Abstract
Background:
The TOPCAT trial suggested clinical benefits of spironolactone treatment among patients with Heart Failure with Preserved Ejection Fraction (HFpEF) enrolled in the Americas. However, a comprehensive assessment of biologic pathways impacted by spironolactone therapy in HFpEF has not been performed.
Methods:
We conducted aptamer-based proteomic analysis utilizing 5,284 modified aptamers to 4,928 unique proteins on plasma samples from TOPCAT participants from the Americas (n=164 subjects with paired samples at baseline and 1 year) to identify proteins and pathways impacted by spironolactone therapy in HFpEF. Mean percentage change from baseline was calculated for each protein. Additionally, we conducted pathway analysis of proteins altered by spironolactone.
Results:
Spironolactone therapy was associated with proteome-wide significant changes in 7 proteins. Amongst these, caspase recruitment domain-containing protein 18 (CARD18), polycystin 2 (PKD2), and pregnancy-specific glycoprotein 2 (PSG2) were upregulated, whereas hepatic growth factor (HGF), phospholipid-transfer protein (PLTP), insulin growth factor 2 receptor (IGF2R), and switch associated protein 70 (SWP70) were downregulated. CARD18, a caspase-1 inhibitor, was the most up-regulated protein by spironolactone (−0.5% with placebo versus +66.5% with spironolactone, p<0.0001). The top canonical pathways that were significantly associated with spironolactone were apelin signaling, stellate cell activation, glycoprotein 6 signaling, atherosclerosis signaling, liver x receptor activation, and farnesoid x receptor activation. Amongst the top pathways, collagens were a consistent theme that increased in patients receiving placebo but decreased in patients randomized to spironolactone.
Conclusions:
Proteomic analysis in the TOPCAT trial revealed proteins and pathways altered by spironolactone, including the caspase inhibitor CARD18 and multiple pathways that involved collagens. In addition to effects on fibrosis, our studies suggest potential anti-apoptotic effects of spironolactone in HFpEF, a hypothesis that merits further exploration.
Keywords: heart failure, spironolactone, CARD18, HFpEF
Introduction
Heart failure with preserved ejection fraction (HFpEF) is an important cause of cardiovascular morbidity and mortality 1,2, and there is an urgent need for the development of pharmacological interventions that can improve clinical outcomes in HFpEF patients. The utilization of spironolactone in HFpEF is based on the results of TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial), which suggested beneficial effects among patients enrolled in the Americas. Spironolactone is a mineralocorticoid receptor inhibitor that improves diastolic dysfunction in HFpEF 3,4, reduces fibrosis 5–7, and reduces blood pressure 8–12, although the effects on blood pressure do not explain the effects in HFpEF13.
Broad proteomic scans of plasma, such as aptamer-based assays, have been successfully utilized to identify novel biomarkers involved in HF clinical outcomes and to provide a more detailed biological understanding of HF phenotypes 14–17. In addition, proteomic strategies can be utilized to broadly investigate potential mechanisms of drug action, which may contribute to the discovery of downstream pathways or molecules for therapeutic targeting. In the present study, we performed proteomic analysis of samples from the American subset of the TOPCAT Biorepository to further explore and understand pathways altered by spironolactone therapy.
Methods
Study population
Plasma protein quantification was performed from paired baseline and one-year follow-up samples available from the TOPCAT trial 18, which have been previously described. The raw data and analytical methods of this paper are not publicly available for purposes of reproducing the results or replicating the procedures. This data might be available subject to the establishment of appropriate data-sharing agreements and regulatory approvals. The parent TOPCAT trial data are available through the US National Institutes of Health BioLINCC website. From 2006–2012, TOPCAT randomized patients with HFpEF, defined as symptomatic HF with an EF greater than 45%, to either spironolactone or placebo. From HFpEF patients, a subset (n=218, or 6.3%) had available frozen plasma samples for de novo protein quantification along with clinical data, which was obtained through the National Institutes of Health BioLINCC repository. Patients from the American subset with paired samples at baseline and one-year post-randomization (n=164) were included in the present analysis. All study participants provided written informed consent, and all participating institutions received approval from local Institutional Review Boards.
Plasma Protein Quantification
All plasma samples were analyzed using the SomaScan® assay version 4, which is a multiplexed, modified aptamer-based binding assay. The SomaScan® assay utilizes Slow-Off-rate Modified Aptamer (SOMAmer) reagents, which are chemically modified nucleotides, to bind and quantify target proteins in relative fluorescent units directly proportional to the amount of target protein in the sample. Assay details have been previously described 19. This assay includes 5,284 modified aptamer reagents to 4,928 unique protein targets. A Detailed description of the assay methodology is provided in the supplementary material.
Statistical analysis
Participant characteristics were summarized using mean (SD) for continuous variables with symmetric distribution and median (interquartile range) for continuous variables with a skewed distribution. Categorical variables are expressed as counts (percentages). Analysis of variance (ANOVA) was used to compare normally-distributed continuous variables, whereas the Kruskal-Wallis test was used for non-normally distributed variables, and the chi-square or Fisher’s exact test, as appropriate, were used for categorical data.
The percentage change from baseline was computed for each protein and each participant, and these changes were compared between the 2 treatment arms. We implemented alpha correction for multiple comparisons based on the number of principal components underlying the variability of all measured proteins, as previously described 20,21. There were 111 principal components corresponding to a nominal p value threshold of 0.00045. This method avoids alpha error overcorrection that may occur with the Bonferroni method due to the intrinsic correlation structure between proteins. Differences between the treatment arms were considered statistically significant if the multiplicity corrected p-value for the comparison was <0.05. All probability values presented are 2-tailed. Analyses were performed using the MATLAB statistics and machine learning toolbox (Matlab 2016b, the Mathworks; Natwick, MA).
Pathway analyses
Between-arm differences in plasma proteins between the baseline and 1-year samples were utilized to perform pathway analyses, using Ingenuity® Pathway Analysis (IPA) software (Qiagen; Hilden, Germany; www.qiagen.com/ingenuity) 22–24. Proteins were identified according to their unique protein Identifier (UniProt ID) annotation. The totality of proteins included in the SomaScan® assay was used as the reference set and both direct and indirect experimentally confirmed relationships from all species were included. The ‘Core analysis’ module in IPA was used to perform pathway analysis on the differentially expressed proteins. This analysis identifies specific canonical pathways in which the changes induced by spironolactone are significantly overrepresented.
Results
Study population
The baseline characteristics of patients with available paired samples for analysis vs those who were not represented in the biorepository are shown in Table 1. Compared to the overall TOPCAT population, our subset (164 patients) was older (median [IQR]=73.5 [66,80] vs 69 [61,76]; p<0.0001), more frequently male (57% vs 48%; p=0.02), exhibited a higher BMI (median [IQR] = 32.7 [28.1,36.8] vs 30.8 [27.1,35.7] kg/m2; p=0.01), and was more likely to report a history of smoking (60% vs 40%; p<0.0001). Our subset also exhibited a higher prevalence of diabetes (45% vs 31%; p=0.0004) and atrial fibrillation (53% vs 34%; p<0.0001), as well as higher rates of percutaneous coronary interventions (25% vs 14%; p<0.0001) and coronary artery bypass grafts (27% vs 12%; p<0.0001). Beta-blocker (85% vs 77%; p=0.009) and statin (76% vs 51%, p<0.0001) use was more prevalent, whereas ACEI/ARB (76% vs 84%, p=0.003) use was less prevalent. Baseline characteristics of patients in our subset randomized to spironolactone vs placebo are shown in Table 2. These groups were similar with respect to most clinical characteristics, although more patients in the spironolactone group required insulin therapy at baseline (13.1% vs 26.2% in the placebo vs spironolactone group, respectively, p=0.0478).
Table 1.
General Characteristics of American TOPCAT trial participants included vs. not included in this analysis.
| TOPCAT America Participants not included in this analysis. (n=1601) Median (IQR), Mean (SD), or n (%) |
TOPCAT America Participants included in this analysis. (n=164) Median (IQR), Mean (SD), or n (%) |
P value | |
|---|---|---|---|
| Age | 72 (64,79) | 73.5 (66,80) | 0.12 |
| Male sex | 789 (49%) | 94 (57%) | 0.05 |
| Body Mass Index (BMI) | 32.9 (27.9,38.6) | 32.7 (28.1,36.8) | 0.42 |
| Race | 0.04 | ||
| White | 1241 (77%) | 141 (85%) | |
| Black | 283 (17%) | 19 (11%) | |
| Asian | 77 (5%) | 4 (2%) | |
| LVEF | 58 (52,64) | 60 (53.5,65) | 0.35 |
| Smoking History | 802 (54%) | 96 (61%) | 0.09 |
| Myocardial infarction | 321 (20%) | 38 (23%) | 0.34 |
| Stroke | 147 (9%) | 11 (6%) | 0.28 |
| Coronary artery bypass graft (CABG) | 291 (18%) | 45 (27%) | 0.004 |
| Percutaneous coronary intervention (PCI) | 303 (19%) | 41 (25%) | 0.06 |
| Chronic obstructive pulmonary disease (COPD) | 274 (17%) | 17 (10%) | 0.02 |
| Hypertension (HTN) | 1432 (90%) | 155 (95%) | 0.04 |
| Atrial fibrillation (AF) | 655 (41%) | 87 (53%) | 0.002 |
| Diabetes Mellitus (DM) | 714 (45%) | 74 (45%) | 0.9 |
| Glomerular filtration rate (GFR) | 60.6 (48.8,77) | 63.1 (51.7,75.6) | 0.43 |
| Hematocrit (HCT) | 38.5 (35.4,41.9) | 39 (35.9,41.7) | 0.65 |
| B-natriuretic peptide (BNP) | 396 (188,794) | 489 (190,1068) | 0.13 |
| Systolic blood pressure (SBP) | 130 (118,139) | 124 (118,136) | 0.01 |
| Diastolic blood pressure (DBP) | 70 (62,80) | 70 (61,76.5) | 0.01 |
| Insulin | 347 (22%) | 32 (20%) | 0.52 |
| Beta blocker (BBs) | 1246 (78%) | 141 (86%) | 0.01 |
| Calcium channel blocker (CCBs) | 612 (38%) | 69 (42%) | 0.33 |
| Angiotensinogen converting enzyme inhibitor/Angiotensin receptor blocker (ACE/ARBs) | 1269 (80%) | 125 (76%) | 0.35 |
| Aspirin | 927 (58%) | 100 (61%) | 0.45 |
| Statins | 1022 (64%) | 126 (77%) | 0.0009 |
Table 2.
Baseline Characteristics of included participants randomized to placebo or spironolactone.
| Placebo (80) Mean (SD) or n (%) |
Spironolactone (84) Mean (SD) or n (%) |
P value | |
|---|---|---|---|
| Age | 72.4(9.5) | 71.6(9.4) | 0.57 |
| Male sex | 47(56%) | 47(59%) | 0.75 |
| Body Mass Index (BMI) | 32.3(6.7) | 32.6(6.8) | 0.71 |
| Race | 0.43 | ||
| White | 71(85%) | 70(88%) | |
| Black | 10(12%) | 9(11%) | |
| Other | 3(4%) | 1(1%) | |
| Smoking History | 48(59%) | 48(62%) | 0.74 |
| Myocardial infarction | 22(26%) | 16(20%) | 0.36 |
| Stroke | 3(4%) | 8(10%) | 0.12 |
| Coronary artery bypass graft (CABG) | 24(29%) | 21(26%) | 0.86 |
| Percutaneous coronary intervention (PCI) | 20(24%) | 21(26%) | 0.72 |
| Chronic obstructive pulmonary disease (COPD) | 10(12%) | 7(9%) | 0.61 |
| Hypertension (HTN) | 80(95%) | 75(94%) | 0.74 |
| Atrial fibrillation (AF) | 47(56%) | 40(50%) | 0.53 |
| Diabetes Mellitus (DM) | 35(42%) | 39(49%) | 0.43 |
| Glomerular filtration rate (GFR) | 66.4(19) | 62.3(19) | 0.16 |
| Hematocrit (HCT) | 39(4.3) | 38.6(4.3) | 0.55 |
| B-natriuretic peptide (BNP) | 482.7(659.3) | 498.9(665.1) | 0.85 |
| Systolic blood pressure (SBP) | 124.3(14.2) | 124.8(14.2) | 0.82 |
| Diastolic blood pressure (DBP) | 69.3(10.8) | 68.8(10.8) | 0.77 |
| Insulin | 11(13%) | 21(26%) | 0.04 |
| Beta blocker (BBs) | 74(88%) | 67(84%) | 0.5 |
| Calcium channel blocker (CCBs) | 33(39%) | 36(45%) | 0.52 |
| Angiotensinogen converting enzyme inhibitor/Angiotensin receptor blocker (ACE/ARBs) | 66(79%) | 59(74%) | 0.58 |
| Aspirin | 51(61%) | 49(61%) | 1 |
| Statins | 67(80%) | 59(74%) | 0.45 |
Changes in the plasma proteome associated with spironolactone therapy.
We identified significant changes in 7 proteins between the placebo and spironolactone groups, shown in Figure 1. Baseline levels of these 7 proteins did not differ and are presented in Table S1. Spironolactone induced upregulation of caspase recruitment domain-containing protein 18 (CARD18: −0.5 vs +66.5 % change in placebo and spironolactone groups, respectively; corrected p<0.0001), polycystin 2 (PKD2; −8 vs +20 %, corrected p=0.001), and pregnancy specific glycoprotein 2 (PSG2; −11.4 vs +13.8 %, corrected p=0.02), as well as downregulation of hepatic growth factor (HGF; +6.6 vs −5 %, corrected p=0.009), phospholipid transfer protein (PLTP; +5.6 vs −4.7 %, corrected p=0.01), switch associated protein 70 (SWP70; +4.5 vs −4.7 %, corrected p=0.04), and insulin growth factor 2 receptor (IGF2R; +0.3 vs −8.2 %, corrected p=0.03). Genetic validation of aptamer specificity for these proteins is provided in the supplement. The CARD18 aptamer specificity is demonstrated by Western blotting (Supplementary Figure 1).
Figure 1. Mean percent change between placebo vs spironolactone group for proteins that demonstrated multiplicity corrected p-value <0.05.
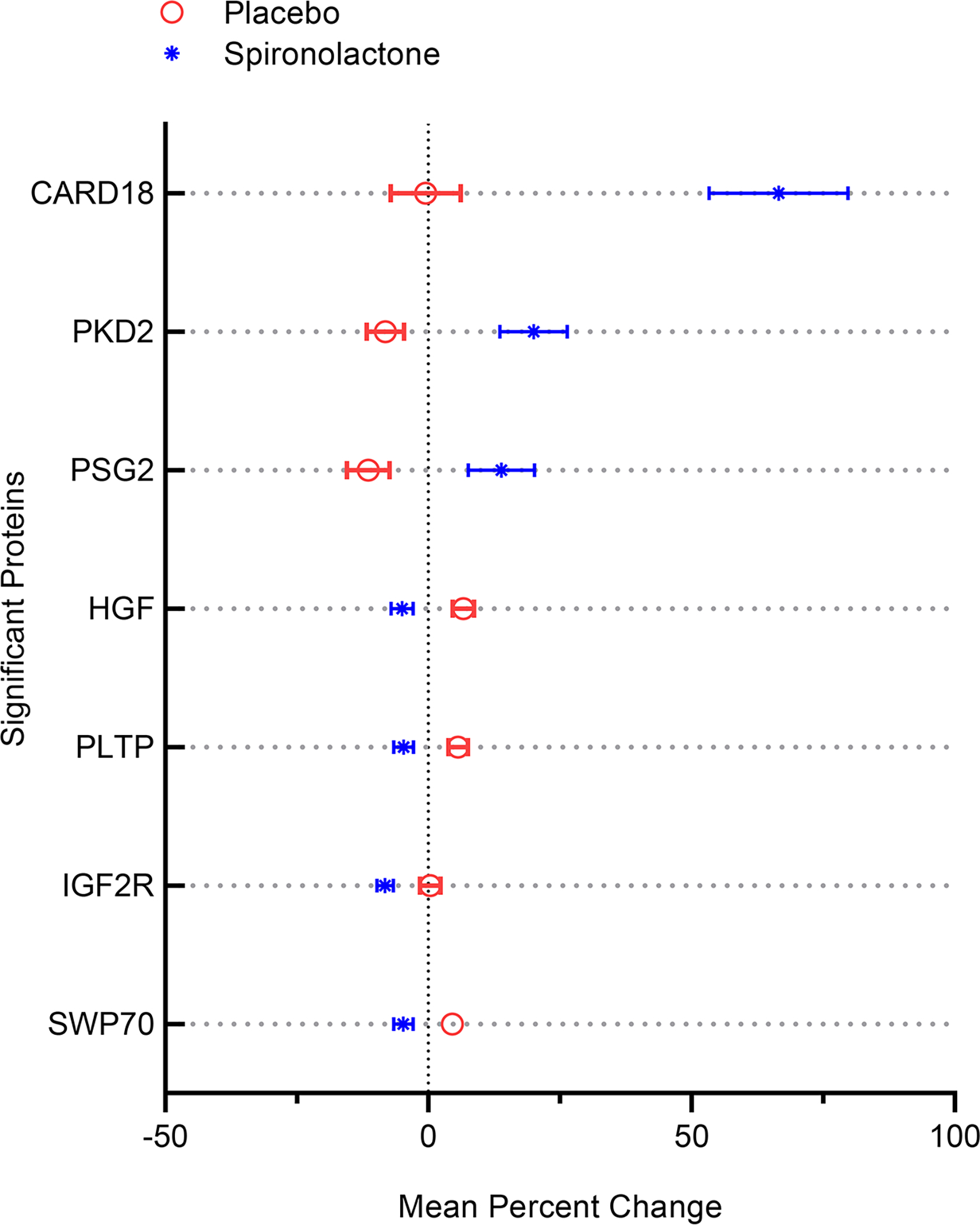
Data shown include mean percentage change +/− SEM in the placebo arm (red) vs spironolactone arm (blue). CARD18: Caspase Associated Recruitment Domain 18. PKD2: Polycystin-2/Polycystic Kidney Disease 2. PSG2: Pregnancy Specific Beta-1-Glycoprotein 2. HGF: Hepatocyte Growth Factor. PLTP: Phospholipid Transfer Protein. IGF2R: Insulin-like Growth Factor 2 Receptor. SWP70: Switch-Associated Protein 70.
Additionally, because spironolactone is known to affect renal function, we performed a secondary analysis after adjustment for changes in cystatin C and found no changes in the levels of the seven significant proteins compared to our primary analysis (Table S2).
Pathways associated with spironolactone therapy.
Pathway analysis revealed 6 pathways that were differentially expressed between the spironolactone arm and the control arm. These significant canonical pathways were apelin liver signaling (p=0.00006), stellate cell activation (p=0.0003), Glycoprotein 6 (GP6) signaling (p=0.005), atherosclerosis signaling (p=0.008), LXR/RXR activation (p=0.008), and FXR/RXR activation (p=0.02) (Figure 2A). For the proteins in each significant pathway, heatmaps (Figures 2B–G) highlight individual protein changes in spironolactone vs placebo arms over the follow-up period. Notably, the top 4 canonical pathways were enriched for multiple collagens that increased in the placebo group but decreased with spironolactone.
Figure 2. Pathway analysis stratified by arm.
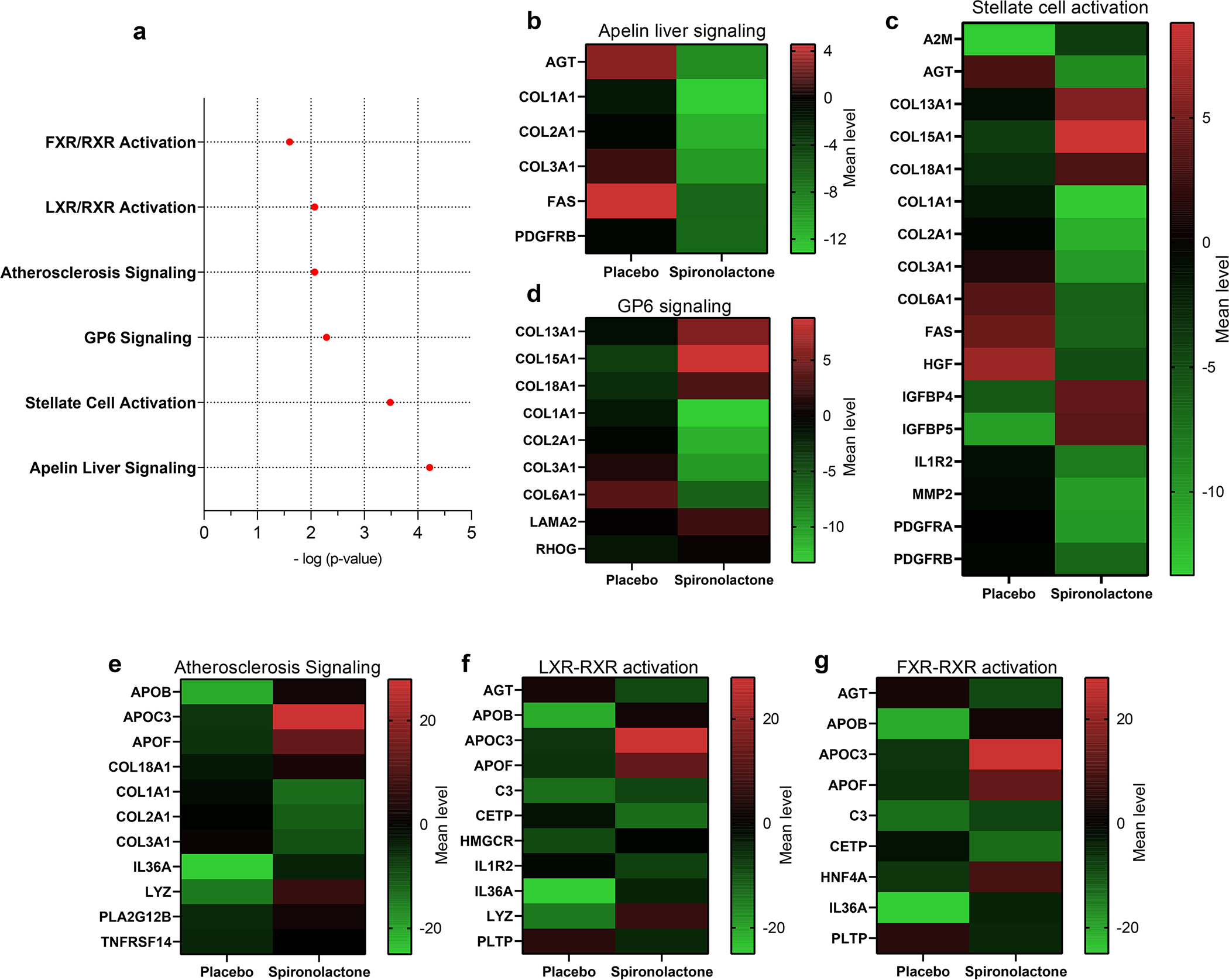
A) Pathways that demonstrated interaction p-value < 0.05 with randomization to spironolactone arm. B-G) Heatmaps of the mean percent change for proteins involved in the pathways identified in (A) including B) apelin liver signaling pathway, C) stellate cell activation, (D) GP6 signaling, E) atherosclerosis signaling, F) liver X Receptor-Retinoid X Receptor (LXR/RXR), and G) farnesoid X Receptor-Retinoid X Receptor (FXR/RXR). A2M: alpha 2 microglobulin, AGT: Angiotensin, ApoB: Apolipoprotein B, ApoC3: Apolipoprotein C3, ApoF: Apolipoprotein F, C3: Complement protein 3, CETP: Cholesterol ester transfer protein, COL: Collagen, FAS: Fas death receptor, GP6 Signaling: Glycoprotein 6 signaling, HGF: Hepatocyte growth factor, HMGCR: Hydroxy-3-Methylglutaryl-CoA Reductase, HNF4A: Hepatocyte Nuclear Factor 4 Alpha, IGFBP: Insulin-like growth factors binding protein, IL1R2: interleukin 1 receptor type 2, IL36A: Interleukin 36 alpha, LAMA2: Laminin subunit alpha-2, LYZ: Lysozyme, MMP2: Matrix metalloproteinase 2, PDGFRA: Platelet derived growth factor receptor alpha, PDGFRB: Platelet derived growth factor receptor beta, PLA2G12B: Phospholipase A2 Group 12 B, PLTP: Phospholipid transfer protein, RHOG: Ras Homolog Family Member G, TNRFSF14: TNF Receptor Superfamily Member 14.
Discussion
We report for the first time a comprehensive proteomic analysis of the effect of spironolactone therapy in the TOPCAT trial. We identified previously unknown proteins and pathways altered by spironolactone in HFpEF, including proteome-wide significant changes in CARD18, PKD2, PSG2, HGF, PLTP, IGF2R, and SWP70. These changes, along with corresponding pathway analyses, indicate the effects of spironolactone on caspase signaling, fibrosis, growth factors, and lipoprotein biology (Figure 3).
Figure 3. Plasma proteins altered by spironolactone in TOPCAT:
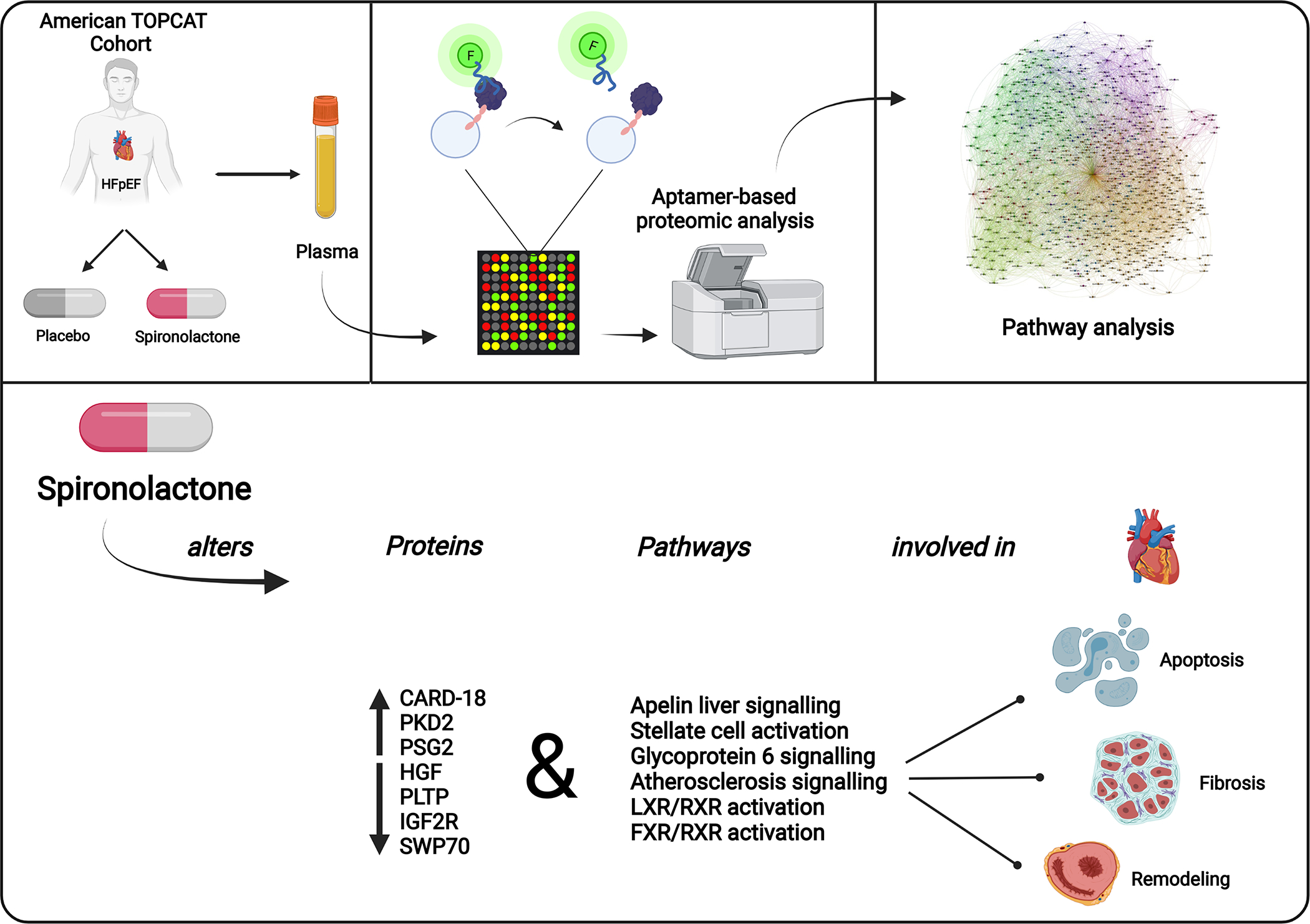
In HFpEF patients, spironolactone alters proteins and pathways involved in myocardial apoptosis, fibrosis, and remodeling. CARD18: Caspase Associated Recruitment Domain 18. FXR/RXR: Farnesoid X Receptor-Retinoid X Receptor. LXR/RXR: Liver X Receptor-Retinoid X Receptor. PKD2: Polycystin-2/Polycystic Kidney Disease 2. PSG2: Pregnancy Specific Beta-1-Glycoprotein 2. HGF: Hepatocyte Growth Factor. PLTP: Phospholipid Transfer Protein. IGF2R: Insulin-like Growth Factor 2 Receptor. SWP70: Switch-Associated Protein 70.
In our analysis, the most abundantly, significantly upregulated protein in the spironolactone group was CARD18, also known as iceberg. CARD18 is a small caspase recruitment domain-containing decoy molecule induced by pro-inflammatory stimuli, which inhibits caspase-1 oligomerization and activation and subsequent generation of IL-1β 25–27. Caspase-1 is a pro-apoptotic molecule implicated in cardiomyopathy via its role in Ang II-induced cardiomyocyte hypertrophy and up-regulation of IL-1β 28. Caspase-1 triggers the activation of the NOD-like receptor family pyrin domain containing 3 (NLRP-3) inflammasome, causing pyroptotic cell death of cardiomyocytes, a key process in HF 29. Furthermore, spironolactone reduces levels of caspase-1 and IL-1β in a murine diabetic model 30. Similarly, multiple recent reports have indicated a cardioprotective role of other caspase recruitment domain-containing proteins. For example, the apoptosis repressor with caspase recruitment domain (ARC), is an anti-apoptotic protein that reduces myocardial cell death in response to biomechanical and ischemic stress 31–34. Strikingly, low-dose spironolactone could decrease infarct size and apoptosis in the reperfused myocardium of rats by preventing the degradation of ARC 35. In the context of the present work, larger studies should determine whether changes in CARD18 are causally involved in the therapeutic response to spironolactone or represent a biomarker of its efficacy, rather than representing a less relevant downstream effect of the drug.
Spironolactone randomized therapy, compared with placebo, was also associated with significant increases in the protein PKD2. PKD2, or polycystin 2, is commonly known as the one of two most commonly mutated genes in autosomal dominant polycystic kidney disease (ADPKD) 36. In a randomized trial of patients with ADPKD, spironolactone therapy reduced blood pressure without affecting markers of endothelial dysfunction 37. In the myocardium, PKD2 regulates cardiac diastolic function via its interaction with Ryanodine Receptor 2, which is involved in calcium handling 38. PKD2 loss-of-function mutations impair diastolic function and predispose to HF 39. Similarly, in PKD−/− mice, the release of natriuretic peptides (e.g., BNP) is significantly reduced compared to controls in response to β adrenergic stress 40. However, it remains unclear whether effects on PKD2 are involved in the therapeutic response to spironolactone in HFpEF. Further mechanistic studies are needed to explore whether positive clinical effects of spironolactone in HFpEF might be, at least in part, mediated by PKD2.
A third novel finding of our study is that PLTP, a protein important for transferring phospholipids and free cholesterol from triglyceride-rich lipoproteins into HDL, was significantly downregulated in the spironolactone group. Prior studies support a potential mechanistic role for PLTP in cardiac dysfunction. High PLTP is a strong positive predictor of coronary artery disease (CAD) 41, a very common cause of HF. However, a direct mechanistic role for PLTP on HF is also possible. High PLTP activity is positively associated with LV dysfunction independent of PLTP effect on CAD 42,43. Moreover, PLTP is linked to increased insulin resistance 44–47 and the development of diabetes 48 which can also contribute to ventricular dysfunction. Finally, PLTP has also been associated with inflammation. PLTP deficient mice fed a high-fat diet had reduced IL-6 compared to controls 49, while IL-6 dependent-induction of TNFα required PLTP 50. Moreover, PLTP deficient mice exhibited reduced ability of LDL to induce monocyte chemotactic activity and improved the anti-inflammatory activity of HDL 51. PLTP also impairs the reverse cholesterol transport process, as increased systemic PLTP activity decreased cholesterol efflux and from macrophages 52. Given the possible roles for PLTP beyond atherosclerosis and CAD, our finding that spironolactone therapy is associated with a reduction in PLTP protein levels also merits further exploration. In particular, determining whether spironolactone might significantly alter PLTP activity in addition to PLTP protein levels will be a critical step forward.
Another protein downregulated by spironolactone and previously implicated in HF survival is HGF, or hepatocyte growth factor. In two cohorts of patients, circulating HGF was positively associated with mortality in patients with stable congestive HF 53 and advanced HF 54. Since several murine studies indicate protective roles for HGF in the acute setting 55,56, HGF might be an important counter-regulatory of the cardiac stress response 57. Given that the absolute percentage changes we observed in HGF were small, it is certainly possible that the changes are reflective of underlying regulatory effects that are only weakly represented by changes in HGF levels.
The last two proteome-wide significant proteins downregulated by spironolactone were IGF2R and SWP70. IGF2R has been implicated in cardiomyocyte hypertrophy, fibrosis, and myocardial remodeling 58–61. Serum IGF2R levels were increased in patients with end-stage heart failure compared to controls 62. These findings, combined with our present data, lead to the hypothesis that spironolactone-induced reductions in IGF2R may be causally involved in its cardioprotective effects in HFpEF. Finally, SWP-70 is a guanine nucleotide exchange factor 63, with no known roles in cardiovascular biology.
Spironolactone is known to affect renal function, changes in which impact the circulating proteome. To investigate whether changes in renal function could be mediating the effect of spironolactone on circulating proteins, we adjusted for cystatin C, which is a valid surrogate for glomerular filtration rate 64,65. In these adjusted analyses, the same 7 proteins were significantly associated with randomization to spironolactone, again with CARD18 exhibiting the most clinically and statistically significant change. These data suggest that spironolactone affected circulating CARD18 independent of effects on glomerular filtration.
We utilized pathway analysis to identify six significant pathways altered by spironolactone. One pathway is the apelin signaling pathway. Apelin is an endogenous ligand to the APJ receptor, found in myocardial tissue. Apelin has both a potent inotropic and an arterial vasodilator effect and is highly expressed in the left ventricular tissue of HF patients 66. In a randomized trial, acute administration of apelin in HF patients produced peripheral and coronary vasodilation and improved cardiac output 67. Furthermore, some studies have suggested a role for apelin in attenuating post-infarction remodeling 68,69, possibly by an antioxidant mechanism 70,71. Despite these intriguing observations, our findings only establish a correlation between spironolactone and apelin signaling, without implying that changes in this pathway mediate any of the potential benefits of the drug in HFpEF. The value of apelin as a therapeutic target remains unclear and is the focus of various ongoing studies.
Two additional pathways associated with spironolactone were the liver X receptor (LXR) and the farnesoid X receptor (FXR) signaling pathways. LXR is a receptor expressed in the heart, which is activated after myocardial infarction and associated with protection against myocardial ischemia-reperfusion injury 72,73, as well as protection against pathological myocardial fibrosis and hypertrophy 74,75. On the other hand, FXR, a regulator of apoptosis in cardiomyocytes 76, contributes to myocardial ischemia-reperfusion injury, and its knockout reduces apoptosis, fibrosis, and post-infarction remodeling in mice 77. Evaluation of the individual components of these pathways revealed that multiple collagens changed in each pathway. Fibrillar collagen chains such as COL1A1, COL2A1, and COL3A1 were consistently downregulated with spironolactone in agreement with reports about a potential anti-fibrotic role for spironolactone 78–80. Interestingly, spironolactone also decreases serum markers of collagen synthesis in patients with HFrEF and HFpEF 81,82.
Recently, Ferriera et al. analyzed protein biomarkers in baseline and 9-month samples of patients from the “Heart ‘Omics’ in Aging” HOMAGE trial 83. This trial investigated the effect of spironolactone on cardiovascular function and markers of fibrosis in patients with risk factors for HF (e.g., CAD, hypertension, diabetes).7 Consistent with our findings, Ferriera et al. reported reduced circulating markers of fibrosis and extracellular matrix metabolism in patients treated with spironolactone. In addition, while they reported several changes in markers of inflammation and insulin signaling, they did not observe any effect of spironolactone on apoptosis or apelin signaling. Differences between the present study and the work of Ferriera et al. may be related to different study populations and divergent proteomic strategies. Ferriera et al. investigated plasma from patients who had risk of developing heart failure in contrast to our population with established HFpEF. Moreover, they utilized a targeted 164 protein Olink Proseek-multiplex® cardiovascular and inflammation assay; in comparison, our aptamer-based proteomic strategy included 4,928 protein targets. The strength of the present approach is that it lends itself to a less biased discovery approach, including unexpected targets such as CARD18, but may increase the risk of type II error due to correct the alpha value for a larger number of comparisons
Our study should be interpreted in the context of its strengths and limitations. Strengths of our study include the relatively unbiased approach to interrogating plasma proteomics, which included ~5000 proteins, the randomized, double-blinded nature of the parent trial, and the highly systematic prospective data collection. Our study also has limitations. Proteins in the SomaScan® platform have been selected based on their previous identification and the ad hoc development of aptamer-based detection for incorporation into the SomaScan®. Given the large number of proteins interrogated, we applied correction for alpha error to minimize false-positive findings, which inevitably leads to a loss in power relative to analyses that utilize nominal statistical significance. In addition, since we are examining circulating factors, we cannot be certain of the tissue of origin of these factors in this clinical context. Whether the observed changes are a direct effect downstream of mineralocorticoid receptor antagonism or due to secondary alterations in the HFpEF phenotype cannot be ascertained by our analysis. It should be noted that plasma levels do not necessarily reflect in vivo compartmentalized activity; this is particularly true for neural activation pathways in which metabolomics or plasma levels of specific neurotransmitters may be more informative than proteomics. Finally, our study was based on a subset of TOPCAT participants with available plasma samples, rather than the TOPCAT population at large. The subsample included in this study exhibited some clinical differences compared to the subsample not included, which limits the generalizability of the findings. This also resulted in a smaller sample size, which does not provide sufficient statistical power to correlate the proteomics changes induced by spironolactone therapy with the risk of subsequent events. As such, our study is unable to establish whether any of the reported changes are actually involved in potential therapeutic or other clinically relevant effects of the drug in HFpEF.
In summary, we present a proteome-wide analysis of the effects of randomized spironolactone therapy in HFpEF and identify various plasma proteins that are impacted by this drug. Spironolactone altered proteins (CARD18, PKD2, PSG2, HGF, PLTP, IGF2R, SWP70) and pathways (apelin liver signaling, stellate cell activation, Glycoprotein 6 (GP6) signaling, atherosclerosis signal, LXR-RXR activation, FXR-RXR activation) are involved in myocardial apoptosis, fibrosis, and remodeling. Whether the effects of spironolactone on these proteins and pathways are mechanistic drivers of its clinical efficacy or side effects, versus representing epiphenomena unrelated to these effects, will need to be examined through additional studies.
Supplementary Material
Clinical Perspective.
What is new?
Spironolactone increases levels of the anti-apoptotic protein (CARD18).
Spironolactone alters multiple pathways including apelin liver signaling, stellate cell activation, Glycoprotein 6 signaling, atherosclerosis signaling, LXR/RXR activation, and FXR/RXR activation).
Proteomics can be utilized to yield unexpected targets or markers of drugs in randomized controlled clinical trials.
What are the clinical implications?
Spironolactone appears to exert broad effects in HFpEF, including alterations in anti-apoptotic pathways and pathways regulating collagens.
Whether these effects are direct consequences of mineralocorticoid antagonism that mediate the effects of spironolactone, or markers of drug efficacy, should be explored in larger studies.
Acknowledgments
This manuscript was prepared using TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial research materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TOPCAT Trial or the National Heart, Lung, and Blood Institute. We appreciate the scientific input and technical support from Robert McDowell, Leon Carayannopoulos, Jean-Francois Tamby, Matt Mealiffe, Maria Borentain, Sheerin Latham, Leonard Adam, Joseph Luettgen, Ellen Kick, Terrye Delmonte, Matt Bunn from Bristol Myers Squibb.
Sources of Funding:
This work was funded by a research grant from Bristol-Myers Squibb (J.A.C.). J.A.C. is also supported by NIH grants R01-HL 121510, U01-TR003734, 3U01TR003734–01W1, U01-HL160277, R33-HL-146390, R01-HL153646, K24-AG070459, R01-AG058969, R01-HL104106, P01-HL094307, R03-HL146874, R56-HL136730, R01 HL155599, R01 HL157264, and 1R01HL153646–01. A.J. is supported by K08 HL138262 and R01HL155344.
Non-standard abbreviations and acronyms:
- HFpEF
Heart failure with preserved ejection fraction
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial
- CARD18
Caspase Associated Recruitment Domain 18
- PKD2
Polycystin-2/Polycystic Kidney Disease 2
- PSG2
Pregnancy Specific Beta-1-Glycoprotein 2
- HGF
Hepatocyte Growth Factor
- PLTP
Phospholipid Transfer Protein
- IGF2R
Insulin-like Growth Factor 2 Receptor
- SWP70
Switch-Associated Protein 70
Footnotes
Disclosures:
JAC has recently consulted for Bayer, Sanifit, Fukuda-Denshi, Bristol-Myers Squibb, JNJ, Edwards Life Sciences, Merck, NGM Biopharmaceuticals and the Galway-Mayo Institute of Technology. He received University of Pennsylvania research grants from National Institutes of Health, Fukuda-Denshi, Bristol-Myers Squibb, Microsoft and Abbott. He is named inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction and a patent application for the use of novel fibrosis biomarkers in HFpEF (not included in this study). He has received payments for editorial roles from the American Heart Association, the American College of Cardiology, Elsevier and Wiley. He has received research device loans from Atcor Medical, Fukuda-Denshi, Uscom, NDD Medical Technologies, Microsoft and MicroVision Medical. A.J. has received funding from AstraZeneca and is co-inventor on a patent utilizing fusion protein nanodiscs for the treatment of heart failure and eye diseases, and is on the scientific advisory board of Mobius, Scientific. AMR has received public contestable grants from Health Research Council (HRC) of New Zealand (NZ), NZ Heart Foundation, Tertiary Education Commission of NZ; the National Medical Council (NMRC) of Singapore, the Agency for Science Technology and Research (ASTaR) Singapore; grants, advisory board fees and/or support in kind from Roche Diagnostics, Abbott Laboratories, Thermo Fisher, AstraZeneca, Novartis, Medtronic, Sphingotec and Critical Diagnostics.
References
- 1.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, Treatments, and Outcomes of Patients With Preserved Systolic Function Hospitalized for Heart Failure. A Report From the OPTIMIZE-HF Registry. J Am Coll Cardiol [Internet]. 2007. [cited 2021 Mar 6];50:768–777. Available from: https://pubmed.ncbi.nlm.nih.gov/17707182/ [DOI] [PubMed] [Google Scholar]
- 2.Bhatia RS, Tu J v., Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of Heart Failure with Preserved Ejection Fraction in a Population-Based Study. New England Journal of Medicine [Internet]. 2006. [cited 2021 Mar 6];355:260–269. Available from: https://pubmed.ncbi.nlm.nih.gov/16855266/ [DOI] [PubMed] [Google Scholar]
- 3.Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation [Internet]. 2004. [cited 2021 Mar 6];110:558–565. Available from: https://pubmed.ncbi.nlm.nih.gov/15277317/ [DOI] [PubMed] [Google Scholar]
- 4.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA - Journal of the American Medical Association [Internet]. 2013. [cited 2021 Mar 6];309:781–791. Available from: https://pubmed.ncbi.nlm.nih.gov/23443441/ [DOI] [PubMed] [Google Scholar]
- 5.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, O’Moore-Sullivan T, Marwick TH. A randomized study of the beneficial effects of aldosterone antagonism on lv function, structure, and fibrosis markers in metabolic syndrome. JACC: Cardiovascular Imaging. 2011;4:1239–1249. [DOI] [PubMed] [Google Scholar]
- 6.Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: A pilot study. Circulation [Internet]. 2005. [cited 2021 Mar 29];112:2940–2945. Available from: https://pubmed.ncbi.nlm.nih.gov/16275882/ [DOI] [PubMed] [Google Scholar]
- 7.Cleland JGF, Ferreira JP, Mariottoni B, Pellicori P, Cuthbert J, Verdonschot JAJ, Petutschnigg J, Ahmed FZ, Cosmi F, Brunner La Rocca H-P, et al. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart ‘OMics’ in AGEing (HOMAGE) randomized clinical trial. European Heart Journal [Internet]. 2021. [cited 2021 Mar 29];42:684–696. Available from: https://academic.oup.com/eurheartj/article/42/6/684/5993916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman N, Dobson J, Wilson S, Dahlöf B, Sever PS, Wedel H, Poulter NR. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension [Internet]. 2007. [cited 2021 Mar 29];49:839–845. Available from: http://www.hypertensionaha.org [DOI] [PubMed] [Google Scholar]
- 9.Lim PO, Jung RT, MacDonald TM. Raised aldosterone to renin ratio predicts antihypertensive efficacy of spironolactone: A prospective cohort follow-up study. British Journal of Clinical Pharmacology [Internet]. 1999. [cited 2021 Mar 29];48:756–760. Available from: https://pubmed.ncbi.nlm.nih.gov/10594479/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeunemaitre X, Chatellier G, Kreft-Jais C, Charru A, Devries C, Plouin PF, Corvol P, Menard J. Efficacy and tolerance of spironolactone in essential hypertension. The American Journal of Cardiology [Internet]. 1987. [cited 2021 Mar 29];60:820–825. Available from: https://pubmed.ncbi.nlm.nih.gov/3661395/ [DOI] [PubMed] [Google Scholar]
- 11.Johnston LC, Grieble HG. Treatment of arterial hypertensive disease with diuretics. V. Spironolactone, an aldosterone antagonist – Arch Int Med [Internet]. [cited 2021 Mar 29]; 1967. Mar;119(3):225–31. Available from: https://pubmed.ncbi.nlm.nih.gov/6020067/ [PubMed] [Google Scholar]
- 12.Wolf RL, Mendlowitz M, Roboz J, Styan GP, Kornfeld P, Weigl A. Treatment of hypertension with spironolactone. JAMA [Internet]. [cited 2021 Mar 29]; 1966. Dec 12;198(11):1143–9. Available from: https://pubmed.ncbi.nlm.nih.gov/5332539/ [PubMed] [Google Scholar]
- 13.Selvaraj S, Claggett B, Shah SJ, Anand I, Rouleau JL, Desai AS, Lewis EF, Pitt B, Sweitzer NK, Pfeffer MA, et al. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: an analysis of the TOPCAT trial. European Journal of Heart Failure [Internet]. 2018. [cited 2021 Mar 29];20:483–490. Available from: https://pubmed.ncbi.nlm.nih.gov/29148144/ [DOI] [PubMed] [Google Scholar]
- 14.Chirinos JA, Cohen JB, Zhao L, Hanff T, Sweitzer N, Fang J, Corrales-Medina V, Anmar R, Morley M, Zamani P, et al. Clinical and proteomic correlates of plasma ACE2 (angiotensin-converting enzyme 2) in human heart failure. Hypertension [Internet]. 2020. [cited 2021 May 26];1526–1536. Available from: 10.1161/HYPERTENSIONAHA.120.15829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamo L, Yu J, Rocha-Resende C, Javaheri A, Head RD, Mann DL. Proteomic Signatures of Heart Failure in Relation to Left Ventricular Ejection Fraction. J Am Coll Cardiol. 2020;76:1982–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gui H, She R, Luzum J, Li J, Bryson TD, Pinto Y, Sabbah HN, Williams LK, Lanfear DE. Plasma Proteomic Profile Predicts Survival in Heart Failure with Reduced Ejection Fraction. Circ Genom Precis Med [Internet]. 2021 [cited 2021 May 26]; 2021. Jun;14(3):e003140. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33999650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan MY, Efthymios M, Tan SH, Pickering JW, Troughton R, Pemberton C, Ho H-H, Prabath J-F, Drum CL, Ling LH, et al. Prioritizing Candidates of Post–Myocardial Infarction Heart Failure Using Plasma Proteomics and Single-Cell Transcriptomics. Circulation [Internet]. 2020. [cited 2021 Oct 19];1408–1421. Available from: 10.1161/CIRCULATIONAHA.119.045158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 19.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, Carter J, Dalby AB, Eaton BE, Fitzwater T, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE. 2010. Dec 7;5(12):e15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auro K, Joensuu A, Fischer K, Kettunen J, Salo P, Mattsson H, Niironen M, Kaprio J, Eriksson JG, Lehtimäki T, et al. A metabolic view on menopause and ageing. Nature Communications 2014 5:1 [Internet]. 2014. [cited 2021 Jul 5];5:1–11. Available from: https://www.nature.com/articles/ncomms5708 [DOI] [PubMed] [Google Scholar]
- 21.Tromp J, Khan MAF, Klip IjT, Meyer S, Boer RA de, Jaarsma T, Hillege H, Veldhuisen DJ van, Meer P van der, Voors AA. Biomarker Profiles in Heart Failure Patients With Preserved and Reduced Ejection Fraction. J Am Heart Assoc [Internet]. 2017. [cited 2021 Oct 17];6. Available from: https://www.ahajournals.org/doi/abs/10.1161/JAHA.116.003989 [DOI] [PMC free article] [PubMed]
- 22.Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, Burgess S, Jiang T, Paige E, Surendran P, et al. Genomic atlas of the human plasma proteome. Nature 2018 558:7708 [Internet]. 2018. [cited 2021 Oct 17];558:73–79. Available from: https://www.nature.com/articles/s41586-018-0175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, Sterling DG, Williams SA. Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients With Stable Coronary Heart Disease. JAMA [Internet]. 2016. [cited 2021 Oct 17];315:2532–2541. Available from: https://jamanetwork.com/journals/jama/fullarticle/2529627 [DOI] [PubMed] [Google Scholar]
- 24.Emilsson V, Ilkov M, Lamb JR, Finkel N, Gudmundsson EF, Pitts R, Hoover H, Gudmundsdottir V, Horman SR, Aspelund T, et al. Co-regulatory networks of human serum proteins link genetics to disease. Science (1979) [Internet]. 2018. [cited 2021 Oct 17];361:2021. Available from: 10.1126/science.aaq1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humke EW, Shriver SK, Starovasnik MA, Fairbrother WJ, Dixit VM. ICEBERG: A novel inhibitor of interleukin-1β generation. Cell. 2000;103:99–111. [DOI] [PubMed] [Google Scholar]
- 26.Thome M, Hofmann K, Burns K, Martinon F, Bodmer JL, Mattmann C, Tschopp J. Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Current Biology. 1998;8:885–889. [DOI] [PubMed] [Google Scholar]
- 27.Druilhe A, Srinivasula SM, Razmara M, Ahmad M, Alnemri ES. Regulation of IL-1β generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death and Differentiation [Internet]. 2001. [cited 2021 Mar 15];8:649–657. Available from: www.nature.com/cdd [DOI] [PubMed] [Google Scholar]
- 28.Bai Y, Sun X, Chu Q, Li A, Qin Y, Li Y, Yue E, Wang H, Li GY, Zahra SM, et al. Caspase-1 regulates Ang II-induced cardiomyocyte hypertrophy via up-regulation of IL-1ß. Bioscience Reports [Internet]. 2018. [cited 2021 Mar 15];38:20171438. Available from: /pmc/articles/PMC5857903/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng C, Duan F, Hu J, Luo B, Huang B, Lou X, Sun X, Li H, Zhang X, Yin S, et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biology. 2020;34:101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira NS, Bruder-Nascimento T, Pereira CA, Zanotto CZ, Prado DS, Silva JF, Rassi DM, Foss-Freitas MC, Alves-Filho JC, Carlos D, et al. NLRP3 Inflammasome and Mineralocorticoid Receptors Are Associated with Vascular Dysfunction in Type 2 Diabetes Mellitus. Cells [Internet]. 2019 [cited 2021 Mar 29]; 2019. Dec 8;8(12):1595. Available from: /pmc/articles/PMC6952964/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donath S, Li P, Willenbockel C, Al-Saadi N, Gross V, Willnow T, Bader M, Martin U, Bauersachs J, Wollert KC, Dietz R, et al. Apoptosis repressor with caspase recruitment domain is required for cardioprotection in response to biomechanical and ischemic stress. Circulation [Internet]. 2006. [cited 2021 May 31];113:1203–1212. Available from: http://www.circulationaha.org [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson ÅB, Tsai JG, Logue SE, Crow MT, Gottlieb RA. Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. Journal of Biological Chemistry [Internet]. 2004. [cited 2021 May 31];279:21233–21238. Available from: https://pubmed.ncbi.nlm.nih.gov/15004034/ [DOI] [PubMed] [Google Scholar]
- 33.Koseki T, Inohara N, Chen S, Núñez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci U S A [Internet]. 1998. [cited 2021 May 31];95:5156–5160. Available from: https://pubmed.ncbi.nlm.nih.gov/9560245/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Li C, Zhang D, Shi D, Qi M, Feng J, Yuan T, Xu X, Liang D, Xu L et al. SNX13 reduction mediates heart failure through degradative sorting of apoptosis repressor with caspase recruitment domain. Nature Communications [Internet]. 2014. [cited 2021 May 31];5:1–13. Available from: www.nature.com/naturecommunications [DOI] [PubMed] [Google Scholar]
- 35.Loan Le TY, Mardini M, Howell VM, Funder JW, Ashton AW, Mihailidou AS. Low-dose spironolactone prevents apoptosis repressor with caspase recruitment domain degradation during myocardial infarction. Hypertension [Internet]. 2012. [cited 2021 Jun 24];59:1164–1169. Available from: https://pubmed.ncbi.nlm.nih.gov/22508833/ [DOI] [PubMed] [Google Scholar]
- 36.González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A [Internet]. 2001. [cited 2021 Mar 15];98:1182–1187. Available from: https://pubmed.ncbi.nlm.nih.gov/11252306/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nowak KL, Gitomer B, Farmer-Bailey H, Wang W, Malaczewski M, Klawitter J, You Z, George D, Patel N, Jovanovich A, et al. Mineralocorticoid Antagonism and Vascular Function in Early Autosomal Dominant Polycystic Kidney Disease: A Randomized Controlled Trial. American Journal of Kidney Diseases [Internet]. 2019. [cited 2021 Mar 29];74:213–223. Available from: https://pubmed.ncbi.nlm.nih.gov/30803706/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anyatonwu GI, Estrada M, Tian X, Somlo S, Ehrlich BE. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci U S A [Internet]. 2007. [cited 2021 Mar 15];104:6454–6459. Available from: https://pubmed.ncbi.nlm.nih.gov/17404231/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paavola J, Schliffke S, Rossetti S, Kuo IYT, Yuan S, Sun Z, Harris PC, Torres VE, Ehrlich BE. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. Journal of Molecular and Cellular Cardiology [Internet]. 2013. [cited 2021 Mar 15];58:199–208. Available from: https://pubmed.ncbi.nlm.nih.gov/23376035/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giehl E, Lemos FO, Huang Y, Giordano FJ, Kuo IY, Ehrlich BE. Polycystin 2-dependent cardio-protective mechanisms revealed by cardiac stress. Pflugers Archiv European Journal of Physiology [Internet]. 2017. [cited 2021 Mar 15];469:1507–1517. Available from: /pmc/articles/PMC5792378/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlitt A, Bickel C, Thumma P, Blankenberg S, Rupprecht HJ, Meyer J, Jiang XC. High plasma phospholipid transfer protein levels as a risk factor for coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology [Internet]. 2003. [cited 2021 Mar 4];23:1857–1862. Available from: http://www.atvbaha.org [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Sun A, Zou Y, Ge J, Kamran H, Jiang XC, Lazar JM. High PLTP activity is associated with depressed left ventricular systolic function. Atherosclerosis [Internet]. 2013. [cited 2021 Jun 24];228:438–442. Available from: https://pubmed.ncbi.nlm.nih.gov/23545183/ [DOI] [PubMed] [Google Scholar]
- 43.Cavusoglu E, Marmur JD, Chhabra S, Chopra V, Eng C, Jiang XC. Relation of baseline plasma phospholipid transfer protein (PLTP) activity to left ventricular systolic dysfunction in patients referred for coronary angiography. Atherosclerosis [Internet]. 2009. [cited 2021 Jun 24];207:261–265. Available from: /pmc/articles/PMC4351717/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan KCB, Shiu SWM, Wong Y. Plasma phospholipid transfer protein activity and small, dense LDL in type 2 diabetes mellitus. European Journal of Clinical Investigation [Internet]. 2003. [cited 2021 Mar 4];33:301–306. Available from: 10.1046/j.1365-2362.2003.01132.x [DOI] [PubMed] [Google Scholar]
- 45.Riemens SC, van Tol A, Sluiter WJ, Dullaart RPF. Plasma phospholipid transfer protein activity is lowered by 24-h insulin and Acipimox administration: Blunted response to insulin in type 2 diabetic patients. Diabetes [Internet]. 1999. [cited 2021 Mar 4];48:1631–1637. Available from: https://diabetes.diabetesjournals.org/content/48/8/1631 [DOI] [PubMed] [Google Scholar]
- 46.Riemens SC, van Tol A, Sluiter WJ, Dullaart RPF. Plasma phospholipid transfer protein activity is related to insulin resistance: Impaired acute lowering by insulin in obese type II diabetic patients [Internet]. In: Diabetologia. Springer; 1998. [cited 2021 Mar 4]. p. 929–934.Available from: 10.1007/s001250051009 [DOI] [PubMed] [Google Scholar]
- 47.Tan KCB, Shiu SWM, Wong Y, Tam S. Plasma phospholipid transfer protein activity and subclinical inflammation in type 2 diabetes mellitus. Atherosclerosis. 2005;178:365–370. [DOI] [PubMed] [Google Scholar]
- 48.Shelly L, Royer L, Sand T, Jensen H, Luo Y. Phospholipid transfer protein deficiency ameliorates diet-induced hypercholesterolemia and inflammation in mice. Journal of Lipid Research. 2008;49:773–781. [DOI] [PubMed] [Google Scholar]
- 49.Schlitt A, Liu J, Yan D, Mondragon-Escorpizo M, Norin AJ, Jiang XC. Anti-inflammatory effects of phospholipid transfer protein (PLTP) deficiency in mice. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids. 2005;1733:187–191. [DOI] [PubMed] [Google Scholar]
- 50.Yan D, Navab M, Bruce C, Fogelman AM, Jiang XC. PLTP deficiency improves the anti-inflammatory properties of HDL and reduces the ability of LDL to induce monocyte chemotactic activity. Journal of Lipid Research. 2004;45:1852–1858. [DOI] [PubMed] [Google Scholar]
- 51.Samyn H, Moerland M, van Gent T, van Haperen R, Grosveld F, van Tol A, de Crom R. Elevation of systemic PLTP, but not macrophage-PLTP, impairs macrophage reverse cholesterol transport in transgenic mice. Atherosclerosis. 2009;204:429–434. [DOI] [PubMed] [Google Scholar]
- 52.Mishra M, Muthuramu I, de Geest B. HDL dysfunction, function, and heart failure [Internet]. Aging. 2019. [cited 2021 Mar 4];11:293–294. Available from: /pmc/articles/PMC6366992/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamblin N, Susen S, Dagorn J, Mouquet F, Jude B, van Belle E, Bauters C, de Groote P. Prognostic significance of circulating levels of angiogenic cytokines in patients with congestive heart failure. American Heart Journal [Internet]. 2005. [cited 2021 Mar 16];150:137–143. Available from: https://pubmed.ncbi.nlm.nih.gov/16084160/ [DOI] [PubMed] [Google Scholar]
- 54.Rychli K, Richter B, Hohensinner PJ, Ali KM, Neuhold S, Zorn G, Berger R, Mörtl D, Huber K, Pacher R, et al. Hepatocyte growth factor is a strong predictor of mortality in patients with advanced heart failure. Heart [Internet]. 2011. [cited 2021 Mar 16];97:1158–1163. Available from: https://pubmed.ncbi.nlm.nih.gov/21572126/ [DOI] [PubMed] [Google Scholar]
- 55.Okayama K, Azuma J, Dosaka N, Iekushi K, Sanada F, Kusunoki H, Iwabayashi M, Rakugi H, Taniyama Y, Morishita R. Hepatocyte growth factor reduces cardiac fibrosis by inhibiting endothelial-mesenchymal transition. Hypertension [Internet]. 2012. [cited 2021 Mar 29];59:958–965. Available from: 10.1161/HYPERTENSIONAHA. [DOI] [PubMed] [Google Scholar]
- 56.Jin H, Wyss J, Yang R, Schwall R. The Therapeutic Potential of Hepatocyte Growth Factor for Myocardial Infarction and Heart Failure. Current Pharmaceutical Design [Internet]. 2005. [cited 2021 Mar 29];10:2525–2533. Available from: https://pubmed.ncbi.nlm.nih.gov/15320761/ [DOI] [PubMed] [Google Scholar]
- 57.Daniels LB, Maisel AS. Natriuretic Peptides [Internet]. J Am Coll Cardiol. 2007. [cited 2021 Mar 16];50:2357–2368. Available from: https://pubmed.ncbi.nlm.nih.gov/18154959/ [DOI] [PubMed] [Google Scholar]
- 58.Lee S da, Chu CH, Huang EJ, Lu MC, Liu JY, Liu CJ, Hsu HH, Lin JA, Kuo WW, Huang CY. Roles of insulin-like growth factor II in cardiomyoblast apoptosis and in hypertensive rat heart with abdominal aorta ligation. American Journal of Physiology - Endocrinology and Metabolism [Internet]. 2006. [cited 2021 Mar 29];291 (2): E306–14. Available from: https://pubmed.ncbi.nlm.nih.gov/16825605/ [DOI] [PubMed] [Google Scholar]
- 59.Chu CH, Tzang BS, Chen LM, Kuo CH, Cheng YC, Chen LY, Tsai FJ, Tsai CH, Kuo WW, Huang CY. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Gq interaction and protein kinase Cα-/CaMKII activation in H9c2 cardiomyoblast cells. Journal of Endocrinology [Internet]. 2008. [cited 2021 Mar 29];197:381–390. Available from: https://pubmed.ncbi.nlm.nih.gov/18434368/ [DOI] [PubMed] [Google Scholar]
- 60.Huang CY, Hao LY, Buetow DE. Hypertrophy of cultured adult rat ventricular cardiomyocytes induced by antibodies against the insulin-like growth factor (IGF)-I or the IGF-I receptor is IGF-II-dependent. Molecular and Cellular Biochemistry [Internet]. 2002. [cited 2021 Mar 29];233:65–72. Available from: https://pubmed.ncbi.nlm.nih.gov/12083381/ [DOI] [PubMed] [Google Scholar]
- 61.Lau MMH, Stewart CEH, Liu Z, Bhatt H, Rotwein P, Stewart CL. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes and Development [Internet]. 1994. [cited 2021 Mar 29];8:2953–2963. Available from: https://pubmed.ncbi.nlm.nih.gov/8001817/ [DOI] [PubMed] [Google Scholar]
- 62.Wei Y, Li J, Huang J, Zhang X, Zhao H, Cui C, Li Y, Hu S. Elevation of IGF-2 receptor and the possible underlying implications in end-stage heart failure patients before and after heart transplantation. Journal of Cellular and Molecular Medicine [Internet]. 2012. [cited 2021 Mar 29];16:1038–1046. Available from: /pmc/articles/PMC4365882/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature [Internet]. 2002. [cited 2021 Mar 29];416:759–763. Available from: https://pubmed.ncbi.nlm.nih.gov/11961559/ [DOI] [PubMed] [Google Scholar]
- 64.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med [Internet]. 2012. [cited 2022 Feb 28];367:20–29. Available from: https://pubmed.ncbi.nlm.nih.gov/22762315/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grams ME, Juraschek SP, Selvin E, Foster MC, Inker LA, Eckfeldt JH, Levey AS, Coresh J. Trends in the prevalence of reduced GFR in the United States: a comparison of creatinine- and cystatin C-based estimates. Am J Kidney Dis [Internet]. 2013. [cited 2022 Feb 28];62:253–260. Available from: https://pubmed.ncbi.nlm.nih.gov/23619125/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Földes G, Horkay F, Szokodi I, Vuolteenaho O, Ilves M, Lindstedt KA, Mäyränpää M, Sármán B, Seres L, Skoumal R, et al. Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochemical and Biophysical Research Communications [Internet]. 2003. [cited 2021 Jun 1];308:480–485. Available from: https://pubmed.ncbi.nlm.nih.gov/12914775/ [DOI] [PubMed] [Google Scholar]
- 67.Japp AG, Cruden NL, Barnes G, van Gemeren N, Mathews J, Adamson J, Johnston NR, Denvir MA, Megson IL, Flapan AD, et al. Acute cardiovascular effects of apelin in humans: Potential role in patients with chronic heart failure. Circulation [Internet]. 2010. [cited 2021 Jun 1];121:1818–1827. Available from: https://pubmed.ncbi.nlm.nih.gov/20385929/ [DOI] [PubMed] [Google Scholar]
- 68.Wang W, McKinnie SMK, Patel VB, Haddad G, Wang Z, Zhabyeyev P, Das SK, Basu R, McLean B, Kandalam V, et al. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic Apelin analogues. J Am Heart Assoc [Internet]. 2013. [cited 2021 Jun 1]; 2(4):e000249. Available from: https://pubmed.ncbi.nlm.nih.gov/23817469/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li L, Zeng H, Chen JX. Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction. American Journal of Physiology - Heart and Circulatory Physiology [Internet]. 2012. [cited 2021 Jun 1];303(5):H605–18. Available from: https://pubmed.ncbi.nlm.nih.gov/22752632/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Azizi Y, Faghihi M, Imani A, Roghani M, Nazari A. Post-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damagethrough reduction of oxidative injury and nitric oxide enhancement in the ratmodel of myocardial infarction. Peptides (NY) [Internet]. 2013. [cited 2021 Jun 1];46:76–82. Available from: https://pubmed.ncbi.nlm.nih.gov/23727032/ [DOI] [PubMed] [Google Scholar]
- 71.Foussal C, Lairez O, Calise D, Pathak A, Guilbeau-Frugier C, Valet P, Parini A, Kunduzova O. Activation of catalase by apelin prevents oxidative stress-linked cardiac hypertrophy. FEBS Letters [Internet]. 2010. [cited 2021 Jun 1];584:2363–2370. Available from: https://pubmed.ncbi.nlm.nih.gov/20398658/ [DOI] [PubMed] [Google Scholar]
- 72.He Q, Pu J, Yuan A, Lau WB, Gao E, Koch WJ, Ma XL, He B. Activation of liver-X-receptor α but not liver-X-receptor β protects against myocardial ischemia/reperfusion injury. Circulation: Heart Failure [Internet]. 2014. [cited 2021 Jun 1];7:1032–1041. Available from: https://pubmed.ncbi.nlm.nih.gov/25277999/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei P, Baysa A, Nebb HI, Valen G, Skomedal T, Osnes JB, Yang Z, Haugen F. Activation of Liver X receptors in the heart leads to accumulation of intracellular lipids and attenuation of ischemia-reperfusion injury. Basic Research in Cardiology [Internet]. 2013. [cited 2021 Jun 1];108(1):323. Available from: https://pubmed.ncbi.nlm.nih.gov/23266787/ [DOI] [PubMed] [Google Scholar]
- 74.He Q, Pu J, Yuan A, Yao T, Ying X, Zhao Y, Xu L, Tong H, He B. Liver X receptor agonist treatment attenuates cardiac dysfunction in type 2 diabetic db/db mice. Cardiovascular Diabetology [Internet]. 2014. [cited 2021 Jun 1];13:149. Available from: https://pubmed.ncbi.nlm.nih.gov/25416469/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cannon M v., Yu H, Candido WM, Dokter MM, Lindstedt EL, Silljé HHW, van Gilst WH, de Boer RA. The liver X receptor agonist AZ876 protects against pathological cardiac hypertrophy and fibrosis without lipogenic side effects. European Journal of Heart Failure [Internet]. 2015. [cited 2021 Jun 1];17:273–282. Available from: https://pubmed.ncbi.nlm.nih.gov/25684370/ [DOI] [PubMed] [Google Scholar]
- 76.Pu J, Yuan A, Shan P, Gao E, Wang X, Wang Y, Lau WB, Koch W, Ma XL, He B. Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. European Heart Journal [Internet]. 2013. [cited 2021 Jun 1];34:1834–1845. Available from: /pmc/articles/PMC3689100/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao J, Liu X, Wang B, Xu H, Xia Q, Lu T, Wang F. Farnesoid X receptor deletion improves cardiac function, structure and remodeling following myocardial infarction in mice. Molecular Medicine Reports [Internet]. 2017. [cited 2021 Jun 1];16:673–679. Available from: /pmc/articles/PMC5482148/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lijnen P, Petrov V. Antagonism of the renin-angiotensin-aldosterone system and collagen metabolism in cardiac fibroblasts [Internet]. Methods and Findings in Experimental and Clinical Pharmacology. 1999. [cited 2021 Jun 6];21:215–227. Available from: https://pubmed.ncbi.nlm.nih.gov/10389125/ [DOI] [PubMed] [Google Scholar]
- 79.Fullerton MJ, Funder JW. Aldosterone and cardiac fibrosis: In vitro studies. Cardiovascular Research [Internet]. 1994. [cited 2021 Jun 6];28:1863–1867. Available from: https://academic.oup.com/cardiovascres/article/28/12/1863/327875 [DOI] [PubMed] [Google Scholar]
- 80.Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. Journal of Molecular and Cellular Cardiology [Internet]. 1993. [cited 2021 Jun 6];25:563–575. Available from: https://pubmed.ncbi.nlm.nih.gov/8377216/ [DOI] [PubMed] [Google Scholar]
- 81.Pellicori P, Ferreira JP, Mariottoni B, Brunner-La Rocca HP, Ahmed FZ, Verdonschot J, Collier T, Cuthbert JJ, Petutschnigg J, Mujaj B, et al. Effects of spironolactone on serum markers of fibrosis in people at high risk of developing heart failure: rationale, design and baseline characteristics of a proof-of-concept, randomised, precision-medicine, prevention trial. The Heart OMics in AGing (HOMAGE) trial. European Journal of Heart Failure [Internet]. 2020. [cited 2021 Jun 6];22:1711–1723. Available from: 10.1002/ejhf.1716 [DOI] [PubMed] [Google Scholar]
- 82.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: Insights from the Randomized Aldactone Evaluation Study (RALES). Circulation [Internet]. 2000. [cited 2021 Jun 6];102:2700–2706. Available from: http://www.circulationaha.org [DOI] [PubMed] [Google Scholar]
- 83.Ferreira JP, Verdonschot J, Wang P, Pizard A, Collier T, Ahmed FZ, Brunner-La-Rocca HP, Clark AL, Cosmi F, Cuthbert J, et al. Proteomic and Mechanistic Analysis of Spironolactone in Patients at Risk for HF. JACC: Heart Failure. 2021;9:268–277. [DOI] [PubMed] [Google Scholar]
- 84.Williams SA, Kivimaki M, Langenberg C, Hingorani AD, Casas JP, Bouchard C, Jonasson C, Sarzynski MA, Shipley MJ, Alexander L, et al. Plasma protein patterns as comprehensive indicators of health. Nature medicine [Internet]. 2019. [cited 2022 Feb 28];25:1851–1857. Available from: https://pubmed.ncbi.nlm.nih.gov/31792462/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grams ME, Surapaneni A, Chen J, Zhou L, Yu Z, Dutta D, Welling PA, Chatterjee N, Zhang J, Arking DE, et al. Proteins Associated with Risk of Kidney Function Decline in the General Population. Journal of the American Society of Nephrology : JASN [Internet]. 2021. [cited 2022 Feb 28];32:2291–2302. Available from: https://pubmed.ncbi.nlm.nih.gov/34465608/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Technology - SomaLogic [Internet]. [cited 2022 Feb 28];Available from: https://somalogic.com/technology/
- 87.Pietzner M, Wheeler E, Carrasco-Zanini J, Cortes A, Koprulu M, Wörheide MA, Oerton E, Cook J, Stewart ID, Kerrison ND, et al. Mapping the proteo-genomic convergence of human diseases. Science (New York, NY: ) [Internet]. 2021. [cited 2022 Feb 28];12:374. Available from: https://pubmed.ncbi.nlm.nih.gov/34648354/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferkingstad E, Sulem P, Atlason BA, Sveinbjornsson G, Magnusson MI, Styrmisdottir EL, Gunnarsdottir K, Helgason A, Oddsson A, Halldorsson B v, et al. Large-scale integration of the plasma proteome with genetics and disease. Nature genetics [Internet]. 2021. [cited 2022 Feb 28];53:1712–1721. Available from: https://pubmed.ncbi.nlm.nih.gov/34857953/ [DOI] [PubMed] [Google Scholar]
- 89.Emilsson V, Gudmundsdottir V, Gudjonsson A, Jonmundsson T, Jonsson BG, Karim MA, Ilkov M, Staley JR, Gudmundsson EF, Launer LJ, et al. Coding and regulatory variants are associated with serum protein levels and disease. Nature Communications 2022. 13:1 [Internet]. 2022 [cited 2022 Feb 28];13:1–11. Available from: https://www.nature.com/articles/s41467-022-28081-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gudjonsson A, Gudmundsdottir V, Axelsson GT, Gudmundsson EF, Jonsson BG, Launer LJ, Lamb JR, Jennings LL, Aspelund T, Emilsson V, et al. A genome-wide association study of serum proteins reveals shared loci with common diseases. Nature Communications 2022. 13:1 [Internet]. 2022 [cited 2022 Feb 28];13:1–13. Available from: https://www.nature.com/articles/s41467-021-27850-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, Burgess S, Jiang T, Paige E, Surendran P, et al. Genomic atlas of the human plasma proteome. Nature [Internet]. 2018. [cited 2022 Feb 28];558:73–79. Available from: https://pubmed.ncbi.nlm.nih.gov/29875488/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Emilsson V, Ilkov M, Lamb JR, Finkel N, Gudmundsson EF, Pitts R, Hoover H, Gudmundsdottir V, Horman SR, et al. Co-regulatory networks of human serum proteins link genetics to disease. Science (New York, NY: ) [Internet]. 2018. [cited 2022 Feb 28]; 361(6404):769–773. Available from: https://pubmed.ncbi.nlm.nih.gov/30072576/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giudice V, Biancotto A, Wu Z, Cheung F, Candia J, Fantoni G, Kajigaya S, Rios O, Townsley D, Feng X, et al. Aptamer-based proteomics of serum and plasma in acquired aplastic anemia. Experimental hematology [Internet]. 2018. [cited 2022 Feb 28];68:38. Available from: /pmc/articles/PMC6748047/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Russell TM, Green LS, Rice T, Kruh-Garcia NA, Dobos K, de Groote MA, Hraha T, Sterling DG, Janjic N, Ochsner UA. Potential of High-Affinity, Slow Off-Rate Modified Aptamer Reagents for Mycobacterium tuberculosis Proteins as Tools for Infection Models and Diagnostic Applications. Journal of clinical microbiology [Internet]. 2017. [cited 2022 Feb 28];55:3072–3088. Available from: https://pubmed.ncbi.nlm.nih.gov/28794178/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kraemer S, Vaught JD, Bock C, Gold L, Katilius E, Keeney TR, Kim N, Saccomano NA, Wilcox SK, et al. From SOMAmer-Based Biomarker Discovery to Diagnostic and Clinical Applications: A SOMAmer-Based, Streamlined Multiplex Proteomic Assay. PLOS ONE [Internet]. 2011. [cited 2022 Feb 28];6:e26332. Available from: 10.1371/journal.pone.0026332 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


