Abstract
Background: Multiple Sclerosis treatment with B-cell targeted therapies may be associated with an increased incidence of headache. We aimed to find and compare the association of B-cell targeted therapies with the incidence of headache in patients with Multiple Sclerosis. Methods: In a systematic based approach, the following databases were searched from inception until the 6th of June 2020: Pubmed/MEDLINE, ClinicalTrials.gov, EU Clinical Trials Register. Only randomized clinical trials (RCTs) enrolling patients with Multiple Sclerosis comparing B-cell targeted therapies (Rituximab, Ocrelizumab, Ofatumumab, Ublituximab or Cladribine) with placebo were selected for the systematic review and further meta-analysis. PRISMA guidelines were followed at all stages of the systematic review. The primary outcome was an all-cause headache of B-cell targeting therapy in patients with Multiple Sclerosis. Results: Nine RCTs were included. Compared with placebo, treatment with B-cell targeting therapies revealed a trend in headache risk, but it was not statistically significant (Relative Risk 1.12 [95% Confidence Interval 0.96–1.30]; p = 0.15; I2 = 9.32%). Surprisingly, in a sub-group analysis, Cladribine was statistically significant for an increase in headache risk (RR 1.20 [95% CI 1.006–1.42]; p = 0.042; I2 = 0%; 3 studies with 2107 participants). Conclusions: Even though a trend is shown, B-cell targeted therapies do not correlate with an increased incidence of headache as an adverse effect. Sub-analyses revealed a significant association between Cladribine alone and an increased incidence of headache. Whereas a purinergic signaling cascade is proposed as a mechanism of action, further research is needed to unravel the underlying pathogenetic mechanism of headache induction and establish headache prevention strategies.
Keywords: B-cell targeted therapies, headache incidence, cladribine, ocrelizumab, ofatumumab, ublituximab, rituximab, purinergic signaling
1. Introduction
Headache disorders are ranked as the fourth most common among neurological disorders in terms of disease prevalence (age standardized) and years lived with the disease (YLD) in both sexes [1]. Headache disorders usually affect both young and older adults (40%), thus resulting in the loss of many working hours (absenteeism) and lower productivity (presenteeism) [2]. Among all headache disorders, migraine has the third highest prevalence of all medical illnesses. According to the World Health Organization (WHO), disability due to migraine is greater even than disability attributed to cardiovascular disorders [3,4,5,6].
Multiple Sclerosis (MS) is an inflammatory disorder of the central nervous system with a high prevalence, ranking sixth in frequency among neurological disorders. Not only MS as a primary disease but also comorbidities frequently associated with MS, affect the quality of life of these patients, resulting in reduced productivity, as measured in disability-adjusted life years (DALYs) [7].
A definite association between headache disorders, and specifically migraine and MS, is not yet proven. Recent studies evaluated the incidence of headache disorders to be up to 64% in MS patients [8,9,10,11]. Many hypotheses have been speculated about the pathophysiological mechanism of headache in MS patients and the various co-factors involved. Experimental cortical demyelination that accelerates cortical spreading depression, the presence of meningeal and cortical B-cell follicles, and the specific location of lesions attempt to explain headache incidence in MS patients [12,13,14,15]. Freedman and Gray, who have studied the presence of headache in patients with MS during an attack, showed that nearly half of the patients had brain stem involvement [16]. Epidemiological studies confirm the aforementioned results and imaging studies have indicated that midbrain/periaqueductal (PAG) MS lesions are associated with an increased incidence of headache [17,18,19]. PAG as well as other midbrain structures and their connections to the rostral ventromedial medulla and the dorsolateral pontomesencephalic tegmentum have been associated with the occurrence of headache, by decreasing the firing of the nociresponsive neurons of the dorsal horn [17,20]. Additionally, retrospective observational studies that included patients who underwent deep brain electrical stimulation showed that migraine-like attacks were generated via the stimulation of PAG [21,22]. Other locations that have also been related to headache are the substantia nigra, the red nucleus and the hypothalamus, all of which are linked to PAG by afferent and efferent signaling [17]. Among the co-factors, MS therapies are considered to play a role.
With the development of research regarding B-cell implications in the pathophysiology of MS, new depleting therapies that target either B-cells alone or both T- and B-cells have been introduced in MS clinical practice (Rituximab, Ocrelizumab, Ofatumumab, Cladribine and Ublituximab). Common adverse events of B-cell therapies usually include lymphopenia, susceptibility to infections and an increased incidence of malignancies. The majority of clinical trials focus on severe adverse events (SAEs), such as the aforementioned. Minor side effects such as headache and dizziness are usually not systematically reported and are often underrated, resulting in missing data.
B-cell therapies that have been used in MS as well as in many other diseases (e.g., lymphoma, rheumatoid arthritis, chronic lymphocytic leukemia, pemphigus, etc.), have been insufficiently related to headache as an adverse event. However, this finding occurred mostly as an Infuse Related Reaction (IRR), as reported by the Summary of Product Characteristics (SmPC) of each drug [23,24,25,26,27,28,29,30,31,32,33]. Headache was noted largely in the real-world evidence status and not in the pre-market clinical studies, resulting in missing data regarding pain characteristics that would allow classification.
Headache as an adverse event has been assessed, but not meticulously reported, in many clinical trials among the different available MS therapies. A recent meta-analysis, which included all studies on interferon-beta (INF-β) in MS [34], showed increased headache incidence over placebo in patients receiving INF-β (Relative Risk 1.16 [95% Confidence Interval 1.02–1.33], p-value = 0.02), who have reported de novo headaches, the aggravation of pre-existing headaches or change in the clinical features. INF-β was one of the most widely prescribed immunomodulatory MS treatment until now. According to the International Classification of Headache Disorders 3rd edition (ICHD-3) criteria [35], this type of headache is categorized as secondary (§ 8.1.10 Headache attributed to long-term use of non-headache medication) and studies speculate potential underlying pathogenetic mechanisms related to INF-β, as the headache attack has a close temporal relation to the subcutaneous injection of the drug. In vitro studies show that IFN-β influences the neuronal excitability in neocortical pyramidal neurons [36,37], which seems to play an important role in the pathogenesis of primary headaches [38]. Cytokine level changes (e.g., serum tumor necrosis factor alfa and IL-10) should also be evaluated, as high serum levels are reported in headache patients [37,39,40]. Patients receiving fingolimod in a FREEDOMS trial had an increased incidence of headache compared to placebo (26.6% vs. 23%, p-value = 0.007) [41]. Furthermore, fingolimod is associated with posterior reversible encephalopathy syndrome (PRES) and there are several cases that support a temporal correlation of fingolimod treatment initiation with new and persistent headaches [42,43]. A direct endothelial modulatory effect has been hypothesized by the authors as a mechanism of action [43,44,45,46].
A possible common denominator might be the increase in IL-10 levels. Munno et al. [39] showed that IL-10 levels were increased in patients during migraine attacks and subsequently decreased after sumatriptan treatment. Both INF-β and fingolimod result in an increase in IL-10 expression in circulating B-cells [47,48,49] and myeloid cells [50].
Taking the above into consideration together with the higher IL-10 levels produced by the reconstituted B-cells particularly following anti-CD20 treatment [50], we hypothesize that anti-B-cell therapies might thereby contribute to increased headache incidence in patients with MS.
However, to date, no previous studies exist that review the incidence of headache as an adverse event in MS patients receiving different anti-B-cell therapies. To provide additional evidence, we conducted a systematical review and meta-analysis to investigate the association of B-cell targeted therapies with headache incidence in MS patients.
2. Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA) statement [51,52,53]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020199534). No institutional review board approval was required because all the study data were published previously and this study did not include individual patient data.
2.1. Eligibility Criteria, Literature Search and Study Selection
A systematic literature search was conducted through June 2020 to identify randomized placebo-controlled trials enrolling adult patients (>18 years of age) with Multiple Sclerosis treated with B-cell targeted therapies (Rituximab, Ocrelizumab, Cladribine, Ofatumumab and Ublituximab). The following electronic bibliographic databases were searched from inception until the 6th of June 2020 using a comprehensive search strategy: MEDLINE and PubMed (non-MEDLINE records only). ClinicalTrials.gov and EU Clinical Trials Register (clinicltrialsregister.eu) were also searched for all registered clinical trials and RCTs. The search strategy was structured according to the Peer Review of Electronic Search Strategies (PRESS) 2015 guidelines [54]. No limits were applied to sex or race. Only English language articles were considered. The full texts of articles identified as relevant during the title and abstract screening stage were obtained and reviewed. The comprehensive search strategy, detailed information about the inclusion and exclusion criteria and the PRISMA statement along with the screening/eligibility process are described in Figure 1 and in the Appendix BAppendix C and Appendix D and Supplement Table S1.
Figure 1.
PRISMA Flow Diagram.
2.2. Outcomes
The primary outcome was the incidence in headache (all types) reported as an adverse event throughout the whole duration of the studies selected.
2.3. Data Extraction and Risk of Bias
Two reviewers (T.M. and M.B.) independently extracted individual study data and four reviewers (T.M., M.B., N.P., A.D. and A.L.) evaluated studies for the risk of bias using the Cochrane Risk of Bias tool for RCTs (ROB-II) [55]. The following domains of each of the primary studies were assessed: random sequence generation, allocation concealment, blinding of study participants, incomplete outcome data, selective reporting and other biases. Based on these domains, the overall risk of bias for each included study was assessed.
2.4. Statistical Analyses
We applied random-effects meta-analysis to Risk Ratio (RR) estimating the association of headache with the B-cell targeted treatment of Multiple Sclerosis. Random-effects models were a priori preferred over fixed-effects models due to the expected heterogeneity between studies with regard to intervention and outcome definition. The significance level for the overall effect was set at p < 0.05. Between-study heterogeneity was assessed by the I2 and the Cochran’s Q test; the significance level was defined by a p value < 0.05 or I2 > 50%. A sub-group analysis was performed for the different pharmacological interventions. All analyses were performed using R v. 4.0 (R foundation, Vienna, Austria) with the ‘metafor’ package [56,57,58].
3. Results
We identified 258 records that were eligible for inclusion, including 30 RCTs (Figure 1). The included trials evaluated four interventions (Rituximab, Ocrelizumab, Cladribine, Ofatumumab). There were no data available concerning headache as an adverse event in clinical trials of Ublituximab.
Summary of Study Retrieval and Identification for Meta-analysis according to PRISMA statement.
In total, nine studies [59,60,61,62,63,64,65,66,67] (3785 patients) were included in the final analysis, whose characteristics are shown in Table 1.
Table 1.
Main characteristics of the included trials.
| NCT | Trial Name | Sponsor | Intervention | Age, Mean, y (SD) | # of Participants Included in the Safety Population Analysis | # Treated with H/Treated | # Placebo with H/Placebo |
|---|---|---|---|---|---|---|---|
| NCT00213135 [65] | CLARITY | EMD Serono | CLA | 38.6 (10.0) | 1319 | 198/884 | 75/435 |
| NCT00725985 [61] | ORACLE-MS | EMD Serono | CLA | 31.9 (8.7) | 616 | 122/410 | 57/206 |
| NCT00436826 [64] | ONWARD | EMD Serono | CLA | 38.9 (10.2) | 172 | 31/124 | 10/48 |
| NCT01194570 [60] | ORATORIO | Hoffmann-La Roche | OCR | 44.6 (8.0) | 702 | 68/486 | 33/216 |
| NCT01457924 [59] | MIRROR | GlaxoSmithKline | OFA | 37.2 (9.36) | 231 | 12/164 | 7/67 |
| NCT00097188 [66] | HERMES | Genentech, Inc. | RTX | 40 | 104 | 13/69 | 7/35 |
| NCT00087529 [67] | OLYMPUS | Genentech, Inc. | RTX | 49.9 (8.90) | 439 | 77/292 | 27/147 |
| NCT00676715 [62] | - | Genentech, Inc. | OCR | 37.6 (8.8) | 164 | 19/110 | 13/54 |
| NCT00640328 [63] | OMS115102 | GlaxoSmithKline | OFA | 36.3 (7.9) | 38 | 2/26 | 1/12 |
NCT: clinicaltrails.gov registration number, #: number, CLA: Cladribine, OCR: Ocrelizumab, RTX: Rituximab, OFA: Ofatumumab, CLARITY: A Safety and Efficacy Study of Oral Cladribine in Subjects With Relapsing-remitting Multiple Sclerosis (RRMS), ORACLE-MS: Oral Cladribine in Early Multiple Sclerosis (MS), ONWARD: A Phase 2 Study of Cladribine Add-on to Interferon-beta (IFN-beta) Therapy in Multiple Sclerosis (MS) Subjects With Active Disease, ORATORIO: A Study of Ocrelizumab in Participants With Primary Progressive Multiple Sclerosis, MIRROR: Ofatumumab Subcutaneous Administration in Subjects With Relapsing-Remitting Multiple Sclerosis, HERMES: A Study to Evaluate Rituximab in Adults With Relapsing Remitting Multiple Sclerosis, OLYMPUS: A Study to Evaluate the Safety and Efficacy of Rituximab in Adults With Primary Progressive Multiple Sclerosis, OMS115102: Ofatumumab Dose-finding in Relapsing Remitting Multiple Sclerosis (RRMS) Patients.
Cladribine (a synthetic purine nucleoside analog that inhibits DNA synthesis and ribonucleotide reductase) was assessed in three studies (2107 participants), Ocrelizumab (a humanized anti-CD20 monoclonal antibody) in two studies (866 participants), Ofatumumab (a fully human anti-CD20 monoclonal antibody) in two studies (269 participants) and Rituximab, a chimeric anti-CD20 monoclonal antibody, also in two studies (543 participants), as presented in Table 2.
Table 2.
Main characteristics of study results by intervention.
| Intervention | # of Trials | # of Patients | RR | 95% CI | p-Value | I2 |
|---|---|---|---|---|---|---|
| Cladribine | 3 | 2107 | 1.20 | 1.006–1.42 | 0.042 | 0% |
| Ocrelizumab | 2 | 866 | 0.857 | 0.93–1.89 | 0.115 | 0% |
| Ofatumumab | 2 | 269 | 0.728 | 0.33–1.66 | 0.448 | 0% |
| Rituximab | 2 | 543 | 1.33 | 0.62–1.19 | 0.354 | 0% |
| Total | 9 | 3785 | 1.12 | 0.96–1.30 | 0.15 | 9.32% |
#: Number, RR: Risk Ratio for the incidence of headache, CI: Confidence Interval, I2 Study Heterogeneity.
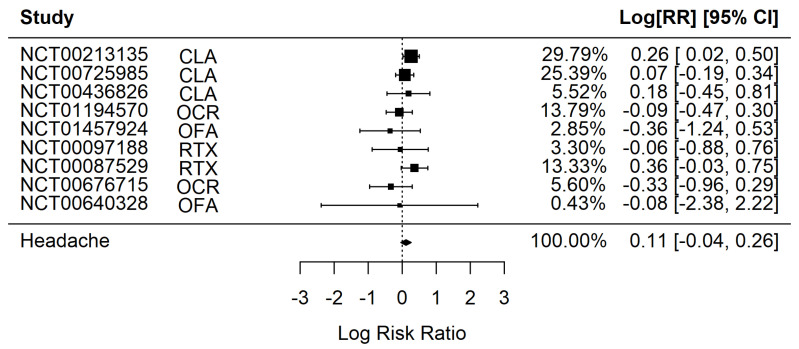
The presence of headache was not associated, in general, with B-cell depleting therapy (RR 1.12 [95% CI 0.96–1.30], p-value = 0.15, I2 = 9.32%, Q = 7.42, p = 0.492) (Figure 2).
Figure 2.
Forest plot for the association of headache with B-Cell modifying therapies. NCT: clinicaltrials.gov registration number, CLA: Cladribine, OCR: Ocrelizumab, RTX: Rituximab, OFA: Ofatumumab, RR: Risk Ratio, CI: Confidence Interval.
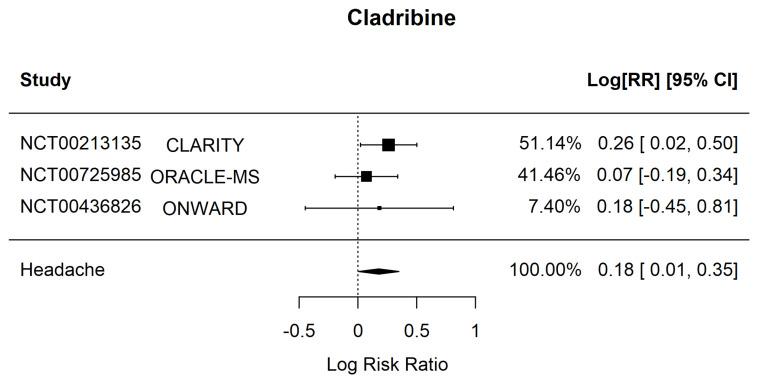
By extracting the Risk Ratio of headache incidence from the included randomized control trials for each drug, a statistically significant association between headache and treatment with Cladribine was uncovered (RR 1.20, [95% CI 1.006–1.42], p-value = 0.042, I2 = 0%, Q = 1.07, p = 0.586) (Table 2, Figure 3).
Figure 3.
Forest plot of sub-group analysis for the association of headache with Cladribine treatment. NCT: clinicaltrials.gov registration number, RR: Risk Ratio, CI: Confidence Interval.
A sub-group analysis of the included studies for every monoclonal antibody against the B-cell line (i.e., Ofatumumab, Rituximab and Ocrelizumab) and for each drug separately showed no apparent connection between the development of headache and the different treatment options (Supplement Figures S1–S4).
According to ROB-II, all studies fall into the category of some concerns of bias. The main reason is the absence of a finalized pre-specified analysis plan before unblinding the outcome data that were available for headache assessment analysis. As a result, there were some concerns in the domain of bias in the selection of the reported result. In all other ROB-II domains there was low risk of bias (Supplement Figure S5 and S6).
Most of the headache-related data we present here were extracted from clinicaltrials.gov and were not available in the respective trial’s publications. There is a concern of possible publication bias, especially since headaches are considered secondary adverse effects, but this concern is minimized due to the obligatory reporting in clinicaltrials.gov. The analysis of our results indicates that it probably does not have a big effect in this case (Egger’s test p-value = 0.18, Supplement Figure S7).
4. Discussion
We performed a meta-analysis and compared the relative risk of headache in patients with MS under treatment with B-cell depletion therapies to those receiving placebo and found that B-cell targeted therapies are not associated overall with an increased risk of developing secondary headache. Furthermore, we conducted a Risk Ratio analysis separately for each drug included in the original analysis. No apparent correlation was found between the development of headache and monoclonal antibodies against B-cells (Supplement Figures S1–S4). Interestingly, an association of Cladribine and headache incidence was identified (Figure 3). As comparison between B-cell therapies and other MS treatments (head-to-head clinical trials) was unfeasible, due to cross-over studies and many confounding factors. A placebo comparator was a priori decided for the study analysis.
The route of administration plays an important role, especially when it involves parenteral administration, injection related adverse events and high frequency dosing regimen. B-cell therapy studies are more appropriate for investigating headache incidence, as they do not bear the risk of injection-associated headache, as opposed to interferons that are administered subcutaneously with a high frequency. Thus, the above results depict the active-drug’s action more, rather than the route of administration.
Although headache is among the commonly reported side effects of Cladribine, an induction mechanism has not been proposed so far. The modulation of nociception by perturbations introduced in purinergic signaling cascades may explain headaches. The contribution of purinergic signaling in pain conduction encompasses both vasomotor, neuronal and cortical processes, closely following the dissemination of purinergic receptors in each tissue [68]. Of note, the ATP-mediated migrainogenic activation of trigeminal nerves has been shown to be regulated by the calcitonin gene-related peptide (CGRP) [69]. Experimental models have furthermore determined that the purinergic modulation of nociception or the algogenic activation of the trigeminal ganglia may occur either directly, i.e., via activation of algogenic P2-calcium receptors [70], or indirectly by modulating the nitroxidergic system peripherally [71].
In addition, early studies of radioligand binding affinity demonstrated that Cladribine binds as an agonist to the adenosine receptor sub-types A1 and A2A [72]. This may be relevant to the emergence of headache in Cladribine-treated patients, as A2A receptor-mediated signaling is thought to be central to the pathophysiology of headaches [73]. Interestingly, A1 receptor mRNA and protein levels were found to be reduced in MS brain tissue [74] and A1 receptor deficiency increased proinflammatory responses and aggravated experimental allergic encephalomyelitis in mice [75]. This may suggest that transcriptional control or the transcript degradation of the A1 receptor gene is perturbed during MS-associated neuroinflammation. It is therefore conceivable that A2A receptors are disproportionately expressed in the CNS of MS patients, resulting in excess A2A receptor-mediated signaling upon treatment with Cladribine. A possible purinergic signaling cascade and disproportional expression of A2A over A1 receptors, as mechanism of action, should be investigated through further studies.
This study is not without caveats. Limitations of this study include the heterogeneity of the drugs under investigation, the different categorization of headache disorders and MS criteria and variability in methods used. More specifically, the chronological spectrum of the included studies in the analysis ranges from 2008 to 2019. During this period, different diagnostic criteria for both MS and headache disorders were applied. Furthermore, all the included studies share in common that they report every type of headache together as one group, named in general as “head pain”, without sub-dividing into further categories such as migraine, TTH or other type of primary or secondary headache disorder.
As stated previously, minor adverse events such as headache were not the primary study interest of the clinical trials. For this reason, several studies have disclosed that the data were collected by a non-systematic assessment. Consequently, there were missing data in the clinical trials of our interest.
We should also note that even though all the treatments analyzed in this study have a reported direct or indirect B-cell mediated mechanism of action, they do not share similar pharmacodynamics. Even the three so called “anti-CD20 monoclonal antibodies” (Rituximab, Ocrelizumab, Ofatumumab), despite sharing the “same” target (B-cells that express CD20), differ in the exact neuroimmunomodulatory effects. Moreover, Cladribine is a synthetic purine nucleoside analog that inhibits DNA synthesis and ribonucleotide reductase. Once inside the cell, Cladribine is activated mostly in lymphocytes, after being triphosphorylated by the enzyme deoxyadenosine kinase (dCK). Activated, the triphosphorylated Cladribine is incorporated into mitochondrial and nuclear DNA, which triggers apoptosis [76]. Due to the extremely specific ratio of dCK to 5′-NTase needed to activate and accumulate enough Cladribine to induce apoptosis, only lymphocytes are uniquely vulnerable [77]. Within the lymphocyte pool, Cladribine targets B-cells more than T cells. The CD3+ T cells remain suppressed longer than the CD19+ B-cells, and the CD4+ cells are affected more than the CD8+ cells.
Overall, the shift to B-cell therapies in the management of MS provided more and better tolerated treatment options. However, as newly introduced players emerge in the field of MS therapeutics, increased pharmacovigilance is needed. With every new treatment introduced, the expectation of a more individualized, targeted and better tolerated medical care raises.
5. Conclusions
In this study, we show for the first time that contrary to the rationale of being expected, B-cell targeted MS therapies are not significantly associated with the incidence of headache as an adverse effect. However, a statistically significant association (RR 1.20, [95% CI 1.006–1.42], p-value = 0.042) between Cladribine and an increased incidence of headache was observed. Physicians, apart from severe adverse events (SAEs), such as lymphopenia, hepatotoxicity, carcinogenesis and opportunistic infections, also need to keep in mind those minor adverse effects to achieve treatment adherence through patient education. Further research is needed to elucidate the pathogenetic mechanism of headache induction in B-cell targeted MS therapies, as well as to identify headache prevention strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm12091474/s1.
Appendix A
| Name | Location | Role | Contribution |
|---|---|---|---|
| Theodoros Mavridis | First Department of Neurology, Eginition Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece | Author | Design and conceptualized study; acquisition of data; interpreted the data; drafted the manuscript for intellectual content |
| Nikolaos Papagiannakis | First Department of Neurology, Eginition Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece | Author | Acquisition of data; methodology; curation and interpretation of data; formal analysis; visualization data; investigation; review and editing |
| Marianthi Breza | First Department of Neurology, Eginition Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece | Author | Acquisition of data; methodology; curation and interpretation of data; formal analysis; visualization data; investigation; review and editing |
| Georgios Vavougios | Scientific Research Associate, Neuroimmunology Laboratory, Department of Neurology, Athens Naval Hospital, Athens, Greece | Author | Acquisition of data; methodology; curation and interpretation of data; formal analysis; visualization data; investigation; review and editing |
| Kostas Patas | First Department of Neurology, Eginition Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece | Author | Acquisition of data; methodology; curation and interpretation of data; formal analysis; visualization data; investigation; review and editing |
| Ariadne Daponte | First Department of Neurology, Eginition Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece | Author | Acquisition of data; methodology; curation and interpretation of data; formal analysis; visualization data; investigation; review and editing |
| Achilleas Laskaratos | National and Kapodistrian University of Athens, Medical School, Athens, Greece | Author | Acquisition of data; revised the manuscript for intellectual content |
| Paraschos Archontakis-Barakakis | Northeast Internal Medicine Associates, LaGrange, Indiana, USA. | Author | Formal analysis; revised the manuscript for intellectual content; editing |
| Ioannis Pantazopoulos | Department of Emergency Medicine, Faculty of Medicine, University of Thessaly, Biopolis, 41500, Larissa, Greece | Author | Revised the manuscript for intellectual content; supervision of the entire process; project administration |
| Dimos D. Mitsikostas | First Department of Neurology, Eginition Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece | Author | Revised the manuscript for intellectual content; supervision of the entire process; project administration |
Appendix B. Systematic Review Search Strategy
Database searching:
| Name of Database (incl. Interface) | Why Is It Relevant (to the Topic)? | Does It Offer a Controlled Vocabulary/Subject Headings? | Do You Have Access to This Database? |
|---|---|---|---|
| MEDLINE (PubMed) | MEDLINE is the U.S. National Library of Medicine® (NLM) premier bibliographic database that contains more than 26 million references to journal articles in life sciences with a concentration on biomedicine. | Yes, MeSH terms | Yes |
Structure of the search strategy:
| Concept | Importance |
|---|---|
| Rituximab | High—search terms for this have to be included |
| Ocrelizumab | High—search terms for this have to be included |
| Ofatumumab | High—search terms for this have to be included |
| Ublituximab | High—search terms for this have to be included |
| Cladribine | High—search terms for this have to be included |
| Multiple Sclerosis | High—search terms for this have to be included |
| Clinical Trials | High—search terms for this have to be included |
Supplementary searches: What other information sources are relevant for to this topic? Choose at least two and explain why you would use them.
| Name of Source/Technique | Why Is It Relevant (to the Topic)? |
|---|---|
| Reference list | It may find studies using the “snowball” technique, that may not appear in the first search results. |
| Clinicaltrials.gov | It may find studies that may not appear in the first search results. |
| Clinicaltrialsregister.eu | It can help identify more studies than clinicaltrials.gov on this specific subject. |
Appendix B.1. Pubmed/MEDLINE Search Strategy
Time of search: Last search on 6 June 2020.
Appendix B.2. Boolean Search String
((((((rituximab) OR ocrelizumab) OR ofatumumab) OR cladribine) OR ublituximab) AND multiple sclerosis) AND clinical trial
Appendix B.3. Actual MeSH Term Search
(((((((“rituximab”[MeSH Terms] OR “rituximab”[All Fields]) OR “rituximab s”[All Fields]) OR (“ocrelizumab”[Supplementary Concept] OR “ocrelizumab”[All Fields])) OR (“ofatumumab”[Supplementary Concept] OR “ofatumumab”[All Fields])) OR ((“cladribin”[All Fields] OR “cladribine”[MeSH Terms]) OR “cladribine”[All Fields])) OR (“ublituximab”[Supplementary Concept] OR “ublituximab”[All Fields])) AND ((“multiple sclerosis”[MeSH Terms] OR (“multiple”[All Fields] AND “sclerosis”[All Fields])) OR “multiple sclerosis”[All Fields])) AND ((“clinical trial”[Publication Type] OR “clinical trials as topic”[MeSH Terms]) OR “clinical trial”[All Fields])
Results: 228 entries.
Appendix B.4. Additional/Supplementary Search
Search through reference lists and clinicaltrials.gov (“snowball technique”).
Results: 30 clinical trials.
Appendix C. Inclusion/Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Additional Information Regarding Exclusion Criteria
The monoclonal antibody against the surface glycoprotein CD52, alemtuzumab, was not included to the initial search. Alemtuzumab is a humanized anti-CD52 IgG1 monoclonal antibody that depletes CD52-expressing cells from the circulation. Data derived from experimental and clinical trials and long-term observational studies indicate that alemtuzumab induces a marked immunosuppression related to the depletion of circulating T and B lymphocytes (non-selective immune reconstitution). While it targets both T and B-cells, it induces a deep T cell and a rather milder B-cell depletion, in contrast to the Cladribine that was included. Thus, its non-selectivity and the preference towards the T cell linage were the main reasons for excluding it.
Appendix D. PRISMA Statement and Screening/Eligibility Strategy
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews were applied in form of the PRISMA 2009 checklist (Table S1) and flow diagram (Figure S1) [51,52].
The first screening of the 258 reports resulted in 124 full articles that were assessed for eligibility. According to the above inclusion and exclusion criteria, 115 full-text articles and registered clinical trials were excluded with reasoning. The screening and eligibility phase was blindly conducted by two individual researchers (T.M. and M.B.). For the interrater reliability of selection, a test was performed on older data collection studies by calculating the statistical k (>90%). In case of a disagreement (<5%), two other researchers (G.V. and A.L.) carried out an audit of these studies based on the same inclusion and exclusion criteria. During the eligibility process, studies without provided results of adverse events were identified and raw data from clinicaltrials.gov and clinicaltrialsregister.eu were collected. An attempt was made to communicate to the corresponding author of one study meeting the above criteria without success. We subsequently further hand searched the reference lists of eligible articles and relevant reviews (the “snowball” procedure). All studies were evaluated for potential population overlap based on geographical setting, recruitment periods and same registration numbers. In case of overlapping populations, we retained the study with the largest sample size. In total, nine studies were finally included in the qualitative synthesis of this systematical review and subsequent meta-analysis. Our primary outcome of interest was all-cause headache as a side effect, as defined by standard clinical criteria.
Author Contributions
Author Contributions are available in Appendix A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available in Clinicaltrials.gov at https://clinicaltrials.gov/ (assessed on 6 June 2020).
Conflicts of Interest
T.M. received travel grants from Merck and Sanofi-Genzyme. M.B. received travel grants from Merck, Teva-Specifar, Genesis Pharma and Pfizer. N.P. declares no conflict of interest. K.P. declares no conflict of interest. A.D. declares no conflict of interest. A.L. declares no conflict of interest. G.V. declares no conflict of interest. A.B.P. declares no conflict of interest. I.P. declares no conflict of interest. D.D.M. received consulting, research, speaking fees and/or travel grants from Allergan, Amgen, Bayer, Biogen, Cefaly, ElectroCore, Eli-Lily, Genesis Pharma, Merck-Serono, Merz, Mylan, Novartis, Roche, Sanofi-Genzyme and Teva-Specifar.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiner T.J., Jensen R., Katsarava Z., Linde M., MacGregor E.A., Osipova V., Paemeleire K., Olesen J., Peters M., Martelletti P. Aids to Management of Headache Disorders in Primary Care (2nd Edition): On Behalf of the European Headache Federation and Lifting the Burden: The Global Campaign against Headache. J. Headache Pain. 2019;20:57. doi: 10.1186/s10194-018-0899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner J.T., Stovner L.J., Vos T., Jensen R., Katsarava Z. Migraine Is First Cause of Disability in under 50s: Will Health Politicians Now Take Notice? J. Headache Pain. 2018;19:17. doi: 10.1186/s10194-018-0846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner T.J., Alliance World Headache Lifting the Burden: The Global Campaign against Headache. Lancet Neurol. 2004;3:204–205. doi: 10.1016/S1474-4422(04)00703-3. [DOI] [PubMed] [Google Scholar]
- 5.Murray L.C.J., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., et al. Disability-Adjusted Life Years (Dalys) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 6.Harwood H.R., Sayer A.A., Hirschfeld M. Current and Future Worldwide Prevalence of Dependency, Its Relationship to Total Population, and Dependency Ratios. Bull. World Health Organ. 2004;82:251–258. [PMC free article] [PubMed] [Google Scholar]
- 7.Wallin T.M., Culpepper W.J., Nichols E., Bhutta Z.A., Gebrehiwot T.T., Hay S.I., Khalil I.A., Krohn K.J., Liang X., Naghavi M., et al. Global, Regional, and National Burden of Multiple Sclerosis 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:269–285. doi: 10.1016/S1474-4422(18)30443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husain F., Pardo G., Rabadi M. Headache and Its Management in Patients with Multiple Sclerosis. Curr. Treat. Options Neurol. 2018;20:10. doi: 10.1007/s11940-018-0495-4. [DOI] [PubMed] [Google Scholar]
- 9.Mantia L.L., Prone V. Headache in Multiple Sclerosis and Autoimmune Disorders. Neurol. Sci. 2015;36((Suppl. S1)):75–78. doi: 10.1007/s10072-015-2146-9. [DOI] [PubMed] [Google Scholar]
- 10.Kister I., Caminero A.B., Monteith T.S., Soliman A., Bacon T.E., Bacon J.H., Kalina J.T., Inglese M., Herbert J., Lipton R.B. Migraine Is Comorbid with Multiple Sclerosis and Associated with a More Symptomatic Ms Course. J. Headache Pain. 2010;11:417–425. doi: 10.1007/s10194-010-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kister I., Caminero A.B., Herbert J., Lipton R.B. Tension-Type Headache and Migraine in Multiple Sclerosis. Curr. Pain Headache Rep. 2010;14:441–448. doi: 10.1007/s11916-010-0143-5. [DOI] [PubMed] [Google Scholar]
- 12.Möhrke J., Kropp P., Zettl U.K. Headaches in Multiple Sclerosis Patients Might Imply an Inflammatorial Process. PLoS ONE. 2013;8:e69570. doi: 10.1371/journal.pone.0069570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pakpoor J., Handel A.E., Giovannoni G., Dobson R., Ramagopalan S.V. Meta-Analysis of the Relationship between Multiple Sclerosis and Migraine. PLoS ONE. 2012;7:e45295. doi: 10.1371/journal.pone.0045295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magliozzi R., Howell O.W., Reeves C., Roncaroli F., Nicholas R., Serafini B., Aloisi F., Reynolds R. A Gradient of Neuronal Loss and Meningeal Inflammation in Multiple Sclerosis. Ann. Neurol. 2010;68:477–493. doi: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- 15.Merkler D., Klinker F., Jürgens T., Glaser R., Paulus W., Brinkmann B.G., Sereda M.W., Stadelmann-Nessler C., Guedes R.C., Brück W., et al. Propagation of Spreading Depression Inversely Correlates with Cortical Myelin Content. Ann. Neurol. 2009;66:355–365. doi: 10.1002/ana.21746. [DOI] [PubMed] [Google Scholar]
- 16.Freedman M.S., Gray T.A. Vascular Headache: A Presenting Symptom of Multiple Sclerosis. Can. J. Neurol. Sci. 1989;16:63–66. doi: 10.1017/S0317167100028523. [DOI] [PubMed] [Google Scholar]
- 17.Gee J.R., Chang J., Dublin A.B., Vijayan N. The Association of Brainstem Lesions with Migraine-Like Headache: An Imaging Study of Multiple Sclerosis. Headache. 2005;45:670–677. doi: 10.1111/j.1526-4610.2005.05136.x. [DOI] [PubMed] [Google Scholar]
- 18.Tortorella P., Rocca M.A., Colombo B., Annovazzi P., Comi G., Filippi M. Assessment of Mri Abnormalities of the Brainstem from Patients with Migraine and Multiple Sclerosis. J. Neurol. Sci. 2006;244:137–141. doi: 10.1016/j.jns.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Kurtzke J.F., Beebe G.W., Nagler B., Auth T.L., Kurland L.T., Nefzger M.D. Studies on Natural History of Multiple Sclerosis: 4. Clinical Features of the Onset Bout. Acta Neurol Scand. 1968;44:467–494. doi: 10.1111/j.1600-0404.1968.tb05587.x. [DOI] [PubMed] [Google Scholar]
- 20.Fields H.L. Textbook of Pain. Churchill Livingstone; London, UK: 1999. Central Nervous System Mechanisms of Pain Modulation. [Google Scholar]
- 21.Raskin N.H., Hosobuchi Y., Lamb S. Headache May Arise from Perturbation of Brain. Headache. 1987;27:416–420. doi: 10.1111/j.1526-4610.1987.hed2708416.x. [DOI] [PubMed] [Google Scholar]
- 22.Veloso F., Kumar K., Toth C. Headache Secondary to Deep Brain Implantation. Headache. 1998;38:507–515. doi: 10.1046/j.1526-4610.1998.3807507.x. [DOI] [PubMed] [Google Scholar]
- 23.Celltrion Healthcare Rituximab (Blitzima) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/blitzima.
- 24.Celltrion Healthcare Rituximab (Truxima) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/truxima.
- 25.Celltrion Healthcare Rituximab (Ritemvia) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ritemvia.
- 26.Lipomed Cladribine (Litak) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/litak.
- 27.Merck Cladribine (Mavenclad) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/mavenclad.
- 28.Novartis Ofatumumab (Kesimpta) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kesimpta.
- 29.Novartis Ofatumumab (Arzerra) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/arzerra#product-information-section.
- 30.Roche Ocrelizumab (Ocrevus) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ocrevus.
- 31.Roche Rituximab (Mabthera) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/mabthera.
- 32.Sandoz Rituximab (Rixathon) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/rixathon.
- 33.Sandoz Rituximab (Riximyo) [(accessed on 31 August 2022)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/riximyo.
- 34.Filippini G., Munari L., Incorvaia B., Ebers G.C., Polman C., D’Amico R., Rice P.G. Interferons in Relapsing Remitting Multiple Sclerosis: A Systematic Review. Lancet. 2003;361:545–552. doi: 10.1016/S0140-6736(03)12512-3. [DOI] [PubMed] [Google Scholar]
- 35.Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd Edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 36.Hadjilambreva G., Mix E., Rolfs A., Muller J., Strauss U. Neuromodulation by a Cytokine: Interferon-Beta Differentially Augments Neocortical Neuronal Activity and Excitability. J. Neurophysiol. 2005;93:843–852. doi: 10.1152/jn.01224.2003. [DOI] [PubMed] [Google Scholar]
- 37.La Mantia L., D’Amico D., Rigamonti A., Mascoli N., Bussone G., Milanese C. Interferon Treatment May Trigger Primary Headaches in Multiple Sclerosis Patients. Mult. Scler. 2006;12:476–480. doi: 10.1191/1352458506ms1298oa. [DOI] [PubMed] [Google Scholar]
- 38.Lang E., Kaltenhauser M., Neundorfer B., Seidler S. Hyperexcitability of the Primary Somatosensory Cortex in Migraine—A Magnetoencephalographic Study. Pt 11Brain. 2004;127:2459–2469. doi: 10.1093/brain/awh295. [DOI] [PubMed] [Google Scholar]
- 39.Munno I., Marinaro M., Bassi A., Cassiano M.A., Causarano V., Centonze V. Immunological Aspects in Migraine: Increase of Il-10 Plasma Levels During Attack. Headache. 2001;41:764–767. doi: 10.1046/j.1526-4610.2001.01140.x. [DOI] [PubMed] [Google Scholar]
- 40.Covelli V., Massari F., Fallacara C., Munno I., Pellegrino N.M., Jirillo E., Savastano S., Ghiggi M.R., Tommaselli A.P., Lombardi G. Increased Spontaneous Release of Tumor Necrosis Factor-Alpha/Cachectin in Headache Patients. A Possible Correlation with Plasma Endotoxin and Hypothalamic-Pituitary-Adrenal Axis. Int. J. Neurosci. 1991;61:53–60. doi: 10.3109/00207459108986270. [DOI] [PubMed] [Google Scholar]
- 41.Kappos L., Radue E.W., O’Connor P., Polman C., Hohlfeld R., Calabresi P., Selmaj K., Agoropoulou C., Leyk M., Zhang-Auberson L., et al. A Placebo-Controlled Trial of Oral Fingolimod in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 42.Fragoso Y.D., Adoni T., Gomes S., Goncalves M.V., Matta A.P., Mendes M.F., Siquineli F. Persistent Headache in Patients with Multiple Sclerosis Starting Treatment with Fingolimod. Headache. 2015;55:578–579. doi: 10.1111/head.12526. [DOI] [PubMed] [Google Scholar]
- 43.Linda H., von Heijne A. A Case of Posterior Reversible Encephalopathy Syndrome Associated with Gilenya((R)) (Fingolimod) Treatment for Multiple Sclerosis. Front. Neurol. 2015;6:39. doi: 10.3389/fneur.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundblad C., Axelberg H., Grande P.O. Treatment with the Sphingosine-1-Phosphate Analogue Fty 720 Reduces Loss of Plasma Volume During Experimental Sepsis in the Rat. Acta Anaesthesiol. Scand. 2013;57:713–718. doi: 10.1111/aas.12130. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez T., Estrada-Hernandez T., Paik J.H., Wu M.T., Venkataraman K., Brinkmann V., Claffey K., Hla T. Phosphorylation and Action of the Immunomodulator Fty720 Inhibits Vascular Endothelial Cell Growth Factor-Induced Vascular Permeability. J. Biol. Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 46.Tolle M., Levkau B., Keul P., Brinkmann V., Giebing G., Schonfelder G., Schafers M., Lipinski K.v., Jankowski J., Jankowski V., et al. Immunomodulator Fty720 Induces Enos-Dependent Arterial Vasodilatation Via the Lysophospholipid Receptor S1p3. Circ. Res. 2005;96:913–920. doi: 10.1161/01.RES.0000164321.91452.00. [DOI] [PubMed] [Google Scholar]
- 47.Grutzke B., Hucke S., Gross C.C., Herold M.V., Posevitz-Fejfar A., Wildemann B.T., Kieseier B.C., Dehmel T., Wiendl H., Klotz L. Fingolimod Treatment Promotes Regulatory Phenotype and Function of B Cells. Ann. Clin. Transl. Neurol. 2015;2:119–130. doi: 10.1002/acn3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyazaki Y., Niino M., Fukazawa T., Takahashi E., Nonaka T., Amino I., Tashiro J., Minami N., Fujiki N., Doi S., et al. Suppressed Pro-Inflammatory Properties of Circulating B Cells in Patients with Multiple Sclerosis Treated with Fingolimod, Based on Altered Proportions of B-Cell Subpopulations. Clin. Immunol. 2014;151:127–135. doi: 10.1016/j.clim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura M., Matsuoka T., Chihara N., Miyake S., Sato W., Araki M., Okamoto T., Lin Y., Ogawa M., Murata M., et al. Differential Effects of Fingolimod on B-Cell Populations in Multiple Sclerosis. Mult. Scler. 2014;20:1371–1380. doi: 10.1177/1352458514523496. [DOI] [PubMed] [Google Scholar]
- 50.Li R., Patterson K.R., Bar-Or A. Reassessing B Cell Contributions in Multiple Sclerosis. Nat. Immunol. 2018;19:696–707. doi: 10.1038/s41590-018-0135-x. [DOI] [PubMed] [Google Scholar]
- 51.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The Prisma Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The Prisma Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. Straus. Prisma Extension for Scoping Reviews (Prisma-Scr): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 54.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. Press Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Sterne J.A.C., Savovic J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. Rob 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 56.R Core Team . R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing. R Core Team; Vienna, Austria: 2021. [Google Scholar]
- 57.Douglas B., Mächler M., Bolker B., Walker S. Fitting Linear Mixed-Effects Models Usinglme4. J. Stat. Softw. 2015;67:48. [Google Scholar]
- 58.Viechtbauer W. Conducting Meta-Analyses Inrwith Themetaforpackage. J. Stat. Softw. 2010;36:48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 59.Ofatumumab Subcutaneous Administration in Subjects with Relapsing-Remitting Multiple Sclerosis. [(accessed on 31 August 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01457924.
- 60.A Study of Ocrelizumab in Participants with Primary Progressive Multiple Sclerosis. [(accessed on 31 August 2022)]; Available online: https://ClinicalTrials.gov/show/NCT01194570.
- 61.Oral Cladribine in Early Multiple Sclerosis (Ms) [(accessed on 31 August 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00725985.
- 62.A Study of the Efficacy and Safety of Ocrelizumab in Patients with Relapsing-Remitting Multiple Sclerosis. [(accessed on 31 August 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00676715.
- 63.Ofatumumab Dose-Finding in Relapsing Remitting Multiple Sclerosis (Rrms) Patients. [(accessed on 31 August 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00640328.
- 64.A Phase 2 Study of Cladribine Add-on to Interferon-Beta (Inf-Beta) Therapy in Multiple Sclerosis (Ms) Subjects with Active Disease (Onward) [(accessed on 31 August 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00436826.
- 65.A Safety and Efficacy Study of Oral Cladribine in Subjects with Relapsing-Remitting Multiple Sclerosis (Rrms) [(accessed on 31 August 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00213135.
- 66.A Study to Evaluate Rituximab in Adults with Relapsing Remitting Multiple Sclerosis. [(accessed on 31 August 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00097188.
- 67.A Study to Evaluate the Safety and Efficacy of Rituximab in Adults with Primary Progressive Multiple Sclerosis. [(accessed on 31 August 2022)]; Available online: https://ClinicalTrials.gov/show/NCT00087529.
- 68.Cieslak M., Czarnecka J., Roszek K., Komoszynski M. The Role of Purinergic Signaling in the Etiology of Migraine and Novel Antimigraine Treatment. Purinergic Signal. 2015;11:307–316. doi: 10.1007/s11302-015-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yegutkin G.G., Guerrero-Toro C., Kilinc E., Koroleva K., Ishchenko Y., Abushik P., Giniatullina R., Fayuk D., Giniatullin R. Nucleotide Homeostasis and Purinergic Nociceptive Signaling in Rat Meninges in Migraine-Like Conditions. Purinergic Signal. 2016;12:561–574. doi: 10.1007/s11302-016-9521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ceruti S., Fumagalli M., Villa G., Verderio C., Abbracchio M.P. Purinoceptor-Mediated Calcium Signaling in Primary Neuron-Glia Trigeminal Cultures. Cell Calcium. 2008;43:576–590. doi: 10.1016/j.ceca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Borsani E., Albertini R., Labanca M., Lonati C., Rezzani R., Rodella L.F. Peripheral Purinergic Receptor Modulation Influences the Trigeminal Ganglia Nitroxidergic System in an Experimental Murine Model of Inflammatory Orofacial Pain. J. Neurosci. Res. 2010;88:2715–2726. doi: 10.1002/jnr.22420. [DOI] [PubMed] [Google Scholar]
- 72.Jensen K., Johnson L.A., Jacobson P.A., Kachler S., Kirstein M.N., Lamba J., Klotz K.N. Cytotoxic Purine Nucleoside Analogues Bind to A1, A2a, and A3 Adenosine Receptors. Naunyn Schmiedebergs Arch. Pharm. 2012;385:519–525. doi: 10.1007/s00210-011-0719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fried N.T., Elliott M.B., Oshinsky M.L. The Role of Adenosine Signaling in Headache: A Review. Brain Sci. 2017;7:30. doi: 10.3390/brainsci7030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnston J.B., Silva C., Gonzalez G., Holden J., Warren K.G., Metz L.M., Power C. Diminished Adenosine A1 Receptor Expression on Macrophages in Brain and Blood of Patients with Multiple Sclerosis. Ann. Neurol. 2001;49:650–658. doi: 10.1002/ana.1007. [DOI] [PubMed] [Google Scholar]
- 75.Tsutsui S., Schnermann J., Noorbakhsh F., Henry S., Yong V.W., Winston B.W., Warren K., Power C. A1 Adenosine Receptor Upregulation and Activation Attenuates Neuroinflammation and Demyelination in a Model of Multiple Sclerosis. J. Neurosci. 2004;24:1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leist T.P., Weissert R. Cladribine: Mode of Action and Implications for Treatment of Multiple Sclerosis. Clin. Neuropharmacol. 2011;34:28–35. doi: 10.1097/WNF.0b013e318204cd90. [DOI] [PubMed] [Google Scholar]
- 77.Beutler E. Cladribine (2-Chlorodeoxyadenosine) Lancet. 1992;340:952–956. doi: 10.1016/0140-6736(92)92826-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in Clinicaltrials.gov at https://clinicaltrials.gov/ (assessed on 6 June 2020).