Abstract
Early reports indicate an association between the severity of the COVID-19 infection and the widespread 25-hydroxy vitamin D deficiency known to exist in populations around the world. Vitamin D deficiency is extremely common among African American (AA) communities, where the COVID-19 infection rate is three-fold higher, and the mortality rate nearly six-fold higher, compared with rates in predominantly white communities. COVID-19 infection primarily affects the lungs and airways. Previous reports have linked 25-hydroxy vitamin D deficiency with subclinical interstitial lung disease. AA are at risk for lower cellular glutathione (GSH) levels and GSH deficiency epigenetically impairs VD biosynthesis pathway genes. Compared with vitamin D alone, co-supplementation of vitamin D and L-cysteine (a GSH precursor) showed a better efficacy in improving levels of GSH and VD-regulatory genes at the cellular/tissue level, and increasing 25(OH) vitamin D levels and reducing inflammation biomarkers in the blood in mice studies. We propose that randomized clinical trials are needed to examine the potential of co-supplementation with anti-inflammatory antioxidants, vitamin D and L-cysteine in correcting the 25(OH)VD deficiency and preventing the ‘cytokine storm,’ one of the most severe consequences of infection with COVID-19, thereby preventing the adverse clinical effects of COVID-19 infection in the vulnerable AA population.
Introduction:
The Centers for Disease Control and Prevention (CDC) has reported that African Americans (AA) have the highest age-adjusted case rate for contracting coronavirus disease (COVID-19), a higher rate of hospitalization, and are more likely to die compared to white Americans (Caucasians) [1, 2]. Studies from different parts of the world demonstrate how the association between 25(OH) vitamin D deficiency [3–12] and elevated pro-inflammatory cytokine levels (cytokine storm) [13–18] affects the severity and outcome in subjects infected with COVID-19.
High risk of 25(OH) vitamin D deficiency in AA:
Two-thirds of the US population, particularly African Americans (AA), are at risk for inadequate or deficient levels of 25-hydroxy vitamin D (25(OH)D) [5, 19, 20]. This is caused in part due to their increased skin pigmentation, which functions not only as a natural sunscreen, but also significantly reduces the ability of the skin to produce vitamin D from sun exposure. The bioavailability of 25(OH)VD in response to ingesting VD supplements varies significantly among individual subjects and is dependent on the status of the VD-metabolism genes [21–24]. Acquired risk factors for vitamin D deficiency include race, higher BMI, winter season, higher geographic latitudes, and inadequate dietary intake [21]. 25(OH)VD biosynthesis mainly occurs in the liver by the action of VD-25hydroxylase (CYP2R1, CYP27A1) on cholecalciferol consumed from the diet or formed during the skin exposure to Ultraviolet B from sunlight. 25(OH)VD is transported into the circulation bound to VDBP/GC. 25(OH)VD conversion to its active metabolite (1,25(OH)2VD) is catalyzed by CYP27B1 present in both renal (primary site) and non-renal tissues. Catabolic inactivation of 25(OH)VD and 1,25(OH)2D3 by CYP24A1 is thought to limit 1,25(OH)2D3 signaling. The circulating and cellular levels of 1,25(OH)2VD (calcitriol) are regulated by cellular CYP27B1, CYP24A1 and circulating PTH concentrations. The biological actions of 1,25(OH)2 VD are directly related to the status of VDR in target tissues where translocation of 1,25(OH)2VD/VDR to the nucleus regulates transcription of target genes. The incidence of vitamin D deficiency or inadequacy is on the rise because of the increasing prevalence of metabolic syndrome disorders such as obesity, and diabetes, as well as inadequate sensible sun exposure. Circulating 25(OH)VD is considered a comprehensive and stable metabolite to diagnose 25(OH)VD deficiencies and monitor VD consumption. According to the clinical practice guidelines recommended by the Endocrine Society, vitamin D deficiency was defined as 25(OH)D<50 nmol/l and vitamin D-inadequacy as 50≤25(OH)D<75 nmol/l. Clinical studies have demonstrated an association between better health outcomes and higher blood levels of 25(OH)D [5, 19, 20, 23, 24].
Role of glutathione (GSH) in VD metabolites biosynthesis and metabolism:
Human studies from different laboratories have reported the existence of a positive correlation between blood levels of GSH and those of 25(OH)D in adults, children, and diabetic patients and AA subjects [25–27]. Consumption of dietary antioxidants plays a beneficial role by increasing serum 25(OH)D [28]. Preclinical studies have shown that low levels of GSH negatively affect the vitamin D regulatory and glucose-metabolism genes in the liver and muscle of high fat diet-fed mice and diabetic rats [27, 29–31]. Improved GSH status following co-supplementation with vitamin D and L-cysteine (a GSH precursor) demonstrated a significantly greater increase in circulating 25(OH)VD and a significantly greater decrease in the oxidative stress, TNF and insulin resistance levels compared with supplementation with vitamin D alone in a vitamin D deficient mouse model (27). The mechanism may result from the dual action of GSH-mediated reduction in oxidative stress and upregulation of the vitamin D regulatory genes ((VDBP/CYP2R1/CYP27A1/VDR), which are required for the efficient transport and hydroxylation of vitamin D in the liver, as well as the activation of the VDR/PGC-1α/GLUT-4 pathway responsible for the metabolic actions of 1,25(OH)2D in target tissues [27, 29, 30]. Indeed, lower levels of GSH, impaired vitamin D responsive genes, and vitamin D deficiency have been reported in obesity and diabetes in general, and in AA subjects in particular [25–27]. These preclinical studies provide strong evidence for a previously undiscovered mechanism by which a deficiency or inadequacy in 25-hydroxy vitamin D is linked to lower GSH levels. The combination of vitamin D and L-cysteine has been found effective for improving clinical outcomes in animal studies and this needs to be examined in human studies and has not been studied in the clinical setting of COVID-19.
Reduced GSH levels in AA:
GSH is formed from L-cysteine (LC), glycine, and glutamate by the enzymatic action of glutamate-cysteine ligase and glutathione synthetase [32]. LC is a rate-limiting factor in GSH synthesis [32]. GSH is a major antioxidant, and reflects the in vivo defense against oxidative stress [32]. GSH is oxidized to GSSG during its antioxidative function. glucose-6-phosphate dehydrogenase (G6PD) catalyzes the production of nicotinamide adenine dinucleotide phosphate reduced form (NADPH). NADPH is needed by glutathione reductase for the recycling of oxidized glutathione (GSSG) to GSH. Blood levels of GSH are lower in African Americans, presumably due to lower consumption of L-cysteine and a deficiency of G6PD. GSH deficiency increases oxidative stress and oxidative modification of endogenous enzymes and proteins, which can result in impaired cell function [27]. A link has been established between impaired immunity associations and reduced cellular levels of GSH [33]. GSH or its precursor L-cysteine has been used to replenish intracellular GSH levels in anti-viral therapy [34]. An imbalance in both GSH homeostasis and oxidative stress is an essential component of the inflammation and respiratory distress common not only to aging, but also to a variety of diseases, such as diabetes, chronic obstructive pulmonary disease, acute respiratory distress syndrome, tuberculosis, neurodegenerative diseases, and several viral infections, including HIV (in humans) and SIV (in rhesus macaques) [33, 35, 36]. The incidence of G6PD is nearly 11% in AA, compared with 1% in Caucasians [37–39]. Diabetes per se results in lower GSH levels in diabetic animals and patients [26, 40, 41]. Under stressful situations, such as diabetes, G6PD deficient cells are unable to regenerate enough NADPH, which exacerbates GSH deficiency and oxidative stress [42, 43], and can contribute to GSH and 25(OH)VD deficiencies in AA.
Role of 25(OH)D in boosting immunity and lung functions:
Vitamin D supplementation upregulates and induces innate antimicrobial and anti-viral defense mechanisms and reduces the insult caused by both viral and bacterial stimuli [44, 45]. The benefits of vitamin D supplementation in lowering the risk of viral infection and providing protection against acute respiratory tract infections have been reviewed previously [46]. Improvement in vitamin D status reduces the incidence and infectivity of influenza A, retrovirus, and dengue virus infection [45, 47]. The potential mechanisms by which vitamin D reduces the risk of viral infection and respiratory illness include induction of the antimicrobial peptide cathelicidin and IL-17 and suppression of the CD26 cell receptors that facilitate virus entry into the host [45, 47]. Vitamin D upregulates glutamate-cysteine ligase, increases GSH, lowers oxidative stress, and pro-inflammatory cytokines levels and hereby can prevent built up of so-called cytokine storm [48–50]. Low levels of serum 25(OH)D have been independently associated with subclinical interstitial lung disease and COPD. Alpha-1-antitrypsin (AAT) is a protease inhibitor. The primary function of AAT is to inhibit neutrophil elastase and prevent elastin degradation in the lungs. AAT deficiency and excess elastin degradation impair the recoiling of elastin and make breathing difficult, as observed in chronic obstructive pulmonary disease (COPD). Vitamin D deficiency has been shown to result in significantly lower AAT expression in the lungs and emphysema in mice exposed to cigarette smoke [35, 51]. ATT synthesis by the CD4+ T cells is required in mediating the immune regulatory system controlled by vitamin D [52]. Both vitamin D deficiency/insufficiency and AATD are extensively linked to decreased lung function. A positive correlation between low blood levels of 25(OH)D and lower AAT levels has been observed in type 2 diabetic patients [53].
Justification for co-supplementation with vitamin D and L-cysteine.
VD is essential for the regulation of numerous vital genes [54]. Epidemiological studies demonstrate an association between better health outcomes and higher blood levels of 25(OH)VD [55–58]. Randomized controlled clinical trials have shown that, while supraphysiological doses of VD are needed to achieve adequate blood levels of 25(OH)VD, not all subjects respond to them [59–62]. Recent studies clinical trials have also questioned the therapeutic effects of high-dose VD supplementation [61, 63, 64]. The disconnect between the limited success of VD supplementation therapy in clinical trials, despite the convincing association between low 25(OH)VD levels and the poor health outcomes associated with chronic diseases, is puzzling. The co-supplementation approach using vitamin D and L-cysteine is superior to supplementation with vitamin D alone because an improvement in cellular GSH status due to added LC will be beneficial in several important ways. First, it will upregulate VD-metabolism genes (VDBP/CYP2R1/CYP27A1/VDR), which are required for the efficient transport and hydroxylation of cholecalciferol, and activation of the VDR/PGC-1α/GLUT-4 pathway responsible for the metabolic actions of 1,25(OH)2VD [27]. Second, both lipids and proteins are integral constituents of the membrane bilayer and are essential in the maintenance of the structure and specialized physiological functions of various organs in the body. These two micronutrients are complementary: VD is lipophilic, and LC is hydrophilic. Thus, co-supplementation with VD and L-cysteine/GSH will be more effective in neutralizing oxidative injury at both lipid and protein sites and provide stronger antioxidative and anti-inflammatory protection from the oxidative stress induced by the COVID-19 infection. Thus, combined consumption of GSH precursors and VD, rather than solely using high-dose VD, is both novel and a potentially effective strategy to achieve a more efficient bioavailability in response to cholecalciferol alone consumption. Animal studies have shown that, compared with vitamin D alone, co-supplementation of vitamin D and L-cysteine (a GSH precursor) in deed showed a greater benefit in increasing both the levels of GSH and VD-regulatory genes at the cellular/tissue level, and in increasing 25(OH) vitamin D levels and in reducing oxidative stress, TNF and inflammation biomarkers in the circulation. Clinical trials are needed to investigate whether co-supplementation of vitamin D and L-cysteine can provide a low-cost strategy to optimize circulating levels of 25(OH)VD and boost body’s immunity and defense in protecting from the adverse clinical effects of COVID-19 infection in our population.
Summary:
An association between a high incidence of 25(OH) vitamin D deficiency and the severity of COVID-19 infection has been reported. Both GSH and 25(OH) vitamin D deficiencies and insufficiencies are prevalent in people of color, especially African Americans [5, 25–27, 31, 65–68]. GSH or its precursor L-cysteine has been shown to stimulate and correct levels of GSH and improve VD-regulatory genes at the cellular/tissue level, and increase 25(OH) vitamin D levels and reduce inflammation biomarkers in the blood. GSH deficiency increases the risk of various diseases, including impairment of the activities of specialized immune cells and thus the body’s ability to fight infection. As a group, African Americans have a higher incidence of 25(OH) vitamin D deficiency or inadequacy. We believe that combined supplementation using vitamin D with the GSH precursor L-cysteine could potentially correct the status of the vitamin D metabolism genes by increasing GSH and the antioxidant capacity. Upregulation of the intracellular glutathione redox status and 25(OH)D may provide a new therapeutic option for preventing inflammation and impaired immunity in subjects exposed to COVID-19. Figure 1 illustrates that both excess vitamin D deficiency and excess adverse clinical effects of COVID-19 occur in African American communities. The treatment of widespread 25(OH) vitamin D deficiency or inadequacy with co-supplementation using a combination of vitamin D and a GSH precursor (L-cysteine) has the potential to help prevent or reduce the adverse effects of COVID-19 infection, particularly in the AA population.
Figure 1.
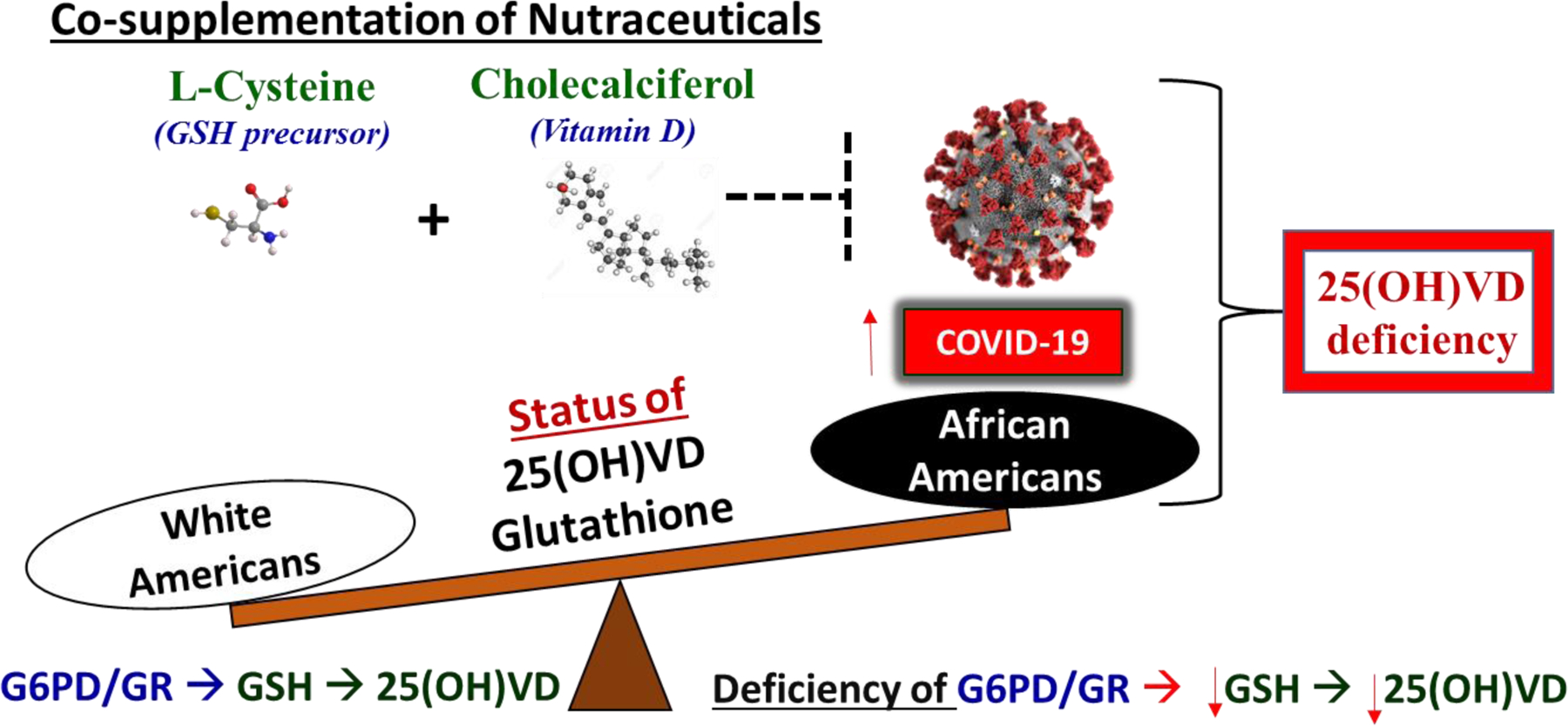
Potential of L-cysteine and vitamin D co-supplementation to reduce vitamin D deficiency and mortality associated with COVID-19 in African Americans.
Acknowledgments:
The Malcolm W. Feist Endowed Chair in Diabetes provided support to SKJ. RP is supported by the cardiovascular Research Fellowship from the Center for Cardiovascular Diseases and Sciences (CCDS) LSUHSC-Shreveport. SKJ also received support from grants from the National Institutes of Health/National Center for Complementary and Integrative Health (RO1 AT007442, 1R33 AT010637). The authors thank Ms. Georgia Morgan for excellent editing.
Footnotes
Competing interests: The authors declare no competing interests.
References
- 1.Thebault R, Ba Tran A, Williams V. The coronavirus is infecting and killing black americans at an alarmingly high rate Washington Post; Accessed April 30, 2020 https://www.washingtonpost.com/nation/2020/04/07/coronavirus-is-infecting-killing-black-americans-an-alarmingly-high-rate-post-analysis-shows/, April 7, 2020. [Google Scholar]
- 2.Age-adjusted rates of lab confirmed covid-19 nonhospitalized cases, estimated non-fatal hospitalized cases,and patients known to have died 100,000 by race/ethnicity group as of april16,2020 https://www1.nyc.gov/assets/doh/downloads/pdf/imm/covid-19-deaths-race-ethnicity-04162020-1.pdf April16,2020(Accessed 05/11/2020).
- 3.D’Avolio A, Avataneo V, Manca A, Cusato J, De Nicolo A, Lucchini R, Keller F, Cantu M. 25-hydroxyvitamin d concentrations are lower in patients with positive pcr for sars-cov-2. Nutrients 12(5), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glicio E. Vitamin d level of mild and severe elderly cases of covid-19: A preliminary report Available at SSRN: https://ssrn.com/abstract=3593258, 2020. [Google Scholar]
- 5.Grant WB, Al Anouti F, Moukayed M. Targeted 25-hydroxyvitamin d concentration measurements and vitamin d3 supplementation can have important patient and public health benefits. Eur J Clin Nutr 74(3):366–376, 2020. [DOI] [PubMed] [Google Scholar]
- 6.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients 12(4), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL, Jani BD, Welsh P, Mair FS, Gray SR, O’Donnell CA, Gill JM, Sattar N, Pell JP. Vitamin d concentrations and covid-19 infection in uk biobank. Diabetes Metab Syndr 14(4):561–565, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilie PC, Stefanescu S, Smith L. The role of vitamin d in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.. Kara M, Ekiz T, Ricci V, Kara O, Chang KV, Ozcakar L. ‘Scientific strabismus’ or two related pandemics: Covid-19 & vitamin d deficiency. Br J Nutr:1–20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laird E, Rhodes J, Kenny R. Vitamin d and inflammation: Potential implications for severity of covid-19. Ir Med J 113(5):81, 2020. [PubMed] [Google Scholar]
- 11.Lau F, Majumder R, Torabi A, Saeg F, Hoffman R, Cirillo J, Greiffenstein P. Vitamin d insufficiency is prevalent in severe covid-19. MedRxiv 10.1101/2020.04.24.20075838, 2020. [DOI] [Google Scholar]
- 12.Meltzer D, Best T, Zhang H, Vokes T, Arora V, Solway J . Association of vitamin d deficiency and treatment with covid-19 incidence. MedRxiv 10.1101/2020.05.08.20095893, 2020. [DOI] [Google Scholar]
- 13.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodriguez L. Sars-cov-2 infection: The role of cytokines in covid-19 disease. Cytokine Growth Factor Rev, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duret PM, Sebbag E, Mallick A, Gravier S, Spielmann L, Messer L. Recovery from covid-19 in a patient with spondyloarthritis treated with tnf-alpha inhibitor etanercept. Ann Rheum Dis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korakas E, Ikonomidis I, Kousathana F, Balampanis K, Kountouri A, Raptis A, Palaiodimou L, Kokkinos A, Lambadiari V. Obesity and covid-19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo W, Zhang JW, Zhang W, Lin YL, Wang Q. Circulating levels of il-2, il-4, tnf-alpha, ifn-gamma and c reactive protein are not associated with severity of covid-19 symptoms. J Med Virol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soy M, Keser G, Atagunduz P, Tabak F, Atagunduz I, Kayhan S. Cytokine storm in covid-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol 39(7):2085–2094, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe sars-cov-2 infection: Review of 3939 covid-19 patients in china and emerging pathogenesis and therapy concepts. J Leukoc Biol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holick MF. The vitamin d deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 18(2):153–165, 2017. [DOI] [PubMed] [Google Scholar]
- 20.. Ginde AA, Mansbach JM, Camargo CA Association between serum 25-hydroxyvitamin d level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch Intern Med 169(4):384–390, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin d status, adiposity, and lipids in black american and caucasian children. J Clin Endocrinol Metab 96(5):1560–1567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thacher TD, Clarke BL. Vitamin d insufficiency. Mayo Clin Proc 86(1):50–60, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD. Circulating 25-hydroxy-vitamin d and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 5(6):819–829, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao P, Bennett D, Mafham M, Lin X, Chen Z, Armitage J, Clarke R. Vitamin d and calcium for the prevention of fracture: A systematic review and meta-analysis. JAMA Netw Open 2(12):e1917789, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez JA, Chowdhury R, Jones DP, Martin GS, Brigham KL, Binongo JN, Ziegler TR, Tangpricha V. Vitamin d status is independently associated with plasma glutathione and cysteine thiol/disulphide redox status in adults. Clin Endocrinol (Oxf) 81(3):458–466, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain SK, Micinski D, Huning L, Kahlon G, Bass PF, Levine SN. Vitamin d and l-cysteine levels correlate positively with gsh and negatively with insulin resistance levels in the blood of type 2 diabetic patients. Eur J Clin Nutr 68(10):1148–1153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.. Jain SK, Parsanathan R, Achari AE, Kanikarla-Marie P, Bocchini JA Jr., Glutathione stimulates vitamin d regulatory and glucose-metabolism genes, lowers oxidative stress and inflammation, and increases 25-hydroxy-vitamin d levels in blood: A novel approach to treat 25-hydroxyvitamin d deficiency. Antioxidants & redox signaling 29(17):1792–1807, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afshari L, Amani R, Soltani F, Haghighizadeh MH, Afsharmanesh MR. The relation between serum vitamin d levels and body antioxidant status in ischemic stroke patients: A case-control study. Adv Biomed Res 4:213, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsanathan R, Jain SK. Glutathione deficiency induces epigenetic alterations of vitamin d metabolism genes in the livers of high-fat diet-fed obese mice. Scientific reports 9(1):14784, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsanathan R, Jain SK. Glutathione deficiency alters the vitamin d-metabolizing enzymes cyp27b1 and cyp24a1 in human renal proximal tubule epithelial cells and kidney of hfd-fed mice. Free radical biology & medicine 131:376–381, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Jain SK, Kanikarla-Marie P, Warden C, Micinski D. L-cysteine supplementation upregulates glutathione (gsh) and vitamin d binding protein (vdbp) in hepatocytes cultured in high glucose and in vivo in liver, and increases blood levels of gsh, vdbp, and 25-hydroxy-vitamin d in zucker diabetic fatty rats. Mol Nutr Food Res 60(5):1090–1098, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu SC. Glutathione synthesis. Biochimica et biophysica acta 1830(5):3143–3153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Droge W, Schulze-Osthoff K, Mihm S, Galter D, Schenk H, Eck HP, Roth S, Gmunder H. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 8(14):1131–1138, 1994. [PubMed] [Google Scholar]
- 34.Crinelli R, Zara C, Smietana M, Retini M, Magnani M, Fraternale A. Boosting gsh using the co-drug approach: I-152, a conjugate of n-acetyl-cysteine and beta-mercaptoethylamine. Nutrients 11(6), 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crane-Godreau MA, Black CC, Giustini AJ, Dechen T, Ryu J, Jukosky JA, Lee HK, Bessette K, Ratcliffe NR, Hoopes PJ, Fiering S, Kelly JA, Leiter JC. Modeling the influence of vitamin d deficiency on cigarette smoke-induced emphysema. Front Physiol 4:132, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamide Y, Utsugi M, Dobashi K, Ono A, Ishizuka T, Hisada T, Koga Y, Uno K, Hamuro J, Mori M. Intracellular glutathione redox status in human dendritic cells regulates il-27 production and t-cell polarization. Allergy 66(9):1183–1192, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan M, Herschel M, Hammerman C, Hoyer JD, Stevenson DK. Hyperbilirubinemia among african american, glucose-6-phosphate dehydrogenase-deficient neonates. Pediatrics 114(2):e213–219, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Parsanathan R, Jain SK. Glucose-6-phosphate dehydrogenase (g6pd) deficiency is linked with cardiovascular disease. Hypertens Res 43(6):582–584, 2020. [DOI] [PubMed] [Google Scholar]
- 39.Parsanathan R, Jain SK. G6pd deficiency shifts polarization of monocytes/macrophages towards a proinflammatory and profibrotic phenotype. Cell Mol Immunol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekhar RV, McKay SV, Patel SG, Guthikonda AP, Reddy VT, Balasubramanyam A, Jahoor F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 34(1):162–167, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan KS, Lee KO, Low KC, Gamage AM, Liu Y, Tan GY, Koh HQ, Alonso S, Gan YH. Glutathione deficiency in type 2 diabetes impairs cytokine responses and control of intracellular bacteria. J Clin Invest 122(6):2289–2300, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg J, Tymoczko J, Stryer L. Glucose 6-phosphate dehydrogenase plays a key role in protection against reactive oxygen species. Biochemistry 5th edition New York: W H Freeman; 2002. Section 205, Available from: https://www.ncbi.nlm.nih.gov/books/NBK22389/, 2002. [Google Scholar]
- 43.Tang HY, Ho HY, Wu PR, Chen SH, Kuypers FA, Cheng ML, Chiu DT. Inability to maintain gsh pool in g6pd-deficient red cells causes futile ampk activation and irreversible metabolic disturbance. Antioxidants & redox signaling 22(9):744–759, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gombart AF. The vitamin d-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 4(9):1151–1165, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruber-Bzura BM. Vitamin d and influenza-prevention or therapy? Int J Mol Sci 19(8), 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunville CF, Mourani PM, Ginde AA. The role of vitamin d in prevention and treatment of infection. Inflamm Allergy Drug Targets 12(4):239–245, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beard JA, Bearden A, Striker R. Vitamin d and the anti-viral state. J Clin Virol 50(3):194–200, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dzik K, Skrobot W, Flis DJ, Karnia M, Libionka W, Kloc W, Kaczor JJ. Vitamin d supplementation attenuates oxidative stress in paraspinal skeletal muscles in patients with low back pain. Eur J Appl Physiol 118(1):143–151, 2018. [DOI] [PubMed] [Google Scholar]
- 49.Jain SK, Micinski D. Vitamin d upregulates glutamate cysteine ligase and glutathione reductase, and gsh formation, and decreases ros and mcp-1 and il-8 secretion in high-glucose exposed u937 monocytes. Biochem Biophys Res Commun 437(1):7–11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee WC, Mokhtar SS, Munisamy S, Yahaya S, Rasool AHG. Vitamin d status and oxidative stress in diabetes mellitus. Cell Mol Biol (Noisy-le-grand) 64(7):60–69, 2018. [PubMed] [Google Scholar]
- 51.Heulens N, Korf H, Cielen N, De Smidt E, Maes K, Gysemans C, Verbeken E, Gayan-Ramirez G, Mathieu C, Janssens W. Vitamin d deficiency exacerbates copd-like characteristics in the lungs of cigarette smoke-exposed mice. Respir Res 16:110, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimeloe S, Rice LV, Chen H, Cheadle C, Raynes J, Pfeffer P, Lavender P, Richards DF, Nyon MP, McDonnell JM, Kemper C, Gooptu B, Hawrylowicz CM. Vitamin d (1,25(oh)2d3) induces alpha-1-antitrypsin synthesis by cd4(+) t cells, which is required for 1,25(oh)2d3-driven il-10. J Steroid Biochem Mol Biol 189:1–9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindley VM, Bhusal K, Huning L, Levine SN, Jain SK. Reduced 25(oh) vitamin d association with lower alpha-1-antitrypsin blood levels in type 2 diabetic patients. J Am Coll Nutr:1–6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neme A, Seuter S, Malinen M, Nurmi T, Tuomainen TP, Virtanen JK, Carlberg C. In vivo transcriptome changes of human white blood cells in response to vitamin d. J Steroid Biochem Mol Biol, 2018. [DOI] [PubMed] [Google Scholar]
- 55.Budhathoki S, Hidaka A, Yamaji T, Sawada N, Tanaka-Mizuno S, Kuchiba A, Charvat H, Goto A, Kojima S, Sudo N, Shimazu T, Sasazuki S, Inoue M, Tsugane S, Iwasaki M, Japan Public Health Center-based Prospective Study G. Plasma 25-hydroxyvitamin d concentration and subsequent risk of total and site specific cancers in japanese population: Large case-cohort study within japan public health center-based prospective study cohort. BMJ 360:k671, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitri J, Muraru MD, Pittas AG. Vitamin d and type 2 diabetes: A systematic review. Eur J Clin Nutr 65(9):1005–1015, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pilz S, Verheyen N, Grubler MR, Tomaschitz A, Marz W. Vitamin d and cardiovascular disease prevention. Nat Rev Cardiol 13(7):404–417, 2016. [DOI] [PubMed] [Google Scholar]
- 58.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin d and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 348:g2035, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrett-Mayer E, Wagner CL, Hollis BW, Kindy MS, Gattoni-Celli S. Vitamin d3 supplementation (4000 iu/d for 1 y) eliminates differences in circulating 25-hydroxyvitamin d between african american and white men. Am J Clin Nutr 96(2):332–336, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis RD, Laing EM. Conflicting reports on vitamin d supplementation: Evidence from randomized controlled trials. Mol Cell Endocrinol 410:11–18, 2015. [DOI] [PubMed] [Google Scholar]
- 61.Lin KW. Vitamin d screening and supplementation in primary care: Time to curb our enthusiasm. Am Fam Physician 97(4):226–227, 2018. [PubMed] [Google Scholar]
- 62.Sacheck JM, Van Rompay MI, Chomitz VR, Economos CD, Eliasziw M, Goodman E, Gordon CM, Holick MF. Impact of three doses of vitamin d3 on serum 25(oh)d deficiency and insufficiency in at-risk schoolchildren. J Clin Endocrinol Metab 102(12):4496–4505, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, Group VR. Vitamin d supplements and prevention of cancer and cardiovascular disease. N Engl J Med 380(1):33–44, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R, Desouza C, Dolor R, Foreyt J, Fuss P, Ghazi A, Hsia DS, Johnson KC, Kashyap SR, Kim S, LeBlanc ES, Lewis MR, Liao E, Neff LM, Nelson J, O’Neil P, Park J, Peters A, Phillips LS, Pratley R, Raskin P, Rasouli N, Robbins D, Rosen C, Vickery EM, Staten M, Group DdR. Vitamin d supplementation and prevention of type 2 diabetes. N Engl J Med 381(6):520–530, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deo SH, Holwerda SW, Keller DM, Fadel PJ. Elevated peripheral blood mononuclear cell-derived superoxide production in healthy young black men. Am J Physiol Heart Circ Physiol 308(5):H548–552, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jain SK, McVie R. Effect of glycemic control, race (white versus black), and duration of diabetes on reduced glutathione content in erythrocytes of diabetic patients. Metabolism 43(3):306–309, 1994. [DOI] [PubMed] [Google Scholar]
- 67.Szanton SL, Rifkind JM, Mohanty JG, Miller ER 3rd, Thorpe RJ, Nagababu E, Epel ES, Zonderman AB, Evans MK. Racial discrimination is associated with a measure of red blood cell oxidative stress: A potential pathway for racial health disparities. Int J Behav Med 19(4):489–495, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams SK, Fiscella K, Winters P, Martins D, Ogedegbe G. Association of racial disparities in the prevalence of insulin resistance with racial disparities in vitamin d levels: National health and nutrition examination survey (2001–2006). Nutr Res 33(4):266–271, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


