Abstract
Enterococcus species are Gram-positive bacteria that are normal gastrointestinal tract inhabitants that play a beneficial role in the dairy and meat industry. However, Enterococcus species are also the causative agents of health care-associated infections that can be found in dairy and fermented food products. Enterococcal infections are led by strains of Enterococcus faecalis and Enterococcus faecium, which are often resistant to antibiotics and biofilm formation. Enterococci virulence factors attach to host cells and are also involved in immune evasion. LC-MS/MS-based methods offer several advantages compared with other approaches because one can directly identify microbial peptides without the necessity of inferring conclusions based on other approaches such as genomics tools. The present study describes the use of liquid chromatography–electrospray ionization tandem mass spectrometry (LC–ESI–MS/MS) to perform a global shotgun proteomics characterization for opportunistic pathogenic Enterococcus from different dairy and fermented food products. This method allowed the identification of a total of 1403 nonredundant peptides, representing 1327 proteins. Furthermore, 310 of those peptides corresponded to proteins playing a direct role as virulence factors for Enterococcus pathogenicity. Virulence factors, antibiotic sensitivity, and proper identification of the enterococcal strain are required to propose an effective therapy. Data are available via ProteomeXchange with identifier PXD036435. Label-free quantification (LFQ) demonstrated that the majority of the high-abundance proteins corresponded to E. faecalis species. Therefore, the global proteomic repository obtained here can be the basis for further research into pathogenic Enterococcus species, thus facilitating the development of novel therapeutics.
Keywords: LC–ESI–MS/MS, proteomics, mass spectrometry, antibiotic resistance peptides, antibiotic production, virulence factors, Enterococcus spp., dairy, fermented food products
1. Introduction
Enterococcus species include a ubiquitous group of Gram-positive organisms present in natural environments, playing a beneficial role in the dairy and meat industry for dairy and fermented food products, and in the gastrointestinal (GI) tract of humans and other animals. These bacteria are also one of the major causative agents of health care-associated infections. Enterococcal infections are often resistant to antibiotics and biofilm production [1,2].
Enterococcus faecalis and Enterococcus faecium cause the majority of enterococcal infections (urinary tract, catheterized urinary tract, bloodstream, wounds and surgical sites, and heart valves in endocarditis), while E. faecalis is responsible for 80–90% of enterococcal-associated nosocomial infections, followed by E. faecium (10–15%). As common inhabitants of the human GI tract, these organisms may be considered opportunistic pathogens and are involved in polymicrobial diseases [3,4].
Thus, the pathogenesis of Enterococci may be achieved by their production of virulence factors, in addition to their resistance to antimicrobials. Virulence factors play a role in attachment to host cells or extracellular matrix (ECM) proteins and are also involved in immune evasion [5]. Specifically, the production of cell surface proteins, the capacity for maintaining the cell envelope integrity, the adaptation to whatever nutrient sources are available, and potentially the formation of biofilms readily promote GI colonization and have important projections of their ability to participate in HGT processes [6]. Moreover, the presence of chromosomally integrated bacteriophages and plasmids contributes to the plasticity of enterococcal genomes and thus the evolution of multidrug-resistant enterococci [7]. In addition, to promote their competitive fitness with bacterial commensals in the GI tract, they may produce antimicrobials often genetically encoded on conjugative plasmids [6].
Enterococcus detection and identification have usually focused on culture and biochemical tests [8,9]. The methodology involved is time-consuming and inappropriate for short shelf-life foodstuffs that require fast and unequivocal methods to quickly detect and identify pathogens. Although several procedures that include molecular-based techniques, such as polymerase chain reaction (PCR), whole genome sequencing, and enzyme-linked immunosorbent assay (ELISA), all of them requiring long enrichment times, expensive chemicals and specialized equipment, have been developed [10,11,12], other methods, including biosensors, can be applied [13]. Furthermore, several studies that dig deeper into the Enterococcus proteome analysis have been proposed as alternative methods of fast enterococcal identification, as occurs with the ability to form biofilms [14,15]. In addition, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) [16,17] was used to establish antimicrobial resistance classification among the Enterococcus spp. Another approach has been performed by liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) to analyze the proteome of a dairy-isolated E. faecalis [18].
The detection of antimicrobial resistance mechanisms and virulence factors in enterococci has been traditionally performed using molecular genetic methods. However, while this information provides the repertoire of genetic mechanisms present in the bacterial genome, further proteomic analyses could offer information on the proteins produced in a given environment and proteomic fingerprinting for microbial identification. Techniques such as liquid chromatography–electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) have been used successfully to specifically identify pathogenic bacterial strains [19,20]. Additional studies involve the use of LC–ESI–MS/MS to research the proteomics of antibiotic resistance and the production of antimicrobials and other virulence factors to correctly identify different streptococcal and Listeria species [21,22].
This study aims to identify bacterial peptides related to virulence factors, antimicrobials, antimicrobial resistance mechanisms, and toxins using advanced shotgun proteomic methods (LC-ESI-MS/MS). This proteomic characterization of dairy-related enterococci would be important for detecting disease outbreaks quickly, providing a broad context for epidemiological investigations from a One Health approach.
2. Results
2.1. Enterococcus spp. Proteomics Data Repository
Fourteen Enterococcus strains were studied (Table 1). Bacterial peptides were prepared by treating the protein mixtures with trypsin and analyzed by LC-ESI-MS/MS, as described previously [19,21,22,23,24,25]. A total of 1403 nonredundant peptides were identified, which corresponded to 1327 annotated proteins from the Enterococcus UniProt/TrEMBL database (431,881 protein sequence entries in September 2020). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [26] partner repository with the dataset identifier PXD036435. The depth of the proteome results corresponded to surface-associated proteins due to the fact that the majority of virulence factors are localized in the surface of bacteria, either secretory or membrane associated [27,28], and we prepared the protein extraction to increase its identification. Accordingly, the virulence factors were identified by comparison with both the “Virulence Factors of Pathogenic Bacteria Database” (VFDB; http://www.mgc.ac.cn/VFs/, accessed on 20 May 2022) and with previously reported data [1,6,29,30,31].
Table 1.
Enterococcus strains used in this study. Total virulence factor peptides represent the number of peptides identified by LC–ESI-MS/MS. CECT—Spanish Type Culture Collection. F3 lacks a GenBank accession number because the sequencing chromatogram did not show enough quality.
| Sample | Species | Strain | Source | GenBank Accession Number | Total Virulence Factor Peptides |
|---|---|---|---|---|---|
| F1 | Enterococcus faecalis | ISPA118 | Milk | OP113834 | 39 |
| F2 | Enterococcus faecalis | ISPA 10 | Dairy product | KC510240 | 44 |
| F3 | Enterococcus faecalis | ISPA T07 | Milk | - * | 37 |
| F4 | Enterococcus durans | CECT 411 | Milk powder | KC510230 | 5 |
| F5 | Enterococcus faecalis | ISPA 28 | Cheese | OP113835 | 50 |
| F7 | Enterococcus faecium | ISPA FRP2 | Cheese | KC510246 | 14 |
| F9 | Enterococcus faecium | ISPA ID114 | Milk | KC510249 | 19 |
| F10 | Enterococcus faecium | ISPA G07 | Cheese | KC510247 | 4 |
| F11 | Enterococcus faecalis | ISPA ID119 | Dairy product | KC510243 | 7 |
| F12 | Enterococcus faecium | ISPA G010 | Cheese | KC510248 | 2 |
| F13 | Enterococcus faecalis | MORA 13 | Dairy product | KC510244 | 32 |
| F14 | Enterococcus faecalis | ISPA ID 116 | Milk | OP113836 | 12 |
| F15 | Enterococcus faecalis | ISPA 148 | Dairy product | KC510245 | 25 |
| F16 | Enterococcus faecalis | ISPA 89 | Dairy product | KC510242 | 20 |
| Total peptides | 310 | ||||
* F3 lacks a GenBank accession number because the sequencing chromatogram was not of a high enough quality.
In the group of nonredundant peptides, 310 were unequivocally identified as virulence factors and included proteins such bacteriocins, multidrug transporters, and phage-associated proteins. Additional polypeptides (toxins and antitoxins), together with polypeptides involved in antibiotic resistance, were also found. The 310 virulent factors identified for the studied strains are displayed in Tables S1–S6 in Supplemental Data and organized into groups according to the main role they play; these virulent factors include toxins, antibiotic resistance peptides, and other tolerance proteins involved in resistance to toxic substances, colonization and immune evasion factors, antimicrobial compounds, ATP-binding cassette (ABC), and other transporters associated with virulence factors. In addition, the main antibiotic resistance proteins, antimicrobial-related proteins, and other virulence factors are summarized in Table 2. Table 3 displays the total number of peptides corresponding to virulence factors organized into groups according to their main role.
Table 2.
Proteins corresponding to bacterial resistance to antibiotics, antimicrobial-related proteins, and other virulence factors, identified in the Enterococcus strains analyzed.
| Function | Protein |
|---|---|
| Antibiotic resistance | TetR family transcriptional regulator |
| TetR family transcriptional regulator | |
| N-acetyltransferase domain-containing protein | |
| PadR domain-containing protein | |
| GNAT family acetyltransferase | |
| Cass2 domain-containing protein | |
| MarR family transcriptional regulator | |
| Penicillin-binding protein | |
| Daunorubicin resistance protein DrrC | |
| OmpR/PhoB-type domain-containing protein | |
| PASTA domain-containing protein | |
| VanY D-Ala-D-Ala carboxypeptidase | |
| Additional resistances and tolerances | Cold-shock protein |
| General stress protein | |
| CsbD-like protein | |
| YitT family protein | |
| Tellurite resistance protein TelA | |
| MerR family transcriptional regulator | |
| SugE protein | |
| Chaperone protein DnaK | |
| Antimicrobial compounds | Lantibiotic biosynthesis protein |
| Type 2 lantipeptide synthetase LanM | |
| LanM family lanthionine synthetase | |
| Thiopeptide-type bacteriocin biosynthesis domain protein | |
| Bacteriocin Protein | |
| Radical SAM additional 4Fe4S-binding SPASM domain-containing protein | |
| Toxin | Type II toxin–antitoxin system PemK/MazF family toxin |
| Type II toxin–antitoxin system RelE/ParE family toxin | |
| Toxin–antitoxin system, antitoxin component, AbrB family | |
| Toxin PIN | |
| Exfoliative toxin A/B | |
| Prevent-host-death family antitoxin (Phd antitoxin) | |
| LXG domain-containing protein | |
| Colonization and immune evasion factors | Internalin |
| Sortase | |
| LPXTG cell wall anchor domain-containing protein | |
| Adhesin BspA | |
| Fibronectin/fibrinogen-binding protein | |
| Collagen-binding protein | |
| Ig domain-containing protein | |
| DUF4097 domain-containing protein | |
| Flagellar hook-associated protein 2 | |
| Fn3-like domain-containing protein | |
| Fimbrial isopeptide formation D2 domain-containing protein | |
| SpaA domain-containing protein | |
| Endopeptidase NlpC/P60 family protein | |
| M24/M78/M28/M20/M25/M40 family peptidases | |
| Peptidase T | |
| DD-transpeptidase | |
| Dipeptidase PepV | |
| Endopeptidase La | |
| S8/S9/S74 Family Peptidases | |
| Dipeptidyl aminopeptidase | |
| Peptidase U32 | |
| Peptidase S74 | |
| Proline dipeptidase | |
| Oligoendopeptidase PepF/M3 family protein | |
| Peptidase C51 | |
| ImmA/IrrE family metallo-endopeptidase | |
| Isoaspartyl dipeptidase | |
| Signal peptidase I | |
| Immune inhibitor A | |
| ClpA protease | |
| ClpC protease | |
| Zinc protease | |
| Capsular polysaccharide biosynthesis protein CpsC | |
| N-acetylmuramoyl-L-alanine amidase | |
| LysM domain protein | |
| Hemolysin III family channel protein | |
| Autolysin modifier protein | |
| Mga domain-containing protein | |
| Toxin secretion/phage lysis holin | |
| Type VII secretion protein EssC | |
| LysR family transcriptional regulator | |
| LytTR family transcriptional regulator | |
| HTH-type transcriptional regulator KdgR | |
| ArpU family transcriptional regulator | |
| Competence protein ComEA helix-hairpin-helix repeat region | |
| Control of competence regulator ComK, YlbF/YmcA | |
| Regulatory protein YlbF | |
| Spore coat protein | |
| Sporulation protein YjcZ | |
| Restriction endonuclease type IV, Mrr | |
| SfeI restriction endonuclease | |
| Type-2 restriction enzyme | |
| O-antigen ligase | |
| Methyl-accepting chemotaxis protein (MCP) signaling domain | |
| N-acetylglucosamine-6-phosphate deacetylase | |
| Glycosyl/glycerophosphate transferases involved in teichoic acid biosynthesis TagF/TagB/EpsJ/RodC | |
| ABC transporters | Copper ABC transporter permease |
| Ferrichrome ABC transporter FhuC | |
| Cobalt ABC transporter permease | |
| Lantibiotic protection ABC transporter | |
| Multidrug ABC transporter | |
| Spermidine/putrescine ABC transporter | |
| Glycine betaine ABC transporter | |
| Peptide ABC transporter | |
| Amino acid ABC transporter | |
| Excinuclease ABC subunit A | |
| Sulfate ABC transporter ATP-binding protein | |
| C4-dicarboxylate ABC transporter | |
| Multiple sugar ABC transporter substrate-binding protein | |
| Sugar ABC transporter | |
| Carbohydrate ABC transporter substrate-binding protein | |
| Heme ABC transporter | |
| Thiol reductant ABC exporter subunit CydC | |
| Other transporters | Major facilitator superfamily (MFS) transporter |
| Multidrug resistance MFS transporter | |
| Cation diffusion facilitator family transporter | |
| EamA/RhaT family transporter | |
| Copper-transporting ATPase CopB | |
| Alternative virulence factors | Transposase |
| Tnp-DDE superfamily | |
| IS4 family transposase | |
| Transposase InsI for insertion sequence element IS30C | |
| IS30/IS4/IS6 family transposaseS | |
| Conjugative transposon protein | |
| Conjugal transfer protein TraG | |
| Mutator family transposase | |
| Tyrosine-type recombinase/integrase | |
| Enterococcus faecalis plasmid pPD1 bacI | |
| Putative plasmid replication protein | |
| PrgI family protein | |
| Pheromone response system RNA-binding regulator PrgU | |
| Regulatory protein RecX | |
| CRISPR-associated endonuclease Cas9 | |
| CRISPR-associated endonuclease Cas10 | |
| CRISPR-associated endoribonuclease Cas2 | |
| YqaJ domain-containing protein | |
| Luciferase family oxidoreductase, group 1 | |
| Phage proteins | Phage capsid proteins |
| Phage tail proteins | |
| Phage portal protein | |
| Phage/plasmid primase, P4 family domain protein | |
| PBSX family phage terminase | |
| Phage terminase | |
| Phage integraseç | |
| Cro/CI family transcriptional regulator | |
| BppU family phage baseplate upper protein | |
| phage infection protei YhgE |
Table 3.
Total number of peptides corresponding to virulence factors organized in groups according to the main role they play, identified in the Enterococcus strains analyzed.
| Function | Total Virulence Factor Peptides |
|---|---|
| Colonization and immune evasion factors | 104 |
| ABC transporters | 70 |
| Other transporters | 8 |
| Alternative virulence factors | 32 |
| Phage proteins | 49 |
| Antibiotic resistance | 21 |
| Additional resistances and tolerances | 11 |
| Antimicrobial compounds | 6 |
| Toxins | 9 |
| Total Peptides | 310 |
Strains F5, F2, F1, F3, and F13 contained the highest number of peptides related to virulence, with 50, 44, 39, 37, and 32 peptides of virulence, respectively. Taken together, these results suggest that these strains are probably the most pathogenic strains within the species E. faecalis. However, for strains F4, F10, and F12, fewer than 50 nonredundant total peptides were identified (see the complete nonredundant dataset in Excel Supplemental Data).
2.2. Label-Free Quantification (LFQ) of Enterococcus Species
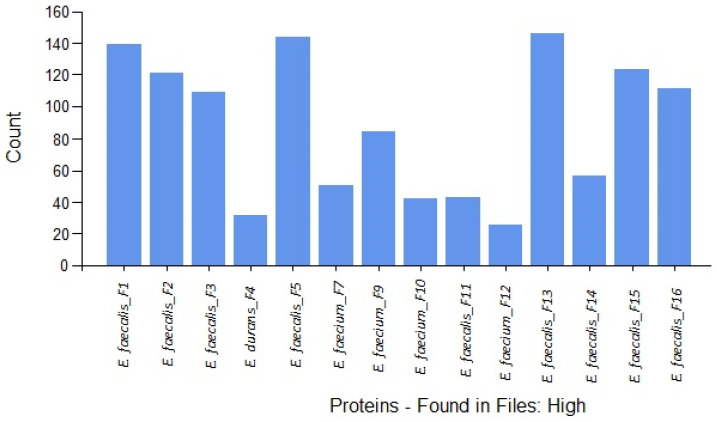
Relative label-free quantification of each Enterococcus species (E. faecalis, E. faecium and E. durans) was also performed to determine the protein abundance of each sample. Supplementary Data 3 contains these results. High-abundance proteins for each species and strains were compared. Figure 1 shows the distribution of the high-abundance proteins detected for each strain.
Figure 1.
Distribution of the high-abundance proteins for each Enteroccocus strain determined by LFQ.
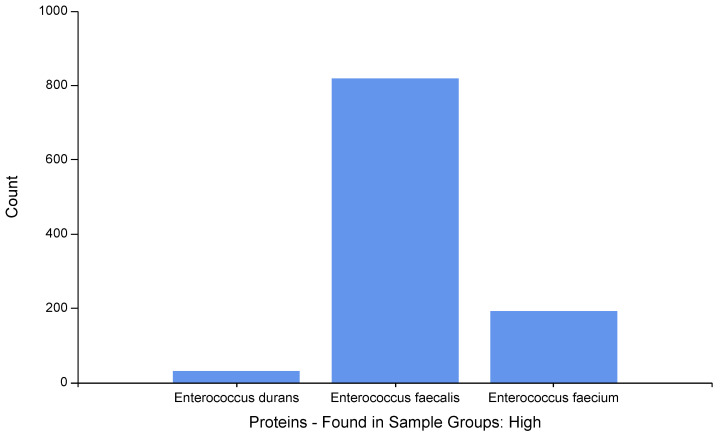
According to the different species, Figure 2 shows the high-abundance proteins for each Enterococcus species (E. durans, E. faecalis, and E. faecium).
Figure 2.
Distribution of the high-abundance proteins for each Enteroccocus species determined by LFQ.
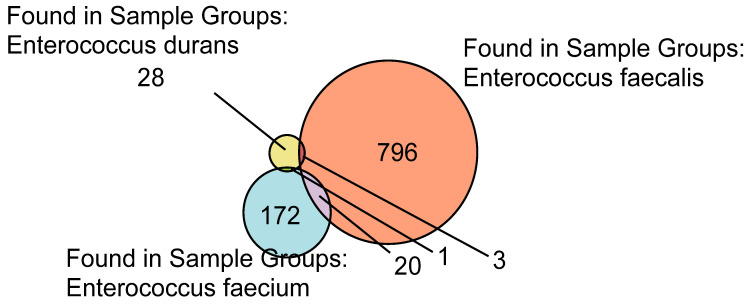
Using a Venn diagram, Figure 3 shows the distribution and overlapping of the high-abundance proteins for all the Enterococcus samples analyzed by LFQ.
Figure 3.
Venn diagram of the high-abundance proteins for all the Enteroccocus samples determined by LFQ.
As was demonstrated in Figure 1, Figure 2 and Figure 3, the majority of the high-abundance proteins were detected in E. faecalis species.
2.3. Proteins Involved in Bacterial Resistance to Antibiotics and Other Toxic Substances
This study identified 32 enterococcal peptides related to antimicrobial resistance or toxic substances (Table S1). Twenty-one of the proteins determined were associated with antibiotic resistance, whereas the remaining eleven peptides were related to other tolerances.
Two peptides were characterized as belonging to the MarR family of transcriptional regulators, and a set of three peptides was characterized as penicillin-binding proteins, which exhibit high affinity for the antibiotic [32]. MarR acts as a regulator for proteins involved in resistance against several antibiotics [33]. Three peptides belonging to the TetR family of regulators (TFRs) were identified; TetR proteins play a role in regulating genes encoding small-molecule exporters and antibiotic resistance. They also contribute to both antibiotic and quorum-sensing production [34]. Another peptide has been identified as a PASTA domain (penicillin-binding protein and serine/threonine kinase associated domain), which is found mainly in Gram-positive bacteria [35,36]. A transmembrane Ser/Thr kinase (IreK) in the PASTA kinase protein family is involved in cell envelope integrity and antimicrobial resistance in E. faecalis [37].
Three additional peptides were found to correspond to the GCN-2-related N-acetyl transferase (GNAT) family of acetyltransferases, which confer antibiotic resistance by catalyzing the acetylation of amino groups in aminoglycoside antibiotics [38]. VanY D-Ala-D-Ala carboxypeptidase was also identified by analysis of their peptides, and vanY is necessary for the synthesis of the vancomycin-inducible D,D-carboxypeptidase. VanY D-Ala-D-Ala peptidases provide resistance to the antibiotic vancomycin in some strains of enterococci [39,40]. One peptide interestingly related to a DrrC protein, which is involved in resistance to daunorubicin, was also identified [41]. Moreover, a peptide has been found to belong to the OmpR/PhoB subfamily of proteins. Members of the OmpR/PhoB subfamily include diverse transcriptional regulators, such as Enterococcus faecium VanR, which controls resistance to the antibiotic vancomycin [42]. One of the peptides involved in the stress caused by phenolic acids was also identified, PadR, an environmental sensor that acts as a repressor of padA gene expression in the phenolic acid stress response [43]. An additional peptide was characterized as aminoglycoside N(3)-acetyltransferase [44]. This enzyme can provide resistance against a variety of antibiotics, including gentamicin, kanamycin, tobramycin, neomycin, and apramycin, which contain 2-deoxystreptamine rings; these rings act as acceptors for acetyltransferase activity.
Bacterial tolerance refers to the ability of bacterial populations to survive harsh environments, such as the environment created by the use of antimicrobial agents, without developing resistance, since many of the mechanisms governing bacterial tolerance directly influence the virulence of the strain. This study revealed peptides with a role in bacterial resistance and tolerance to such conditions. One peptide was identified as TelA, a protein belonging to the toxic anionic resistance family, which confers tellurite resistance [45]. Another further peptide corresponded to a Cass 2 protein. Cass 2 is an integron-associated protein that binds cationic drug compounds with submicromolar affinity [46]. Two peptides were related to mercury resistance MerR proteins; these peptides are metal ion sensing regulators that create a mercury-resistant phenotype via transcription of several Mer genes [47]. A peptide of a SugE protein was also determined; SugE is a small multidrug resistance protein [48].
Additional peptides related to other important bacterial tolerances, including thermotolerance and osmotolerance, were identified. Regardless, stress tolerances play a role in facilitating bacterial persistence in the environment. Accordingly, this study identified two peptides belonging to heat shock proteins, such as DnaJ, a chaperone involved in thermotolerance phenotypes. Another peptide was identified as belonging to the cold shock-like proteins (CSPs) that provide tolerances in extreme temperatures by cellular physiology modifications, including decreased membrane fluidity, reduced mRNA transcription and translation due to the stabilization of secondary structures, inefficient folding of some proteins, and reduced enzyme activity [49]. Two peptides, CspD and CspA, were identified. In addition, three peptides of bacterial general stress response protein, one of them characterized as CsbD [50] and another one as YitT, are involved in general stress protein and are particularly required for protection against paraquat stress [51].
Many bacterial transporters, such as the ABC transporter, play a role in either antibiotic and other resistances or tolerances. Some transporter proteins are described in Section 2.6.
2.4. Antibacterial Compounds and Proteins Involved in Antibacterial Production
This study identified six antibacterial proteins from all the bacterial strains analyzed, which are present in the proteomic repository for Enterococcus spp. (Table S2). Bacteriocin-related peptides were discovered in the F1, F2, F5, F11, and F13 strains. Four peptides were identified with homology to lantibiotic biosynthesis proteins such as LanM, which is involved in posttranslational modifications [52]. Furthermore, one bacteriocin peptide was identified. The last bacteriocin-related peptide belongs to the radical S-adenosylmethionine (SAM enzymes, which tend to be involved in the maturation of subtilisin, anaerobic sulfatase-maturing enzyme, pyrroloquinoline quinolone (PQQ), and mycofactocin) [53].
2.5. Proteins Involved in Bacterial Toxicity
The present study identified nine peptides involved in bacterial toxicity by LC-ESI-MS/MS (Table S3). The peptides correspond to PIN toxin domains; Type II toxin–antitoxin systems, such as Phd repressor/antitoxin, PemK/MazF family toxin, and RelE/ParE family toxin; and LXG domain-containing protein, AbrB family toxin, and exfoliative toxin A/B. Exfoliative toxins (ETs) are serine proteases that hydrolyze desmoglein 1 (Dsg1), which causes dissociation of keratinocytes in both human and animal skin, thus helping S. aureus colonize the skin of mammals [54]. AbrB family members are transcription factors that act as antitoxins [55]. RelE/ParE family toxin is a member of the Type II toxin–antitoxin system that was also identified in this study. RelE toxins are mRNA interferases, while ParE toxins inhibit gyrase activity [56]. One of the characterized peptides corresponds to a Type II toxin–antitoxin system PemK/MazF family toxin; this family of proteins contains a toxin and an antitoxin gene pair as part of a postsegregation killing system, where gene loss results in the toxin attacking the cell. MazE is the antitoxin that inhibits toxin MazF, and under stress conditions, mazEF transcription is reduced, leading to the degradation of MazE, thus inhibiting cell division and resulting in cell death [57]. Two peptides that belong to an LXG domain-containing protein were identified in the Enterococcus strains (F5 and F13); this domain is present in the N-terminal region of a group of polymorphic toxin proteins. In prokaryotes, PIN domains are the toxic components of toxin–antitoxin (TA) systems, and their toxicity is produced by their ribonuclease activity. The PIN domain TA systems are now called VapBC Tas (virulence associated proteins), where VapB is the inhibitor and VapC is the PIN-domain ribonuclease toxin [58].
2.6. Proteins Involved in Host Colonization and Immune Evasion
A total of 104 peptides were identified that belonged to proteins that play a role in Enterococcus colonization and immune evasion (Table S4).
Enterococcal cell surface proteins are important for bacterial internalization into the mammalian host, playing a role in the maintenance of cell envelope integrity, adaptation to whatever nutrients are available, and formation of biofilms. To date, this study identified one peptide corresponding to a homolog of various Listeria monocytogenes virulence factors called internalin, a cell adhesion protein involved in host colonization [59]. Bacterial surface proteins are a group with important functions, such as adherence, invasion, signaling and interaction with the host immune system or environment in general. In Gram-positive bacteria, many surface proteins belonging to the “LPxTG” family are anchored to the peptidoglycan (PG) by an enzyme known as sortase [60]. Other adhesion peptides are indeed homologs to proteins involved in cell adhesion, such as thirteen peptides that corresponded to LPXTG-domain-containing protein, cell wall anchor domain, and one peptide assimilable to a sortase. LPXTG surface adhesin is involved in E. faecium biofilm formation [61]. Sortases are polypeptides that covalently attach secreted proteins to their cell wall to assemble pili; they play a key role during the infection process and represent potential drug targets [62]. Sortase A (SrtA) has been described as a membrane-associated enzyme that anchors surface proteins to enterococcal cell wall components, thus promoting biofilm formation [63,64].
Four additional peptides were found to be homologs to BspA, one peptide to Fibronectin/fibrinogen-binding protein, and one peptide to a collagen-binding protein. BspA is an antigen I/II family polypeptide that confers adhesion in Group B Streptococcus and is therefore linked to pathogenesis [65]. Adhesion pili are virulence factors present on the surface of bacteria; they are usually required for biofilm formation, enabling the bacteria to bind and adhere to target cells [66]. Four peptides were determined to have a fimbrial isopeptide-formation D2 domain. Proteins with this domain include fimbrial proteins with lectin-like adhesion functions, and most characterized members are involved in surface adhesion to host structure [67]. A peptide of flagellar hook-associated protein 2 (HAP2 or FliD) and an Fn3-like domain-containing protein peptide have also been identified. HAP2 forms the distal end of the flagella and plays a role in mucin-specific bacterial adhesion [68]. Fn3 is a fibronectin type III glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins [69]. Finally, an uncharacterized adhesion protein was identified together with DUF4097, a domain-containing protein and an Ig domain-containing protein. Proteins that contain an Ig domain are found in a variety of bacterial and phage surface proteins, such as intimins, as well as in some uncharacterized eukaryote proteins. Intimin is a bacterial cell-adhesion molecule that mediates the intimate interaction between bacteria and host cells [70,71]. However, the DUF4097 domain-containing protein has a putative all-beta structure with a twenty-residue repeat with a highly conserved repeating GD, gly-asp, motif, and it may form part of a bacterial adhesion [72]. A pilin subunit SpaA was also identified, which is composed of the shaft pilin SpaA, the tip adhesion SpaC, and the base pilin SpaB anchored to the cell wall.
A total of 33 peptides were identified as peptidases and proteases, including members of the P60, M20, M74, M24, dipeptidase PepV and T peptidase families, CLp proteases, and oligoendopeptidase PepF/M3 family protein. In particular, the 60-kDa extracellular protein (p60) is a member of the P60 family that is encoded by the iap gene and participates in the host invasion process [73]. Moreover, the dipeptidase PepV (an enzyme located in the final stage of the intracellular proteolytic system) has been demonstrated to be distributed widely in lactic acid bacteria, especially in lactococci [74]. Additionally, a peptide that belongs to an oligoendopeptidase pepF/M3 family has been determined, and it can participate in the regulation of sporulation [75] (Kanamaru et al., 2002). ImmA/IrrE proteases, which are involved in bacterial resistance to hostile environments, have been identified in the analyzed strains [76] (Gómez et al., 2020). Moreover, an immune inhibitor A peptide has been identified; this protein belongs to the MEROPS peptidase family M6 (immune inhibitor A family). B. thuringiensis has two proteins belonging to this group (InhA and InhA2), and InhA2 has been shown to have a vital role in virulence when the host is orally infected. The B. cereus member has been found to be an exosporium component from endospores [77].
Moreover, two peptides belonging to the CLp ATase family were detected (CLpA and CLpC); CLp proteins are formed by a CLp ATase and a peptidase; the latter hydrolyzes the proteins controlling the modulation of virulence factors, such as biofilm formation [78,79].
This study characterized two additional peptides related to capsular polysaccharides (CPSs). CPS contributes to pathogenesis by inhibiting the entrapment of pneumococci in neutrophil extracellular traps; the cpsABCD locus is involved in the modulation of CPS biosynthesis [80,81]. In addition, one N-acetylmuramoyl-L-alanine amidase peptide was identified. Cbp proteins include an N-acetylmuramoyl-L-alanine amidase in their biological module, which is involved in peptidoglycan release, proinflammatory teichoic acid formation, cell division and bacterial colonization [82]. N-Acetylmuramoyl-L-alanine amidases are autolysins involved in bacterial adherence to eukaryotic cells. Additional lysis proteins identified three listerial peptides; the one contained an autolysin modifier protein, and two had peptides belonged to the LysM domain; this last one is a protein module, originally found in enzymes that degrade bacterial cell walls, present in many bacterial proteins thus far involved in pathogenesis [83]. Additionally, a peptide of a hemolysin III family channel protein was identified; this protein is an integral membrane protein with hemolytic activity and is found in different Enterococcus species [84].
An additional group of eleven peptides corresponded to an Mga protein, a DNA-binding protein that regulates the expression of virulence genes; MafR is a newly described member of the Mga/AtxA family of global transcriptional regulators found in Enterococcus [85]. The peptides found included the M protein family of polypeptides (emm, mrp, and enn), C5a peptidase (scpA), and collagen-like protein 1 (scl1), which play an important role in colonization and immune evasion [86]. Three of the peptides identified belonged to the well-characterized type VII secretion systems (ESSs). This system facilitates the secretion of extracellular proteins across the cytoplasmic membrane and is involved in host infection, which is associated with virulence in S. aureus [87].
Transcriptional regulators controlling virulence factors were also identified in Enterococcus species; they include one peptide identified as LysR, two peptides corresponding to LytR, and one peptide representing the LytTR transcriptional regulators. LysR contributes to virulence by controlling multiple pathways that include cationic antimicrobial peptide (CAMP) resistance, fructose and mannose metabolism, and beta-lactam resistance [88,89]. LytR and LytTR proteins regulate additional virulence factors, such as extracellular polysaccharides, toxins, and bacteriocins, and polypeptides bind specific DNA sequences and act as transcriptional activators [90,91,92].
One peptide belonging to the kdgR transcriptional regulator was also detected. kdgR is a LysR family regulator, a repressor linked to the synthesis of enzymes involved in pectin degradation, as well as pectinase(s) secretion and catabolism thereof, but mostly overall affecting the production of cell wall-degrading enzymes. These are secreted mainly through the Type II secretion system (T2SS) and are directed toward the breakdown of the host plant cell wall [93]. In addition, a peptide was identified as belonging to the ArpU family transcriptional regulator, which represents a group of proteins that includes the putative autolysin regulatory protein ArpU. ArpU was originally described as a regulator of cellular muramidase-2 of Enterococcus hirae but appears to have been cloned from a prophage [94].
One peptide belonging to the toxin secretion/phage lysis holin was also detected. Toxin secretion/phage lysis, holin, includes, in addition to phage holins, the protein TcdE/UtxA, a holin-like protein encoded by toxigenic isolates of Clostridioides difficile and related species. TcdE mediates the release of the large clostridial glucosylating toxins (LCGTs) that act in combination with lytic enzymes in bacterial lysis (Tan et al., 2001; Vidor et al., 2022).
A peptide obtained from the analyzed Enterococcus strains was identified as ComEA, and two others were identified as ComK; they are part of the Com system, which is involved in escaping from the host phagosome and reaching the cytoplasm. This role is facilitated by the competence (Com) system proteins [95]. The ComK regulator includes YlbF and YmcA proteins, which are required for competence development, sporulation and the formation of biofilms. Furthermore, additional peptides included methyl-/ethyl-accepting chemotaxis. These proteins are a family of bacterial receptors that mediate chemotaxis to diverse signals, responding to changes in the concentration of attractants and repellents in the environment, which results in the alteration of swimming behavior [96].
Two peptides were determined to be sporulation-related proteins; one peptide was a spore coat protein, and the other was the sporulation TjcZ protein, which is involved in spore germination. Proteins in this entry are found only in endospore-forming bacterial species. A Gly-rich variable region is followed by a strongly conserved, highly hydrophobic region, predicted to form a transmembrane helix, ending with an invariant Gly [97].
Three peptides of restriction enzymes have been determined, a type-2 restriction enzyme, a Sfel restriction endonuclease, and a Mrr, a type IV restriction endonuclease, both involved in the acceptance of modified foreign DNA and with the ability to restrict both adenine- and cytosine-methylated DNA. It constitutes an essential mechanism for the generation of genetic variability that in turn mediates adaptations to the environment in bacterial populations. Mrr spurs the SOS response after high-pressure stress in Escherichia coli [98].
Enterococcal polysaccharide antigen (EPA) is required for normal cell growth and division and for resistance to cephalosporins, playing a critical role in host colonization. EPA residues consist of phosphopolysaccharide chains corresponding to teichoic acids [99]. In addition, lipoteichoic acid (LTA) has been reported to be involved in a wide range of inflammatory diseases; LTA is recognized by eukaryotic Toll-like receptor 2 (TLR2), which triggers innate immune responses [100]. A peptide of glycosyl-/glycerophosphate transferases involved in teichoic acid biosynthesis TagF/TagB/EpsJ/RodC was identified among the peptides obtained from Enterococcus strains.
Deacetylase enzymes were also among the proteins identified in Enterococcus spp. strains; these enzymes include a peptidoglycan-N-acetylglucosamine deacetylase, which plays a role in peptidoglycan deacetylation, avoiding eukaryotic lysozyme recognition during infection [101,102].
Finally, one peptide that exhibits weak similarity to O-antigen ligases was identified. This protein is involved in the synthesis of O-antigen, a side chain of the lipopolysaccharide found in the outer membrane in Gram-negative bacteria. Similar findings have been made in other Gram-positive bacteria, such as Bacillus subtilis [103].
2.7. Transporters Associated with Virulence Factors
Several ABC-type transporters are involved in virulence and play an important role in bacterial propagation during infection [104,105]. Seventy putative ABC transporters representing virulence factor peptides were identified in all Enterococcus strains, in addition to eight peptides corresponding to a variety of transporters that facilitate bacterial virulence strategies (Table S5).
Bacteria, including pathogenic strains, are well-established to have a variety of strategies to survive harsh conditions, such as the conditions generated upon nutrient deprivation, responding in such cases with the release of several stress proteins as well as by immune evasion mechanisms. The LC-ESI-MS/MS analyses carried out here identified several peptides corresponding to proteins that are required for the uptake of metals, such as cobalt, copper and ferrochromium. Furthermore, some peptides identified as ABC transporters are involved in the transport of lantibiotic oligopeptides and peptides, amino acids, glycine/betaine [104], spermidine, putrescine, and multidrug transporters. One peptide of a lantibiotic ABC transporter was identified for the F13 strain, and three peptides of multidrug ABC transporters were identified for the F2 and F3 strains. Multidrug ABC efflux transporters extrude antibiotics out of the bacterial cells, thus allowing pathogenic bacteria to resist antimicrobial treatment [104,106].
Additionally, nonABC transporters related to virulence were also identified in the peptide analysis. These peptides belong to a variety of transporters, including the EamA/RhaT family transporter, cation diffusion facilitator family transporter, copper-transporting, MFS and drug efflux MFS transporters. The MFS (major facilitator superfamily) is one of the largest groups of solute transporters [107]. However, the cation diffusion facilitator family (CDF) is designed to resist increasing concentrations of divalent metal ions such as cadmium, zinc, and cobalt, facilitating their removal from the cells [108]. Moreover, many members of the EamA family proteins are classified as drug/metabolite transporters [109].
2.8. Other Bacterial Virulence Factors
Bacteriophages are well known to encode genes as bacterial virulence factors, including Panton-Valentine leucocidin, staphylokinase, enterotoxins, chemotaxis-inhibitory proteins and exfoliative toxins in S. aureus [24,110] and Streptococcus species [22,111,112]. These viruses are usually integrated into bacterial chromosomes as prophages, wherein they encode new properties in the host, or vice versa, as transcription may hardly be affected by gene disruptions [112]. Phage-encoded recombinases, rather than the host recombinase RecA, are involved in bacterial genome excisions and integrations [113,114]. In addition, bacteriophage and bacteria interactions may substantially alter the variability of the bacterial population [115,116].
Likewise, the presence of mobile genetic elements is considered a major mechanism to produce bacterial variability related to a possible mechanism(s) of horizontal plasmid transfer (HGT) among bacteria [117]. These mobile elements may be either plasmids or viral DNA fragments and provide a wide range of genes that encode proteins involved in antibiotic resistance, virulence determinants, and additional polypeptides playing important roles in a variety of metabolic pathways [23,118]. Many peptides corresponding to proteins involved in the acquisition of these mobile elements were identified in the current analysis, including recombinases and integrases. Additional peptides represented specific plasmid proteins, such as E. faecalis plasmid pPD1 bacI and transposases corresponding to different transposons.
Eleven of the characterized peptides (Table S6) are indeed a part of different transposases. Among these peptides, several transposon insertion sequence (IS4, IS6, and IS30) peptides and three peptides of the transposase DDE superfamily proteins have been determined. These ISs have been associated with macrolide resistance genes in Enterococcus isolates [119]. Peptides belonging to recombinases and integrases, conjugative transposon protein, and one conjugal transfer protein, TraG, have been identified. TraG is an essential gene within the Tra operon for DNA transfer in bacterial conjugation [120].
Three peptides corresponding to a mutator family protein were also identified (Table S6); this polypeptide belongs to the class II DNA transposable element (TE) family that can exchange ectopic genomic sequences, leading to the formation of new gene arrangements [121].
In addition, peptides that correspond to a PrgI family protein, a putative plasmid replication protein, and another corresponding to the Enterococcus faecalis plasmid pPD1 bacI were identified in this study, and collaborators observed that the bacteriocin-encoding plasmid pPD1 enhances the ability of E. faecalis to colonize the GIT. Strains harboring such a plasmid of pPD1 easily displace preexisting enterococcal strains lacking the plasmid, including vancomycin-resistant enterococci (VRE). Importantly, the pPD1-mediated colonization advantage required the resident bacteriocin synthesis operon [122]. Moreover, PrgI is encoded in plasmids of E. faecalis, but its function is still largely unknown [123]. A pheromone response system RNA-binding regulator PrgU was determined in this study. rgU is a protein of plasmid origin expressed mainly in Enterococcus bacteria. PrgU has been postulated to probably act as an RNA-binding regulator, mitigating the toxicity accompanying overproduction of PrgB-like adhesins, which are involved in conjugative transfer [124].
In addition, more proteins of phage origin and involved in genetic modifications were also identified, such as three peptides for clustered regularly interspersed short palindromic repeats (CRISPR)-associated endonuclease Cas proteins, a peptide of a YqaJ domain, and two peptides belonging to regulatory protein RecX, which might be regulators of RecA activity by interaction with the RecA protein or filament [125]. YqaJ forms part of the two-component SynExo viral recombinase functional unit [126].
A large number of phage peptides from structural proteins were identified (Table S6). Peptides from proteins such as the major and minor capsid proteins, tail tape measure protein, phage portal protein, and phage tail fiber proteins were determined. The tape measure protein (TMP) determines the tail length and facilitates DNA entry into the cell during infection, and some TMPs have been reported to carry lysozyme-like and peptidase domains [127,128].
Several peptides of transposases, integrases, recombinases, and terminases were also identified. In this sense, a phage/plasmid primase P4 family domain protein and a PBSX family phage terminase peptide were determined, with the second involved in double-stranded DNA binding, DNA packaging, endonuclease, and ATPase activities [129].
Nine peptides from repressor-type Cro/CI were also determined. CI and Cro are encoded in the lysogeny module of lambdoid bacteriophages, particularly λ bacteriophages. Together, CII and CIII (which are formed through the antiterminator role of protein N) act as inducers that favor the first expression of the cI gene from the appropriate promoter; if the CI repressor predominates, the phage remains in the lysogenic state, but if the Cro predominates, the phage transitions into the lytic cycle, helped by the late Q regulator. The xenobiotic XRE regulator is extended in bacteria and has similarity to the Croλ repressor, exhibiting a helix-turn-helix (HTH) conformation [129]. Peptides of the CI/Cro-repressor types are usually named XRE family proteins in the National Center for Biotechnology Information (NCBI) database for bacteria.
Two peptides of a BppU family phage baseplate upper protein, several uncharacterized proteins that belong to bacteriophages and a phage infection protein YhgE were also found. BppU, also known as ORF48, is the N-terminal domain of baseplate upper proteins, which is found in bacteriophages [130]. Baseplate proteins are multiprotein molecular machines that control host cell recognition, attachment, tail sheath contraction, and viral DNA ejection [131].
Among phage peptides found in this analysis, some peptides identified when compared to the UniProtKB database were determined to be homologs to proteins found in different Enterococcus bacteriophages (Table S7); other peptides were determined to be phage peptides found in Enterococcus bacteria. Proteins that we found of bacteriophage origin were either structural proteins, such as phage head protein gp7, phage tail sheath, tail length tape-measure protein, or altogether uncharacterized proteins (Table S7).
3. Discussion
Due to the increased incidence of Enterococcus spp. in clinical microbiology and their presence in the food chain, the development of sensitive, rapid, and automated methods is required for the accurate identification and characterization of strains that can be implicated in food spoilage and food poisoning [132]. The LC-ESI-MS/MS proteome obtained from fourteen Enterococcus strains from dairy products identified 1403 nonredundant peptides. A total of 1327 peptides represent proteins that act as either virulence factors, toxins, or antibiotic resistance peptides, as well as tolerance proteins involved in bacterial resistance to toxic substances. Other identified proteins included colonization and immune evasion factors, polypeptides associated with antimicrobial production, ABC transporters, and other transporters related to virulence factors. The Enterococcus strains isolated from dairy products were previously characterized by MALDI-TOF-MS and phylogenetic analyses based on the 16S rRNA gene, and the genetic results were compared to the proteomic data. In addition, the analyses reported here involved an in-depth study of the antimicrobial and virulence factors present in the strains using shotgun proteomics tools. The rapid and accurate identification and potential pathogenicity characterization of pathogenic bacteria, including Enterococcus species, is an essential issue to maintain a good quality of the food chain.
As mentioned above, F5, F2, F1, F3, and F13 are enterococcal strains containing the highest number of virulence-related peptides identified (with 50, 44, 39, 37, and 32 peptides of virulence, respectively), indicating that these strains represent the most pathogenic bacteria compared with the other strains analyzed. The methodology described here allows determination of specific virulence factors as displayed by the bacteria in situ, as given in a particular environment and timing, hence allowing determination of the actual virulence status of the bacteria present in the food chain. This technology is a remarkable property. The technology may be used without previous growth of the bacterial strains that often do not show the same characteristics as the parental strains as they occur in the foodstuff.
All pathogen adaptations to either harsh environments or sublethal concentrations of antimicrobial agents contribute to the development of antimicrobial resistance, and, unfortunately, the last decades have seen a constant increase in antibiotic resistance phenotypes in enterococcal strains thus far isolated from foodstuffs [133,134].
The Enterococcus strains studied here contain many peptides involved in their resistome, including resistance to penicillin that is mediated by penicillin-binding proteins and other antibiotics, such as the MarR family of transcriptional regulators, TetR proteins, the PASTA domain, and the GNAT family of acetyltransferases. A variety of different drug transport pathways were also found, such as the multidrug efflux MFS transporter.
There is no doubt that, today, it is even more essential than ever that pathogenic bacterial strains be quickly identified both in foodstuffs and in clinics to provide the appropriate antimicrobial treatment. The present report shows that bacterial characterization can be quickly achieved by the use of LC-ESI-MS/MS. In fact, there is an urgent need for novel therapies for both the treatment and prevention of diseases caused by pathogenic species of enterococci. Bacteriocins are active against antibiotic-resistant bacterial strains, and, accordingly, they can be used, either independently or in combination with other antimicrobials, to cope with these infections. Indeed, bacteriocin and related peptides have been found here, and a deeper analysis should be performed to ascertain their effectiveness as antimicrobials.
Some reports have demonstrated how strains isolated from food and/or food production environments that harbor plasmids and bacteriophages in their genome provide important advantages for survival in food or associated environments, and, notably, the presence of mobile genetic elements is indeed considered a major mechanism of antibiotic resistance acquisition [6,7].
Finally, many peptides corresponding to proteins that play a role in the colonization and immune evasion of pathogenic enterococcal strains were identified in this study. These proteins play a crucial role in bacterial internalization into mammalian cells during the course of infection; thus, the identification of microbial peptides may provide a positive way to characterize the pathogen [4].
The precise proteomic method implemented in the present study represents a useful step for further analyses of pathogenic bacteria, as it offers considerable advantages over traditional approaches in terms of speed and reliability, without the need for full genomic sequencing and analysis or pregrowth of the bacterial strains [19,21,22,24,135].
4. Materials and Methods
4.1. Bacterial Strains
Fourteen Enterococcus strains isolated from dairy products were used in this study (Table 1). Strains were previously characterized by MALDI-TOF-MS and 16S rRNA sequencing [132]. The Enterococcus strains were grown in brain heart infusion (BHI, Oxoid Ltd., Hampshire, UK) at 31 °C for 24 h. Bacterial cultures were then transferred to plate count agar (PCA, Oxoid Ltd., Hampshire, UK) and subjected to further incubation at 31 °C for 24 h.
4.2. Protein Extraction
Protein extraction was performed as indicated previously [19]. In short, a fresh inoculation loop of bacterial culture was resuspended in 100 μL of a solution with 50% acetonitrile (ACN; Merck, Darmstadt, Germany) and 1% aqueous trifluoroacetic acid (TFA; Acros Organics, Bridgewater, NJ, USA). After vortexing and centrifuging, the supernatant was further treated with lysis buffer consisting of 60 mM Tris-HCl pH 7.5, 1% lauryl maltoside, 5 mM phenylmethanesulfonyl fluoride (PMSF), and 1% dithiothreitol (DTT). The supernatant was transferred to a new tube, and the amount of protein was determined by the bicinchoninic acid method (Sigma Chemical Co., St. Louis, MO, USA). Due to the difficulties to achieve the main 14 different Enterococcus strains that affect to dairy products, Enterococcus faecalis (9 different strains), Enterococcus durans (1 strain), and Enterococcus faeceium (4 different strains), only one biological replicate for each strain was used in the manuscript.
4.3. Peptide Sample Preparation
Protein extracts were solubilized and further digested with trypsin, as reported previously [136]. To do so, 100 μg of protein was dried in a SpeedVac (CentriVap, Labconco Co., Kansas City, MO, USA), resuspended in 25 μL of denaturation buffer (8 M urea in 25 mM ammonium bicarbonate, pH 8.0), and sonicated for 5 min. Then, the addition of DTT followed, at a final concentration of 10 mM, and incubation at 37 °C for 1 h. Alkylation was easily achieved by addition of the appropriate amount of iodoacetamide (IAA) to a final concentration of 50 mM; the solution was dark-incubated for 1 additional hour at room temperature. The sample was diluted with 4 volumes of 25 mM ammonium bicarbonate (pH 8.0) to reduce the urea concentration. The final step included trypsin digestion (Promega, Madison, WI, USA) with a protease:protein ratio of 1:100. The incubation was performed overnight at 37 °C.
4.4. Shotgun LC–ESI–MS/MS Analysis
The peptide digests prepared as shown before were acidified with formic acid (FA) (~pH 2), desalted in a C18 MicroSpin™ column (The Nest Group, Southborough, MA, USA), and finally analyzed by LC-ESI-MS/MS using a Proxeon EASY-nLC II Nanoflow system (Thermo Fisher Scientific, San Jose, CA, USA) coupled to an LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific) [19,137]. Peptide separation (2 μg) was performed in a reverse-phase (RP) column (EASY-Spray column, 50 cm × 75 μm ID, PepMap C18, 2 μm particles, 100 Å pore size, Thermo Fisher Scientific) equipped with a 10 mm precolumn (Accucore XL C18, Thermo Fisher Scientific). Elution from the column was performed by means of a linear gradient from 5 to 35% solvent B (solvent A: 98% water, 2% ACN, 0.1% FA; solvent B: 98% ACN, 2% water, 0.1% fatty acid (FA)) for 120 min at a flow rate of 300 nL/min. Electrospray ionization was carried out with a spray voltage of 1.95 kV at a capillary temperature of 230 °C. Peptides were analyzed in positive mode (1 μscan; 400 to 1600 amu), followed by 10 data-dependent collision-induced dissociation (CID) MS/MS scans (1 μscan), using an isolation width of 3 amu and a normalized collision energy of 35%. After the second fragmentation event, dynamic exclusion was set for 30 s, and ions with an unassigned charge state were excluded from MS/MS analysis.
4.5. LC-ESI-MS/MS Data Processing
The MS/MS spectra obtained by LC-ESI-MS/MS were analyzed using the program SEQUEST-HT (Proteome Discoverer 2.4, Thermo Fisher Scientific) and compared to the Enterococcus UniProt/TrEMBL protein database (containing 431,881 protein sequence entries, September 2020). MS/MS spectra were searched using fully tryptic cleavage constraints, and up to two missed cleavage sites were allowed. Tolerance windows were set at 10 ppm for precursor ions and 0.06 Da for MS/MS fragment ions. The variable modifications allowed were (M*) methionine oxidation (+15.99 Da) and protein N-terminal acetylation (+42.0106 Da). Carbamidomethylation of cysteine (Cys) (+57.02 Da) (C*) was considered a fixed modification. The percolator algorithm (Käll et al., 2007) was used to validate the results as well as for statistical analysis. The peptide false discovery rate (FDR) was always kept at less than 1%. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [26] partner repository with the dataset identifier PXD036435.
Label-free quantification (LFQ) of relative protein abundance for each strain were performed using the Proteome Discover 2.4 program (Thermo Fisher Scientific), using the Minora Feature Detector node and the ANOVA (individual proteins) approach. Peak areas of ion features corresponding to the same peptide in different charge forms were summed up to one value.
Acknowledgments
The authors wish to thank Stefano Morandi from ISPA-CNR, Italy, for providing several of the microbial strains of dairy origin considered in this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231810971/s1.
Author Contributions
A.G.A. wrote the manuscript; A.G.A., M.Q.-B., T.G.V. and M.C. conceptualized, revised and corrected the paper. P.C.-M., J.B.-V. and M.C. co-supervised the work. M.C. obtained the funding. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors declare no conflict of interest.
Funding Statement
A.G.A. thanks the USC for his “Convocatoria de Recualificación do Sistema Universitario Español-Margarita Salas” postdoc grant under the “Plan de Recuperación Transformación” program funded by the Spanish Ministry of Universities with European Union’s NextGeneration EU funds. This work has received financial support from the Xunta de Galicia and the European Union (European Social Fund—ESF), the Spanish Ministry of Economy and Competitivity Project AGL 2.013-48.244-R and the European Regional Development Fund (ERDF) (2007–2013). The study was also supported by the GAIN-Xunta de Galicia Project (IN607D 2017/01) and the Spanish AEI/EU-FEDER PID2019-103845RB-C21 project.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.García-Solache M., Rice L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019;32:e00058-18. doi: 10.1128/CMR.00058-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stagliano D.R., Susi A., Adams D.J., Nylund C.M. Epidemiology and Outcomes of Vancomycin-Resistant Enterococcus Infections in the U.S. Military Health System. Mil. Med. 2021;186:100–107. doi: 10.1093/milmed/usaa229. [DOI] [PubMed] [Google Scholar]
- 3.Devoe C., Segal M.R., Wang L., Stanley K., Madera S., Fan J., Schouest J., Graham-Ojo R., Nichols A., Prasad P.A., et al. Increased rates of secondary bacterial infections, including Enterococcus bacteremia, in patients hospitalized with coronavirus disease 2019 (COVID-19) Infect. Control Hosp. Epidemiol. 2021:1–8. doi: 10.1017/ice.2021.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goh H.M.S., Yong M.H.A., Chong K.K.L., Kline K.A. Model systems for the study of Enterococcal colonization and infection. Virulence. 2017;8:1525–1562. doi: 10.1080/21505594.2017.1279766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garsin D.A., Frank K.L., Silanpää J., Ausubel F.M., Hartke A., Shankar N., Murray B.E. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Massachusetts Eye and Ear Infirmary; Boston, MA, USA: 2014. Pathogenesis and Models of Enterococcal Infection. [PubMed] [Google Scholar]
- 6.Banla L.I., Salzman N.H., Kristich C.J. Colonization of the mammalian intestinal tract by enterococci. Curr. Opin. Microbiol. 2019;47:26–31. doi: 10.1016/j.mib.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer K.L., Kos V.N., Gilmore M.S. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 2010;13:632–639. doi: 10.1016/j.mib.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamzah A.M., Kadim H.K. Isolation and identification of Enterococcus fecalis from cow milk samples and vaginal swab from human. J. Entomol. Zool. Stud. 2018;6:218–222. [Google Scholar]
- 9.Sorescu I., Dumitru M., Ciurescu G. Lactobacillus spp. and Enterococcus faecium strains isolation, identification, preservation and quantitative determinations from turkey gut content. Rom. Biotechnol. Lett. 2019;24:41–49. doi: 10.25083/rbl/24.1/41.49. [DOI] [Google Scholar]
- 10.Hayat Z., Shahzad K., Ali H., Casini R., Naveed K., Hafeez A., El-Ansary D.O., Elansary H.O., Fiaz S., Abaid-Ullah M., et al. 16S rRNA gene flow in Enterococcus spp. and SNP analysis: A reliable approach for specie level identification. Biochem. Syst. Ecol. 2022;103:104445. doi: 10.1016/j.bse.2022.104445. [DOI] [Google Scholar]
- 11.Rogers L.A., Strong K., Cork S.C., McAllister T.A., Liljebjelke K., Zaheer R., Checkley S.L. The Role of Whole Genome Sequencing in the Surveillance of Antimicrobial Resistant Enterococcus spp.: A Scoping Review. Front. Public Health. 2021;9:656. doi: 10.3389/fpubh.2021.599285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waters S.M., Doyle S., Murphy R.A., Power R.F.G. Development of solution phase hybridisation PCR-ELISA for the detection and quantification of Enterococcus faecalis and Pediococcus pentosaceus in Nurmi-type cultures. J. Microbiol. Methods. 2005;63:264–275. doi: 10.1016/j.mimet.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 13.La Rosa S.L., Solheim M., Diep D.B., Nes I.F., Brede D.A. Bioluminescence based biosensors for quantitative detection of Enterococcal peptide–pheromone activity reveal inter-strain telesensing in vivo during polymicrobial systemic infection. Sci. Rep. 2015;5:8339. doi: 10.1038/srep08339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suriyanarayanan T., Qingsong L., Kwang L.T., Mun L.Y., Seneviratne C.J. Quantitative proteomics of strong and weak biofilm formers of Enterococcus faecalis reveals novel regulators of biofilm formation. Mol. Cell. Proteom. 2018;17:643–654. doi: 10.1074/mcp.RA117.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giard J.-C., Auffray Y., Benachour A., Hartke A., Laplace J.-M., Rince A., Verneuil N., Pichereau V. Proteomics Analysis: A Powerful Tool to Identify Proteome Phenotype and Proteome Signature in Enterococcus faecalis. Curr. Proteom. 2012;1:273–282. doi: 10.2174/1570164043152795. [DOI] [Google Scholar]
- 16.Brackmann M., Leib S.L., Tonolla M., Schürch N., Wittwer M. Antimicrobial resistance classification using MALDI-TOF-MS is not that easy: Lessons from vancomycin-resistant Enterococcus faecium. Clin. Microbiol. Infect. 2020;26:391–393. doi: 10.1016/j.cmi.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Holzknecht B.J., Dargis R., Pedersen M., Pinholt M., Christensen J.J., Hammerum A.M., Littauer P., Worning P., Westh H., Moser C., et al. Typing of vancomycin-resistant Enterococci with MALDI-TOF mass spectrometry in a nosocomial outbreak setting. Clin. Microbiol. Infect. 2018;24:1104.e1–1104.e4. doi: 10.1016/j.cmi.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Cirrincione S., Neumann B., Zühlke D., Riedel K., Pessione E. Detailed soluble proteome analyses of a dairy-isolated Enterococcus faecalis: A possible approach to assess food safety and potential probiotic value. Front. Nutr. 2019;6:71. doi: 10.3389/fnut.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrera M., Böhme K., Gallardo J.M., Barros-Velázquez J., Cañas B., Calo-Mata P. Characterization of foodborne strains of Staphylococcus aureus by shotgun proteomics: Functional networks, virulence factors and species-specific peptide biomarkers. Front. Microbiol. 2017;8:1–15. doi: 10.3389/fmicb.2017.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfrunder S., Grossmann J., Hunziker P., Brunisholz R., Gekenidis M.T., Drissner D. Bacillus cereus Group-Type Strain-Specific Diagnostic Peptides. J. Proteome Res. 2016;15:3098–3107. doi: 10.1021/acs.jproteome.6b00216. [DOI] [PubMed] [Google Scholar]
- 21.Abril A.G., Carrera M., Böhme K., Barros-Velázquez J., Calo-Mata P., Sánchez-Pérez A., Villa T.G. Proteomic Characterization of Antibiotic Resistance in Listeria and Production of Antimicrobial and Virulence Factors. Int. J. Mol. Sci. 2021;22:8141. doi: 10.3390/ijms22158141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abril A.G., Carrera M., Böhme K., Barros-Velázquez J., Rama J.L.R., Calo-Mata P., Sánchez-Pérez A., Villa T.G. Proteomic Characterization of Antibiotic Resistance, and Production of Antimicrobial and Virulence Factors in Streptococcus Species Associated with Bovine Mastitis. Could Enzybiotics Represent Novel Therapeutic Agents Against These Pathogens? Antibiotics. 2020;9:302. doi: 10.3390/antibiotics9060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coelho C., Brown L., Maryam M., Vij R., Smith D.F.Q., Burnet M.C., Kyle J.E., Heyman H.M., Ramirez J., Prados-Rosales R., et al. Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J. Biol. Chem. 2019;294:1202–1217. doi: 10.1074/jbc.RA118.006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abril A.G., Carrera M., Böhme K., Barros-Velázquez J., Cañas B., Rama J.L.R., Villa T.G., Calo-Mata P. Proteomic Characterization of Bacteriophage Peptides from the Mastitis Producer Staphylococcus aureus by LC-ESI-MS/MS and the Bacteriophage Phylogenomic Analysis. Foods. 2021;10:799. doi: 10.3390/foods10040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abril A.G., Villa T.G., Calo-Mata P., Barros-Velázquez J., Carrera M. Food Proteom: Technological Advances, Current Applications and Future Perspectives. Academic Press; New York, NY, USA: 2022. Application of proteomics to the identification of foodborne pathogens; pp. 337–362. [Google Scholar]
- 26.Perez-Riverol Y., Bai J., Bandla C., García-Seisdedos D., Hewapathirana S., Kamatchinathan S., Kundu D.J., Prakash A., Frericks-Zipper A., Eisenacher M., et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–D552. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma A.K., Dhasmana N., Dubey N., Kumar N., Gangwal A., Gupta M., Singh Y. Bacterial Virulence Factors: Secreted for Survival. Indian J. Microbiol. 2017;57:1–10. doi: 10.1007/s12088-016-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chajęcka-Wierzchowska W., Zadernowska A., Łaniewska-Trokenheim Ł. Virulence factors of Enterococcus spp. presented in food. LWT. 2017;75:670–676. doi: 10.1016/j.lwt.2016.10.026. [DOI] [Google Scholar]
- 29.Wagner T., Joshi B., Janice J., Askarian F., Škalko-Basnet N., Hagestad O.C., Mekhlif A., Wai S.N., Hegstad K., Johannessen M. Enterococcus faecium produces membrane vesicles containing virulence factors and antimicrobial resistance related proteins. J. Proteom. 2018;187:28–38. doi: 10.1016/j.jprot.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Ghaziasgar F.S., Poursina F., Hassanzadeh A. Virulence factors, biofilm formation and antibiotic resistance pattern in Enterococcus faecalis and Enterococcus faecium isolated from clinical and commensal human samples in Isfahan, Iran. Ann. Ig. 2019;31:156–164. doi: 10.7416/ai.2019.2268. [DOI] [PubMed] [Google Scholar]
- 31.Najafi K., Ganbarov K., Gholizadeh P., Tanomand A., Rezaee M.A., Mahmood S.S., Asgharzadeh M., Kafil H.S. Oral cavity infection by Enterococcus faecalis: Virulence factors and pathogenesis. Rev. Res. Med. Microbiol. 2020;31:51–60. doi: 10.1097/MRM.0000000000000168. [DOI] [Google Scholar]
- 32.Dowson C.G., Hutchison A., Brannigan J.A., George R.C., Hansman D., Linares J., Tomasz A., Smith J.M., Spratt B.G. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA. 1989;86:8842–8846. doi: 10.1073/pnas.86.22.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grove A. MarR family transcription factors. Curr. Biol. 2013;23:R142–R143. doi: 10.1016/j.cub.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Cuthbertson L., Nodwell J.R. The TetR Family of Regulators. Microbiol. Mol. Biol. Rev. 2013;77:440–475. doi: 10.1128/MMBR.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeats C., Finn R.D., Bateman A. The PASTA domain: A β-lactam-binding domain. Trends Biochem. Sci. 2002;27:438–440. doi: 10.1016/S0968-0004(02)02164-3. [DOI] [PubMed] [Google Scholar]
- 36.Alabdali Y.A.J., Oatley P., Kirk J.A., Fagan R.P. A cortex-specific penicillin-binding protein contributes to heat resistance in Clostridioides difficile spores. Anaerobe. 2021;70:102379. doi: 10.1016/j.anaerobe.2021.102379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kristich C.J., Wells C.L., Dunny G.M. A eukaryotic-type Ser/Thr kinase in Enterococcus faecalis mediates antimicrobial resistance and intestinal persistence. Proc. Natl. Acad. Sci. USA. 2007;104:3508–3513. doi: 10.1073/pnas.0608742104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetting M.W., Luiz L.P., Yu M., Hegde S.S., Magnet S., Roderick S.L., Blanchard J.S. Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 2005;433:212–226. doi: 10.1016/j.abb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Arthur M., Molinas C., Courvalin P. Sequence of the vanY gene required for production of a vancomycin-inducible D,D-carboxypeptidase in Enterococcus faecium BM4147. Gene. 1992;120:111–114. doi: 10.1016/0378-1119(92)90017-J. [DOI] [PubMed] [Google Scholar]
- 40.Kim H.S., Hahn H., Kim J., Jang D.M., Lee J.Y., Back J.M., Im H.N., Kim H., Han B.W., Suh S.W. Structural basis for the substrate recognition of peptidoglycan pentapeptides by Enterococcus faecalis VanYB. Int. J. Biol. Macromol. 2018;119:335–344. doi: 10.1016/j.ijbiomac.2018.07.081. [DOI] [PubMed] [Google Scholar]
- 41.Erlandson A., Gade P., Menikpurage I.P., Kim C.Y., Mera P.E. The UvrA-like protein Ecm16 requires ATPase activity to render resistance against echinomycin. Mol. Microbiol. 2022;117:1434–1446. doi: 10.1111/mmi.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viswanath L.S., Sugumar M., Peela S.C.M., Walia K., Sistla S. Detection of vancomycin variable enterococci (VVE) among clinical isolates of Enterococcus faecium collected across India-first report from the subcontinent. Indian J. Med. Microbiol. 2022;40:285–288. doi: 10.1016/j.ijmmb.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Park S.C., Kwak Y.M., Song W.S., Hong M., Yoon S. Il Structural basis of effector and operator recognition by the phenolic acid-responsive transcriptional regulator PadR. Nucleic Acids Res. 2017;45:13080–13093. doi: 10.1093/nar/gkx1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luque-Sastre L., Arroyo C., Fox E.M., McMahon B.J., Bai L., Li F., Fanning S. Antimicrobial Resistance in Bacteria from Livestock and Companion Animals. ASM Press; Washington, DC, USA: 2018. Antimicrobial Resistance in Listeria Species; pp. 237–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suprenant K.A., Bloom N., Fang J., Lushington G. The major vault protein is related to the toxic anion resistance protein (TelA) family. J. Exp. Biol. 2007;210:946–955. doi: 10.1242/jeb.001800. [DOI] [PubMed] [Google Scholar]
- 46.Deshpande C.N., Harrop S.J., Boucher Y., Hassan K.A., Leo R.D., Xu X., Cui H., Savchenko A., Chang C., Labbate M., et al. Crystal Structure of an Integron Gene Cassette-Associated Protein from Vibrio cholerae Identifies a Cationic Drug-Binding Module. PLoS ONE. 2011;6:e16934. doi: 10.1371/journal.pone.0016934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hobman J.L., Wilkie J., Brown N.L. A design for life: Prokaryotic metal-binding MerR family regulators. BioMetals. 2005;18:429–436. doi: 10.1007/s10534-005-3717-7. [DOI] [PubMed] [Google Scholar]
- 48.Son M.S., Del Castilho C., Duncalf K.A., Carney D., Weiner J.H., Turner R.J. Mutagenesis of SugE, a small multidrug resistance protein. Biochem. Biophys. Res. Commun. 2003;312:914–921. doi: 10.1016/j.bbrc.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Ermolenko D.N., Makhatadze G.I. Bacterial cold-shock proteins. Cell. Mol. Life Sci. C. 2002;59:1902–1913. doi: 10.1007/PL00012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barcarolo M.V., Gottig N., Ottado J., Garavaglia B.S. Participation of two general stress response proteins from Xanthomonas citri subsp. citri in environmental stress adaptation and virulence. FEMS Microbiol. Ecol. 2020;96:138. doi: 10.1093/femsec/fiaa138. [DOI] [PubMed] [Google Scholar]
- 51.Reuß D.R., Altenbuchner J., Mäder U., Rath H., Ischebeck T., Sappa P.K., Thürmer A., Guérin C., Nicolas P., Steil L., et al. Large-scale reduction of the Bacillus subtilis genome: Consequences for the transcriptional network, resource allocation, and metabolism. Genome Res. 2017;27:289–299. doi: 10.1101/gr.215293.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Begley M., Cotter P.D., Hill C., Ross R.P. Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins. Appl. Environ. Microbiol. 2009;75:5451–5460. doi: 10.1128/AEM.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montalbán-López M., Scott T.A., Ramesh S., Rahman I.R., Van Heel A.J., Viel J.H., Bandarian V., Dittmann E., Genilloud O., Goto Y., et al. New developments in RiPP discovery, enzymology and engineering. Nat. Prod. Rep. 2021;38:130–239. doi: 10.1039/D0NP00027B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abril A.G., Villa T.G., Barros-Velázquez J., Cañas B., Sánchez-Pérez A., Calo-Mata P., Carrera M. Staphylococcus aureus Exotoxins and Their Detection in the Dairy Industry and Mastitis. Toxins. 2020;12:537. doi: 10.3390/toxins12090537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brielle R., Pinel-Marie M.L., Felden B. Linking bacterial type I toxins with their actions. Curr. Opin. Microbiol. 2016;30:114–121. doi: 10.1016/j.mib.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Lima-Mendez G., Alvarenga D.O., Ross K., Hallet B., Van Melderen L., Varani A.M., Chandler M. Toxin-Antitoxin Gene Pairs Found in Tn3 Family Transposons Appear To Be an Integral Part of the Transposition Module. MBio. 2020;11:e00452-20. doi: 10.1128/mBio.00452-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szczepanowski R., Braun S., Riedel V., Schneiker S., Krahn I., Pühler A., Schlüter A. The 120 592 bp IncF plasmid pRSB107 isolated from a sewage-treatment plant encodes nine different antiobiotic-resistance determinants, two iron-acquisition systems and other putative virulence-associated functions. Microbiology. 2005;151:1095–1111. doi: 10.1099/mic.0.27773-0. [DOI] [PubMed] [Google Scholar]
- 58.Arcus V.L., Mckenzie J.L., Robson J., Cook G.M. The PIN-domain ribonucleases and the prokaryotic VapBC toxin–antitoxin array. Protein Eng. Des. Sel. 2011;24:33–40. doi: 10.1093/protein/gzq081. [DOI] [PubMed] [Google Scholar]
- 59.Nes I.F., Diep D.B., Holo H. Bacteriocin diversity in Streptococcus and Enterococcus. J. Bacteriol. 2007;189:1189–1198. doi: 10.1128/JB.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dramsi S., Bierne H. Spatial organization of cell wall-anchored proteins at the surface of gram-positive bacteria. Curr. Top. Microbiol. Immunol. 2017;404:177–201. doi: 10.1007/82_2016_4. [DOI] [PubMed] [Google Scholar]
- 61.Hendrickx A.P.A., Van Luit-Asbroek M., Schapendonk C.M.E., Van Wamel W.J.B., Braat J.C., Wijnands L.M., Bonten M.J.M., Willems R.J.L. SgrA, a nidogen-binding LPXTG surface adhesin implicated in biofilm formation, and EcbA, a collagen binding MSCRAMM, are two novel adhesins of hospital-acquired Enterococcus faecium. Infect. Immun. 2009;77:5097–5106. doi: 10.1128/IAI.00275-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobitz A.W., Kattke M.D., Wereszczynski J., Clubb R.T. Sortase Transpeptidases: Structural Biology and Catalytic Mechanism. Adv. Protein Chem. Struct. Biol. 2017;109:223–264. doi: 10.1016/bs.apcsb.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guiton P.S., Hung C.S., Kline K.A., Roth R., Kau A.L., Hayes E., Heuser J., Dodson K.W., Caparon M.G., Hultgren S.J. Contribution of autolysin and sortase A during Enterococcus faecalis DNA-dependent biofilm development. Infect. Immun. 2009;77:3626–3638. doi: 10.1128/IAI.00219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kristich C.J., Nguyen V.T., Le T., Barnes A.M.T., Grindle S., Dunny G.M. Development and use of an efficient system for random mariner transposon mutagenesis to identify novel genetic determinants of biofilm formation in the core Enterococcus faecalis genome. Appl. Environ. Microbiol. 2008;74:3377–3386. doi: 10.1128/AEM.02665-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rego S., Heal T.J., Pidwill G.R., Till M., Robson A., Lamont R.J., Sessions R.B., Jenkinson H.F., Race P.R., Nobbs A.H. Structural and Functional Analysis of Cell Wall-anchored Polypeptide Adhesin BspA in Streptococcus agalactiae. J. Biol. Chem. 2016;291:15985–16000. doi: 10.1074/jbc.M116.726562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Epler Barbercheck C.R., Bullitt E., Andersson M. Subcellular Biochemistry. Volume 87. Springer; New York, NY, USA: 2018. Bacterial adhesion pili; pp. 1–18. [DOI] [PubMed] [Google Scholar]
- 67.Spraggon G., Koesema E., Scarselli M., Malito E., Biagini M., Norais N., Emolo C., Barocchi M.A., Giusti F., Hilleringmann M., et al. Supramolecular Organization of the Repetitive Backbone Unit of the Streptococcus pneumoniae Pilus. PLoS ONE. 2010;5:e10919. doi: 10.1371/journal.pone.0010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arora S.K., Ritchings B.W., Almira E.C., Lory S., Ramphal R. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect. Immun. 1998;66:1000–1007. doi: 10.1128/IAI.66.3.1000-1007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pozzo T., Pasten J.L., Karlsson E.N., Logan D.T. Structural and Functional Analyses of β-Glucosidase 3B from Thermotoga neapolitana: A Thermostable Three-Domain Representative of Glycoside Hydrolase 3. J. Mol. Biol. 2010;397:724–739. doi: 10.1016/j.jmb.2010.01.072. [DOI] [PubMed] [Google Scholar]
- 70.Campellone K.G. Cytoskeleton-modulating effectors of enteropathogenic and enterohaemorrhagic Escherichia coli: Tir, EspFU and actin pedestal assembly. FEBS J. 2010;277:2390–2402. doi: 10.1111/j.1742-4658.2010.07653.x. [DOI] [PubMed] [Google Scholar]
- 71.Ben Said L., Jouini A., Fliss I., Torres C., Klibi N. Antimicrobial resistance genes and virulence gene encoding intimin in Escherichia coli and Enterococcus isolated from wild rabbits (Oryctolagus cuniculus) in Tunisia. Acta Vet. Hung. 2019;67:477–488. doi: 10.1556/004.2019.047. [DOI] [PubMed] [Google Scholar]
- 72.Andreevskaya M., Jääskeläinen E., Johansson P., Ylinen A., Paulin L., Björkroth J., Auvinen P. Food spoilage-associated Leuconostoc, Lactococcus, and Lactobacillus species display different survival strategies in response to competition. Appl. Environ. Microbiol. 2018;84:554–572. doi: 10.1128/AEM.00554-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schubert K., Bichlmaier A.M., Mager E., Wolff K., Ruhland G., Fiedler F. P45, an extracellular 45 kDa protein of Listeria monocytogenes with similarity to protein p60 and exhibiting peptidoglycan lyric activity. Arch. Microbiol. 2000;173:21–28. doi: 10.1007/s002030050003. [DOI] [PubMed] [Google Scholar]
- 74.Mori S., Mori K., Suzuki I., Kasumi T. Phylogenetic Analysis of Lactococcus lactis subspecies Based on Decoding the Sequence of the pepT Tripeptidase Gene, the pepV Dipeptidase Gene and 16S rRNA. Syst. Appl. Microbiol. 2004;27:414–422. doi: 10.1078/0723202041438400. [DOI] [PubMed] [Google Scholar]
- 75.Kanamaru K., Stephenson S., Perego M. Overexpression of the PepF oligopeptidase inhibits sporulation initiation in Bacillus subtilis. J. Bacteriol. 2002;184:43–50. doi: 10.1128/JB.184.1.43-50.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gómez L.A., Alvarez F.I., Molina R., Soto R., Daza-Castro C., Flores M., León Y., Oñate A.A. A Zinc-dependent metalloproteinase in the intracellular adaptation of Brucella abortus in macrophages. bioRxiv. 2020 doi: 10.3389/fmicb.2020.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Livingston E.T., Mursalin M.H., Coburn P.S., Astley R., Miller F.C., Amayem O., Lereclus D., Callegan M.C. Immune inhibitor a metalloproteases contribute to virulence in Bacillus endophthalmitis. Infect. Immun. 2021;89:IAI0020121. doi: 10.1128/IAI.00201-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwon H.Y., Ogunniyi A.D., Choi M.H., Pyo S.N., Rhee D.K., Paton J.C. The ClpP protease of Streptococcus pneumoniae modulates virulence gene expression and protects against fatal pneumococcal challenge. Infect. Immun. 2004;72:5646–5653. doi: 10.1128/IAI.72.10.5646-5653.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kajfasz J.K., Martinez A.R., Rivera-ramos I., Abranches J., Koo H., Quivey R.G. Role of Clp Proteins in Expression of Virulence Properties of Streptococcus mutans. J. Bacteriol. 2009;191:2060–2068. doi: 10.1128/JB.01609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paton J.C., Trappetti C. Gram-Positive Pathogens. ASM Press; Washington, DC, USA: 2019. Streptococcus pneumoniae Capsular Polysaccharide; pp. 304–315. [Google Scholar]
- 81.Palmer K.L., Godfrey P., Griggs A., Kos V.N., Zucker J., Desjardins C., Cerqueira G., Gevers D., Walker S., Wortman J., et al. Comparative genomics of Enterococci: Variation in Enterococcus faecalis, clade structure in E. faecium, and defining characteristics of E. gallinarum and E. casseliflavus. MBio. 2012;3:e00318-11. doi: 10.1128/mBio.00318-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Domingos R.M., Teixeira R.D., Zeida A., Agudelo W.A., Alegria T.G.P., Da Silva Neto J.F., Vieira P.S., Murakami M.T., Farah C.S., Estrin D.A., et al. Substrate and product-assisted catalysis: Molecular aspects behind structural switches along organic hydroperoxide resistance protein catalytic cycle. ACS Catal. 2020;10:6587–6602. doi: 10.1021/acscatal.0c01257. [DOI] [Google Scholar]
- 83.Bateman A., Bycroft M. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD) J. Mol. Biol. 2000;299:1113–1119. doi: 10.1006/jmbi.2000.3778. [DOI] [PubMed] [Google Scholar]
- 84.Rehman M.A., Yin X., Zaheer R., Goji N., Amoako K.K., McAllister T., Pritchard J., Topp E., Diarra M.S. Genotypes and Phenotypes of Enterococci Isolated From Broiler Chickens. Front. Sustain. Food Syst. 2018;2:83. doi: 10.3389/fsufs.2018.00083. [DOI] [Google Scholar]
- 85.Ruiz-Cruz S., Moreno-Blanco A., Espinosa M., Bravo A. Transcriptional activation by MafR, a global regulator of Enterococcus faecalis. Sci. Rep. 2019;9:6146. doi: 10.1038/s41598-019-42484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ribardo D.A., Lambert T.J., McIver K.S. Role of Streptococcus pyogenes two-component response regulators in the temporal control of Mga and the Mga-regulated virulence gene emm. Infect. Immun. 2004;72:3668–3673. doi: 10.1128/IAI.72.6.3668-3673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Warne B., Harkins C.P., Harris S.R., Vatsiou A., Stanley-Wall N., Parkhill J., Peacock S.J., Palmer T., Holden M.T.G. The Ess/Type VII secretion system of Staphylococcus aureus shows unexpected genetic diversity. BMC Genom. 2016;17:222. doi: 10.1186/s12864-016-2426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdelhamed H., Ramachandran R., Narayanan L., Ozdemir O., Cooper A., Olivier A.K., Karsi A., Lawrence M.L. Contributions of a LysR transcriptional regulator to listeria monocytogenes virulence and identification of its regulons. J. Bacteriol. 2020;202:e00087-20. doi: 10.1128/JB.00087-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torres C., Alonso C.A., Ruiz-Ripa L., León-Sampedro R., del Campo R., Coque T.M. Antimicrobial Resistance in Bacteria from Livestock and Companion Animals. ASM Press; Washington, DC, USA: 2018. Antimicrobial Resistance in Enterococcus spp. of animal origin; pp. 185–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trost M., Wehmhöner D., Kärst U., Dieterich G., Wehland J., Jänsch L. Comparative proteome analysis of secretory proteins from pathogenic and nonpathogenic Listeria species. Proteomics. 2005;5:1544–1557. doi: 10.1002/pmic.200401024. [DOI] [PubMed] [Google Scholar]
- 91.McGowan S., Lucet I.S., Cheung J.K., Awad M.M., Whisstock J.C., Rood J.I. The FxRxHrS Motif: A Conserved Region Essential for DNA Binding of the VirR Response Regulator from Clostridium perfringens. J. Mol. Biol. 2002;322:997–1011. doi: 10.1016/S0022-2836(02)00850-1. [DOI] [PubMed] [Google Scholar]
- 92.Del Papa M.F., Perego M. Enterococcus faecalis virulence regulator FsrA binding to target promoters. J. Bacteriol. 2011;193:1527–1532. doi: 10.1128/JB.01522-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang H., Wang Y., Humphris S., Nie W., Zhang P., Wright F., Campbell E., Hu B., Fan J., Toth I. Pectobacterium atrosepticum KDPG aldolase, Eda, participates in the Entner–Doudoroff pathway and independently inhibits expression of virulence determinants. Mol. Plant Pathol. 2021;22:271–283. doi: 10.1111/mpp.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Del Mar Lleo M., Fontana R., Solioz M. Identification of a gene (arpU) controlling muramidase-2 export in Enterococcus hirae. J. Bacteriol. 1995;177:5912–5917. doi: 10.1128/jb.177.20.5912-5917.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Molloy S. A competent escape for Listeria. Nat. Rev. Microbiol. 2012;10:670. doi: 10.1038/nrmicro2885. [DOI] [PubMed] [Google Scholar]
- 96.Ud-Din A.I.M.S., Roujeinikova A. Methyl-accepting chemotaxis proteins: A core sensing element in Prokaryotes and Archaea. Cell. Mol. Life Sci. 2017;74:3293–3303. doi: 10.1007/s00018-017-2514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuwana R., Yamamura S., Ikejiri H., Kobayashi K., Ogasawara N., Asai K., Sadaie Y., Takamatsu H., Watabe K. Bacillus subtilis spoVIF (yjcC) gene, involved in coat assembly and spore resistance. Microbiology. 2003;149:3011–3021. doi: 10.1099/mic.0.26432-0. [DOI] [PubMed] [Google Scholar]
- 98.Aertsen A., Michiels C.W. Mrr instigates the SOS response after high pressure stress in Escherichia coli. Mol. Microbiol. 2005;58:1381–1391. doi: 10.1111/j.1365-2958.2005.04903.x. [DOI] [PubMed] [Google Scholar]
- 99.Guerardel Y., Sadovskaya I., Maes E., Furlan S., Chapot-Chartier M.P., Mesnage S., Rigottier-Gois L., Serror P. Complete structure of the Enterococcal polysaccharide antigen (EPA) of vancomycin-resistant Enterococcus faecalis v583 reveals that EPA decorations are teichoic acids covalently linked to a rhamnopolysaccharide backbone. MBio. 2020;11:e00277-20. doi: 10.1128/mBio.00277-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang S.S., Sim J.R., Yun C.H., Han S.H. Lipoteichoic acids as a major virulence factor causing inflammatory responses via Toll-like receptor 2. Arch. Pharm. Res. 2016;39:1519–1529. doi: 10.1007/s12272-016-0804-y. [DOI] [PubMed] [Google Scholar]
- 101.Benachour A., Ladjouzi R., Le Jeune A., Hébert L., Thorpe S., Courtin P., Chapot-Chartier M.P., Prajsnar T.K., Foster S.J., Mesnage S. The Lysozyme-Induced Peptidoglycan N-Acetylglucosamine Deacetylase pgda (EF1843) Is Required for Enterococcus faecalis Virulence. J. Bacteriol. 2012;194:6066–6073. doi: 10.1128/JB.00981-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vollmer W., Tomasz A. Peptidoglycan N-acetylglucosamine deacetylase, a putative virulence factor in Streptococcus pneumoniae. Infect. Immun. 2002;70:7176–7178. doi: 10.1128/IAI.70.12.7176-7178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meeske A.J., Riley E.P., Robins W.P., Uehara T., Mekalanos J.J., Kahne D., Walker S., Kruse A.C., Bernhardt T.G., Rudner D.Z. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature. 2016;537:634–638. doi: 10.1038/nature19331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Price C.T.D., Bukka A., Cynamon M., Graham J.E. Glycine betaine uptake by the proXVWZ ABC transporter contributes to the ability of Mycobacterium tuberculosis to initiate growth in human macrophages. J. Bacteriol. 2008;190:3955–3961. doi: 10.1128/JB.01476-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanaka K.J., Song S., Mason K., Pinkett H.W. Selective substrate uptake: The role of ATP-binding cassette ( ABC ) importers in pathogenesis. BBA Biomembr. 2018;1860:868–877. doi: 10.1016/j.bbamem.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orelle C., Mathieu K., Jault J.M. Multidrug ABC transporters in bacteria. Res. Microbiol. 2019;170:381–391. doi: 10.1016/j.resmic.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 107.Kumar S., Mukherjee M.M., Varela M.F. Modulation of Bacterial Multidrug Resistance Efflux Pumps of the Major Facilitator Superfamily. Int. J. Bacteriol. 2013;2013:204141. doi: 10.1155/2013/204141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cotrim C.A., Jarrott R.J., Martin J.L., Drew D. A structural overview of the zinc transporters in the cation diffusion facilitator family. Acta Crystallogr. Sect. D Struct. Biol. 2019;75:357–367. doi: 10.1107/S2059798319003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jack D.L., Yang N.M., Saier M.H. The drug/metabolite transporter superfamily. Eur. J. Biochem. 2001;268:3620–3639. doi: 10.1046/j.1432-1327.2001.02265.x. [DOI] [PubMed] [Google Scholar]
- 110.Xia G., Wolz C. Phages of Staphylococcus aureus and their impact on host evolution. Infect. Genet. Evol. 2014;21:593–601. doi: 10.1016/j.meegid.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 111.Romero P., López R., García E. Genomic organization and molecular analysis of the inducible prophage EJ-1, a mosaic myovirus from an atypical Pneumococcus. Virology. 2004;322:239–252. doi: 10.1016/j.virol.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 112.Fortier L.C., Sekulovic O. Importance of prophages to evolution and virulence of bacterial pathogens. Virulence. 2013;4:354–365. doi: 10.4161/viru.24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Menouni R., Hutinet G., Petit M.A., Ansaldi M. Bacterial genome remodeling through bacteriophage recombination. FEMS Microbiol. Lett. 2015;362:1–10. doi: 10.1093/femsle/fnu022. [DOI] [PubMed] [Google Scholar]
- 114.Deghorain M., Van Melderen L. The Staphylococci phages family: An overview. Viruses. 2012;4:3316–3335. doi: 10.3390/v4123316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feiner R., Argov T., Rabinovich L., Sigal N., Borovok I., Herskovits A.A. A new perspective on lysogeny: Prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 2015;13:641–650. doi: 10.1038/nrmicro3527. [DOI] [PubMed] [Google Scholar]
- 116.Penadés J.R., Chen J., Quiles-Puchalt N., Carpena N., Novick R.P. Bacteriophage-mediated spread of bacterial virulence genes. Curr. Opin. Microbiol. 2015;23:171–178. doi: 10.1016/j.mib.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 117.Hall R.J., Whelan F.J., McInerney J.O., Ou Y., Domingo-Sananes M.R. Horizontal Gene Transfer as a Source of Conflict and Cooperation in Prokaryotes. Front. Microbiol. 2020;11:1569. doi: 10.3389/fmicb.2020.01569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robinson K.M., Dunning Hotopp J.C. Mobile elements and viral integrations prompt considerations for bacterial DNA integration as a novel carcinogen. Cancer Lett. 2014;352:137–144. doi: 10.1016/j.canlet.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fatoba D.O., Amoako D.G., Akebe A.L.K., Ismail A., Essack S.Y. Genomic analysis of antibiotic-resistant Enterococcus spp. reveals novel Enterococci strains and the spread of plasmid-borne Tet(M), Tet(L) and Erm(B) genes from chicken litter to agricultural soil in South Africa. J. Environ. Manag. 2022;302:114101. doi: 10.1016/j.jenvman.2021.114101. [DOI] [PubMed] [Google Scholar]
- 120.Lu J., Wong J.J.W., Edwards R.A., Manchak J., Frost L.S., Glover J.N.M. Structural basis of specific TraD–TraM recognition during F plasmid-mediated bacterial conjugation. Mol. Microbiol. 2008;70:89–99. doi: 10.1111/j.1365-2958.2008.06391.x. [DOI] [PubMed] [Google Scholar]
- 121.Wang J., Yu Y., Tao F., Zhang J., Copetti D., Kudrna D., Talag J., Lee S., Wing R.A., Fan C. DNA methylation changes facilitated evolution of genes derived from Mutator-like transposable elements. Genome Biol. 2016;17:92. doi: 10.1186/s13059-016-0954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kommineni S., Bretl D.J., Lam V., Chakraborty R., Hayward M., Simpson P., Cao Y., Bousounis P., Kristich C.J., Salzman N.H. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature. 2015;526:719–722. doi: 10.1038/nature15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li F., Alvarez-Martinez C., Chen Y., Choi K.J., Yeo H.J., Christie P.J. Enterococcus faecalis PrgJ, a VirB4-Like ATPase, Mediates pCF10 conjugative transfer through substrate binding. J. Bacteriol. 2012;194:4041–4051. doi: 10.1128/JB.00648-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhatty M., Camacho M.I., Gonzalez-Rivera C., Frank K.L., Dale J.L., Manias D.A., Dunny G.M., Christie P.J. PrgU: A suppressor of sex pheromone toxicity in Enterococcus faecalis. Mol. Microbiol. 2017;103:398–412. doi: 10.1111/mmi.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alekseev A., Pobegalov G., Morozova N., Vedyaykin A., Cherevatenko G., Yakimov A., Baitin D., Khodorkovskii M. A new insight into RecA filament regulation by RecX from the analysis of conformation-specific interactions. bioRxiv. 2022 doi: 10.1101/2022.03.14.484239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vellani T.S., Myers R.S. Bacteriophage SPP1 Chu is an alkaline exonuclease in the SynExo family of viral two-component recombinases. J. Bacteriol. 2003;185:2465–2474. doi: 10.1128/JB.185.8.2465-2474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodríguez-Rubio L., Gutiérrez D., Martínez B., Rodríguez A., Götz F., García P. The tape measure protein of the Staphylococcus aureus bacteriophage vB_SauS-phiIPLA35 has an active muramidase domain. Appl. Environ. Microbiol. 2012;78:6369–6371. doi: 10.1128/AEM.01236-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mahony J., Alqarni M., Stockdale S., Spinelli S., Feyereisen M., Cambillau C., Van Sinderen D. Functional and structural dissection of the tape measure protein of Lactococcal phage TP901-1. Sci. Rep. 2016;6:36667. doi: 10.1038/srep36667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Durante-Rodríguez G., Mancheño J.M., Díaz E., Carmona M. Refactoring the λ phage lytic/lysogenic decision with a synthetic regulator. Microbiologyopen. 2016;5:575–581. doi: 10.1002/mbo3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Veesler D., Spinelli S., Mahony J., Lichière J., Blangy S., Bricogne G., Legrand P., Ortiz-Lombardia M., Campanacci V., Van Sinderen D., et al. Structure of the phage TP901-1 1.8 MDa baseplate suggests an alternative host adhesion mechanism. Proc. Natl. Acad. Sci. USA. 2012;109:8954–8958. doi: 10.1073/pnas.1200966109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kostyuchenko V.A., Leiman P.G., Chipman P.R., Kanamaru S., Van Raaij M.J., Arisaka F., Mesyanzhinov V.V., Rossmann M.G. Three-dimensional structure of bacteriophage T4 baseplate. Nat. Struct. Mol. Biol. 2003;10:688–693. doi: 10.1038/nsb970. [DOI] [PubMed] [Google Scholar]
- 132.Quintela-Baluja M., Böhme K., Fernández-No I.C., Morandi S., Alnakip M.E., Caamaño-Antelo S., Barros-Velázquez J., Calo-Mata P. Characterization of different food-isolated Enterococcus strains by MALDI-TOF mass fingerprinting. Electrophoresis. 2013;34:2240–2250. doi: 10.1002/elps.201200699. [DOI] [PubMed] [Google Scholar]
- 133.Shridhar P.B., Amachawadi R.G., Tokach M., Patel I., Gangiredla J., Mammel M., Nagaraja T.G. Whole genome sequence analyses-based assessment of virulence potential and antimicrobial susceptibilities and resistance of Enterococcus faecium strains isolated from commercial swine and cattle probiotic products. J. Anim. Sci. 2022;100:skac030. doi: 10.1093/jas/skac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yuksekdag Z., Ahlatcı N.S., Hajikhani R., Darilmaz D.O., Beyatli Y. Safety and metabolic characteristics of 17 Enterococcus faecium isolates. Arch. Microbiol. 2021;203:5683–5694. doi: 10.1007/s00203-021-02536-8. [DOI] [PubMed] [Google Scholar]
- 135.Abril A.G., Carrera M., Böhme K., Barros-Velázquez J., Cañas B., Rama J.L.R., Villa T.G., Calo-Mata P. Characterization of Bacteriophage Peptides of Pathogenic Streptococcus by LC-ESI-MS/MS: Bacteriophage Phylogenomics and Their Relationship to Their Host. Front. Microbiol. 2020;11:1241. doi: 10.3389/fmicb.2020.01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carrera M., Cañas B., Gallardo J.M. The sarcoplasmic fish proteome: Pathways, metabolic networks and potential bioactive peptides for nutritional inferences. J. Proteom. 2013;78:211–220. doi: 10.1016/j.jprot.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 137.Calo-Mata P., Carrera M., Böhme K., Barros-Velázquez J. Novel Peptide Biomarker Discovery for Detection and Identification of Bacterial Pathogens by Bioactives compounds from seafood by-products. Artic. J. Anal. Bioanal. Tech. 2016;7:296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.