, DIAL diabetes lifetime-perspective prediction
Discussion
In this predefined sub-study we found that multiple cardiovascular parameters improved in patients with T2DM 6 months after replacing their insulin therapy with the combination of DMR and GLP-1RA. In line with this, the ASCVD risk score decreased significantly from 13.6% to 11.5% and in five patients the risk score even lowered by a category. This risk score provides an estimate of 10-year risk of cardiovascular events and is a recognized risk assessment for patients with T2DM [16]. In addition, by using the DIAL model, it was estimated that ASCVD-free life years increased by 1 year. The DIAL model was developed specifically for patients with T2DM [17]. Since cardiovascular complications are the leading cause of morbidity and mortality in individuals with T2DM, finding better ways to decrease this risk is desirable. In this article we demonstrated a positive impact of the combination of a single DMR and GLP-1RA on cardiovascular parameters.
The observed improvements in parameters of cardiovascular health in our patients are probably the result of two important changes that are part of our study intervention. Firstly, exogenous insulin therapy was discontinued. Hyperinsulinemia often leads to weight gain and further deterioration of metabolic health [6]. Secondly, the DMR procedure has been found to improve insulin sensitivity, also leading to lower levels of endogenous insulin [12–14, 18, 19]. The exact mechanism behind the insulin sensitizing effect of DMR has yet to be elucidated. We hypothesize that its insulin sensitizing effect can occur due to changes in either the gut-brain axis or local signalling to the liver and pancreas, cellular or histological changes in the duodenal mucosa, bile acid composition and microbiota diversity. In patients included in this INSPIRE study, we observed changes in bile acid composition [20] and minor changes in gut microbiota diversity [21]. We have taken duodenal biopsies before and 3 months after DMR to assess histological changes, this data is under evaluation.
We observed a significant relative reduction of abdominal VAT volume of 24%. Currently, there are no reference values available for VAT. However, excessive VAT is associated with cardiovascular morbidity [22, 23]. In a population at high risk, a lower VAT is therefore a logical positive outcome.
In addition, we assessed 24 h blood pressure. We found a significant decrease in daytime values at 6 months. Other values did not change, this could be due to the small sample size and the fact that night time values were already within normal ranges.
Plasma lipid optimization is an important part of cardiovascular risk management in patients with T2DM [24]. We observed that lipid levels improved in our study population 6 months after DMR. HDL remained stable, which is a positive finding as HDL levels are inversely correlated with cardiovascular disease [25].
Next, we observed that urine microalbumin decreased significantly. Urine microalbumin is an important gauge for renovascular health. We deem it unlikely that a major effect on nephrosclerosis was established in only 6 months. This decrease in microalbumin probably results from a decrease in hyperfiltration, which is common in patients with inadequately controlled T2DM.
This study has some limitations. Firstly, this is an observational uncontrolled proof-of-concept study with a limited sample size. Secondly, due to the design of the study, it is difficult to determine the effect of DMR or GLP-1RA separately. GLP-1RA therapy has been found non-inferior to glargine therapy in improving glycaemia, but solely in insulin-naïve patients [26]. Besides a significant short-term reduction of glycaemia, GLP-1RA therapy has been associated with a significant reduction of cardiovascular events in patients with T2DM, but only with a hazard ratio of 0.87 after 4 years [27]. Since the glycaemic and some of the metabolic effects of DMR and GLP-1RA appear to act synergistically, the combination may also lead to a greater and clinically more meaningful reduction of cardiovascular events. Larger randomized controlled trials are needed to confirm these findings and to evaluate the effect of DMR alone on parameters of cardiovascular health. Thirdly, VAT was measured at the level of L2, in contrast to what is advised in literature (L3-L4), however these slices were not available.
In conclusion, multiple parameters of cardiovascular health improved significantly in our study patients, 6 months after starting the combination of DMR and GLP-1RA to eliminate exogenous insulin therapy. Individual effects of these parameters might not be very impressive, but together they show a pattern of improvement in overall cardiovascular health, supported by absolute improvements in ASCVD and DIAL scores. In patients with T2DM with high risk of developing cardiovascular disease (based on the ASCVD algorithm), this combination treatment might be beneficial. It is not yet clear whether these improvements translate to a true reduction in cardiovascular events, but when controlled studies find similar results, this combination treatment could be a paradigm-shifting treatment approach in patients with T2DM at high risk of developing cardiovascular disease.
Supplementary Information
Additional file 1: Figure S1. Individual ASCVD risk scores for estimated 10-year risk of heart disease or stroke per patient at baseline and 6 months after DMR. (16) Data are expressed as %. Calculator can be found on (ACC/AHA ASCVD Risk Calculator (cvriskcalculator.com). ASCVD: atherosclerotic cardiovascular disease, DMR: duodenal mucosal resurfacing. Patient baseline characteristics and medication use at study entry. Data is expressed as median (Q1-Q3). T2D: type 2 diabetes mellitus, BMI: body mass index, HOMA-IR: homeostatic model assessment for insulin resistance.
Acknowledgements
Not applicable.
Abbreviations
- ABPM
Ambulatory 24 h blood pressure
- ASCVD
Atherosclerotic cardiovascular Disease
- BMI
Body mass index
- DMR
Duodenal mucosal resurfacing
- DIAL
Diabetes lifetime-perspective prediction
- FPG
Fasting plasma glucose
- GLP-1RA
Glucagon-like-peptide-1 receptor agonist
- HbA1c
Hemoglobin A1c
- HDL
High density lipoprotein
- HOMA-IR
Homeostatic model of insulin resistance
- ILMA
Immunoluminometric assay
- LDL
Low density lipoprotein
- L3
Lumbar vertebra 3
- MAP
Mean arterial pressure
- MMTT
Mixed meal tolerance test
- MRI
Magnetic resonance imaging
- RYGB
Roux-en-Y gastric bypass
- SAT
Subcutaneous adipose tissue
- SST
Serum-separating tube
- T2DM
Type 2 diabetes mellitus
- VAT
Visceral adipose tissue
Author contributions
Conceptualization: AB, FH, MN and JB. Investigation: SM and AB. MRI assessment: RH. Formal statistical analysis: SM and AB. Writing manuscript: SM, CB and AB. Editing: CB, AB, FH, MN and JB. Reviewing: CB, AB, RH, FH, MN and JB. All authors read and have approved the final manuscript. All authors read and approved the final manuscript.
Funding
Amsterdam UMC received an unrestricted research grant from Fractyl Laboratories.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the medical ethics committee of the Amsterdam University Medical Center. The study was conducted in accordance with ICH Good Clinical Practice Guidelines and the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
MN is supported by a personal ZONMW-VICI grant 2020 [09150182010020]. JB received consultancy fees for participation in advisory board meeting for Fractyl Laboratories in 2019, and consultancy for Endogenex and Digma in 2021.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Diabetes F. IDF Diabetes Atlas. Ninth edition ed. www.diabetesatlas.org2019.
- 2.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88(11):1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes A 10. cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(1):S103–S23. doi: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 4.Boyle JG, Livingstone R, Petrie JR. Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: a comparative review. Clin Sci (Lond) 2018;132(15):1699–1709. doi: 10.1042/CS20171299. [DOI] [PubMed] [Google Scholar]
- 5.Noakes M, Clifton PM. Changes in plasma lipids and other cardiovascular risk factors during 3 energy-restricted diets differing in total fat and fatty acid composition. Am J Clin Nutr. 2000;71(3):706–712. doi: 10.1093/ajcn/71.3.706. [DOI] [PubMed] [Google Scholar]
- 6.Lebovitz HE. Insulin: potential negative consequences of early routine use in patients with type 2 diabetes. Diabetes Care. 2011;34(2):S225–S230. doi: 10.2337/dc11-s225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruban A, Uthayakumar A, Ashrafian H, Teare JP. Endoscopic interventions in the treatment of obesity and diabetes. Dig Dis Sci. 2018;63(7):1694–1705. doi: 10.1007/s10620-018-5117-1. [DOI] [PubMed] [Google Scholar]
- 8.van Baar ACG, Nieuwdorp M, Holleman F, Soeters MR, Groen AK, Bergman J. The duodenum harbors a broad untapped therapeutic potential. Gastroenterology. 2018;154(4):773–777. doi: 10.1053/j.gastro.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Alsabrook GD, Goodman HR, Alexander JW. Gastric bypass for morbidly obese patients with established cardiac disease. Obes Surg. 2006;16(10):1272–1277. doi: 10.1381/096089206778663779. [DOI] [PubMed] [Google Scholar]
- 10.McCloskey CA, Ramani GV, Mathier MA, Schauer PR, Eid GM, Mattar SG, et al. Bariatric surgery improves cardiac function in morbidly obese patients with severe cardiomyopathy. Surg Obes Relat Dis. 2007;3(5):503–507. doi: 10.1016/j.soard.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14(3):198–202. doi: 10.1016/j.cardfail.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Rajagopalan H, Cherrington AD, Thompson CC, Kaplan LM, Rubino F, Mingrone G, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes: 6-month interim analysis from the first-in-human proof-of-concept study. Diabetes Care. 2016;39(12):2254–2261. doi: 10.2337/dc16-0383. [DOI] [PubMed] [Google Scholar]
- 13.van Baar ACG, Holleman F, Crenier L, Haidry R, Magee C, Hopkins D, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut. 2019 doi: 10.1136/gutjnl-2019-318349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Baar ACG, Meiring S, Smeele P, Vriend T, Holleman F, Barlag M, et al. Duodenal mucosal resurfacing combined with GLP-1RA to discontinue insulin in type 2 diabetes: a feasibility study. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Murray TE, Williams D, Lee MJ. Osteoporosis, obesity, and sarcopenia on abdominal CT: a review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist. Abdom Radiol (NY) 2017;42(9):2376–2386. doi: 10.1007/s00261-017-1124-5. [DOI] [PubMed] [Google Scholar]
- 16.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129(2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 17.Berkelmans GFN, Gudbjornsdottir S, Visseren FLJ, Wild SH, Franzen S, Chalmers J, et al. Prediction of individual life-years gained without cardiovascular events from lipid, blood pressure, glucose, and aspirin treatment based on data of more than 500 000 patients with Type 2 diabetes mellitus. Eur Heart J. 2019;40(34):2899–2906. doi: 10.1093/eurheartj/ehy839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aroda VR. A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20(1):22–33. doi: 10.1111/dom.13162. [DOI] [PubMed] [Google Scholar]
- 19.Mingrone G, van Baar AC, Deviere J, Hopkins D, Moura E, Cercato C, et al. Safety and efficacy of hydrothermal duodenal mucosal resurfacing in patients with type 2 diabetes: the randomised, double-blind, sham-controlled, multicentre REVITA-2 feasibility trial. Gut. 2021 doi: 10.1136/gutjnl-2020-323608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meiring S, Meessen ECE, van Baar AC, Holleman F, Nieuwdorp M, Olde Damink SW, et al. Duodenal mucosal resurfacing with GLP-1 receptor agonism increases postprandial unconjugated bile acids in patients with insulin-dependent type 2 diabetes. Am J Physiol Endocrinol Metab. 2021 doi: 10.1152/ajpendo.00337.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meiring S, van Baar ACG, Sørensen N, Holleman F, Soeters MR, Nieuwdorp M and Bergman JJGHM. A Changed Gut Microbiota Diversity Is Associated With Metabolic Improvements After Duodenal Mucosal Resurfacing With Glucagon-Like-Peptide-1 Receptor Agonist in Type 2 Diabetes in a Pilot Study. Front. Clin. Diabetes Healthc. 2022;3:856661. 10.3389/fcdhc.2022.856661. [DOI] [PMC free article] [PubMed]
- 22.Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233(1):104–112. doi: 10.1016/j.atherosclerosis.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Miazgowski T, Kucharski R, Soltysiak M, Taszarek A, Miazgowski B, Widecka K. Visceral fat reference values derived from healthy European men and women aged 20–30 years using GE healthcare dual-energy x-ray absorptiometry. PLoS ONE. 2017;12(7):e0180614. doi: 10.1371/journal.pone.0180614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diabetes A American Addendum 10. cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(1):S111–S134. doi: 10.2337/dc20-S010. [DOI] [PubMed] [Google Scholar]
- 25.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384(9943):618–625. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 26.D'Alessio D, Haring HU, Charbonnel B, de Pablos-Velasco P, Candelas C, Dain MP, et al. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17(2):170–178. doi: 10.1111/dom.12406. [DOI] [PubMed] [Google Scholar]
- 27.Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96(1133):156–161. doi: 10.1136/postgradmedj-2019-137186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Individual ASCVD risk scores for estimated 10-year risk of heart disease or stroke per patient at baseline and 6 months after DMR. (16) Data are expressed as %. Calculator can be found on (ACC/AHA ASCVD Risk Calculator (cvriskcalculator.com). ASCVD: atherosclerotic cardiovascular disease, DMR: duodenal mucosal resurfacing. Patient baseline characteristics and medication use at study entry. Data is expressed as median (Q1-Q3). T2D: type 2 diabetes mellitus, BMI: body mass index, HOMA-IR: homeostatic model assessment for insulin resistance.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


