Abstract
Several studies, although with conflicting results, have sought to determine the concentration of soluble CTLA4 antigens in peripheral blood plasma and peritoneal fluid in patients with endometriosis-related infertility. A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) through a search of the following databases: MEDLINE, EMBASE, Global Health, The Cochrane Library, Health Technology Assessment Database and Web of Science, and Clinical Trials research register. We included observational or prospective human and animal studies with any features related to endometriosis and/or infertility studies involving CTLA4-related pathogenesis published in English. The results of studies in which the size and characteristics of the observed groups were not stated were excluded. From the initial pool of 73 publications identified and screened, we finally included 5 articles to summarize the most recent knowledge about CTLA4-linked autoimmunity in the pathogenesis of endometriosis and related infertility. Evidence from clinical studies shows that CTLA4-based autoimmunity is involved in the maintenance of chronic inflammation in the peritoneal environment, with pre-clinical evidence of anti-CTLA antibodies as a potential novel target therapy for endometriosis. However, CTLA4 gene analyses do not support findings of CTLA4-linked autoimmunity as a primary determinant of the pathogenesis of endometriosis. These findings underlie the role of complex interactions within the family of immune checkpoint molecules involved. Further studies are needed to investigate the clinical relevance of anti-CTLA target therapy, taking into account the potential adverse events and repercussions of novel immunologic therapy modalities. However, with the general scarcity of studies investigating this topic, the clinical importance of CTLA4 autoimmunity still remains unclear.
Keywords: endometriosis, infertility, CTLA4, reproductive immunology, pathophysiology, systematic review
1. Introduction
Endometriosis presents one of the most common chronic gynecological conditions associated with cyclic pelvic pain, subfertility or both [1,2]. It affects approximately 20% of women hospitalized for pelvic pain and is associated with infertility in 50% of these cases [3,4]. The symptoms of endometriosis vary from mild to severe, but in most cases, women suffering from endometriosis need an important monthly intake of analgesics and generally have a poor health-related quality of life [5,6,7]. Furthermore, the significant diagnostic delay of endometriosis, as well as the search for effective pharmacological strategies, causes a substantial economic burden [8]. Women with endometriosis are also at an increased risk of developing several cancers [9,10,11] and autoimmune disorders.
The exact pathogenesis of endometriosis remains elusive, but a number of theories have been proposed that integrate several genetic and epigenetic theories [12]. More recent work emphasizes the importance of dysregulation of the immune system in the progression of the disease [13,14]. Thus, the widely accepted Sampson’s theory can be applied to the majority of women, but it has been hypothesized that endometriosis develops in women whose dysregulated immune mechanisms cannot overcome the appropriate response to refluxing endometrial debris [15]. In the last decade, studies on apoptosis [16,17,18] and immune checkpoint molecules [19,20] have focused on reproductive immunology. Those molecules act as inhibitory signaling mediators to maintain immune tolerance by several mechanisms: regulation of T-cell homeostasis, promoting T regulatory (Treg) cell development, inhibition of autoreactive T cells and inhibition of the effector T-cell differentiation and cytokine production leading to immunosuppression [21,22]. One of the immune checkpoint molecules, cytotoxic T lymphocyte–associated antigen-4 (CTLA4), particularly its soluble form (sCTLA4), has been correlated with several autoimmune diseases, such as autoimmune thyroid disease, type 1 diabetes mellitus, and immune thrombocytopenia and has been linked with endometriosis-related infertility [23]. Inhibitory in its nature, CTLA4 is a critical immunoregulatory molecule that belongs to the family of type I membrane receptors [24]. CTLA4 is an important structure in signaling between cells of the immune system, downregulating T-cell activation and favoring the anergic state of lymphocytes [25].
Several studies, although with conflicting results, have sought to determine the concentration of the sCTLA4 antigen in peripheral blood plasma and peritoneal fluid in patients with endometriosis-related infertility [23,26,27,28,29]. Data suggest that significantly higher percentages of CD4+/CTLA4 and CD8+/CTLA4 T lymphocytes have been observed in patients with endometriosis and intraoperative adhesions, implicating that there is a possible correlation between the ratio of CD8+ T/CD4+ T and endometriosis-related infertility [28]. These studies have expanded the general knowledge of the pathophysiology of endometriosis and may open up new potential diagnostic and treatment options for endometriosis-related infertility. Considering the increasing attention to the problem, in this article, we aimed to summarize the most recent knowledge about the topic.
2. Materials and Methods
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [30], through a search of the following databases: MEDLINE, EMBASE, Global Health, The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register), Health Technology Assessment Database and Web of Science, and Clinical Trials research register. The aim was to summarize data from relevant articles regarding the correlation between CTLA4 and endometriosis-related infertility. The search included the following keywords, from the inception of respective databases to 25 July 2022: CTLA4, anti-CTLA4 antibody, endometriosis, autoimmunity, pathogenesis, female infertility and immune therapy. EndNote 20 reference manager was used to combine and remove duplicate results. Three authors (M.M., S.D. and A.S.L.) independently screened the titles and abstracts of the studies obtained by the search strategy. The full text of each potentially relevant study was obtained and assessed for inclusion independently by the two authors (I.B. and G.V.). They also independently extracted data from the included studies using a pre-piloted standard form to ensure consistency of the data extraction. Three other authors (M.Š.G., V.C. and M.Ć.) independently reviewed the selection and data extraction processes.
We included observational or prospective human and animal studies with any features related to endometriosis and/or infertility studies involving CTLA4-related pathogenesis published in English. The results of studies in which the size and characteristics of the observed groups were not stated were excluded. Studies without original data, including reviews, comments, editorials and meta-analyses, were excluded (Table S1). The results were compared, and any disagreement was discussed and resolved by consensus. Studies providing ambiguous or insufficient, low-quality data or not quantifiable outcomes were also excluded. Due to the nature of the findings, we opted for a narrative synthesis of the results from the selected articles.
3. Results
3.1. Literature Search
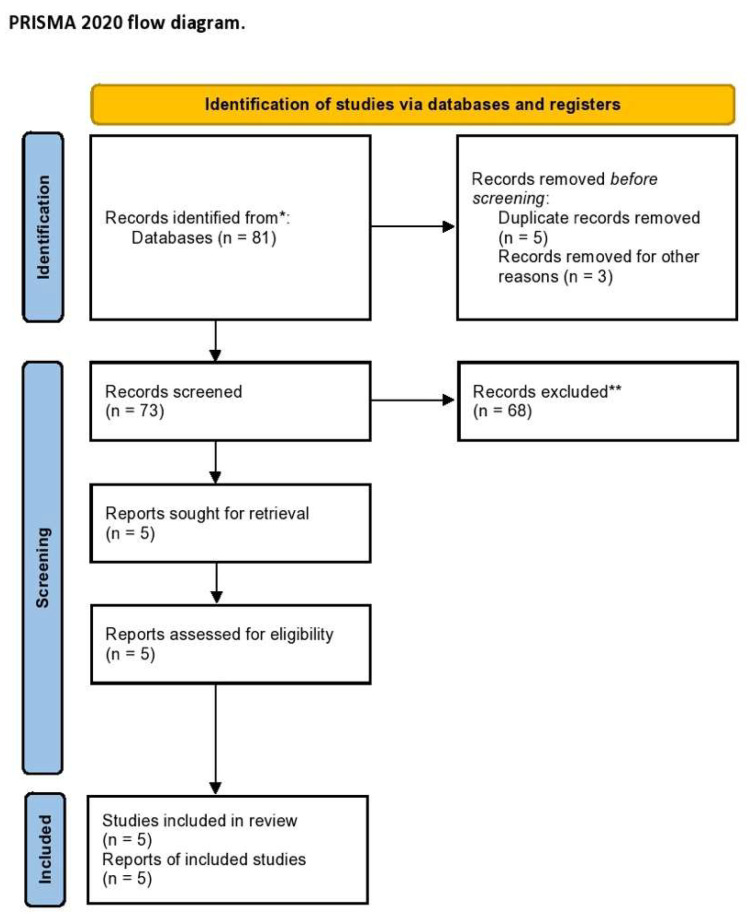
The electronic searches, after duplicate records were removed, provided a total of 73 citations. Of these, 68 were excluded after title/abstract screening (not relevant to the review inclusion criteria). We examined the full text of 5 publications remaining to summarize the most recent knowledge about CTLA4-linked autoimmunity in the pathogenesis of endometriosis and related infertility [23,26,27,28,31]. The PRISMA flow chart of the literature search is reported in Figure 1.
Figure 1.
PRISMA flow diagram of the systematic literature search. * MEDLINE, EMBASE, Global Health, The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register), Health Technology Assessment Database and Web of Science, and Clinical Trials; ** Records excluded by a human and automation tools.
3.2. Study Characteristics
Table 1 shows the detailed characteristics of the included studies after the literature search [23,26,27,28,31]. Only one study has been conducted on animal models, specifically, in the peritoneal fluid of a mouse endometriosis model [31], while other four studies were case-control studies comparing either infertile women with or without endometriosis or women with histologically confirmed endometriosis and women without any sign of disease [23,26,27,28].
Table 1.
Characteristics of the included studies.
| Study | Study Type | Study Population | Results | |
|---|---|---|---|---|
| Cases | Controls | |||
| Viganò et al. (2005) [26] | Case-control study | 150 women 1 | 168 women 2 |
|
| Lerner et al. (2011) [27] | Case-control study | 244 infertile women
|
172 fertile women 2 |
|
| Santoso et al. (2020) [23] | Case-control study | 44 infertile women 1 | 44 infertile women 2 |
|
| Abramiuk et al. (2021) [28] | Case-control study | 54 women 1 | 20 women 2 |
|
| Liu et al. (2016) [31] | Animal in vitro study | 30 mouse endometriosis models (LPTM transplanted with autologous uterine endometrium) | / |
|
1 Women with endometriosis diagnosed by laparoscopy and confirmed by histopathological examination. 2 Women without signs of endometriosis observed by laparoscopy; a Observed by ORs—calculated using allele frequencies and G variants of the polymorphisms as risk factors for the disease; (s)CTLA4 (soluble) cytotoxic T-lymphocyte antigen 4, EFI Endometriosis Fertility Index, rASRM revised American Society for Reproductive Medicine score, ORs odds ratios, LPTM laparotomy, PGLA poly(lactic-co-glycolic acid).
4. Discussion
4.1. Membrane-Bound and Soluble CTLA4 Antigen Involved in Endometriosis and/or Infertility
CTLA4 antigen is found in two forms: membrane-bound protein CTLA4 and free, sCTLA4 form found in serum and peritoneal fluid [32]. The latter is considered one of the immune checkpoint molecules with roles in carcinogenesis, inflammation and pregnancy [23]. Membrane-bound CTLA4 directly competes with CD28 by binding to the same ligands, CD80 (B7.1) and CD86 (B7.2), and thus is one of the inhibitory regulators of lymphocyte activation [33].
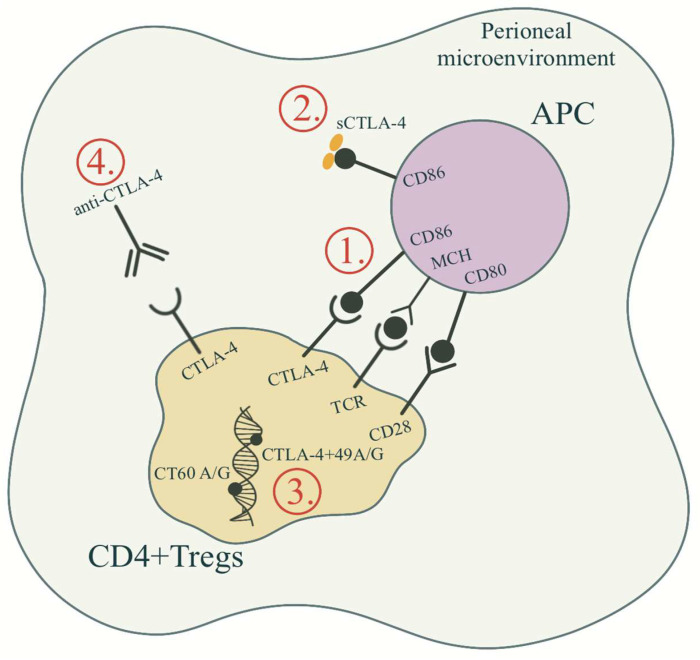
The role of CTLA4 in endometriosis pathophysiology is summarized in Figure 2. Abramiuk et al. [28] aimed to evaluate the expression of CTLA4 on the surface of CD4+, CD8+ and B19+ lymphocytes and to determine the concentration of sCTLA4 antigen in serum and peritoneal fluid in patients. A similar study was conducted by Santoso and colleagues [23], who hypothesized that soluble immune checkpoint molecules are elevated during endometriosis in infertile women in peripheral serum and peritoneal fluid. CD8+ T cells without CTLA4 expression result in significantly higher IFN-gamma and granzyme B production and enhanced cytolysis in an animal model, which can be in concordance with significantly higher CTLA4 antigen expression among CTLA4 T cells in the serum of patients with endometriosis, with a positive correlation of the percentage of CD4+ and CD8+ T cells and the endometriosis stage [28]. A recent study also showed that there is a higher Treg cell percentage in the serum of patients with endometriosis, without similar findings in peritoneal fluid [23]. Moreover, Abramiuk et al. [28] showed the role of immunosuppressive mechanisms in the development of endometriosis-related infertility: CD4+ and CD8+ T lymphocytes negatively correlated with the percentage of NK and NKT-like cells (confirming previous findings [34]), while CD8+ T cells positively correlated with the percentage of CD4+CD25+Foxp3high Treg cells.
Figure 2.
The mechanisms of CTLA4 involvement in the pathogenesis of endometriosis. (1) The CTLA4 expressed on the surface of T cells involved in inhibitory co-stimulatory signal in T cell proliferation; (2) Higher serum and peritoneal soluble form of CTLA4 (sCTLA4) found in advanced endometriosis and related infertility; (3) CTLA4 gene polymorphisms not associated with the pathogenesis of endometriosis; (4) Anti-CTLA4 antibodies in mouse endometriosis model found to reduce the number of Treg cells and thus inhibit proliferation and invasion of ectopic endometrium. (Original illustration).
Santoso and coworkers found that immune checkpoint molecules, with involvement of sCTLA4 in the peritoneum, act as immune regulators in the pathogenesis of endometriosis [23]. They found significantly higher serum sCTLA4 in the late-stages (III and IV) compared to early-stages (I and II) endometriosis and the control group, which can indicate the peripheral tolerogenic role of sCTLA4 on immune function in endometriosis patients. Furthermore, higher levels of sCTLA4 in the serum of advanced-stage endometriosis might be the outcome of increased proteolytic cleavage of membrane forms from local endometrial-like cell lesions, which then circulate into the periphery. This study also found higher concentrations of sCTLA4 in the peritoneal fluid compared to their concentration in serum, as well in the peritoneal fluid of women with endometriosis-related infertility compared to their gynecologic control [23]. Finally, serum and peritoneal concentrations of sCTLA4 were positively correlated, which can indicate that serum levels originate from the local peritoneal cavity.
4.2. CTLA4 Gene Variants in the Pathogenesis of Endometriosis
CTLA4 gene, mapped on the 2q33 chromosome, is associated with susceptibility to autoimmunity, with the CTLA4 region as an important locus for autoimmune diseases [35]. For this reason, several genetic polymorphisms of CTLA4 gene, with prominent CTLA4+49A/G polymorphism (exon 1) and CT60 A/G dimorphism (3′-UTR), were already investigated [36].
Two research groups [26,27] conducted case-control studies to investigate the genotype frequencies of the latter variants in patients with endometriosis compared to healthy controls. Both groups found that CTLA4 gene variants do not play a role in the pathogenesis of endometriosis, with or without related female infertility. Viganò et al. formed two hypotheses of autoimmune etiology of endometriosis [26]; on the one hand, autoimmunity can underlie endometriosis development without CTLA4-dependent predisposition; on the other hand, endometriosis is not associated with autoimmune etiology but can predispose the development of autoimmune reactions. Nevertheless, further studies are needed to investigate genotype frequencies between different severity and manifestations of endometriosis. Indeed, Viganò et al. did not find any association of the variants’ distribution with parameters indicative of a specific manifestation of the disease [26]. However, Lerner et al. [27] also found no differences in the frequency of CTLA4+49A/G polymorphism in patients with minimal-to-mild and moderate-to-severe endometriosis compared to controls. These findings may underlie the fact that endometriosis etiology, irrespective of related infertility, is not primarily associated with the development of CTLA4-related autoimmunity.
4.3. Anti-CTLA4 Antibodies as Target Therapy for Endometriosis?
Although there are substantial improvements in hormonal and non-hormonal therapies [37,38,39], majority of the currently available treatment options for endometriosis suppress ovarian function, are partially or totally contraceptive, and do not present a final solution for patients [40]. Accumulating evidence suggests that immunotherapy may open new scenarios for the treatment of refractory and recurrent cases of endometriosis [41]. Liu et al. conducted an experimental study with endometriosis mouse model [31]. They hypothesized that PLGA/anti-CTLA4 suppress ectopic endometrial cell proliferation and invasion by reducing IL-10 and TGF-β production secreted by CD4+CD25+Treg cells in a sustained-release manner [31].
Pathological immune response in the peritoneal environment is responsible for the implantation of ectopic endometrium [42] and maintenance of immunological self-tolerance mediated by CD4+CD25+ Treg cells, which were found in the peritoneal fluid of women with endometriosis in higher concentrations. The latter subpopulation of T cells is activated via CTLA4-modified binding of CD80/CD86 ligands to the TCR of T cells, which can stimulate endometrial stromal cell proliferation and invasion by secreted suppressive cytokines IL-10 and TGF-β (Figure 2).
Liu et al. formulated a drug delivery system of anti-CTLA4 antibody encapsulated with polylactic-co-glycolic acid (PLGA), which ensured sustained release of the antibody in an in vitro micro-environment [31]. They found that both proliferation and invasion of ectopic endometrial cells were suppressed with PLGA/anti-CTLA4, reducing IL-10 and TGF-β secreted by CD4+CD25+Treg cells. Thus, this study provided preliminary experimental results for anti-CTLA-based therapy for endometriosis. However, treatment with the anti-CTLA4 antibody results in a broad spectrum of adverse events, at least in the investigated mouse model, such as the suppressive influence on Tregs. Although this finding from the mouse model cannot be translated directly into humans, it is possible that the anti-CTLA4 antibody may exert the same suppressive effect on Tregs in humans, leading to potential severe side effects due to reduction of self-tolerance and thus increased autoimmunity [43].
5. Conclusions
This systematic review provides a summary of the available pieces of evidence about CTLA4-based autoimmunity in the pathogenesis of endometriosis and related infertility. Data from clinical studies show that CTLA4-based autoimmunity is involved in the maintenance of chronic inflammation in the peritoneal environment, with pre-clinical evidence of anti-CTLA antibodies as a potential novel target therapy for endometriosis. However, CTLA4 gene analyses do not support findings of CTLA4-linked autoimmunity as a primary determinant of the pathogenesis of endometriosis. These findings underlie the role of complex interactions within the family of immune checkpoint molecules involved. Further studies are needed to investigate the clinical relevance of anti-CTLA target therapy, taking into account the potential adverse events and repercussions of novel immunologic therapy modalities. However, due to the low number of studies investigating this topic, the clinical importance of CTLA4 autoimmunity in endometriosis remains unclear.
Abbreviations
CD—cluster of differentiation; CTLA4—cytotoxic T-lymphocyte-associated protein 4; FOXP3—Forkhead box P3 (scurfin); IFN—interferon; IL-10—interleukin-10; NKT—Natural killer T cells; PLGA—Poly Lactic-co-Glycolic Acid; PRISMA—Preferred Reporting Items for Systematic Reviews and Meta-Analyses; sCTLA4—soluble cytotoxic T-lymphocyte-associated protein 4; TGF-β—Transforming growth factor-β; Tregs—Regulatory T cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231810902/s1.
Author Contributions
Conceptualization, M.M. and A.S.L.; methodology S.D.; software, S.D.; validation, M.M., M.Š.G. and I.B.; formal analysis, A.S.L. and V.C. investigation, M.M., G.V. and M.Ć. resources, M.M. and S.D.; data curation, A.S.L. and M.M. writing—original draft preparation, M.M. and I.B.; writing—review and editing, A.S.L. and M.Ć. visualization, G.V.; supervision, A.S.L. and M.Ć. project administration, M.Š.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laganà A.S., Garzon S., Götte M., Viganò P., Franchi M., Ghezzi F., Martin D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019;20:5615. doi: 10.3390/ijms20225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laganà A.S., Vitagliano A., Chiantera V., Cicinelli E. Diagnosis and Treatment of Endometriosis and Endometriosis-Associated Infertility: Novel Approaches to an Old Problem. J. Clin. Med. 2022;11:3914. doi: 10.3390/jcm11133914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor H.S., Kotlyar A.M., Flores V.A. Endometriosis Is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet. 2021;397:839–852. doi: 10.1016/S0140-6736(21)00389-5. [DOI] [PubMed] [Google Scholar]
- 4.Chapron C., Marcellin L., Borghese B., Santulli P. Rethinking Mechanisms, Diagnosis and Management of Endometriosis. Nat. Rev. Endocrinol. 2019;15:666–682. doi: 10.1038/s41574-019-0245-z. [DOI] [PubMed] [Google Scholar]
- 5.Škegro B., Bjedov S., Mikuš M., Mustač F., Lešin J., Matijević V., Ćorić M., Elveđi Gašparović V., Medić F., Sokol Karadjole V. Endometriosis, Pain and Mental Health. Psychiatr. Danub. 2021;33:632–636. [PubMed] [Google Scholar]
- 6.Mikuš M., Matak L., Vujić G., Škegro B., Škegro I., Augustin G., Lagana A.S., Ćorić M. The Short Form Endometriosis Health Profile Questionnaire (EHP-5): Psychometric Validity Assessment of a Croatian Version. Arch. Gynecol. Obstet. 2022 doi: 10.1007/s00404-022-06691-1. [DOI] [PubMed] [Google Scholar]
- 7.Vitale S.G., La Rosa V.L., Rapisarda A.M.C., Laganà A.S. Impact of Endometriosis on Quality of Life and Psychological Well-Being. J. Psychosom. Obstet. Gynaecol. 2017;38:317–319. doi: 10.1080/0167482X.2016.1244185. [DOI] [PubMed] [Google Scholar]
- 8.The members of the Endometriosis Guideline Core Group. Becker C.M., Bokor A., Heikinheimo O., Horne A., Jansen F., Kiesel L., King K., Kvaskoff M., Nap A., et al. ESHRE Guideline: Endometriosis. Hum. Reprod. Open. 2022;2022:hoac009. doi: 10.1093/hropen/hoac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetvicka V., Fiala L., Garzon S., Buzzaccarini G., Terzic M., Laganà A.S. Endometriosis and Gynaecological Cancers: Molecular Insights behind a Complex Machinery. Prz. Menopauzalny. 2021;20:201–206. doi: 10.5114/pm.2021.111276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terzic M., Aimagambetova G., Kunz J., Bapayeva G., Aitbayeva B., Terzic S., Laganà A.S. Molecular Basis of Endometriosis and Endometrial Cancer: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021;22:9274. doi: 10.3390/ijms22179274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Králíčková M., Laganà A.S., Ghezzi F., Vetvicka V. Endometriosis and Risk of Ovarian Cancer: What Do We Know? Arch Gynecol. Obstet. 2020;301:1–10. doi: 10.1007/s00404-019-05358-8. [DOI] [PubMed] [Google Scholar]
- 12.Koninckx P.R., Fernandes R., Ussia A., Schindler L., Wattiez A., Al-Suwaidi S., Amro B., Al-Maamari B., Hakim Z., Tahlak M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021;12:745548. doi: 10.3389/fendo.2021.745548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Symons L.K., Miller J.E., Kay V.R., Marks R.M., Liblik K., Koti M., Tayade C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018;24:748–762. doi: 10.1016/j.molmed.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Laganà A.S., Vitale S.G., Salmeri F.M., Triolo O., Ban Frangež H., Vrtačnik-Bokal E., Stojanovska L., Apostolopoulos V., Granese R., Sofo V. Unus pro Omnibus, Omnes pro Uno: A Novel, Evidence-Based, Unifying Theory for the Pathogenesis of Endometriosis. Med. Hypotheses. 2017;103:10–20. doi: 10.1016/j.mehy.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Hogg C., Horne A.W., Greaves E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front. Endocrinol. 2020;11:7. doi: 10.3389/fendo.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vetvicka V., Laganà A.S., Salmeri F.M., Triolo O., Palmara V.I., Vitale S.G., Sofo V., Králíčková M. Regulation of Apoptotic Pathways during Endometriosis: From the Molecular Basis to the Future Perspectives. Arch. Gynecol. Obstet. 2016;294:897–904. doi: 10.1007/s00404-016-4195-6. [DOI] [PubMed] [Google Scholar]
- 17.Salmeri F.M., Laganà A.S., Sofo V., Triolo O., Sturlese E., Retto G., Pizzo A., D’Ascola A., Campo S. Behavior of Tumor Necrosis Factor-α and Tumor Necrosis Factor Receptor 1/Tumor Necrosis Factor Receptor 2 System in Mononuclear Cells Recovered from Peritoneal Fluid of Women with Endometriosis at Different Stages. Reprod. Sci. 2015;22:165–172. doi: 10.1177/1933719114536472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sturlese E., Salmeri F.M., Retto G., Pizzo A., De Dominici R., Ardita F.V., Borrielli I., Licata N., Laganà A.S., Sofo V. Dysregulation of the Fas/FasL System in Mononuclear Cells Recovered from Peritoneal Fluid of Women with Endometriosis. J. Reprod. Immunol. 2011;92:74–81. doi: 10.1016/j.jri.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Strauß T., Greve B., Gabriel M., Achmad N., Schwan D., Espinoza-Sanchez N.A., Laganà A.S., Kiesel L., Poutanen M., Götte M., et al. Impact of Musashi-1 and Musashi-2 Double Knockdown on Notch Signaling and the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2022;23:2851. doi: 10.3390/ijms23052851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filipchiuk C., Laganà A.S., Beteli R., Ponce T.G., Christofolini D.M., Martins Trevisan C., Fonseca F.L.A., Barbosa C.P., Bianco B. BIRC5/Survivin Expression as a Non-Invasive Biomarker of Endometriosis. Diagnostics. 2020;10:E533. doi: 10.3390/diagnostics10080533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian S.L., Taube J.M., Anders R.A., Pardoll D.M. Mechanism-Driven Biomarkers to Guide Immune Checkpoint Blockade in Cancer Therapy. Nat. Rev. Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B., Chan H.L., Chen P. Immune Checkpoint Inhibitors: Basics and Challenges. Curr. Med. Chem. 2019;26:3009–3025. doi: 10.2174/0929867324666170804143706. [DOI] [PubMed] [Google Scholar]
- 23.Santoso B., Sa’adi A., Dwiningsih S.R., Tunjungseto A., Widyanugraha M.Y.A., Mufid A.F., Rahmawati N.Y., Ahsan F. Soluble Immune Checkpoints CTLA-4, HLA-G, PD-1, and PD-L1 Are Associated with Endometriosis-Related Infertility. Am. J. Reprod. Immunol. 2020;84:e13296. doi: 10.1111/aji.13296. [DOI] [PubMed] [Google Scholar]
- 24.Liu J.-N., Kong X.-S., Huang T., Wang R., Li W., Chen Q.-F. Clinical Implications of Aberrant PD-1 and CTLA4 Expression for Cancer Immunity and Prognosis: A Pan-Cancer Study. Front. Immunol. 2020;11:2048. doi: 10.3389/fimmu.2020.02048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marable J., Ruiz D., Jaiswal A.K., Bhattacharya R., Pantazes R., Agarwal P., Suryawanshi A.S., Bedi D., Mishra A., Smith B.F., et al. Nanobody-Based CTLA4 Inhibitors for Immune Checkpoint Blockade Therapy of Canine Cancer Patients. Sci. Rep. 2021;11:20763. doi: 10.1038/s41598-021-00325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viganó P., Lattuada D., Somigliana E., Abbiati A., Candiani M., Di Blasio A.M. Variants of the CTLA4 Gene That Segregate with Autoimmune Diseases Are Not Associated with Endometriosis. Mol. Hum. Reprod. 2005;11:745–749. doi: 10.1093/molehr/gah225. [DOI] [PubMed] [Google Scholar]
- 27.Lerner T.G., Bianco B., Teles J.S., Vilarino F.L., Christofolini D.M., Barbosa C.P. Analysis of CTLA4 Gene Variant in Infertile Brazilian Women with and without Endometriosis. Int. J. Immunogenet. 2011;38:259–262. doi: 10.1111/j.1744-313X.2011.01000.x. [DOI] [PubMed] [Google Scholar]
- 28.Abramiuk M., Bębnowska D., Hrynkiewicz R., Polak P.N.-R.G., Kotarski J., Roliński J., Grywalska E. CLTA-4 Expression Is Associated with the Maintenance of Chronic Inflammation in Endometriosis and Infertility. Cells. 2021;10:487. doi: 10.3390/cells10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H., Zhao J., Lu J., Sun X. Ovarian Endometrioma Infiltrating Neutrophils Orchestrate Immunosuppressive Microenvironment. J. Ovarian Res. 2020;13:44. doi: 10.1186/s13048-020-00642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q., Ma P., Liu L., Ma G., Ma J., Liu X., Liu Y., Lin W., Zhu Y. Evaluation of PLGA Containing Anti-CTLA4 Inhibited Endometriosis Progression by Regulating CD4+CD25+Treg Cells in Peritoneal Fluid of Mouse Endometriosis Model. Eur. J. Pharm. Sci. 2017;96:542–550. doi: 10.1016/j.ejps.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Pai C.-C.S., Simons D.M., Lu X., Evans M., Wei J., Wang Y.-H., Chen M., Huang J., Park C., Chang A., et al. Tumor-Conditional Anti-CTLA4 Uncouples Antitumor Efficacy from Immunotherapy-Related Toxicity. J. Clin. Investig. 2019;129:349–363. doi: 10.1172/JCI123391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosseini A., Gharibi T., Marofi F., Babaloo Z., Baradaran B. CTLA-4: From Mechanism to Autoimmune Therapy. Int. Immunopharmacol. 2020;80:106221. doi: 10.1016/j.intimp.2020.106221. [DOI] [PubMed] [Google Scholar]
- 34.Laganà A.S., Triolo O., Salmeri F.M., Granese R., Palmara V.I., Ban Frangež H., Vrtčnik Bokal E., Sofo V. Natural Killer T Cell Subsets in Eutopic and Ectopic Endometrium: A Fresh Look to a Busy Corner. Arch. Gynecol. Obstet. 2016;293:941–949. doi: 10.1007/s00404-015-4004-7. [DOI] [PubMed] [Google Scholar]
- 35.Ghaderi A. CTLA4 Gene Variants in Autoimmunity and Cancer: A Comparative Review. Iran. J. Immunol. 2011;8:127–149. [PubMed] [Google Scholar]
- 36.Gough S.C.L., Walker L.S.K., Sansom D.M. CTLA4 Gene Polymorphism and Autoimmunity. Immunol. Rev. 2005;204:102–115. doi: 10.1111/j.0105-2896.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 37.Garzon S., Laganà A.S., Barra F., Casarin J., Cromi A., Raffaelli R., Uccella S., Franchi M., Ghezzi F., Ferrero S. Novel Drug Delivery Methods for Improving Efficacy of Endometriosis Treatments. Expert Opin. Drug Deliv. 2021;18:355–367. doi: 10.1080/17425247.2021.1829589. [DOI] [PubMed] [Google Scholar]
- 38.Garzon S., Laganà A.S., Barra F., Casarin J., Cromi A., Raffaelli R., Uccella S., Franchi M., Ghezzi F., Ferrero S. Aromatase Inhibitors for the Treatment of Endometriosis: A Systematic Review about Efficacy, Safety and Early Clinical Development. Expert Opin. Investig. Drugs. 2020;29:1377–1388. doi: 10.1080/13543784.2020.1842356. [DOI] [PubMed] [Google Scholar]
- 39.Dababou S., Garzon S., Laganà A.S., Ferrero S., Evangelisti G., Noventa M., D’Alterio M.N., Palomba S., Uccella S., Franchi M., et al. Linzagolix: A New GnRH-Antagonist under Investigation for the Treatment of Endometriosis and Uterine Myomas. Expert Opin. Investig. Drugs. 2021;30:903–911. doi: 10.1080/13543784.2021.1957830. [DOI] [PubMed] [Google Scholar]
- 40.Zajec V., Mikuš M., Vitale S.G., D’alterio M.N., Gregov M., Šarić M.J., Carugno J., Angioni S., Ćorić M. Current Status and Challenges of Drug Development for Hormonal Treatment of Endometriosis: A Systematic Review of Randomized Control Trials. Gynecol. Endocrinol. 2022:1–8. doi: 10.1080/09513590.2022.2109145. [DOI] [PubMed] [Google Scholar]
- 41.Hoogstad-van Evert J., Paap R., Nap A., van der Molen R. The Promises of Natural Killer Cell Therapy in Endometriosis. Int J. Mol. Sci. 2022;23:5539. doi: 10.3390/ijms23105539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szegeczki V., Fazekas L., Kulcsár M., Reglodi D., Török P., Orlik B., Laganà A.S., Jakab A., Juhasz T. Endometrium as Control of Endometriosis in Experimental Research: Assessment of Sample Suitability. Diagnostics. 2022;12:970. doi: 10.3390/diagnostics12040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar P., Bhattacharya P., Prabhakar B.S. A Comprehensive Review on the Role of Co-Signaling Receptors and Treg Homeostasis in Autoimmunity and Tumor Immunity. J. Autoimmun. 2018;95:77–99. doi: 10.1016/j.jaut.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.