Abstract
Serum beta-D-glucan (BDG) determination plays an important role in the diagnosis of candidemia among critically ill patients admitted to the intensive care unit (ICU). However, BDG levels measured may be lower in the case of infections caused by some non-albicans species, such as C. parapsilosis and C. auris. The aim of this single-center study was to investigate the sensitivity of serum BDG for the diagnosis of candidemia stratified according to causative Candida species in ICU patients. This was a single-center, retrospective study, including all adult patients admitted to ICU during the period 2018–2021. All episodes of candidemia with a determination of BDG available within 3 days before or after positive blood culture were recorded. The preplanned primary objective was to investigate the sensitivity of serum BDG to detect candidemia early and the effect of different Candida species. The secondary objective was to measure serum BDG in patients with candidemia from different Candida species. In total, 146 candidemia episodes in 118 patients were analyzed. Median BDG value for C. albicans candidemia (182 pg/mL) was higher than that observed for C. parapsilosis (78 pg/mL, p = 0.015) and C. auris (48 pg/mL, p = 0.022). The overall sensitivity of BDG for the diagnosis of candidemia was low (47%, 95% CI 39–55%). In conclusion, in critically ill patients admitted to ICU, serum BDG levels for candidemia were different among species, with lower levels confirmed for C. parapsilosis and C. auris. Serum BDG sensitivity for early detection of candidemia was lower than previously reported in other ICU populations.
Keywords: ICU, sensitivity, BDG, Candida
1. Introduction
1,3-beta-D-glucan (BDG) is a key component of the cell wall of fungi, and its determination in serum samples is an indirect microbiological tool used to support the diagnosis of invasive fungal diseases [1].
Serum BDG has different sensitivity and specificity according to the characteristics of the baseline patient population considered [2]. Among critically ill patients admitted to the intensive care unit (ICU) and non-hematological patient population, the main limitation of serum BDG to detect candidemia has been a low specificity (with acceptable sensitivity) [3,4], while low sensitivity (but high specificity) has been reported in patients with hematological malignancies [5,6]. In the ICU, even with reported rather good sensitivity and specificity (respectively, 86% and 71%), low positive predictive value (PPV) has been reported at low prevalences of candidemia, with high negative predictive value (NPV) [3,7]. The use of a higher than standard cut-off for positivity has been suggested as potentially useful in ICU patients to increase PPV [8]. Despite these concerns, BDG has been supported for early diagnosis of invasive fungal infections and pre-emptive therapy in critically ill patients admitted to the ICU by international guidelines [9,10]. In a recently published study, good NPV and sensitivity were described among critically ill patients [11]. Although the evaluation of such parameters was not the primary aim of the study, the authors concluded that a negative value of serum BDG might be used as the only tool to discontinue empirical antifungal therapy in the ICU. However, serum BDG values might also be influenced by the fungal species responsible for the infection. A lower sensitivity of serum BDG in C. parapsilosis candidemia has been previously described [11], and growing evidence suggests a similar lower sensitivity in the detection of C. auris invasive infection [12,13,14]. Accordingly, we hypothesized that, in ICU patients, BDG sensitivity to detect candidemia early could be different for different Candida species. Consequently, the major aim of the present study was to assess the sensitivity of serum BDG in a population of critically ill patients diagnosed with candidemia and to evaluate possible differences in sensitivity according to the Candida species responsible for the infection.
2. Materials and Methods
2.1. Study Design and Patient Selection
This is a single-center, retrospective study conducted in the ICUs in Policlinico San Martino Hospital, Genoa, Italy, in the period between 1 January 2018 and 30 September 2021. Inclusion criteria for the present study were: (i) age ≥18 years, (ii) admission to any of the ICUs in our hospital, (iii) at least one blood culture positive for Candida spp., and (iv) a value of serum BDG available within 3 days before or after positive blood culture collection. In the case of multiple episodes of candidemia caused by the same species in the same patient during the study period, a new episode was considered only when at least 30 days elapsed after the last positive culture of the previous one. During the whole study period, four ICUs served our hospital, and a fifth was added during the novel coronavirus-19 disease (COVID-19) pandemic. The preplanned primary objective was to investigate the sensitivity of serum BDG to detect candidemia early and the effect of different Candida species on BDG sensitivity. The secondary objective was to descriptively compare serum BDG levels in patients with candidemia caused by different Candida species.
2.2. Microbiology
Blood cultures and BDG sampling were performed according to the caring clinicians’ judgment. Blood samples were cultured following standard recommendations by the automated Bactec method with both aerobic and anaerobic media (Bactec FX; Becton-Dickinson Microbiology Systems, Franklin Lakes, NJ, USA). Candida species identification in blood cultures was performed with matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS—VITEK MS; bioMérieux, Marcy-l’Etoile, France), using VITEK MS v4.0 software. BDG levels were tested with Fungitell assay (Associates of Cape Cod, Falmouth, MA, USA), according to the manufacturer’s instructions. The established cut-off for positivity was 80 pg/mL.
2.3. Statistical Analysis
Categorical variables are expressed as an absolute number, percentage, and 95% confidence interval (95% CI) and confronted with the Chi-square test. Continuous variables are expressed as median values and interquartile range (IQR) or mean values ± standard deviation (SD). Serum BDG values were compared among the Candida species using the Kruskal–Wallis test, while the Student’s t-test was used to compare the mean times of BDG sampling. Statistical analyses were carried out with Stata (v.16; StataCorp; Collage Station, TX, USA).
2.4. Ethical Considerations
This study was performed in accordance with the guidelines of the Declaration of Helsinki. It was approved by the Liguria Ethics Committee (approval number 43/2022).
3. Results
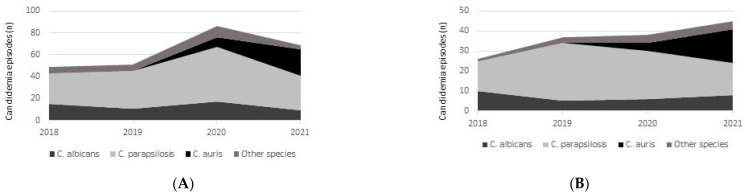
During the study period, 255 episodes of candidemia occurred in patients admitted to the ICUs. Overall, 146 candidemia (57.3%) episodes of candidemia occurring in 118 critically ill patients met the inclusion criteria of concomitant BDG testing and were included in the present study. In total, 72 patients (61.0%) were males, and the median age of the cohort was 66 years (IQR 60–74 years). The representativity of the included candidemia episodes with regard to all the episodes occurring during the study period was similar for each Candida species considered: 29/52 episodes for C. albicans (55.8%), 84/144 episodes for C. parapsilosis (58.3%), 21/33 episodes for C. auris (63.6%) and 12/26 episodes for other species (46.2%). Change in species epidemiology over time is shown in Figure 1, both for the overall population of the ICU (Figure 1A) and for patients included in the present study (Figure 1B). Serum BDG levels and sensitivity are summarized in Table 1. Median serum BDG values were higher in the case of C. albicans compared to C. auris (p = 0.022) and to C. parapsilosis (p = 0.015), while no difference in BDG levels was present between C. auris and C. parapsilosis (p = 0.57). No statistically significant difference was observed in serum BDG sensitivity among different species: neither C. albicans vs. C. auris (p = 0.18) nor C. albicans vs. C. parapsilosis (p = 0.09). When restricting the analysis only to BDG values available after the first positive blood culture, overall sensitivity was 52.5% (95% CI 39.6–65.1%), while the sensitivity according to species was 71.4% (95% CI 41.1–90.0%) for C. albicans, 14.3% (95% CI 1.2–70.1%) for C. auris, 51.5% (95% CI 34.3–68.4%) for C. parapsilosis, and 60% (95% CI 10.6–95.0%) for other Candida species.
Figure 1.
Distribution of Candida species responsible for candidemia over time, both in the overall ICU population (A) and among included patients (B).
Table 1.
Serum BDG values, overall sensitivity, and sensitivity stratified according to Candida species.
| Candida Species (Total Number of Episodes = 146; Total Number of BDG Samples = 187) § | Median BDG Value (IQR), in pg/mL §§ | Median Time from Candidemia Onset to First BDG Determination (IQR), in Days | Sensitivity (95% CI) |
|---|---|---|---|
| For all samples (n = 187) | 84 (21–314) | −0.06 (−1.28, 0.83) | 51.3% (44.1–58.5%) |
| For all episodes (n = 146) | 47.3% (39.0–55.0%) | ||
| For C. albicans (n = 40) samples | 182 (30.5–523) | 0 (−1.16–0.78) | 65.0% (48.7–78.4%) |
| For C. albicans (n = 29) episodes | 62.1% (42.8–78.2%) | ||
| For C. parapsilosis (n = 105) samples | 78 (19–290) | −0.08 (−1.29–1.00) | 48.6% (39.1–58.2%) |
| For C. parapsilosis (n = 84) episodes | 44.0% (33.7–54.9%) | ||
| For C. auris (n = 26) samples | 48 (15–159) | 0 (−1.99–0.83) | 42.3% (24.5–62.4%) |
| For C. auris (n = 21) episodes | 42.9% (23.0–65.3%) | ||
| For other species (n = 16) samples * | 81 (29–195) | −0.62 (−1.99–0.53) | 50.0% (25.6–74.4%) |
| For other species (n = 12) episodes ** | 41.7% (16.4–72.2%) |
§ Number of episodes refers to the number of candidemia episodes; §§ Referred to total number of BDG samples; * C. glabrata n = 11, C. tropicalis n = 4, C. lusitaniae n = 1; ** C. glabrata n = 7, C. tropicalis n = 4, C. lusitaniae n = 1; BDG: b-D-glucan, 95% CI: 95% confidence interval; IQR: interquartile range.
Among 37 candidemia episodes, 2 or more serum BDG values were available, and a description of concordance or discordance among values per episode is reported in Table 2. The mean difference observed between the lowest and the highest BDG value available per episode was 79.1 pg/mL (median 33 pg/mL, IQR 9–108 pg/mL). When stratifying these results according to Candida species, a different concordance between serial BDG samples was noted. Indeed, the observed median difference of BDG values for C. albicans episodes (n = 10) was 13 pg/mL (IQR 0–60 pg/mL), 86 pg/mL (IQR 39–91 pg/mL) for C. auris episodes (n = 5), and of 87.4 pg/mL (IQR 9–124 pg/mL) for C. parapsilosis episodes (n = 18). An increase over time in the values of serum BDG available for each episode was noted in 4 episodes (40%) of C. albicans candidemia, 6 episodes (33%) of C. parapsilosis candidemia, and 2 episodes (40%) of C. auris candidemia (see Supplementary Figure S1 for more details).
Table 2.
Description of serum BDG concordance/discordance in 37 candidemia episodes with ≥2 BDG results available.
| BDG Values * | All (n = 37) | C. albicans (n = 10) | C. auris (n = 5) | C. parapsilosis (n = 18) | Other Species (n = 4) |
|---|---|---|---|---|---|
| Discordant values | 7 (18.9) | 1 (10) | 2 (40) | 3 (16.7) | 1 (25) |
| Concordant values | 30 (81.1) | 9 (90) | 3 (60) | 15 (83.3) | 3 (75) |
* Concordant values are defined as 2 or more serum BDG results all either negative (<80 pg/mL) or positive (≥80 pg/mL), while discordant values are defined as 2 values belonging to different categories: positive and negative. No differences were observed between groups: C. albicans vs. C. auris (p = 0.24), C. albicans vs. C. parapsilosis (p = 0.99), C. auris vs. C. parapsilosis (p = 0.29). BDG: b-D-glucan, 95% CI: 95% confidence interval; IQR: interquartile range.
4. Discussion
In critically ill patients admitted to the ICU, serum BDG values in temporal proximity of C. albicans candidemia were higher when compared either with C. parapsilosis or C. auris candidemia. Serum BDG sensitivity for early detection of candidemia in our study was lower than previously reported in ICU populations.
A lower release of serum BDG in the case of C. parapsilosis [11] or C. auris [12,13] candidemia has been previously reported. While we did not observe a statistically significant difference in sensitivity of a positive BDG value for the diagnosis of candidemia sustained by different species (although it was numerically higher for C. albicans), a general consideration stemming from our population of critically ill patients is that the sensitivity of BDG was generally low, ranging from 40% to 60%.
The sensitivity observed in the present study is lower than the 81% (95% CI 74 to 86%) sensitivity reported in a recent meta-analysis including 10 studies for ICU patients at risk for candidemia and candidiasis [15,16]. However, stratification of serum BDG sensitivity by Candida species was not performed in previous studies. The most recent randomized clinical trial on the use of serum BDG for guiding antifungal therapy in critically ill patients [7] reported a sensitivity of 64.3% for candidemia, despite C. albicans being the most frequently isolated Candida species responsible for infection. The data on sensitivity based on species distribution have not been reported in previous studies. A recent randomized trial reported 100% sensitivity, but it was not designed to specifically investigate this aspect, carrying the limitation of the low sample size for this question and that only 33% (i.e., 2 of 6 episodes) of documented infections of the bloodstream were caused by non-albicans species [17]. Our study showed that lower absolute serum BDG levels were observed in candidemia caused by C. auris, followed by C. parapsilosis, and higher values were observed for C. albicans [11,12,13,14]. High variability was observed in serum BDG values in candidemia episodes sustained by non-albicans species. Overall, the low sensitivity of serum BDG for the diagnosis of candidemia and the high variability of BDG values might be explained by the pathogenesis of candidemia in patients with central venous catheters (CVCs) due to species that are known skin colonizers, such as C. auris and C. parapsilosis. BDG was not helpful in anticipating the diagnosis of candidemia, but, on the contrary, higher levels of serum BDG were detected after the onset of invasive infection. This might be explained by a sudden inoculation of high fungal load through colonized or infected CVCs, in contrast to the progressive increase in fungal burden released in the bloodstream in the case of abdominal origin of candidemia. Unfortunately, this hypothesis could not be confirmed by our data as exact time-to-positivity for positive blood cultures drawn from CVC and peripheral vein, CVC tip cultures, and timing of CVC removal were not available for all the included subjects. An important aspect to be considered is that a reduced sensitivity may cast further doubts on the usefulness of BDG in hospitals where certain non-albicans species are more prevalent. In this regard, we think an interesting research question is to assess whether combinations of diagnostic biomarkers could improve the diagnostic accuracy of BDG in similar scenarios [18,19,20].
Limitations of our study are its retrospective nature and the predominance of C. parapsilosis infections, although the sample included is representative of the overall epidemiology of candidemia in our ICUs. Only 21 episodes of C. auris candidemia were included, and this might have impaired the power of our observation in detecting a difference in sensitivity of serum BDG. Moreover, repeated serum BDG values were available only for 1 in 4 episodes, thus impairing our ability to clearly evaluate the trend of BDG values over time. Some potential additional causes of high BDG levels (e.g., the administration of intravenous immunoglobulins) in the days preceding candidemia might have occurred, likely contributing to an increase in the observed sensitivity of BDG. Finally, as mentioned above, the lack of complete data on the rate of CVC-related infections and their management is a limitation in interpreting the findings of our study.
In conclusion, in critically ill patients admitted to the ICU, serum BDG levels were lower in C. parapsilosis and C. auris candidemia compared to episodes caused by C. albicans, with an overall rather low sensitivity of BDG for the diagnosis of bloodstream infections. Local epidemiology, with particular attention to emerging non-albicans species of Candida, should be carefully considered when assessing the role of BDG in the diagnosis and management of empirical therapy in ICU patients with suspected candidemia.
Acknowledgments
We would like to thank all the colleagues working in the Infectious Diseases Unit and in all the Intensive Care Units for their daily dedication to the care of patients and their support during the conduction of the present study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8090921/s1, Figure S1: Trend over time of serum BDG values stratified according to Candida species causing candidemia, when two or more samples were available.
Author Contributions
Conceptualization and methodology: M.M., L.M. and D.R.G.; formal analysis and investigation: A.S. and L.M.; writing—original draft preparation, L.M. and M.M.; writing—review and editing, M.M., L.M., A.S., C.S., S.D., S.T., A.V., F.M., N.U., P.M., L.B., P.P., A.M., D.R.G. and M.B.; resources: L.M., P.M., F.M., N.U. and A.M.; supervision: M.B., P.P., A.M. and M.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was performed in accordance with the guidelines of the Declaration of Helsinki. It was approved by the Liguria Ethics Committee (approval number 43/2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Outside the submitted work, M.B. reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Bayer, BioMérieux, Cidara, Cipla, Gilead, Menarini, MSD, Pfizer, and Shionogi. Outside the submitted work, D.R.G. reports investigator-initiated grants from Pfizer Inc and Gilead Italia and speaker and/or advisory board fees from Pfizer and Tillotts Pharma. Outside the submitted work, A.M. reports an investigator-initiated grant from Gilead Italia. The other authors have no conflict of interest to disclose.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patterson T.F., Donnelly J.P. New Concepts in Diagnostics for Invasive Mycoses: Non-Culture-Based Methodologies. J. Fungi. 2019;5:9. doi: 10.3390/jof5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karageorgopoulos D.E., Vouloumanou E.K., Ntziora F., Michalopoulos A., Rafailidis P.I., Falagas M.E. β-D-Glucan Assay for the Diagnosis of Invasive Fungal Infections: A Meta-Analysis. Clin. Infect. Dis. 2011;52:750–770. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 3.Rouzé A., Estella Á., Timsit J.-F. Is (1,3)-β-d-Glucan Useless to Guide Antifungal Therapy in ICU? Intensive Care Med. 2022;48:930–932. doi: 10.1007/s00134-022-06766-2. [DOI] [PubMed] [Google Scholar]
- 4.Martín-Mazuelos E., Loza A., Castro C., Macías D., Zakariya I., Saavedra P., Ruiz-Santana S., Marín E., León C. β-d-Glucan and Candida Albicans Germ Tube Antibody in ICU Patients with Invasive Candidiasis. Intensive Care Med. 2015;41:1424–1432. doi: 10.1007/s00134-015-3922-y. [DOI] [PubMed] [Google Scholar]
- 5.Lamoth F., Cruciani M., Mengoli C., Castagnola E., Lortholary O., Richardson M., Marchetti O. Third European Conference on Infections in Leukemia (ECIL-3) β-Glucan Antigenemia Assay for the Diagnosis of Invasive Fungal Infections in Patients with Hematological Malignancies: A Systematic Review and Meta-Analysis of Cohort Studies from the Third European Conference on Infections in Leukemia (ECIL-3) Clin. Infect. Dis. 2012;54:633–643. doi: 10.1093/cid/cir897. [DOI] [PubMed] [Google Scholar]
- 6.Mikulska M., Balletto E., Castagnola E., Mularoni A. Beta-D-Glucan in Patients with Haematological Malignancies. J. Fungi. 2021;7:1046. doi: 10.3390/jof7121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloos F., Held J., Kluge S., Simon P., Kogelmann K., de Heer G., Kuhn S.-O., Jarczak D., Motsch J., Hempel G., et al. (1 → 3)-β-d-Glucan-Guided Antifungal Therapy in Adults with Sepsis: The CandiSep Randomized Clinical Trial. Intensive Care Med. 2022;48:865–875. doi: 10.1007/s00134-022-06733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.León C., Ruiz-Santana S., Saavedra P., Castro C., Loza A., Zakariya I., Úbeda A., Parra M., Macías D., Tomás J.I., et al. Contribution of Candida Biomarkers and DNA Detection for the Diagnosis of Invasive Candidiasis in ICU Patients with Severe Abdominal Conditions. Crit. Care. 2016;20:149. doi: 10.1186/s13054-016-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornely O.A., Bassetti M., Calandra T., Garbino J., Kullberg B.J., Lortholary O., Meersseman W., Akova M., Arendrup M.C., Arikan-Akdagli S., et al. ESCMID* Guideline for the Diagnosis and Management of Candida Diseases 2012: Non-Neutropenic Adult Patients. Clin. Microbiol. Infect. 2012;18((Suppl. S7)):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Loeches I., Antonelli M., Cuenca-Estrella M., Dimopoulos G., Einav S., De Waele J.J., Garnacho-Montero J., Kanj S.S., Machado F.R., Montravers P., et al. ESICM/ESCMID Task Force on Practical Management of Invasive Candidiasis in Critically Ill Patients. Intensive Care Med. 2019;45:789–805. doi: 10.1007/s00134-019-05599-w. [DOI] [PubMed] [Google Scholar]
- 11.Mikulska M., Giacobbe D.R., Furfaro E., Mesini A., Marchese A., Del Bono V., Viscoli C. Lower Sensitivity of Serum (1,3)-β-d-Glucan for the Diagnosis of Candidaemia Due to Candida Parapsilosis. Clin. Microbiol. Infect. 2016;22:646.e5–646.e8. doi: 10.1016/j.cmi.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Chibabhai V., Fadana V., Bosman N., Nana T. Comparative Sensitivity of 1,3 Beta-D-Glucan for Common Causes of Candidaemia in South Africa. Mycoses. 2019;62:1023–1028. doi: 10.1111/myc.12982. [DOI] [PubMed] [Google Scholar]
- 13.Farooqi J., Niamatullah H., Irfan S., Zafar A., Malik F., Jabeen K. Comparison of β-d-Glucan Levels between Candida Auris and Other Candida Species at the Time of Candidaemia: A Retrospective Study. Clin. Microbiol. Infect. 2021;27:1519.e1–1519.e5. doi: 10.1016/j.cmi.2021.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Mikulska M., Furfaro E., Magnasco L., Codda G., Giacobbe D.R., Dentone C., Vena A., Marchese A., Bassetti M. Levels of Beta-D-Glucan in Candida Auris Supernatants, an in Vitro and in Vivo Preliminary Study. Clin. Microbiol. Infect. 2022;28:1154.e1–1154.e3. doi: 10.1016/j.cmi.2022.02.045. [DOI] [PubMed] [Google Scholar]
- 15.Kritikos A., Poissy J., Croxatto A., Bochud P.-Y., Pagani J.-L., Lamoth F. Impact of the Beta-Glucan Test on Management of Intensive Care Unit Patients at Risk for Invasive Candidiasis. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01996-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haydour Q., Hage C.A., Carmona E.M., Epelbaum O., Evans S.E., Gabe L.M., Knox K.S., Kolls J.K., Wengenack N.L., Prokop L.J., et al. Diagnosis of Fungal Infections. A Systematic Review and Meta-Analysis Supporting American Thoracic Society Practice Guideline. Ann. Am. Thorac. Soc. 2019;16:1179–1188. doi: 10.1513/AnnalsATS.201811-766OC. [DOI] [PubMed] [Google Scholar]
- 17.De Pascale G., Posteraro B., D’Arrigo S., Spinazzola G., Gaspari R., Bello G., Montini L.M., Cutuli S.L., Grieco D.L., Di Gravio V., et al. (1,3)-β-d-Glucan-Based Empirical Antifungal Interruption in Suspected Invasive Candidiasis: A Randomized Trial. Crit. Care. 2020;24:550. doi: 10.1186/s13054-020-03265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacobbe D.R., Asperges E., Cortegiani A., Grecchi C., Rebuffi C., Zuccaro V., Scudeller L., Bassetti M., The FUNDICU investigators Performance of Existing Clinical Scores and Laboratory Tests for the Diagnosis of Invasive Candidiasis in Critically Ill, Nonneutropenic, Adult Patients: A Systematic Review with Qualitative Evidence Synthesis. Mycoses. 2022 doi: 10.1111/myc.13515. [DOI] [PubMed] [Google Scholar]
- 19.Giacobbe D.R., Mikulska M., Tumbarello M., Furfaro E., Spadaro M., Losito A.R., Mesini A., De Pascale G., Marchese A., Bruzzone M., et al. Combined Use of Serum (1,3)-β-d-Glucan and Procalcitonin for the Early Differential Diagnosis between Candidaemia and Bacteraemia in Intensive Care Units. Crit. Care. 2017;21:176. doi: 10.1186/s13054-017-1763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Jiménez M.C., Muñoz P., Valerio M., Alonso R., Martos C., Guinea J., Bouza E. Candida Biomarkers in Patients with Candidaemia and Bacteraemia. J. Antimicrob. Chemother. 2015;70:2354–2361. doi: 10.1093/jac/dkv090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.