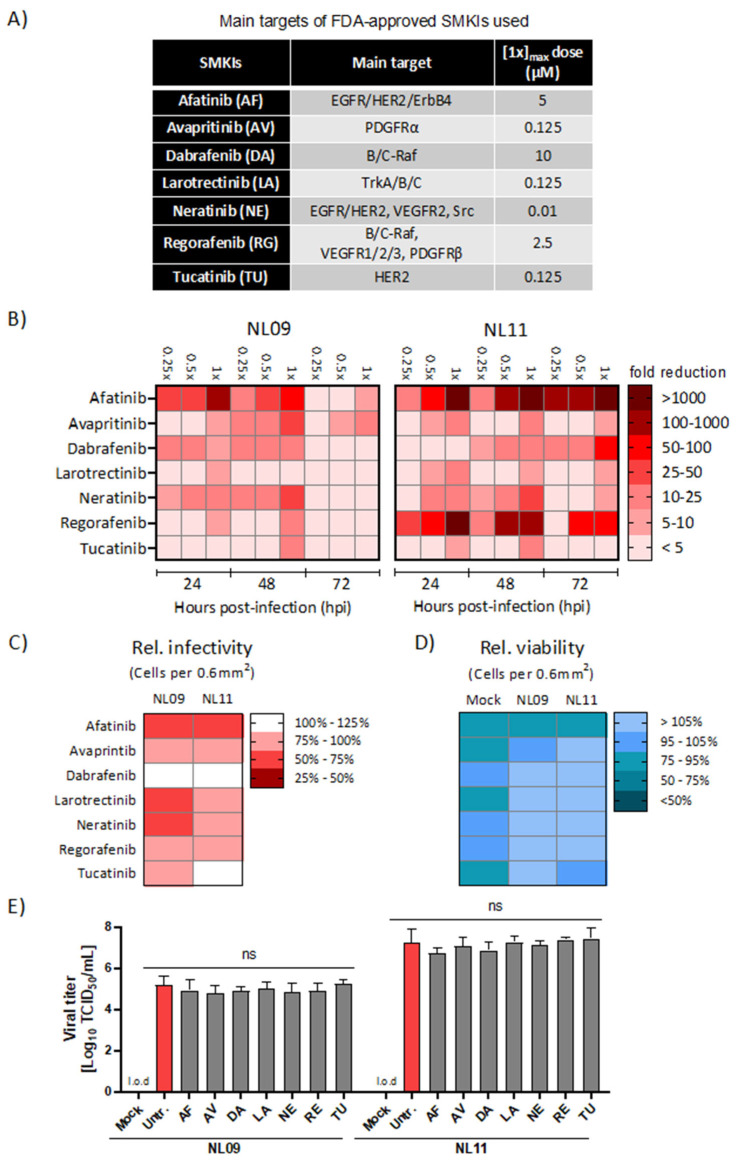
Figure 1.
Effect of SMKI treatment on IAV replication, infectivity and viability. (A) Main targets and applied dosages of FDA-approved SMKIs used in this study. (B) A549 cells were infected with NL09 or NL11 at MOI = 1 +/− the indicated SMKIs at [0.25×, 0.5× or 1×]max concentration up to 72 hpi. Viral titers were quantified by a TCID50/mL assay at 24, 48 and 72 hpi and visualized using a heatmap of the fold-change in viral titers relative to DMSO treatment (n = 4/condition). Additionally, see Figure S2. (C,D) A549 cells were infected with NL09 or NL11 at MOI = 1 and incubated for 48 h in the presence of SMKIs ([0.5×]max concentration). Fluorescence microscopy images were acquired from cells stained for infected cells by anti-IAV NP antibody (red), and nuclei by using NucBlue Live ReadyProbes (blue). Data visualized in the heatmap are % infectivity (C) and % cell viability (D) relative to untreated infected cells or mock-infected treated cells (n = 4/condition). Additionally, see Figure S3. Images were quantified using ImageJ software. (E) NL09 and NL11 virus stocks were preincubated with the control (DMSO) or the [1×]max concentration of the respective SMKI for 4 h at 37 °C. A549 cells were then infected using a 1:1000 dilution. Viral titers were determined at 72 h by a TCID50/mL assay (n = 3). Means ± SDs are shown. l.o.d.: limit of detection. ns, not significant. p-values were determined by Welch t-tests compared to untreated cells.