Abstract
The gonococcal pilus, a member of the type IV family of pili, is composed of numerous monomers of the pilin protein and plays an important role in the initiation of disease by providing the primary attachment of the bacterial cell to human mucosal tissues. Piliation also correlates with efficient DNA transformation. To investigate the relationships between these pilus-related functions, the piliation state, and the availability of pilin, we constructed a derivative of MS11-C9 (ΔpilE1) in which the lacIOP regulatory sequences control pilE transcription. In this strain, MS11-C9.10, the steady-state levels of pilin mRNA and protein directly correlate with the concentration of IPTG (isopropyl-β-d-thiogalactopyranoside) in the growth medium and can reach near-wild-type levels of expression. Transmission electron microscopy (TEM) demonstrated that the number of pili per cell correlated with the steady-state expression levels: at a low level of transcription, single long pili were observed; at a moderate expression level, many singular and bundled pili were expressed; and upon full gene expression, increased lateral association between pili was observed. Analysis of pilus assembly by TEM and epithelial cell adherence over a time course of induction demonstrated that pili were expressed as early as 1 h postinduction. Analysis at different steady-state levels of transcription demonstrated that DNA transformation efficiency and adherence of MS11-C9.10 to transformed and primary epithelial cells also correlated with the level of piliation. These data show that modulation of the level of pilE transcription, without a change in pilE sequence, can alter the number of pili expressed per cell, pilus bundling, DNA transformation competence, and epithelial cell adherence of the gonococcus.
Neisseria gonorrhoeae is a gram-negative diplococcus and the causative agent of the sexually transmitted disease gonorrhea. Pili are long, filamentous appendages composed primarily of the protein subunit pilin (48). The pilin protein undergoes antigenic variation at a high frequency (21, 42), allowing escape from the host immune response (53, 60) and alteration of adherence properties (22, 23, 36, 52). Pilin sequence changes occur predominantly by nonreciprocal recombination events (14). Variant sequences from silent copies of potential pilin coding sequences (pilS) transfer to the pilin expression gene (pilE) (13, 39) in a RecA (21)-, RecO-, and RecQ (26)-dependent fashion.
The gonococcal pilus is a member of the type IV family of pili, which are found on a variety of gram-negative bacteria including Neisseria meningitidis, Pseudomonas aeruginosa, Vibrio cholerae, Escherichia coli, and Myxococcus spp. (56). Many type IV pilus assembly genes are homologous to genes involved in the general secretion pathway as well as genes implicated in DNA binding and uptake in Bacillus subtilis and Haemophilus influenzae (46). Pili are required for full natural DNA transformation efficiency of N. gonorrhoeae (43). Gonococci (Gc) that do not express pilin (41, 61) or are nonpiliated due to a mutation in a pilus assembly gene (5, 9, 49) are greatly reduced in competence. The presence of the PilC protein, which copurifies with pili (18), has also been shown to be required for efficient DNA transformation (34). Some pilus phase variants demonstrate intermediate levels of transformation competence (10, 23). A 10-bp transformation uptake sequence is essential for efficient gonococcal transformation (6, 11), although uptake sequence-independent transformation can occur at a low frequency (4, 44).
Pilus-mediated adherence of Gc has been studied extensively in vitro. Piliation has been shown to enhance adherence to epithelial cells both in tissue culture (31, 51) and in organ culture (25). Cell culture systems using primary cells derived from the human urogenital tract have recently been developed to aid in further investigation of gonococcal adherence and invasion mechanisms (15, 28). Studies with cultured cells and tissue sections have shown that antigenic variation of the pilin protein can alter the adherence properties of the pilus and may confer tissue tropism (17, 36). Antigenic variation may also subsequently modify gonococcal adherence by causing a change in the number and aggregation properties of the pili expressed by the bacterial cell (23). The gonococcal PilC protein is also important for pilus-mediated adherence (33, 58); PilC has been proposed as the pilus tip-located adhesin (35) and has been localized at the bacterial cell surface (32). Human membrane cofactor protein (MCP or CD46) has been shown to be a cellular gonococcal pilus receptor (19).
To investigate how altering the availability of pilin would affect pilus biogenesis and pilus-related functions, we created a derivative of gonococcal strain MS11 in which the expressed pilin gene is under the control of lacIOP regulatory sequences. In this strain, MS11-C9.10, the steady-state levels of pilin mRNA and pilin protein correlate directly with the levels of IPTG (isopropyl-β-d-thiogalactopyranoside) in the growth medium. This allowed examination of the relationships between pilE transcription and piliation phenotype, DNA transformation competence, and adherence to epithelial cells. Furthermore, the lac-regulatable pilE gene allowed study of the kinetics of pilus growth and expression upon addition of IPTG to the growth medium.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli DH10B (F−mcrA mcrB mrr recA endA) (Gibco-BRL) was grown at 37°C on Luria-Bertani medium (Difco) and selected for antibiotic resistance at 40 μg of kanamycin (KAN) and 200 μg of erythromycin (ERM) (Sigma) per ml. Gc strains MS11-A, MS11-B2, MS11-C9 (38), and their derivatives were grown on Gc Medium Base (Difco) plus Kellogg Supplements (20) (GCB) at 37°C in 5% CO2, with or without up to 5 mM IPTG (Diagnostic Chemicals Ltd.). Gc were selected for antibiotic resistance at 10 μg of ERM per ml.
Construction of the regulatable pilE strain C9.10.
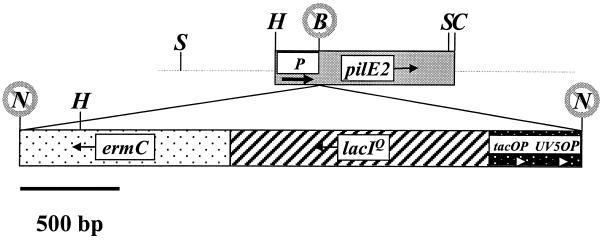
All enzymes used were supplied by New England Biolabs unless otherwise noted and were used under conditions recommended by the supplier. pNG3049 (41) was linearized with Bsu36I, which cuts between the pilE2 promoter and the pilE2 coding region in the 5′ untranslated portion (Fig. 1). pHSX-ermC-lacIOP (40) was digested with NotI to release a 3.1-kb fragment containing ermC, lacIq, and the tandem lac operator promoter sequences, tacOP and UV5OP. The ends of the pNG3049 and pHSX-ermC-lacIOP fragments were filled with the Klenow fragment of polymerase I, the blunt ends were ligated, and the ligation was used to transform E. coli DH10B. Clones were selected for ERM and KAN resistance. A resultant cloned plasmid DNA of the correct size and orientation was linearized with PstI and used to transform Gc strain MS11-A. Gc chromosomal DNA was isolated from transformants as described by Boyle-Vavra and Seifert (3) and analyzed by Southern blotting as described by Sambrook et al. (37) using a pilE gene probe. DNA from one Gc transformant which had incorporated the construct into the correct location was used to transform Gc strain MS11-C9 (ΔpilE1), and transformants were selected on ERM. Transformants were analyzed by Southern blotting, and one transformant that had incorporated the construct into the pilE2 locus, C9.10, was used throughout the remainder of this study.
FIG. 1.
Schematic of the regulatable pilE gene of C9.10. Shaded boxes indicate genes and are drawn to scale. Arrows extended from the gene names indicate the direction of transcription. Thick arrows indicate the operators and promoters. P, pilE2 promoter. Restriction sites are abbreviated in capital letters as follows: B, Bsu36I; C, ClaI, H, HpaI, N, NotI, S, SmaI. Crossed-out restriction sites were destroyed during construction.
Construction of MS11-C9.11 recA6.
To generate an MS11 derivative with a wild-type pilE promoter and the pilE coding sequence of C9.10, C9.10 was first transformed with pVD300recA6 DNA to introduce the IPTG-regulated recA6 allele (40) to aid in the maintenance of the C9.10 pilE coding sequence. C9.10 recA6 was then transformed in the presence of IPTG with pNG3049 DNA, which was originally used to create C9.10 and contains the C9.10 pilE coding sequence under the control of a wild-type pilE promoter. Gc from the transformation mix were plated on GCB medium in the absence of antibiotics and IPTG and then screened for a piliated morphology. If the regulatable pilE construct was maintained, Gc would have a nonpiliated morphology, but if the wild-type promoter from pNG3049 had recombined into the chromosome, transformed Gc would display a piliated morphology in the absence of IPTG. The pilE sequence of piliated colonies were determined, and transformants that had the C9.10 pilE sequence and were ERM sensitive were subjected to Southern analysis to confirm proper recombination into the chromosome and loss of ermC. This strain, MS11-C9.11 recA6, was used in the time course experiments.
RNA purification and analysis.
Gc were grown in 10 ml of GC Liquid (GCL) medium (1.5% Proteose Peptone no. 3 [Difco], 0.4% K2HPO4, 0.1% KH2PO4, 0.1% NaCl) with Kellogg Supplements, 0.042% sodium bicarbonate, and 0, 0.005, 0.01, 0.02, 0.05, 0.1, 0.5, or 1.0 mM IPTG at 37°C for 4 to 6 h with shaking. Cells were sedimented at 5,000 × g for 15 min, and the total RNA was purified using the TRIzol reagent (Gibco-BRL) according to the manufacturer's protocol.
Equal amounts of each RNA sample were analyzed by Northern blot as described by Sambrook et al. (37). Membranes were fixed by UV cross-linking and drying. Prehybridization took place for 2 h at 50°C in 50% formamide, as described previously (37). A pilE gene probe was isolated from plasmid pNG3100 (41) after digestion with EcoRI and HindIII and separation on a 1.0% agarose TAE gel. The ∼1-kb fragment containing pilE was random primer labeled with [α32P]dCTP (Amersham) (7), hybridized overnight, washed three times for 5 min each time at room temperature with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and twice for 15 min each time at 65°C with 0.1× SSC–0.5% sodium dodecyl sulfate (SDS), and exposed to X-Omat-AR film (Kodak) with an intensifying screen. Densitometric analysis was performed using the AlphaImager 2000 (Alpha Innotech Corp.).
Analysis of pilin and PilC production.
To analyze pilin production, Gc were grown as described for RNA purification. Cells were sedimented at 5,000 × g for 15 min and resuspended in 5× protein sample buffer (2). Equal amounts of the cell lysates were separated on SDS–15% polyacrylamide gel electrophoresis (PAGE) gels and transferred to a nitrocellulose membrane (Micron Separations, Inc.) using a Trans-Blot Cell (Bio-Rad). The membranes were blocked with MegaBlocI (CEL Associates) as recommended by the manufacturer and probed with the T36 anti-pilin polyclonal antiserum, which was raised against a pilin peptide spanning amino acids 37 to 56 (CILAEGQKSAVTEYYLNNGK) (a gift from M. So) (8) at a dilution of 1:1,000. Alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G secondary antibody (Promega) was used at a dilution of 1:7,500, and the immunoblots were developed colorimetrically as described previously (23).
Analysis of PilC production was performed as described for pilin analysis, except that cell lysates were separated on SDS–7.5% PAGE gels, and membranes were probed with preabsorbed polyclonal PilC antiserum (a gift from J. Pfeifer and S. Normark).
Electron microscopy.
To analyze piliation at steady-state levels of induction, Gc were grown with 0, 0.005, 0.01, 0.02, 0.05, 0.5, or 5.0 mM IPTG for 18 to 20 h on plates, and poly-l-lysine-treated (1 μg ml−1) Formvar-coated grids (Ladd Research Industries, Inc.) were used to lift cells from colonies. The grids were then fixed and negatively stained as described previously (23).
To perform the time course of piliation induction with C9.10 and C9.11 recA6, Gc were grown overnight on GCB medium in the absence of IPTG and were suspended in 8 ml of HEPES-buffered medium (10 mM HEPES [pH 7.4], 145 mM NaCl, 5 mM KCl, 5 mM glucose, 1 mM CaCl2, 1 mM MgCl2, 1.5% Proteose Peptone no. 3, 1% Iso VitaleX) at an optical density at 550 nm of approximately 0.1. Gc were grown with continuous rotation in the presence of 1 mM IPTG or without IPTG. At regular intervals over a 16-h period, aliquots were transferred to Parlodion-coated 300-mesh copper grids. Samples were allowed to adsorb for 1 h, and then the supernatant was removed. Grids were washed thrice with double-distilled H2O for 5 min to remove the residual medium and salts. Grids were then air dried and negatively stained with 1% ammonium molybdate for 30 s. Excess stain was removed, and grids were air dried under vacuum prior to examination and photography in a Hitachi Hu11-E-1 electron microscope at 75 kV and with SO-163 electron image film (Kodak).
Determination of gonococcal transformation efficiency.
Gc were grown in the absence of IPTG on plates for 16 to 18 h, collected with Dacron swabs, and suspended to a density of 108 CFU per ml in GCL. Then, 20 μl of cells was added to 200 μl of GCL containing 0 to 1 mM IPTG, 5 mM MgSO4 (41), and 100 ng of pSY6 DNA (44), which, when recombined into the Gc chromosome, confers resistance to nalidixic acid (NAL) (Sigma). After 15 min at 37°C, the transformation mixes were diluted into 2 ml of GCL plus Kellogg Supplements and the same IPTG concentration as previously and then incubated at 37°C in 5% CO2 for 5 h. The transformation mixes were diluted and plated on GCB medium with 2 μg of NAL per ml to select transformants and on GCB medium to determine CFU.
Cell culture conditions and adherence assays.
Opa− Gc were used for all adherence assays. The Opa status of each strain was determined by Western analysis as previously described (23), except that membranes were probed with the 4B12 anti-Opa monoclonal antibody (a gift from M. Blake) (1).
Primary cultures of human corneal epithelial cells were established as described previously (50). For use in adherence assays, epithelial cells were grown on 12-mm circular Thermanox coverslips in 1 ml of MCDB 153 medium. Before the start of the infection, the medium was replaced with 1 ml of RPMI supplemented with 5% fetal calf serum and 0.1% Iso VitaleX. Gc, grown on Gc agar plates in the presence of the appropriate antibiotics (37°C, 5% CO2), were added to the cells at a multiplicity of infection of 100. When appropriate, 0.5 mM IPTG was present during the assay. At 0 to 2 h of incubation (37°C, 5% CO2), the infection was stopped by rinsing the cells three times with 1 ml of Dulbecco phosphate-buffered saline (PBS) to remove unbound bacteria, followed by fixation in 0.1% glutaraldehyde–1% paraformaldehyde in Dulbecco PBS. Specimens were stained with crystal violet (0.007% in distilled water), and the bacterial adherence was scored with an Olympus (New Hyde Park, N.Y.) BH-2 microscope.
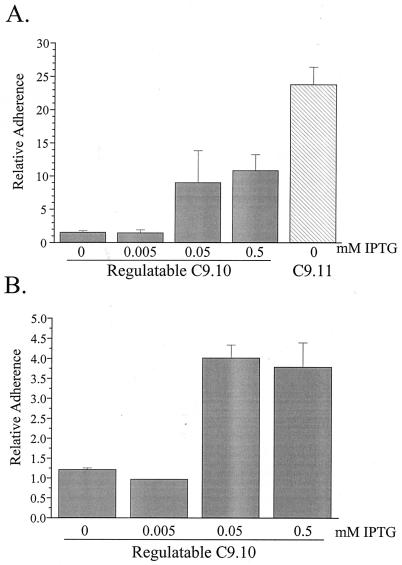
The Chang conjunctival cell line (ATCC CCL 20.2) was maintained, and adherence assays were performed as previously described (23). The data presented in Fig. 7 were calculated by dividing the number of adherent CFU of each strain by the number of adherent CFU of the nonpiliated strain MS11-B2.
FIG. 7.
Adherence of C9.10 and C9.11 recA6 to epithelial cells. For 18 h prior to the assay, Gc were induced with IPTG as indicated below the x axis. Gc were incubated with the cultured cells for 2.5 h. Monolayers were washed, disrupted, and plated to allow the counting of cell-associated CFU. The number of cell-associated CFU for each strain was divided by the number of cell-associated CFU of nonpiliated control strain MS11-B2 to yield the relative adherence. Values represent the mean and standard error of at least three identical experiments. (A) Adherence to Chang conjunctival epithelial cells. (B) Adherence to primary urethral epithelial cells.
The primary urethral epithelial cells were collected and maintained as described by Harvey et al. (15). Five days prior to the adherence assay, 2 × 104 viable primary urethral epithelial cells were plated per well in 24-well culture dishes containing collagen-coated coverslips in hormonally defined growth medium (Clonetics). At the time of the assay, each well contained ∼3.3 × 104 viable cells in a confluent monolayer. The adherence assay was performed as described for the Chang cells.
RESULTS
Construction and characterization of the regulatable pilE strain C9.10.
We constructed a Gc strain in which the wild-type pilE promoter was displaced with lac regulatory sequences to investigate the relationships between pilE transcription, pilus assembly, and pilus function. The first step in constructing a regulatable pilE strain was to insert an ERM resistance cassette and lacIOP regulatory sequences upstream of the pilE coding sequence in the 5′ untranslated region. The plasmid pHSX-ermC-lacIOP (40) contains two tandem lac promoter-operator sequences (tac-UV5), which are regulated more effectively by lacIQ than single operator promoter regions (27). The ermC and lacIQ genes are transcribed in the opposite orientation of the lac promoter-operator sequences and therefore do not influence transcription of genes downstream of these sequences. The lac promoter-operator sequences, the lacIQ gene, and the ermC gene were inserted into pilE2, just upstream of the pilE2 ribosomal binding site and downstream of pilE2 promoter sequences, in pNG3049 (41) (Fig. 1). This construct was transformed into MS11-C9, which carries the pilE2 gene, and nonpiliated (P−) ERM-resistant Gc transformants were grown with 0.5 M IPTG on solid medium to identify transformants that exhibited a piliated (P+) colony morphology. These transformants were analyzed by Southern blot to ensure that the construct was in the pilE2 locus and that no other pilin loci had changed (data not shown). One transformant (MS11-C9.10, or C9.10) was chosen for further analysis.
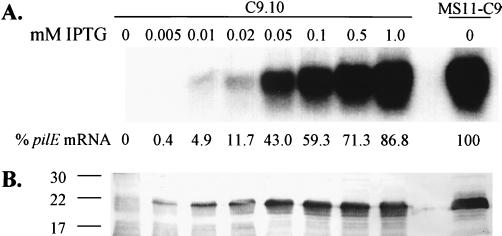
The regulatable pilE strain C9.10 was characterized by Northern and Western analysis to determine the relative levels of pilin mRNA and protein produced with respect to the parental strain MS11-C9. C9.10 was grown in liquid medium for 4 to 6 h without IPTG or grown in media containing IPTG at 0.005 to 1.0 mM. Total RNA was isolated and probed with a pilE gene probe. Pilin mRNA was not detectable when C9.10 was grown without IPTG, and pilin mRNA levels gradually increased when cells were grown with increasing concentrations of IPTG in the growth medium, ultimately reaching an expression level of 86.8% compared to the wild type (Fig. 2A). Whole-cell lysates were prepared from the same bacterial cultures as the RNA samples and were probed with a pilin anti-peptide antiserum that binds to a conserved pilin epitope (see Materials and Methods) (8). No detectable pilin was produced when C9.10 was grown without IPTG (Fig. 2B). The level of pilin protein steadily increased as the IPTG in the growth medium increased to 0.05 mM, and then pilin expression leveled off despite further increases in pilE mRNA expression. We have previously shown that monoclonal antibodies that bind to conserved epitopes on pilin react with different affinities to some pilin variants (23), and therefore the levels of C9.10 variant pilin expression cannot be directly compared to the C9 pilin variant. These Northern and Western analyses demonstrated that in C9.10, the regulatable pilE gene allows for control of pilE transcription and pilin production by altering the concentration of IPTG.
FIG. 2.
Northern and Western analyses of C9.10. The concentration of IPTG used for induction is indicated above each lane. (A) Pilin mRNA was detected with a pilE gene probe. (B) Pilin protein was detected with the T36 anti-pilin polyclonal antiserum. The mobilities of molecular mass markers are indicated to the left of the blot (in kilodaltons).
Pilus expression by C9.10.
We have previously shown that changes in the pilin amino acid sequence due to antigenic variation alter the level of pilin produced, the number of pili per cell, the extent of pilus bundling, and the percentage of cells expressing pili in a given population (23). We used C9.10 to determine how varying the expression level of a given variant pilin protein affected pilus assembly and pilus bundling. Transmission electron microscopy (TEM) was used to determine the piliation phenotype of C9.10 when grown in the presence of 0, 0.005, 0.01, 0.02, 0.05, 0.5, or 5.0 mM IPTG for 18 to 20 h on solid media. No pili were detected on negatively stained cells when IPTG was not added to the growth medium (Fig. 3A). At the lowest level of induction (0.005 mM IPTG), most cells did not express detectable pili, but 5 to 10% of cells expressed one or two long pili (Fig. 3B). At 0.01 and 0.02 mM IPTG, a number of singular pili were present on most cells (Fig. 3C and D). Many singular pili, as well as small bundles and networks of pili, were observed at 0.05 mM IPTG (Fig. 3E). At 0.5 and 5.0 mM IPTG, single pili and pili in larger bundles and networks were detected (Fig. 3F and G).
FIG. 3.
TEM images of negatively stained C9.10. (A) C9.10 in the absence of IPTG. (B) IPTG at 0.005 mM. (C) IPTG at 0.01 mM. (D) IPTG at 0.02 mM. (E) IPTG at 0.05 mM. (F) IPTG at 0.5 mM. (G) IPTG at 5.0 mM. A typical cell from the grid is shown for each level of induction except for panel B, which represents a minority of the population. Scale bars in panels A and F represent 0.5 μm; panel B to E and panel G images were obtained at the same magnification as for panel A. The arrow in panel B points to a pilus.
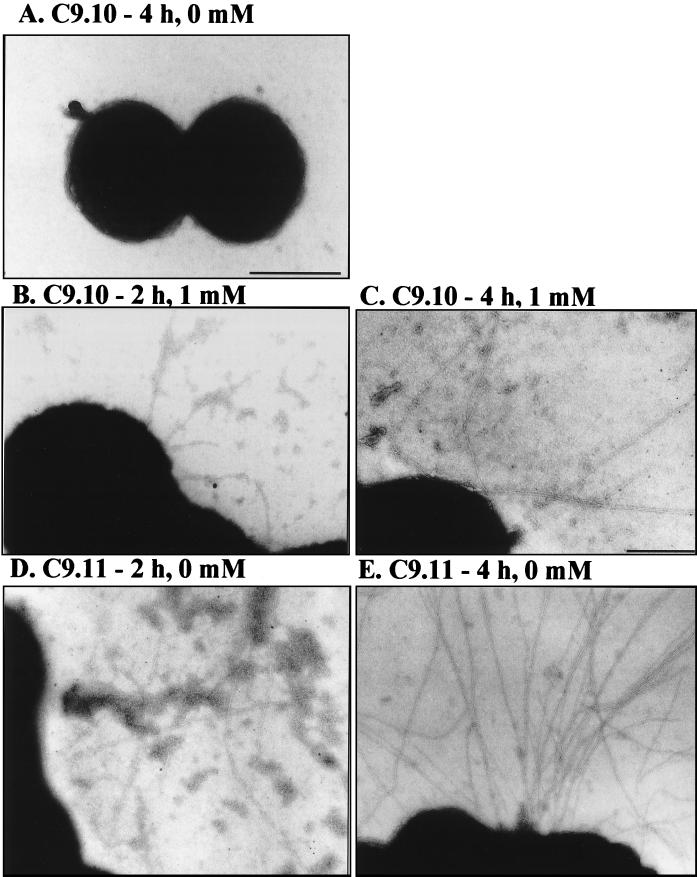
In addition to determining the steady-state pilus expression at different levels of IPTG, we analyzed the dynamics of pilus expression over a period of 16 h of growth in liquid medium, with pilE induction beginning at t0. Gc were grown overnight on solid medium in the absence of IPTG. Gc were then inoculated into liquid medium containing no or 1 mM IPTG; samples were taken at 2, 4, 8, 12, and 16 h and processed for analysis of piliation by TEM. Pili were never observed when C9.10 was not induced with IPTG (Fig. 4A). After 2 h of growth in the presence of IPTG, 60% of the C9.10 cells expressed a few long pili per cell (Fig. 4B). After 4 h of growth in IPTG, 5 to 10 pili per organism were detected on greater than 95% of the cells (Fig. 4C). The piliation of C9.10 after 8 h of induction was similar to that at 4 h, with approximately 10 pili per cell on the majority of cells in the population (data not shown). After 12 and 16 h of growth, a substantial portion of the cells in the culture was dead (presumably due to autolysis), and a few pili were detectable on the intact cells. This analysis demonstrates that pilus production is rapidly induced upon exposure of C9.10 to IPTG and that a threshold level of pilus expression is achieved after 4 h of IPTG induction. Interestingly, C9.10 is significantly less piliated when grown in liquid medium compared to growth on solid medium (compare with Fig. 3F). This reduction in piliation is not due to the heterologous promoter since, we have also observed decreased piliation during liquid growth for a wild-type pilE variant (data not shown).
FIG. 4.
TEM images of negatively stained C9.10 and C9.11 over a time course of pilus induction. (A) C9.10 after 4 h of growth without IPTG. (B) C9.10 after 2 h of growth with 0.5 mM IPTG. (C) C9.10 after 4 h of growth with 1.0 mM IPTG. (D) C9.11 after 2 h of growth without IPTG. (E) C9.11 after 4 h of growth without IPTG. A typical cell from the grid is shown in each panel. The bar represents 0.5 μm in panels A, B, D, and E and 0.25 μm in panel C.
The kinetics of pilus expression was also examined by assaying the adherence of C9.10 to primary corneal epithelial cells. Primary corneal cells were inoculated with Gc upon induction with 0.5 mM IPTG at t0. At 0, 15, 30, 60, 90, and 120 min postinoculation, the primary cells were washed to remove nonadherent Gc and observed to determine the adherence. No adherent Gc were observed during the first 30 min of IPTG induction (Fig. 5A to C) or when the primary cells were inoculated with a nonpiliated control (data not shown). Gc began to adhere to the primary corneal cells after 60 min of IPTG induction (Fig. 5D), and the number of Gc per cell increased after 90 and 120 min of IPTG induction (Fig. 5E and F). These results corroborate the TEM data (Fig. 4B) and indicate that functional pili are expressed as early as 1 h post-IPTG induction.
FIG. 5.
Adherence of C9.10 to primary corneal cells over a time course of pilus induction. Gc were grown overnight in the absence of IPTG. Gc were incubated with 0.5 mM IPTG and the cultured cells as indicated in each panel. Monolayers were then washed, fixed, stained with crystal violet, and viewed with a light microscope.
For accurate comparison between C9.10 and a strain with a wild-type pilE promoter, a strain which has a wild-type pilE promoter and expresses the same pilE sequence as C9.10 was generated (see Materials and Methods). This strain (MS11-C9.11 recA6) was then analyzed for piliation over a 16-h time course in liquid medium. During the first 8 h of growth, MS11-C9.11 recA6 expressed numerous pili per cell that were aggregated into bundles (Fig. 4D and E). At the 12- and 16-h time points, similar to what was seen with the regulatable pilE strain, a significant reduction in viability was observed, but many pili were still present. The number of pili expressed per cell and the extent of pilus bundling by C9.10 did not reach that expressed by MS11-C9.11 recA6.
DNA transformation efficiency of C9.10.
Gc readily take up their own DNA, and piliated organisms possess much higher transformation efficiencies than their nonpiliated counterparts (43). Studies with pilus biogenesis mutants have shown that the mere presence of pilin protein that cannot be assembled into pili is not sufficient to maintain a high level of transformation competence (5, 9, 49). However, Gc that express a few pili have been shown to exhibit transformation efficiencies between that of piliated and nonpiliated (ΔpilE) variants (10, 23). We therefore utilized the regulatable pilE strain to investigate the relationship between pilin expression levels and DNA transformation efficiency.
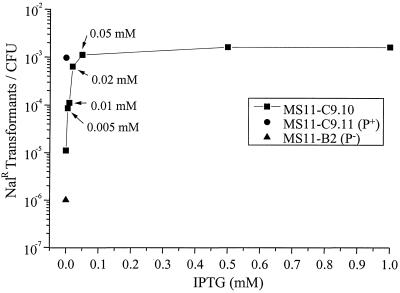
Gc strains C9.10, C9.11 recA6, and MS11-B2 (ΔpilE1 ΔpilE2) (38) were grown in the absence of IPTG overnight on solid medium. Gc were suspended in liquid medium containing 0, 0.005, 0.01, 0.02, 0.05, 0.5, or 1.0 mM IPTG and pSY6 plasmid DNA that, when recombined into the chromosome, confers resistance to NAL (44). After 5 h of incubation, Gc were selected on NAL to assess the transformation efficiency. As shown in Fig. 6, the transformation efficiency of C9.10 directly correlated with the number of single long pili up to an intermediate level of pilE transcription (0.05 mM IPTG). The point at which the transformation competence of C9.10 reached a plateau was similar to the competence level of C9.11 recA6, which expresses the same pilin sequence under the control of the wild-type pilE promoter, showing that full transformation competence is reached at a pilin expression level that is ca. 50% that of the wild type.
FIG. 6.
DNA transformation competence of C9.10, C9.11 recA6, and MS11-B2. Strains were incubated in the absence or presence of IPTG as indicated for 5 h with plasmid pSY6, which confers resistance to NAL (NalR) when recombined into the chromosome. The transformation efficiency was expressed as the number of NAL-resistant transformants per CFU. A representative of three identical experiments is shown.
Adherence of C9.10 to epithelial cells.
Gonococcal pili have been suggested to play a crucial role in the initiation of disease by providing the primary attachment of the bacterial cell to human mucosal tissues (47). Previous investigations into how the level of piliation affects adherence demonstrated that both the number of pili per cell and the extent of pilus bundling influence gonococcal adherence to epithelial cells (23). To study the relationship between the level of piliation and adherence, we used two different types of epithelial cells. The transformed cell line that we chose was the Chang conjunctival epithelial cell line, which has been used in numerous studies of gonococcal adherence (17, 36, 54). A second type of epithelial cell culture that we used was the primary urethral epithelial cell system established by Harvey et al. (15).
Gc were grown in the presence of 0, 0.005, 0.05, or 0.5 mM IPTG for 18 to 20 h on solid medium. Gc were incubated with nearly confluent monolayers of Chang cells or primary urethral epithelial cells at a ratio of 100 Gc to 1 cultured cell for 2.5 h. When C9.10 was not induced or was induced with a low level of IPTG (0.005 mM), its adherence was not significantly different from that of the nonpiliated control MS11-B2 (Fig. 7). However, at intermediate (0.05 mM IPTG) and high (0.5 mM IPTG) induction levels, adherence of C9.10 to both epithelial cell types increased significantly over that of uninduced C9.10. Adherence of C9.10 to Chang cells, even when induced at 0.5 mM IPTG, never reached the adherence level of the positive control C9.11 recA6 (Fig. 7A). Furthermore, C9.10 exhibited an approximately threefold-greater adherence to Chang cells than primary urethral cells relative to the P− control strain (Fig. 7). These data indicate that, unlike DNA transformation competence, a low level of pilus expression does not promote a significant level of adherence over that of a nonpiliated strain and that an intermediate level of piliation is necessary to significantly increase adherence to cultured epithelial cells.
DISCUSSION
We used the lac regulatory system to modulate Gc pilin expression in N. gonorrhoeae. By altering the steady-state level of pilin transcription with different levels of IPTG, the relationships between pilin expression, pilus expression, and pilus-related functions (DNA transformation and epithelial cell adherence) were examined. Importantly, this was accomplished without the complications brought about when pilin sequence changes pilus expression through the process of antigenic variation. Moreover, by adjusting pilin expression through transcription, new insights into pilus assembly processes were obtained. Finally, a time course of pilus expression allowed the determination of the time required for pilus expression and the development of pilus-mediated cell adherence.
The regulatable pilE strain C9.10 is the first reported strain in which a change in pilus bundling has been observed without a change in pilin amino acid sequence. At low to intermediate levels of IPTG, strain C9.10 expresses singular pili, and the number of pili per cell increases as the IPTG concentration increases. At 0.05 mM IPTG pili begin to associate into loose networks, and at 0.5 and 5 mM IPTG pili are aggregated into substantial bundles as well as remaining singular. A change in pilus expression from singular pili to bundled pili upon the increase of pilin expression indicates that the extent of pilus bundling is at least partially determined by the concentration of pili and not solely by the primary pilin amino acid sequence. Our previous study of a panel of strain FA1090 pilin variants (23), as well as numerous other studies (24, 55), showed that a change in the pilin amino acid sequence is often associated with a change in the extent of pilus bundling. Together, these studies indicate that both the level of pilin expression and the primary pilin amino acid sequence influence the aggregation of gonococcal pili. It has not been determined whether the individual pili within a pilus bundle all originate from the same gonococcal cell or whether they contain pili from multiple cells.
One of the most striking observations made with the regulatable pilE strain was its piliation phenotype when induced at low levels of IPTG. Most Gc were nonpiliated at the lowest induction level (0.005 mM IPTG), but a minority of the cells expressed one or two detectable pili of approximately wild-type length. This expression phenotype was unexpected and in contrast to the phenotype of an E. coli strain containing an IPTG-inducible type I pilus system. When a lacUV5-regulated fim operon was maximally induced for 30 min, the majority of cells expressed a few short pili; the number of pili per cell increased over the following 1.5 h, but the pili never reached wild-type length (59). In contrast, we observed that long pili were expressed after 2 h of induction, and the number of long pili increased over the next 2 h. One significant difference between these two bacterial species is that E. coli expresses a lactose permease (lacY), whereas the Gc do not carry any of the lac operon genes. Another inherent difference between these two systems is that many of the type I pilus biogenesis genes are encoded in an operon with the main pilus subunit, whereas the gonococcal pilus biogenesis genes are not. In the type I pilus system, the number of pili assembled per cell is directly correlated with the level of FimD, an outer membrane protein required for pilus assembly (reviewed in reference 16). Therefore, it seems that during type I pilus assembly, all “centers” equally compete for pilin and assemble pilin into pili at similar rates. However, when pilin is rate limiting in the Gc type IV pilus system, the pilin that is available is assembled into a pilus of wild-type length at only one or two sites, if at all. Perhaps a threshold level of pilin must accumulate, conceivably in the inner membrane (57), prior to assembly of a pilus. Another possibility is that when the level of pilin is low, the level of one or more additional pilus biogenesis genes is also low, allowing for only a limited number of pilus assembly sites, as seen in the type I pilus system. This does not seem to be the case with the pilus biogenesis proteins PilQ and PilC, which are amply expressed in nonpiliated strains (18, 29). Our favored hypothesis is that Gc pilin is targeted to the inner membrane, and only one pilus assembly site has access to this pilin pool. If a localized threshold level of pilin accumulates, a single pilus is assembled. When there is a greater amount of pilin available, the threshold level of pilin can be accessible to additional sites of pilus assembly throughout the membrane, and the number of pili per cell consequently increases. This implies that the apparatus through which a pilus is assembled is regulated, since pilus assembly only occurs when enough pilin has accumulated to form a pilus of wild-type length.
The DNA transformation experiments with the regulatable pilE strain revealed that only a small amount of pilin is necessary to significantly increase the transformation competence of the Gc over that of a nonpiliated strain. Even when the regulatable pilE strain was not induced, its transformation efficiency was 10-fold higher than that of the nonpiliated strain, MS11-B2, which has deletions of pilE1 and pilE2 (38). One explanation for this phenomenon could be that in a small proportion of cells, the lac promoter is transiently or genetically derepressed. This would cause the small proportion of cells to produce enough pilin to significantly increase the competence of the population, but not enough pilin to be easily detected. Alternatively, we have found that the regulatable pilE construct is minimally transcribed when uninduced (40). However, we have never detected pili when C9.10 was not induced with IPTG. This suggests that small amounts of pilin may play a role in DNA transformation even when not incorporated into a detectable pilus. Additionally, small increases in pilE transcriptional activity correlated with relatively large increases in transformation competence. Comparable results were seen with another gonococcal inducible pilin strain (34). In this inducible pilin strain (N456), pilE was under control of a less tightly regulated lac regulatory construct than that used for C9.10. The DNA transformation efficiency of N456 increased significantly after 1 and 12 h of IPTG induction compared to the same strain when uninduced, even though very little pilin was produced (34). By studying a panel of different regulatable pilE variants and pilus assembly mutants in strain FA1090, we propose that pili are not required for transformation competence but that a pilin-dependent change of the pilus assembly apparatus into a transport competent state is required for DNA internalization (C. D. Long and H. S. Seifert, unpublished results).
The maximal transformation efficiency of C9.10, which was equal to that of the wild-type control C9.11 recA6, was attained at the intermediate induction level of 0.05 mM IPTG. As mentioned above, it is at this level of induction that the pili of C9.10 began to aggregate and, at higher levels of induction, the extent of pilus bundling increased. The increase in transformation competence directly correlated with the number of single pili present but not with the total number of pili. This suggests that single, unbundled pili may be important in the process of DNA uptake. This hypothesis is further supported by the observation that other minimally piliated gonococcal strains exhibit intermediate DNA transformation efficiencies (10, 23).
One interesting observation from the adherence experiments is that piliated Gc exhibited greater adherence to the immortalized Chang cell line than the primary urethral epithelial cells relative to the adherence of a nonpiliated control. The different adherence profiles between the Chang and primary urethral cells indicate that Chang cells may have altered the expression of CD46 or other putative downstream effector molecules involved in gonococcal adherence, express CD46 in a more accessible manner, or altered the expression of different receptors or coreceptors for pilus-mediated adherence. Alternatively, although the conjunctiva and urethra are both primary sites of gonococcal infection, perhaps the distribution of pilus receptor(s) is different at each site.
The data from the adherence experiments also lend insight into the level of piliation required by Gc for sufficient adherence to epithelial cells. A significant increase in the adherence of the regulatable pilE strain to both Chang conjunctival and primary urethral epithelial cells was seen when steady-state induction of pilE was increased from 0.005 to 0.05 mM IPTG and a greater number of singular pili, as well as loose “networks” of pili, were present. However, there was no significant increase in adherence when IPTG was increased from 0.05 to 0.5 mM and pili became more bundled. A related phenomenon has been observed with a panel of meningococcus (Mc) carrier and disease isolates of various serogroups (12). A majority of the Mc isolates expressing aggregated pili exhibited low adherence to human buccal epithelial cells, and most isolates expressing unaggregated pili exhibited medium to high levels of adherence (12). Interestingly, these data contrast the report from Marceau et al., who found that the adherence of encapsulated Mc to an epithelial cell line is strongly promoted by bundled pili and that variants which express singular pili exhibit a low level of adherence (24). However, the classification of all Mc into “aggregated” and “unaggregated” categories may not accurately reflect the true piliation state of a particular pilin variant. In the present study, and in our previous study of a panel of gonococcal pilE variants (23), TEM revealed that both aggregated and singular pili can be expressed by a given variant. The number of pili per cell and the percentage of cells expressing pili can also differ between pilE variants (23). It is possible that singular pili may be expressed by the “aggregated” Mc variants, but the pili may be difficult to detect due to pilus fragility and shearing during electron microscopy sample preparation. Conversely, the presence of capsule, differential glycosylation (45), and/or the decreased number of possible pilin variants available to the Mc (due to fewer pilS copies in its genome [30]) may limit the overall piliation state to only a few phenotypes. Further investigation into the relationships between pilin sequence, piliation state, and pilus-mediated adherence in Gc and Mc is clearly needed in order to draw conclusions regarding the effect of pilus bundling on adherence.
In this study, the effects of a wide range of pilin production and piliation levels on gonococcal pilus-related functions were examined without changing the pilE sequence. The DNA transformation and adherence data generated through use of the regulatable pilE strain mirror our previous observations using a panel of pilE variants which express various levels of singular and aggregated pili (23). The expression of a few singular pili correlates with an intermediate level of DNA transformation efficiency. Furthermore, variants that expressed the greatest number of singular pili per cell exhibited the highest levels of adherence to Chang cells, whereas the adherence of a variant expressing highly bundled pili was significantly decreased (23). By taking into account the findings of both studies, we conclude that it is the piliation state and not the pilE sequence per se which directly affects the pilus-related functions of adherence and DNA transformation.
ACKNOWLEDGMENTS
We thank M. So for the T36 anti-pilin antiserum, J. Pfeifer and S. Normark for the PilC antiserum, and M. Blake for the 4B12 anti-Opa monoclonal antibody. Finally, we thank Eric Skaar and Eric Sechman for critical reading of the manuscript.
This work was supported by PHS grants AI31494 and AI33493 to H.S.S. and grants AI18384 and AI38515 to M.A.A. C.D.L. was partially supported by PHS grant T32 GM08061.
REFERENCES
- 1.Blake M S, Blake C M, Apicella M A, Mandrell R E. Gonococcal opacity: lectin-like interactions between Opa proteins and lipooligosaccharide. Infect Immun. 1995;63:1434–1439. doi: 10.1128/iai.63.4.1434-1439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollag D M, Edelstein S J. Protein methods. New York, N.Y: John Wiley & Sons, Inc.; 1991. [Google Scholar]
- 3.Boyle-Vavra S, Seifert H S. Shuttle mutagenesis: two mini-transposons for gene mapping and for lacZ transcriptional fusions in Neisseria gonorrhoeae. Gene. 1993;129:51–57. doi: 10.1016/0378-1119(93)90695-y. [DOI] [PubMed] [Google Scholar]
- 4.Boyle-Vavra S, Seifert H S. Uptake-sequence-independent DNA transformation exists in Neisseria gonorrhoeae. Microbiology. 1996;142:2839–2845. doi: 10.1099/13500872-142-10-2839. [DOI] [PubMed] [Google Scholar]
- 5.Drake S L, Koomey M. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol Microbiol. 1995;18:975–986. doi: 10.1111/j.1365-2958.1995.18050975.x. [DOI] [PubMed] [Google Scholar]
- 6.Elkins C, Thomas C E, Seifert H S, Sparling P F. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J Bacteriol. 1991;173:3911–3913. doi: 10.1128/jb.173.12.3911-3913.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 8.Forest K T, Bernstein S L, Getzoff E D, So M, Tribbick G, Geysen H M X, Deal C D, Tainer J A. Assembly and antigenicity of the Neisseria gonorrhoeae pilus mapped with antibodies. Infect Immun. 1996;64:644–652. doi: 10.1128/iai.64.2.644-652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freitag N E, Seifert H S, Koomey M. Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol Microbiol. 1995;95:575–586. doi: 10.1111/j.1365-2958.1995.tb02420.x. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs C P, Reimann B Y, Schultz E, Kaufmann A, Haas R, Meyer T F. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature. 1989;338:651–652. doi: 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- 11.Goodman S D, Scocca J J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1988;85:6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenblatt J J, Floyd K, Philipps M E, Frasch C E. Morphological differences in Neisseria meningitidis pili. Infect Immun. 1988;56:2356–2362. doi: 10.1128/iai.56.9.2356-2362.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas R, Meyer T F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986;44:107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- 14.Hagblom P, Segal E, Billyard E, So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985;315:156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- 15.Harvey H A, Ketterer M R, Preston A, Lubaroff D, Williams R, Apicella M A. Ultrastructural analysis of primary human urethral epithelial cell cultures infected with Neisseria gonorrhoeae. Infect Immun. 1997;65:2420–2427. doi: 10.1128/iai.65.6.2420-2427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hultgren S J, Normark S, Abraham S N. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson A-B, Ilver D, Falk P, Pepose J, Normark S. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol Microbiol. 1994;13:403–416. doi: 10.1111/j.1365-2958.1994.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson A B, Nyberg G, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kallstrom H, Liszewski M K, Atkinson J P, Jonsson A B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 20.Kellogg D S, Jr, Peacock W L, Deacon W E, Brown L, Pirkle C I. Neisseria gonorrhoeae. I. Virulence genetically linked to clonial variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koomey M, Gotschlich E C, Robbins K, Bergstrom S, Swanson J. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics. 1987;117:391–398. doi: 10.1093/genetics/117.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambden P R, Robertson J N, Watt P J. Biological properties of two distinct pilus types produced by isogenic variants of Neisseria gonorrhoeae P9. J Bacteriol. 1980;141:393–396. doi: 10.1128/jb.141.1.393-396.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long C D, Madraswala R N, Seifert H S. Comparisons between colony phase variation of Neisseria gonorrhoeae FA1090 and pilus, pilin, and S-pilin expression. Infect Immun. 1998;66:1918–27. doi: 10.1128/iai.66.5.1918-1927.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marceau M, Beretti J L, Nassif X. High adhesiveness of encapsulated Neisseria meningitidis to epithelial cells is associated with the formation of bundles of pili. Mol Microbiol. 1995;17:855–863. doi: 10.1111/j.1365-2958.1995.mmi_17050855.x. [DOI] [PubMed] [Google Scholar]
- 25.McGee Z A, Johnson A P, Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981;143:413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- 26.Mehr I J, Seifert H S. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation, and DNA repair. Mol Microbiol. 1998;30:697–710. doi: 10.1046/j.1365-2958.1998.01089.x. [DOI] [PubMed] [Google Scholar]
- 27.Morales V M, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 28.Mosleh I M, Boxberger H J, Sessler M J, Meyer T F. Experimental infection of native human ureteral tissue with Neisseria gonorrhoeae: adhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect Immun. 1997;65:3391–3398. doi: 10.1128/iai.65.8.3391-3398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newhall W J, Wilde C E, Sawyer W D, Haak R A. High-molecular-weight antigenic protein complex in the outer membrane of Neisseria gonorrhoeae. Infect Immun. 1980;27:475–482. doi: 10.1128/iai.27.2.475-482.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry A C, Nicolson I J, Saunders J R. Neisseria meningitidis C114 contains silent, truncated pilin genes that are homologous to Neisseria gonorrhoeae pil sequences. J Bacteriol. 1988;170:1691–1697. doi: 10.1128/jb.170.4.1691-1697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Punsalang A P, Jr, Sawyer W D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973;8:255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman M, Kallstrom H, Normark S, Jonsson A B. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol Microbiol. 1997;25:11–25. doi: 10.1046/j.1365-2958.1997.4601823.x. [DOI] [PubMed] [Google Scholar]
- 33.Rudel T, Boxberger H J, Meyer T F. Pilus biogenesis and epithelial cell adherence of Neisseria gonorrhoeae pilC double knock-out mutants. Mol Microbiol. 1995;17:1057–1071. doi: 10.1111/j.1365-2958.1995.mmi_17061057.x. [DOI] [PubMed] [Google Scholar]
- 34.Rudel T, Facius D, Barten R, Scheuerpflug I, Nonnenmacher E, Meyer T F. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1995;92:7986–7990. doi: 10.1073/pnas.92.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudel T, Scheurerpflug I, Meyer T F. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 36.Rudel T, van Putten J P M, Gibbs C P, Haas R, Meyer T F. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol. 1992;6:3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Segal E, Billyard E, So M, Storzbach S, Meyer T F. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell. 1985;40:293–300. doi: 10.1016/0092-8674(85)90143-6. [DOI] [PubMed] [Google Scholar]
- 39.Segal E, Hagblom P, Seifert H S, So M. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc Natl Acad Sci USA. 1986;83:2177–2181. doi: 10.1073/pnas.83.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seifert H S. Insertionally inactivated and inducible recA alleles for use in Neisseria. Gene. 1997;188:215–220. doi: 10.1016/s0378-1119(96)00810-4. [DOI] [PubMed] [Google Scholar]
- 41.Seifert H S, Ajioka R S, Paruchuri D, Heffron F, So M. Shuttle mutagenesis of Neisseria gonorrhoeae: pilin null mutations lower DNA transformation competence. J Bacteriol. 1990;172:40–46. doi: 10.1128/jb.172.1.40-46.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serkin C D, Seifert H S. Frequency of pilin antigenic variation in Neisseria gonorrhoeae. J Bacteriol. 1998;180:1955–1958. doi: 10.1128/jb.180.7.1955-1958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sparling P F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966;92:1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein D C. Transformation of Neisseria gonorrhoeae: physical requirements of the transforming DNA. Can J Microbiol. 1991;37:345–349. doi: 10.1139/m91-056. [DOI] [PubMed] [Google Scholar]
- 45.Stimson E, Virji M, Makepeace K, Dell A, Morris H R, Payne G, Saunders J R, Jennings M P, Barker S, Panico M, Blench I, Moxon E R. Meningococcal pilin: a glycoprotein substituted with digalactosy1–2,4-diacetamido-2,4,6-trideoxyhexose. Mol Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- 46.Strom M S, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 47.Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973;137:571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanson J, Kraus S J, Gotschlich E C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971;134:886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tonjum T, Freitag N E, Namork E, Koomey M. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol Microbiol. 1995;95:451–464. doi: 10.1111/j.1365-2958.1995.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 50.van Putten J P, Paul S M. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virji M, Everson J S. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect Immun. 1981;31:965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virji M, Everson J S, Lambden P R. Effect of anti-pilus antisera on virulence of variants of Neisseria gonorrhoeae for cultured epithelial cells. J Gen Microbiol. 1982;128:1095–1100. doi: 10.1099/00221287-128-5-1095. [DOI] [PubMed] [Google Scholar]
- 53.Virji M, Heckels J E. Antigenic cross-reactivity of Neisseria pili: investigations with type- and species-specific monoclonal antibodies. J Gen Microbiol. 1983;129:2761–2768. doi: 10.1099/00221287-129-9-2761. [DOI] [PubMed] [Google Scholar]
- 54.Virji M, Heckels J E. The role of common and type-specific pilus antigenic domains in adhesion and virulence of gonococci for human epithelial cells. J Gen Microbiol. 1984;130:1089–1095. doi: 10.1099/00221287-130-5-1089. [DOI] [PubMed] [Google Scholar]
- 55.Virji M, Saunders J R, Sims G, Makepeace K, Maskell D, Ferguson D J. Pilus-facilitated adherence of Neisseria meningitidis to human epithelial and endothelial cells: modulation of adherence phenotype occurs concurrently with changes in primary amino acid sequence and the glycosylation status of pilin. Mol Microbiol. 1993;10:1013–1028. doi: 10.1111/j.1365-2958.1993.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 56.Wall D, Kaiser D. Type IV pili and cell motility. Mol Microbiol. 1999;32:1–10. doi: 10.1046/j.1365-2958.1999.01339.x. [DOI] [PubMed] [Google Scholar]
- 57.Watts T H, Worobec E A, Paranchych W. Identification of pilin pools in the membranes of Pseudomonas aeruginosa. J Bacteriol. 1982;152:687–691. doi: 10.1128/jb.152.2.687-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolfgang M, Park H S, Hayes S F, van Putten J P, Koomey M. Suppression of an absolute defect in type IV pilus biogenesis by loss- of-function mutations in pilT, a twitching motility gene in Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1998;95:14973–14978. doi: 10.1073/pnas.95.25.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodall L D, Russell P W, Harris S L, Orndorff P E. Rapid, synchronous, and stable induction of type 1 piliation in Escherichia coli by using a chromosomal lacUV5 promoter. J Bacteriol. 1993;175:2770–2778. doi: 10.1128/jb.175.9.2770-2778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zak K, Diaz J-L, Jackson D, Heckels J E. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984;149:166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q Y, DeRyckere D, Lauer P, Koomey M. Gene conversion in Neisseria gonorrhoeae: evidence for its role in pilus antigenic variation. Proc Natl Acad Sci USA. 1992;89:5366–5370. doi: 10.1073/pnas.89.12.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]