Abstract
It has long been recognized that there are significant differences between the sexes affecting prevalence, incidence, and severity over a broad range of diseases. Until the early 1990s, the limited research conducted on women’s health focused primarily on diseases affecting fertility and reproduction, and women were excluded from most clinical trials. For these reasons, the prevention, diagnosis, and treatment of serious chronic diseases such as cardiovascular disease in women continue to be based primarily on findings in men, and sex-specific clinical guidelines are mostly lacking. Hypertension, obesity, and diabetes, interrelated risk factors for cardiovascular disease, differ by sex in terms of prevalence and adverse effects as well as by genetics and biology. Research is needed to understand sex differences in hypertension, obesity, and diabetes to optimally inform sex-specific prevention, diagnosis, and treatment strategies for women and men. In this way, sex-specific clinical guidelines can be developed where warranted.
Keywords: cardiovascular risk factors, sex differences
The landmark 2001 Institute of Medicine report, “Exploring the Biological Contributions to Human Health: Does Sex Matter?”1 confirmed that significant differences between the sexes affect prevalence, incidence, and severity over a broad range of diseases. However, women were excluded from most clinical trials until the early 1990s, and sex differences have not been widely studied until more recently. As a result, prevention, diagnosis and treatment of serious chronic diseases, such as cardiovascular disease (CVD) in women, continue to be based primarily on findings in men, and sexspecific clinical guidelines are mostly lacking. Optimal care for women should include consideration and integration of sex differences into therapeutic guidelines (Figure 1).2
FIGURE 1. Intersection of Sex and Gender Across the Lifespan.

Sex and gender have an intersectional influence on disease risk, treatment, and complications across the lifespan. Adapted from “The Trans-NIH Strategic Plan for Women’s Health Research.”2 NIH = National Institutes of Health.
As stated by the Canadian Institute of Health Research in 2020, “sex refers to a set of biological attributes . . . including chromosomes, gene expression, hormone levels and function, and reproductive/sexual anatomy.”3 Sex differences may affect health and should be considered in trial design4 (Figure 2). Identifying and understanding sex differences is the first step in establishing sex-specific treatment guidelines where warranted.
FIGURE 2. Embedding Sex and Gender Into Clinical Research4.

Model for the prospective incorporation of sex and gender into clinical investigation. This model proposes a strategy to investigate the critical role of gender identity and sex in the planning, analysis, and conduct of clinical trials
As noted, a major challenge in the exploration of sex as a biological variable related to clinical guidelines is that fewer women were included in studies and the resulting lack of incorporation of sex-specific analyses. Therefore, in 2022, knowledge about CVD, including critical CVD risk factors such as hypertension, obesity, and diabetes, still does not include information about treatment options, treatments, and outcomes in women vs men based on biological sex differences.4–7
The purpose of this manuscript is to focus on sex differences in 3 key interrelated risk factors for CVD: hypertension, obesity, and diabetes. Although concepts related to gender, which refers to psychosociocultural factors, are also of importance, they are not the focus of this manuscript.
BACKGROUND: SEX DIFFERENCES IN HYPERTENSION, OBESITY, AND DIABETES
Sex differences exist in hypertension, obesity, and diabetes.8 Although it is known that sex modifies the incidence and risks posed by diabetes, obesity, and hypertension, there must be prospective studies to guide evidence-based, sex-specific, goal-directed therapy to improve cardiovascular health outcomes. Education is also needed so that women understand that hypertension (including hypertensive disorders of pregnancy), obesity, and diabetes place them at risk for CVD and to promote the prioritization of selfcare. To clarify what is known and what remains to be studied, we examine hypertension, obesity, and diabetes through the lens of sex differences, including the current epidemiologic data and differences in clinical care, while highlighting the lack of clinical practice guideline recommendations that incorporate sex differences (Figure 2). This paper is not meant to be a comprehensive review of these differences but will serve to highlight what is known and, importantly, serve as a call to action for future research in the area.
DESCRIPTIVE EPIDEMIOLOGY OF SEX DIFFERENCES
HYPERTENSION.
The prevalence of hypertension is higher in men than in women before age 60 years. National Health and Nutrition Examination Surveys (NHANES) data demonstrate a lower prevalence of hypertension in younger women as defined by a blood pressure >130/80 mm Hg9,10 (Figure 3). This difference in prevalence may, in part, be explained by specific impacts of estrogen on the vasculature and the sympathetic nervous system.9 Mortality related to hypertension is greater in women (with the exception of chronic kidney disease [CKD]).8 Overall, non-Hispanic (NH) Black people have a higher blood pressure than NH-White or Hispanic people. In NH-Black women, hypertension prevalence equals that of NH-Black men. Age-adjusted prevalence in hypertension in the United States decreased in women overall between 1999 and 2018, whereas overall it has not decreased in men.10 Hypertension has the highest impact on mortality of all pharmacologically modifiable cardiovascular risk factors.11 It is estimated that elimination of hypertension could reduce CVD mortality by 30.4% among men and 38.0% among women. However, hypertension remains effectively undertreated. In the United States, based on NHANES 2015 to 2018 data, women have greater awareness, treatment, and control of hypertension with the exception of NH Asian women.8
FIGURE 3. Hypertension Prevalence by Sex, Age, Race/Ethnicity, and Educational Status8.
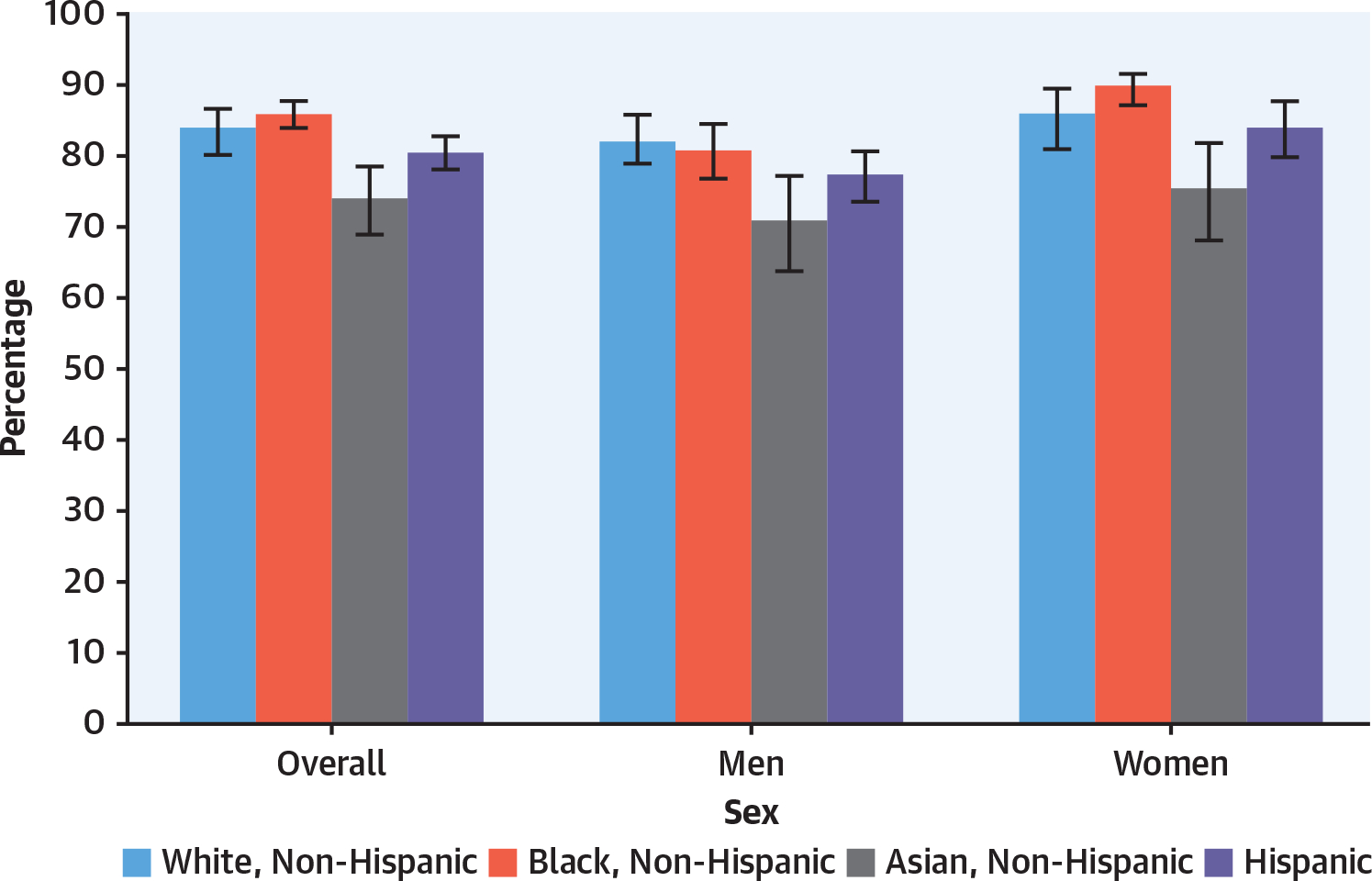
Age-adjusted prevalence of hypertension among adults aged ≥18 years by sex, race, and Hispanic origin in the United States. Hypertension is defined as systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥80 mm Hg, or currently taking medication to lower blood pressure.
Globally, with new guidelines, there is an increase in the prevalence of hypertension (exceeding 1.4 billion), including untreated hypertension.12,13 In South Korea and China, hypertension and its impact on mortality are greater in lower-income groups. The combined impact of hypertension and lower income level on cardiovascular outcomes is male predominant in China10 and India,14 whereas this difference is not reported in South Korea.15 In contrast, a small study from East Africa (using self-report) indicated twice the incidence of hypertension in women compared to men.16
Disentangling the cardiovascular effects of hypertension from those of other risk factors and comorbidities is challenging because hypertension is common in those who have obesity, type 2 diabetes (T2D), or type 1 diabetes.6 Wenger et al17 reported that 20% to 30% of hypertension is associated with overweight/obesity, with a 2- to 6-fold increase in prevalence of hypertension in the presence of overweight/obesity. Early studies of hypertension and diabetes included relatively few or no women. Data generated in the past 10 years are beginning to provide clarifying insights. For instance, premenopausal women typically have lower blood pressure than men8 (Figure 3). With advancing age (and postmenopausal status), hypertension rates are similar in women and men yet are associated with greater mortality risk and poorer achievement of guideline-directed therapy in women.8,18,19 Emerging evidence suggests that hypertension may be a more important risk factor for acute coronary syndrome and heart failure with preserved ejection fraction in women than men.18,20 Conditions that occur with pregnancy, such as preeclampsia, are associated with a short-term increased risk of postpartum hypertension and a longer-term risk of CVD.21 The relationship between blood pressure and CVD risk may also differ for the sexes in nuanced ways. Ji et al22 noted that CVD risk increased beginning at lower thresholds of systolic blood pressure for women than for men. Other large studies have reported that stroke is a complication of hypertension in women, whereas coronary heart disease and heart failure are more commonly associated with hypertension in men.20 Kringeland et al23 found that women with stage 1 hypertension in their early 40s doubled the risk of acute coronary syndromes during midlife, whereas the association was nonsignificant in men when adjusted for confounding cardiovascular risk factors. These epidemiologic reports support the need for interventional studies for the development of sex-specific hypertension management strategies. An examination of the effects of sex on the differences in hypertension observed in men and women highlights key differences. For example, analysis of the Stockholm Regional Healthcare Data Warehouse noted that men and women were treated with different antihypertensive medications and that fewer medications were dispensed for women.24 Genetics, age, race, and menopausal status—potential markers of sex differences—are implicated in some of the differences seen, as noted earlier. However, factors that are not biological also play a role. SPRINT-2015 (Systolic Blood Pressure Intervention Trial) reported that intensive blood pressure targets were associated with 27% lower all-cause mortality than the standardized targets (systolic blood pressure: 140) and were expected to lead to sex-specific guidelines.24 Only 30% of SPRINT participants were women, and the follow-up time was reduced in women. Sex-specific guidelines did not result, in part because of a lack of statistical significance. However, the ability to formally study sex and racial differences is growing, with increased sophistication in studies that could lead to sex- or race-specific approaches to pharmacotherapy. For instance, Sherwood et al25 studied the role of sympathetic activation, a mediator of increased blood pressure, in normotensive and hypertensive African American men and women compared to NH-White men and women. They found that β-adrenergic receptor responsiveness was reduced in men, African American people of both sexes, and people with higher body mass index (BMI). In contrast, α1-adrenergic receptor responsiveness was increased in women, people with hypertension, and African American people.25 More intentional study design with prespecified endpoints is needed to sort out the influences of sex appropriately (Figure 2).
CKD is one of the most serious sequelae of hypertension.26 A recent meta-analysis exploring the relationship between hypertension and CKD demonstrated a stronger relationship between hypertension and CKD progression in men than women.26 A regional study in India that focused on sex differences in the relationship between hypertension and the development of CKD progression supported increased risk in men.14 A cohort analysis followed individuals with hypertension over 10.7 years comparing ambulatory blood pressure monitoring to office blood pressure monitoring. Ambulatory blood pressure monitoring revealed a greater burden of hypertension in men than women, which was associated with greater progression to CKD and excess mortality.27 Taken together, prevalence of hypertension is higher and confers a greater risk of CKD progression in men than women, although the specific role of sex remains unclear.
OBESITY.
The prevalence of obesity in the United States increased from 30.5% in 1999 to more than 40% in 2017 to 2018 (as did severe obesity).28 The latest NHANES data reflect epidemiologic changes in obesity in the United States. Hales et al,28 using NHANES data, reported for the first time a similar prevalence of obesity in men and women (43% in men and 41.9% in women) (Figure 4).28,29 Obesity was formerly more common in women than men in middle age but is now similar between men and women across the lifespan.27,28 Severe obesity, which affects 9.2% of people, is still greatest between ages 40 and 59 years and is more prevalent in women than men (6.2% in men and 10.5% in women).8,28 NH-Black women have the highest prevalence of obesity, at 56.9%. Hispanic men and women and NH-Black men have an obesity prevalence between 41% and 45%. In both men and women, NH-Asian descent predicts a lower prevalence of obesity with no difference by sex.28 Obesity is a leading modifiable cause of morbidity and mortality from heart disease, stroke, and T2D for both men and women.17 Obesity is increased in youth aged 6 to 19 years. It is greater in boys than girls between ages 6 and 11 years but greater in girls than boys aged 12 to 19 years.28 The impact of race and ethnicity in youth is similar to that in adults, with greater obesity in NH-Black and Hispanic individuals. However, in youth younger than 19 years of age, there is no sex difference detected by race or ethnicity.28
FIGURE 4. Obesity Prevalence by Sex, Age, and Race/Ethnicity28.
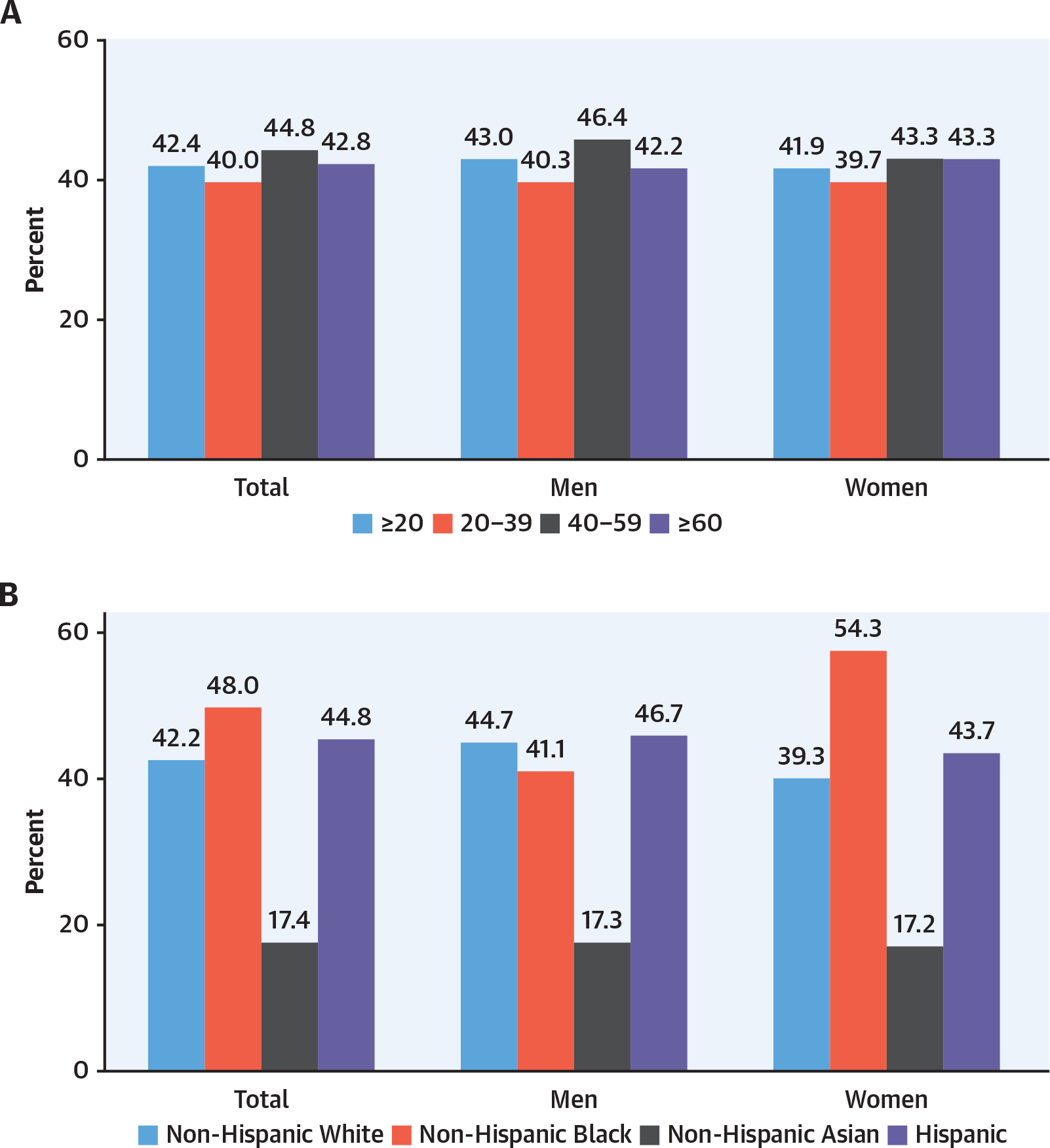
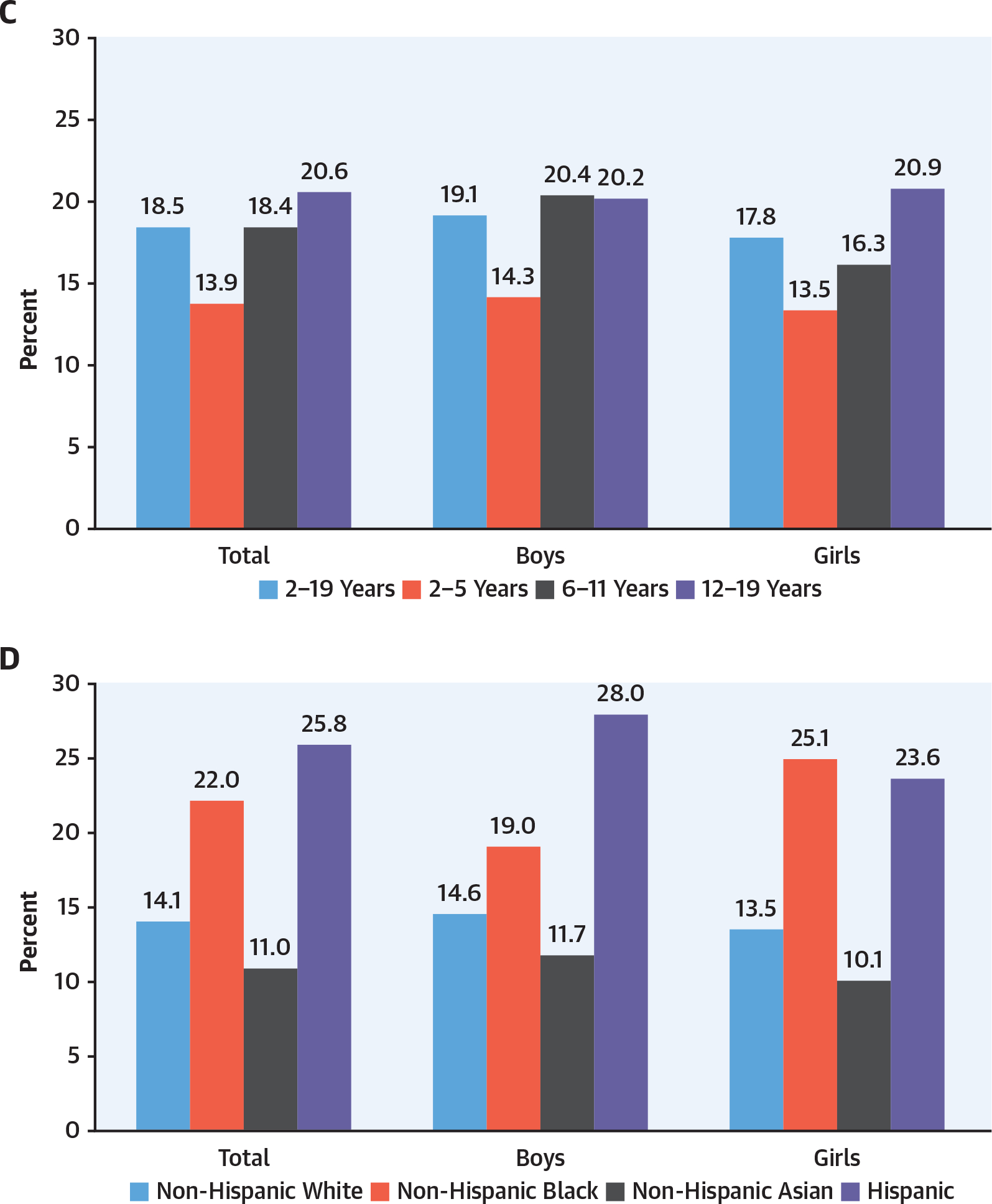
(A) Prevalence of obesity among adults aged ≥20 years by sex and age: United States, 2015 to 2016. (B) Age-adjusted prevalence of obesity among adults aged ≥20 years by sex, race, and Hispanic origin. (C) Prevalence of obesity among youth aged 2–19 years by sex and age. (D) Prevalence of obesity among youth aged 2–19 years by sex, race, Hispanic origin.
There are sex differences in the manifestations of obesity (Figure 4). For instance, men and women deposit adipose tissue differently, with men having greater central adiposity and women (especially before menopause) having greater peripheral fat depots.30 These differences are frequently depicted as the central/visceral adiposity (apple shape) distribution in men vs the hip/subcutaneous (pear shape) distribution in women. This adipose distribution has physiologic implications. For example, with menopause and the accompanying loss of estrogen, there is a redistribution of adipose tissue to the visceral adipose depots in women, which are classically less insulin sensitive and associated with increased cardiometabolic risk. In addition, estrogen replacement in menopause decreases visceral adipose tissue, and preclinical studies demonstrate estrogen regulation of fat cell proliferation and differentiation.31 By comparison, men have higher skeletal muscle mass and lower fat mass than women; however, the adipose distribution in men is less subcutaneous and more central, and this distribution can be associated with lower insulin sensitivity in men. All of these sex-based observations regarding adiposity are greatly modified by diet, physical activity, and family history/genetic background.
A global analysis of 10 million people from 239 studies originating from Asia, Australia and New Zealand, Europe, and North America evaluated the impact of sex and BMI on mortality.32 Men had a greater HR for mortality with increasing BMI (1.51 vs 1.30 per 5 kg/m2 compared with women) (Figure 5). This mortality risk has prompted a global call for obesity prevention and treatment. Interestingly, a large-scale analysis to define genetic loci that influence body size identified single nucleotide polymorphisms associated with obesity risk by sex and age together as well as individually, consistent with the interaction of genetics and sex.33 These observations offer insights into sex differences in obesity-related complications and comorbidities; they do not yet define parameters useful in clinical practice. In children, obesity is more highly associated with hepatic steatosis in boys and with early pubarche and menarche in girls.34 Obesity is an epidemiologic predictor of premature mortality, likely stemming from its association with comorbid conditions (reviewed by Bray et al35) including hypertension, diabetes, depression, sleep apnea, nonalcoholic steatohepatitis, and breast and endometrial cancer as well as sex difference constructs.
FIGURE 5. Body Mass Index and All-Cause Mortality in Men and Women32.
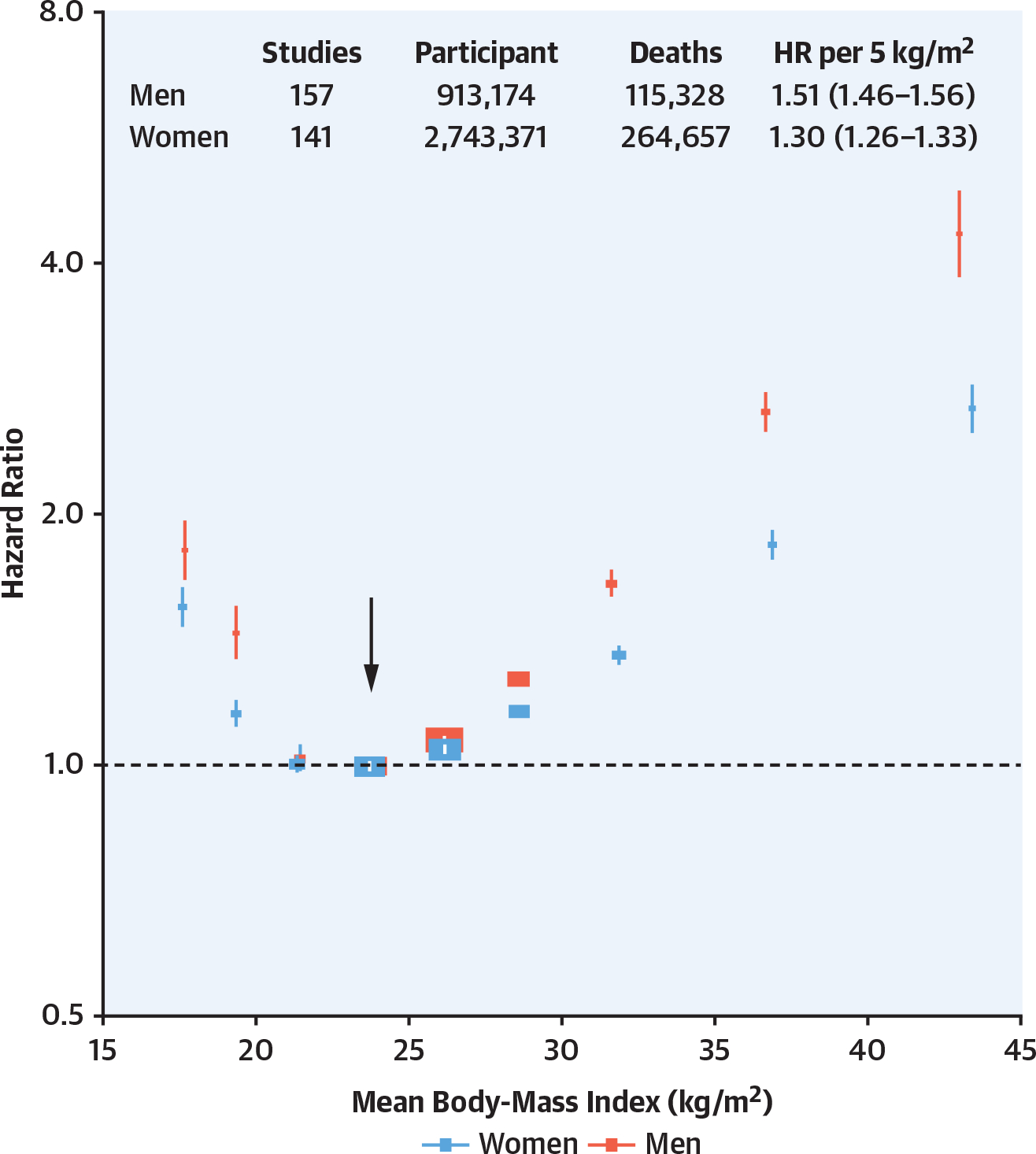
Association of body mass index with all-cause mortality, by sex, based on an individual-participant-data meta-analysis of 239 prospective studies on 4 continents. Analyses were restricted to 3.9 million never-smokers without pre-existing chronic disease.
DIABETES.
A total of 34.1 million people live with diabetes in the United States (12% of the population older than 18 years of age). Thirteen percent of men and 11% of women are affected36 (Table 1). Diabetes-related hospitalization and mortality are greater for men than women.8 However, in 2 different metaanalyses, the data were split on sex differences in diabetes related to all-cause mortality.8 The largest population affected by diabetes is the Hispanic population, followed by NH-Black and NH-Asian people. The effects of sex on diabetes risk were not robustly evaluated in the Centers for Disease Control and Prevention NHANES report.36 Youth-onset T2D is rapidly increasing (although still rare), and with the exception of Chinese boys, there is a 2:1 female predominance37 (reviewed by Huebschmann et al38 and Kautzky-Willer et al39); in contrast, male youth are more likely to have prediabetes than female youth.8 The prevalence of gestational diabetes was highest in Hispanic females (9.3%) and lower among NH-White and NH-Black females.8
TABLE 1.
Diabetes Demographics36
| Diagnosed Diabetes | Undiagnosed Diabetes | Total Diabetes Percentage | |
|---|---|---|---|
|
| |||
| Total | 9.4 (8.6–10.2) | 2.6 (2.2–3.1) | 12.0 (11.1–12.9) |
| Sex | |||
| Men | 10.4 (9.2–11.7) | 3.0 (2.2–4.0) | 13.3 (12.0–14.8) |
| Women | 8.6 (7.7–9.S) | 2.2 (1.8–2.8) | 10.8 (9.9–11.8) |
| Race/ethnicity | |||
| White, Non-Hispanic | 7.9 (7.2–8.7) | 202 (1.6–2.9) | 10.0 (9.2–11.0) |
| Black, Non-Hispanic | 13.7 (12.S-1S.1) | 3.0 (2.0–4.S) | 16.8 (1S.4–18.1) |
| Asian, Non-Hispanic | 11.3 (9.2–13.7) | 4.7 (3.0–7.3) | 16.0 (13.7–18.S) |
| Hispanic | 13.7 (12.1–1S.6) | 4.1 (3.1-S.4) | 17.9 (16.0–19.9) |
| Education | |||
| Less than high school | 12.7 (11.4–14.2) | 3.9 (2.S-S.8) | 16.6 (14.8–18.6) |
| High school | 9.7 (8.S-11.1) | 3.0 (2.1–4.4) | 12.8 (11.1–14.7) |
| More than high schoo | 8.3 (7.3–9.S) | 2.2 (1.6–2.8) | 10.S (9.4–11.8) |
Values are % (95% CI). Age-adjusted prevalence of diagnosed, undiagnosed, and total diabetes among adults aged 18 years or older, United States, 2013 to 2016. Data source: 2013 to 2016 National Health and Nutrition Examination Survey.36
In the United States, there is a consistent relationship between increases in obesity with increased incidence of diabetes (with obesity accounting for 30%–53% of the attributable risk), highest in NHWhite women.40 European studies have indicated that men develop diabetes at a younger age with lower BMI than women.39 Girls and women have lower physical activity and more sedentary time than boys and men.8 A secondary analysis of global data sets tested the relationships between obesity and physical activity and diabetes prevalence by sex. In men, the relationship between increased obesity prevalence and increased diabetes was inconsistent. In women there was a stronger relationship between obesity and diabetes than in men, but certain regions did not follow this pattern.41 It is likely that different populations have different genetic backgrounds that regulate insulin secretion and insulin sensitivity. Additional multinational examination of genetic loci associated with clinical characteristics such as insulin sensitivity, adiposity, and fasting glucose reinforces the sexual-dimorphic impact of genetic variants and highlights the need to examine genetic risk by sex across populations.42
The cardiovascular consequences of diabetes differ in men and women, with women having greater cardiovascular sequelae.43 Reviews have highlighted epidemiologic data and physiologic mechanisms potentially contributing to poorer CVD outcomes in women compared to men.7,35,38–40,44,45 Premenopausal women without diabetes have fewer heart attacks than men. The reasons for this cardioprotection are not entirely clear but are likely multifactorial, with contributions from physiologic differences, including the impact of sex hormones, differences in cardiovascular risk factors, and differences between the sexes in the diagnosis and treatment of diabetes and CVD. A striking sex difference is the loss of cardioprotection in premenopausal women with diabetes relative to age-similar women without diabetes.38 Even short diabetes exposure, such as gestational diabetes, is a sex-specific risk factor for CVD. Not mentioned earlier, hypertension in pregnancy or preeclampsia increases diabetes risk compared to normotensive pregnancy.8 Many reviews have highlighted how sex influences in diabetes likely contribute to poor outcomes in women and offspring of diabetic pregnancies.8,35,38–40,44,45 Despite the differences, there are no sex-specific therapeutic guidelines for preventing or treating diabetes at present.
SEX DIFFERENCES IN CLINICAL CARE FOR HYPERTENSION, OBESITY, AND DIABETES
Despite the significant sex differences revealed by the literature in hypertension, obesity, and diabetes, only recently has the idea of sex-specific clinical cardiovascular care emerged. To date, no sex-specific recommendations for the management of hypertension, obesity, and diabetes have been drafted, largely because of a lack of prospective evidence-based support for such guidance. One reason for the lack of sex-specific care recommendations stems from inadequate inclusion of women in clinical trials until relatively recently. Many of the landmark cardiovascular outcomes trials on which current guidelines are formulated included significantly fewer women than men (or no women), thereby limiting sex-specific evidence. However, there are many examples that illustrate situations in which there are signals that differentiating care between the sexes might lead to better outcomes. Importantly, clinical trials must be designed prospectively to include adequate numbers of women to support an examination of sex differences (Figure 2). This section serves as a call to action for research efforts addressing this important topic.
Sex differences in therapeutic response to antihypertensive agents were recently reviewed by Kalibala et al.46 Pharmacokinetics are known to differ between women and men because of differing gastrointestinal acid composition and motility as well as differences in volume of distribution, organ perfusion, and hepatic metabolism.46 Additionally, because of concerns for pregnancy risk, men are more often prescribed goal-directed angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and beta-blockers in a setting where women receive diuretics and calcium-channel blockers.47 Biological factors, including salt sensitivity in women and lower renin-angiotensin aldosterone system in women before menopause, reinforce these prescribing patterns. Sex differences in cardiovascular outcomes across the largest hypertension trials to date were also recently reviewed, and a few differences were noted.46 In the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, stroke rate in women was higher with angiotensin-converting enzyme inhibitors compared to either diuretics or calcium-channel blockers; in the Valsartan Antihypertensive Long-Term Use Evaluation Trial, women had a better cardiovascular morbidity and mortality outcome with amlodipine than valsartan, whereas the majority of studies (representing more than 150,000 person-years) demonstrated no sex difference in outcome with antihypertensive therapy. Women appear to have lower clearance of and response to beta-blockers and, therefore, a narrower therapeutic index.47 Even the relatively recent SPRINT trial failed to include enough women and follow them for long enough to gain conclusive results that could have led to sexspecific guidelines.24,48 Hence, across clinical trial data, there is no clear message as to evidence-based sex-specific guidelines.
Obesity is an increasing problem changing the global cardiometabolic health landscape. In contrast to other cardiovascular outcomes studies, most participants in weight loss clinical trials and lifestyle interventions are women. For example, 2 of the largest, most well-known weight loss cohorts highlighting behavior change were predominantly female. These include the National Weight Control Registry (a cohort of more than 10,000 individuals—80% of them women—who have successfully lost at least 30 lb and maintained that weight loss for >1 year)49 and a younger cohort, the MedWeight study (69% women, with 15%–25% weight loss with the regain over the first year as the comparator).50 In these studies, differences were noted in behavior and food choices between men and women, but no clear sex differences in maintenance or weight regain emerged by sex.50 Similarly, new agents to treat obesity have been studied in female-predominant interventions, although no sex differences in outcomes have been widely disseminated. The recently approved semaglutide 2.4-mg preparation for obesity has been methodically studied across populations with and without diabetes. Prespecified analysis of the full suite of Semaglutide Treatment Effect in People With Obesity program and A Heart Disease Study of Semaglutide in Patients with Type 2 Diabetes clinical trials may offer the opportunity for well-powered analyses to define sex differences in medication tolerance, weight loss, and cardiovascular benefit.51 In another example, women currently undergo bariatric surgery more commonly than men. Approximately 80% of bariatric surgery patients are women, and 20% are men.52 Reasons for this discrepancy remain unclear and likely reflect social context beyond sex. Men also have worse complications secondary to bariatric surgery and yet express more satisfaction with the treatment.52 Although significant behavioral and pharmacologic weight loss data are available (predominantly in women), rigorous data to support sex-specific treatment approaches are not established.
Similarly, there are no sex-specific recommendations for diabetes prevention and treatment, although there are sex differences in the effects of diabetes and CVD and medication effects in women compared to men (Table 2). For instance, the loss of cardioprotection in younger women with diabetes is not well addressed in cardiovascular risk engines that support the initiation of risk factor interventions. There is literature reporting on sex differences in the response to drugs that treat T2D.67 Dennis et al68 studied 22,379 patients starting sulfonylurea or thiazolidinedione therapy in the U.K. Clinical Practice Research Datalink. They found that there were different benefits as well as complications according to sex and BMI. For hemoglobin A1c lowering, men had greater response to sulfonylureas, and women responded better to thiazolidinediones (although there was weight gain in obese women). Several studies demonstrated greater cardiovascular benefit from drugs to treat diabetes in men vs women.68,69 Raparelli et al69 reported that among a total of 167,254 adults with T2D, including 46% women, newer glucose-lowering drugs such as glucagon-like peptide-1 receptor agonists were associated with a lower risk of cardiovascular events than sulfonylureas. The effects were stronger in women than men. Gouni-Berthold et al70 reported that women with diabetes and CVD had poorer control of CVD risk factors including lipids, blood pressure, and low-density lipoprotein cholesterol, as well as hemoglobin A1c, than men. These findings identify the need for prospectively designed studies to investigate sex differences in pharmacologic, interventional, and behavioral treatments for diabetes. The finding that women with T2D are less aggressively treated than men also requires further study.53,69
TABLE 2.
Contemporary Guidelines for Treatment of Hypertension, Obesity, and Diabetes
| Screening of Targets Differs by Sex or Gender | Sex Specific | |
|---|---|---|
|
| ||
| Hypertension: | ||
| • Primary change in the hypertension guidelines (AHA/ACC) is a lower threshold for pharmacotherapy at a blood pressure of 130/80 mm Hg | ||
| • Not included in the ADA or European guidance | ||
| • Sex and gender not addressed | ||
| “Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines”53 | No | Pregnancy Hypertensive disorders of pregnancy |
| “2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines”54 | No | Pregnancy Hypertensive disorders of pregnancy |
| “International Society of Hypertension Global Hypertension Practice Guidelines”55 | No | Pregnancy Hypertensive disorders of pregnancy |
| “ACC/AHA Versus ESC/ESH on Hypertension Guidelines: JACC Guideline Comparison”56 | No | Pregnancy Hypertensive disorders of pregnancy |
| “Hypertension Canada’s 2020 Comprehensive Guidelines for the Prevention, Diagnosis, Risk Assessment, and Treatment of Hypertension in Adults and Children”57 | No | Management of hypertension in women planning pregnancy |
| “2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8)”58 | No | Pregnancy Hypertensive disorders of pregnancy |
| “Estimating the Association of the 2017 and 2014 Hypertension Guidelines With Cardiovascular Events and Deaths in US Adults: An Analysis of National Data”59 | No | Pregnancy Hypertensive disorders of pregnancy |
| Obesity: | ||
| • Increased number of effective medications (focus on GLP-1 receptor ligands) | ||
| • Sex and gender not addressed | ||
| “American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients With Obesity”60 | No | Different calorie goals Preconception Pregnancy Lactation |
| “AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society”61 | No | Different calorie goals Preconception Pregnancy Lactation |
| “Obesity in Adults: A Clinical Practice Guideline”29 | No | Different calorie goals Preconception Pregnancy Lactation |
| “Pediatric Obesity–Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline”34 | No | Preconception Pregnancy Lactation |
| “Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline”62 | No | Different calorie goals Preconception Pregnancy Lactation goals |
| Diabetes: | ||
| •Incorporation of cardiovascular outcomes trials with GLP-1 and SGLT-2 inhibitor into glucose-lowering therapy | ||
| • ADA/ACC/AHA and ESC guidance concordance is strong | ||
| • Sex and gender not addressed | ||
| “Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021”63 | No | No |
| “Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021”64 | No | Preconception Pregnancy Lactation |
| “Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2021”65 | No | Preconception Pregnancy Lactation |
| “Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes: A Report of the American College of Cardiology Solution Set Oversight Committee”66 | No | No |
AAPA = American Academy of Physician Assistants; ABC = Association of Black Cardiologists; ACC = American College of Cardiology; ACPM = American College of Preventive Medicine; ADA = American Diabetes Association; AGS = American Geriatrics Society; AHA = American Heart Association; APhA = American Pharmacists Association; ASH = American Society of Hypertension; ASPC = American Society for Preventive Cardiology; ESC = European Society of Cardiology; ESH = European Society of Hypertension; GLP-1 = glucagon-like peptide-1; NMA = National Medical Association; PCNA = Preventive Cardiovascular Nurses Association; SGLT-2 = sodium-glucose cotransporter-2; TOS = The Obesity Society.
To summarize, sex differences must be included in the approach to optimize the clinical management of hypertension, obesity, and diabetes. Healthy behaviors differ in women vs men and girls vs boys; these differences are reported by sex in some cases, although not all. One key example with clinically relevant sex differences is physical activity.71 Physical activity is beneficial for the prevention and treatment of hypertension, obesity, and diabetes. Most people in the United States are sedentary, and women and girls are less active than men across all ages, races, and ethnicities for reasons that are unclear.8 Therefore, useful physical activity interventions must address evidence-based approaches to increase physical activity for women and girls.
CLINICAL PRACTICE GUIDELINE RECOMMENDATIONS
Increasingly, the sex differences observed in hypertension, obesity, and diabetes are leading to evidence-based reports of sex differences in outcomes. Much more research is needed to understand the biological basis for the influence of sex and to bring these findings to establishing sexspecific clinical guidelines. Women are now included in clinical studies in greater numbers (although this remains an area of concern), and data need to be prospectively analyzed, disaggregated, and reported by sex. Findings of sex differences in epidemiologic and interventional studies should be analyzed as a routine aspect of prespecified study design supported by U.S. Food and Drug Administration guidance to inform evidence-based recommendations that are specific to women and men (summarized in Table 2).
FUTURE DIRECTIONS
Much more research is needed to understand and incorporate sex differences in studies of the prevention, diagnosis, and treatment of hypertension, obesity, and diabetes, especially because they are associated with CVD (Central Illustration). However, in addition to understanding the influences of sex differences, it is essential that much more research also evaluate and incorporate gender constructs, which may also strongly affect optimal care for both sexes and all genders72 (Figure 2). Women are more likely to be deleteriously affected by gender-related issues such as lower socioeconomic status, depression, education, power, and physical activity as well as reduced access to medical care; these factors demonstrate a positive interaction with obesity as well as hypertension and diabetes.24 The intersection of sex and gender may underlie the observation that women receive less goal-directed therapy than men. It is crucial to consider, for instance, that control of cardiovascular risk factors was better predicted by gender than by biological sex, showing the importance of understanding gender as well as sex differences.7 Future studies must therefore incorporate both sex and gender constructs.
CENTRAL ILLUSTRATION. A Call to Action for Studying Sex Differences.
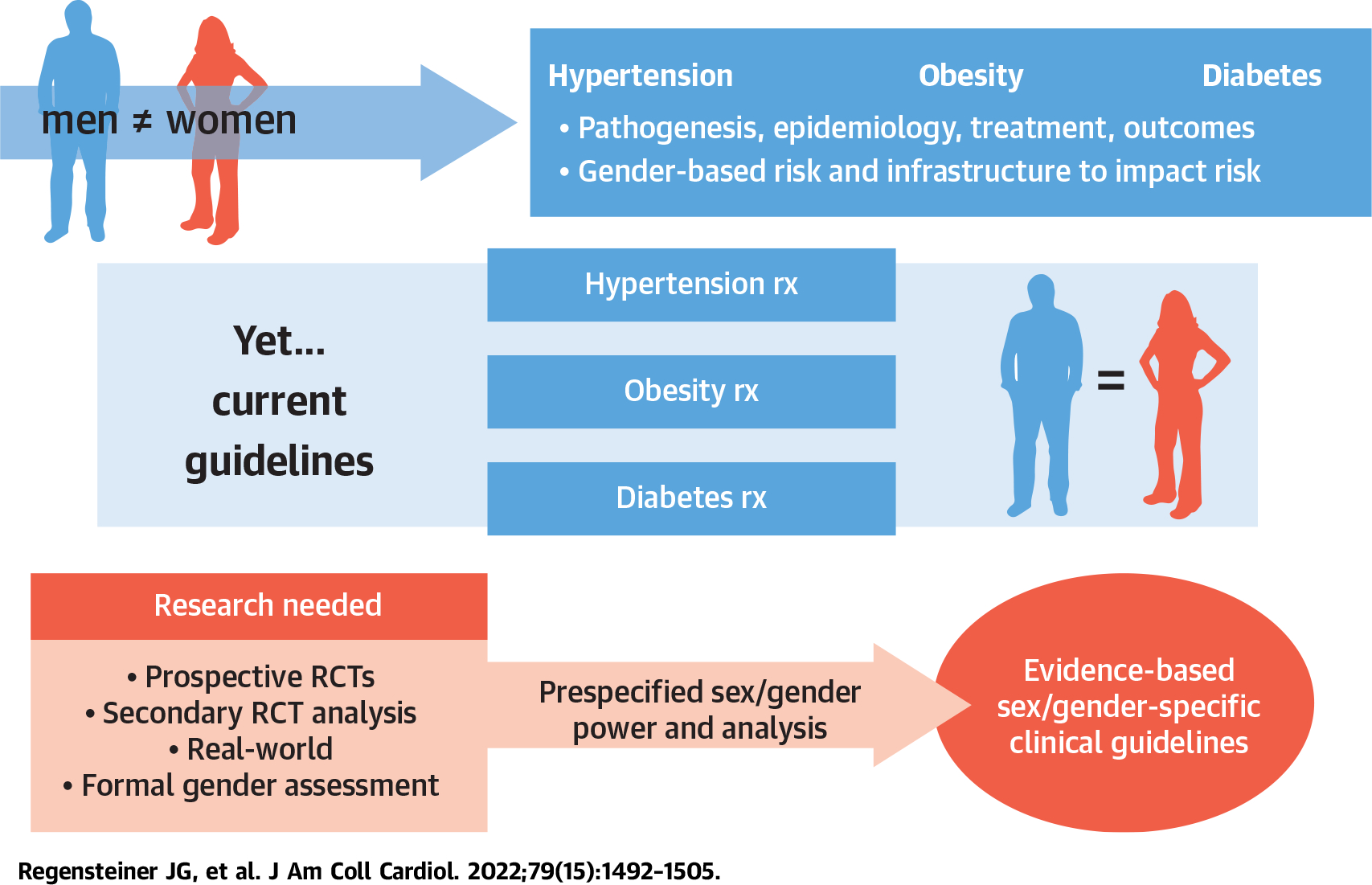
The pathogenesis, epidemiology, and outcomes for hypertension, obesity, and diabetes differ in women and men and in boys and girls, demonstrating the intersection of sex and gender. However, because of a lack of specifically designed studies to examine therapeutic sexspecific responses to therapy, we lack a robust evidence base to present well-defended sex-specific guidance. Future studies should address sex and gender as outcome variables incorporated into the design of the study. Currently available data from completed and in-process studies should be explored for sex differences. RCT = randomized controlled trial; rx = treatment.
CONCLUSIONS
Biological sex differences such as genetic predisposition, gene expression, and hormone levels likely have a powerful and understudied impact on the presentation of hypertension, obesity, and diabetes.73 These differences are not well understood because of a combination of the inadequate inclusion of women in clinical trials and a lack of prespecified analyses of these factors. Biological sex differences in hypertension are supported by studies in preclinical models and specifically demonstrated in the case of hypertensive disorders of pregnancy and preeclampsia.11,26,74 Menopause and the resulting changes in hormone levels also likely play a role in age-related increases in hypertension. The interaction between obesity, body fat distribution, genes, and environment also differs by sex.33,35,42,74 Genetic and biological differences in the risk for developing diabetes and cardiovascular mortality are established.7,33,39,42 Prospective clinical trials are lacking to support sex-specific guidelines for hypertension, obesity, or diabetes except screening and management in anticipation of pregnancy and during pregnancy as well as with postpartum breastfeeding. Understanding the influences of sex to inform optimal clinical guidance and improve the health of men and women will require comprehensive analysis of available data dedicated to these issues and thoughtful design of further prospective studies to formally address sex and gender (Figure 2).
HIGHLIGHTS.
A lack of sex-specific clinical guidelines can adversely affect patient care, especially for women with hypertension or diabetes, for whom therapy often falls short of goals.
Carefully designed studies of the influence of sex on responses to clinical interventions could improve care for all patients.
Sex and gender should be incorporated into the design of prospective trials to ensure that outcomes and the implementation of findings are broadly and appropriately applicable to patient care.
ACKNOWLEDGMENT
The authors thank Ms. Regina Daly for her excellent assistance with the manuscript.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- NH
non-Hispanic
- NHANES
National Health and Nutrition Examination Surveys
- T2D
type 2 diabetes
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Exploring the Biological Contributions to Human Health: Does Sex Matter? Institute of Medicine Committee on Understanding the Biology of Sex and Gender Differences; 2001. [Google Scholar]
- 2.National Institutes of Health. Advancing science for the health of women: the trans-NIH strategic plan for women’s health research. Office of Research on Women’s Health. Accessed December 14, 2021. https://orwh.od.nih.gov/sites/orwh/files/docs/ORWH_Strategic_Plan_2019_508C_0.pdf [Google Scholar]
- 3.Canadian Institute of Health Research. What is gender? What is sex? Government of Canada. Accessed December 14, 2021. https://cihr-irsc.gc.ca/e/48642.html [Google Scholar]
- 4.Vasquez-Avila K, Pacheco-Barrios K, de Melo PS, Fregni F. Addressing the critical role of gender identity and sex in the planning, analysis, and conduct of clinical trials. Princ Pract Clin Res. 2021;7:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regensteiner JG, Golden S, Huebschmann AG, et al. Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2015;132:2424–2447. [DOI] [PubMed] [Google Scholar]
- 6.Hamulyak EN, Brockmeier AJ, Killas JD, Ananiadou S, Middeldorp S, Leroi AM. Women’s health in The BMJ: a data science history. BMJ Open. 2020;10:e039759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396:565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins WS, Richardson C, Williams A, Williams-DeVane CR. Creating a metabolic syndrome research resource using the National Health and Nutrition Examination Survey. Database (Oxford). 2020:baaa103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.QuickStats: Age-adjusted prevalence of adults aged ≥18 years with hypertension who are aware they have hypertension, by sex and race/ethnicity—National Health and Nutrition Examination Survey, United States, 2011–2014. MMWR Morb Mortal Wkly Rep. 2016;65:525. [DOI] [PubMed] [Google Scholar]
- 11.Kawasoe S, Kubozono T, Ojima S, et al. Sex differences in the effects of weight reduction on future blood pressure elevation in a mildly obese middle-aged population. Circ Rep. 2020;2:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tocci G, Presta V, Ferri C, Redon J, Volpe M. Blood pressure targets achievement according to 2018 ESC/ESH guidelines in three European excellence centers for hypertension. High Blood Press Cardiovasc Prev. 2020;27:51–59. [DOI] [PubMed] [Google Scholar]
- 13.Egan BM, Kjeldsen SE, Grassi G, Esler M, Mancia G. The global burden of hypertension exceeds 1.4 billion people: should a systolic blood pressure target below 130 become the universal standard? J Hypertens. 2019;37:1148–1153. [DOI] [PubMed] [Google Scholar]
- 14.Gummidi B, John O, Ghosh A, et al. A systematic study of the prevalence and risk factors of CKD in Uddanam, India. Kidney Int Rep. 2020;5:2246–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin JH, Jung MH, Kwon CH, et al. Disparities in mortality and cardiovascular events by income and blood pressure levels among patients with hypertension in South Korea. J Am Heart Assoc. 2021;10(7):e018446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngaruiya C, Wambua M, Mutua TK, et al. The last frontier for global non-communicable disease action: the emergency department—a cross-sectional study from East Africa. PLoS One. 2021;16:e0248709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenger NK, Arnold A, Bairey Merz CN, et al. Hypertension across a woman’s life cycle. J Am Coll Cardiol. 2018;71:1797–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muiesan ML, Salvetti M, Rosei CA, Paini A. Gender differences in antihypertensive treatment: myths or legends? High Blood Press Cardiovasc Prev. 2016;23:105–113. [DOI] [PubMed] [Google Scholar]
- 19.Geraghty L, Figtree GA, Schutte AE, Patel S, Woodward M, Arnott C. Cardiovascular disease in women: from pathophysiology to novel and emerging risk factors. Heart Lung Circ. 2021;30:9–17. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull F, Woodward M, Anna V. Effectiveness of blood pressure lowering: evidence-based comparisons between men and women. Expert Rev Cardiovasc Ther. 2010;8:199–209. [DOI] [PubMed] [Google Scholar]
- 21.Veerbeek JH, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015;65:600–606. [DOI] [PubMed] [Google Scholar]
- 22.Ji H, Niiranen TJ, Rader F, et al. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation. 2021;143:761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kringeland E, Tell GS, Midtbo H, Igland J, Haugsgjerd TR, Gerdts E. Stage 1 hypertension, sex, and acute coronary syndromes during midlife: the Hordaland Health Study. Eur J Prev Cardiol. 2022;29(1):147–154. [DOI] [PubMed] [Google Scholar]
- 24.Reckelhoff JF. Gender differences in hypertension. Curr Opin Nephrol Hypertens. 2018;27:176–181. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood A, Hill LK, Blumenthal JA, Johnson KS, Hinderliter AL. Race and sex differences in cardiovascular α-adrenergic and β-adrenergic receptor responsiveness in men and women with high blood pressure. J Hypertens. 2017;35:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weldegiorgis M, Woodward M. The impact of hypertension on chronic kidney disease and end-stage renal disease is greater in men than women: a systematic review and meta-analysis. BMC Nephrol. 2020;21:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minutolo R, Gabbai FB, Agarwal R, et al. Sex difference in ambulatory blood pressure control associates with risk of ESKD and death in CKD patients receiving stable nephrology care. Nephrol Dial Transplant. 2021;36(11):2000–2007. [DOI] [PubMed] [Google Scholar]
- 28.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;(360):1–8. [PubMed] [Google Scholar]
- 29.Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192:E875–E891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zore T, Palafox M, Reue K. Sex differences in obesity, lipid metabolism, and inflammation—a role for the sex chromosomes? Mol Metab. 2018;15:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol. 2021;17:47–66. [DOI] [PubMed] [Google Scholar]
- 32.Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkler TW, Justice AE, Graff M, et al. The influence of age and sex on genetic associations with adult body size and shape: a large-scale genome-wide interaction study. PLoS Genet. 2015;11:e1005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity—assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an Endocrine Society scientific statement. Endocr Rev. 2018;39:79–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Diabetes Statistics Report. Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 37.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG, Reusch JEB. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia. 2019;62:1761–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37:278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cameron NA, Petito LC, McCabe M, et al. Quantifying the sex-race/ethnicity-specific burden of obesity on incident diabetes mellitus in the United States, 2001 to 2016: MESA and NHANES. J Am Heart Assoc. 2021;10:e018799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alkaf B, Blakemore AI, Järvelin MR, Lessan N. Secondary analyses of global datasets: do obesity and physical activity explain variation in diabetes risk across populations? Int J Obes (Lond). 2021;45:944–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lagou V, Mägi R, Hottenga JJ, et al. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nat Commun 2021:12:24. Erratum in: Nat Commun. 2021;12(1):995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Salameh A, Chanson P, Bucher S, Ringa V, Becquemont L. Cardiovascular disease in type 2 diabetes: a review of sex-related differences in predisposition and prevention. Mayo Clin Proc. 2019;94:287–308. [DOI] [PubMed] [Google Scholar]
- 44.Glechner A, Harreiter J, Gartlehner G, et al. Sex-specific differences in diabetes prevention: a systematic review and meta-analysis. Diabetologia. 2015;58:242–254. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Liu J, Shao S, et al. Sex differences in the prevalence of and risk factors for abnormal glucose regulation in adults aged 50 years or older with normal fasting plasma glucose levels. Front Endocrinol (Lausanne). 2020;11:531796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalibala J, Pechère-Bertschi A, Desmeules J. Gender differences in cardiovascular pharmacotherapy-the example of hypertension: a mini review. Front Pharmacol. 2020;11:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cadeddu C, Franconi F, Cassisa L, et al. Arterial hypertension in the female world: pathophysiology and therapy. J Cardiovasc Med (Hagerstown). 2016;17:229–236. [DOI] [PubMed] [Google Scholar]
- 48.Wenger NK, Ferdinand KC, Bairey Merz CN, Walsh MN, Gulati M, Pepine CJ. Women, hypertension, and the systolic blood pressure intervention trial. Am J Med. 2016;129:1030–1036. [DOI] [PubMed] [Google Scholar]
- 49.Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17–23. [DOI] [PubMed] [Google Scholar]
- 50.Poulimeneas D, Anastasiou CA, Santos I, Hill JO, Panagiotakos DB, Yannakoulia M. Exploring the relationship between the Mediterranean diet and weight loss maintenance: the MedWeight study. Br J Nutr. 2020;124:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. [DOI] [PubMed] [Google Scholar]
- 52.Kochkodan J, Telem DA, Ghaferi AA. Physiologic and psychological gender differences in bariatric surgery. Surg Endosc. 2018;32:1382–1388. [DOI] [PubMed] [Google Scholar]
- 53.Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):2176–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 55.Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38:982–1004. [DOI] [PubMed] [Google Scholar]
- 56.Bakris G, Ali W, Parati G. ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J Am Coll Cardiol. 2019;73:3018–3026. [DOI] [PubMed] [Google Scholar]
- 57.Rabi DM, McBrien KA, Sapir-Pichhadze R, et al. Hypertension Canada’s 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. 2020;36:596–624. [DOI] [PubMed] [Google Scholar]
- 58.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 59.Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the association of the 2017 and 2014 hypertension guidelines with cardiovascular events and deaths in US adults: an analysis of national data. JAMA Cardiol. 2018;3:572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1–203. [DOI] [PubMed] [Google Scholar]
- 61.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. [DOI] [PubMed] [Google Scholar]
- 62.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362. [DOI] [PubMed] [Google Scholar]
- 63.Cardiovascular disease and risk management: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44:S125–S150. [DOI] [PubMed] [Google Scholar]
- 64.Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44:S111–S124. [DOI] [PubMed] [Google Scholar]
- 65.Obesity management for the treatment of type 2 diabetes: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44:S100–S110. [DOI] [PubMed] [Google Scholar]
- 66.Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fourny N, Beauloye C, Bernard M, Horman S, Desrois M, Bertrand L. Sex differences of the diabetic heart. Front Physiol. 2021;12:661297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dennis JM, Henley WE, Weedon MN, et al. Sex and BMI alter the benefits and risks of sulfonylureas and thiazolidinediones in type 2 diabetes: a framework for evaluating stratification using routine clinical and individual trial data. Diabetes Care. 2018;41:1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raparelli V, Elharram M, Moura CS, et al. Sex differences in cardiovascular effectiveness of newer glucose-lowering drugs added to metformin in type 2 diabetes mellitus. J Am Heart Assoc. 2020;9:e012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gouni-Berthold I, Berthold HK, Mantzoros CS, Bohm M, Krone W. Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care. 2008;31:1389–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robinson AT, Wenner MM, Charkoudian N. Differential influences of dietary sodium on blood pressure regulation based on race and sex. Auton Neurosci. 2021;236:102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson JL, Greaves L, Repta R. Better science with sex and gender: facilitating the use of a sex and gender-based analysis in health research. Int J Equity Health. 2009;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Connelly PJ, Azizi Z, Alipour P, Delles C, Pilote L, Raparelli V. The importance of gender to understand sex differences in cardiovascular disease. Can J Cardiol. 2021;37:699–710. [DOI] [PubMed] [Google Scholar]
- 74.Sabbatini AR, Kararigas G. Estrogen-related mechanisms in sex differences of hypertension and target organ damage. Biol Sex Differ. 2020;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


