Abstract
Simple Summary
The genus Trichinella includes roundworm parasites with a wide geographical spread that can cause illness in humans and animals. In this context, an epidemiological study of Trichinella infection was carried out in the northeastern part of Romania to investigate for the first time its prevalence in pigs, horses, wild boars and bears, the geographical distribution of Trichinella species and the natural reservoir of the parasites. Between 2010 and 2015, a total of 166,270 animals were examined by specific methods in order to calculate the prevalence of Trichinella infection, the involved species, and their geographical distribution. The overall prevalence of Trichinella infection in animals was 0.188%. But the specific prevalence varied as follows: in pigs 0.096%, horses 0.021%, wild boar 1.46% and bears 36.76%. The geographical distribution showed that T. spiralis was dominant, occupying the entire northeastern part of Romania, being identified in pigs, horses, wild boars and bears. T britovi occupied five mountain counties, being identified only in wild boars and bears. These results validate the presence of T. spiralis and T. britovi in domestic and game animals in northeast Romania and warn about the risk of human infection in the region.
Abstract
The genus Trichinella includes species with a wide geographical spread that cause pathology in humans and animals. In this context, an epidemiological study of Trichinella infection was carried out in the northeastern part of Romania to investigate for the first time the prevalence of this infection in pigs, horses, wild boars and bears, the geographical distribution of Trichinella species and the natural reservoir of Trichinella infection. Between 2010 and 2015, a total of 166,270 animals were examined by the method of artificial digestion, in order to calculate the annual and general prevalence of Trichinella infection, according to the host and the Trichinella species involved, the Pearson correlation coefficient (r), trendline and geographical distribution of species of the genus Trichinella. Taxonomic framing was performed by the multiplex PCR method. The overall prevalence of Trichinella infection in animals was 0.188%. Within the host species, the prevalence varied as follows: in pigs 0.096%, horses 0.021%, wild boar 1.46% and bears 36.76%. The geographical distribution showed that T. spiralis was dominant, occupying the entire northeastern part of Romania, being identified in pigs, horses, wild boars and bears. T britovi occupied five mountain counties, being identified only in wild boars and bears. These results validate the presence of T. spiralis and T. britovi in domestic and game animals in the northeastern part of Romania.
Keywords: Trichinella spiralis, T. britovi, prevalence, pig, horse, wild boar, bear, northeast Romania
1. Introduction
Trichinellosis is a severe parasitic zoonosis caused by species of the genus Trichinella [1], with a wide geographical spread [2], affecting a wide range of hosts (mammals, birds, reptiles) [3]. The geographical distribution of the genus Trichinella is influenced by human intervention in the habitat of domestic and wild animals [4]. Trichinella spp. belong to the phylum Nematoda, class Enoplea, order Trichocephalida, family Trichinellidae, genus Trichinella [5,6]. Currently, the genus Trichinella includes 10 species and 3 genotypes divided into two clades: encapsulated species such as Trichinella spiralis (T1), Trichinella nativa (T2), Trichinella britovi (T3), Trichinella murrelli (T5), Trichinella nelsoni (T7), Trichinella patagoniensis (T12), T. chancalensis (T13), and three genotypes (Trichinella T6, T8 and T9), and non-encapsulated species such as Trichinella pseudospiralis (T4), Trichinella papuae (T10), Trichinella zimbabwensis (T11) [5,7]. In Europe, there are four prevalent species: T. spiralis, T. britovi, T. nativa, and T. pseudospiralis [8,9]. Trichinella spiralis has the highest prevalence in domestic animals (pig, horse) and is also identified in game (wild boar, bear) [10], whereas T. britovi is the more widespread among wild carnivores but also infects domestic and wild pigs [11]. In humans, trichinellosis is transmitted by eating raw or incompletely cooked meat and meat products from domestic animals (pig, horse) and game (wild boar, bear), parasitized with larvae of Trichinella spp. [12,13,14,15]. Over time, the social and economic impact of Trichinella species has greatly influenced the epidemiological view of this zoonosis [4]. Currently, human population growth and socioeconomic changes have led to people moving to new ecological regions and changes in animal husbandry practices, which could have an impact on the occurrence of trichinellosis in humans and Trichinella infections in animals. [16]. The control of trichinellosis, regulated by the normative acts in force (EU regulation 2075/2005, Codex Alimentarius: CAC, 2015; Health Code for terrestrial animals OEI), is rigorously applied in the European Union and is estimated at an annual cost between 25 and 400 million [17]. Previous studies on Trichinella infection in animals and the impact of this zoonosis on humans in Romania have been performed by Blaga et al. [18], Iacob and Tășchină-Nicolae [19], Neghină et al. [20] and Nicorescu et al. [21] emphasizing the importance of Trichinella infection in domestic and game animals as a source of trichinellosis in humans.
The current paper is an epidemiological study on the prevalence and geographical distribution of Trichinella species in domestic animals (pigs and horses) and game (wild boars and bears) in northeastern Romania to elucidate the current Trichinella infection status in Romania and become a useful working tool in comparative processing of data by region.
2. Materials and Methods
2.1. Epidemiological Study
2.1.1. Geographical Area
The study was based on the analysis of data from 2010 to 2015 provided by the Veterinary and Food Safety Laboratories in all counties in North-East Romania. The area investigated comprises 36,850 km2 with a total population of 3,674,367 inhabitants ranging from 44.78–48.24° north latitude and between 28.05–28.24° east longitude, respectively [22]. Geographically, all the natural features are present (plain, plateau, hill, mountain), ensuring a different climate with varied fauna and flora. The counties of Suceava (SV), Neamț (NT), Bacău (BC), Vrancea (VN) and Buzău (BZ) are located in the mountainous area, being populated with wild boars, bears, and other wild carnivores (wolf, fox, lynx, wild cat, etc.). Meanwhile, the counties of Botoșani (BT), Iași (IS), Vaslui (VS), Galați (GL) are located in hilly, plateau and plain areas, being populated with wild boar. The variety of the natural features favors the circulation of wild animals from one area to another, complicating the epidemiological surveillance of Trichinella infection. The geographical distribution of the host animals highlights that bears inhabited the territory of five neighboring counties (SV, NT, BC, VN, BZ), and the wild boar inhabited all counties. Pigs raised in an industrial or extensive household system were present in all counties, and horses in three counties (SV, BT and BZ).
2.1.2. Collection and Examination of Samples-Identification of Trichinella Species
During the analyzed period, a total number of 166,270 samples of muscle tissue from domestic animals (pig: 131,759; horse: 23,748) and wild animals (wild boar: 10,695; bear: 68) were examined. They were examined after slaughter in the slaughterhouse and in the households of the population or after collection by shooting during the hunting season. An average sample of 50 g of muscle tissue (diaphragm, intercostal muscles and tongue) was taken from each carcass. The examination of samples was done in specialized laboratories by artificial digestion, according to the protocol developed by the European Commission [23]. Positive cases were sent to the Institute of Veterinary Hygiene and Public Health (IISPV) Bucharest and the European Reference Laboratory in Rome for the molecular identification of Trichinella species. The identification of Trichinella species was made by the multiplex PCR method, according to the protocol established by Pozio and La Rosa (2003). Five pairs of primers were used: Primer pair I: 5′GTTCCATGTGAACAGCAGT-3′; 5′-CGAAAACATACGACAACTGC-3′; Primer pair II: 5′-GCTACATCCTTTTGATCTGTT-3′; 5′-AGACACAATATCAACCACAGTACA-3′; Primer pair III: 5′-GCGGAAGGATCATTATCGTGT-3′; 5′-ATGGATTA CAAAGAAAACCATCACT-3′; Primer pair IV: 5′-GTGAGCGTAATAAAGGTGCAG-3′; 5′-TTCATCACACATCTTCCACTA-3′; Primer pair V: 5′-CAATTGAAAACCGCTTAGCGTGTTT-3′; 5′TGATCTGAGGTCGACATTTCC-3′ were designated to amplify the internal transcribed spacers ITS1 and ITS2, and the expansion segment V (ESV) region of the ribosomal DNA. 10 µL of total DNA were subjected to multiplex PCR in a 30 µL mixture reaction. The mix for the detection of the target sequence contained 1× PCR buffer, 3 mM MgCl2, 0.2 mM of each deoxynucleotide triphosphate, 0.3 µM of each primer and 1 U of Taq polymerase. Amplification was carried out as follows: initial denaturation at 95 °C for 4 min; 40 cycles of 95 °C for 10 s, 55 °C for 30 s and 72 °C for 30 s; and a final elongation step at 72 °C for 3 min. DNA fragments were analyzed by electrophoresis in a 2% agarose gel in 1× TAE buffer (40 mmol/l Tris–HCl, 2 mmol/l acetate, 1 mmol/lEDTA) and stained with ethidium bromide. The bands in the gel were visualized and photographed under UV light [24].
2.2. Statistical Analysis
The statistical analysis was performed using MS EXCEL 2016 software. Confidence intervals (CI) were calculated, and α = 0.05 was considered statistically significant [25]. The annual and general prevalence of Trichinella spp. infection in pigs, horses, wild boar and bears was evaluated in each county and cumulatively throughout the northeast, as well as the geographical distribution of Trichinella and host species. The Pearson correlation coefficient (R2) and the Trichinella infection trendline were calculated. The results obtained were framed in tables and represented graphically (trendline and correlation coefficient R2) but also distributed in the maps.
3. Results
3.1. The Prevalence and Dynamics of Trichinella Infection in North-Eastern Romania
The data study reveals that the overall prevalence of Trichinella infection in the examined animals was 0.188% (313/166,270 samples).
The prevalence varied depending on the host species as follows: in pigs 0.096% (127/131,759); in horses 0.021% (5/23,748) in wild boar 1.46% (156/10,695) and in bears 36.76% (25/68), (Table 1).
Table 1.
The annual and general prevalence of Trichinella infection in animals from North-Eastern Romania.
| Year | Total Samples Examined | Pig | Horse | Wild Boar | Bear | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | |
| 2010 | 17/30,645 | 0.0476–0.0524 | 7/26,939 | 0.0183–0.0217 | 1/2807 | 0.0282–0.0418 | 8/896 | 0.2750–1.5050 | 1/3 | 0.0000–86.6731 |
| 2011 | 32/16,615 | 0.1840–0.1960 | 18/8200 | 0.2110–0.2290 | 1/7144 | 0.0113–0.0167 | 13/1270 | 0.4674–1.5726 | 0/1 | 0.0000–0.0000 |
| 2012 | 54/22,240 | 0.2344–0.2456 | 24/14,248 | 0.1638–0.1762 | 3/6733 | 0.0391–0.0489 | 24/1251 | 1.1515–2.6685 | 3/8 | 3.9520–71.0480 |
| 2013 | 76/34,036 | 0.2156–0.2244 | 25/26,093 | 0.0964–0.1036 | 0/5702 | 0.0000–0.0000 | 44/2218 | 1.4002–2.5598 | 7/23 | 11.6258–49.2342 |
| 2014 | 59/36,542 | 0.1562–0.1638 | 18/33,770 | 0.0477–0.0523 | 0/610 | 0.0000–0.0000 | 34/2147 | 1.0525–2.1075 | 7/15 | 21.4227–71.9173 |
| 2015 | 75/26,192 | 0.2746–0.2854 | 35/22,509 | 0.1552–0.1648 | 0/752 | 0.0000–0.0000 | 33/2913 | 0.7462–1.5138 | 7/18 | 16.3686–61.4114 |
| Total | 313/16,6270 | 0.1861–0.1899 | 127/131,759 | 0.0944–0.0976 | 5/23,748 | 0.0192–0.0228 | 156/10,695 | 1.2327–1.6873 | 25/68 | 25.3000–48.2200 |
| General prevalence (%): 0.188 | 0.096 | 0.021 | 1.46 | 36.76 | ||||||
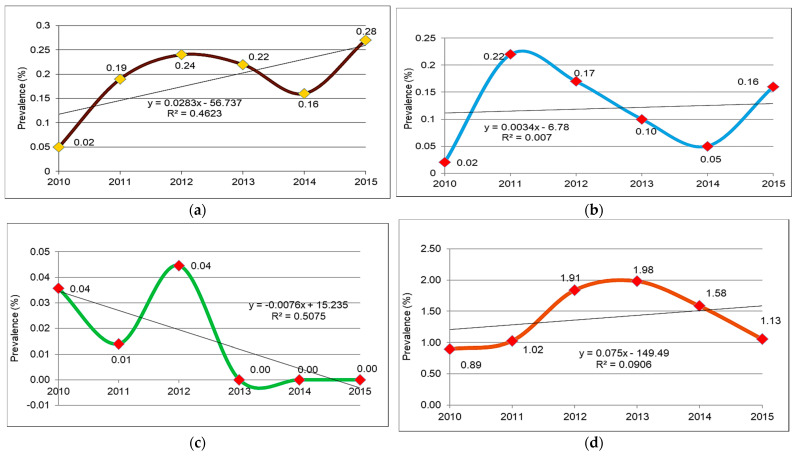
The dynamics of the general prevalence of Trichinella infection, the Pearson correlation coefficient (R2) and the trendline are presented in Figure 1a.
Figure 1.
(a) Dynamics of the general prevalence (%) of Trichinella infection in animals in Northeastern Romania (2010–2015); (b) Specific dynamics of Trichinella infection in pigs (2010–2015); (c) Specific dynamics of Trichinella infection in horses (2010–2015); (d) Specific dynamics of Trichinella infection in wild boar (2010–2015); (e) Specific dynamics of Trichinella infection in bears (2010–2015).
The general prevalence of Trichinella infection in animals shows close values over the entire period studied (2010–2015), with oscillating dynamics. It started at 0.02% in 2010 and reached a maximum of 0.28% in 2015. In this case, there is an ascending trend, also defined by the line of predictability, and the Pearson correlation coefficient reveals an average correlation (R2 = 0.4623). Prevalence values, although low, suggest a persistent Trichinella infection in the population of domestic animals (pigs and horses) and wild animals (wild boars and bears) spread throughout the northeast.
The prevalence dynamics, the Pearson correlation coefficient (R2) and the trendline are indicated in Figure 1b.
In pigs, the prevalence of Trichinella infection indicates minimal values (0.02%) in 2010, an increase (0.22%) in the following year (2011), to subsequently register a descending trend until 2014 (0.05%), with a new trend increase (0.16%) in 2015. In fact, there is a tendency to equalize the predictability line and a very, very weak correlation (R2 = 0.007) of the infection. The prevalence of Trichinella infection, even with low values, confirms the persistence of parasites in the pig population throughout the analyzed period in northeastern Romania.
The prevalence dynamics of Trichinella infection in horses, the Pearson correlation coefficient (R2) and the trendline are shown in Figure 1c.
In horses, the specific dynamics of Trichinella infection are particular and are due to a small number of cases compared to a large number of animals examined. Thus, in 2010, the prevalence was 0.035%, decreased in the following year (2011) to 0.014% and increased (0.044%) in 2012. Subsequently, it fell steadily over the next three years to 0.00%. The clearly descending aspect of the predictability line and an average correlation (R2 = 0.5075) of the infection was noticed.
The prevalence dynamics of Trichinella infection in wild boar, the Pearson correlation coefficient (R2) and the trendline are demonstrated in Figure 1d.
The dynamics of the prevalence of Trichinella infection in wild boars describe a simple line, starting with 0.89% in 2010, reaching a peak of 1.98% in 2013 and decreasing to 1.13% in 2015. In wild boars, the annual value of prevalence was higher (0.89–1.98%) than in pigs and horses, recorded in the same analyzed period. The predictability line of Trichinella infection is slightly ascending, and the Pearson correlation coefficient (R2 = 0.0906) indicates a very weak correlation of the infection.
The prevalence dynamics of Trichinella infection in bears, the Pearson correlation coefficient (R2), and the trendline are indicated in Figure 1e.
In bears, the dynamics of prevalence are sinuous and are positioned on both sides of the predictability line. The annual prevalence has higher values compared to wild boars and oscillates from 0.00% in 2011 to 46.67% in 2014. The predictability line has an ascending aspect, revealing a weak correlation (R2 = 0.2803) of the infection.
3.2. Geographical Distribution of Trichinella Infection in Animals in North-Eastern Romania
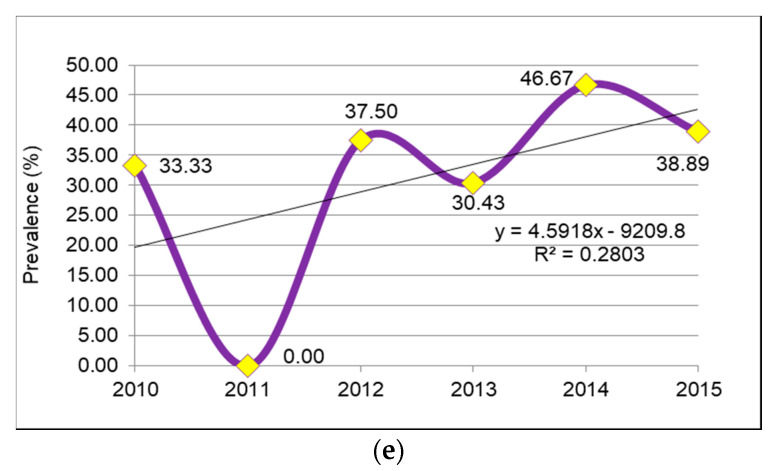
The geographical spread of Trichinella infection in northeastern Romania is included in Table 2 and illustrated in Figure 2.
Table 2.
Geographical distribution of Trichinella infection in animals in North-Eastern Romania.
| County | Pig | Horse | Wild Boar | Bear | ||||
|---|---|---|---|---|---|---|---|---|
| Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | |
| Suceava (SV) | 0/1109 | 0.0000–0.0000 | 5/18,407 | 0.0248–0.0295 | 34/2397 | 0.9450–1.8918 | 6/19 | 10.6777–52.4802 |
| Botoșani (BT) | 1/12,308 | 0.0065–0.0097 | 0/5244 | 0.00000–0.00000 | 1/145 | 0.0000–0.7650 | 0 | 0.0000–0.0000 |
| Neamț (NT) | 1/107,675 | 0.0007–0.0011 | 0/0 | 0.00000–0.00000 | 0/211 | 0.0000–0.0000 | 0/3 | 0.0000–0.0000 |
| Iași (IS) | 0/183 | 0.0000–0.0000 | 0/0 | 0.00000–0.00000 | 15/500 | 1.5047–4.4953 | 0 | 0.0000–0.0000 |
| Bacău (BC) | 3/125 | 0.0000–5.0831 | 0/0 | 0.00000–0.00000 | 67/3279 | 1.5591–2.5276 | 6/20 | 9.9160–50.0840 |
| Vaslui (VS) | 12/65 | 9.0293–27.8938 | 0/0 | 0.00000–0.00000 | 4/338 | 0.0306–2.3363 | 0 | 0.0000–0.0000 |
| Vrancea (VN) | 3/7825 | 0.0341–0.0426 | 0/0 | 0.00000–0.00000 | 19/950 | 1.1097–2.8903 | 5/13 | 12.0149–64.9082 |
| Galați (GL) | 96/1063 | 7.3080–10.7541 | 0/0 | 0.00000–0.00000 | 6/542 | 0.2261–1.9879 | 0/1 | 0.0000–0.0000 |
| Buzău (BZ) | 11/1406 | 0.7608–0.8039 | 0/97 | 0.00000–0.00000 | 10/2333 | 0.4086–0.4487 | 8/12 | 39.9944–93.3389 |
| Total | 127/131,759 | 0.0948–0.0980 | 5/23,748 | 0.01923–0.02288 | 156/10,695 | 1.2314–1.6858 | 25/68 | 25.3044–48.2250 |
C.I. 95% (Confidence Interval; α = 0.05 was considered as statistically significant).
Figure 2.
Geographical distribution and prevalence of positive samples for Trichinella infection in animals in North-Eastern Romania (2010–2015).
The estimation of the prevalence of infection in geographically distributed animals was made using a confidence interval because it covers the real value of prevalence with a given probability.
The geographical distribution of Trichinella infection in pigs shows that the infection was caused only by T. spiralis with a prevalence of 0.096% (127/131,759), but with huge variability. The minimum prevalence of 0.000098% (1/107,675) was found in Neamț county, and the maximum prevalence of 18.46% (12/65) was registered in Vaslui county. The geographical distribution of Trichinella infection in horses indicates that the infection was caused only by T. spiralis with a general prevalence of 0.021% (5/23,748), ranging from zero in Botoșani (0/5244) and Buzău (0/97) counties to 0.03% (5/18,407) in the Suceava county. The geographical distribution of Trichinella infection in wild boar reveals that the infection was caused by T. spiralis and T. britovi, with a general prevalence of 1.46% (156/10,695), ranging from zero in Neamț (0/211), to 3.00% (15/500) in Iași county, without co-infection. The geographical distribution of Trichinella infection in bears illustrates the infection was caused by T. spiralis and T. britovi, with a prevalence of 36.76% (25/68), ranging from zero (0/3) in Neamț and Galați (0/1) to 66.66% (8/12) in Buzău county, without co-infection.
3.3. Geographical Distribution and Prevalence of T. spiralis and T. britovi, in Animals, in North-Eastern Romania
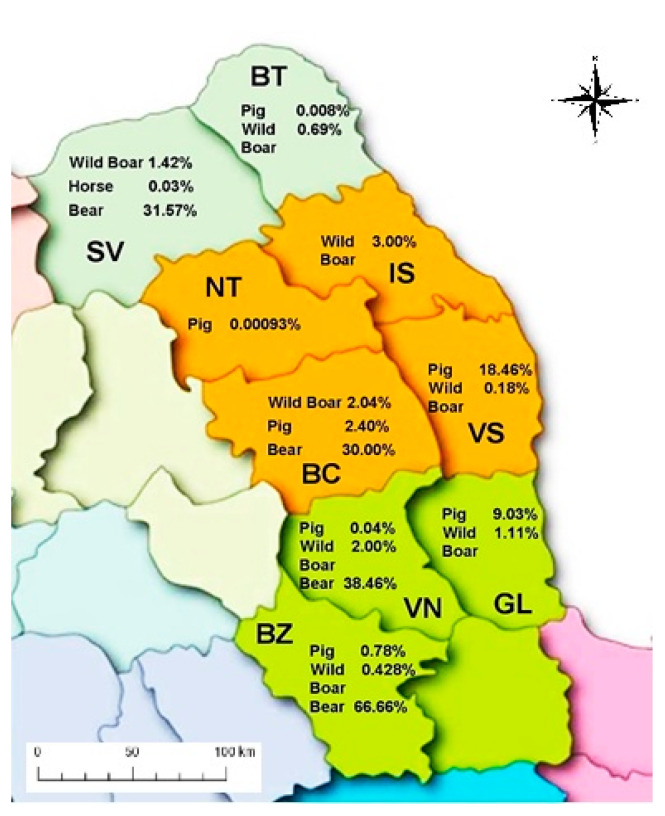
The geographical distribution of T. spiralis and T. britovi species is different and unequal and is influenced by a multitude of factors. T. spiralis has a wide geographical spread, being identified in all (nine) counties, in pigs, horses, wild boar, and bears, with varying prevalence (Table 3). The geographical distribution of the T. spiralis species in North-Eastern Romania is illustrated in Figure 3A.
Table 3.
Geographical prevalence of T. spiralis in pigs, horses, wild boars and bears, in North-Eastern Romania.
| County | Pig | Horse | Wild Boar | Bear | ||||
|---|---|---|---|---|---|---|---|---|
| Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | |
| Suceava (SV) | 0/1109 | 0.0000–0.0000 | 5/18,407 | 0.00003–0.00051 | 20/34 | 42.2804–75.3666 | 4/6 | 28.9379–100.000 |
| Botoșani (BT) | 1/12,308 | 0.0000–0.0002 | 0/5244 | 0.00000–0.00000 | 1/145 | 0.6144–0.7650 | 0 | 0.00000–0.00000 |
| Neamț (NT) | 1/107,675 | 0.00000–0.00003 | 0/0 | 0.00000–0.00000 | 0/211 | 0.0000–0.0000 | 0 | 0.00000–0.00000 |
| Iași (IS) | 0/183 | 0.0000–0.0000 | 0/0 | 0.00000–0.00000 | 15/500 | 1.5047–4.4956 | 0 | 0.00000–0.00000 |
| Bacău (BC) | 3/125 | 0.0000–0.0508 | 0/0 | 0.00000–0.00000 | 56/67 | 74.7119–92.4523 | 5/6 | 53.5072–100.0000 |
| Vaslui (VS) | 12/65 | 0.0903–0.2789 | 0/0 | 0.00000–0.00000 | 0/338 | 0.0000–0.0000 | 0 | 0.00000–0.00000 |
| Vrancea (VN) | 3/7825 | 0.0000–0.0008 | 0/0 | 0.00000–0.00000 | 11/19 | 35.6940–80.0955 | 2/5 | 0.0000–82.9414 |
| Galați (GL) | 96/1063 | 0.0731–0.1075 | 0/0 | 0.00000–0.00000 | 6/542 | 0.2261–1.9879 | 0 | 0.00000–0.00000 |
| Buzău (BZ) | 11/1406 | 0.0032–0.0124 | 0/97 | 0.00000–0.00000 | 10/2333 | 0.1635–06937 | 5/8 | 28.9520–96.0480 |
| Total | 127/131,759 | 0.0008–0.0011 | 5/23,748 | 0.00003–0.00040 | 119/4189 | 2.3377–3.3439 | 16/25 | 45.1840–82.8160 |
Figure 3.
Geographical distribution of positive samples for Trichinella spiralis (A) and Trichinella britovi (B).
The data in Table 3 emphasize that T. spiralis is geographically widespread in all counties of northeast Romania, with a different prevalence in both synanthropic and sylvanic environments. So, in the synanthropic environment (pigs: 127/131,759 and horses: 5/23,748), the prevalence of T. spiralis infection was 0.085% (132/155,507). In the sylvatic environment (wild boar (119/4189) and bears (16/25), T. spiralis had a prevalence of 3.20% (135/4218). Within the total number of Trichinella spp infections, T. spiralis had an overall prevalence of 74.58% (135 T. spiralis/181 (156 + 25, Table 1, total Trichinella infections), where the prevalence of T. spiralis in the wild boar was 76.28% (119 T. spiralis/156–total Trichinella infections), and in bears was 64% (16 T. spiralis/25 total Trichinella infections).
T. britovi is geographically restricted to the mountainous area, counties SV, BC, VN and BZ, where it has been identified in bears, and counties SV, BC, VN and VS, where it has been identified in wild boars (Table 4). The distribution of T. britovi in both bears and wild boars in the same mountain counties (SV, BC, VN) is noticeable, which confirms the sylvatic maintenance of the Trichinella infection and a source of infection for other hosts.
Table 4.
Geographical prevalence of T. britovi in wild boar and bears in North-Eastern Romania.
| County. | Wild Boar | Bears | ||
|---|---|---|---|---|
| Positive/ Tested |
C.I. [a–b] | Positive/ Tested |
C.I. [a–b] | |
| Suceava (SV) | 14/34 | 24.6334–57.7196 | 2/6 | 0.0000–71.0536 |
| Bacău (BC) | 11/67 | 7.5477–25.2881 | 1/6 | 0.0000–46.4871 |
| Vrancea (VN) | 8/19 | 19.9045–64.3060 | 3/5 | 17.0586–100.000 |
| Buzău (BZ) | 0/2333 | 0.0000–0.0000 | 3/8 | 3.9520–71.0480 |
| Vaslui (VS) | 4/338 | 0.0306–2.3363 | 0 | 0.0000–0.0000 |
| Total | 37/2791 | 0.9014–1.7500 | 9/25 | 17.1840–54.8160 |
The geographical distribution of T. britovi species in bear and wild boar is illustrated in Figure 3B.
Within the genus Trichinella, T britovi was identified only in the sylvatic environment, in game (bear and wild boar) with a prevalence of 25.41% (46 T britovi/181 total game positive (156 wild boar + 25 bears + Table 1). In wild boars, the prevalence of T. britovi was 23.72% (37/156), and in bears, it was 36% (9 T. britovi/25 total Trichinella positive). No co-infections of the two species were reported in either bears or the wild boar. The overall prevalence of T. britovi was 0.43% (46 T. britovi/10,763 total samples examined, comprising 10,695 wild boars and 68 bears).
4. Discussion
The northeastern part of Romania, historically known as Moldova, includes nine counties arranged from northeast to south, as follows: SV-BT, NT-IS, BC-VS, VN-GL and BZ, defining the distribution area of the host animals and the origin of the samples examined.
The prevalence of T. spiralis and T. britovi species and the geographical distribution in the North-Eastern part of Romania were influenced by numerous factors, including natural features, forested areas, wild animals, agricultural areas, rural population preference for extensive pig breeding, education and public awareness regarding the veterinary sanitary control of meat obtained from the household or from game meat.
Numerous studies on the prevalence of Trichinella infection in animals have been undertaken in Romania’s neighboring countries. In this regard, research conducted by Lalkovski (2017) in Bulgaria during the same period (2010–2016) reveals that Trichinella infection was caused by T. britovi (94.17%) and T. spiralis (5.83%). Both species were identified in pigs and wild boars in a ratio of 45:1 in wild boars and 1:1 in pigs. Trichinella britovi was the most widespread geographically, being identified throughout the country, while T. spiralis was identified only in a few areas [26]. In Hungary, research conducted by Szell et al. (2012) [15] show that Trichinella infection was identified in wild boars, with a very low prevalence of 0.0077%. The species identified were T. britovi (64.7%), T. spiralis (29.4%) and T. pseudospiralis (5.9%), and their geographical distribution shows that the level of risk differs from one area to another.
In a recent study by Klun et al. [27] it was shown that in Serbia, T. spiralis had a prevalence of 77.8% in wild carnivores, respectively in red fox and wild cat, and T. britovi, in the same hosts, had a prevalence of 22.2%. The predominance of T. spiralis in wild animals in Serbia indicates the transition of this species from domestic to wild animals [27].
The geographical distribution of Trichinella species in Europe differs from country to country. Thus, T. spiralis-the most pathogenic species to humans has an uneven distribution with important foci in Eastern countries [28]. In most countries, T. britovi is more widespread (62.5–100%) than T. spiralis (0.0–37.5%), although in Finland, Germany, Poland and Spain, T. spiralis is more widespread (56.3–84.2%) [29]. In Poland, Trichinella infection in animals is caused by T. spiralis and T. britovi species, but recently Bilska-Zając et al. [12] identified T. nativa in wild boar, confirming the spread of this species in new regions of Europe. In Greece, Trichinella infection is caused by T. britovi, with a prevalence of 0.29% in pigs and 6.4% in wild boars [30]. In Italy, Trichinella infections are caused by T. spiralis and T. britovi-species identified in domestic and wild animals [31]. A case of simultaneous parasitism with both species has been reported in horses [32]. Trichinella pseudospiralis is also present in Italy, and this species was reported in two owls (Strix aluco and Athene noctua), one red kite (Milvus milvus), five wild boars (Sus scrofa), one wolf (Canis lupus italicus), and one red fox (Vulpes vulpes) [33]
Prevalence studies conducted by Serrano et al. [34] and Boadella et al. [35] show that in Spain, Trichinella infection is caused by T. britovi and T. spiralis species. In the Extremadura region, T. britovi has been found in wild boar in more than a quarter of cases of Trichinella infection, with a higher level of infection than T. spiralis [34]. In the central part of the country, the average prevalence of Trichinella infection in wild boars was 0.2% [35]. Research by Deksne et al. (2016) [36] show that in Latvia, Trichinella infection is caused by T. britovi, T. nativa and T. spiralis species, with an overall prevalence of 2.5% in wild boars [11]. T britovi had a maximum prevalence of 94.0%; native Trichinella was detected in single (1.1%) or mixed (4.4%) infection with T. britovi; T. spiralis has been detected in mixed infection with T. britovi [36].
Regarding the environmental conditions, there is no difference between the two species of Trichinella, although T. britovi prefers habitats at higher altitudes than T. spiralis [29]. Some studies show that T. spiralis (T1) has the highest prevalence (43.3%) of all species and genotypes of the genus Trichinella, followed by T. britovi (T3) (41.2%) [37]. Other studies show that the species T. britovi (T3) has a higher prevalence (44.8%) compared to T. spiralis (T1), 39.9% [4]. The two species dispute their primacy according to numerous factors, including identification methods. The combined use of serological ELISA and Western blot methods is 31.4 times more sensitive than digestion (32/1462 vs. 1/1462), suggesting their potential use for epidemiological surveillance of Trichinella infection in wild boar populations and other host animals [13,38].
Wild animals are the most important reservoir for the genus Trichinella and an important source of infection for domestic animals and humans [39,40]. The high prevalence of Trichinella spp. in wildlife suggests that they are indicators for assessing the risk of infection with Trichinella spp. [36].
In Romania, Blaga et al. [18] reported similar values of the prevalence of T. spiralis species (49.2%), compared to T. britovi (50.8%), due to the numerous household outbreaks associated with pig herds.
Nicorescu et al. [21] conducted an epidemiological study on the prevalence of Trichinella spp. in pigs, wild boars and bears throughout Romania, reporting that in bears, the prevalence was highest (12.93%), followed by wild boar (1.66%) and pigs (0.20%). Multiplex PCR analysis of Trichinella-positive isolates revealed that T. spiralis had a prevalence of 74.49% compared to 22.45% in T. britovi; the mixed infection with the two Trichinella species was 3.0%. The authors reported that Trichinella infections were widespread in all areas but with a different prevalence. Thus, in the south and southeast of Romania, T. spiralis was identified at 98.25% and 87.88%, respectively, compared to T. britovi, identified at 1.75% and 12.12%, respectively. The same authors show that in the North-East, the prevalence of Trichinella infection was 2.8% in game and 0.01% in pigs. In the northwest, the Trichinella infection was 2.48% in game and 1.52% in pigs. The authors note that, geographically, T. spiralis covers the entire territory of Romania, being identified in pigs and game, while T. britovi was present in game in all areas, and in pigs, only in the central, southwestern, and northwest areas [21]. Our study confirms the data communicated by Nicorescu et al. by identifying T. spiralis in pigs, wild boar, bears, and in addition, in horses, while T. britovi was identified only in wild boar and bear, with a very different prevalence from one species to another.
From studies on Trichinella infection in animals, it is observed that in European countries, the prevalence values are very different, being either in favor of T. spiralis or in favor of T. britovi, without being a common regulatory-equalizing factor. Each country or area has its own specifics, including climate factors, relief, vegetation, the presence of forests, agricultural areas, domestic animals, wildlife and the human population with traditions, customs, level of culture and civilization.
Thus, the prevalence of Trichinella infection in animals is close in value in some areas and very different in others. There is no uniformity in the presence, dynamics and distribution of Trichinella infection in animals. Our epidemiological study covers a Romanian geographical area that, until now, has been studied only partially and never in its entirety as part of the northeast.
From this point of view, our study demonstrates that, in the northeastern part of Romania, T. spiralis is present in all counties, being identified in all examined animal species. The highest prevalence was in game (wild boar: 83.58%; bears: 83.33%), followed by domestic animals (pigs: 18.46% and horses: 0.027%).
T. britovi was geographically present in five mountain counties, identified only in game, with the maximum prevalence in bears (60.00%), followed by wild boar (42.10%). The mountainous relief and the forested areas with different altitudes offer favorable conditions for the bear and wild boar population development. This aspect is present in three counties SV, BC and VN (Table 4), where bears and wild boars coexist, contributing to developing and maintaining a natural reservoir of Trichinella spp. The number of bears and wild boars is regulated in the hunting season, but in abundant feeding conditions, wild boar populations grow much faster, exceeding the ability of hunters to regulate the number of individuals in a forest area. In hilly areas, with deciduous forests and sufficient food, abundant populations of invading wild boars and agricultural regions are developing, causing economic damage. Our results are consistent with data from the literature [18,20,21,41].
In humans, a retrospective analysis of trichinellosis shows that worldwide, between 1986 and 2009, 65,818 cases and 42 deaths were reported in 41 countries, a context in which Europe accounted for 86% of cases (56,912). Of these cases, 28,564 (50%) were reported in Romania between 1990 and 1999 [40,42]. However, in the last 16 years (from 2002 to 2017), in the European Union, there has been a decrease in the incidence of trichinellosis, with 5518 cases reported. However, Bulgaria and Romania reported, in 2017, more than half of the confirmed cases and outbreaks [40].
According to data reported by the National Institute of Public Health (INSP) and the National Center for Surveillance and Control of Communicable Diseases (CNSCBT) in Romania, between 2010 and 2017, human trichinellosis showed a variable incidence per 100,000 inhabitants. Thus, the incidence was 0.9%000 in 2010; 0.7%000 in 2011; 1.3%000 in 2012; 0.94%000 in 2013; 1.61%000 in 2014; 0.48%000 in 2015; 0.44%000, in 2016 and 0.69%000, in 2017, suggesting a downward trend of infection among the population. The annual fluctuation in the number of cases is probably the consequence of the consumption of pork and wild boar products during the winter holidays, during the hunting season, the culinary habits, the tradition of preparing meat dishes, the consumption of raw dishes and the lack of veterinary examination [43].
It is known that humans become infected with all species of the genus Trichinella, but T. spiralis is the most pathogenic to humans. In most cases, infection is manifested by allergic reactions (facial edema), muscle pain, gastrointestinal disorders, heart disorders, and non-specific clinical signs which develop variously, sometimes fatally, depending on different factors such as the source of infection and the number of larvae ingested [29].
Trichinellosis is continuously reported in humans in Romania. Therefore, the assessment and monitoring of risk factors should be improved in both domestic and game animals and other wild species to monitor the presence and prevalence of these parasites [41].
Sustained epidemiological surveillance in the prevention of trichinellosis in humans leads to a decrease in the incidence and impact of this disease on the population’s health [30]. Important elements of this activity include the allocation of economic funds, the improvement of animal husbandry practices, meat inspection, consumer education, medical care and the constant updating of prophylaxis measures [43].
5. Conclusions
T. spiralis was found prevalent in North-Eastern Romania, being present in all nine counties and in all hosts examined, which included pigs, horses, wild boars, and bears, with a general prevalence of 0.18% (283/159,750). T. britovi was dominant in wild boar and bear, being present in five counties, with a general prevalence of 0.43% (46/10,763). No mixed infections with the two species were reported in the same host animal. The prevalence of Trichinella infection in the North-Eastern part of Romania, particularly in the game animals, confirms the presence of a well-preserved sylvatic reservoir of the parasite. This, constitutes a greater risk of infection to humans and of dispersion to synanthropic animals, suggesting increased attention should be paid to consumers that occasionally purchase meat or meat products without veterinary examination.
Acknowledgments
The authors express their gratitude to Bruja Sabina (Suceava), Harabagiu Theodor (Botoșani), Zugun Maria (Neamț), Conoro Constantin (Bacău), Ișan Elena (Iași), Niță Costel (Vaslui), Ciornohac Mona (Vrancea), Chircă Adrian (Buzău), and Călinescu Steluța (Galați) for providing the raw data for this study.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by O.I., C.C. and M.M. The first draft of the manuscript was written by O.I. and all authors commented on previous earlier versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of the Faculty of Veterinary Medicine Iasi (protocol code 1071/06,09,2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petrović J., Grgić Ž., Radulović J.P., Ratajac R., Urošević M., Pustahija T., Medić S. Epidemiology of human trichinellosis in Vojvodina province, Serbia from 2005 to 2016. Acta Vet. Hung. 2019;67:40–50. doi: 10.1556/004.2019.005. [DOI] [PubMed] [Google Scholar]
- 2.Korhonen P., Pozio E., La Rosa G., Chang B.C.H., Koehler A., Hoberg E.P., Boag P., Tan P., Jex A., Hofmann A., et al. Phylogenomic and biogeographic reconstruction of the Trichinella complex. Nat. Commun. 2016;7:10513. doi: 10.1038/ncomms10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pozio E. Adaptation of Trichinella spp. for survival in cold climates. Food Waterborne Parasitol. 2016;4:4–12. doi: 10.1016/j.fawpar.2016.07.001. [DOI] [Google Scholar]
- 4.Pozio E. The opportunistic nature of Trichinella-Exploitation of new geographies and habitats. Vet. Parasitol. 2013;194:128–132. doi: 10.1016/j.vetpar.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 5.Pozio E., Zarlenga D.S. Trichinella and Trichinellosis. Elsevier Inc.; Amsterdam, The Netherlands: Academic Press; Cambridge, MA, USA: 2021. pp. 35–76. [Google Scholar]
- 6.Blaxter M. Nematodes: The worm and its relatives. PLoS Biol. 2011;9:e1001050. doi: 10.1371/annotation/083d39ea-2269-4915-9297-bc6d9a9f7c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stroehlein A.J., Young N., Korhonen P., Chang B.C.H., Sternberg P.W., La Rosa G., Pozio E., Gasser R.B. Analysis of compact Trichinella kinomes reveal a MOS-like protein kinase with a unique N-terminal Domain. G3 Genes Genomes Genet. 2016;6:2847–2856. doi: 10.1534/g3.116.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pozio E., Zarlenga D.S. New pieces of the Trichinella puzzle. Int. J. Parasitol. 2013;43:983–997. doi: 10.1016/j.ijpara.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Bilska-Zając E., Różycki M., Chmurzyńska E., Marucci G., Cencek T., Karamon J., Bocian Ł. Trichinella species circulating in wild boar (Sus scrofa) populations in Poland. Int. J. Parasitol. Parasites Wildl. 2013;2:211–213. doi: 10.1016/j.ijppaw.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guenther S., Nöckler K., von Nickisch-Rosenegk M., Landgraf M., Ewers C., Wieler L.H., Schierack P. Detection of Trichinella spiralis, T. britovi and T. pseudospiralis in muscle tissue with real-time PCR. J. Microbiol. Methods. 2008;75:287–292. doi: 10.1016/j.mimet.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Kirjušina M., Deksne G., Marucci G., Bakasejevs E., Jahundoviča I., Daukšte A., Zdankovska A., Bērziņa Z., Esīte Z., Bella A., et al. A 38-year study on Trichinella spp. in wild boar (Sus scrofa) of Latvia shows a stable incidence with an increased parasite biomass in the last decade. Parasit Vectors. 2015;8:137. doi: 10.1186/s13071-015-0753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ilska-Zając E., Różycki M., Chmurzyńska E., Antolak E., Próchniak M., Grądziel-Krukowska K., Karamon J., Sroka J., Zdybel J., Cencek T. First case of Trichinella nativa infection in wild boar in Central Europe-molecular characterization of the parasite. Parasitol. Res. 2017;116:1705–1711. doi: 10.1007/s00436-017-5446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y., Cai Y.N., Tong M.W., Sun N., Xuan Y.H., Kang Y.J., Vallée I., Boireau P., Cheng S.P., Liu M.Y. Serological tools for detection of Trichinella infection in animals and humans. One Health. 2016;2:25–30. doi: 10.1016/j.onehlt.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rostami A., Gamble J.H., Dupouy-Camet J., Khazan H., Bruschi F. Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol. 2017;64:65–71. doi: 10.1016/j.fm.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Szell Z., Marucci G., Ludovisi A., Gomez-Morales M.A., Sréter T., Pozio E. Spatial distribution of Trichinella britovi, T. spiralis and T. pseudospiralis of domestic pigs and wild boars (Sus scrofa) in Hungary. Vet. Parasitol. 2012;183:393–396. doi: 10.1016/j.vetpar.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Torgerson P.R., Macpherson C.N.L. The socioeconomic burden of parasitic zoonoses: Global trends. Vet. Parasitol. 2011;182:79–95. doi: 10.1016/j.vetpar.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Franssen F., Swart A., Giessen J., Havelaar A., Takumi K. Parasite to patient: A quantitative risk model for Trichinella spp. in pork and wild boar meat. Int. J. Food Microbiol. 2017;241:262–275. doi: 10.1016/j.ijfoodmicro.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Blaga R., Gherman C., Cozma V., Zocevic A., Pozio E., Boireau P. Trichinella species circulating among wild and domestic animals in Romania. Vet. Parasitol. 2009;159:218–221. doi: 10.1016/j.vetpar.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Iacob O.C., Tășchină-Nicolae P.M. Epidemiological aspects regarding the trichinellosis in wild boar (Sus scrofa, Linnaeus, 1758) and the risck of transmission to human. Lucr. Stiintifice-Univ. Stiinte Agric. Banat. Timis. Med. Vet. 2009;42:38–46. [Google Scholar]
- 20.Neghină R., Neghină A.M., Marincu I. Trichinellosis in hospitalized patients from a Romanian endemic area, 2007–2009. Clin. Microbiol. Infect. 2012;18:86–90. doi: 10.1111/j.1469-0691.2011.03573.x. [DOI] [PubMed] [Google Scholar]
- 21.Nicorescu D.M.L., Ioniță M., Ciupescu L., Buzatu V.C., Tănăsuică R., Mitrea I.L. New insights into the molecular epidemiology of Trichinella infection in domestic pigs, wild boars, and bears in Romania. Vet. Parasitol. 2015;212:257–261. doi: 10.1016/j.vetpar.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Latitude and Longitude Finder on Map Get Coordinate. [(accessed on 20 April 2021)]. Available online: http://www.latlong.net.
- 23.Kapel O.M.C. Changes in the EU legislation on Trichinella inspection-New challenges in the epidemiology. Vet. Parasitol. 2005;132:189–194. doi: 10.1016/j.vetpar.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 24.Pozio E., La Rosa G. PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol. Biol. 2003;216:299–309. doi: 10.1385/1-59259-344-5:299. [DOI] [PubMed] [Google Scholar]
- 25.Reiczigel J., Rózsa L. Quantitative Parasitology. Qpweb; Budapest, Hungary: 2005. v. 3.0. [Google Scholar]
- 26.Lalkovski N. Species composition of Trichinella in domestic and wild animals in Bulgaria. Bulg. J. Vet. Med. 2019;22:99–104. doi: 10.15547/bjvm.2038. [DOI] [Google Scholar]
- 27.Klun I., Cosic N., Cirovic D., Vasilev D., Teodorovic V., Djurkovic-Djakovic O. Trichinella spp. in wild mesocarnivores in an endemic setting. Acta Vet. Hung. 2019;67:34–39. doi: 10.1556/004.2019.004. [DOI] [PubMed] [Google Scholar]
- 28.Garbarino C., Interisano M.M., Chiatante A., Marucci G., Merli E., Arrigoni N., Cammi G., Ricchi M., Tonanzi D., Tamba M., et al. Trichinella spiralis a new alien parasite in Italy and the increased risk of infection for domestic and wild swine. Vet. Parasitol. 2017;246:1–4. doi: 10.1016/j.vetpar.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Pozio E., Rinaldi L., Marucci G., Musella V., Galati F., Cringoli G., Boireaue B., La Rosaa G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int. J. Parasitol. 2009;39:71–79. doi: 10.1016/j.ijpara.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Dimzas D., Chassalevris T., Ozolina Z., Dovas C.I., Diakou A. Investigation of the Food-Transmitted Parasites Trichinella spp. and Alaria spp. in Wild Boars in Greece by Classical and Molecular Methods and Development of a Novel Real-Time PCR for Alaria spp. Detection. Animals. 2021;11:2803. doi: 10.3390/ani11102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turiac I.A., Cappelli M.G., Olivieri R., Angelillis R., Martinelli D., Prato R., Fortunato F. Trichinellosis outbreak due to wild boar meat consumption in southern Italy. Parasit Vectors. 2017;10:107. doi: 10.1186/s13071-017-2052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liciardi M., Marucci G., Addis G., Ludovisi A., Gomez Morales M.A., Deiana B., Cabajd W., Poziob E. Trichinella britovi and Trichinella spiralis mixed infection in a horse from Poland. Vet. Parasitol. 2009;161:345–348. doi: 10.1016/j.vetpar.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Ricchiuti L., Petrini A., Interisano M., Ruberto A., Salucci S., Marino L., Del Riccio A., Cocco A., Badagliacca P., Pozio E. First report of Trichinella pseudospiralis in a wolf (Canis lupus italicus) Int. J. Parasitol. Parasites. Wildl. 2021;15:195–198. doi: 10.1016/j.ijppaw.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serrano F.J., Perez-Martin E., Reina D., Nieto G.C., Navarrete I., Murrell D.K. Intensity of natural Trichinella spiralis and T. britovi infections in animal hosts of Extremadura (Spain) and its repercussion for diagnosis by direct methods. Res. Rev. Parasitol. 1998;58:117–120. [Google Scholar]
- 35.Boadella M., Barasona J.A., Pozio E., Montoro V., Vicente J., Gortazar C., Acevedo P. Spatio-temporal trends and risk factors for Trichinella species infection in wild boar (Sus scrofa) populations of central Spain: A long-term study. Int. J. Parasitol. 2012;42:739–745. doi: 10.1016/j.ijpara.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Deksne G., Segliņa Z., Jahundoviča I., Esīte Z., Bakasejevs E., Bagrade G., Keidāne D., Interisano M., Marucci G., Tonanzi D., et al. High prevalence of Trichinella spp. in sylvatic carnivore mammals of Latvia. Vet. Parasitol. 2016;231:118–123. doi: 10.1016/j.vetpar.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Feidas H., Kouam K.M., Kantzoura V., Theodoropoulos G. Global geographic distribution of Trichinella species and genotypes. Infect. Genet. Evol. 2014;26:255–266. doi: 10.1016/j.meegid.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Gómez-Morales M.A., Ludovisi A., Amati M., Bandino E., Capelli G., Corrias F., Gelmini L., Nardi A., Sacchi C., Cherchi S., et al. Indirect versus direct detection methods of Trichinella spp. infection in wild boar (Sus scrofa) Parasit Vectors. 2014;7:171. doi: 10.1186/1756-3305-7-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keuling O., Baubet E., Duscher A., Ebert C., Fischer C., Monaco A., Podgórski T., Prevot C., Ronnenberg K., Sodeikat G., et al. Mortality rates of wild boar Sus scrofa L. in central Europe. Eur. J. Wildl. Res. 2013;59:805–814. doi: 10.1007/s10344-013-0733-8. [DOI] [Google Scholar]
- 40.Pozio E. Trichinella and trichinellosis in Europe. Vet. Glas. 2019;73:65–84. doi: 10.2298/VETGL190411017P. [DOI] [Google Scholar]
- 41.Boros Z., Vallee I., Panait L.C., Gherman C.M., Chevillot A., Boireau P., Cozma V. Seroprevalance of Trichinella spp. in wild boars (Sus scrofa) from Bihor county, western Romania. Helminthologia. 2020;57:235–240. doi: 10.2478/helm-2020-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murell K.D., Pozio E. Worldwide occurrence and impact of human Ttrichinellosis, 1986–2009. Emerg. Infect. Dis. 2011;17:2194–2202. doi: 10.3201/eid1712.110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Institute of Public Health (INSP) National Center for Surveillance and Control of Communicable Diseases (CNSCBT) Analysis of the Evolution of Communicable Disease under Surveillance-Reports for 2010, 2011, 2012, 2013, 2014, 2015, 2016, 2017. National Institute of Public Health (INSP); Cuernavaca, Mexico: National Center for Surveillance and Control of Communicable Diseases (CNSCBT); Bucharest, Romania: 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.