Abstract
Fungal infections by Candida spp. are opportunistic and most often occur in individuals with some predisposing factor. Essential oils (EO) have anti-Candida potential, being a therapeutic alternative to be explored, especially for superficial and mucosal candidiasis. The objective was to analyze the synergistic potential between the EO of Citrus limon, Cupressus sempervirens, Litsea cubeba and Melaleuca alternifolia, and each of them with clotrimazole, to inhibit in vitro the formation and eradication of Candida spp. biofilms. Added to this, the survival of Caenorhabditis elegans was evaluated after exposure to EO, clotrimazole and their synergistic combinations. Anti-Candida activity was determined by microdilution for the substances alone and in EO–EO and EO–clotrimazole combinations. The combinations were performed by the checkerboard method, and the reduction in the metabolic activity of biofilms was determined by the viability of MTT/menadione. C. elegans larvae survival was evaluated after 24 h of exposure to EO, clotrimazole and synergistic combinations. The minimum inhibitory concentration (MIC) of EO ranged from 500 to >4000 µg/mL. The lowest MIC (500 µg/mL) was for C. sempervirens and L. cubeba on a C. krusei isolate; for clotrimazole, the MIC ranged from 0.015 to 0.5 µg/mL. Biofilm inhibition and eradication both ranged from 1000 to >4000 µg/mL. The lethal concentration (LC50) of C. limon, L. cubeba and M. alternifolia was 2000 µg/mL for C. elegans, while for C. sempervirens and clotrimazole, it was not determined within the concentration limits tested. In combination, more than 85% of the larvae survived M. alternifolia–clotrimazole, M. alternifolia–L. cubeba, C. sempervirens–clotrimazole and C. sempervirens–C. limon combinations. This study is the first, to our knowledge, to present a synergistic relationship of EO–EO and EO–clotrimazole combinations on Candida spp. biofilms.
Keywords: essential oils, clotrimazole, Candida spp., synergy, biofilm, toxicity, Caenorhabditis elegans
1. Introduction
Infections caused by species of the genus Candida are opportunistic and more severe in immunocompromised, hospitalized individuals, using invasive devices and with comorbidities [1]. Superficial candidiasis affects the oral and vaginal mucosa, skin and nails; and factors external to the individual, such as climatic conditions in tropical and subtropical regions, and factors inherent to it, such as local humidity, use of immunosuppressive or antibacterial drugs and some comorbidities, such as diabetes, facilitate the development of the disease [2]. In superficial infections, the most frequent Candida species is C. albicans; however, in recent years, Candida non-albicans species have shown relevance among the causative agents of vulvovaginal candidiasis (VVC) and recurrent VVC (RVVC), including the species C. glabrata, C. krusei and C. tropicalis [2,3,4].
The azole antifungals have been one of the therapeutic options for the treatment of superficial mycoses since the 1960s–1970s [5,6]. In this sense, topical formulations containing azoles are attractive for VVC and RVVC due to the lower incidence of adverse effects compared to the same drug class for oral use and systemic action [3]. Clotrimazole has a cure rate of between 73% and 100% of infections, similar to other topical antifungals such as nystatin [6,7].
In the last decades, the report of new Candida species and in vitro resistant isolates to traditional antifungals has been an incentive for the search and development of new ways of managing these infections [8]. Resistance is a result of multiple factors that include structural changes in the drug target and the ability of Candida spp. to form biofilms [9,10,11]. In this sense, the community structure and firm adherence between the microorganisms of the biofilm allow a barrier condition that makes penetration of drugs difficult and consequently reduces the effectiveness of the treatment [8,10,11].
Essential oils are plant-derived products with potential activity against microorganisms, attributable to the complex mixture of chemotypes [12,13,14]. Recently, combinations of multiple agents have optimized antifungal activity against clinically relevant fungi. Thus, a new therapeutic approach combining conventional antifungal drugs, such as clotrimazole, and natural products with antifungal activity may have the potential for clinical use [15,16,17,18].
The in vivo screening of compounds with proven in vitro antimicrobial action is one of the necessary steps within the current safety context to identify the toxicity of new anti-infective agents [19]. In this context, in vivo studies using alternative animal models such as Drosophila melanogaster, Galleria mellonella and Caenorhabditis elegans have been proposed to assess the preliminary toxicity of new health products [20,21]. Thus, the free-living nematode C. elegans can be an alternative predictive model option, being of low cost, fast cultivation and not very complex laboratory handling, and lending itself to the evaluation and screening of acute toxicity for use in animals, including humans, and contamination of the environment [11,19,22]. In this sense, the evident anti-Candida action of isolated essential oils could mean they present lower inhibitory values when combined with other essential oils or antifungal substances, such as clotrimazole, and its acute toxic repercussions.
Thus, in this study, the in vitro inhibitory activity of the essential oils of Cupressus sempervirens, Citrus limon, Litsea cubeba and Melaleuca alternifolia, alone and in combination, and associated with clotrimazole, against Candida species biofilms were analyzed. Furthermore, the in vivo toxicity of these essential oils against C. elegans was also evaluated.
2. Materials and Methods
2.1. Essential Oils and Candida Species
The essential oils (EO) of Citrus limon, Cupressus sempervirens, Litsea cubeba and Melaleuca alternifolia (FERQUIMA®; Vargem Grande Paulista, SP, Brazil) were included in this study. The analysis of the EO, carried out by chromatography, was informed by the supplier company and is shown in Table 1. Four reference strains, Candida albicans ATCC 90028, Candida glabrata ATCC 2001, Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019, and four clinical isolates from the vaginal mucosa (Candida albicans SV 01, Candida glabrata SV 02, Candida krusei SV 03 and Candida parapsilosis SV 04) obtained from previous studies were included in this study [23]. All microorganisms were stored in brain heart infusion (BHI)–glycerol broth at −20 °C and subcultured on Sabouraud dextrose agar (SDA; Disco, Detroit, MI, USA) and CHROMagar Candida medium (Becton Dickinson and Company, Sparks, MD, USA), to evaluate the viability and purity, and even to confirm the identification of the species.
Table 1.
Main components of essential oils from Citrus limon, Cupressus sempervirens, Litsea cubeba and Melaleuca alternifolia, according to the supplier company.
| Essential Oil | Part of the Plant | Extraction Method | Main Components |
|---|---|---|---|
| C. limon | Fruit | Cold pressing | Limonene (65.6%), β-pinene (15.06%), γ-terpinene (7.93%), α-pinene (2.34%), sabinene (1.76%) and myrcene (1.55%) |
| C. sempervirens | Leaf | NI | α-Pinene (52.4%), δ-3-carene (22%), limonene (3.5%), terpinolene (3.4%), myrcene (2.4%), terpenyl acetate (1.7%), cedrol (1.4%), β-pinene (1.2%) and terpinen-4-ol (1%) |
| L. cubeba | Fruit | Steam distillation | Geranyl acetate (42%), neral (30%) and limonene (13%) |
| M. alternifolia | Leaf | Steam distillation | Terpinen-4-ol (41%), γ-terpinene (20.5%), α-terpinene (9.63%), α-terpinolene (3.37%), α-terpineol (2.78%), α-pinene (2.59%), ρ-cymene (2.39%), aromadendrene (2%), vidiflorene (1.81%), δ-cadinene (1.54%) and 1,8-cineol (1.50%) |
NI: not informed. Chemical analysis of the oils by chromatography was provided by the supplier company.
2.2. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
To determine the MIC of EO and antifungal agents, the broth microdilution method was used [24], with some adaptations. Flat-bottomed 96-well plates (Kasvi, PR, Brazil) and RPMI-1640 broth with glutamine and without sodium bicarbonate (Corning Incorporated, Corning, NY, USA) were used, plus 18 g/L of glucose (Sigma-Aldrich, St. Louis, MO, USA), buffered with MOPS at pH 7 (Sigma-Aldrich, St. Louis, MO, USA) as a culture medium, and yeast suspension at a resulting final concentration of 0.5–2.5 × 103 CFU/mL. The concentration ranges varied from 7.81 to 4000 µg/mL for EO, from 0.03 to 16 µg/mL for amphotericin B (Sigma-Aldrich, St. Louis, MO, USA) and from 0.125 to 64 µg/mL for fluconazole and clotrimazole (Sigma-Aldrich, St. Louis, MO, USA). Amphotericin B and fluconazole were used as controls [24].
EO and amphotericin B were solubilized in DMSO (dimethyl sulfoxide; 2%), fluconazole and clotrimazole in water and later diluted in RPMI. C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 strains were used to validate the tests [24]. The MIC was determined in a spectrophotometer at 490 nm [24]. The cut-off point for defining susceptibility was set at 80% inhibition of fungal growth compared to azole-free growth and 90% inhibition for amphotericin B [24] and EO [25]; cultures were incubated for 24 h at 35 °C. The tests were performed in triplicate.
The MFC was determined by transferring 5 μL of cell suspension from each well to a plate containing SDA, followed by incubation for 48 h at 30 °C. The MFC was the one corresponding to the concentration of the well where the growth of yeast colonies was no longer evident [26]. The ratio of the MFC and MIC of EO and clotrimazole was used to interpret the results, defining the drug as fungistatic (MFC/MIC: >4) or fungicidal (MFC/MIC: ≤4) [27].
2.3. Evaluation of the Activity of EO and Clotrimazole against Candida spp. Biofilms
2.3.1. Determination of the Minimum Biofilm-Inhibiting Concentration (MBIC)
Inhibition of biofilm formation was determined in 96-well, flat-bottomed plates [28], to which 100 µL of cell suspension in RPMI-1640 was added (1 to 5 × 106 cells/mL, adjusted to a turbidity equivalent to 0.5 McFarland scale), as well as 100 µL of the drug (EO and/or clotrimazole), at concentrations of 4 × MIC, 2 × MIC, 1 × MIC, 0.5 × MIC and 0.25 × MIC. The culture was incubated at 35 °C for 48 h. Then, non-adherent cells were removed, and the wells were washed three times with PBS (10 mM phosphate buffer, 2.7 mM potassium chloride, 137 mM sodium chloride, pH 7.4). Then, 100 µL of MTT solution (5 mg/mL; 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide; Sigma-Aldrich, St. Louis, MO, USA) plus 10 µL of phytomenadione (1 µM; 2-methyl-3-[(E,7R,11R)-3,7,11,15-tetramethylhexadec-2-enyl]naphthalene-1, 4-dione; Sigma-Aldrich, St. Louis, MO, USA) was added to each well. The plate was incubated at 35 °C for 24 h in the dark. Subsequently, the supernatant was removed, and 100 µL of DMSO was added to each well, and the plate was incubated at 35 °C for 15 min, protected from light. Then, 80 µL of solvent was removed from each well and transferred to another plate, and the reading was performed at 490 nm [29]. Growth and sterility controls were included for each plate in the experiment. The tests were performed in triplicate.
2.3.2. Determination of the Minimum Biofilm-Eradication Concentration (MBEC)
The biofilm was previously formed in 96-well, flat-bottomed plates. Two hundred microliters of the yeast cell suspension were added to each well (1 to 5 × 106 cells/mL, adjusted to 0.5 McFarland in RPMI-1640), and the plates were incubated at 35 °C for 48 h [28]. Then, non-adherent cells were removed, and the wells were washed three times with PBS. Then, 100 µL of the drug (EO and/or clotrimazole) was added at concentrations of 4 × MIC, 2 × MIC, 1 × MIC, 0.5 × MIC and 0.25 × MIC and incubated at 35 °C for 24 h in the dark. The procedures for revealing the biofilm were the same as described in the previous item.
2.4. Evaluation of the Synergistic Potential of EO–EO and EO–Clotrimazole Associations against Planktonic Growth and on Biofilms
To evaluate the combined effect of EO and clotrimazole on planktonic cells, the checkerboard technique was used [30,31]. The synergistic potential of the combination of the EO of C. sempervirens, C. limon, L. cubeba and M. alternifolia among themselves and of each one of them with clotrimazole on the Candida biofilm was made according to the results obtained for the MIC of the planktonic cells, as provided in the Supplementary Material (Table S1). Concentrations of 0.25 × MIC, 0.5 × MIC, 1 × MIC, 2 × MIC and 4 × MIC were used for drug testing. Then, 100 µL of drug A (EO) horizontal and 100 μL of drug B (EO/clotrimazole) vertical, and 100 μL of cell suspension (1 to 5 × 103 cells/mL) were added to all wells of a 96-well flat-bottomed plate. Growth (containing drug-free yeast suspension) and sterility (RPMI-1640) controls were included in each plate. The plates were incubated at 35 °C for 48 h, and the reading was performed at 490 nm; results were considered capable of reducing ≥ 90% of optical density (OD) in relation to the control free of EO and clotrimazole [30]. The results were interpreted according to the fractional inhibitory concentration index (FICI), determined as follows:
| (1) |
The interpretation was conducted according to the classification of the substance interaction score, where antagonism was considered when the score was greater than 4.0, indifference at a score greater than 1, additivity at a score between 0.5 and 1.0, and synergism at a score less than 0.5 [31]. One hundred microliters of the combined solution “A” (EO) and “B” (EO or clotrimazole) was added to each well, starting from column 11 and column 2. Briefly, the first and last wells received the highest concentrations of each of the two compounds evaluated, resulting in decreasing concentrations from one end of the plate to the other. Growth and sterility controls were included in each of the plates, and each experiment was conducted in triplicate.
2.5. Toxicity Assay for Caenorhabditis elegans
The toxicity test was performed by exposing C. elegans larvae (AU37 [glp-4 (bn2) I; mutant strain sek-1 (km4) X) to EO and clotrimazole [25].
C. elegans larvae were transferred to nematode growth medium (NGM), contained in Petri dishes, which contained a previous mat of Escherichia coli OP50 (E. coli). The plates were incubated at 16 °C for 72 h. Then, synchronization of the larvae in stage L2 was performed by treating the larvae with sodium hypochlorite. Then, the larvae were transferred to another plate containing NGM medium without E. coli OP50 and incubated at 16 °C for 24 h [20,32].
For the experiment, a solution medium, composed of 40% BHI broth, plus cholesterol (10 µg/mL), kanamycin (90 µg/mL), ampicillin (200 µg/mL) and 60% 50 mM NaCl, was used. The assay was performed using 96-well flat-bottomed plates. Then, 180 µL of solution medium and 20 µL of the suspension of synchronized larvae in stage L4 were added to each well of the plate so that 10 to 20 C. elegans larvae were placed in each well, evaluated in final serial concentrations ranging from 4000 to 250 µg/mL diluted in solution medium. As a survival control, solution medium plus the larvae, without drug, was used, and as a test control, solution medium and DMSO were used. The plates were incubated for 24 h at 35 °C in a humid chamber.
The results were interpreted considering the survival rate of the larvae and the 50% lethal dose (LD50), determined by the concentration of the drug that was able to kill 50% of the larvae [33,34]. Each experiment was performed twice in triplicate.
3. Results
3.1. Determination of the MIC and MFC of Essential and Antifungal Oils against Planktonic Growth
The lowest MIC (500 µg/mL) found was for C. krusei SV 03, with the EO of C. sempervirens and L. cubeba. The MIC of the EO ranged from 500 to >4000 µg/mL, considering the different oils and the eight isolates tested. The EO of C. limon presented an MIC that ranged from 1000 to 4000 µg/mL, C. sempervirens from 500 to >4000 µg/mL, L. cubeba from 500 to 2000 µg/mL and M. alternifolia from 1000 to 2000 µg/mL. For clotrimazole, the MIC ranged from 0.015 to 0.5 µg/mL (Table 1). The MIC of fluconazole and amphotericin B (test validation controls) were, respectively, 32 and 1 µg/mL; those for C. krusei ATCC 6258 and C. parapsilosis ATCC 2019 were 0.25 and 0.25 µg/mL, respectively.
The lowest fungicidal concentrations (1000 µg/mL) were found for the EO of C. limon against C. albicans ATCC 90028, C. sempervirens against C. krusei SV 03 and L. cubeba against C. albicans ATCC 90028 and C. krusei SV 03; for clotrimazole, the lowest fungicidal concentration (0.030 µg/mL) was found for the isolate of C. glabrata ATCC 2001.
Evaluation of the fungicidal activity (MFC/MIC: ≤4) showed that the EO of L. cubeba and the antifungal clotrimazole were fungicidal for all the tested isolates; however, all the other EO evaluated presented fungicidal activity dependent on the isolate, as can be seen in Table 2. Thus, fungicidal activity was found for the EO of C. limon, M. alternifolia and C. sempervirens, respectively, for four, five and six isolates.
Table 2.
Minimum inhibitory concentration (µg/mL) and minimum fungicidal concentration (µg/mL) of essential oils and clotrimazole tested with Candida species.
| Candida spp. Isolates | C. limon | C. sempervirens | L. cubeba | M. alternifolia | Clotrimazole | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| C. albicans ATCC 90028 | 2000 | 1000 | 2000 | 4000 | 1000 | 1000 | 2000 | 4000 | 0.25 | 0.25 |
| C. albicans SV 01 | 4000 | >4000 | 4000 | >4000 | 2000 | 4000 | 4000 | >4000 | 0.125 | 0.25 |
| C. glabrata ATCC 2001 | 4000 | 4000 | 2000 | 4000 | 2000 | 4000 | 4000 | 4000 | 0.015 | 0.030 |
| C. glabrata SV 02 | 4000 | >4000 | 1000 | 4000 | 2000 | 4000 | 4000 | >4000 | 0.25 | 0.5 |
| C. krusei ATCC 6258 | 4000 | >4000 | 2000 | 4000 | 1000 | 2000 | 4000 | 4000 | 0.5 | 1 |
| C. krusei SV 03 | 1000 | 4000 | 500 | 1000 | 500 | 1000 | 2000 | 4000 | 0.5 | 1 |
| C. parapsilosis ATCC 22019 | 1000 | 4000 | 4000 | 4000 | 1000 | 4000 | 4000 | 4000 | 0.25 | 0.5 |
| C. parapsilosis SV 04 | 4000 | >4000 | >4000 | >4000 | 2000 | 4000 | 4000 | >4000 | 0.125 | 0.25 |
MIC: minimum inhibitory concentration (µg/mL); MFC: minimum fungicidal concentration (µg/mL). Fungicide: (MFC/MIC: <4) in bold.
3.2. Assessment of the Development of Candida spp. Biofilms
The activity of EO and clotrimazole to inhibit (MBIC) and eradicate (MBEC) the biofilm formed by Candida species is shown in Table 3. Most EO presented an MBIC greater than or equal to 4000 µg/mL. The lowest MBIC was 1000 µg/mL, found for C. sempervirens (C. krusei SV 03), L. cubeba (C. albicans ATCC 90028) and M. alternifolia (C. krusei SV 03). The lowest MBEC was 1000 µg/mL for L. cubeba (C. albicans ATCC 90028). Clotrimazole demonstrated MBIC and MBEC values ranging from 0.125 to 2 µg/mL and 0.25 to 4 µg/mL, respectively.
Table 3.
Activity of essential oils and clotrimazole against the formation of biofilms and preformed biofilms of Candida species.
| Species | C. limon | C. sempervirens | L. cubeba | M. alternifolia | Clotrimazole | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBIC | MBEC | MIC | MBIC | MBEC | MIC | MBIC | MBEC | MIC | MBIC | MBEC | MIC | MBIC | MBEC | |
| C. albicans ATCC 90028 | 2000 | 4000 | 4000 | 2000 | 4000 | >4000 | 1000 | 1000 | 1000 | 2000 | 2000 | 4000 | 0.25 | 1 | 1 |
| C. albicans SV 01 | 4000 | >4000 | >4000 | 4000 | 4000 | 4000 | 2000 | 2000 | 4000 | 4000 | 4000 | 4000 | 0.125 | 0.5 | 1 |
| C. glabrata ATCC 2001 | 4000 | >4000 | >4000 | 2000 | >4000 | 4000 | 2000 | 2000 | >4000 | 4000 | 4000 | >4000 | 0.015 | 0.125 | 0.25 |
| C. glabrata SV 02 | 4000 | >4000 | >4000 | 1000 | >4000 | >4000 | 2000 | >4000 | >4000 | 4000 | 4000 | >4000 | 0.25 | 0.5 | 1 |
| C. krusei ATCC 6258 | 4000 | >4000 | >4000 | 2000 | 2000 | 2000 | 1000 | >4000 | >4000 | 2000 | 4000 | >4000 | 0.5 | 1 | 2 |
| C. krusei SV 03 | 1000 | 2000 | 4000 | 500 | 1000 | >4000 | 500 | 4000 | >4000 | 1000 | 1000 | >4000 | 0.5 | 1 | 1 |
| C. parapsilosis ATCC 22019 | 1000 | >4000 | >4000 | 4000 | 4000 | >4000 | 1000 | 4000 | >4000 | 4000 | 4000 | >4000 | 0.25 | 2 | 4 |
| C. parapsilosis SV 04 | 4000 | >4000 | >4000 | >4000 | 4000 | >4000 | 2000 | >4000 | >4000 | 4000 | >4000 | >4000 | 0.125 | 0.25 | 0.5 |
Isolated MBIC and MBEC (µg/mL): capable of reducing ≥ 90% of optical density (OD) compared to control free of EO and clotrimazole. Results in bold: ≤1000 µg/mL.
3.3. Evaluation of Synergism of EO and Clotrimazole
The tests of EO–EO and EO–clotrimazole combinations resulted in 80 combinations; of these, 13 (16.25%) showed antagonism, 42 (52.5%) showed indifference, 17 (21.25%) had an additive effect and 8 (10%) showed synergism. The OE–OE and OE–clotrimazole combinations performed and their results related to inhibition of Candida spp. are provided in the Supplementary Materials (Table S1). The synergistic effect was variable, depending on the combination (EO–EO or EO–clotrimazole) and the Candida strain. The EO–EO and EO–clotrimazole combinations that showed synergism in the evaluation of MIC were selected for evaluation of inhibition (MBIC) and eradication (MBEC) of biofilms (Table 4).
Table 4.
Minimum inhibitory, biofilm-inhibitory and biofilm-eradication concentrations of EO–EO and EO–clotrimazole combinations against Candida species.
| Species | Combination | MIC (µg/mL) | Biofilm (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| Isolated MIC * | Combined MIC ** | Isolated MBIC * | Combined MBIC ** | Isolated MBEC * | Combined MBEC ** | ||
| C. albicans ATCC 90028 | M. alternifolia | 2000 | 250 | 2000 | 62.5 | 4000 | 62.5 |
| Clotrimazole | 0.25 | 0.063 | 1 | 0.25 | 1 | 0.25 | |
| C. albicans SV 01 | L. cubeba | 2000 | 250 | 2000 | 125 | 4000 | 250 |
| M. alternifolia | 4000 | 1000 | 4000 | 2000 | 4000 | 1000 | |
| C. glabrata ATCC 2001 | L. cubeba | 2000 | 500 | 2000 | 2000 | >4000 | 2000 |
| C. limon | 4000 | 1000 | >4000 | 250 | >4000 | 250 | |
| C. glabrata SV 02 | L. cubeba | 2000 | 250 | >4000 | 1000 | >4000 | >1000 |
| M. alternifolia | 4000 | 1000 | 4000 | 250 | >4000 | >250 | |
| C. limon | 4000 | 1000 | >4000 | 4000 | >4000 | 4000 | |
| M. alternifolia | 4000 | 1000 | 4000 | 250 | >4000 | 250 | |
| C. krusei ATCC 6258 | C. sempervirens | 2000 | 1000 | 2000 | 4000 | 2000 | >4000 |
| C. limon | 4000 | 250 | >4000 | 62.5 | >4000 | >250 | |
| C. limon | 1000 | 1000 | >4000 | 4000 | >4000 | 2000 | |
| M. alternifolia | 2000 | 1000 | 4000 | 250 | >4000 | 500 | |
| C. parapsilosis SV 04 | C. sempervirens | >4000 | 250 | 4000 | 500 | >4000 | 125 |
| Clotrimazole | 0.125 | 0.032 | 0.25 | 0.015 | 0.5 | 0.063 | |
Isolated MBIC and MBEC (µg/mL): able to reduce by ≥90% optical density (OD) compared to control free of EO and clotrimazole. * Isolated: only one substance (EO or clotrimazole). ** Combined: MIC of the combination (EO–EO or EO–clotrimazole) that resulted in synergism.
3.4. In Vivo Assay in Caenorhabditis elegans
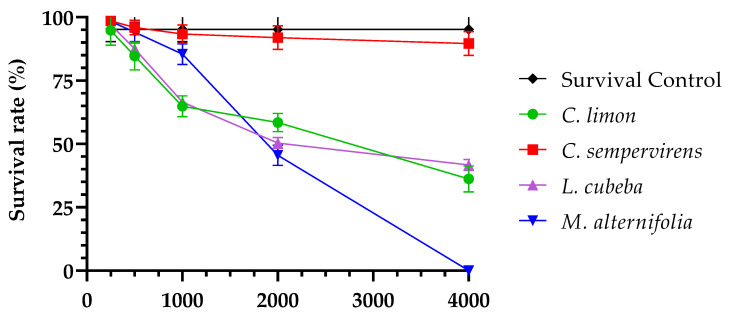
The test of acute toxicity of EO and clotrimazole against C. elegans showed average survival greater than 90% of larvae for the concentration of 250 µg/mL of all tested EO; and for clotrimazole, 100% of C. elegans larvae survived at all concentrations within the evaluated range (0.125–4 µg/mL). It was not possible to determine the LC50 of C. sempervirens because at all concentrations evaluated, survival occurred in more than 90% of the larvae; the LC50 for the EO of M. alternifolia was 2000 µg/mL and for C. limon and L. cubeba it was 4000 µg/mL (Figure 1; Table 5). The test controls showed that DMSO concentrations ≤5% did not affect the survival of C. elegans larvae, and the untreated control showed a mean survival of 96% at 24 h.
Figure 1.
Survival rate (%) of C. elegans larvae in 24 h, evaluated at different concentrations of essential oils of C. limon, C. sempervirens, L. cubeba and M. alternifolia.
Table 5.
Mean survival rate (%) of C. elegans tested at different concentrations of essential oils from C. limon, C. sempervirens, L. cubeba and M. alternifolia.
| Concentration (µg/mL) | C. limon | C. sempervirens | L. cubeba | M. alternifolia |
|---|---|---|---|---|
| 250 | 95.83 | 97.83 | 97.83 | 97.82 |
| 500 | 85.96 | 95.35 | 87.76 | 94.00 |
| 1000 | 64.58 | 93.48 | 67.50 | 85.11 |
| 2000 | 58.82 | 92.86 | 51.11 * | 46.15 * |
| 4000 | 37.25 * | 88.64 | 40.43 | 0 |
* LC50: lethal concentration responsible for the mortality of 50% of C. elegans larvae.
The EO–EO and EO–clotrimazole combinations that showed synergism in the evaluation of the MIC were selected to evaluate the survival of C. elegans larvae. Overall, the combinations showed a mean survival of 90% of the larvae for the combinations L. cubeba–M. alternifolia, C. sempervirens–C. limon, M. alternifolia–clotrimazole and C. sempervirens–clotrimazole (Table 6). Survival of less than 20% of larvae, demonstrating greater acute toxicity, was found for the combinations C. limon–M. alternifolia and L. cubeba–C. limon.
Table 6.
Survival rate (%) of C. elegans subjected to combinations of essential oil and clotrimazole after 24 h of exposure.
| Compound “A” | Concentration (µg/mL) | Compound “B” | Concentration (µg/mL) | Survival (% Average) |
|---|---|---|---|---|
| M. alternifolia | 250 | Clotrimazole | 0.063 | 88.89 |
| L. cubeba | 250 | M. alternifolia | 1000 | 93.3 |
| C. sempervirens | 250 | Clotrimazole | 0.032 | 100.00 |
| C. sempervirens | 1000 | C. limon | 250 | 90.00 |
| C. limon | 1000 | M. alternifolia | 1000 | 18.52 |
| L. cubeba | 500 | C. limon | 1000 | 13.79 |
4. Discussion
EO have been extensively studied nowadays and can be a complementary alternative for the treatment of infections caused by Candida species, especially mucocutaneous infections. This study investigated the activity of the EO of C. limon, C. sempervirens, L. cubeba and M. alternifolia, alone and in combination with each other and with clotrimazole, on four species of the genus Candida, to determine in vitro the MIC, MBIC and MBEC; in addition to this, an in vivo toxicity assessment for the nematode C. elegans was performed.
The EO extracted from plants of the studied species are products that have a complex chemical composition and may have more than 20 identified compounds [12,13,14,15,18]. Our study used EO that presented limonene (65.6%), α-pinene (52.4%), terpinen-4-ol (41%) and geranyl acetate (42%) as the main component, respectively, for C. limon, C. sempervirens, M. alternifolia and L. cubeba. These constituents are like those described for EO of these plants in other studies [15,18,29,35,36,37,38,39,40,41]. Terpene derivatives, a class that includes the mentioned constituents, are closely related to the antimicrobial biological action of these EO, as already demonstrated for Candida spp. in other studies [13,15,16,41,42,43].
In the present study, the MIC varied according to the isolate and according to the EO, but the EO of C. sempervirens and L. cubeba presented the lowest MIC (500 µg/mL) for the same species (C. krusei SV 03), while M. alternifolia and clotrimazole combined (62.5–0.25 µg/mL) inhibited C. albicans ATCC 90028 at lower concentrations than in isolation. In this sense, the ranges of results for the EO showed the effectiveness of plant-derived products in inhibiting microorganisms [42,43], a significant finding for the genus, given the recognized adaptive antifungal arsenal associated with C. albicans and the intrinsic fluconazole resistance of C. krusei [9,20,44].
This variability of MIC can be observed in the literature [9,18] and is due to the characteristics of each isolate, which may be related to virulence factors and the origin of the isolate (blood, feces, respiratory tract or environment). In addition to this, storage and the constant activation and reactivation of cells, which occur in repeated cultures, may have generated adaptive changes in the phenotypic profile of the reference strains [45].
The MFC were, on average, 2 × MIC for most isolates and EO, but for some, it was not possible to make this determination, as the values were greater than 4000 µg/mL, that is, greater than the limits of concentrations tested. Still, MIC and MFC of 2000 and 1000 µg/mL, respectively, were observed for C. albicans ATCC 90028 when evaluated for the EO of C. limon. This fact may be explained if the growth curve of cells in contact with this EO is evaluated, as it is possible that the fungicidal effect occurs through mechanisms that involve the depletion of some essential intracellular constituent for growth, such as ergosterol reserves, associated with other mechanisms of enzymatic inhibition, or action on the membrane or cell wall [46] which is time-dependent, but other assays need to be performed to elucidate this finding.
The anti-Candida activity of EO may be a direct result of the interaction of the various chemical components present and the association of different mechanisms, which may explain the fungistatic and fungicidal effects. The characteristics common to EO, such as lipophilicity and ability to cause damage to vital structures, membrane, and cell wall, result in increased membrane permeability and release of intracellular contents, with consequent death of Candida spp. cells [13,37].
The fungistatic action of clotrimazole at low concentrations is due to structural changes in ergosterol; at high concentrations, it has a fungicidal effect [6]. Thus, it can be assumed that the potentiation of the effects, demonstrated by the synergism observed in the association of clotrimazole with different proportions of EO, is the result of the multiplicity of mechanisms resulting from the various constituents of the EO, leading to the fungicidal effect [47]. The activity of EO against biofilm [10,13,17,25,26,48,49,50,51] is another factor that contributes to the need for studies that evaluate the combination of other drugs and a greater number of EO [26,47,51,52].
The application of a product with simultaneous inhibition of microbial growth and biofilm is advantageous since it allows for more efficient satisfactory results in different structures of Candida spp. The present study demonstrated that there was inhibition of biofilm formation and a reduction in the viability of the cells of previously formed biofilm, with MIC up to five times lower for the synergistic combination when compared to the same MIC found for the drugs evaluated alone. This study is the first, to our knowledge, to present a synergistic relationship of EO–EO and EO–clotrimazole on Candida spp., evaluating their action on biofilms.
The initial assessment of a substance, such as toxicity and antifungal activity, is a preliminary step in the design of new drug and health product candidates [32]. Our study sought to evaluate the safety of EO and clotrimazole alone, as well as in combinations, exposed for 24 h to the in vivo model C. elegans. It was observed that more than 80% of C. elegans larvae survived at concentrations of 500 µg/mL for three of the evaluated EO. For C. sempervirens, 80% of the larvae survived at the concentration of 4000 µg/mL, and for clotrimazole, the survival of 100% of the larvae was observed at all concentrations.
Among the EO evaluated, the biological activity of the EO of C. limon and M. alternifolia is better known when compared with those of L. cubeba and C. sempervirens [18]. As observed in Table 4, the LC50 was not determined for the EO of C. sempervirens (LC50: >4000 µg/mL), suggesting that it is the least toxic for C. elegans larvae among the four evaluated. Our study demonstrated that lethal toxicity of L. cubeba EO against C. elegans larvae was at 2000 µg/mL; however, lower concentrations such as 0.120–0.525 mg/mL (120–525 µg/mL) were found previously for the nematode Bursaphelenchus xylophilus [37]. In our study, we found lower toxicity of C. sempervirens EO alone; however, it was moderate and high for other combinations (OE–OE and OE–clotrimazole). Some studies have provided other models for assessing toxicity by evaluating different cell cultures, showing that in vitro inhibitory concentrations (IC50) for MCF-7 and MDA-MB-231 mammary tumor cells were lower than 34.5 and 65.2 μg/mL, respectively [53], and that C. sempervirens is lethal at higher concentrations in human promyelocytic leukemia strains (HL-60 and NB4) (LC50: 333.79 to 365.41 µg/mL) [38] and in experimental animal Ehrlich ascitic carcinoma (LC50: 372.43 µg/mL) [38]. In the larvae of Culex quinquefasciatus, a non-vertebrate model and vector of filariasis, the LC50 was 16.1 μg/mL after 24 h of exposure [39].
The complexity of factors intrinsic to EO, such as the variability and concentration of chemotypes, which can vary in the same plant species according to the part of the plant used for extraction, region of cultivation and stage of development, may be, in part, responsible for the different results obtained in the same toxicity model used. In different models, this variability of constituents can be even greater, as can be seen in some studies [12,15,18,41,49,53]. Thus, it is suggested that toxicity is evaluated in different models to obtain evidence of greater safety and definition of the best drug concentrations that may have biological action and an absence or reduction of damage.
Our study focused on the preliminary assessment of EO–EO and EO–clotrimazole combinations, using concentration ranges applied predictively to planktonic cells and subsequently to biofilm and C. elegans after 24 h. Therefore, the totality of combinations that the checkerboard provides for the biofilm was not explored, nor was the influence of different exposure times of the substances for inhibition, eradication and toxicity. Our study used evaluation in the C. elegans model; therefore, it is important to evaluate correlation with the results in other models for a better understanding of the mechanism related to toxicity, including the use of EO in biocompatible pharmaceutical applications in nanosystems to improve aspects of physicochemical and biological agents against Candida spp.
The complexity of the composition of EO allows wide use in alternative and complementary medicine. The exploration of antimicrobial activity may enable new strategies and therapeutic alternatives for infectious diseases, especially mucocutaneous ones, where topical application is possible. The association of EO makes it possible for some constituents, even though they are not in the majority, to interact, enhancing or evidencing biological effects and reducing toxicity. In this context, studies still need to be carried out to determine the practical relevance of the combinations, better concentrations of each one of them, and the economic and market viability, in addition to the advantages over existing products.
5. Conclusions
The EO–EO and EO–clotrimazole combinations showed synergistic activity in vitro, dependent on the isolate and on the Candida species, and of the combined drugs, when evaluating the inhibition of planktonic growth in vitro and the inhibition of biofilm formation and eradication. The combinations M. alternifolia–clotrimazole, L. cubeba–M. alternifolia, C. sempervirens–clotrimazole and C. sempervirens–C. limon were the most efficient against planktonic cells and biofilm. In addition, they demonstrated low or negligible toxicity to C. elegans larvae. Thus, our results suggest that the drug combinations evaluated here show promising activity in the control and treatment of vaginal infections caused by Candida species, for topical application through different devices, for example, local nanorelease systems, such as mucoadhesive formulations.
Acknowledgments
To Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the master’s scholarship awarded to R.A.S.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics14091872/s1, Table S1: Combinations between OE–OE and OE–clotrimazole in Candida species.
Author Contributions
Concept, research, writing, review and editing, R.A.d.S., N.B.S.S., R.H.P., C.H.G.M., D.V.D.d.B.R. and R.d.S.P.; supervision, D.V.D.d.B.R. and R.d.S.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pfaller M.A., Diekema D.J., Turnidge J.D., Castanheira M., Jones R.N. Open Forum Infectious Diseases. Oxford University Press; Oxford, UK: 2019. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species from 1997–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva-Rocha W.P., Azevedo M.F., Chaves G.M. Epidemiology and fungal species distribution of superficial mycoses in Northeast Brazil. J. Mycol. Méd. 2017;27:57–64. doi: 10.1016/j.mycmed.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Gonçalves B., Ferreira C., Alves C.T., Henriques M., Azeredo J., Silva S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016;42:905–927. doi: 10.3109/1040841X.2015.1091805. [DOI] [PubMed] [Google Scholar]
- 4.Willems H.M.E., Ahmed S.S., Liu J., Xu Z., Peters B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi. 2020;6:27. doi: 10.3390/jof6010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denning D.W., Kneale M., Sobel J.D., Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018;18:e339–e347. doi: 10.1016/S1473-3099(18)30103-8. [DOI] [PubMed] [Google Scholar]
- 6.Soriano-Ruiz J.L., Calpena-Capmany A.C., Canadas-Enrich C., Febrer N.B., Suner-Carbo J., Souto E.B., Clares-Naveros B. Biopharmaceutical profile of a clotrimazole nanoemulsion: Evaluation on skin and mucosae as anticandidal agent. Int. J. Pharm. 2019;554:105–115. doi: 10.1016/j.ijpharm.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Taudorf E.H., Jemec G.B.E., Hay R.J., Saunte D.M.L. Cutaneous candidiasis–an evidence-based review of topical and systemic treatments to inform clinical practice. J. Eur. Acad. Dermatol. Venereol. 2019;33:1863–1873. doi: 10.1111/jdv.15782. [DOI] [PubMed] [Google Scholar]
- 8.Silva S., Rodrigues C.F., Araújo D., Rodrigues M., Henriques M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi. 2017;3:8. doi: 10.3390/jof3010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos G.C.D.O., Vasconcelos C.C., Lopes A.J.O., dos Cartágenes M., Filho A.K.D.B., do Nascimento F.R.F., Ramos R.M., Pires E.R.R.B., de Andrade M.S., Rocha F.M.G., et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018;9W:1351. doi: 10.3389/fmicb.2018.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sardi J.C.O., Scorzoni L., Bernardi T., Fusco-Almeida A.M., Giannini M.M. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013;62:10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 11.Scorzoni L., de Paula e Silva A.C.A., Marcos C.M., Assato P.A., de Melo W.C.M.A., de Oliveira H.C., Costa-Orlandi C.B., Mendes-Giannini M.J.S., Fusco-Almeida A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017;8:36. doi: 10.3389/fmicb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutlu-Ingok A., Devecioglu D., Dikmetas D.N., Karbancioglu-Guler F., Capanoglu E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules. 2020;25:4711. doi: 10.3390/molecules25204711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannas S., Usai D., Tardugno R., Benvenuti S., Pellati F., Zanetti S., Molicotti P. Chemical composition, cytotoxicity, antimicrobial and antifungal activity of several essential oils. Nat. Prod. Res. 2016;30:332–339. doi: 10.1080/14786419.2015.1060592. [DOI] [PubMed] [Google Scholar]
- 14.Tariq S., Wani S., Rasool W., Shafi K., Bhat M.A., Prabhakar A., Shalla A.H., Rather M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents Against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019;134:103580. doi: 10.1016/j.micpath.2019.103580. [DOI] [PubMed] [Google Scholar]
- 15.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Baz A., Mosbah R., Goda R., Mansour B., Sultana T., Dahms T., El-Ganiny A. Back to Nature: Combating Candida albicans Biofilm, Phospholipase and Hemolysin Using Plant Essential Oils. Antibiotics. 2021;10:81. doi: 10.3390/antibiotics10010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jafri H., Ahmad I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact sinergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida Tropicalis. J. Mycol. Med. 2020;30:100911. doi: 10.1016/j.mycmed.2019.100911. [DOI] [PubMed] [Google Scholar]
- 18.Silva R.A., Antonieti F.M.P.M., Röder D.V.D.B., Pedroso R.S. Essential oils of Melaleuca, Citrus, Cupressus, and Litsea for the management of infections caused by Candida Species: A Systematic Review. Pharmaceutics. 2021;13:1700. doi: 10.3390/pharmaceutics13101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkabti A.B., Issi L., Rao R.P. Caenorhabditis elegans as a Model Host to Monitor the Candida Infection Processes. J. Fungi. 2018;4:123. doi: 10.3390/jof4040123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scorzoni L., de Lucas M.P., Mesa-Arango A.C., Fusco-Almeida A.M., Lozano E., Cuenca-Estrella M., Mendes-Giannini M.J., Zaragoza O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS ONE. 2013;8:e60047. doi: 10.1371/journal.pone.0060047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segal E., Frenkel M. Experimental in vivo models of candidiasis. J. Fungi. 2018;4:21. doi: 10.3390/jof4010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernando-Ortiz A., Mateo E., Perez-Rodriguez A., de Groot P.W.J., Quindós G., Eraso E. Virulence of Candida auris from different clinical origins in Caenorhabditis elegans and Galleria mellonella Host Models. Virulence. 2021;12:1063–1075. doi: 10.1080/21505594.2021.1908765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felix T.C., Araújo L.B., Brito Röder D.V.D., Pedroso R.S. Evaluation of vulvovaginitis and hygiene habits of women attended in primary health care units of the Family. Int. J. Women’s Health. 2020;12:49–57. doi: 10.2147/IJWH.S229366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute (CLSI) M27-S4: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Third Informational Supplement. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012. [Google Scholar]
- 25.Pedroso R.S., Balbino B.L., Andrade G., Dias M.C.P.S., Alvarenga T.A., Pedroso R.C.N., Pires R.H. In Vitro and In Vivo Anti-Candida spp. Activity of plant-derived products. Plants. 2019;11:494. doi: 10.3390/plants8110494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolić M.M., Jovanović K.K., Marković T.L., Marković D.L., Gligorijević N.N., Radulović S.S., Kostić M., Glamočlija J.M., Soković M.D. Antimicrobial synergism and cytotoxic properties of Citrus limon L.; Piper nigrum L. and Melaleuca alternifolia (Maiden and Betche) Cheel essential oils. J. Pharm. Pharmacol. 2017;69:1606–1614. doi: 10.1111/jphp.12792. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui Z.N., Farooq F., Musthafa T.N.M., Ahmad A., Khan A.U. Synthesis, characterization and antimicrobial evaluation of novel halopyrazole derivatives. J. Saudi Chem. Soc. 2013;17:237–243. doi: 10.1016/j.jscs.2011.03.016. [DOI] [Google Scholar]
- 28.Pierce C.G., Uppuluri P., Tristan A.R., Wormley F.L., Jr., Mowat E., Ramage G., Lopez-Ribot J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Prot. 2008;3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cota B.B., Oliveira D.B.C., Borges T.C., Catto A.C., Serafim C.V., Rodrigues A.R.A., Kohlhoff M., Zani C.L., Andrade A.A. Antifungal activity of extracts and purified saponins from the rhizomes of Chamaecostus cuspidatus against Candida and Trichophyton species. J. Appl. Microbiol. 2020;130:61–75. doi: 10.1111/jam.14783. [DOI] [PubMed] [Google Scholar]
- 30.American Society for Microbiology . Synergism testing: Broth microdilution checkerboard and broth macrodilution methods. In: Isenberg H.D., editor. Clinical Microbiology Procedures Handbook. ASM Press; Washington, DC, USA: 1992. pp. 1–28. [Google Scholar]
- 31.Odds F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 32.Tampakakis E., Okoli I., Mylonakis E.A. C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat. Protoc. 2008;3:1925–1931. doi: 10.1038/nprot.2008.193. [DOI] [PubMed] [Google Scholar]
- 33.Lu L., Shu C., Chen L., Yang Y., Ma S., Zhu K., Shi B. Insecticidal activity and mechanism of cinnamaldehyde in C. elegans. Fitoterapia. 2020;146:104687. doi: 10.1016/j.fitote.2020.104687. [DOI] [PubMed] [Google Scholar]
- 34.Medina-Alarcón K.P., Singulani J.L., Voltan A.R., Sardi J.C., Petrônio M.S., Santos M.B., Polaquini C.R., Regasini L.O., Bolzani V.S., da Silva D.H., et al. Alkyl Protocatechuate loaded nanostructured lipid systems as a treatment strategy for Paracoccidioides brasiliensis and Paracoccidioides litizii in vitro. Front. Microbiol. 2017;8:1048. doi: 10.3389/fmicb.2017.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo M., Jiang L.K., Zou G.L. Acute and genetic toxicity of essential oil extracted from Litsea cubeba (Lour.) Pers. J. Food Prot. 2005;68:581–588. doi: 10.4315/0362-028X-68.3.581. [DOI] [PubMed] [Google Scholar]
- 36.Hammer K.A., Carson C.F., Riley T.V. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2004;12:1081–1085. doi: 10.1093/jac/dkh243. [DOI] [PubMed] [Google Scholar]
- 37.Park I.K., Kim J., Lee S.G., Shin S.C. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophius) J. Nematol. 2007;39:275–279. [PMC free article] [PubMed] [Google Scholar]
- 38.Fayed S.A. Chemical Composition, Antioxidant, Anticancer Properties and Toxicity Evaluation of Leaf Essential Oil of Cupressus sempervirens. Not. Bot. Horti Agrobot. Cluj-Napoca. 2015;43:320–326. doi: 10.15835/nbha43210070. [DOI] [Google Scholar]
- 39.Almadiy A.A., Nenaah G.E. Bioactivity and safety evaluations of Cupressus sempervirens essential oil, its nanoemulsion and main terpenes against Culex quinquefasciatus Say. Environ. Sci. Pollut. Res. 2022;29:13417–13430. doi: 10.1007/s11356-021-16634-z. [DOI] [PubMed] [Google Scholar]
- 40.Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Altern. Med. 2016;2016:3012462. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahizan N.A., Yang S.-K., Moo C.-L., Song A.A.-L., Chong C.-M., Chong C.-W., Abushelaibi A., Lim S.-H.E., Lai K.-S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules. 2019;24:2631. doi: 10.3390/molecules24142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Algiannis N., Kalpotzakis K., Mitaku S., Chinou L.B. Composition and antimicrobial of essential oils two Origanum species. J. Agric. Food Chem. 2001;49:4168–4170. doi: 10.1021/jf001494m. [DOI] [PubMed] [Google Scholar]
- 43.Holetz F.B., Pessini G.L., Sanches N.R., Cortez D.A., Nakamura C.V., Filho B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz. 2002;97:1027–1031. doi: 10.1590/S0074-02762002000700017. [DOI] [PubMed] [Google Scholar]
- 44.Jamiu A.T., Albertyn J., Sebolai O.M., Pohl C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2020;59:14–30. doi: 10.1093/mmy/myaa031. [DOI] [PubMed] [Google Scholar]
- 45.Bacelo K.L., Costa K.R.C., Ferreira J.C., Candido R.C. Biotype stability of Candida albicans isolates after culture storage determined by randomly amplified polymorphic DNA and phenotypical methods. Mycoses. 2010;53:468–474. doi: 10.1111/j.1439-0507.2009.01741.x. [DOI] [PubMed] [Google Scholar]
- 46.Mukherjee P.K., Chandra J., Kuhn D.M., Ghannoum M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003;71:4333–4340. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carbone C., Teixeira M.D.C., Sousa M.D.C., Martins-Gomes C., Silva A.M., Souto E.M.B., Musumeci T. Clotrimazole-Loaded Mediterranean Essential Oils NLC: A Synergic Treatment of Candida Skin Infections. Pharmaceutics. 2019;11:231. doi: 10.3390/pharmaceutics11050231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmeira-de-Oliveira A., Gaspar C., Palmeira-de-Oliveira R., Silva-Dias A., Salgueiro L., Cavaleiro C., Pina-Vaz C., Martinez-de-Oliveira J., Queiroz J.A., Rodrigues A.G. The anti-Candida activity of Thymbra capitata essential oil: Effect upon pre-formed biofilm. J. Ethnopharmacol. 2012;140:379–383. doi: 10.1016/j.jep.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 49.Ahmad A., Khan A., Manzoor N. Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur. J. Pharm. Sci. 2013;48:80–86. doi: 10.1016/j.ejps.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Abdullahi T.J., Jacobus A., Olihile S., Onele G., Carolina H.P. Inhibitory effect of polyunsaturated fatty acids alone or in combination with fluconazole on Candida krusei biofilms in vitro and in Caenorhabditis elegans. Med. Myc. 2021;59:1225–1237. doi: 10.1093/mmy/myab055. [DOI] [PubMed] [Google Scholar]
- 51.Stringaro A., Vavala E., Colone M., Pepi F., Mignogna G., Garzoli S., Cecchetti S., Ragno R., Angiolella L. Effects of Mentha suaveolens essential oil alone or in combination with other drugs in Candida albicans. Evid. Based Complement. Altern. Med. 2014;2014:12590. doi: 10.1155/2014/125904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pires R.H., Montanari L.B., Martins C.H., Zaia J.E., Almeida A.M., Matsumoto M.T., Mendes-Giannini M.J. Anticandidal efficacy of cinnamon oil against planktonic and biofilm cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia. 2011;172:453–464. doi: 10.1007/s11046-011-9448-0. [DOI] [PubMed] [Google Scholar]
- 53.Powers C.N., Osier J.L., McFeeters R.L., Brazell C.B., Olsen E.L., Moriarity D.M., Satyal P., Setzer W.N. Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules. 2018;23:1549. doi: 10.3390/molecules23071549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.