Abstract
Rice is a staple cereal crop worldwide, and increasing its yields is vital to ensuring global food security. Salinity is a major factor that affects rice yield. Therefore, it is necessary to investigate salt tolerance mechanisms in rice. Proteins containing WD40 repeats play important roles in eukaryotic development and environmental adaptation. Here, we showed that overexpression of OsABT, a gene encoding a WD40-repeat protein, enhanced salt tolerance in rice seedlings by regulating root activity, relative conductivity, malondialdehyde and H2O2 content, and O2•− production rate. Root ion concentrations indicated that OsABT overexpression lines could maintain lower Na+ and higher K+/Na+ ratios and upregulated expression of salt-related genes OsSOS1 and OsHAK5 compared with the wild-type (WT) Nipponbare plants. Furthermore, Overexpression of OsABT decreased the abscisic acid (ABA) content, while downregulating the ABA synthesis genes OsNCED3 and OsNCED4 and upregulating the ABA catabolic gene OsABA8ox2. The yeast two-hybrid and bimolecular fluorescence complementation analyses showed that OsABT interacted with the ABA receptor proteins OsPYL4, OsPYL10, and PP2C phosphatase OsABIL2. A transcriptome analysis revealed that the differentially expressed genes between OsABT overexpression lines and WT plants were enriched in plant hormone signal transduction, including ABA signaling pathway under salt stress. Thus, OsABT can improve the salt tolerance in rice seedling roots by inhibiting reactive oxygen species accumulation, thereby regulating the intracellular Na+/K+ balance, ABA content, and ABA signaling pathway.
Keywords: rice, root, salt stress, WD40 proteins, ABA, Na+ and K+, ion toxicity, transcriptome analysis
1. Introduction
Abiotic stresses, such as salt stress, drought, and extreme temperatures, seriously affect the growth and productivity of rice [1]. Salinity stress is one of the major constraints to rice growth, affecting over 20% of all arable land globally [2]. As an essential organ for rice growth and development, roots not only anchor plants and take up water and nutrients from the soil but also synthesize many substances [3]. Roots are the primary site for salt stress signal perception and can act as an early warning system [4].
Salt stress can cause ion toxicity in plants and interfere with K+ and Na+ homeostasis in the cytoplasm, thereby damaging root cell membrane selectivity [5]. Plants use the salt overly sensitive (SOS) pathway for salt stress signaling and development of Na+ tolerance [6,7]. OsSOS1 mediates Na+ influx and Na+ redistribution at the xylem parenchyma boundary and regulates Na+ efflux in the roots [8]. Vacuolar compartmentalization of Na+ can also reduce its accumulation in the cytoplasm, where the vacuolar Na+/H+ antiporter OsNHXs play an important role in regulating rice salt tolerance [9,10,11,12]. Potassium plays a key role in regulating plant growth and cellular salt tolerance [13]. Several genes encoding root K+ uptake channels have been identified in rice. OsAKT1, OsKAT1, OsHAK1, OsHAK5, and OsHAK21 participate in salt stress responses by increasing K+ uptake and maintaining a low Na+/K+ ratio [14,15,16,17,18].
Reactive oxygen species (ROS) have been identified as important signaling molecules that regulate root development, cell differentiation, elongation, and hair formation as well as lateral root emergence [19,20,21,22]. However, a large number of intracellular ROS are produced under stress, causing oxidative damage to proteins, RNA, and DNA molecules. Therefore, the ability to scavenge ROS is of great significance in improving stress tolerance [23]. It was reported that a drought-sensitive mutant 1 (dsm1) regulates the response to drought stress by regulating ROS scavenging in rice [24]. OsVTC1-3 RNAi rice accumulated a large amount of O2•− and H2O2 in the roots under salt stress, and the tolerance to salt stress was reduced [25]. Overexpression of OsMADS25 can reduce the level of ROS in rice roots by enhancing the activity of antioxidant enzymes, regulating root growth, and conferring salt tolerance [26]. OsR3L1 maintains ROS homeostasis under salt stress by regulating the activities of ROS-scavenging enzymes in the early stages of root development, thereby improving salt tolerance [27]. These results show that ROS play an important role in the growth and development of rice roots.
Abscisic acid (ABA) plays a key role in plant development by regulating a series of physiological processes, such as seed germination and dormancy, root development, stomatal regulation, and abiotic stress resistance [28]. ABA is biosynthesized in the root phloem and transported to the leaves and other tissues to perform its function [29]. ABA accumulates significantly under osmotic stress conditions such as drought and salinity [30,31,32]. Under salt stress conditions, ABA binds to receptors that regulate the expression of ABA-responsive genes through the ABA signaling pathway [33,34,35,36,37] that has three core components: PYR/PYL/RCAR ABA receptors (PYLs), type 2C protein phosphatases (PP2Cs), and SNF1-related protein kinase 2 (SnRK2s) [38]. In recent years, some candidate genes related to the ABA signaling pathway have been identified that play a key role in promoting root stress response in rice [39,40,41,42,43,44].
WD40 proteins are extremely abundant and highly conserved in eukaryotes [45]. They act as important flexible scaffolds that mediate protein–protein or protein-DNA interactions and play pivotal roles in diverse cellular processes, including RNA processing, immune responses, signal transduction, gene regulation, microtubule organization, and hormone response [46,47,48,49]. The ABA signaling terminator gene (ABT), which encodes a WD40-repeat protein in Arabidopsis, is critical for seed germination and seedling establishment [50]. In our previous studies, we identified OsABT (Os03g0738700) and found that its overexpression significantly enhanced salt tolerance in rice seedlings [51]. However, the mechanism underlying salt tolerance mediated by OsABT remains unknown. In this study, we demonstrate that OsABT can improve the salt tolerance of rice roots at the seedling stage by inhibiting ROS accumulation, maintaining intracellular Na+ and K+ balance, and regulating ABA content. Meanwhile, the OsABT protein interacts with OsABIL2, OsPYL4, and OsPYL10 to mediate root salt tolerance by affecting the ABA signaling pathway and the expression of ABA-related genes at the rice seedling stage.
2. Results
2.1. Identification of OsABT Overexpression Rice
To identify the functions of OsABT, we transformed it into wild-type (WT) Nipponbare plants and identified two-week-old T6 generation transgenic rice OsABT–OE lines (OE–3 and OE–4) using reverse transcription-polymerase chain reaction (RT-PCR) and quantitative real-time polymerase chain reaction (qRT-PCR) (Figure S1). The transgenic rice lines OE–3 and OE–4 showed significantly higher OsABT expression of roots than the WT plants and thus could be deemed as OsABT overexpression lines, which were used for subsequent experiments.
2.2. Overexpression of OsABT Enhances the Salt Tolerance of Rice Seedlings
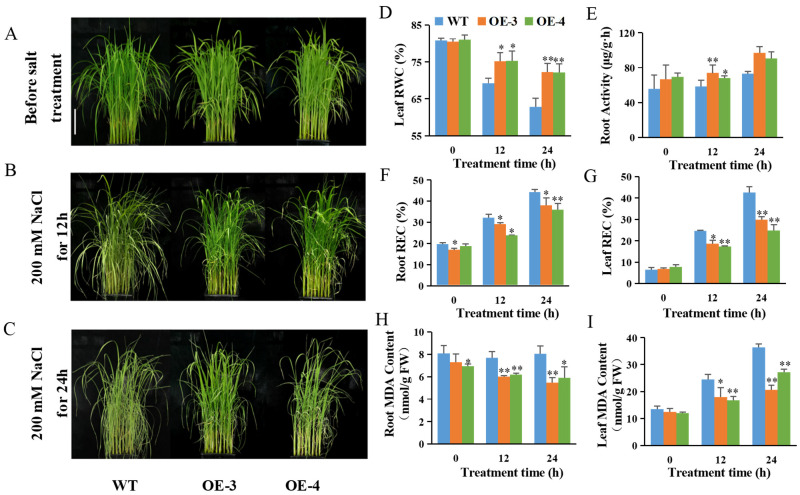
To further investigate the salt tolerance mechanism mediated by OsABT, the OsABT–OE lines (OE–3 and OE–4) and WT plants were exposed to 200 mM NaCl. Before salt treatment, the OsABT–OE and WT phenotypes did not significantly differ from each other (Figure 1A). After salt treatment for 12 and 24 h, the OsABT–OE lines showed significantly less leaf curling and higher relative leaf water content than the WT plants (Figure 1B–D). These results indicate that the OsABT overexpression lines had a better salt-tolerant phenotype at the seedling stage.
Figure 1.
Overexpression of OsABT enhances the salt tolerance of rice seedlings. (A–C) Phenotypes of two-week-old OsABT–OE lines (OE–3 and OE–4) and wild-type (WT) plants under 200 mM NaCl for 0, 12 and 24 h. Scale Bar: 5 cm. (D–I) Relative leaf water content; root activity; relative root and leaf conductivity; root and leaf malondialdehyde (MDA) content of OsABT–OE lines and WT under 200 mM NaCl for 0, 12, and 24 h. Data are means ± SD of three independent experiments. Asterisks indicate significant differences compared to WT plants at * p < 0.05 and ** p < 0.01 (Student’s t-test).
The two-week-old rice seedlings were in a rapid growth period, and their root activity gradually increased. Salt treatment inhibited this increase in root activity. Before salt treatment, no significant difference in root activity was observed between the OsABT–OE lines and WT plants. At 24 h of salt treatment, the root activity of OsABT–OE lines was significantly higher than that of WT plants (Figure 1E). The relative conductivity of roots and leaves increased gradually with the extension of salt treatment time. Under salt stress, the relative conductivity of the roots and leaves of the OsABT–OE lines was significantly lower than that of WT, especially at 24 h of salt treatment (Figure 1F,G). The malondialdehyde (MDA) content in the roots and leaves of the OsABT–OE lines was also lower than that of the WT plants (Figure 1H,I). These results suggest that OsABT can improve the salt tolerance of rice seedlings by increasing root activity and reducing relative conductivity and MDA content.
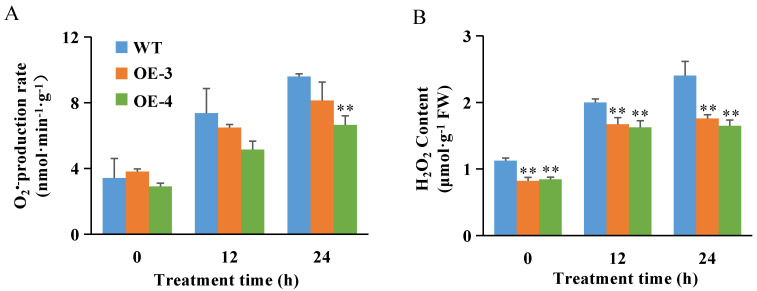
Salt stress leads to ROS accumulation that adversely affects plant tissues [23]. Although the O2•− production rate and H2O2 content in rice roots increased in all plant lines following 12-h and 24-h salt treatments, these indicators were significantly lower in OsABT–OE lines than in WT plants at the same time of salt treatment (Figure 2). These results show that the OsABT overexpression lines may enhance the salt tolerance of rice by inhibiting O2•− production and reducing H2O2 content in roots, indicating that OsABT has an inhibitory effect on ROS accumulation under salt stress.
Figure 2.
Changes of reactive oxygen species (ROS) in roots of various rice plants under salt stress. O2•− production rate (A) and H2O2 content (B) in roots of two–week–old OsABT–OE lines (OE–3 and OE–4) and WT plants under 200 mM NaCl for 0, 12, and 24 h. Data are means ± SD of three independent experiments. Asterisks indicate significant differences compared to WT plants at ** p < 0.01 (Student’s t–test).
2.3. Overexpression of OsABT Regulates Na+ and K+ Levels under Salt Stress
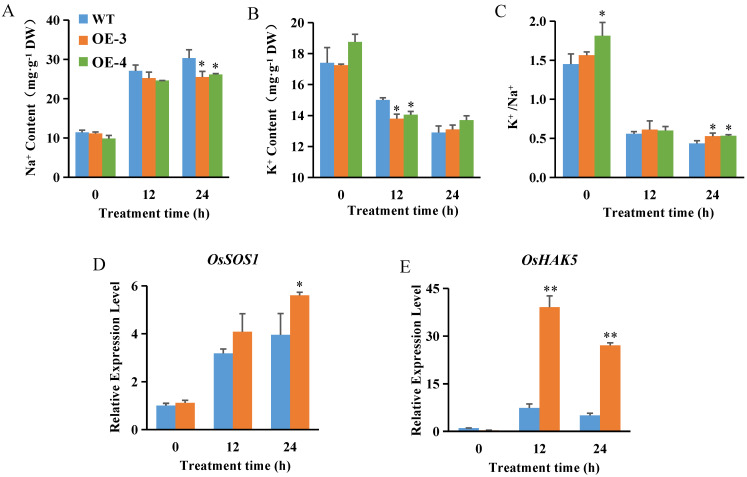
An important aspect of salt tolerance is the avoidance of Na+ accumulation and K+ homeostasis [52]. Two-week-old hydroponically grown OsABT–OE lines and WT plants were exposed to 200 mM NaCl. The level of Na+ in the roots increased continuously in all plant lines under salt stress. However, the OsABT–OE lines accumulated significantly less Na+ than the WT plants, especially after 24 h of salt treatment (Figure 3A). In contrast to Na+ levels, K+ levels declined in the roots of all plant lines during salt stress. At 24 h of salt treatment, the K+ content in OsABT–OE lines was higher than that in WT plants (Figure 3B). A decrease in the K+/Na+ ratio was detected in all plant lines under salt stress. However, a markedly higher K+/Na+ ratio was observed in the OsABT–OE lines than in the WT plants after 24 h of salt treatment (Figure 3C). These results imply that OsABT can inhibit Na+ accumulation and maintain a high K+/Na+ ratio in the roots, thereby maintaining the balance between Na+ and K+ in rice root cells.
Figure 3.
Overexpression of OsABT regulates Na+ and K+ levels under salt stress. (A–C) Na+ content, K+ content, and K+/Na+ ratio in the roots of two–week–old OsABT–OE lines (OE–3 and OE–4) and wild type (WT) plants exposed to salt stress (200 mM NaCl) for 0, 12, and 24 h. (D,E) Expression of OsSOS1 and OsHAK5 in the roots of two–week–old OsABT–OE line (OE–3) and WT plants. Root RNA was isolated and used for quantitative real–time polymerase chain reaction (qRT–PCR). Actin was used as an internal control. The relative expression levels were represented by fold change relative to the expression of WT before salt treatment (0 h). Data are means ± SD of three independent experiments. Asterisks indicate significant differences compared to WT plants at * p < 0.05 and ** p < 0.01 (Student’s t–test).
Next, we determined the root expression of OsSOS1 and OsHAK5 by qRT-PCR. Before salt treatment, the expression of OsSOS1 and OsHAK5 was not significantly different between the OsABT–OE line (OE–3) and WT plants. Salt stress significantly induced the expression of these genes in all plant lines; however, this tendency was enhanced in OE–3 plants. After 24 h of salt treatment, the root expression levels of OsSOS1 and OsHAK5 in OE–3 were 1.42- and 5.41-fold of that in WT, respectively (Figure 3D,E). These results reveal that overexpression of OsABT regulates Na+ and K+ levels in rice roots under salt stress by enhancing the expression of OsSOS1 and OsHAK5 genes.
2.4. Overexpression of OsABT Inhibits ABA Synthesis under Salt Stress
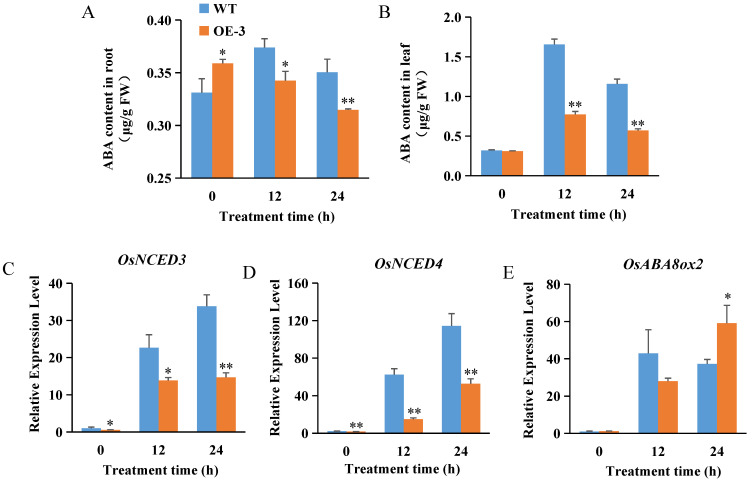
Abscisic acid (ABA) greatly contributes to plant growth and development and plays a key role in controlling the adaptive response of plants to environmental stresses. ABA accumulation and biosynthesis genes are upregulated under salt stress [53]. We found that the ABA content in the roots of the OsABT–OE line (OE–3) was lower than that of the WT plants. Meanwhile, the leaf ABA content showed first an increasing and then a decreasing trend in all plant lines under salt stress, but this tendency was weakened in OE–3 (Figure 4A,B).
Figure 4.
Overexpression of OsABT inhibits ABA synthesis under salt stress. (A,B) Root and leaf ABA content of the two–week–old OsABT–OE line (OE–3) and WT plants exposed to salt stress (200 mM NaCl) for 0, 12, and 24 h. (C–E) Root expression of the ABA synthesis genes OsNCED3 and OsNCED4, and the ABA catabolic gene OsABA8ox2 in the two–week–old OsABT–OE line (OE–3) and WT plants. Root RNA was isolated and used for qRT–PCR. Actin was used as an internal control. The relative expression levels were represented by fold change relative to the expression in WT plants before salt treatment (0 h). Data are means ± SD of three independent experiments. Asterisks indicate significant differences compared to WT at * p < 0.05 and ** p < 0.01 (Student’s t–test).
The root expression of the ABA synthesis genes OsNCED3 and OsNCED4, and the ABA catabolic gene OsABA8ox2 were further examined. The results showed that the expression of these genes was dramatically induced in all plant lines under salt stress. Compared to the WT plants, the expression of OsNCED3 and OsNCED4 in OE–3 was lower, while that of OsABA8ox2 in OE–3 was higher. At 24 h of salt treatment, the expression levels of OsNCED3 and OsNCED4 in OE–3 was only 43.55% and 46.24% of WT, while the expression of OsABA8ox2 was 1.59-fold of WT (Figure 4C–E). ABA is synthesized in roots and transported to the leaves for accumulation under salt stress [33]. These results indicate that OsABT can inhibit ABA synthesis in rice roots by inhibiting the expression of ABA synthesis genes and promoting the expression of ABA catabolic genes, thereby reducing the leaf ABA content.
2.5. OsABT Interacts with Key Factors of the ABA Signaling Pathway
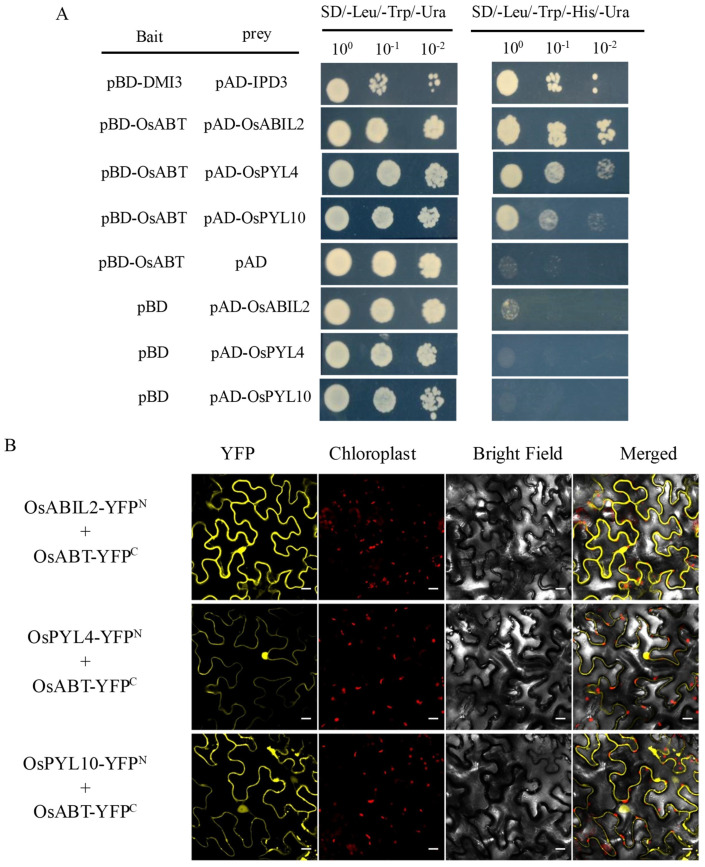
The ABA signaling pathway is regulated by three major components: PYR/PYL/RCAR ABA receptors (PYLs), type 2C protein phosphatases (PP2Cs), and SNF1-related protein kinase 2 (SnRK2s) [38]. Based on the ABA content and ABA biosynthetic gene expression results, we speculated that OsABT likely regulates the ABA signaling pathway. Therefore, we performed a yeast two-hybrid (Y2H) analysis to identify the interaction between OsABT and the ABA receptors OsPYL4, OsPYL10, and the PP2C protein OsABIL2. In the SD/-Leu/-Trp/-His/-Ura medium, OsABT fused to the GAL4-binding domain (BD) interacted with OsABIL2, OsPYL4, and OsPYL10 fused to the GAL4 activation domain (AD) in yeast cells (Figure 5A).
Figure 5.
OsABT interacts with key factors of the ABA signaling pathway. (A) Yeast two hybrid (Y2H) assay of the interactions between OsABT and the three ABA signaling proteins OsABIL2, OsPYL4, and OsPYL10. GAL4 binding domain (BD) and GAL4 activation domain were co–transformed into yeast YRG2, and a series of 2 μL aliquots of the diluted (OD600 = 1, 0.1, 0.01) co–transformed YRG2 culture was spotted on SD/−Leu/−Trp/−Ura and SD/−Leu/−Trp/−His/−Ura media and incubated for 4 days. (B) Bimolecular fluorescence complementation (BiFC) assay of the interactions between OsABT and OsABIL2, OsPYL4, OsPYL10 in Nicotiana benthamiana leaves. Scale bars: 50 μm.
To further confirm the protein-protein interaction between OsABT and OsABIL2, OsPYL4, and OsPYL10 in plant cells, bimolecular fluorescence complementation (BiFC) assays were used. The C- and N-terminal regions of the yellow fluorescent protein (YFP) were coupled to OsABT and the three ABA signaling proteins OsABIL2, OsPYL4, and OsPYL10. These constructs were co-induced into the leaf cells of Nicotiana benthamiana. A reconstituted YFP signal was observed in both cytoplasm and nucleus, whereas no YFP signal was observed in the negative control cells harboring an empty vector (Figure 5B, Figure S2). These data indicate that OsABT physically interacts with OsABIL2, OsPYL4, and OsPYL10 in plant cells.
2.6. Transcriptomic Analysis of OsABT Overexpression Rice Roots under Salt Stress
To elucidate the molecular network underlying OsABT-regulated salt tolerance, we examined changes in gene expression in the roots of a two-week-old OsABT–OE line (OE–3) and WT plants using transcriptome deep sequencing (RNA-seq). We identified 1950 differentially expressed genes (DEGs; 954 upregulated and 996 downregulated) in OE–3 plants compared to the WT before salt treatment and 3499 DEGs (2955 upregulated and 544 downregulated) after 24 h of salt treatment (Figure S3). Considering that OsABT interacts with key factors in the ABA signaling pathway, we analyzed genes associated with plant hormone signal transduction, including ABA signal transduction, in the transcriptome data obtained for plants under salt stress (Figure S4).
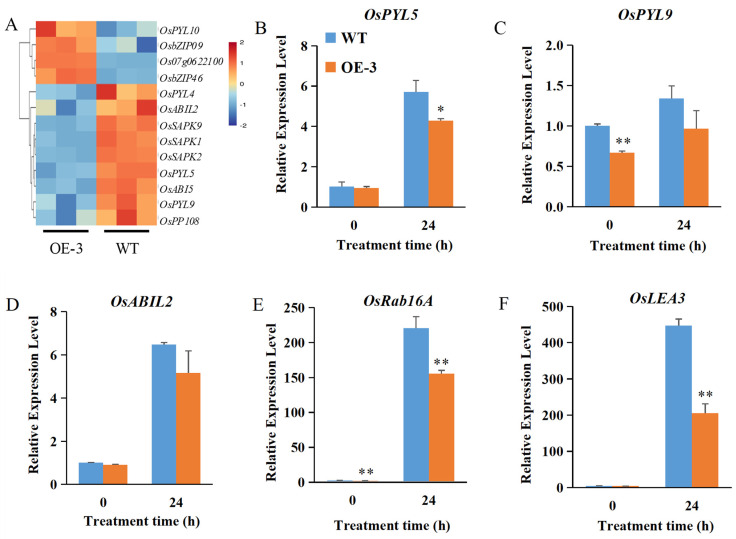
We found that 13 DEGs in the ABA signaling core were regulated by OsABT after 24 h of salt treatment. Compared with WT, nine DEGs related to the ABA signal transduction pathway were downregulated in the roots of the OsABT–OE line, which included ABA receptors OsPYL4, OsPYL9, and OsPYL5; PP2C family proteins OsPP108 and OsABIL2; SnRK2 family proteins OsSAPK1, OsSAPK2, and OsSAPK9; and bZIP transcription factor OsABI5 (Figure 6). In contrast to the expression of ABA signal transduction genes, most DEGs in the other seven plant hormone signal transduction pathways were upregulated in the roots of the OsABT–OE line (Figure S5). These results suggest that OsABT negatively regulates the ABA signal transduction pathway.
Figure 6.
OsABT negatively regulates ABA signal transduction pathway. (A) Cluster analysis of differentially expressed genes (DEGs) in ABA signal transduction between OE–3 and WT at 24 h of salt treatment. Each column in the figure represents a sample, and each row represents a gene. Log2 processing was performed on the fragments per kilobase of exon per million mapped fragments (FPKM) value of these DEGs. (B–F) Root expression levels of ABA–related genes in 2–week–old OsABT–OE line (OE–3) and WT plants. Root RNA was isolated and used for qRT–PCR. Actin was used as an internal control. The relative expression levels were represented by fold change relative to the WT expression levels before salt treatment (0 h). Data are means ± SD of three independent experiments. Asterisks indicate significant differences compared to WT at * p < 0.05 and ** p < 0.01 (Student’s t–test).
To confirm these results, we detected the expression of DEGs using qRT-PCR. The expression of the ABA signal transduction-related genes OsPYL5, OsPYL9, and OsABIL2; ABA-responsive genes OsLEA3 and OsRAB16A was significantly induced by salt stress. Compared to the WT roots, the OE–3 roots had a lower expression of these genes. At 24 h of salt treatment, the expression of the ABA-responsive genes OsLEA3 and OsRAB16 decreased by 54.10% and 29.58% compared with the WT plant, while ABA signal transduction-related genes OsPYL5, OsPYL9, and OsABIL2 decreased by 26.38%, 28.01%, and 20.41%, respectively. These results are consistent with the transcriptome data and show that OsABT negatively regulates the expression of ABA-related genes.
3. Discussion
The root is a vital organ for absorbing water and other nutrients. More importantly, several studies have reported that modulating root development improves stress tolerance and increases yield in crops [3,4,54]. In this study, we found that OsABT overexpression lines showed an obvious and stable salt-tolerant phenotype compared to WT under 200 mM NaCl treatment (Figure 1A–C). At the same time, the relative conductivity, MDA content, O2•− production rate, and H2O2 content in the roots of OsABT overexpression lines were lower than those of WT (Figure 1F–I and Figure 2). It is well known that ROS homeostasis is related to oxidative stress. At low cellular concentrations, ROS act as signaling molecules. Under severe biotic and abiotic stresses, ROS overproduction leads to impaired cell growth [23]. Therefore, plants have evolved complex systems to regulate ROS homeostasis and protect themselves from oxidative stress [24,25,26,27]. OsABT can enhance salt tolerance in rice by inhibiting the production of O2•− and H2O2 in the roots of rice seedlings.
Salt stress can cause cytoplasmic Na+ accumulation and cell damage in rice. In addition, it causes reduction in the cellular K+ content, changes in the intracellular membrane potential, and hyperpolarization of the cell membrane. Therefore, maintaining the balance between Na+ and K+ in cells is essential for rice growth under salt stress [55]. Studies have shown that salt stress can lead to an increase in the Na+ content and a decrease in the K+ content of the roots and leaves [56], consistent with the changes in Na+ and K+ concentrations under salt stress in this study. Meanwhile, the roots of OsABT overexpression lines had lower Na+ content, lower K+ content, and higher K+/Na+ ratios than those of WT (Figure 3A–C). Furthermore, the root expression of OsSOS1 and OsHAK5 in OsABT overexpression lines was significantly upregulated compared with the WT plants under salt stress (Figure 3D,E), indicating that OsABT may regulate the expression of OsSOS1 and OsHAK5 to reduce the accumulation of Na+ and maintain the balance of Na+ and K+ in root cells under salt stress, thereby improving salt tolerance in rice.
Plant hormones, particularly ABA, can control gene expression through signal transduction and increase plant stress resistance [28]. Huang et al. [57] demonstrated that 9-cis-epoxycarotenoid dioxygenase (NCED) is a rate-limiting enzyme in ABA biosynthesis. OsABA8ox encodes ABA 8′-hydroxylase that catalyzes the committed step of ABA catabolism [58]. In this study, ABA content in the roots and leaves of OsABT overexpression lines was lower than that in WT plants (Figure 4A,B). Meanwhile, the root expression of OsNCED3 and OsNCED4 in the OsABT overexpression lines was lower than that in the WT plants under salt treatment, whereas the ABA catabolic gene OsABA8ox2 had the opposite effect (Figure 4C–E). It is well known that ABA is biosynthesized in the root phloem and transported to the leaves and other tissues to function [33], showing that OsABT regulates the ABA content by inhibiting the expression of ABA synthesis genes and promoting the expression of ABA catabolic genes in rice roots, thereby affecting the accumulation of ABA in leaves.
As scaffold protein, proteins containing WD40 repeats may function as a component of protein complexes [47]. The WD40 protein XIW1 is a nucleocytoplasmic shuttle protein and plays an active role in ABA responses by interacting with and maintaining stability of ABI5 in the nucleus [59]. OsRACK1A (a WD40 type protein) can interacted with many salt-stress suppressed proteins directly [52]. ABT is a protein containing seven WD40 repeats in Arabidopsis. Wang et al. [50] found ABT interacts with PYR1/PYL/RCAR (PYR1, PYL4) and PP2C proteins (ABI1, ABI2), and hampers the inhibition of ABI1/ABI2 by ABA-bound PYR1/PYL4, thereby terminating ABA signaling. A rice ortholog of AtABI1 and AtABI2, named OsABI-LIKE2 (OsABIL2), is localized in both the nucleus and cytosol. OsABIL2 play an important role in regulating root development. In the presence of ABA, OsABIL2 can interact with OsPYL1, redistributing from nucleus to the cytosol and releasing the inhibition on SAPK8/10. Phosphorylated SAPK8/10 can activate downstream transcription factors to regulate the expression of ABA-responsive gene expression [60]. In this study, we showed that OsABT interacts with OsABIL2, OsPYL4, and OsPYL10 via Y2H and BiFC analyses (Figure 5). The transcriptome and qRT-PCR analyses showed that several core ABA signaling and ABA-responsive genes were obviously regulated by OsABT, such as OsPYL5, OsPYL9, OsABIL2, OsLEA3 and OsRAB16A (Figure 6), indicating that OsABT regulates salt tolerance in rice seedling roots through the ABA signaling pathway, and is particularly important for OsABIL2-OsPYLs complexes.
Therefore, the relationship between ABA and salt tolerance deserves further attention. Xu et al. [61] found that the accumulation of ABA and the expression of the ABA synthesis gene OsNCED3 were upregulated in SiMYB19 transgenic rice, which showed improved salt tolerance compare with WT rice. Zhang et al. [62] found that OsNAC45 positively regulates the ABA signaling pathway and confers salt tolerance in rice. Interestingly, OsSAE1 negatively regulates the ABA signaling pathway and positively regulates salt tolerance in rice seedlings by inhibiting the expression of OsABI5 [63]. Chen et al. [51] found that OsABT inhibits the ABA signaling pathway by interacting with OsABI2, and the tolerance to salt stress was higher in OsABT overexpressing Arabidopsis than in wild type plants. In this study, OsABT negatively regulated ABA synthesis and signaling pathways and conferred salt tolerance in rice. Salt tolerance is a complex trait involving many genes and pathways, but accumulating evidence has revealed multifaceted crosstalk between the ABA signaling pathway and the SOS signaling pathway. For example, SOS2 is able to physically interact with ABI2 and it is disrupted by the abi2-1 mutation, which causes increased tolerance to salt shock and ABA insensitivity in plants [64]. Meanwhile, there is an inseparable connection between the ABA signaling and ROS. ABA regulates ROS production through plasma membrane and mitochondria-localized NADPH oxidases. These ROS act as important secondary messengers in regulating root growth, stomatal movement, and seed germination through the ABA signaling pathway [65]. In vitro analyses indicated that H2O2 could in turn inactivate the activities of the PP2C enzymes ABI1 and ABI2 [66,67]. In the future, it will be interesting to know whether OsABT interacts with salt stress-related proteins, especially Na+/K+ transporters, and reduces the ROS levels in rice roots by negatively regulating the ABA signaling pathway.
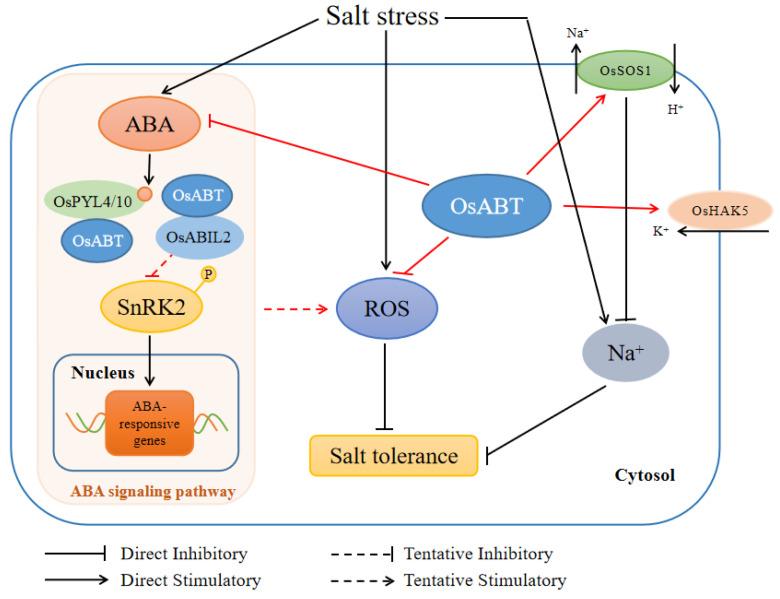
In conclusion, based on our experimental results and relevant literature, we proposed a working model for the OsABT gene to improve the salt tolerance of roots in rice seedlings (Figure 7). Salt stress causes ROS accumulation in rice roots, which seriously affects root salt tolerance in rice seedlings. OsABT can inhibit ROS accumulation, thereby reducing the damage caused by ROS to cells under salt stress. Moreover, OsABT maintains the intracellular Na+ and K+ balance by enhancing the expression of OsSOS1 and OsHAK5 in roots. OsABT also inhibits the expression of the ABA synthesis genes OsNCED3 and OsNCED4 and promotes the expression of the ABA catabolic gene OsABA8ox2, thereby inhibiting the accumulation of ABA. Meanwhile, OsABT interacts with OsPYL4/10 and OsABIL2, and negatively regulates the expression of ABA signal transduction-related genes and ABA responsive genes, resulting in the attenuation of ABA signaling. Furthermore, it was reported the ABA signaling pathway is related to ROS and SOS signaling pathway. Thus, OsABT may affect the ROS levels and Na+/K+ balance through the ABA signaling pathway to regulate root salt tolerance in rice seedlings.
Figure 7.
A proposed model illustrating the mechanism of the OsABT gene to improve the salt tolerance of roots at the rice seedling stage. OsABT confers salt tolerance to rice seedling roots by inhibiting ROS accumulation, maintaining intracellular Na+ and K+ balance, and regulating the ABA content. Meanwhile, OsABT interacts with OsPYL4/10 and OsABIL2, and negatively regulates the expression of ABA signal transduction-related genes and ABA responsive genes, resulting in the attenuation of ABA signaling. OsABT may affect the ROS levels and Na+/K+ balance through the ABA signaling pathway to regulate root salt tolerance in rice seedlings.
4. Materials and Methods
4.1. Plant Materials and Grown Conditions
Rice (Oryza sativa L. cv. Nipponbare) was used as the wild-type control for physiological experiments and as a recipient for genetic transformation in this study. The 35S::OsABT overexpression rice lines (OsABT–OE) were used as experimental materials [51]. Rice seeds were soaked in water for 2 days and germinated on a damp gauze for 2 days. The rice seeds were then transferred into 96-well plates and grown in a growth room at temperatures of 30 °C with a 14 h light/10 h dark photoperiod. After culturing in water for 3 days, 1/2 of Hoagland’s solution (pH 5.5–6.0) for 3 days, and then the full Hoagland’s solution (pH 5.5–6.0) every 3 days. For the NaCl treatment, two-week-old rice seedlings were exposed to 200 mM NaCl for 0, 12, and 24 h. The roots were quickly frozen in liquid nitrogen and stored in at −80 °C.
4.2. Measurements of Physiological Index
Root activity was measured using the α-naphthylamine oxidation method [68], and relative conductivity was measured using a DDS-307 conductivity meter (LeiCi, Shanghai, China). The MDA content was determined using the thiobarbituric acid method [69]. The O2•− production rate was determined using the hydroxylamine oxidation method [70]. The H2O2 content was determined using a kit (Grace, Jiangsu, China). To measure the content of Na+ and K+, the roots were washed three times with deionized water and dried at 80 °C for 3 days. All samples were weighed and digested with 5 mL of concentrated HNO3. Subsequently, the sample was diluted with deionized water to 25 mL, kept for 1–2 days, and then analyzed by Inductively coupled plasma atomic emission spectroscopy (ICP-AES; ICP9000, Shimadzu, Japan) [71]. The ABA content in rice roots and leaves was measured using high-performance liquid chromatography (HPLC; Waters 2695, USA) [72].
4.3. RNA Extraction and qRT-PCR Analyses
Total RNA was extracted from rice roots using an RNA extraction kit (CWBIO, Jiangsu, China), and the first strand of cDNA was synthesized using a HiFiScript cDNA Synthesis Kit (CWBIO, Jiangsu, China). qRT-PCR was performed using the SYBR Premix Ex TaqTM II kit (TaKaRa, Dalian, China), and the amplification reaction was performed using a CFX96TM fluorescence quantitative PCR instrument (Bio-Rad, CA, USA). Each reaction was conducted in three biological and technical replicates. The rice Actin gene (Os03g0718100) was used as an internal standard. The relative expression levels of genes were calculated using the 2-ΔΔCT method. The primer sets used for the qRT-PCR are listed in Table S1.
4.4. Y2H Analysis
The full-length coding sequence (CDS) of OsABT was fused into a pBD-GAL4 Cam vector, and the full CDSs of OsABIL2, OsPYL4, OsPYL10 were fused with the pAD-GAL4-2.1 vector. Each construct was transformed into the yeast strain YRG2. The transformed yeast clones were first grown on the SD/-Leu/-Trp/-Ura medium and then transferred to the SD/-Leu/-Trp/-His/-Ura medium for 4 days at 30 °C. The primer sequences used for Y2H analysis are listed in Table S1.
4.5. BiFC Assay
To produce a fusion with either the N- or C-terminal fragment of YFP, the full-length coding regions of OsABT were subcloned into pCAMBIA1300-YFPC vectors, and OsABIL2, OsPYL4, and OsPYL10 were subcloned into the pCAMBIA1300-YFPN vectors. The corresponding BiFC plasmids and negative controls were co-expressed in N. benthamiana leaves. The cells were visualized 48 h after transformation, and images were captured using a confocal laser scanning microscope (Leica SP8; Leica Microsystems, Wetzlar, Germany).
4.6. Transcriptome Analysis
Total RNA was isolated in triplicate from the roots of OE–3 and WT treated with 200 mM NaCl for 0 and 24 h. Total root RNA was isolated and purified using the TRIzol reagent (Invitrogen, CA, USA) to obtain mRNA. A cDNA library was established using a reverse transcriptase and DNA polymerase, and paired-end sequencing was performed using Illumina Novaseq ™6000. Significant difference analysis was performed using the DEGseq2 software. Genes with a fold difference of more than two times, that is, fold change ≥ 2 or fold change ≤ 0.5 and p < 0.05, were defined as differentially expressed genes (DEGs) and were annotated using the GO and KEGG databases.
4.7. Statistical Analysis
The averages and standard deviations (SD) of all results were calculated, and Student’s t-tests was performed to generate p-values. All analyses were performed using the SPSS 20.0 software.
Acknowledgments
We thank Xia Li and Zhijuan Wang from Huazhong Agricultural University for their help in experimental ideas and methods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231810656/s1.
Author Contributions
Conceptualization, B.S.; Data curation, D.W. and E.C.; Investigation, D.W., L.B., X.H. and X.Q.; Project administration, B.S.; Writing—original draft, D.W. and E.C.; Writing—review & editing, B.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Zhejiang Science and Technology Major Program on Agricultural New Variety Breeding, grant number 2021C02063-6-4.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.He M., He C.Q., Ding N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018;9:1771. doi: 10.3389/fpls.2018.01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Zelm E., Zhang Y., Testerink C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020;71:403–433. doi: 10.1146/annurev-arplant-050718-100005. [DOI] [PubMed] [Google Scholar]
- 3.Seo D.H., Seomun S., Choi Y.D., Jang G. Root development and stress tolerance in rice: The key to improving stress tolerance without yield penalties. Int. J. Mol. Sci. 2020;21:1807. doi: 10.3390/ijms21051807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saini S., Kaur N., Pati P.K. Reactive oxygen species dynamics in roots of salt sensitive and salt tolerant cultivars of rice. Anal. Biochem. 2018;550:99–108. doi: 10.1016/j.ab.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Negrão S., Schmöckel S.M., Tester M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017;119:1–11. doi: 10.1093/aob/mcw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Ishitani M., Halfter U., Kim C.S., Zhu J.K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA. 2000;97:3730–3734. doi: 10.1073/pnas.97.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H., Ishitani M., Kim C., Zhu J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Shabala S., Shabala L., Zhou M., Meinke H., Venkataraman G., Chen Z., Zeng F., Zhao Q. Tissue-specific regulation of Na+ and K+ transporters explains genotypic differences in salinity stress tolerance in rice. Front. Plant Sci. 2019;10:1361. doi: 10.3389/fpls.2019.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda A., Nakamura A., Tanaka Y. Molecular cloning and expression of the Na+/H+ exchanger gene in Oryza sativa. Biochim. Biophys. Acta. 1999;1446:149–155. doi: 10.1016/S0167-4781(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda A., Nakamura A., Hara N., Toki S., Tanaka Y. Molecular and functional analyses of rice NHX-type Na+/H+ antiporter genes. Planta. 2011;233:175–188. doi: 10.1007/s00425-010-1289-4. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda A., Nakamura A., Tagiri A., Tanaka H., Miyao A., Hirochika H., Tanaka Y. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004;45:146–159. doi: 10.1093/pcp/pch014. [DOI] [PubMed] [Google Scholar]
- 12.Liu S., Zheng L., Xue Y., Zhang Q., Wang L., Shou H. Overexpression of OsVP1 and OsNHX1 increases tolerance to drought and salinity in rice. J. Plant Biol. 2010;53:444–452. doi: 10.1007/s12374-010-9135-6. [DOI] [Google Scholar]
- 13.Wu H., Zhang X., Giraldo J.P., Shabala S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil. 2018;431:1–17. doi: 10.1007/s11104-018-3770-y. [DOI] [Google Scholar]
- 14.Fuchs I., Stölzle S., Ivashikina N., Hedrich R. Rice K+ uptake channel OsAKT1 is sensitive to salt stress. Planta. 2005;221:212–221. doi: 10.1007/s00425-004-1437-9. [DOI] [PubMed] [Google Scholar]
- 15.Obata T., Kitamoto H.K., Nakamura A., Fukuda A., Tanaka Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiol. 2007;144:1978–1985. doi: 10.1104/pp.107.101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G., Hu Q., Luo L., Yang T., Zhang S., Hu Y., Yu L., Xu G. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015;38:2747–2765. doi: 10.1111/pce.12585. [DOI] [PubMed] [Google Scholar]
- 17.Yang T., Zhang S., Hu Y., Wu F., Hu Q., Chen G., Cai J., Wu T., Moran N., Yu L., et al. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 2014;166:945–959. doi: 10.1104/pp.114.246520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y., Shen L., Shen Z., Jing W., Ge H., Zhao J., Zhang W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ. 2015;38:2766–2779. doi: 10.1111/pce.12586. [DOI] [PubMed] [Google Scholar]
- 19.Yang L., Zhang J., He J., Qin Y., Hua D., Duan Y., Chen Z., Gong Z. ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet. 2014;10:e1004791. doi: 10.1371/journal.pgen.1004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukagoshi H., Busch W., Benfey P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Orman-Ligeza B., Parizot B., de Rycke R., Fernandez A., Himschoot E., Van Breusegem F., Bennett M.J., Périlleux C., Beeckman T., Draye X. RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development. 2016;143:3328–3339. doi: 10.1242/dev.136465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundaravelpandian K., Chandrika N.N.P., Schmidt W. PFT1, a transcriptional mediator complex subunit, controls root hair differentiation through reactive oxygen species (ROS) distribution in Arabidopsis. New Phytol. 2013;197:151–161. doi: 10.1111/nph.12000. [DOI] [PubMed] [Google Scholar]
- 23.Mittler R., Blumwald E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell. 2015;27:64–70. doi: 10.1105/tpc.114.133090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning J., Li X., Hicks L.M., Xiong L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010;152:876–890. doi: 10.1104/pp.109.149856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Zhao H., Qin H., Li Z., Liu H., Wang J., Zhang H., Quan R., Huang R., Zhang Z. The synthesis of ascorbic acid in rice roots plays an important role in the salt tolerance of rice by scavenging ROS. Int. J. Mol. Sci. 2018;19:3347. doi: 10.3390/ijms19113347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu N., Chu Y., Chen H., Li X., Wu Q., Jin L., Wang G., Huang J. Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA-mediated regulatory pathway and ROS scavenging. PLoS Genet. 2018;14:e1007662. doi: 10.1371/journal.pgen.1007662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao W., Wang K., Chang Y., Zhang B., Li F., Meng Y., Li M., Zhao Q., An S. OsHyPRP06/R3L1 regulates root system development and salt tolerance via apoplastic ROS homeostasis in rice (Oryza sativa L.) Plant Cell Environ. 2022;45:900–914. doi: 10.1111/pce.14180. [DOI] [PubMed] [Google Scholar]
- 28.Watkins J.M., Chapman J.M., Muday G.K. Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol. 2017;175:1807–1825. doi: 10.1104/pp.17.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z.Y., Kim D.H., Hwang I. ABA homeostasis and signaling involving multiple subcellular compartments and multiple receptors. Plant Cell Rep. 2013;32:807–813. doi: 10.1007/s00299-013-1396-3. [DOI] [PubMed] [Google Scholar]
- 30.Lalk I., Dörffling K. Hardening, abscisic acid, proline and freezing resistance in two winter wheat varieties. Physiol. Plant. 1985;63:287–292. doi: 10.1111/j.1399-3054.1985.tb04267.x. [DOI] [Google Scholar]
- 31.Singh N.K., Larosa P.C., Handa A.K., Hasegawa P.M., Bressan R.A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc. Natl. Acad. Sci. USA. 1987;84:739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo M., Liu J.H., Mohapatra S., Hill R.D., Mohapatra S.S. Characterization of a gene family encoding abscisic acid- and environmental stress-inducible proteins of alfalfa. J. Biol. Chem. 1992;267:15367–15374. doi: 10.1016/S0021-9258(19)49543-4. [DOI] [PubMed] [Google Scholar]
- 33.Leng P., Yuan B., Guo Y. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014;65:4577–4588. doi: 10.1093/jxb/eru204. [DOI] [PubMed] [Google Scholar]
- 34.Xie Y., Mao Y., Duan X., Zhou H., Lai D., Zhang Y., Shen W. Arabidopsis HY1-modulated stomatal movement: An integrative hub is functionally associated with ABI4 in dehydration-induced ABA responsiveness. Plant Physiol. 2016;170:1699–1713. doi: 10.1104/pp.15.01550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Y., Hao Q., Li W., Yan C., Yan N., Yin P. Identification and characterization of ABA receptors in Oryza sativa. PLoS ONE. 2014;9:e95246. doi: 10.1371/journal.pone.0095246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khadri M., Tejera N.A., Lluch C. Alleviation of salt stress in common bean (Phaseolus vulgaris) by exogenous abscisic acid supply. J. Plant Growth Regul. 2006;25:110–119. doi: 10.1007/s00344-005-0004-3. [DOI] [Google Scholar]
- 37.Etehadnia M., Waterer D.R., Tanino K.K. The method of ABA application affects salt stress responses in resistant and sensitive potato lines. J. Plant Growth Regul. 2008;27:331–341. doi: 10.1007/s00344-008-9060-9. [DOI] [Google Scholar]
- 38.Hubbard K.E., Nishimura N., Hitomi K., Getzoff E.D., Schroeder J.I. Early abscisic acid signal transduction mechanisms: Newly discovered components and newly emerging questions. Genes Dev. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu H., Dai M., Yao J., Xiao B., Li X., Zhang Q., Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu H., You J., Fang Y., Zhu X., Qi Z., Xiong L. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol. Biol. 2008;67:169–181. doi: 10.1007/s11103-008-9309-5. [DOI] [PubMed] [Google Scholar]
- 41.Gao F., Xiong A., Peng R., Jin X., Xu J., Zhu B., Chen J., Yao Q. OsNAC52, a rice NAC transcription factor, potentially responds to ABA and confers drought tolerance in transgenic plants. Plant Cell Tissue Organ Cult. 2010;100:255–262. doi: 10.1007/s11240-009-9640-9. [DOI] [Google Scholar]
- 42.Yuan X., Wang H., Cai J., Bi Y., Li D., Song F. Rice NAC transcription factor ONAC066 functions as a positive regulator of drought and oxidative stress response. BMC Plant Biol. 2019;19:1–19. doi: 10.1186/s12870-019-1883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H., Xu N., Wu Q., Yu B., Chu Y., Li X., Huang J., Jin L. OsMADS27 regulates the root development in a NO3−—Dependent manner and modulates the salt tolerance in rice (Oryza sativa L.) Plant Sci. 2018;277:20–32. doi: 10.1016/j.plantsci.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H., Liu X.-L., Zhang R.-X., Yuan H.-Y., Wang M.-M., Yang H.-Y., Ma H.-Y., Liu D., Jiang C.-J., Liang Z.-W. Root damage under alkaline stress is associated with reactive oxygen species accumulation in rice (Oryza sativa L.) Front. Plant Sci. 2017;8:1580. doi: 10.3389/fpls.2017.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stirnimann C.U., Petsalaki E., Russell R.B., Müller C.W. WD40 proteins propel cellular networks. Trends Biochem. Sci. 2010;35:565–574. doi: 10.1016/j.tibs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Smith T.F. The Coronin Family of Proteins. Springer; Berlin/Heidelberg, Germany: 2008. Diversity of WD-repeat proteins; pp. 20–30. [Google Scholar]
- 47.Xu C., Min J. Structure and function of WD40 domain proteins. Protein Cell. 2011;2:202–214. doi: 10.1007/s13238-011-1018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith T.F., Gaitatzes C., Saxena K., Neer E.J. The WD repeat: A common architecture for diverse functions. Trends Biochem. Sci. 1999;24:181–185. doi: 10.1016/S0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 49.Van Nocker S., Ludwig P. The WD-repeat protein superfamily in Arabidopsis: Conservation and divergence in structure and function. BMC Genom. 2003;4:1–11. doi: 10.1186/1471-2164-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z., Ren Z., Cheng C., Wang T., Ji H., Zhao Y., Deng Z., Zhi L., Lu J., Wu X. Counteraction of ABA-mediated inhibition of seed germination and seedling establishment by ABA signaling terminator in Arabidopsis. Mol. Plant. 2020;13:1284–1297. doi: 10.1016/j.molp.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Eryong C., Bo S. OsABT, a rice WD40 domain-containing protein, is involved in abiotic stress tolerance. Rice Sci. 2022;29:247–256. doi: 10.1016/j.rsci.2021.07.012. [DOI] [Google Scholar]
- 52.Zhang D., Wang Y., Shen J., Yin J., Li D., Gao Y., Xu W., Liang J. OsRACK1A, encodes a circadian clock-regulated WD40 protein, negatively affect salt tolerance in rice. Rice. 2018;11:1–15. doi: 10.1186/s12284-018-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cutler S.R., Rodriguez P.L., Finkelstein R.R., Abrams S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 54.Lee D.K., Chung P.J., Jeong J.S., Jang G., Bang S.W., Jung H., Kim Y.S., Ha S.H., Choi Y.D., Kim J.K. The rice OsNAC6 transcription factor orchestrates multiple molecular mechanisms involving root structural adaptions and nicotianamine biosynthesis for drought tolerance. Plant Biotechnol. J. 2017;15:754–764. doi: 10.1111/pbi.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adem G.D., Chen G., Shabala L., Chen Z.H., Shabala S. GORK channel: A master switch of plant metabolism? Trends Plant Sci. 2020;25:434–445. doi: 10.1016/j.tplants.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi R., Nishio T., Ichizen N., Takano T. Cloning and functional analysis of the K+ transporter, PhaHAK2, from salt-sensitive and salt-tolerant reed plants. Biotechnol. Lett. 2007;29:501–506. doi: 10.1007/s10529-006-9246-9. [DOI] [PubMed] [Google Scholar]
- 57.Huang Y., Jiao Y., Xie N., Guo Y., Zhang F., Xiang Z., Wang R., Wang F., Gao Q., Tian L. OsNCED5, a 9-cis-epoxycarotenoid dioxygenase gene, regulates salt and water stress tolerance and leaf senescence in rice. Plant Sci. 2019;287:110188. doi: 10.1016/j.plantsci.2019.110188. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Wang X., Luo Y., Zhang L., Yao Y., Han L., Chen Z., Wang L., Li Y. OsABA8ox2, an ABA catabolic gene, suppresses root elongation of rice seedlings and contributes to drought response. Crop. J. 2020;8:480–491. doi: 10.1016/j.cj.2019.08.006. [DOI] [Google Scholar]
- 59.Xu X., Wan W., Jiang G., Xi Y., Huang H., Cai J., Chang Y., Duan C.G., Mangrauthia S.K., Zhu J.K., et al. Nucleocytoplasmic trafficking of the Arabidopsis WD40 repeat protein XIW1 regulates ABI5 stability and abscisic acid responses. Mol. Plant. 2019;12:1598–1611. doi: 10.1016/j.molp.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Li C., Shen H., Wang T., Wang X. ABA regulates subcellular redistribution of OsABI-LIKE2, a negative regulator in ABA signaling, to control root architecture and drought resistance in Oryza sativa. Plant Cell Physiol. 2015;56:2396–2408. doi: 10.1093/pcp/pcv154. [DOI] [PubMed] [Google Scholar]
- 61.Xu C., Luo M., Sun X., Yan J., Shi H., Yan H., Yan R., Wang S., Tang W., Zhou Y. SiMYB19 from foxtail millet (Setaria italica) confers transgenic rice tolerance to high salt stress in the field. Int. J. Mol. Sci. 2022;23:756. doi: 10.3390/ijms23020756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X., Long Y., Huang J., Xia J. OsNAC45 is involved in ABA response and salt tolerance in rice. Rice. 2020;13:1–13. doi: 10.1186/s12284-020-00440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y., Zhou J., Li Z., Qiao J., Quan R., Wang J., Huang R., Qin H. SALT AND ABA RESPONSE ERF1 improves seed germination and salt tolerance by repressing ABA signaling in rice. Plant Physiol. 2022;189:1110–1127. doi: 10.1093/plphys/kiac125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohta M., Guo Y., Halfter U., Zhu J.K. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc. Natl. Acad. Sci. USA. 2003;100:11771–11776. doi: 10.1073/pnas.2034853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwak J.M., Mori I.C., Pei Z.-M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D., Schroeder J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meinhard M., Grill E. Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett. 2001;508:443–446. doi: 10.1016/S0014-5793(01)03106-4. [DOI] [PubMed] [Google Scholar]
- 67.Meinhard M., Rodriguez P.L., Grill E. The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta. 2002;214:775–782. doi: 10.1007/s00425-001-0675-3. [DOI] [PubMed] [Google Scholar]
- 68.Gao J.F. Plant Physiology Experiment Guide. Higher Education Press; Beijing, China: 2006. pp. 221–223. [Google Scholar]
- 69.Velikova V., Yordanov I., Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 70.Chaitanya K.S.K., Naithani S.C. Role of superoxide lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Gaertn. f. New Phytol. 1994;126:623–627. doi: 10.1111/j.1469-8137.1994.tb02957.x. [DOI] [Google Scholar]
- 71.Yang C., Ma B., He S.J., Xiong Q., Duan K.X., Yin C.C., Chen H., Lu X., Chen S.Y., Zhang J.S. MAOHUZI6/ETHYLENE INSENSITIVE3-LIKE1 and ETHYLENE INSENSITIVE3-LIKE2 regulate ethylene response of roots and coleoptiles and negatively affect salt tolerance in rice. Plant Physiol. 2015;169:148–165. doi: 10.1104/pp.15.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao H.M., Cai W.J., Ye T.T., Ding J., Feng Y.Q. Spatio-temporal profiling of abscisic acid, indoleacetic acid and jasmonic acid in single rice seed during seed germination. Anal. Chim. Acta. 2018;1031:119–127. doi: 10.1016/j.aca.2018.05.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon request from the corresponding author.