Abstract
Melanoma is the most aggressive type of skin cancer, the incidence and mortality of which are increasing worldwide. Its extensive degree of heterogeneity has limited its response to existing therapies. For many years the therapeutic strategies were limited to surgery, radiotherapy, and chemotherapy. Fortunately, advances in knowledge have allowed the development of new therapeutic strategies. Despite the undoubted progress, alternative therapies are still under research. In this context, nanotechnology is also positioned as a strong and promising tool to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve photothermal and photodynamic therapies outcomes. This review describes the latest advances in nanotechnology field in the treatment of melanoma from 2011 to 2022. The challenges in the translation of nanotechnology-based therapies to clinical applications are also discussed. To sum up, great progress has been made in the field of nanotechnology-based therapies, and our understanding in this field has greatly improved. Although few therapies based on nanoparticulate systems have advanced to clinical trials, it is expected that a large number will come into clinical use in the near future. With its high sensitivity, specificity, and multiplexed measurement capacity, it provides great opportunities to improve melanoma treatment, which will ultimately lead to enhanced patient survival rates.
Keywords: melanoma, treatment, nanotechnology, drug delivery, photothermal therapy, photodynamic therapy, regulation, clinical trials
1. Introduction
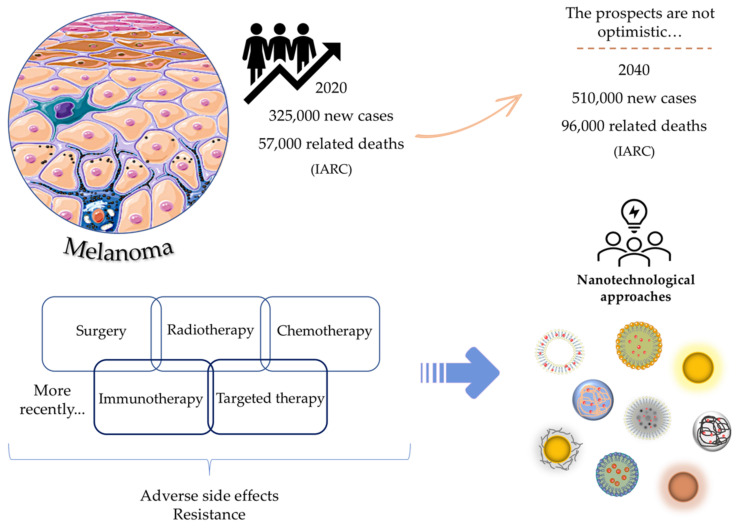
Today, cancer is undoubtably one of the biggest public health problems around the world. According to the World Health Organization, it is the leading cause of death in people under 70 years old in 57 countries, and the second-leading cause in another 55 countries [1]. Along with cancer, cardiovascular diseases also have a great impact on global mortality [2]. However, although today these diseases are still the leading cause of premature death in 70 countries, the predicted decrease in their mortality rate indicates that cancer will surpass them as the leading cause of death in many countries [1,2]. The International Agency for Research on Cancer (IARC) estimated that in 2020, cancer affected around 19.3 million new people and claimed the lives of around 10 million worldwide. Furthermore, the projections are not encouraging. The growth trend of recent years with regard to the number of new cases is expected to continue and an increase of almost 50% is predicted for 2040, totaling 28.4 million cases [1].
In this context, skin cancers are no exception, and among them, melanoma, despite having a much lower incidence compared to others, is the most aggressive form, responsible for most skin cancer-related deaths [3,4,5]. The IARC estimates that in 2020 there were about 325,000 new cases and 57,000 deaths associated with cutaneous melanoma, and increases of more than 50% for both indicators are expected within 20 years [6,7]. Air pollution, depletion of the atmospheric ozone layer and global warming are some of the factors that contribute to this increase [8,9].
Melanoma is characterized as a complex and unpredictable malignancy that derives from the malignant transformation and consequently uncontrolled proliferation of melanocytes [3,10]. Melanocytes are melanin-producing cells, which, in addition to providing color to the skin, play an important role in protecting it from ultraviolet radiation [11]. Its location is predominantly in the basal layer of the epidermis [8,9], and therefore, cutaneous melanoma comprises the vast majority of diagnosed cases [5,12]. However, melanocytes can still be present in a wide variety of other tissues, such as hair follicles, the eye, the inner ear, the mucosa surfaces, or even the central nervous system. These cases comprise the non-cutaneous forms of melanoma [9,10,11,12].
In terms of therapeutic strategies currently available for the treatment of melanoma, surgery, radiotherapy, chemotherapy and, more recently, immunological and targeted therapies are included [13]. The approval of these last two therapeutic classes has completely revolutionized the therapeutic arsenal. However, these therapies are still far from being considered satisfactory [14]. The resistance and significant adverse side effects, together with the complexity, and consequent high morbidity and mortality associated with melanoma, continue to capture the interest of the scientific community. The investment in the search for greater knowledge about the underlying pathophysiological mechanisms, as well as the development of more selective, effective and safer treatments, still remains a priority [15,16]. Herein, nanotechnology appears as a potential strategy to improve treatment outcomes while reducing adverse side effects. The use of lipid-based systems, polymeric, metallic, and hybrid nanoparticles (NPs) as carriers of one or more anticancer agents as well as its possible combination with physical methods, such as photothermal (PTT) and photodynamic therapies (PDT), have shown promising results in this field [17,18,19,20]. Thus, this review aims to provide an updated approach to the latest nanotechnological advances as innovative therapeutic strategies in the management of melanoma by using different types of nanosystems, as schematically represented in Figure 1.
Figure 1.
Melanoma: epidemiological data, current therapies and new alternative strategies for improving clinical outcomes.
2. Melanoma
As already reported, melanoma comprises a malignant transformation of melanin-producing cells. This transformation is a highly complex and multifactorial process that arises from the interaction of several modifiable and non-modifiable risk factors [12]. Exposure to ultraviolet radiation, either through sunlight or the use of tanning devices, is the modifiable risk factor with the greatest impact on the number of new diagnosed cases of cutaneous melanoma, being responsible for 60–70% of cases [21,22]. In addition, sunburn at an early age, certain medications, such as immunosuppressive drugs, or environmental exposure to some chemicals, such as pesticides or heavy metals, complete the range of modifiable risk factors [3,23]. Regarding non-modifiable risk factors, age, sex, ethnicity, a high number of common nevi or atypical nevi, a personal or family history of skin cancer, diseases that compromise the immune system, genetic alterations, specific genetic conditions such as albinism and even some individual phenotypic traits such as the presence of freckles or light eyes and skin are all factors associated with an increased risk of melanoma [12,23,24,25]. Skin color and its response to UV radiation are parameters that allow the categorization of different skin phototypes. The Fitzpatrick scale is the most used numerical classification for human skin color. Herein, skin types vary from type I to VI. The higher the phototype, the lower the associated risk of melanoma [5,24].
In addition to the etiological heterogeneity mentioned above, the clinical presentation is also quite variable, with distinct epidemiological, dermatological, and histopathological characteristics associated with each subtype. Among the main types of cutaneous melanoma, and in increasing order of incidence, are superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, and acral lentiginous melanoma [4]. Therefore, the selection of the most suitable therapeutic option takes into account, among other individual aspects, the anatomical location and the stage and genetic profile of the tumor [13]. The five-year overall survival of a patient with advanced stage melanoma does not exceed 15–20%. On the contrary, if detected at early stages, this value increases exponentially to 99% [24]. These numbers emphasize the importance of a combined strategy in the prevention and early detection of this type of malignancy to improve the prognosis and survival rates of patients [26,27].
3. Nanotechnology Applied to Melanoma Therapy
Nanotechnology is a concept created in 1959 by the Nobel Prize laureate in Physics, Richard Feynman. Since then it had an extraordinary evolution, being even considered one of the most promising technologies of this century [28]. Nanotechnology comprises the design, development, production, characterization, and application of materials at the nanoscale [29,30]. The definition of nanoparticle is not completely consensual, because although some authors defend its variation between few nanometers and 100 nm, sizes up to 1000 nm can also be considered in drug-delivery fields [31,32,33,34,35].
The application of nanotechnology to medicine, called nanomedicine, has aroused growing interest from researchers around the world. It is present not only in the treatment of various diseases, but also in diagnosis and monitoring, as well as in immunization and vaccine development [20,36,37,38,39].
The behavior NPs in in vitro and in vivo systems, and consequently the rate of drug release, is closely related to several physicochemical characteristics of the nanosystems. Adhesion to the cell surface, phagocytosis and degradation profile are dependent on the particle size, charge, morphology and surface chemistry of the NPs as well as on the hemodynamic properties and density of the drug binding sites [40,41].
Concerning the drug release from nanodelivery systems, it generally occurs through different mechanisms, depending on how the drug is associated within the nanosystem, through covalent linkage at their surface, incorporated in the matrix, following complexation, via interaction or physical encapsulation of unmodified drug molecules. Regarding the cleavage of covalently conjugated drugs, this can be done by ester, amide, or hydrazone hydrolysis, disulfide exchange, hypoxia activation, self-immolation reaction (enzyme, thiol-disulfide exchange, low pH and light-promoted), photochemistry and thermolysis [41,42]. On the other hand, non-covalently bound drugs are released by controlling the pore size, the effective volume of the carrier as well as the internal conformation and permeability. Furthermore, the release of associated drugs can be promoted by external stimuli such as pH, temperature, and use of ultrasound [41,42]. Moreover, the type of coating and the thickness of the encapsulating material affect the release rate [41].
Additionally, the fate of NPs in the human body might also raise some safety issues. In general, NPs intravenously administered circulate in the bloodstream until they are cleared and eliminated. Renal and hepatobiliary elimination are the two main mechanisms. The pathway is different between biodegradable nanocarriers and nonbiodegradable NPs like gold nanoparticles (Au NPs). It has been shown that small metallic NPs (smaller than 5.5 nm) can be mostly cleared by renal filtration and urinary excretion and, in some studies, by feces. Larger NPs can prevent the renal excretion and can be accumulated in the liver and spleen. Several studies have suggested that nanomaterials can be eliminated in a hepatobiliary manner, through transcytosis in the hepatocytes, resulting in transport to the biliary system, followed by the gastrointestinal tract, and eventually eliminated in feces. In this case, hepatobiliary elimination is normally slow, ranging from hours to months or even longer. The liver sinusoidal endothelial cell fenestrae size in humans was reported to be 107.5 ± 1.5 nm [43]. Particles with dimensions that are larger than these fenestrae cannot directly enter the space of Disse, but may access it by a less efficient and slower process via transcytosis through the liver sinusoidal endothelial cells and Kupper cells [44].
In terms of treatment, nanotechnology aims to overcome some of the drawbacks of current therapies referred to in the Introduction section. The protection of sensitive therapeutic agents, improvement of pharmacokinetic and pharmacodynamic profiles and, consequently, bioavailability, as well as an increase in selectivity for tumor cells either by passive or active targeting, are some of the promises of this type of nanosystem. Thus, an increase in the safety and efficacy of treatments is expected [45,46,47,48,49]. The success and added value of nanomedicine is confirmed by the various nanomedicines that have already been approved or are undergoing clinical trials [50,51]. The first nanomedicine approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA), in 1995 and 1996, respectively, was Doxil®, precisely in the area of oncology. Doxil® is a liposomal delivery system of doxorubicin commonly used in the treatment of Kaposi’s sarcoma, multiple myeloma, metastatic breast cancer, and ovarian cancer [52]. Since then, several types of nanosystems have been explored for potential application in melanoma. They include systems acting either as carriers of natural compounds, new or existing drugs and genes, or as antimelanoma agents per se. Moreover, the nanosystems can even be activated by external stimuli, such as PTT and PDT [45,53,54,55,56,57,58].
Although phototherapies have experienced an increased interest in recent years [59], the use of light as a therapeutic strategy has already started thousands of years ago [60]. The therapeutic use of sunlight, denominated heliotherapy, is the oldest phototherapeutic strategy and, until the mid-19th century, the only [60] type. Furthermore, for more than 3000 years, ancient Egyptian, Chinese, and Indian civilizations have combined heliotherapy with reactive chemicals to treat a wide range of skin conditions, such as psoriasis, vitiligo or cancer [61,62,63]. At the end of the 19th century and beginning of the 20th century, discoveries made by different researchers such as Hermann von Tappeiner, Oscar Raab, or Niels Finsen paved the way for modern phototherapy [61,64]. In 1960, with the invention of the laser by Theodore H. Maiman, the ablation of tumors through the use of lasers began to be explored. However, the lack of selectivity and the high power densities required raised safety concerns [64]. Phototherapies such as PTT or PDT associated with laser irradiation and involving the administration of photosensitizing agents, are able to trigger a series of chemical, biological, and physiological reactions that culminate in the death of neoplastic cells [65].
PTT is based on the increment of temperature at the tumor site (which can range from 41 to 49 °C) through photothermal enhancers capable of converting the optical energy received from the laser into thermal energy, thus taking advantage of the low resistance of cancer cells to heat. Depending on the temperature reached, mechanisms of apoptosis, necrosis and necroptosis are pointed out [59,66]. In its turn, PDT acts by the chemical damage promoted in the cells, through the production of reactive oxygen species (ROS) due to the activation of a photosensitizing agent by irradiation. An activation of signaling pathways such as apoptosis and necrosis are described. In addition, there is also a collapse of the blood vessels surrounding the tumor, compromising irrigation, also inducing cell death by hypoxia [61,64,65,67]. Moreover, both PTT and PDT have demonstrated a slight ability to stimulate an immune response [68,69].
Although photothermal agents have not yet been tested in large clinical trials and only laser ablation is currently used in clinic, PDT has been applied in the cancer field for over 40 years [64]. The advantage of phototherapies is based on its high efficiency and selectivity in the ablation of cancer cells and minimal invasiveness that allows the reduction of adverse effects and a faster recovery of the patient [65,70,71,72]. However, limited laser penetration restricts phototherapies to the treatment of superficial cancers [72,73]. The additive and synergistic effect of combining PTT or PDT with other therapeutic approaches is increasingly being investigated [19,38,64,70,73,74,75,76,77].
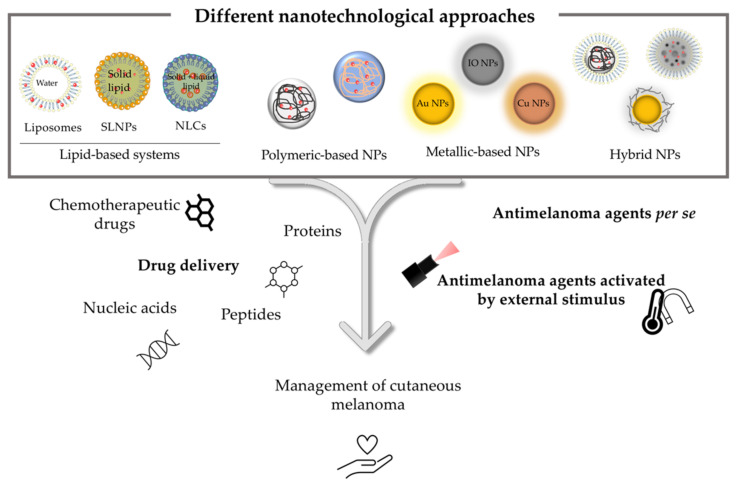
In the following sections, different types of nanotechnology-based strategies are discussed. In melanoma management, several types of nanosystems, as depicted in Figure 2, have been used. Lipid-based systems, such as liposomes, solid lipid nanoparticles (SLNPS) and nanostructured lipid carriers (NLCs), and polymeric, metallic, and hybrid nanoparticles are some examples listed in the present review. The reported outcomes are based on data published by the authors in international journals and do not reflect a personal opinion.
Figure 2.
Schematic illustration of different types of nanosystems: lipidic, polymeric, metallic, and hybrids that can be used for loading chemotherapeutic drugs, proteins, peptides, nucleic acids, DNA, or for activation by external stimuli for melanoma management.
3.1. Lipid-Based Systems
Lipid-based drug-delivery systems are gaining more and more notoriety, either by improving currently available therapies, or by creating new ones. This happens as a result of their ability to transport and allow a controlled release of the most varied molecules. Their high stability, biocompatibility, and biodegradability have been also contributive factors for their widespread use [78,79]. Among these usages, liposomes as vesicular systems or lipid particulate systems such as SLNPs and NLCs have attracted special interest [80]. In the following section, each system and respective in vitro and in vivo examples will be briefly described. Several human and murine melanoma cell lines have been used. In particular, one of the most studied is the murine melanoma B16F10 cell line. This cell line exhibits a morphology of spindle-shaped and epithelial-like cells that was isolated from skin tissue of a mouse with melanoma [15,81]. These cells are highly metastatic and present strong pigmentation. Furthermore, in vivo studies are most often tested in C57BL/6 male mice; that is, the mice strain in which the melanoma cell line B16F10 was created. This strain is widely used in immunocompetent murine melanoma models [15,81,82]. These and more in vitro and in vivo examples are described in Table 1, Table 2 and Table 3.
3.1.1. Liposomes
Liposomes, as drug delivery, are the most well-known, versatile, and represented lipid-based system clinically approved for the treatment of several diseases [83]. The first application in the field of cancer was initiated by Gregory Gregoriadis in the early 1970s [84,85].
Liposomes are spherical vesicles constituted essentially by phospholipids, which are organized in lipid bilayers separated by aqueous compartments. This structure guarantees the loading of hydrophilic drugs in the aqueous compartments and hydrophobic molecules in the lipid bilayers. In addition to phospholipids, liposomes can also include other constituents, such as glycolipids, cholesterol and hydrophilic polymers such as polyethylene glycol (PEG) [86,87]. The latter is widely used to prolong the circulation time of the formulation in the bloodstream. PEG prevents liposome uptake by the reticuloendothelial system, potentiating its extravasation and accumulation at tumor sites through the permeability and retention (EPR) effect [88].
There are some studies reporting the use of liposomes for melanoma management [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103]. A broadly used strategy to improve efficacy has relied on the simultaneously delivery of different liposomal formulations, pursuing a synergistic effect. In this context, Saqr and collaborators [89] proposed the in vitro cytotoxicity assessment of combining hispolon and doxorubicin (DOX) liposomes. DOX is a well-known chemotherapeutic agent with marked adverse side effects. In turn, hispolon, a polyphenol from a natural source, has noticeable in vitro cytotoxic activity, namely in melanoma [104]. Their combination aimed to potentiate the anticancer activity of DOX, while reducing effective dosage and consequently adverse side effects. In vitro assays on the murine melanoma cell line B16BL6 showed a significantly higher cytotoxicity of the conjugation of developed formulations when compared to hispolon-loaded liposomes or DOX-loaded liposomes individually. Furthermore, a significantly higher percentage of in vitro apoptotic cells was observed for the combined strategy, in comparison to control, hispolon-loaded liposomes, and DOX-loaded liposomes.
The application of metal-based complexes in the treatment of cancer has also become relevant [105]. One example is the work developed by Nave et al. and Pinho et al. [92,98], which designed and physicochemically characterized different, long-circulating liposomes incorporating a copper complex for melanoma management, the 1,10-phenanthroline ligand (Cuphen). First, its antiproliferative properties toward tumors cells as well as in vivo safety profile were demonstrated [92]. Liposomal formulations with suitable Cuphen loadings were selected for in vitro and in vivo studies. In vitro antiproliferative properties of copper complex were preserved after incorporation in liposomes. In a murine melanoma model, two selected liposomal formulations significantly reduced tumor progression, and no toxic side effects were observed compared to the control group or animals treated with Cuphen in the free form [98].
In addition to passive targeting via EPR effect, as mentioned above, the active targeting is another strategy whose objective is to enhance the selectivity of the formulation toward tumor sites [106]. In this segment, the group of Merino took precisely this approach and decorated the surface of empty liposomes with monovalent variable fragments (Fab’) of α-PD-L1 [93]. This ligand, in addition to promoting the targeting of the formulation, will also have an immunotherapeutic effect. Thus, after optimizing the immunoliposome production method, DOX was added as a chemotherapeutic agent to the formulation, and its in vitro and in vivo evaluation was performed [99]. Empty liposomes (lip), non-targeted DOX liposomes (DOX lip) and immunoliposomes (Fab’-anti-PD-L1 DOX lip) were tested with respect to cellular uptake efficiency and cytotoxicity in the murine cell line B16OVA, which is known to overexpress PD-L1. Results demonstrated that, in short periods of time (4 h), immunoliposomes significantly increased cellular uptake, and, consequently, its cytotoxicity. An approximately 30-fold decrease in the IC50 value was observed compared to non-targeted DOX liposomes. In the same work, intravenous administration of a single dose or three cycles every 72 h were performed to evaluate the extent of the immune response and the antitumor activity, respectively. C57/BL6 mice were inoculated with the same cell line tested in vitro, and then they were divided into five different groups based on the treatment: control group, DOX in its free form, DOX lip, co-administration of DOX lip with 28 µg/mice of free α-PD-L1, and the developed immunoliposomes. The concentration of DOX, 3 mg/kg, was maintained constant in all formulations. Immunoliposomes have been shown to induce a local and systemic immune response compared to the control group. In addition, a more pronounced and sustained antitumor effect over time, as well as a longer survival rate, were also achieved compared to all other treatment groups. Furthermore, immunoliposomes extended the animals’ life expectancy by almost 1 month versus untargeted formulation.
As reported throughout the work, the combination of two or more different therapeutic strategies has been widely investigated in order to enhance the treatment outcomes [107]. In this sense, Xu and collaborators also investigated the potential of a liposomal formulation that combines immune and photothermal therapies [91]. The developed approach consists of thermally responsive liposomes containing the photoactive dye indocyanine green (ICG) and the immune stimulatory molecule polyinosinic:polycytidylic acid (poly I:C). First, the photothermal efficiency as well as the successful release of poly I:C from the formulation was proven. Next, after a preliminary in vitro efficacy assessment on CT-26 murine carcinoma cells, an in vivo model of melanoma was conducted. This model was divided into two phases. First, subcutaneous inoculation of B16F10 cells into C57BL/6 mice was performed to simulate a primary tumor model. At this point, with appropriate size tumors, empty liposomes, ICG-loaded liposomes, and co-loaded ICG and poly I:C liposomes were intratumorally administrated, and the application or absence of 5 min of PTT was tested. Later, the same animals were subjected to a metastatic model by inoculating the same cell line but intravenously. In terms of results, the combination of PTT with the administration of ICG liposomes or the liposomes co-loaded with ICG and poly I:C promoted anticancer effects. However, poly I:C released from the combination therapy was essential for the activation of tumor antigen-specific immune responses by dendritic cells, enabling the prevention of lung metastases and, consequently, a high survival rate of the animals. These examples and some more are briefly described in Table 1.
Table 1.
In vitro and in vivo assessment of the therapeutic potential of different liposomal formulations in melanoma models.
| Nanosystem Composition | Compound(s) | Model(s) | Summary of Experimental Assays and Conditions | Main Conclusions | Reference |
|---|---|---|---|---|---|
| DSPC:chol:DSPE-PEG | Hispolon or DOX | In vitro: murine B16BL6 cell line | Cell viability assay: MTT (0.5 to 50 μM of hispolon and up to 1 μM of DOX) Cell uptake assay: coumarin-6 Cell death assay: Annexin V/PI |
Combination of hispolon and DOX liposomes exhibited a greater cytotoxicity compared to their use alone. | [89] |
| SPC:chol:DSPE-PEG | Ruthenium (II) triazolopyrimidine complex | In vitro: human A375 and Hs294T cell lines | Cell viability assay: MTT (0.4 to 2.5 μM) | Liposomes allowed a reduction of more than 10-fold in the IC50 value in relation to its free form. | [90] |
| DOTAP:chol:C8-ceramide:DSPE-PEG | DOX | In vitro: murine B16BL6 cell line | Cell viability assay: MTT (0.5 μg/mL of DOX and 10 mg/mL of C8-ceramide) | Co-delivery of DOX and C8-ceramide in a liposomal formulation displayed higher cytotoxicity in comparison with DOX liposomes without ceramide or even DOX solution. | [96] |
| HSPC:DSPE-PEG | Curcumin | In vitro: human MUG-Mel2 cell line | Cell viability assay: MTT (5 and 10 μM) without and with PDT application (380–500 nm; 2.5 J/cm2, 2 min) Cell proliferation assay: wound healing Cell death assays: annexin V-FITC/7-AAD and immunocytochemistry |
After PDT treatment, curcumin liposomes demonstrated increased phototoxicity and decreased motility in melanoma cells. In turn, in healthy cells (HaCat), a reduced toxicity was observed. | [97] |
| DMPC:chol:DSPE-PEG or DMPC:CHEMS:DSPE-PEG |
Copper (II) complex—Cuphen | In vitro: murine B16F10 cell line | Cell viability assay: MTS (0.2 to 12 μM) | In vivo assays demonstrated the safety and high impairment of tumor progression of Cuphen liposomal formulations in comparison to the free form. | [98] |
| In vivo: male C57BL/6 mice; s.c. injection of B16F10 cells | i.v. injection of Cuphen in free form and loaded in two different liposomal formulations (2.5 mg/kg) | ||||
| HSPC:chol:DSPE-PEG | DOX and Fab’-anti-PD-L1 | In vitro: murine B16OVA cell line | Cell viability assay: SRB (0.001 to 100 µM) | Targeted liposomes evidenced the immune system modulation and superior antitumor effect compared to all the other treatments. | [99] |
| In vivo: female C57BL/6 mice; s.c. injection of B16OVA cells | i.v. injection of free DOX, non-targeted DOX liposomes, non-targeted DOX liposomes + free α-PD-L1 and Fab’-anti-PD-L1 DOX liposomes (3 mg/kg) | ||||
| Lecithin:SC:chol:peptide TD | Vemurafenib | In vitro: murine B16F10 and human A375 cell lines | Cell viability assay: MTT (0 to 50 μg/mL of vemurafenib and 0 to 1.25 mg/mL of lecithin) | The modification of vemurafenib liposomes with peptide TD potentiated the transdermal delivery of the compound, resulting in negligible adverse side effects compared to oral and i.v. routes. | [100] |
| In vivo: male BALB/c nude mice; s.c. injection of A375 cells | oral, i.v. and transdermal administration of different formulations of vemurafenib (1.25 and 2.5 mg/mL) | ||||
| DOTAP:DOPE:chol:PEG | Anti-PD-1 siRNA | In vitro: murine B16F0 cell line | Cell viability assay: MTT (20 nM) | Anti-PD-1 siRNA liposomes demonstrated efficacy in silencing PD-1 mRNA expression in T cells, increasing the antitumor immune response. | [101] |
| In vivo: female C57BL/6 mice; s.c. injection of B16F0 cells | i.v. administration of Doxil (5 and 10 mg/kg), liposomal scramble siRNA (5 µg/kg), liposomal siRNA (5 µg/kg), and liposomal siRNA (5 µg/kg) + Doxil (5 mg/kg) | ||||
| DOTAP:DOPE:C6-ceramide:SC | Curcumin and anti-STAT3 siRNA | In vitro: murine B16F10 cell line | Cell viability assay: MTT (250 μM of curcumin and 0.5 nM of siRNA) | Topical iontophoretic administration of a curcumin and anti-STAT3 siRNA nanosystem demonstrated similar tumor inhibition efficacy as observed for i.t. injection, but significantly higher compared to liposomes of each of the compounds individually by either route. | [102] |
| In vivo: female C57BL/6 mice; s.c. injection of B16F10 cells | Topical (iontophoretic and passively) and i.t. administration of curcumin (3 mg/kg) and STAT3 siRNA (0.6 mg/kg) formulations alone or in combination | ||||
| DPPC:chol | 5-ALA | In vitro: murine B16F10 cell line | Cell viability assay: WST-1 (630 nm; 50 J/cm2, 20 min) ROS detection assay: DHE Mitochondrial membrane potential assay: mitotraker |
The combination of PDT and liposomes suggested greater phototoxicity compared to the non-liposomal form. In addition, in vivo assays demonstrated a higher ability to skin penetration in comparison to the free compound. | [103] |
| In vivo: male BALB/c nude mice; s.c. injection of B16F10 cells | Topical administration of free 5-ALA and incorporated in a liposomal formulation for PDT application | ||||
| DPPC:MPPC:DSPE-PEG | ICG and poly I:C | In vivo: C57BL/6 mice; s.c. injection of B16F10 cells, followed by a metastatic model by i.v. injection of B16F10 cells | i.t. administration of empty liposomes, ICG loaded liposomes and co-loaded ICG and poly I:C liposomes for PTT application (808 nm; 1 W/cm2, 5 min) | When submitted to PTT, both ICG liposomes and liposomes co-loaded with ICG and poly I:C promoted anticancer effects. However, only the second formulation prevented lung metastases. | [91] |
Abbreviations: 5-ALA, 5-aminolevulinic acid; CHEMS, cholesteryl hemisuccinate; Chol, cholesterol; DHE, dihydroethidium; DMPC, dimyristoyl phosphatidyl choline; DSPC, distearoyl phosphatidyl choline; DSPE-PEG, distearoyl phosphatidyl ethanolamine covalently linked to polyethylene glycol-2000; DOPE, dioleoyl phosphatidyl ethanolamine; DOTAP, dioleoyl trimethyl ammonium propane; DOX, doxorubicin; DPPC, dipalmitoyl phosphatidyl choline; Fab’, monovalent-variable fragments; HSPC, hydrogenated soya phosphatidyl choline; IC50, half-inhibitory concentration; ICG, indocyanine green; i.t., intratumoral; i.v., intravenous; MPPC, monopalmitoyl phosphatidyl choline; MTS, dimethylthiazol carboxymethoxyphenyl sulfophenyl tetrazolium; MTT, dimethylthiazol diphenyl tetrazolium bromide; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; PDT, photodynamic therapy; PEG, polyethylene glycol-2000; PI, propidium iodide; poly I:C, polyinosinic:polycytidylic acid; PTT, photothermal therapy; ROS, reactive oxygen species; s.c., subcutaneous; SC, sodium cholate hydrate; siRNA, small interference RNA; SPC, soybean phosphatidyl choline; SRB, sulphorodamine B; STAT3, signal transducer and activator of transcription 3; WST-1, water soluble tetrazolium salt.
3.1.2. Solid Lipid Nanoparticles
SLNPs comprise a colloidal drug-delivery system consisting of a solid lipid matrix stabilized by a mixture of surfactants or polymers. This lipid matrix is solid at room and body temperatures [108]. Mono-, di- or triglycerides, fatty acids, fatty alcohols, waxes, and sterols are lipids, and tween 80, lecithin and sodium glycolate are surfactants, commonly used in this type of carrier [109,110]. A low capacity of hydrophilic drug encapsulation, the inability to release the drug uniformly, and its leakage during storage as a result of a decrease in its solubility during the crystallization process, might limit the drug’s use [88,111,112].
Among the various studies reporting the application of this type of lipidic system in the treatment of melanoma [113,114,115,116,117,118,119,120,121], one example is the work developed by Goto et al. [113]. In this work, aluminum chloride phthalocyanine (ClAlPc), a highly hydrophobic photosensitizing agent, was incorporated into the SLNP formulation for further PDT application. This type of therapy is already approved for the treatment of several cancers and the association of photosensitizers with nanotechnology systems has been investigated [122,123]. Therefore, after optimization of the formulation, its in vitro cytotoxicity was evaluated on B16F10 murine melanoma cells in the absence of and after laser irradiation. In the absence of laser irradiation, both ClAlPc SLNPs and ClAlPc in the free form did not show any type of toxicity. After irradiation, a high phototoxicity was observed especially in the case of the nanoformulated compound. This phototoxicity is directly related to the applied energy.
Another example, is a delivery system for temozolomide (TMZ) prepared by Clemente and his colleagues [116]. After optimizing the production method of SLNPs containing TMZ (TMZ SLNPs), cytotoxicity and clonogenicity studies of empty SLNPs, free TMZ and TMZ SLNPs were performed in human (JR8 and A2058) and murine melanoma cell lines (B16F10). These in vitro studies demonstrated a higher cytotoxicity of TMZ SLNPs compared to the free drug. Furthermore, experiments on human umbilical vein endothelial cells have also demonstrated its potential in inhibiting angiogenesis in a concentration-dependent way. Finally, in vivo assays conducted in a xenograft model of C57BL/6J mice, upon inoculation of B16F10 murine melanoma cells, demonstrated the superior ability of TMZ SLNPs on preventing tumor growth progression when compared to animals treated with TMZ in free form or receiving empty SLNPs. No toxic effects or morphological changes in the histological analysis were observed. In addition, an increase in animal survival was also achieved, with 100% of mice treated with nanoformulation surviving until the end of the experiment compared to about 50% of the control group. Furthermore, from the analysis of the tumor samples, an increase in the expression levels of IL-17A as well as a decrease in the expression of the cell proliferation marker ki-67 were found compared to animals treated with non-formulated TMZ. IL-17A is a T cell-derived pro-inflammatory cytokine whose role in carcinogenesis is not fully understood, and even different functions are being related. The potentiation of antitumor immunity is one of the described roles [124,125]. In turn, the high expression of ki-67 is associated to a poor prognosis, reflecting tumor cell proliferation and growth [126,127,128]. Regarding in vivo anti-angiogenic activity, TMZ SLNPs decreased the density of tumor microvessels (assessed by CD31 staining of samples) compared to the control group. However, and contrary to what was observed in vitro, this effect on inhibiting new vascularization was practically similar to that observed in animals treated with free TMZ. Table 2 briefly describes previous and additional examples of in vitro and in vivo experiments.
Table 2.
In vitro and in vivo assessment of the therapeutic potential of different SLNPs in melanoma models.
| Nanosystem Composition | Compound(s) | Model(s) | Summary of Experimental Assays and Conditions | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Glyceryl behenate, sorbitan isostearate and polyoxyethylene-40 hydrogenated | ClAlPc | In vitro: murine B16F10 cell line | Cell viability assay: MTT without (200 and 400 μg/mL) and with (400 μg/mL) application of PDT (670 nm; 0.5, 1 and 2 J/cm2) | ClAlP SLNPs demonstrated remarkable phototoxic effects on melanoma cells compared to free ClAlPc. | [113] |
| Compritol 888, poloxamer 188 and tween 80 | Curcumin and resveratrol | In vitro: murine B16F10 and human SK-MEL-28 cell lines | Cell viability assay: MTT (0.1 to 60 μg/mL of curcumin and 0.03 to 20 μg/mL of resveratrol) Cell proliferation assays: IncuCyte (same concentrations of MTT) and ECIS (60 and 20 μg/mL of curcumin and resveratrol, respectively) |
The combination of the two compounds, either in solution or included in SLNPs, reduced the cancer cells viability compared to their use alone. | [114] |
| Cetyl palmitate, tricaprin and pluronic F68 | PTX and IR-780 | In vitro: murine B16 cell line | Cell viability assay: CCK-8 solution (0.1 to 10 μg/mL of PTX and 0.067 to 6.67 μg/mL of IR-780) after single (808 nm; 1 W/cm2, 5 min) or dual PTT treatment (repeated 24 h later) ROS detection assay: H2DCFDA Cell death assay: annexin V-FITC/PI |
The combination of PTX/IR-780 SLNPs concentrated in DMNs with a dual PTT treatment inhibited dramatically the tumor growth, and a 100% cure rate was achieved. | [115] |
| In vivo: female C57 mice; s.c. injection of B16 cells | Administration of DMNs loading PTX and/or IR-780 SLNPs for single (808 nm; 1 W/cm2, 5 min) and dual (repeated 24 h later) PTT treatment | ||||
| Sodium behenate and PVA | TMZ-C12 | In vitro: human JR8 and A2058 and murine B16F10 melanoma cell lines | Cell viability assay: WST-1 (5 to 50 μM) Clonogenic assay: crystal violet/methanol (5 to 50 μM) |
TMZ SLNPs demonstrated their greater cytotoxicity and anti-angiogenic activity in vitro compared to free TMZ. In vivo performance in terms of tumor growth inhibition and animal survival was also improved. | [116] |
| In vivo: female C57BL6/J mice; s.c. injection of B16F10 cells | i.v. injection of free TMZ, empty SLNPs and TMZ SLNPs (0.5 μmol/g) | ||||
| SLT, GMS, TPGS and tween 20 | DHA-dFdC | In vitro: murine B16F10 cell line | Cell viability assay: MTT (0.0001 to 10 μM) | DHA-dFdC SLNPs increased the chemical stability, plasma half-life and cytotoxicity of the compound in melanoma cells. Also its in vivo antitumor efficacy was improved compared to the free compound. | [117] |
| In vivo: female C57BL/6 mice; s.c. injection of B16F10 cells | i.v. injection of DHA-dFdC solution, empty SLNPs and DHA-dFdC SLNPs (50 mg/kg) |
Abbreviations: ClAlPc, aluminum chloride phthalocyanine; DHA-dFdC, docosahexaenoyl difluorodeoxycytidine; DMNs, dissolving microneedles; ECIS, electrical cell-substrate impedance sensing; GMS, glycerol monostearate; i.v., intravenous; MTT, dimethylthiazol diphenyl tetrazolium bromide; PDT, photodynamic therapy; PTT, photothermal therapy; PTX, paclitaxel; PVA, polyvinyl alcohol; s.c., subcutaneous; SLNP, solid lipid nanoparticle; SLT, soybean lecithin; TMZ-C12, lipophilic prodrug of temozolomide; TPGS, tocopheryl polyethylene glycol 1000 succinate; WST-1, water soluble tetrazolium salt.
3.1.3. Nanostructured Lipid Carriers
NLCs are considered the second generation of lipid NPs as they emerged as a way to overcome the drawbacks associated with SLNPs. NLCs comprise a mixture of solid lipids and liquid lipids, of which isopropyl myristate or oleic acid are examples, resulting in an unstructured lipid matrix. This fact promotes a greater capacity for drug incorporation and more uniform release, as well as greater stability with minimization of drug leakage during storage [88,111,112,129].
Of the various published studies [130,131,132,133,134,135], Malta and co-workers proposed the encapsulation of a new compound, 1-carbaldehyde-3,4-dimethoxyxanthone, in NLCs for further topical application [130]. The compound, previously synthesized by the same group [136], was denominated LEM2. It is a potent activator of the TAp73 with recognized antiproliferative effect on melanoma cells. However, it has a low water solubility and, consequently, low bioavailability. Thus, the compound was encapsulated, and different cytotoxic studies were performed in the human melanoma cell line, A375. Furthermore, in order to confirm whether the nanoformulation did not interfere with the molecular mechanism of the compound, the cell cycle progression and the expression levels of TAp73 protein and other proteins involved (p21, PUMA, BAX and Bcl-2) were evaluated. The results showed that the nanoformulation loading 2 μM of LEM2 promoted a significant increase in cell death, with cell cycle arrest in the G2/M phase, when compared to the empty nanosystem. An increase in the expression levels of TAp73, p21, PUMA, BAX, and MDM2, simultaneous with the reduction of the anti-apoptotic protein Bcl-2, were also achieved. Nevertheless, the validation of the developed NLC formulation was not assessed in vivo.
Liu et al., also proposed this type of system for drug delivery of docetaxel (DTX) [131]. Once again, the nanoformulation of the compound aimed to overcome its lower aqueous solubility. First, the production method of the nanoformulation was optimized and the resulting nanoformulation was characterized. In addition, the sustained release profile over the time of DTX from NLC was verified in vitro, with about 77% of DTX having been released in the first 24 h and the remaining up to 96 h. Then, in vitro cytotoxicity of the developed DTX NLC and Duopafei (DTX associated with high concentrations of Tween 80) was assessed in murine melanoma B16 cells. The results demonstrated a significantly higher cytotoxicity of the nanoformulation compared to Duopafei. Finally, in vivo antitumor efficacy of two different dosages of DTX NLC (10 and 20 mg/kg) in comparison with Duopafei (10 mg/kg) were tested in a melanoma model in Kunming mice. In this regard, although the lower dose has already achieved antitumor efficacy superior to Duopafei, DTX NLC 20 mg/kg was able to further reduce tumor volume even compared to DTX NLC 10 mg/kg. Furthermore, a greater loss of body weight was observed in the group of animals treated with Duopafei compared to any of the tested DTX NLC, suggesting fewer side effects of the nanoformulations. Table 3 depicts the cited examples and few examples more.
Table 3.
In vitro and in vivo assessment of the therapeutic potential of different NLCs in melanoma models.
| Nanosystem Composition | Compound(s) | Model(s) | Summary of Experimental Assays and Conditions | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Precirol ATO 5, OA and tween 80 | LEM2 | In vitro: human A375 cell line | Cell viability assays: SRB (0.010 to 5 μM) and trypan blue (1 and 2 μM) DNA damage assay: cell cycle arrest (PI) (1 and 2 μM) |
LEM2-loaded NLCs potentiate in vitro cell death of cancer cells in a dose-dependent manner, increasing the percentage of cell cycle arrest in the G2/M phase. | [130] |
| Stearic acid, GMS, SLT, OA and pluronic F68 | DTX | In vitro: murine B16 cell line | Cell viability assay: MTT (0.01 to 10 μM) | In vitro and in vivo assays demonstrated the greatest antitumor efficacy of DTX NLCs. In addition, lower in vivo side effects were achieved compared to duopafei. | [131] |
| In vivo: female Kunming mice; s.c. injection of B16 cells | i.v. injection of duopafei (10 mg/kg) and DTX NLCs (10 and 20 mg/kg) | ||||
| Stearylamine, IPM, SLT, TPGS and pluronic F68 | Tripterine | In vitro: murine B16BL6 cell line | Cell viability assay: MTT (2 to 10 μg/mL) Cell uptake assay: HPLC |
Cationic NLCs exhibited greater antitumor activity compared to neutral or anionic ones. | [132] |
| In vivo: male C57BL/6 mice; s.c. injection of B16BL6 cells | Topical administration of compound solution, neutral, anionic and cationic NLCs (6 mg/kg) and i.p. injection of CTX as positive control (20 mg/kg) |
Abbreviations: CTX, cyclophosphamide; DLS, dynamic light scattering; DTIC, dacarbazine; DTX, docetaxel; GMS, glycerol monostearate; HPLC, high performance liquid chromatography; i.p., intraperitoneal; IPM, isopropyl myristate; i.v., intravenous; LEM2, carbaldehyde dimethoxyxanthone; MTT, dimethylthiazol diphenyl tetrazolium bromide; NLC, nanostructured lipid carrier; OA, oleic acid; PI, propidium iodide; s.c., subcutaneous; SLT, soybean lecithin; SRB, sulphorodamine B; TPGS, D-α-tocopheryl polyethylene glycol 1000 succinate.
3.2. Polymeric-Based Nanoparticles
The use of polymers has substantially increased in drug-delivery systems for the treatment of cancer [137]. Their easy production and surface modification, improved pharmacokinetic and pharmacodynamic characteristics, as well as their recognized stability, biocompatibility, and biodegradability are among the main reasons for their growing use [138,139,140,141]. In this context, the polymers commonly used can be divided into three main groups, depending on their origin: from natural sources, such as chitosan, starch, alginate, cellulose, hyaluronic acid, chondroitin sulfate, dextran or albumin; biosynthesized, such as poly β-hydroxybutyrate (PHB); or chemically synthesized, examples of which are polylactic (PLA), poly(lactic-glycolic acid) (PLGA), polyurethane (PU), polymethyl methacrylate resin (PMMA) or poly(ε-caprolactone) (PCL) [142,143,144]. Relevant data are depicted in Table 4.
Among the various polymers used for designing NPs for the treatment of melanoma [145,146,147,148,149,150,151,152,153,154,155,156], the natural polymer chitosan (CS) was proposed by Ferraz’s research team for the encapsulation of S-nitrosomercaptosuccinic acid (S-nitroso-MSA) [145]. The work carried out focused on studying in vitro the cell death mechanism associated with the formulation (S-nitroso-MSA-CS). For this, a B16F10 murine melanoma cell line was used. Regarding cytotoxic assays, a greater impact of S-nitroso-MSA-CS in reducing cell viability was found compared to empty NPs, non-nitroso MSA NPs or free S-nitroso-MSA, which did not demonstrate important cytotoxicity. Interestingly, preferential selectivity was observed toward the cancer cells tested (B16F10) rather than healthy cells (Melan A). Moreover, flow cytometry showed a large percentage of cells in late apoptosis. In parallel, increased caspase 3 activity, as well as increased cell viability, was observed upon incubation of cells with a caspase inhibitor (Boc-D-FMK). The integrity of the cell membrane was also verified by the absence of LDH release and inhibitors of necroptosis and necrosis were tested, not resulting in a decrease in cytotoxicity. Thus, taking all these results together, a caspase-dependent apoptotic mechanism is suggested. Likewise, S-nitroso-MSA-CS also demonstrated ability to increase reactive cellular and mitochondrial oxygen species compared to control. Additionally, it was also proven that the cytotoxic effect of the formulation is not due to the release of nitric oxide (•NO), but due to the direct transfer of the S-nitroso group present in the formulation to free thiol groups of proteins.
PLGA is a copolymer approved by the FDA due to its well-known properties [157,158]. Zhang et al. [152] developed a formulation of polymeric NPs composed of PLGA and poloxamer 407 for the controlled release of apatinib (Apa-PLGA NPs). Apatinib is a small-molecule inhibitor of angiogenesis, acting by supplying oxygen and nutrients to the tumor microenvironment through antagonism of the vascular endothelial growth factor receptor 2 (VEGF-2). The comparative cytotoxicity of Apa in free form or incorporated in PLGA NPs was performed in an in vitro model by using murine melanoma cells (B16) at different concentrations and incubation times. Overall, the antiproliferative properties of the nanoformulation was superior in comparison to Apa in the free form. Subsequently, the antitumor effect of the nanosystem was evaluated in a C57BL/6 mice melanoma model. For this, a first screening to evaluate the intratumoral dose of apatinib was performed. As a result, 6 mg/kg was the dose of apatinib chosen for incorporation into NPs and tested in another group of animals. The results showed that Apa-PLGA NPs promoted the highest reduction on tumor growth progression among all tested animal groups. Statistically significant differences were observed in terms of tumor regression between animals treated with Apa-PLGA NPs or receiving free apatinib. These results were also confirmed by histological analyses which demonstrated higher necrosis levels. In addition, a Western blot analysis detected a reduction in phosphorylation levels of VEGFR-2 and ERK1/2 as well as a reduction in VEGFR-2 protein levels in tumor tissues.
On the other hand, phototherapies such as PTT, in addition to their recognized ability to promote tumor cell death, have also been associated with the development of immunogenicity which, due to not being robust enough for an effective systemic antitumor response, is often combined with other therapeutic strategies [159]. An example in this regard was the work developed by other researchers who combined photothermal and epigenetic therapies in a single nanosystem [151]. The formulation comprises PLGA NPs containing, once again, the photothermal agent ICG and the epigenetic drug Nexturastat A (NextA). The antitumor and immunomodulatory activity of Next A, a histone deacetylase (HDAC) 6 inhibitor, supports its usage. First the photocytotoxic activity of the formulation on the murine melanoma cell line SM1 was evaluated in vitro. A reduction after PTT application of approximately 80% in cell viability were found for an ICG dose of 2 mg/mL. In addition, the effectiveness of inhibiting HDAC 6 by the formulation was also confirmed in SM1 and B16F10 murine cancer cell lines. In parallel, another important factor was the increase in the expression of the major histocompatibility complex Class I, typically downregulated in cancer cells, as well as costimulatory molecules. Lastly, a syngeneic murine melanoma model was established through inoculation of SM1 cells. The animals were distributed in six groups combining both therapies or not. The results showed that the combined therapy allowed to slow tumor progression and increase the survival of the animals. However, it was found that therapeutic efficacy is largely supported by the initial treatment of PTT, with epigenetic therapy not being sufficient to sustain these initially obtained anticancer effects over time. Table 4 summarizes the previous examples and the most representative ones.
Table 4.
In vitro and in vivo assessment of the therapeutic potential of different polymeric-based nanoparticles in melanoma models.
| Nanosystem Composition | Compound(s) | Model(s) | Summary of Experimental Assays and Conditions | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Chitosan | S-nitroso-MSA | In vitro: murine B16F10 cell line | Cell viability assays (5, 10, 20, and 40 μg/mL): MTT, trypan blue and LDH release ROS detection assay: CM-H2DCFDA and MitoSOX Red Cell death assays: annexin V-FITC/PI and caspase-3 activity |
Nanoformulation exhibited high cytotoxicity selectively on cancer cells. | [145] |
| PLGA and PVA | Xanthohumol | In vitro: murine B16F10 cell line | Cell viability assay: MTT (2 to 40 μM) Cell proliferation assay: wound healing |
Loaded PLGA NPs showed high cytotoxicity as well as inhibition of proliferation and migration. | [146] |
| PMMA and sodium lauryl sulfate | α-terpineol | In vitro: murine B16F10 and human SK-MEL-28 cell lines | Cell viability assay: MTT (5, 50 and 500 μg/mL) | Nanosystem exhibited a large and selective cytotoxic effect in both melanoma cell lines tested. | [149] |
| PLA and PVA | DTIC and zinc phthalocyanine | In vitro: human MV3 cell line | Cell viability assay: MTT (20 and 100 μg of DTIC) after PDT application (660 nm; 28 J/cm2, 2.5 min) | In vitro assays demonstrated the added value of combined therapy in reducing cancer cell viability. | [150] |
| PLGA and PVA | ICG and NextA | In vitro: murine SM1 and B16F10 cell lines | Cell viability assay: Cell Titer-Glo ATP (0.5 to 2.0 mg/mL of NPs) with and without application of PTT HDAC activity assay: HDAC-Glo I/II |
The combination of photothermal and epigenetic therapies increased the in vitro expression of immunological markers. Moreover, in an in vivo context, a delayed tumor progression and an improved median survival were achieved. | [151] |
| In vivo: female C57BL/6 mice; s.c. injection of SM1 cells | i.t. administration of different formulation combinations (50 mg/kg of NPs) followed or not by PTT application (808 nm; 0.4 W, 10 min) | ||||
| PLGA and poloxamer 407 | Apatinib | In vitro: murine B16 cell line | Cell viability assay: CCK-8 solution (4, 20 and 40 μg/mL) | In vitro and in vivo experiments demonstrated the high performance of Apa-PLGA NPs. | [152] |
| In vivo: male C57BL/6 mice; injection of B16 cells | i.t. administration of free apatinib at different concentrations (2, 4 and 6 mg/kg), empty PLGA NPs and Apa-PLGA NPs (6 mg/kg) | ||||
| PCL, span 80, caprylic/caprictriglycerides and polysorbate 80 | Resveratrol | In vitro: murine B16F10 cell line | Cell viability assay: MTT (1, 3, 10, 30, 100 and 300 μM) | Confirming the in vitro cytotoxicity results, the in vivo study demonstrated an increase in areas of inflammation and necrosis as well as a reduction of metastases and pulmonary hemorrhage compared to the free compound. | [153] |
| In vivo: male and female C57BL/6J mice; s.c. injection of B16F10 cells | i.p. administration of free resveratrol, empty PCL NPs and resveratrol-PCL NPs (5 mg/kg) | ||||
| Chitosan, sodium alginate and calcium chloride | DOX | In vitro: murine B16F10 and B16OVA cell lines | Cell viability assay: alamar blue solution (1 to 100 μM) | In vitro assays suggested a greater intracellular accumulation and cytotoxicity of the nanosystem compared to the free drug. However, a similar effect between both was observed in the in vivo inhibition of tumor progression. | [154] |
| In vivo: female C57BL/6 mice; s.c. injection of B16OVA cells | i.v. injection of free DOX, empty NPs and DOX NPs (3 mg/kg) |
Abbreviations: Apa, apatinib; CCK-8, cell counting kit-8; CM-H2DCFDA, chloromethyl dichlorodihydrofluorescein diacetate; DOX, doxorubicin; DTIC, dacarbazine; ICG, indocyanine green; FITC, fluorescein isothiocyanate; HDAC, pan-histone deacetylase; i.p., intraperitoneal; i.t., intratumoral; i.v., intravenous; LDH, lactate dehydrogenase; MTT, dimethylthiazol diphenyl tetrazolium bromide; NextA, nexturastat A; NPs, nanoparticles; PCL, poly(ε-caprolactone); PDT, photodynamic therapy; PI, propidium iodide; PLA, polylactic acid; PLGA, poly lactic-co-glycolic acid; PMMA, poly(methyl methacrylate); PTT, photothermal therapy; PVA, polyvinyl alcohol; s.c., subcutaneous; S-nitroso-MSA, S-nitrosomercaptosuccinic acid.
3.3. Metallic-Based Nanoparticles
In recent years, metallic NPs have been attracting the attention of researchers around the world due to their varied unique properties, whether physical, chemical, optical, magnetic, catalytic, or electrical. Furthermore, their biocompatibility, as well as their ease of synthesis and chemical modification are key factors [160,161,162]. Among the metals commonly used in the production of this type of NPs, some stand out: gold (Au), silver (Ag), iron (Fe), copper (Cu), cerium (Ce), platinum (Pt), titanium (Ti), and zinc (Zn) [163,164]. Furthermore, their oxides, hydroxides, sulfides, phosphates, fluorides, and chlorides can be considered [164]. Relevant in vitro and in vivo data are included in Table 5.
There are many studies exploring metallic nanosystems as a strategy for the treatment of melanoma [165,166,167,168,169,170,171,172,173,174,175,176]. An example are the cuprous oxide nanoparticles (Cu2O NPs) developed by Wang and colleagues [171]. Throughout the work, the authors sought to evaluate the antitumor efficacy of the formulation both in in vitro and in vivo melanoma models, as well as to understand the influence of some factors in cell death. First, in vitro cytotoxicity assays in a melanoma cell line (B16F10) showed the dose and time-dependent ability of Cu2O NPs to reduce cell viability. Based on a previous study performed by the same group [177], selectivity for cancer cells was observed. Furthermore, an apoptotic mechanism and significant inhibition of cancer cell migration and invasion was observed when compared to the control. Then, two in vivo melanoma models (subcutaneous and metastatic lung melanoma), through the inoculation of B16F10 cells, were performed. In the first, a significant inhibition of tumor progression of the group treated with Cu2O NPs compared to the control group was observed, leading to a significantly higher survival rate at the end of the experiment than the control group. In its turn, in the metastatic model, the formulation promoted a significant decrease of lung tumor nodules compared to the control group. Moreover, the safety and rapid, essentially hepatic clearance of the formulation was also confirmed in vivo. Finally, in mechanistic terms, it has been proven by the various assays that the cytotoxicity of Cu2O NPs, among other possible factors, is triggered by mitochondrial injury, which is reflected in a decrease of their membrane potential, increase of ROS, release of cytochrome C and activation of caspases 3 and 9.
Au NPs are another example of metallic NPs that have often been used, for example, in PTT [178,179,180,181] or for conjugation with drugs, as explored by other researchers [172,182,183]. Once again, and taking into account that different nanocarriers promote different pharmacokinetic and pharmacodynamic profiles, and consequently different accumulations sites [184,185], drug delivery of doxorubicin was tested, this time associated with Au NPs. The antitumor efficacy of ultra-small Au NPs (~3 nm) conjugated with doxorubicin (Au-DOX) compared to the use of DOX alone was evaluated. In one of their first studies, the ability of this conjugate to reduce IC50 value by a twenty-fold factor in DOX-resistant B16 cells was demonstrated, compared to DOX in its free form [182]. Later, in another study [183], the conclusion was also drawn that the impact that Au-DOX exerts on the malignant cell death is not due to the release of DOX as commonly observed in other approaches, but by the binding of the intact conjugate to cell structures. In its turn, in a more recent study published by the same group [172], the cell viability reduction previously observed for B16 cells, was not observed for the SK-MEL-28 cell line, a cell line sensitive to DOX. However, a two-fold factor reduction of cell viability was still achieved. In addition, here, two different in vivo melanoma models were established in C57BL/6 and nude mice, upon inoculation of a DOX-resistant murine cell line (B16) and a doxorubicin-sensitive human cell line (SK-MEL-28), respectively. For this, the animals were divided into different groups: control, Au NPs, DOX and Au-DOX. Analysis of the results from both in vivo models reveals the greater potential of Au-DOX in inhibiting the progression of tumor volume in a sustained manner over the time. Taking together the histology, as well as the TUNEL staining of the tumor samples from the two in vivo experiments, a greater necrotic component can be seen compared to apoptosis. The treatment effects were more strongly evidenced in the groups of animals submitted to the formulation under study.
In the case of PTT, Pandesh and his team synthesized Fe3O4 NPs surrounded by a gold shell (Fe-Au NPs) [174]. Although the Fe3O4 core gives the NPs the ability to magnetically target the tumor site through a magnet placed under the skin in the tumor region, the gold shells work as a photothermal agent for later application of PTT. First, the thermal conversion capacity of the formulation was tested by using different gold concentrations and laser power densities. The increase in temperature was higher in accord with a higher rise in gold concentration and laser power. Finally, the therapeutic efficacy of the formulation was evaluated through an in vivo model of melanoma. For this, C57BL/6 animals were subcutaneously inoculated with B16F10 cells for primary tumor development. When the desired volume was reached, five treatment groups were established: control, intravenous administration of Fe-Au NPs, intravenous administration of Fe-Au NPs plus laser and intravenous administration of Fe-Au NPs plus magnet plus laser. After two weeks, the mean tumor volume of animals that received the complete treatment (NPs + magnet + laser) increased 7.7-fold compared to 47.3-fold in animals that received no treatment (control group). Thus, an average tumor progression inhibition rate of 83.5% versus control was obtained for this group. The same treatment but in the absence of magnetic targeting of the NPs lowered the inhibition rate to 57%. These and more in vitro and in vivo experiments are briefly described in Table 5.
Table 5.
In vitro and in vivo assessment of the therapeutic potential of different metallic-based nanoparticles in melanoma models.
| Nanosystem Composition | Compound(s) | Model(s) | Summary of Experimental Assays and Conditions | Main Conclusions | Reference |
|---|---|---|---|---|---|
| MoO3 NPs | Non-applicable | In vitro: human G361 cell line | Cell viability assay: MTT (50–400 μg/μL) | MoO3 NPs showed selective cytotoxicity against malignant skin cells compared to healthy cells. | [165] |
| AgPt NPs | Non-applicable | In vitro: human A375 cell line | Cell viability assays: MTS (10–250 µg/mL) | NPs demonstrated antitumor activity in the A375 cell line while being safe for healthy cells. | [166] |
| Pd NPs | Non-applicable | In vitro: human A375 cell line | Cell viability assays (0–40 μg/mL): MTT and NRU DNA damage assays: comet, cell cycle arrest (EtBr) ROS detection assay: H2DCFDA oxidative stress detection assay: BCA Cell death assays: DAPI, AO/EtBr and caspase-3 activity |
In vitro assays demonstrated cytotoxic and genotoxic activity of Pd NPs on melanoma cells. | [169] |
| Cu NPs | Non-applicable | In vitro: human A375 cell line | Cell viability assay: MTT (up to 4.5 μg/mL) Cell membrane fluidity assay: TMA-DPH DNA damage assays: comet (EtBr), chromosomal condensation (DAPI) and cell cycle arrest (PI) ROS detection assay: H2DCFDA Mitochondrial membrane potential assay: JC-1 Cell death assays: annexin V-FITC/PI and caspase-3 activity |
Cu NPs promoted DNA damage, cell cycle arrest in the G2/M phase and depolarization of mitochondrial membrane potential. | [170] |
| Cu2O NPs | Non-applicable | In vitro: murine B16F10 cell line | Cell viability assay: MTT (2.5, 5 and 10 μg/mL) ROS detection assay: H2DCFDA Mitochondrial membrane potential assay: JC-1 Cell death assays: annexin V-FITC/PI and caspase-3 and caspase-9 activities |
Cu2O NPs reduced selectively cancer cell lines viability in vitro. Similarly, in an in vivo context, a significant anti-tumor efficacy, impaired tumor growth progression and inhibition of lung metastasis was observed. | [171] |
| In vivo: subcutaneous and metastatic models; male C57BL/6 mice; s.c. and i.v. injection of B16F10 cells, respectively | i.v. and i.t. administration of Cu2O NPs in subcutaneous (16 mg/kg) and metastatic (2 mg/kg) model, respectively | ||||
| Au NPs | DOX | In vitro: murine B16 and human SK-MEL-28 cell lines | Cell viability assay: SRB | Au-DOX was efficiently internalized and demonstrated great cytotoxicity in melanoma cells. In vivo assays also demonstrated its sustained inhibition of tumor progression over time when compared to DOX alone. | [172] |
| In vivo: male C57BL/6 mice; s.c. injection of B16 cells and female nude mice, s.c. injection of SKMEL-28 cells | Administration of free DOX, Au NPs and Au-DOX conjugation (100 μM of DOX or 4 μL of Au) | ||||
| Ag and TiO2 based NPs | Non-applicable | In vitro: murine B16F10 cell line | Cell viability assay: MTT (75, 100, 150 and 200 μg/mL) | The formulation allied to PTT treatment markedly reduced tumor cells viability in vitro as well as the tumor volume in an in vivo model. | [173] |
| In vivo: male and female C57BL/6J mice; s.c. injection of B16F10 cells | i.t. administration of formulation (100 μg/mL) followed or not by PTT application (808 nm; 2 W/cm2, 1 min) | ||||
| Fe3O4 and Au based NPs | Non-applicable | In vivo: male C57BL/6 mice; s.c. injection of B16F10 cells | i.v. administration of NPs (150 μg Au/mL) with and without PTT treatment (808 nm; 2.5 W/cm2, 6 min) | The magnetically target NPs associated with PTT impaired significantly the tumor growth in comparison to control group. | [174] |
Abbreviations: Ag, silver; AgPt NPs, silver and platinum nanoparticles; AO, acridine orange; Au, gold; Au NPs, gold nanoparticles; BCA, bicinchoninic acid; Cu NPs, copper nanoparticles; Cu2O NPs, cuprous oxide nanoparticles; DAPI, diaminidino phenylindole; DOX, doxorubicin; EtBr, ethidium bromide; Fe3O4, iron(II, III)oxide; FITC, fluorescein isothiocyanate; H2DCFDA, dichlorodihydrofluorescein diacetate; i.t., intratumoral; i.v., intravenous; JC-1, tetrachloro tetraethylbenzimidazolylcarbocyanine iodide; MoO3 NPs, molybdenum trioxide nanoparticles; MTS, dimethylthiazol carboxymethoxyphenyl sulfophenyl tetrazolium; MTT, dimethylthiazol diphenyl tetrazolium bromide; NPs, nanoparticles; NRU, neutral red uptake; Pd NPs, palladium nanoparticles; PI, propidium iodide; POR, porphyrin derivate—Tetrakis-5,10,15,20-(2,4-dihydroxyphenyl)porphyrin; ROS, reactive oxygen species; s.c., subcutaneous; SRB, sulphorodamine B; TiO2, titanium dioxide; TMA-DPH, trimethylamonium diphenyl hexatriene.
3.4. Hybrid Nanoparticles
Hybrid nanosystems have different organic and/or inorganic materials in their composition, which results in unique synergistic functional properties [186,187,188,189,190]. There are several studies that have been carried out by using hybrid NPs in the treatment of melanoma [191,192,193,194,195,196,197,198,199,200,201]. A selection of in vitro and in vivo studies is shown in Table 6. In the liposomes subsection, a study was reported describing the encapsulation of a metallic compound with cytotoxic properties (Cuphen) [98]. Following the notable results in terms of safety and efficacy obtained in vitro and in vivo, researchers from the same group proposed the association of metallic iron oxide NPs with this liposomal nanosystem [191]. The aim was to take advantage of the magnetic targeting of these NPs in order to potentiate the accumulation of the nanoformulation at the tumor microenvironment. Thus, the objectives of this work were to evaluate the influence that the combination of magnetic NPs with Cuphen would have on its cytotoxic properties, as well as to confirm the magnetic properties of the final formulation. The in vitro studies performed in murine (B16F10) and human (MNT-1) melanoma cells, confirmed the maintenance of the cytotoxic activity of Cuphen in the presence of NPs. In addition, and in order to ensure the safety of its parenteral administration, the absence of hemolytic activity of the formulation was also confirmed.
Another type of hybrid NP developed in the context of melanoma management was presented by Lopes and collaborators [197]. The purpose was the use of Au NPs coated with hyaluronic and oleic acids and conjugated with epidermal growth factor (EGF-conjugated HAOA-coated Au NPs) for PTT application. After in vitro assays demonstrating the safety of these Au NPs without laser irradiation, a melanoma xenograft model in severely immunocompromised hairless mice was developed upon inoculation of A375 human melanoma cells. The aim was to evaluate the therapeutic efficacy of in situ administration of Au in combination with near-infrared (NIR) laser irradiation. For this, the animals were divided into four groups (control group, treatment group only submitted to 5 min of laser irradiation; and two other treatment groups submitted to the administration of Au NPs followed by 5 or 10 min of laser irradiation). As a result, in situ administration of Au NPs followed by NIR laser irradiation for 5 min showed the highest tumor volume reduction up to 80%. Moreover, a formation of numerous necrotic foci by histological analysis of tumor samples was observed. Finally, no toxic effects were observed in the different excised organs nor in the skin exposed to the irradiation. Table 6 displays an overview of hybrid nanoparticles.
Table 6.
In vitro and in vivo assessment of the therapeutic potential of different hybrid nanoparticles in melanoma models.
| Nanosystem Composition | Compound(s) | Model(s) | Summary of Experimental Assays and Conditions | Main Conclusions | Reference |
|---|---|---|---|---|---|
| IO NPs loaded liposomes (DMPC: CHEMS:DSPE-PEG) | Copper (II) complex—Cuphen | In vitro: human MNT-1 and murine B16F10 cell lines | Cell viability assay: MTT of free Cuphen (0.5 to 7 μM), free IO NPs (1 to 7.5 mg/mL) and their combination (Cuphen at 1 and 5 µM and IO NPs at 2 mg/mL) | IO NPs did not influence the cytotoxicity of Cuphen and when loaded in liposomes the magnetic properties were verified. | [191] |
| Gold coated loaded liposomes (HSPC) | Curcumin | In vitro: murine B16F10 cell line | Cell viability assays: PI and MTT of free curcumin, curcumin-liposomes and curcumin-lip/Au NPs (100 μg/mL) after PTT (780 nm; 650 mW, 5 min) Cell uptake assay: DAPI |
Curcumin was efficiently internalized by cancer cells when incorporated into the formulation. Furthermore, its adjuvant effect in combination with PTT was shown. | [192] |
| BSA coated Ag NPs | Non-applicable | In vitro: murine B16F10 cell line | Cell viability assay: WST-1 without (10−8 to 10−2 M of Ag) and with (2.7 × 10−3 M of Ag) application of PTT (690 nm; 0.8, 0.9 and 1 W, 10 min) ROS detection assay: H2DCFDA |
The formulation demonstrated its cytotoxicity by increasing the generation of ROS, while also exhibiting its added value as a photothermal agent. | [194] |
| Chitosan coated loaded liposomes (DMPC:chol) | ICG | In vitro: murine B16F10 cell line | Cell viability assay: MTT of free ICG and chitosan coated or uncoated IGC-liposomes (40 μM) without and with application of PDT (785 nm; 100 mW/cm2, 2.5 min) Cell uptake assay: fluorescence intensity |
Chitosan coated ICG-liposomes increased the cellular uptake of ICG and consequently its photocytotoxicity, compared to uncoated ones. | [195] |
| HAOA coated Au NPs | Non-applicable | In vitro: murine B16F10 cell line | Cell viability assay: MTT without (5, 30 and 60 μM) and with (5 μM) application of PTT (811 nm; 0.04 W/cm2, 3 min) | The laser activation of HAOA-coated Au NPs demonstrated a reduction on cancer cell viability compared to observed for healthy cells (HaCat). | [196] |
| EGF-conjugated HAOA coated Au NPs | Non-applicable | In vitro: murine B16F10 and human A375 cell lines | Cell viability assay: MTT (25 to 100 μM) without application of PTT | The safety of the formulation without laser irradiation was confirmed in vitro. In turn, in vivo experiments showed that 5 min of laser irradiation promoted the greatest tumor volume reduction, about 80%. | [197] |
| In vivo: male hairless SCID mice; s.c. injection of A375 cells | i.t. injection of EGF-conjugated coated Au NPs (20 mg/kg) followed by NIR laser irradiation (811 nm; 2.5 W/cm2, 5 or 10 min) | ||||
| Liposome (EPC:chol:DDAB:DSPE-PEG) containing HSA-loaded NPs | CHL | In vitro: murine B16F10 cell line | Cell uptake pathway assay: coumarin-6 | CHL-hybrid NPs exhibited a more pronounced antitumor effect and increased overall survival compared to all the other formulations. | [198] |
| In vivo: male C57BL/6 mice; s.c. injection of B16F10 cells | i.v. injection of CHL solution, CHL-liposomes, CHL-Alb NPs and CHL-Alb/liposome hybrid NPs (5 mg/kg) |
Abbreviations: Ag NPs, silver nanoparticles; Au NPs, gold nanoparticles; BSA, bovine serum albumin; CHEMS, cholesteryl hemisuccinate; CHL, chlorambucil; Chol, cholesterol; DAPI, diaminidino phenylindole; DDAB, dimethyl dioctadecyl ammonium bromide; EGF, epidermal growth factor; EPC, egg phosphatidylcholine; DMPC, dimyristoyl phosphatidyl choline; DSPE-PEG, distearoyl phosphatidyl ethanolamine covalently linked to polyethylene glycol-2000; HAOA, hyaluronic and oleic acids; has, human serum albumin; HSPC, hydrogenated soya phosphatidyl choline; ICG, indocyanine green; IO NPs, iron oxide nanoparticles; i.t., intratumoral; i.v., intravenous; MTT, dimethylthiazol diphenyl tetrazolium bromide; NPs: nanoparticles; NIR, near-infrared; PDT, photodynamic therapy; PI, propidium iodide; PTT, photothermal therapy; ROS, reactive oxygen species; s.c., subcutaneous; SCID, severe combined immune-deficient; WST-1, water soluble tetrazolium salt.
3.5. Examples of Patented Nanomedicine Products
In general, the research and development (R&D) process of a medicine can take up to 20 years. Thus, patenting is one of the main tools that allows investors to safeguard their discoveries, at least for a few years [202,203]. Therefore, and as a result of all the aforementioned pre-clinical research carried out in the field of nanotechnology, numerous product patents have been applied for a wide range of cancers, including melanoma. Some of them are shown in Table 7.
Table 7.
Selected patented nanomedicine products with application to melanoma (not exclusively).
| Nanosystem | Inventor(s) Name(s) | Grant Application Date | Patent Number |
|---|---|---|---|
| Drug loaded Fe(III)-DOPA NPs | Hyung Joon Cha, Bum Jin Kim and Ho gyun Cheong | June 2017 | US9675629B2 |
| Drug loaded PEG-PCL NPs | Adam W. G. Alani | July 2018 | US10016422B2 |
| Silicon dioxide NPs functionalized with an antigen | Markus Weigandt, Andrea Hanefeld, Armin Kuebelbeck and Gregor Larbig | October 2018 | US10111952B2 |
| Iron garnet NPs containing activatable nuclides | Anthony J. Di Pasqua, Kenneth J. Balkus, Imalka S. Munaweera and Yi Shi | February 2019 | US10195297B2 |
| PVP coated silver prussian blue NPs | Sudip Mukherjee and Chitta Ranjan Patra | March 2019 | US10231996B2 |
| Oil or water-in-oil emulsion combining antigens loaded liposomes | Pirouz M. Daftarian, Marc Mansour, Bill Pohajdak, Robert G. Brown and Wijbe M. Kast | April 2019 | US10272042B2 |
| T cell ligands and/or antigens linked to carbon nanotubes | Tarek M. Fahmy, Lisa D. Pfefferle, Gary L. Haller and Tarek R. Fadel | November 2019 | US10485856B2 |
| Complexes of albumin NPs and antibodies | Svetomir N. Markovic and Wendy K. Nevala | September 2020 | US10765741B2 |
| Drug loaded lipid-based NPs decorated with CD47 | Raghu Kalluri and Sónia Melo | March 2021 | US10959952B2 |
| Sensitizer loaded PGA-based polymer/co-polymer/derivate NPs (PTT and SDT) | Nikolitsa Nomikou | June 2021 | US11040101B2 |
| Irinotecan-mesoporous silica NPs coated with a lipid bilayer | Andre E. Nel, Huan Meng and Xiangsheng Liu | August 2021 | US11096900B2 |
| Photosensitizer or drug loaded lipid layer coated NPs (PDT, but not only) |
Wenbin Lin, Xiaopin Duan, Christina Chan and Wenbo Han | February 2022 | US11246877B2 |
| NIR absorbing dye based composite NPs (PTT) | Sehoon Kim, Youngsun Kim, Keunsoo Jeong and Gayoung Kim | April 2022 | US11291726B2 |
Abbreviations: DOPA, 3,4-dihydroxyphenylalanine; NIR, near infrared; NPs, nanoparticles; PCL, poly(ε-caprolactone); PDT, photodynamic therapy; PEG, polyethylene glycol; PGA, polyglutamic acid; PTT, photothermal therapy; PVP, poly(n-vinyl-2-pyrrolidone); SDT, sonodynamic therapy. Data collected from the United States Patent and Trademark Office and Espacenet patent search websites, accessed on 20 July 2022.
3.6. Landscape of Clinical Trials Using Different Types of Nanosystems
Clinical research regarding the application of nanomedicine in the treatment of cutaneous melanoma has been evolving over the last several years. In this context, completed and ongoing clinical trials that explore different types of nanosystems, such as micro- and nanoemulsions, liposomes, polymeric and hybrid NPs, are presented in Table 8.
Table 8.
Examples of the most representative (not all) clinical trials (completed or ongoing) using different types of nanosystems for melanoma treatment.
| Clinical Trial Phase | Clinical Trial Description | Melanoma Stage | Sponsor | Starting Date | Study Completion/Estimated Date | Trial ID |
|---|---|---|---|---|---|---|
| Completed clinical trials | ||||||
| 1 | Pharmacokinetic study of a liposomal vincristine sulfate formulation. | III/IV | Acrotech Biopharma LLC | February 2005 | November 2007 | NCT00145041 |
| Safety and efficacy of a liposomal vaccine targeting dendritic cells (Lipovaxin-MM). | IV | Lipotek Pty Ltd. | September 2009 | March 2012 | NCT01052142 | |
| Safety, pharmacokinetic and pharmacodynamic study of BIND-014 (PSMA-targeted PLA/PEG docetaxel NPs). | Advanced or metastatic | BIND Therapeutics | January 2011 | February 2016 | NCT01300533 | |
| 2 | Safety and efficacy of ABI-007 (nab-paclitaxel). | Unresectable locally recurrent or metastatic | Jonsson Comprehensive Cancer Center | February 2004 | January 2010 | NCT00081042 |
| Safety and efficacy of co-administration of ABI-007 (nab-paclitaxel) and carboplatin. | IV | Alliance for Clinical Trials in Oncology | October 2006 | March 2010 | NCT00404235 | |
| Safety and efficacy analysis of the combination of nab-paclitaxel and bevacizumab versus ipilimumab alone. | IV | Academic and Community Cancer Research United | October 2013 | October 2019 | NCT02158520 | |
| 3 | Safety and efficacy of ABI-007 (nab-paclitaxel) versus dacarbazine. | IV | Celgene | April 2009 | February 2014 | NCT00864253 |
| Ongoing clinical trials | ||||||
| 1 | Safety and efficacy of nab-paclitaxel and bevacizumab. | IV | Mayo Clinic | March 2014 | June 2025 | NCT02020707 |
| Safety and tolerability of a cancer vaccine composed by naked RNA-drug products in a liposomal formulation (Lipo-MERIT). | IIIB/C/IV | BioNTech SE | March 2015 | May 2023 | NCT02410733 | |
| Safety and tolerability of escalating doses of OX40L, IL-23 and IL-36γ encoding human mRNAs encapsulated in a lipid nanoparticle alone or combining with durvalumab. | Advanced or metastatic | ModernaTX, Inc. | November 2018 | January 2023 | NCT03739931 | |
| 2 | Safety and efficacy of the combination of nab-paclitaxel, carboplatin and endostatin after failure of PD-1 therapy. | Advanced | Peking University Cancer Hospital & Institute | March 2019 | September 2022 | NCT03917069 |
Abbreviations: Nab-paclitaxel, nanoparticle albumin-bound paclitaxel; PD-1, programmed cell death protein-1; PEG, polyethylene glycol; PLA, polylactic acid; PSMA, prostate-specific membrane antigen; TLR4, toll-like receptor 4. Data collected from the ClinicalTrials.gov database, accessed on 20 July 2022.
However, and despite all efforts made, no nanotechnology-based product has yet been officially approved for clinical use. In addition, and according to the ClinicalTrials.gov database, accessed on 20 July 2022, there are some clinical trials that were left incomplete, whose recruitment was withdrawn, suspended or terminated for various reasons. According to the same database, recruitment status of closed clinical trials can be classified into four categories: withdrawn, interrupted earlier, even before enrolling any participant; terminated, interrupted earlier and without possibility of restarted; suspended, interrupted earlier, but can be restarted; and completed, completed normally. In this context and accordingly, the information of public domain, phase 1/2 clinical trials, evaluating the pharmacodynamics and pharmacokinetics of liposomal miR-34 (MRX34) (NCT02862145), and a safety and efficacy study of the combination of nab-paclitaxel, temozolomide, and bevacizumab for the treatment of melanoma brain metastases (NCT02065466), are examples of withdrawn clinical trials. In this case, the published reasons were due to serious adverse immune-related side effects and lack of accrual, respectively. In addition to these, and by strategic decision, a phase 2 study which aimed at comparative analysis of the safety and efficacy of the combination of CC-486 (oral azacytidine) with nab-paclitaxel versus nab-paclitaxel alone (NCT01933061) is another example of a withdrawn clinical trial whose study object was nanotechnology in melanoma treatment. In turn, the phase 1 safety and efficacy study of liposomal cytarabine in combination with lomustine and brain radiation therapy for the treatment of leptomeningeal melanoma metastases (NCT01563614) was terminated due to the availability of other therapeutic approaches.
3.7. Regulation of Nano-Based Products
Despite the already wide range of nanodrugs approved for clinical use, regulatory policies remain non-harmonized across different geographic locations around the world [204,205]. It is true that nanomedicines have the same requirements in terms of quality, safety, and efficacy as other medicines. However, due to their small size and large surface area, they require specific considerations, especially with regard to their production and quality control, safety, and efficacy [206,207].
The lack of a unanimous definition or classification of the concept itself is a stumbling block. There is still to be highlighted a high demand in terms of establishing analytical methods depending on the nanomaterial used, as a result of its great heterogeneity, a divergence of the pharmacokinetic profiles from standardized constituent materials as well as the limitations of toxicity studies in assessing the short- and long-term impact in an in vivo situation. In addition, problems with reproducibility between batches, stability when scaling up or even the possible impact they might or might not have on the environment are also pressing issues [204,208,209].
However, there are a few guidelines, not legally binding, representing only their current opinion on the subject [205]. However, the increased number of nanopharmaceutical applications pending approval has pressured regulatory agencies to establish working groups to clearly determine and align some definitions and standards.
In the European Union, the legislation applied by the EMA to nanomedicines is the same applied to other medicines, and its approval or disapproval is supported by a risk/benefit analysis. There is a legal reference dated to 2011 that establishes the definition of nanomaterial (Recommendation 2011/696/EU) [210]. Furthermore, it should be noted that legally (REACH EC 1907/2006) [211], the safety of chemicals, including nanomaterials, is from the responsibility of the European Chemical Agency (ECHA). In the European context, the Nanomedicines Expert Group was one of the established groups, aiming the development of these types of subjects. This group founded projects such as the European Nanomedicine Characterization Laboratory (EUNCL), which is dedicated to the development of methods for the pre-clinical characterization of nanomedicines and the Regulatory Science Framework for Nano(bio)material-based Medical Products and Devices (REFINE), which focus its activity on the development of methods to support regulatory decisions [204,208,209].
Likewise, the FDA has not yet issued specific regulations with regard to nanomedicine. In 2017, a guide for the industry that includes biological products containing nanomaterials was made public; however, it is not legally binding [212]. Thus, approval requests made to the FDA are analyzed on a case-by-case basis and according to concrete product specifications through statutory and regulatory authorities. In addition, the FDA has mechanisms to assist industries in various practical issues during the development of nanodrugs as well as monitoring, soon after they enter the market. Among the groups created by the FDA to deal with underdevelopment in this regulatory area, there is the Nanotechnology Task Force and Nanotechnology Interest Group. Also other external institutes such as the Nanotechnology Characterization Laboratory of the National Cancer Institute (NCL-NCI) and the National Nanotechnology Initiative (NNI) have contributed to the development in the field of nanomedicine regulation [204,208,209].
Furthermore, other joint international efforts have also been made in this area. An example is the Global Summit on Regulatory Science: Nanotechnology Standards and Applications organized in 2019 by the Global Coalition for Regulatory Scientific Research (GCRSR). GCRSR comprises regulatory bodies from various countries who came together to discuss the gaps in nanomedicine regulation and ways to bridge them through global collaborations [207].
4. Conclusions and Future Perspectives
As previously described, cutaneous melanoma is a highly complex, unpredictable and heterogeneous malignancy, associated with increasing incidence and mortality rates. As reported throughout this review, since 2011, with the approval of immunotherapy and targeted therapy, there has been an authentic revolution in the treatment of melanoma with countless other drugs being approved since then. These advances have been crucial to improve the quality of life of patients, as well as to increase their average life expectancy. Even so, the results achieved are still inferior to those observed for other types of cancer, with heterogeneous responses and not always durable. In this context, nanomedicine emerges as a window of opportunities, in an attempt to improve response rates while reducing adverse side effects. In the last decades, nanotechnology has experienced great evolution and nowadays provides several tools capable of overcoming some of the obstacles associated with conventional therapies. Solving solubility and stability problems of several drugs in clinical use, increasing blood circulating half-lives and accumulation at tumor sites, by passive or active delivery, are some of the potentialities offered by this type of strategy. Its value is indisputable, and the proof of this are the nanomedicines already approved for a huge variety of pathologies including cancer, as well as the large number of clinical trials currently ongoing. In the specific case of melanoma, and although several nanosystems have been tested in different animal models, those that have reached clinical trials are so far predominantly liposomes and polymeric NPs. However, there are still pending issues to solve or clarify regarding the approval of novel nano-drug-delivery systems: How to assess potential risks of components of a nanosystem for human health in the short and/or long term? How to ensure reproducibility between batches? How to guarantee the stability of nanoformulations scale up? These are some of the questions that urgently need to be answered and on which the scientific community and regulatory agencies have to concentrate their efforts.
Author Contributions
Writing—original draft preparation, J.L.; writing—review and editing, J.L., C.M.P.R., M.M.G. and C.P.R.; Supervision, C.M.P.R., M.M.G. and C.P.R.; Funding acquisition M.M.G. and C.P.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors acknowledge Fundacão para a Ciência e a Tecnologia (FCT) for financial support through Projects UIDB/00645/2020, UIDB/04138/2020, UIDP/04138/2020, PTDC/BTM-MAT/31794/2017, PTDC/QUI-QIN/0586/2020 and PhD fellowship SFRH/BD/148044/2019.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Laversanne M., Weiderpass E., Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 3.Dimitriou F., Krattinger R., Ramelyte E., Barysch M.J., Micaletto S., Dummer R., Goldinger S.M. The World of Melanoma: Epidemiologic, Genetic, and Anatomic Differences of Melanoma Across the Globe. Curr. Oncol. Rep. 2018;20:87. doi: 10.1007/s11912-018-0732-8. [DOI] [PubMed] [Google Scholar]
- 4.Garbe C., Peris K., Hauschild A., Saiag P., Middleton M., Bastholt L., Grob J.-J., Malvehy J., Newton-Bishop J., Stratigos A.J., et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline—Update 2016. Eur. J. Cancer. 2016;63:201–217. doi: 10.1016/j.ejca.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Davey M.G., Miller N., McInerney N.M. A Review of Epidemiology and Cancer Biology of Malignant Melanoma. Cureus. 2021;13:e15087. doi: 10.7759/cureus.15087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer Cancer Tomorrow—Estimated Number of New Cases from 2020 to 2040 of Melanoma of Skin. [(accessed on 9 August 2022)]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16&single_unit=50000&group_cancers=1&multiple_cancers=1.
- 7.International Agency for Research on Cancer Cancer Tomorrow—Estimated Number of Deaths from 2020 to 2040 of Melanoma of Skin. [(accessed on 9 August 2022)]. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=16&single_unit=5000&group_cancers=1&multiple_cancers=1&types=1.
- 8.Silva G.S., Rosenbach M. Climate change and dermatology: An introduction to a special topic, for this special issue. Int. J. Women’s Dermatol. 2021;7:3–7. doi: 10.1016/j.ijwd.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker E.R. The influence of climate change on skin cancer incidence—A review of the evidence. Int. J. Women’s Dermatol. 2021;7:17–27. doi: 10.1016/j.ijwd.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews N.H., Li W.-Q., Qureshi A.A., Weinstock M.A., Cho E. Epidemiology of Melanoma. In: Ward W.H., Farma J.M., editors. Cutaneous Melanoma, Etiology and Therapy. Volume 6. Codon Publications; Brisbane, Australia: 2017. pp. 139–149. [PubMed] [Google Scholar]
- 11.Baroni A., Buommino E., De Gregorio V., Ruocco E., Ruocco V., Wolf R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012;30:257–262. doi: 10.1016/j.clindermatol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Carr S., Smith C., Wernberg J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. N. Am. 2020;100:1–12. doi: 10.1016/j.suc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Kozar I., Margue C., Rothengatter S., Haan C., Kreis S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim. Biophys. Acta-Rev. Cancer. 2019;1871:313–322. doi: 10.1016/j.bbcan.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowski S.M., Fisher D.E. Biology of Melanoma. Hematol. Oncol. Clin. N. Am. 2021;35:29–56. doi: 10.1016/j.hoc.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Coricovac D., Dehelean C., Moaca E.A., Pinzaru I., Bratu T., Navolan D., Boruga O. Cutaneous melanoma-a long road from experimental models to clinical outcome: A review. Int. J. Mol. Sci. 2018;19:1566. doi: 10.3390/ijms19061566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merlino G., Herlyn M., Fisher D.E., Bastian B.C., Flaherty K.T., Davies M.A., Wargo J.A., Curiel-Lewandrowski C., Weber M.J., Leachman S.A., et al. The state of melanoma: Challenges and opportunities. Pigment Cell Melanoma Res. 2016;29:404–416. doi: 10.1111/pcmr.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgheti-Cardoso L.N., Viegas J.S.R., Silvestrini A.V.P., Caron A.L., Praça F.G., Kravicz M., Bentley M.V.L.B. Nanotechnology approaches in the current therapy of skin cancer. Adv. Drug Deliv. Rev. 2020;153:109–136. doi: 10.1016/j.addr.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Prasad M., Lambe U.P., Brar B., Shah I., Manimegalai J., Ranjan K., Rao R., Kumar S., Mahant S., Khurana S.K., et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018;97:1521–1537. doi: 10.1016/j.biopha.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Xie Z., Fan T., An J., Choi W., Duo Y., Ge Y., Zhang B., Nie G., Xie N., Zheng T., et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020;49:8065–8087. doi: 10.1039/D0CS00215A. [DOI] [PubMed] [Google Scholar]
- 20.Silva C.O., Pinho J.O., Lopes J.M., Almeida A.J., Gaspar M.M., Reis C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics. 2019;11:22. doi: 10.3390/pharmaceutics11010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wróbel S., Przybyło M., Stępień E. The Clinical Trial Landscape for Melanoma Therapies. J. Clin. Med. 2019;8:368. doi: 10.3390/jcm8030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dzwierzynski W.W. Melanoma Risk Factors and Prevention. Clin. Plast. Surg. 2021;48:543–550. doi: 10.1016/j.cps.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Horrell E.M.W., Wilson K., D’Orazio J.A. Melanoma-Epidemiology, Risk Factors, and the Role of Adaptive Pigmentation. In: Murph M., editor. Melanoma-Current Clinical Management and Future Therapeutics. IntechOpen; London, UK: 2015. [Google Scholar]
- 24.O’Neill C.H., Scoggins C.R. Melanoma. J. Surg. Oncol. 2019;120:873–881. doi: 10.1002/jso.25604. [DOI] [PubMed] [Google Scholar]
- 25.Imbernón-Moya A., Podlipnik S., Malvehy J., Puig S. Initial Evaluation of Patients with Pigmented Skin Lesions. Actas Dermosifiliogr. 2016;107:616–618. doi: 10.1016/j.ad.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Apalla Z., Lallas A., Sotiriou E., Lazaridou E., Ioannides D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017;7:1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cormier J., Voss R., Woods T., Cromwell K., Nelson K. Improving outcomes in patients with melanoma: Strategies to ensure an early diagnosis. Patient Relat. Outcome Meas. 2015;6:229. doi: 10.2147/PROM.S69351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules. 2020;25:112. doi: 10.3390/molecules25010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rafique M., Tahir M.B., Rafique M.S., Hamza M. Nanotechnology and Photocatalysis for Environmental Applications. Elsevier; Amsterdam, The Netherlands: 2020. History and fundamentals of nanoscience and nanotechnology; pp. 1–25. [Google Scholar]
- 30.Naves L.B., Dhand C., Venugopal J.R., Rajamani L., Ramakrishna S., Almeida L. Nanotechnology for the treatment of melanoma skin cancer. Prog. Biomater. 2017;6:13. doi: 10.1007/s40204-017-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leon L., Chung E.J., Rinaldi C. Nanoparticles for Biomedical Applications. Fundamental Concepts, Biological Interactions and Clinical Applications. Elsevier; Amsterdam, The Netherlands: 2020. A brief history of nanotechnology and introduction to nanoparticles for biomedical applications; pp. 1–4. [Google Scholar]
- 32.Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., Danquah M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018;9:1050. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allan J., Belz S., Hoeveler A., Hugas M., Okuda H., Patri A., Rauscher H., Silva P., Slikker W., Sokull-Kluettgen B., et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul. Toxicol. Pharmacol. 2021;122:104885. doi: 10.1016/j.yrtph.2021.104885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lövestam G., Rauscher H., Roebben G., Klüttgen B.S., Gibson N., Putaud J.-P., Stamm H. Considerations on a Definition of Nanomaterial for Regulatory Purposes. Publications Office; Luxembourg: 2010. [Google Scholar]
- 35.He Z., Liu K., Byrne H.J., Cullen P.J., Tian F., Curtin J.F. Applications of Targeted Nano Drugs and Delivery Systems. Elsevier; Amsterdam, The Netherlands: 2019. Combination Strategies for Targeted Delivery of Nanoparticles for Cancer Therapy; pp. 191–219. [Google Scholar]
- 36.El-Sayed A., Kamel M. Advances in nanomedical applications: Diagnostic, therapeutic, immunization, and vaccine production. Environ. Sci. Pollut. Res. 2020;27:19200–19213. doi: 10.1007/s11356-019-06459-2. [DOI] [PubMed] [Google Scholar]
- 37.Pelaz B., Alexiou C., Alvarez-Puebla R.A., Alves F., Andrews A.M., Ashraf S., Balogh L.P., Ballerini L., Bestetti A., Brendel C., et al. Diverse Applications of Nanomedicine. ACS Nano. 2017;11:2313–2381. doi: 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng L., Wang X., Gong F., Liu T., Liu Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020;32:1902333. doi: 10.1002/adma.201902333. [DOI] [PubMed] [Google Scholar]
- 39.Wang X., Fan L., Cheng L., Sun Y., Wang X., Zhong X., Shi Q., Gong F., Yang Y., Ma Y., et al. Biodegradable Nickel Disulfide Nanozymes with GSH-Depleting Function for High-Efficiency Photothermal-Catalytic Antibacterial Therapy. iScience. 2020;23:101281. doi: 10.1016/j.isci.2020.101281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halwani A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics. 2022;14:106. doi: 10.3390/pharmaceutics14010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding C., Li Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C. 2017;76:1440–1453. doi: 10.1016/j.msec.2017.03.130. [DOI] [PubMed] [Google Scholar]
- 42.Wong P.T., Choi S.K. Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chem. Rev. 2015;115:3388–3432. doi: 10.1021/cr5004634. [DOI] [PubMed] [Google Scholar]
- 43.Wisse E., Jacobs F., Topal B., Frederik P., De Geest B. The size of endothelial fenestrae in human liver sinusoids: Implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15:1193–1199. doi: 10.1038/gt.2008.60. [DOI] [PubMed] [Google Scholar]
- 44.Poon W., Zhang Y.N., Ouyang B., Kingston B.R., Wu J.L.Y., Wilhelm S., Chan W.C.W. Elimination Pathways of Nanoparticles. ACS Nano. 2019;13:5785–5798. doi: 10.1021/acsnano.9b01383. [DOI] [PubMed] [Google Scholar]
- 45.Beiu C., Giurcaneanu C., Grumezescu A.M., Holban A.M., Popa L.G., Mihai M.M. Nanosystems for Improved Targeted Therapies in Melanoma. J. Clin. Med. 2020;9:318. doi: 10.3390/jcm9020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brys A.K., Gowda R., Loriaux D.B., Robertson G.P., Mosca P.J. Nanotechnology-based strategies for combating toxicity and resistance in melanoma therapy. Biotechnol. Adv. 2016;34:565–577. doi: 10.1016/j.biotechadv.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Gherasim O., Popescu R.C., Gherasim T.G., Grumezescu V., Andronescu E. Nanoparticles in Pharmacotherapy. William Andrew; Norwich, NY, USA: 2019. Pharmacotherapy and nanotechnology; pp. 1–21. [Google Scholar]
- 48.Pautu V., Leonetti D., Lepeltier E., Clere N., Passirani C. Nanomedicine as a potent strategy in melanoma tumor microenvironment. Pharmacol. Res. 2017;126:31–53. doi: 10.1016/j.phrs.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Macedo A.S., Castro P.M., Roque L., Thomé N.G., Reis C.P., Pintado M.E., Fonte P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control. Release. 2020;320:125–141. doi: 10.1016/j.jconrel.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019;4:e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Lázaro I., Mooney D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021;20:1469–1479. doi: 10.1038/s41563-021-01047-7. [DOI] [PubMed] [Google Scholar]
- 52.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016;1:10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishra H., Mishra P.K., Ekielski A., Jaggi M., Iqbal Z., Talegaonkar S. Melanoma treatment: From conventional to nanotechnology. J. Cancer Res. Clin. Oncol. 2018;144:2283–2302. doi: 10.1007/s00432-018-2726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng H., Zhang Z. The application of nanotechnology in immune checkpoint blockade for cancer treatment. J. Control. Release. 2018;290:28–45. doi: 10.1016/j.jconrel.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 55.Pinho J.O., Lopes J., Albino M., Reis C., Matias M., Gaspar M.M. Mitochondrial Dysfunction and Nanotherapeutics. Academic Press; Cambridge, MA, USA: 2021. Advances in nanotechnology-related strategies against melanoma; pp. 385–424. [Google Scholar]
- 56.Chen J., Zhang X.D. Nanoscience in Dermatology. Elsevier; Amsterdam, The Netherlands: 2016. Nanodelivery of Anticancer Agents in Melanoma: Encouraging, But a Long Way to Go; pp. 189–201. [Google Scholar]
- 57.Cassano R., Cuconato M., Calviello G., Serini S., Trombino S. Recent Advances in Nanotechnology for the Treatment of Melanoma. Molecules. 2021;26:785. doi: 10.3390/molecules26040785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang J.Q., Hou X.Y., Yang C.S., Li Y.X., Xin Y., Guo W.W., Wei Z.P., Liu Y.Q., Jiang G. Recent developments in nanomedicine for melanoma treatment. Int. J. Cancer. 2017;141:646–653. doi: 10.1002/ijc.30708. [DOI] [PubMed] [Google Scholar]
- 59.Pivetta T.P., Botteon C.E.A., Ribeiro P.A., Marcato P.D., Raposo M. Nanoparticle Systems for Cancer Phototherapy: An Overview. Nanomaterials. 2021;11:3132. doi: 10.3390/nano11113132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grzybowski A., Sak J., Pawlikowski J. A brief report on the history of phototherapy. Clin. Dermatol. 2016;34:532–537. doi: 10.1016/j.clindermatol.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Correia J.H., Rodrigues J.A., Pimenta S., Dong T., Yang Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics. 2021;13:1332. doi: 10.3390/pharmaceutics13091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitton D., Ackroyd R. A brief overview of photodynamic therapy in Europe. Photodiagn. Photodyn. Ther. 2008;5:103–111. doi: 10.1016/j.pdpdt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Van Straten D., Mashayekhi V., de Bruijn H.S., Oliveira S., Robinson D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers. 2017;9:19. doi: 10.3390/cancers9020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X., Lovell J.F., Yoon J., Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020;17:657–674. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 65.Zhou S., Li D., Lee C., Xie J. Nanoparticle Phototherapy in the Era of Cancer Immunotherapy. Trends Chem. 2020;2:1082–1095. doi: 10.1016/j.trechm.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han H.S., Choi K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines. 2021;9:305. doi: 10.3390/biomedicines9030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Z., Sun Z., Ren Y., Chen X., Zhang W., Zhu X., Mao Z., Shen J., Nie S. Advances in nanomaterials for use in photothermal and photodynamic therapeutics. Mol. Med. Rep. 2019;20:5–15. doi: 10.3892/mmr.2019.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yue J., Mei Q., Wang P., Miao P., Dong W.F., Li L. Light-triggered multifunctional nanoplatform for efficient cancer photo-immunotherapy. J. Nanobiotechnol. 2022;20:181. doi: 10.1186/s12951-022-01388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng C.W., Li J., Pu K. Recent Progresses in Phototherapy-Synergized Cancer Immunotherapy. Adv. Funct. Mater. 2018;28:1804688. doi: 10.1002/adfm.201804688. [DOI] [Google Scholar]
- 70.Wang X., Zhong X., Zha Z., He G., Miao Z., Lei H., Luo Q., Zhang R., Liu Z., Cheng L. Biodegradable CoS2 nanoclusters for photothermal-enhanced chemodynamic therapy. Appl. Mater. Today. 2020;18:100464. doi: 10.1016/j.apmt.2019.100464. [DOI] [Google Scholar]
- 71.Zhao L., Xing Y., Wang R., Yu F.F., Yu F. Self-Assembled Nanomaterials for Enhanced Phototherapy of Cancer. ACS Appl. Bio Mater. 2020;3:86–106. doi: 10.1021/acsabm.9b00843. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X., Wang S., Cheng G., Yu P., Chang J. Light-Responsive Nanomaterials for Cancer Therapy. Engineering. 2022;13:18–30. doi: 10.1016/j.eng.2021.07.023. [DOI] [Google Scholar]
- 73.Wang X., Cheng L. Multifunctional two-dimensional nanocomposites for photothermal-based combined cancer therapy. Nanoscale. 2019;11:15685–15708. doi: 10.1039/C9NR04044G. [DOI] [PubMed] [Google Scholar]
- 74.Liu S., Song R., Li X., Zhou F. Synergistic therapeutic strategies for cancer treatment based on nanophototherapy. Nanophotonics. 2021;10:3391–3395. doi: 10.1515/nanoph-2021-0407. [DOI] [Google Scholar]
- 75.Wang X., Zhong X., Cheng L. Titanium-based nanomaterials for cancer theranostics. Coord. Chem. Rev. 2021;430:213662. doi: 10.1016/j.ccr.2020.213662. [DOI] [Google Scholar]
- 76.Wang X., Zhong X., Lei H., Geng Y., Zhao Q., Gong F., Yang Z., Dong Z., Liu Z., Cheng L. Hollow Cu2Se Nanozymes for Tumor Photothermal-Catalytic Therapy. Chem. Mater. 2019;31:6174–6186. doi: 10.1021/acs.chemmater.9b01958. [DOI] [Google Scholar]
- 77.Wang X., Cheng L. Multifunctional Prussian blue-based nanomaterials: Preparation, modification, and theranostic applications. Coord. Chem. Rev. 2020;419:213393. doi: 10.1016/j.ccr.2020.213393. [DOI] [Google Scholar]
- 78.Layek B., Gidwani B., Tiwari S., Joshi V., Jain V., Vyas A. Recent Advances in Lipid-based Nanodrug Delivery Systems in Cancer Therapy. Curr. Pharm. Des. 2020;26:3218–3233. doi: 10.2174/1381612826666200622133407. [DOI] [PubMed] [Google Scholar]
- 79.None H., Barkat M.A., Das S.S., Pottoo F.H., Beg S., Rahman Z. Lipid-Based Nanosystem As Intelligent Carriers for Versatile Drug Delivery Applications. Curr. Pharm. Des. 2020;26:1167–1180. doi: 10.2174/1381612826666200206094529. [DOI] [PubMed] [Google Scholar]
- 80.Shrestha H., Bala R., Arora S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014;2014:801820. doi: 10.1155/2014/801820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Predoi M.-C., Mîndrilă I., Buteică S., Mărginean O., Mîndrilă B., Niculescu M. Pigmented melanoma cell migration study on murine syngeneic B16F10 melanoma cells or tissue transplantation models. J. Mind Med. Sci. 2019;6:327–333. doi: 10.22543/7674.62.P327333. [DOI] [Google Scholar]
- 82.Fidler I.J., Nicolson G.L. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J. Natl. Cancer Inst. 1976;57:1199–1202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- 83.Gubala V., Johnston L.J., Liu Z., Krug H., Moore C.J., Ober C.K., Schwenk M., Vert M. Engineered Nanomaterials and Human Health: Part 1. Preparation, Functionalization and Characterization (IUPAC Technical Report) Pure Appl. Chem. 2018;90:1283–1324. doi: 10.1515/pac-2017-0101. [DOI] [Google Scholar]
- 84.Gregoriadis G. Liposomes in Drug Delivery: How It All Happened. Pharmaceutics. 2016;8:19. doi: 10.3390/pharmaceutics8020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perrie Y. Gregory Gregoriadis: Introducing liposomes to drug delivery. J. Drug Target. 2008;16:518–519. doi: 10.1080/10611860802228376. [DOI] [PubMed] [Google Scholar]
- 86.Pinho J.O., Matias M., Gaspar M.M. Emergent nanotechnological strategies for systemic chemotherapy against melanoma. Nanomaterials. 2019;9:1455. doi: 10.3390/nano9101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Obeid M.A., Tate R.J., Mullen A.B., Ferro V.A. Lipid-based nanoparticles for cancer treatment. In: Grumezescu A.M., editor. Lipid Nanocarriers for Drug Targeting. William Andrew Publishing; Norwich, NY, USA: 2018. pp. 313–359. [Google Scholar]
- 88.Yingchoncharoen P., Kalinowski D.S., Richardson D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is yet to Come. Pharmacol. Rev. 2016;68:701. doi: 10.1124/pr.115.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al Saqr A., Aldawsari M.F., Alrbyawi H., Poudel I., Annaji M., Mulabagal V., Ramani M.V., Gottumukkala S., Tiwari A.K., Dhanasekaran M., et al. Co-Delivery of Hispolon and Doxorubicin Liposomes Improves Efficacy against Melanoma Cells. AAPS PharmSciTech. 2020;21:304. doi: 10.1208/s12249-020-01846-2. [DOI] [PubMed] [Google Scholar]
- 90.Fandzloch M., Jaromin A., Zaremba-Czogalla M., Wojtczak A., Lewińska A., Sitkowski J., Wiśniewska J., Łakomska I., Gubernator J. Nanoencapsulation of a ruthenium(II) complex with triazolopyrimidine in liposomes as a tool for improving its anticancer activity against melanoma cell lines. Dalton Trans. 2020;49:1207–1219. doi: 10.1039/C9DT03464A. [DOI] [PubMed] [Google Scholar]
- 91.Xu L., Zhang W., Park H.B., Kwak M., Oh J., Lee P.C.W., Jin J.O. Indocyanine green and poly I:C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J. Immunother. Cancer. 2019;7:220. doi: 10.1186/s40425-019-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nave M., Castro R.E., Rodrigues C.M.P., Casini A., Soveral G., Gaspar M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine. 2016;11:1817–1830. doi: 10.2217/nnm-2016-0086. [DOI] [PubMed] [Google Scholar]
- 93.Merino M., Contreras A., Casares N., Troconiz I.F., ten Hagen T.L.M., Berraondo P., Zalba S., Garrido M.J. A new immune-nanoplatform for promoting adaptive antitumor immune response. Nanomed. Nanotechnol. Biol. Med. 2019;17:13–25. doi: 10.1016/j.nano.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 94.Filipczak N., Jaromin A., Piwoni A., Mahmud M., Sarisozen C., Torchilin V., Gubernator J. A Triple Co-Delivery Liposomal Carrier That Enhances Apoptosis via an Intrinsic Pathway in Melanoma Cells. Cancers. 2019;11:1982. doi: 10.3390/cancers11121982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matos C.P., Albino M., Lopes J., Viana A.S., Côrte-Real L., Mendes F., Pessoa J.C., Tomaz A.I., Reis C.P., Gaspar M.M., et al. New iron(III) anti-cancer aminobisphenolate/phenanthroline complexes: Enhancing their therapeutic potential using nanoliposomes. Int. J. Pharm. 2022;623:121925. doi: 10.1016/j.ijpharm.2022.121925. [DOI] [PubMed] [Google Scholar]
- 96.Chen L., Alrbyawi H., Poudel I., Arnold R.D., Babu R.J. Co-delivery of Doxorubicin and Ceramide in a Liposomal Formulation Enhances Cytotoxicity in Murine B16BL6 Melanoma Cell Lines. AAPS PharmSciTech. 2019;20:99. doi: 10.1208/s12249-019-1316-0. [DOI] [PubMed] [Google Scholar]
- 97.Woźniak M., Nowak M., Lazebna A., Więcek K., Jabłońska I., Szpadel K., Grzeszczak A., Gubernator J., Ziółkowski P. The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells. Pharmaceuticals. 2021;14:374. doi: 10.3390/ph14040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pinho J.O., Amaral J.D., Castro R.E., Rodrigues C.M., Casini A., Soveral G., Gaspar M.M. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine. 2019;14:835–850. doi: 10.2217/nnm-2018-0388. [DOI] [PubMed] [Google Scholar]
- 99.Merino M., Lozano T., Casares N., Lana H., Troconiz I.F., ten Hagen T.L.M., Kochan G., Berraondo P., Zalba S., Garrido M.J. Dual activity of PD-L1 targeted Doxorubicin immunoliposomes promoted an enhanced efficacy of the antitumor immune response in melanoma murine model. J. Nanobiotechnol. 2021;19:102. doi: 10.1186/s12951-021-00846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zou L., Ding W., Zhang Y., Cheng S., Li F., Ruan R., Wei P., Qiu B. Peptide-modified vemurafenib-loaded liposomes for targeted inhibition of melanoma via the skin. Biomaterials. 2018;182:1–12. doi: 10.1016/j.biomaterials.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Barati M., Mirzavi F., Nikpoor A.R., Sankian M., Namdar Ahmadabad H., Soleimani A., Mashreghi M., Tavakol Afshar J., Mohammadi M., Jaafari M.R. Enhanced antitumor immune response in melanoma tumor model by anti-PD-1 small interference RNA encapsulated in nanoliposomes. Cancer Gene Ther. 2021;29:814–824. doi: 10.1038/s41417-021-00367-9. [DOI] [PubMed] [Google Scholar]
- 102.Jose A., Labala S., Ninave K.M., Gade S.K., Venuganti V.V.K. Effective Skin Cancer Treatment by Topical Co-delivery of Curcumin and STAT3 siRNA Using Cationic Liposomes. AAPS PharmSciTech. 2018;19:166–175. doi: 10.1208/s12249-017-0833-y. [DOI] [PubMed] [Google Scholar]
- 103.Lin M.W., Huang Y.B., Chen C.L., Wu P.C., Chou C.Y., Wu P.C., Hung S.Y. A formulation study of 5-aminolevulinic encapsulated in DPPC liposomes in melanoma treatment. Int. J. Med. Sci. 2016;13:483–489. doi: 10.7150/ijms.15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al Saqr A., Majrashi M., Alrbyawi H., Govindarajulu M., Fujihashi A., Gottumukkala S., Poudel I., Arnold R.D., Babu R.J., Dhanasekaran M. Elucidating the anti-melanoma effect and mechanisms of Hispolon. Life Sci. 2020;256:117702. doi: 10.1016/j.lfs.2020.117702. [DOI] [PubMed] [Google Scholar]
- 105.Ndagi U., Mhlongo N., Soliman M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Develop. Ther. 2017;11:599–616. doi: 10.2147/DDDT.S119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Riaz M.K., Riaz M.A., Zhang X., Lin C., Wong K.H., Chen X., Zhang G., Lu A., Yang Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018;19:195. doi: 10.3390/ijms19010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gurunathan S., Kang M.H., Qasim M., Kim J.H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018;19:3264. doi: 10.3390/ijms19103264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.García-Pinel B., Porras-Alcalá C., Ortega-Rodríguez A., Sarabia F., Prados J., Melguizo C., López-Romero J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials. 2019;9:638. doi: 10.3390/nano9040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Talegaonkar S., Bhattacharyya A. Potential of Lipid Nanoparticles (SLNs and NLCs) in Enhancing Oral Bioavailability of Drugs with Poor Intestinal Permeability. AAPS PharmSciTech. 2019;20:121. doi: 10.1208/s12249-019-1337-8. [DOI] [PubMed] [Google Scholar]
- 110.Bayón-Cordero L., Alkorta I., Arana L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials. 2019;9:474. doi: 10.3390/nano9030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghasemiyeh P., Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018;13:288. doi: 10.4103/1735-5362.235156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salvi V.R., Pawar P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J. Drug Deliv. Sci. Technol. 2019;51:255–267. doi: 10.1016/j.jddst.2019.02.017. [DOI] [Google Scholar]
- 113.Goto P.L., Siqueira-Moura M.P., Tedesco A.C. Application of aluminum chloride phthalocyanine-loaded solid lipid nanoparticles for photodynamic inactivation of melanoma cells. Int. J. Pharm. 2017;518:228–241. doi: 10.1016/j.ijpharm.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 114.Palliyage G.H., Hussein N., Mimlitz M., Weeder C., Alnasser M.H.A., Singh S., Ekpenyong A., Tiwari A.K., Chauhan H. Novel Curcumin-Resveratrol Solid Nanoparticles Synergistically Inhibit Proliferation of Melanoma Cells. Pharm. Res. 2021;38:851–871. doi: 10.1007/s11095-021-03043-7. [DOI] [PubMed] [Google Scholar]
- 115.Qin W., Quan G., Sun Y., Chen M., Yang P., Feng D., Wen T., Hu X., Pan X., Wu C. Dissolving Microneedles with Spatiotemporally controlled pulsatile release Nanosystem for Synergistic Chemo-photothermal Therapy of Melanoma. Theranostics. 2020;10:8179. doi: 10.7150/thno.44194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clemente N., Ferrara B., Gigliotti C.L., Boggio E., Capucchio M.T., Biasibetti E., Schiffer D., Mellai M., Annovazzi L., Cangemi L., et al. Solid Lipid Nanoparticles Carrying Temozolomide for Melanoma Treatment. Preliminary In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018;19:255. doi: 10.3390/ijms19020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valdes S.A., Alzhrani R.F., Rodriguez A., Lansakara-P D.S.P., Thakkar S.G., Cui Z. A solid lipid nanoparticle formulation of 4-(N)-docosahexaenoyl 2′, 2′-difluorodeoxycytidine with increased solubility, stability, and antitumor activity. Int. J. Pharm. 2019;570:118609. doi: 10.1016/j.ijpharm.2019.118609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tupal A., Sabzichi M., Ramezani F., Kouhsoltani M., Hamishehkar H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 2016;33:372–380. doi: 10.1080/02652048.2016.1200150. [DOI] [PubMed] [Google Scholar]
- 119.Cassano R., Mellace S., Marrelli M., Conforti F., Trombino S. α-Tocopheryl linolenate solid lipid nanoparticles for the encapsulation, protection, and release of the omega-3 polyunsaturated fatty acid: In Vitro anti-melanoma activity evaluation. Colloids Surf. B Biointerfaces. 2017;151:128–133. doi: 10.1016/j.colsurfb.2016.11.043. [DOI] [PubMed] [Google Scholar]
- 120.Athawale R.B., Jain D.S., Singh K.K., Gude R.P. Etoposide loaded solid lipid nanoparticles for curtailing B16F10 melanoma colonization in lung. Biomed. Pharmacother. 2014;68:231–240. doi: 10.1016/j.biopha.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 121.Valizadeh A., Khaleghi A.A., Roozitalab G., Osanloo M. High anticancer efficacy of solid lipid nanoparticles containing Zataria multiflora essential oil against breast cancer and melanoma cell lines. BMC Pharmacol. Toxicol. 2021;22:52. doi: 10.1186/s40360-021-00523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim M.M., Darafsheh A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020;96:280–294. doi: 10.1111/php.13219. [DOI] [PubMed] [Google Scholar]
- 123.Obaid G., Broekgaarden M., Bulin A.L., Huang H.C., Kuriakose J., Liu J., Hasan T. Photonanomedicine: A Convergence of Photodynamic Therapy and Nanotechnology. Nanoscale. 2016;8:12471. doi: 10.1039/C5NR08691D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brevi A., Cogrossi L.L., Grazia G., Masciovecchio D., Impellizzieri D., Lacanfora L., Grioni M., Bellone M. Much More Than IL-17A: Cytokines of the IL-17 Family Between Microbiota and Cancer. Front. Immunol. 2020;11:2914. doi: 10.3389/fimmu.2020.565470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vitiello G.A., Miller G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 2020;217:e20190456. doi: 10.1084/jem.20190456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Q., Peng Z., Shen L., Shen L. Prognostic and Clinicopathological Value of Ki-67 in Melanoma: A Meta-Analysis. Front. Oncol. 2021;11:737760. doi: 10.3389/fonc.2021.737760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tu T.J., Ma M.W., Monni S., Rose A.E., Yee H., Darvishian F., Polsky D., Berman R.S., Shapiro R.L., Pavlick A.C., et al. A High Proliferative Index of Recurrent Melanoma Is Associated with Worse Survival. Oncology. 2011;80:181–187. doi: 10.1159/000328518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li L.T., Jiang G., Chen Q., Zheng J.N. Predic Ki67 is a promising molecular target in the diagnosis of cancer (Review) Mol. Med. Rep. 2015;11:1566–1572. doi: 10.3892/mmr.2014.2914. [DOI] [PubMed] [Google Scholar]
- 129.Subramaniam B., Siddik Z.H., Nagoor N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanopart. Res. 2020;22:141. doi: 10.1007/s11051-020-04848-0. [DOI] [Google Scholar]
- 130.Malta R., Loureiro J.B., Costa P., Sousa E., Pinto M., Saraiva L., Amaral M.H. Development of lipid nanoparticles containing the xanthone LEM2 for topical treatment of melanoma. J. Drug Deliv. Sci. Technol. 2021;61:102226. doi: 10.1016/j.jddst.2020.102226. [DOI] [Google Scholar]
- 131.Liu D., Liu Z., Wang L., Zhang C., Zhang N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B Biointerfaces. 2011;85:262–269. doi: 10.1016/j.colsurfb.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 132.Chen Y., Zhou L., Yuan L., Zhang Z., Liu X., Wu Q. Formulation, characterization, and evaluation of in vitro skin permeation and in vivo pharmacodynamics of surface-charged tripterine-loaded nanostructured lipid carriers. Int. J. Nanomed. 2012;7:3023. doi: 10.2147/IJN.S32476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh P., Singh M., Kanoujia J., Arya M., Saraf S.K., Saraf S.A. Process optimization and photostability of silymarin nanostructured lipid carriers: Effect on UV-irradiated rat skin and SK-MEL 2 cell line. Drug Deliv. Transl. Res. 2016;6:597–609. doi: 10.1007/s13346-016-0317-8. [DOI] [PubMed] [Google Scholar]
- 134.Mohammadian J., Mahmoudi S., Pourmohammad P., Pirouzpanah M., Salehnia F., Maroufi N.F., Samadi N., Sabzichi M. Formulation of Stattic as STAT3 inhibitor in nanostructured lipid carriers (NLCs) enhances efficacy of doxorubicin in melanoma cancer cells. Naunyn-Schmiedebergs Arch. Pharmacol. 2020;393:2315–2323. doi: 10.1007/s00210-020-01942-x. [DOI] [PubMed] [Google Scholar]
- 135.Kamal M.M., Akter S., Lin C.N., Nazzal S. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch. Pharm. Res. 2020;43:371–384. doi: 10.1007/s12272-020-01225-2. [DOI] [PubMed] [Google Scholar]
- 136.Gomes S., Raimundo L., Soares J., Loureiro J.B., Leão M., Ramos H., Monteiro M.N., Lemos A., Moreira J., Pinto M., et al. New inhibitor of the TAp73 interaction with MDM2 and mutant p53 with promising antitumor activity against neuroblastoma. Cancer Lett. 2019;446:90–102. doi: 10.1016/j.canlet.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 137.Martins C., Chauhan V.M., Araújo M., Abouselo A., Barrias C.C., Aylott J.W., Sarmento B. Advanced polymeric nanotechnology to augment therapeutic delivery and disease diagnosis. Nanomedicine. 2020;15:2287–2309. doi: 10.2217/nnm-2020-0145. [DOI] [PubMed] [Google Scholar]
- 138.Banik B.L., Brown J.L. Polymeric Biomaterials in Nanomedicine. In: Kumbar S.G., Laurencin C.T., Deng M., editors. Natural and Synthetic Biomedical Polymers. Elsevier; Amsterdam, The Netherlands: 2014. pp. 387–395. [Google Scholar]
- 139.Banik B.L., Fattahi P., Brown J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8:271–299. doi: 10.1002/wnan.1364. [DOI] [PubMed] [Google Scholar]
- 140.Reis C.P., Neufeld R.J., Ribeiro A.J., Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 141.Pinto Reis C., Neufeld R.J., Veiga F., Ribeiro A.J. Preparation of Drug-Loaded Polymeric Nanoparticles. In: Balogh L.P., editor. Nanomedicine in Cancer. Pan Stanford; Stanford, CA, USA: 2018. pp. 171–214. [Google Scholar]
- 142.Karlsson J., Vaughan H.J., Green J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu. Rev. Chem. Biomol. Eng. 2018;9:105–127. doi: 10.1146/annurev-chembioeng-060817-084055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han J., Zhao D., Li D., Wang X., Jin Z., Zhao K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers. 2018;10:31. doi: 10.3390/polym10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amaral M., Cruz N., Rosa A., Nogueira B., Costa D., Santos F., Brazão M., Policarpo P., Mateus R., Kobozev Y., et al. An update of advanced nanoplatforms for Glioblastoma Multiforme Management. EXCLI J. 2021;20:1544–1570. doi: 10.17179/excli2021-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ferraz L.S., Watashi C.M., Colturato-Kido C., Pelegrino M.T., Paredes-Gamero E.J., Weller R.B., Seabra A.B., Rodrigues T. Antitumor Potential of S-Nitrosothiol-Containing Polymeric Nanoparticles against Melanoma. Mol. Pharm. 2018;15:1160–1168. doi: 10.1021/acs.molpharmaceut.7b01001. [DOI] [PubMed] [Google Scholar]
- 146.Fonseca M., Macedo A.S., Lima S.A.C., Reis S., Soares R., Fonte P. Evaluation of the Antitumour and Antiproliferative Effect of Xanthohumol-Loaded PLGA Nanoparticles on Melanoma. Materials. 2021;14:6421. doi: 10.3390/ma14216421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang B., Liu X., Teng Y., Yu T., Chen J., Hu Y., Liu N., Zhang L., Shen Y. Improving anti-melanoma effect of curcumin by biodegradable nanoparticles. Oncotarget. 2017;8:108624–108642. doi: 10.18632/oncotarget.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baishya R., Nayak D.K., Kumar D., Sinha S., Gupta A., Ganguly S., Debnath M.C. Ursolic Acid Loaded PLGA Nanoparticles: In Vitro and In Vivo Evaluation to Explore Tumor Targeting Ability on B16F10 Melanoma Cell Lines. Pharm. Res. 2016;33:2691–2703. doi: 10.1007/s11095-016-1994-1. [DOI] [PubMed] [Google Scholar]
- 149.Batista F.A., Fontele S.B.C., Santos L.K.B., Alves Filgueiras L., Quaresma Nascimento S., de Castro e Sousa J.M., Gonçalves J.C.R., Mendes A.N. Synthesis, characterization of α-terpineol-loaded PMMA nanoparticles as proposed of therapy for melanoma. Mater. Today Commun. 2020;22:100762. doi: 10.1016/j.mtcomm.2019.100762. [DOI] [Google Scholar]
- 150.Do Reis S.R.R., Helal-Neto E., da Silva de Barros A.O., Pinto S.R., Portilho F.L., de Oliveira Siqueira L.B., Alencar L.M.R., Dahoumane S.A., Alexis F., Ricci-Junior E., et al. Dual Encapsulated Dacarbazine and Zinc Phthalocyanine Polymeric Nanoparticle for Photodynamic Therapy of Melanoma. Pharm. Res. 2021;38:335–346. doi: 10.1007/s11095-021-02999-w. [DOI] [PubMed] [Google Scholar]
- 151.Ledezma D.K., Balakrishnan P.B., Cano-Mejia J., Sweeney E.E., Hadley M., Bollard C.M., Villagra A., Fernandes R. Indocyanine Green-Nexturastat A-PLGA Nanoparticles Combine Photothermal and Epigenetic Therapy for Melanoma. Nanomaterials. 2020;10:161. doi: 10.3390/nano10010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang J., Liu P., Zhang Z., Han J., Yang X., Wang A., Zhang X. Apatinib-loaded nanoparticles inhibit tumor growth and angiogenesis in a model of melanoma. Biochem. Biophys. Res. Commun. 2020;521:296–302. doi: 10.1016/j.bbrc.2019.10.084. [DOI] [PubMed] [Google Scholar]
- 153.Carletto B., Berton J., Ferreira T.N., Dalmolin L.F., Paludo K.S., Mainardes R.M., Farago P.V., Favero G.M. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf. B Biointerfaces. 2016;144:65–72. doi: 10.1016/j.colsurfb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 154.Yoncheva K., Merino M., Shenol A., Daskalov N.T., Petkov P.S., Vayssilov G.N., Garrido M.J. Optimization and in-vitro/in-vivo evaluation of doxorubicin-loaded chitosan-alginate nanoparticles using a melanoma mouse model. Int. J. Pharm. 2019;556:1–8. doi: 10.1016/j.ijpharm.2018.11.070. [DOI] [PubMed] [Google Scholar]
- 155.De Camargo L.E.A., Ludwig D.B., Tominaga T.T., Carletto B., Favero G.M., Mainardes R.M., Khalil N.M. Bovine serum albumin nanoparticles improve the antitumour activity of curcumin in a murine melanoma model. J. Microencapsul. 2018;35:467–474. doi: 10.1080/02652048.2018.1526340. [DOI] [PubMed] [Google Scholar]
- 156.Su Y., Hu J., Huang Z., Huang Y., Peng B., Xie N., Liu H. Paclitaxel-loaded star-shaped copolymer nanoparticles for enhanced malignant melanoma chemotherapy against multidrug resistance. Drug Des. Develop. Ther. 2017;11:659. doi: 10.2147/DDDT.S127328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reis C.P., Gomes A., Rijo P., Candeias S., Pinto P., Baptista M., Martinho N., Ascensão L. Development and evaluation of a novel topical treatment for acne with azelaic acid-loaded nanoparticles. Microsc. Microanal. 2013;19:1141–1150. doi: 10.1017/S1431927613000536. [DOI] [PubMed] [Google Scholar]
- 158.Kapoor D.N., Bhatia A., Kaur R., Sharma R., Kaur G., Dhawan S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015;6:41–58. doi: 10.4155/tde.14.91. [DOI] [PubMed] [Google Scholar]
- 159.Xu P., Liang F. Nanomaterial-Based Tumor Photothermal Immunotherapy. Int. J. Nanomed. 2020;15:9159–9180. doi: 10.2147/IJN.S249252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Da Silva P.B., Machado R.T.A., Pironi A.M., Alves R.C., de Araújo P.R., Dragalzew A.C., Dalberto I., Chorilli M. Recent Advances in the Use of Metallic Nanoparticles with Antitumoral Action—Review. Curr. Med. Chem. 2019;26:2108–2146. doi: 10.2174/0929867325666180214102918. [DOI] [PubMed] [Google Scholar]
- 161.Sharma A., Goyal A.K., Rath G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2018;26:617–632. doi: 10.1080/1061186X.2017.1400553. [DOI] [PubMed] [Google Scholar]
- 162.Sharma H., Mishra P.K., Talegaonkar S., Vaidya B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today. 2015;20:1143–1151. doi: 10.1016/j.drudis.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 163.Attarilar S., Yang J., Ebrahimi M., Wang Q., Liu J., Tang Y., Yang J. The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review From the Biomedical Perspective. Front. Bioeng. Biotechnol. 2020;8:822. doi: 10.3389/fbioe.2020.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Piñón-Segundo E., Mendoza-Muñoz N., Quintanar-Guerrero D. Nanobiomaterials in Clinical Dentistry. Elsevier; Amsterdam, The Netherlands: 2013. Nanoparticles as Dental Drug-Delivery Systems; pp. 475–495. [Google Scholar]
- 165.Indrakumar J., Korrapati P.S. Steering Efficacy of Nano Molybdenum Towards Cancer: Mechanism of Action. Biol. Trace Elem. Res. 2020;194:121–134. doi: 10.1007/s12011-019-01742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ruiz A.L., Garcia C.B., Gallón S.N., Webster T.J. Novel Silver-Platinum Nanoparticles for Anticancer and Antimicrobial Applications. Int. J. Nanomed. 2020;15:169–179. doi: 10.2147/IJN.S176737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yong J.M., Fu L., Tang F., Yu P., Kuchel R.P., Whitelock J.M., Lord M.S. ROS-Mediated Anti-Angiogenic Activity of Cerium Oxide Nanoparticles in Melanoma Cells. ACS Biomater. Sci. Eng. 2022;8:512–525. doi: 10.1021/acsbiomaterials.1c01268. [DOI] [PubMed] [Google Scholar]
- 168.Mukhopadhyay R., Kazi J., Debnath M.C. Synthesis and characterization of copper nanoparticles stabilized with Quisqualis indica extract: Evaluation of its cytotoxicity and apoptosis in B16F10 melanoma cells. Biomed. Pharmacother. 2018;97:1373–1385. doi: 10.1016/j.biopha.2017.10.167. [DOI] [PubMed] [Google Scholar]
- 169.Alarifi S., Ali D., Alkahtani S., Almeer R.S. ROS-Mediated Apoptosis and Genotoxicity Induced by Palladium Nanoparticles in Human Skin Malignant Melanoma Cells. Oxidative Med. Cell. Longev. 2017;2017:8439098. doi: 10.1155/2017/8439098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chakraborty R., Basu T. Metallic copper nanoparticles induce apoptosis in a human skin melanoma A-375 cell line. Nanotechnology. 2017;28:105101. doi: 10.1088/1361-6528/aa57b0. [DOI] [PubMed] [Google Scholar]
- 171.Wang Y., Yang F., Zhang H.X., Zi X.Y., Pan X.H., Chen F., Luo W.D., Li J.X., Zhu H.Y., Hu Y.P. Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria. Cell Death Dis. 2013;4:e783. doi: 10.1038/cddis.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang X., Teodoro J.G., Nadeau J.L. Intratumoral gold-doxorubicin is effective in treating melanoma in mice. Nanomedicine. 2015;11:1365–1375. doi: 10.1016/j.nano.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 173.Nie C., Du P., Zhao H., Xie H., Li Y., Yao L., Shi Y., Hu L., Si S., Zhang M., et al. Ag@TiO2 Nanoprisms with Highly Efficient Near-Infrared Photothermal Conversion for Melanoma Therapy. Chem. Asian J. 2020;15:148–155. doi: 10.1002/asia.201901394. [DOI] [PubMed] [Google Scholar]
- 174.Pandesh S., Haghjooy Javanmard S., Shakeri-Zadeh A., Shokrani P. Targeted Photothermal Therapy of Melanoma in C57BL/6 Mice using Fe3O4@Au Core-shell Nanoparticles and Near-infrared Laser. J. Biomed. Phys. Eng. 2021;11:29. doi: 10.31661/jbpe.v0i0.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arooj S., Nazir S., Nadhman A., Ahmad N., Muhammad B., Ahmad I., Mazhar K., Abbasi R. Novel ZnO:Ag nanocomposites induce significant oxidative stress in human fibroblast malignant melanoma (Ht144) cells. Beilstein J. Nanotechnol. 2015;6:570. doi: 10.3762/bjnano.6.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aplak E., Von Montfort C., Haasler L., Stucki D., Steckel B., Reichert A.S., Stahl W., Brenneisen P. CNP mediated selective toxicity on melanoma cells is accompanied by mitochondrial dysfunction. PLoS ONE. 2020;15:e0227926. doi: 10.1371/journal.pone.0227926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang Y., Zi X.Y., Su J., Zhang H.X., Zhang X.R., Zhu H.Y., Li J.X., Yin M., Yang F., Hu Y.P. Cuprous oxide nanoparticles selectively induce apoptosis of tumor cells. Int. J. Nanomed. 2012;7:2641. doi: 10.2147/IJN.S31133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bagheri S., Yasemi M., Safaie-Qamsari E., Rashidiani J., Abkar M., Hassani M., Mirhosseini S.A., Kooshki H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018;46:462–471. doi: 10.1080/21691401.2018.1430585. [DOI] [PubMed] [Google Scholar]
- 179.Silva C.O., Rijo P., Molpeceres J., Ascensão L., Roberto A., Fernandes A.S., Gomes R., Coelho J.M.P., Gabriel A., Vieira P., et al. Bioproduction of gold nanoparticles for photothermal therapy. Ther. Deliv. 2016;7:287–304. doi: 10.4155/tde-2015-0011. [DOI] [PubMed] [Google Scholar]
- 180.Silva C.O., Petersen S.B., Reis C.P., Rijo P., Molpeceres J., Fernandes A.S., Gonçalves O., Gomes A.C., Correia I., Vorum H., et al. EGF functionalized polymer-coated gold nanoparticles promote EGF photostability and EGFR internalization for photothermal therapy. PLoS ONE. 2016;11:e0165419. doi: 10.1371/journal.pone.0165419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cao Y., Dhahad H.A., El-Shorbagy M.A., Alijani H.Q., Zakeri M., Heydari A., Bahonar E., Slouf M., Khatami M., Naderifar M., et al. Green synthesis of bimetallic ZnO-CuO nanoparticles and their cytotoxicity properties. Sci. Rep. 2021;11:23479. doi: 10.1038/s41598-021-02937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang X., Chibli H., Mielke R., Nadeau J. Ultrasmall gold-doxorubicin conjugates rapidly kill apoptosis-resistant cancer cells. Bioconjug. Chem. 2011;22:235–243. doi: 10.1021/bc100374p. [DOI] [PubMed] [Google Scholar]
- 183.Zhang X., Shastry S., Bradforth S.E., Nadeau J.L. Nuclear uptake of ultrasmall gold-doxorubicin conjugates imaged by fluorescence lifetime imaging microscopy (FLIM) and electron microscopy. Nanoscale. 2014;7:240–251. doi: 10.1039/C4NR04707A. [DOI] [PubMed] [Google Scholar]
- 184.Choi Y.H., Han H.K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018;48:43–60. doi: 10.1007/s40005-017-0370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lopes R.M., Gaspar M.M., Pereira J., Eleutério C.V., Carvalheiro M., Almeida A.J., Cruz M.E.M. Liposomes versus Lipid Nanoparticles: Comparative Study of Lipid-Based Systems as Oryzalin Carriers for the Treatment of Leishmaniasis. J. Biomed. Nanotechnol. 2014;10:3647–3657. doi: 10.1166/jbn.2014.1874. [DOI] [PubMed] [Google Scholar]
- 186.Mukherjee P., Khademhosseini A., Seaberg J., Montazerian H., Hossen M.N., Bhattacharya R. Hybrid Nanosystems for Biomedical Applications. ACS Nano. 2021;15:2099–2142. doi: 10.1021/acsnano.0c09382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ferreira Soares D.C., Domingues S.C., Viana D.B., Tebaldi M.L. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother. 2020;131:110695. doi: 10.1016/j.biopha.2020.110695. [DOI] [PubMed] [Google Scholar]
- 188.Park W., Shin H., Choi B., Rhim W.K., Na K., Keun Han D. Advanced hybrid nanomaterials for biomedical applications. Prog. Mater. Sci. 2020;114:100686. doi: 10.1016/j.pmatsci.2020.100686. [DOI] [Google Scholar]
- 189.Vargas-Bernal R. Introductory Chapter: Hybrid Nanomaterials. In: Vargas-Bernal R., He P., Zhang S., editors. Hybrid Nanomaterials—Flexible Electronics Materials. IntechOpen; London, UK: 2020. [Google Scholar]
- 190.Ananikov V.P. Organic-Inorganic Hybrid Nanomaterials. Nanomaterials. 2019;9:1197. doi: 10.3390/nano9091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cruz N., Pinho J.O., Soveral G., Ascensão L., Matela N., Reis C., Gaspar M.M. A Novel Hybrid Nanosystem Integrating Cytotoxic and Magnetic Properties as a Tool to Potentiate Melanoma Therapy. Nanomaterials. 2020;10:693. doi: 10.3390/nano10040693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Singh S.P., Alvi S.B., Pemmaraju D.B., Singh A.D., Manda S.V., Srivastava R., Rengan A.K. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int. J. Biol. Macromol. 2018;110:375–382. doi: 10.1016/j.ijbiomac.2017.11.163. [DOI] [PubMed] [Google Scholar]
- 193.Labala S., Mandapalli P.K., Kurumaddali A., Venuganti V.V.K. Layer-by-layer polymer coated gold nanoparticles for topical delivery of imatinib mesylate to treat melanoma. Mol. Pharm. 2015;12:878–888. doi: 10.1021/mp5007163. [DOI] [PubMed] [Google Scholar]
- 194.Kim D., Amatya R., Hwang S., Lee S., Min K.A., Shin M.C. BSA-Silver Nanoparticles: A Potential Multimodal Therapeutics for Conventional and Photothermal Treatment of Skin Cancer. Pharmaceutics. 2021;13:575. doi: 10.3390/pharmaceutics13040575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee E.H., Lim S.J., Lee M.K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019;224:115143. doi: 10.1016/j.carbpol.2019.115143. [DOI] [PubMed] [Google Scholar]
- 196.Lopes J., Coelho J.M.P., Vieira P.M.C., Viana A.S., Gaspar M.M., Reis C. Preliminary assays towards melanoma cells using phototherapy with gold-based nanomaterials. Nanomaterials. 2020;10:1536. doi: 10.3390/nano10081536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lopes J., Ferreira-Gonçalves T., Figueiredo I.V., Rodrigues C.M.P., Ferreira H., Ferreira D., Viana A.S., Faísca P., Gaspar M.M., Coelho J.M.P., et al. Proof-of-Concept Study of Multifunctional Hybrid Nanoparticle System Combined with NIR Laser Irradiation for the Treatment of Melanoma. Biomolecules. 2021;11:511. doi: 10.3390/biom11040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang Q., Zhang L., Li Z., Xie X., Gao X., Xu X. Inducing Controlled Release and Increased Tumor-Targeted Delivery of Chlorambucil via Albumin/Liposome Hybrid Nanoparticles. AAPS PharmSciTech. 2017;18:2977–2986. doi: 10.1208/s12249-017-0782-5. [DOI] [PubMed] [Google Scholar]
- 199.Mukherjee S., Kotcherlakota R., Haque S., Bhattacharya D., Kumar J.M., Chakravarty S., Patra C.R. Improved delivery of doxorubicin using rationally designed PEGylated platinum nanoparticles for the treatment of melanoma. Mater. Sci. Eng. 2020;108:110375. doi: 10.1016/j.msec.2019.110375. [DOI] [PubMed] [Google Scholar]
- 200.Shen H., Shi S., Zhang Z., Gong T., Sun X. Coating Solid Lipid Nanoparticles with Hyaluronic Acid Enhances Antitumor Activity against Melanoma Stem-like Cells. Theranostics. 2015;5:755. doi: 10.7150/thno.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shi S., Zhou M., Li X., Hu M., Li C., Li M., Sheng F., Li Z., Wu G., Luo M., et al. Synergistic active targeting of dually integrin αvβ3/CD44-targeted nanoparticles to B16F10 tumors located at different sites of mouse bodies. J. Control. Release. 2016;235:1–13. doi: 10.1016/j.jconrel.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 202.Matias M., Pinho J.O., Penetra M.J., Campos G., Reis C.P., Gaspar M.M. The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval. Cells. 2021;10:3088. doi: 10.3390/cells10113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cockburn I., Long G. The importance of patents to innovation: Updated cross-industry comparisons with biopharmaceuticals. Expert Opin. Ther. Patents. 2015;25:739–742. doi: 10.1517/13543776.2015.1040762. [DOI] [PubMed] [Google Scholar]
- 204.Đorđević S., Gonzalez M.M., Conejos-Sánchez I., Carreira B., Pozzi S., Acúrcio R.C., Satchi-Fainaro R., Florindo H.F., Vicent M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2021;12:500–525. doi: 10.1007/s13346-021-01024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang C., Yan L., Wang X., Zhu S., Chen C., Gu Z., Zhao Y. Progress, challenges, and future of nanomedicine. Nano Today. 2020;35:101008. doi: 10.1016/j.nantod.2020.101008. [DOI] [Google Scholar]
- 206.D’Mello S.R., Cruz C.N., Chen M.L., Kapoor M., Lee S.L., Tyner K.M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017;12:523–529. doi: 10.1038/nnano.2017.67. [DOI] [PubMed] [Google Scholar]
- 207.Halamoda-Kenzaoui B., Baconnier S., Bastogne T., Bazile D., Boisseau P., Borchard G., Borgos S.E., Calzolai L., Cederbrant K., Di Felice G., et al. Bridging communities in the field of nanomedicine. Regul. Toxicol. Pharmacol. 2019;106:187–196. doi: 10.1016/j.yrtph.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sarwal A., Singh G., Singh K., Garg S. Recent Interventions for Nanotechnology Based Drug Products: Insights into the Regulatory Aspects. Curr. Pharm. Des. 2018;24:5219–5228. doi: 10.2174/1381612825666190117094250. [DOI] [PubMed] [Google Scholar]
- 209.Foulkes R., Man E., Thind J., Yeung S., Joy A., Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020;8:4653–4664. doi: 10.1039/D0BM00558D. [DOI] [PubMed] [Google Scholar]
- 210.European Comission Commission Reccomendation of 18 October 2011 on the definition of nanomaterial. Off. J. Eur. Union. 2011;L275:38–40. [Google Scholar]
- 211.European Comission Regulation (EC) No 1907/2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Off. J. Eur. Union. 2006;L396:1–849. [Google Scholar]
- 212.Food and Drud Administration (FDA) Drug Products, Including Biological Products, That Contain Nanomaterials—Guidance for Industry. FDA; Silver Spring, MD, USA: 2017. [Google Scholar]