Abstract
Comamonas spp. are non-fermenting Gram-negative bacilli. They were first discovered in 1894, and since then, twenty-four species have been characterized. The natural habitat of these bacteria is soil, wastewater/sludge, fresh water such as ponds and rivers, and the animal intestinal microbiome. They were also isolated from industrial settings, such as activated sludge and polluted soil, and from the hospital environment and clinical samples, such as urine, pus, blood, feces, and kidney. Comamonas spp. are associated with environmental bioremediation and are considered an important environmental bacterium rather than a human pathogen. However, in the 1980s, they became a concern when several human infections associated with these species were reported. Here, the Comamonas genus was examined in terms of its members, identification techniques, and pathogenicity. Seventy-seven infection cases associated with these microorganisms that have been discussed in the literature were identified and investigated in this project. All relevant information regarding year of infection, country of origin, patient information such as age, sex, underlying medical conditions if any, type of infection caused by the Comamonas species, antibiotic susceptibility testing, treatment, and outcomes for the patient were extracted from case reports. The findings suggest that even though Comamonas spp. are thought of as being of low virulence, they have caused harmful health conditions in many healthy individuals and even death in patients with underlying conditions. Antimicrobial treatment of infections associated with these species, in general, was not very difficult; however, it can become an issue in the future because some strains are already resistant to different classes of antibiotics. Therefore, these pathogens should be considered of such importance that they should be included in the hospital screening programs.
Keywords: Comamonas, nosocomial infection, environmental bacteria
1. Introduction
The growing range of severe infections caused by little-known non-fermenting Gram-negative rods is developing into a major cause of concern. These pathogens are opportunistic, infecting patients undertaking medical treatments in hospital and immunocompromised individuals outside of clinical locations. Bacterial species, including Ralstonia spp., Ochrobactrum spp., Pseudomonas aeruginosa, Sphingomonas paucimobilis, and Brevundimonas spp., all belong to this group [1,2,3,4,5,6]. Other emerging Gram-negative, non-fermenting rod bacteria that can cause potentially severe infections are members of the β-proteobacterial genus Comamonas [7].
Comamonas spp. have been isolated from a broad variety of environments, including water, aircraft water, soil, plants, and animals [8,9,10,11,12]. Several Comamonas spp. have been investigated for their potential to degrade xenobiotic pollutants and for heavy metal detoxification under a variety of environmental conditions [13,14,15,16,17,18,19]. Comamonas spp. are thought to be of low virulence. They have, however, caused infections, including serious infection such as septicemia or endocarditis, in immunocompetent hosts [20,21,22].
Analysis of the scientific/medical literature showed wide-ranging types of infections resulting from Comamonas spp. These were resistant to numerous different antibiotics. The data uncovered that this genus is a more commonplace pathogen than hitherto believed, with numerous infections/conditions caused by Comamonas spp. being severe and incapacitating. The purpose of this study was to give a general summation of infections caused by Comamonas spp., any underlying disorders/illnesses in patients that predispose them to infections with these bacteria and the antibiotic therapies that can be used for the management of these infections to aid medical professionals.
2. Genus Comamonas
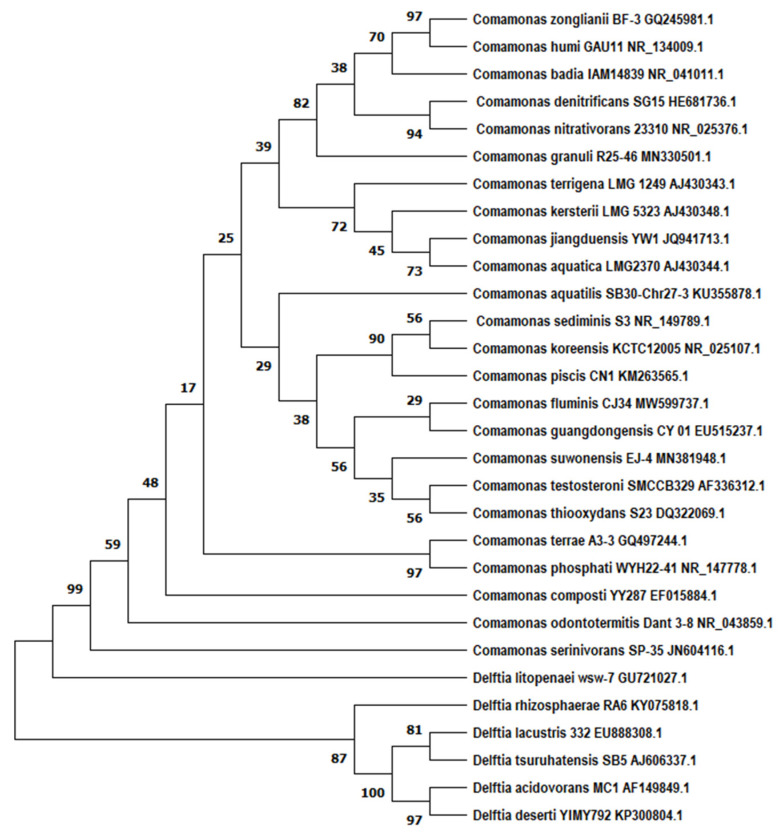
Previously designated as Pseudomonas rRNA homology group III, the family Comamonadaceae now includes the genera Comamonas, Delftia and Acidovorax. The genus Comamonas, assigned to the Comamonadaceae lineage in the β-Proteobacteria, was originally proposed by Davis and Park [23] and the name validly published with the revival of the genus and the type species Comamonas terrigena by De Vos et al. [24]. In 1987, two Pseudomonas species, Pseudomonas acidovorans and Pseudomonas testosterone, were transferred to the genus Comamonas as Comamonas acidovorans and Comamonas testosteroni, respectively [24]. Based on a detailed 16S rRNA gene sequence-based phylogenetic study of the Comamonadaceae C. acidovorans was transferred as a type species to the novel genus Delftia as Delftia acidovorans [25]. Since then, the Comamonas genus has expanded to 24 species (see Table 1). The phylogenetic relationship between all Comamonas spp. described to date is presented in Figure 1.
Table 1.
Listing of validly published Comamonas species.
| Species | Origin/Isolation Site | Genome Sequences | Reference |
|---|---|---|---|
| Comamonas aquatica | China/Freshwater River | Strain: CJG, Size: 3.76 Mb Ref Genome: GCA_000935165.2 (6 genomes) |
Wauters et al., 2000 [28] |
| Comamonas aquatilis | Germany/Garden Pond | No Genome | Kampfer et al., 2018 [29] |
| Comamonas badia | Japan/Activated sludge | Strain: IAM 14839, Size: 3.68 Mb Ref Genome: GCA_000484635.1 |
Tago and Yokota, 2004 [30] |
| Comamonas composti | Taiwan/food waste compost | Strain: YY287T, Size: 4.63 Mb Ref Genome: GCA_000429845.1 |
(Young et al., 2008) [31] |
| Comamonas denitrificans | Sweden/Activated sludge | Strain: 123T Size: 3 Mb Ref Genome: GCA_017368815.1 |
Gumaelius et al., 2001 [32] |
| Comamonas fluminis | China/River water | Strain: CJ34T Size: 4.86 Mb Ref Genome: NZ_CP066783.1 |
Park et al., 2022 [33] |
| Comamonas granuli | Korea/Granules used in wastewater treatment plant | Strain: NBRC 101663T, Size: 3.51 Mb Ref Genome: GCA_003604195.1 |
Kim et al., 2008 [34] |
| Comamonas guangdongensis | China/Subterranean Forest sediment | No Genome | Zhang et al., 2013 [35] |
| Comamonas humi | Japan/Soil | No Genome | Hatayama, 2014 [36] |
| Comamonas jiangduensis | China/Agricultural soil | Strain: YW1T, Size: 2.76 Mb Ref Genome: GCA_902829245.1 |
Sun et al., 2013 [37] |
| Comamonas kerstersii | Dialysis effluent of a patient | Strain: 8943, Size: 3.55 Mb Ref Genome: GCA_002056725.1 |
Wauters et al., 2003 [28] |
| Comamonas koreensis | Korea/Wetland | Strain: YH12T, T50-37 Size: 5.3 Mb Ref Genome: GCA_014076495.1 |
Chang et al., 2002 [38] |
| Comamonas nitrativorans | Uruguay/Denitrifying reactor | Strain: 23310T, Size: 3.36 Mb Ref Genome: SAMN02746010 |
Etchebehere, 2001 [39] |
| Comamonas odontotermitis | Taiwan/Termite Odontotermes formosanus gut | Strain: Dant 3-8T, Size: 4.42 Mb. Ref Genome: GCA_020080045 (For WLL) |
(Chou et al., 2007) [40] |
| Comamonas phosphati | China/Phosphate rock powder—from phosphate mine | Strain: WYH 22-41T, Size: 4.1 Mb Ref Genome: GCA_014637085.1 |
Fuhong et al., 2016 [41] |
| Comamonas piscis | Korea/Korean rockfish intestine | Strain: CN1T, Size: 5.2 Mb Ref Genome: GCA_014109725.1 |
Kang et al., 2016 [42] |
| Comamonas sediminis | USA/Lagoon sediments | Strain: S3T, Size: 4.42 Mb Ref Genome: JAFBFN010000000 (for 4487) |
Subhash et al., 2016 [43] |
| Comamonas serinivorans | China/Wheat straw compost | Strain: SP-35T, Size: 4.52 Mb. Ref Genome: GCA_002158865.1 |
Daochen et al., 2014 [44] |
| Comamonas suwonensis | Republic of Korea/Stream water | Strain: EJ-4 Size: 4.72 Mb Ref Genome: GCA_012844455.2 |
Park et al. 2021 [45] |
| Comamonas terrae | Thailand/Agricultural soil | Strain: A3-3T, Size: 4.7Mb. Ref Genome: GCA_001544075.1 |
Chipirom et al., 2012 [46] |
| Comamonas terrigena | Boston/Hay infusion made from fresh water | Strain: NCIB 8193, Size: 4.7 Mb Ref Genome: AP019749.1 |
De Vos et al., 1985 [24] |
| Comamonas testosteroni | Organic compounds | Strain: KS 0043, Size: 5.41 Mb GCA_000241525.2 (21 Genomes) |
Tamaoka et al., 1987 [47] |
| Comamonas thiooxydans | Sulphur spring | Strain: S23T, Size: 5.27 Mb Ref Genome: GCA_000964545.1 |
Pandey et al., 2009 [48] |
| Comamonas zonglianii | China/Phenol contaminated soil | No Genome | Xin-Yan et al., 2011 [49] |
Figure 1.
Phylogenetic tree of the genus Comamonas (accession numbers are given alongside species name) with the closely related genus Delftia. The tree was built with 16S rDNA genes (partial sequences of ~1400 bp) using neighbor-joining with the Tajma-Nei method utilizing the MEGA 11 software package. Bootstrap values are represented by numbers at nodes. These are based on 1000 resamplings. Bar, 0.0050 substitutions per site [26,27]. It should be remembered that these analyses are based upon 16S rDNA and, as such, are suggestive only.
3. Identification of Comamonas spp.
The Comamonas species are Gram-negative and comprised of straight or slightly curved rods or spirilla. They are usually 0.5 to 2 by 1 to 6 µm. They are generally motile by means of polar or bipolar tufts of 1–5 flagella (excepting C. koreensis). They are aerobic and chemoorganotrophic (De Vos et al., 2015) [50]. Some of the species are non-pigmented, some appear to be cream or yellow-white in color, and some can produce a brown halo around them (Willems and De Vos, 2006) [51], but they do not produce fluorescent pigments. Colonies appear pink-pigmented with a slimy and convex surface on blood agar. No hemolysis was observed on blood and chocolate agar. They are aerobic, oxidase and catalase-positive, non-spore formers, glucose non-fermenters, and chemoorganotrophic. Good growth was observed on media that contained peptone, organic acids, and amino acids (Public Health England, 2015) [52].
4. Comamonas spp. Virulence
Comamonas spp. are believed to be of low virulence. A study of the pangenome of 34 Comamonas genomes, however, showed that they have a diverse array of virulence factors, including polysaccharide biosynthesis for adherence and anti-phagocytosis, a motility system and metabolic enzymes for adaptation in vivo. All sequenced, clinically-isolated Comamonas strains and a number of environmental Comamonas spp. contain hemolysin genes. These analyses indicated that virulence might be species-specific as certain virulence factors are conserved in pathogenic-like strains [53].
5. Comamonas spp. Outbreaks
The overall knowledge gained from research into the scientific and medical literature can be seen in Table 2, Table 3 and Table 4. These tables show the year when the infection happened (if not available, the year of publication was used), country where the infection happened, patient information (age, sex, any reported underlying medical conditions), type of infection caused by the Comamonas infection, antimicrobial testing (susceptibility and resistance), treatment (focusing on the antibiotic therapies used) and patient outcome.
Table 2.
Incidences of Comamonas testosteroni infection from 1987 to 2022. Main characteristics of the case reports.
| Author (Ref.) | Year | Sex/Age | Country | Co-Morbidity | Type of Infection | Susceptible to * | Resistance to * | Antibiotic Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Atkinson et al. 1975 [59] | 1966 | F/31 yr old | USA | Rheumatic heart disease | Septicemia | N/A | N/A | Kanamycin, Tetracycline | Full recovery |
| Grover Smith, 1979 [60] | 1979 | M/48 yr old | USA | Atrophic right leg |
Pyarthrosis Septicemia |
Amikacin, Ampicillin, Carbenicillin, Cephalothin, Chloramphenicol, Colistin, Gentamicin, Kanamycin, Tetracycline, Tobramycin |
N/A | Cephalothin, Gentamicin. Followed by Ampicillin for 21 days. |
Full recovery |
| Barbaro et al., 1987 [54] | 1983 | M/31 yr old | USA | None | Perforated appendix | N/A | N/A | Cefoxitin then drainage, then Ampicillin, Clindamycin, Gentamicin | Full recovery |
| Barbaro et al., 1987 [54] | 1983 | M/11 yr old | USA | None | Perforated appendix | N/A | N/A | Ampicillin, Clindamycin, Tobramycin | Full recovery |
| Barbaro et al., 1987 [54] | 1983 | F/59 yr old | USA | Alcoholic | Cirrhosis | N/A | N/A | Cefoxitin | Full recovery |
| Barbaro et al., 1987 [54] | 1983 | F/24 yr old | USA | Iv drug abuse | Meningitis | N/A | N/A | Moxalactam, Nafcillin | Full recovery |
| Barbaro et al., 1987 [54] | 1984 | F/21 yr old | USA | Pregnant | Perforated appendicitis | Cefoxitin | N/A | Surgery, Iv Cefoxitin for 9 days | Full recovery |
| Barbaro et al., 1987 [54] | 1984 | F/12 yr old | USA | None | Perforated appendicitis | N/A | N/A | Cefoxitin | Full recovery |
| Barbaro et al., 1987 [54] | 1985 | F/84 yr old | USA | Congestive heart failure | Urine tract infection | N/A | N/A | Ampicillin | Full recovery |
| Barbaro et al., 1987 [54] | 1985 | M/24 yr old | USA | None | Perforated appendicitis | N/A | N/A | Cefoxitin | Full recovery |
| Barbaro et al., 1987 [54] | 1985 | F/New-born | USA | Maternal IV drug abuse, Premature birth | Sepsis | N/A | N/A | Ampicillin, amikacin | Died |
| Barbaro et al., 1987 [54] | 1985 | Stillborn | USA | Maternal IV drug abuse, premature birth | Sepsis | N/A | N/A | None | Died |
| Franzetti et al., [61] | 1992 | N/A | Italy | AIDS | Respiratory infection | N/A | N/A | Ceftazidime | Full recovery |
| Le Moal et al., 2001 [62] | 2001 | F/75 yr old | France | Breast cancer | Bacteremia | Aztreonam, Ceftazidime, Piperacillin, Ticarcillin | Amikacin, Ciprofloxacin, Fosfomycin | Ceftazidime, Gentamicin for 10 days | Full recovery |
| Arda et al., 2003 [63] | 2003 | M/50 yr old | Turkey | Undergone cholesteatoma operation | Purulent meningitis | Ceftriaxone, Ceftazidime, Meropenem | N/A | Ceftriaxone (were 3 mg/mL), Ceftazidime (0.75 mg/mL), and Meropenem (0.47 mg/mL), then changed to Meropenem, 3 g/day and operation to remove the cholesteatoma | Full recovery |
| Smith et al., 2003 [64] | 2003 | M/89 yr old | USA | N/A | Bacteremia | N/A | N/A | Levofloxacin | Full recovery |
| Cooper et al., 2005 [22] | 2005 | M/49 yr old | USA | None | Endocarditis | Ampicillin, Gentamicin, first, second, third generation Cephalosporins, Imipenem, Ciprofloxacin, Levofloxacin, Piperacillin, SXT, Tobramycin | N/A | Initially Cefipime, Gentamicin, switched to Ampicillin, then followed by surgery and 6 weeks of IV antibiotic treatment | Full recovery |
| Gul et al., 2007 [65] | 2006 | M/22 yr old | Turkey | None | Bacteremia due to perforated appendicitis | Ampicillin/Sulbactam, Amikacin, Cefazolin, Ceftazidime, Cefepime, Ciprofloxacin, Gentamicin, Imipenem, Levofloxacin, Piperacillin-Tazobactam, Imipenem, Meropenem, SXT, Tobramycin | N/A | Iv Cefazolin 1 g was given before surgery, Iv Cefazolin 1 g every 8 h after surgery | Full recovery |
| Abraham and Simon, 2007 [7] | 2007 | F/54 yr old | USA | Metastatic esophageal cancer, an indwelling central venous catheter | Bacteremia, septic shock | N/A | N/A | Cefepime, Vancomycin, Azithromycin, Drotrecogin alfa, Glucocorticosteroids, Norepinephrine Vasopressin, then was changed to Cefepime and Ciprofloxacin for 16 days | Full recovery |
| Garolo et al., 2007 [66] | 2007 | M/63 yr old | Poland | Lumbar discectomy | Spondylodiscitis | N/A | N/A | Eicoplanine (600 mg e.v./day), Ciprofloxacin (400 mg 2 times/day), then Ciprofloxacin, Cotrimoxazole | Full recovery |
| Jin et al., 2008 [55] | 2008 | M/54 yr old | USA | Alcoholic | Purulent Meningitis | N/A | N/A | Moxifloxacin | Died |
| Reddy et al., 2009 [67] | 2009 | F/82 yr old | India | Diabetes, Cataract surgery | Post-operative endophthalmitis | Ceftazidime, Chloramphenicol, Ciprofloxacin, Gatifloxacin, Moxifloxacin, Ofloxacin | Amikacin, Gentamicin, Tobramycin | Intraocular injection of 1 mg Vancomycin and 1 mg Ceftazidime, Ciprofloxacin (oral and topical), steroids (oral and topical) and Cycloplegics then intravitreal Ceftazidime (1 mg), topical ceftazidime | Full recovery |
| Katırcıoğlu et al., 2010 [68] | 2010 | M/83 yr old | Turkey | Hypertension and ischemic cerebrovascular incident | Sepsis | Amikacin, Ciprofloxacin, Piperacillin-Tazobactam | Aztreonam, Cefepime, Ceftriaxon, Ceftazidime, Cefoperazon-Sulbactam, Tobramycin, Imipenem | Piperacillin-Tazobactam, Amikacin for 10 days | Full recovery |
| Nseir et al., 2011 [69] | 2011 | F/64 yr old | Israel | Diabetes mellitus Patient on hemodialysis | Bacteremia (Catheter-related) | Ceftazidime, Gentamycin, Quinolones | Ampicillin Penicillin, Rocephin. | Vancomycin, ceftriaxone | Died |
| Ozden et al., 2011 [70] | 2011 | M/10 yr old | Turkey | Cerebral palsy, tracheostomy | Infection | N/A | N/A | Ceftriaxone, clarithromycin | Full recovery |
| Tsui et al., 2011 [71] | 2011 | M/73 yr old | Taiwan | Chronic hepatitis B, liver cirrhosis, hepatocellular carcinoma | Bacteremia | N/A | N/A | Radiofrequency ablation for liver tumor, Cefmetazon (1 g every 8 h), Gentamicin (60 mg every 8 h), then changed for IV Levofloxacin (500 mg once a day), oral Levofloxacin (500 mg every day) for 4 days | Full recovery |
| Tsui et al., 2011 [71] | 2011 | M/54 yr old | Taiwan | Alcoholic, Mild obstructive lung disease, replaced hip joints | Bacteremia | N/A | N/A | Iv Oxacillin (2 g every 6 h), Cephalosporin, then IV Ciprofloxacin (400 mg for every 12 h) 8 days | Full recovery |
| Farshad et al., 2012 [72] | 2010 | M/10 yr old | Iran | Brain Medullo-blastoma, chemotherapy | Bacteremia | Amikacin, Ampicillin, Aztreonam Ceftazidime, Ceftriaxone, Cefuroxime, Gentamicin, Cephalexin, Ciprofloxacin, Imipenem, Meropenem, Piperacillin/Tazobactam Tobramycin, Ticarcillin, Tetracycline, | N/A | Iv Ciprofloxacin (10 mg/kg/day for 21 days), Amikacin (15 mg/kg/day for 21 days) | Full recovery |
| Farshad et al., 2012 [72] | 2010 | F/19 yr old | Iran | Osteosarcoma, chemotherapy | Bacteremia, septic shock | Amikacin, Ampicillin, Aztreonam Ceftazidime, Ceftriaxone, Cefuroxime, Gentamicin, Cephalexin, Ciprofloxacin, Imipenem, Meropenem, Piperacillin/Tazobactam Tobramycin, Ticarcillin, Tetracycline | N/A | Iv Vancomycin (60 mg/kg/day for 14 days) and Imipenem (100 mg/kg/day for 14 days), then oral Ciprofloxacin (30 mg/kg/day for three weeks) | Full recovery |
| Al Ramahi et al., 2013 [73] | 2013 | M/47 yr old | Jordan | Renal failure, maintained on hemodialysis | Bacteremia | Cefepime, Ciprofloxacin, Cotrimoxzole, Levofloxacin, Ofloxacin, Polymyxin B, Tigecycline | Amikacin, Gentamicin, Imipenem, Meropenem, Piperacillin/Tazobactam with intermediate sensitivity for Ceftazidime | Cefepime (1 g daily for 14 days), then oral Cyclosporine 200 mg twice daily, Mycophenolate Mofetil 360 mg twice daily Prednisone 30 mg twice daily, oral INH 300 mg once daily | Full recovery |
| Bayhan et al., 2013 [74] | 2013 | M/16 yr old | Turkey | None | Peritonitis due to perforated appendicitis | Amicasin, Ampicillin, Ampicillin-Sulbactam, Ceftazidime, Cefazolin, Ciprofloxacin, Gentamicin, Imipenem, Piperacillin | Ceftriaxone, Cefuroxime, SXT | Removal of appendix, Saline peritoneal lavage, IV Amicasin, Ampicillin, Clindamycin (5 days) | Full recovery |
| Altun et al., 2013 [75] | 2013 | F/29 yr old | Turkey | End-stage renal failure, hypertensive nephrosclerosis, CAPD | Peritonitis | N/A | N/A | Iv Vancomycin, oral Ciprofloxacin (14 days) | Full recovery |
| Orsini et al., 2014 [76] | 2014 | F/80 yr old | USA | Hypertension, diabetes mellitus, hiatal hernia, osteoarthritis, cholelithiasis, obesity | Polymicrobial bacteremia | Ceftazidime, Carbapenems, Piperacillin/Tazobactam, SXT | N/A | Initially Ceftriaxone (2 g IV daily), then Nafcillin (2 g IV every 4 h), Cefazolin (1 g IV every 8 h) and Doripenem (250 mg IV every 8 h) | Full recovery |
| Swain and Rout, 2015 [56] | 2015 | F/50 yr old | India | Diabetes mellitus complicated with chronic renal disease | Bacteremia, septic shock | Ceftazidime, Cefoperazone-Sulbactam, Meropenem | Amikacin, Cefepime, Ciprofloxacin, Gentamicin, Piperacillin-Tazobactam | Piperacillin-Tazobactum (3.375 gm IV 6 hourly), then changed for Cefoperazone-Sulbactam | Died |
| Duran et al., 2015 [77] | 2015 | M/51 yr old | Turkey | Tachycardia | Endocarditis | Amikacin, Ciprofloxacin, Ceftazidime, Cefoperazone-Sulbactam, Cefepime, Colistin Tigecycline | Gentamicin, Imipenem, Meropenem, Netilmicin, Piperacillin-Tazobactam | Cardiovascular surgery, Ciprofloxacin | Full recovery |
| Kim et al., 2015 [21] | 2015 | F/42 yr old | Korea | Meningioma was removed 6 days before infection | Septic shock | N/A | N/A | Initially Piperacillin/Tazobactam, Levofloxacin, Metronidazole iv, renal replacement therapy, Immunoglobulin IV Meropenem/Levofloxacin, then ceftazidime with levofloxacin | Full recovery |
| Khalki et al., 2016 [78] | 2015 | N/A/18 | Morocco | None | Acute appendicitis | Amoxicillin—clavulanic acid, Cefoxitin, 2nd and 3rd generation Cephalosporins, Gentamycin, Amikacin, Carbapenems, Ticarcillin, Piperacillin | Amino-penicillins, Aztreonam, Ciprofloxacin, Nalidixic acid, Norfloxacin, SXT | Surgery, Amoxicillin-clavulanic acid IV for 48 h, then taken orally for 8 days | Full recovery |
| Pekintürk and Akgüneş, 2016 [79] | 2016 | M/62 yr old | Turkey | Left hemiparesis and type II diabetes | Bacteremia | Amikacin, Ceftazidime, Cefepime, Ciprofloxacin, Gentamicin, Imipenem, Levofloxacin, Meropenem, Netilmicin, Piperacillin, Piperacillin-Tazobactam, Tetracycline Tigecycline, Tobramycin, SXT | Aztreonam, Colistin | N/A | Died |
| Parolin et al., 2016 [80] | 2016 | F/4 yr old | Italy | End-stage renal disease, idiopathic epilepsy | Peritonitis | N/A | N/A | Initially IV Ceftazidime, Teicoplanin, then changed to Ciprofloxacin for 3 weeks | Full recovery |
| Hung et al., 2017 [81] | 2017 | F/63 yr old | Taiwan | Hemodialysis patient | Acute Appendicitis | Ceftriaxone, Ceftazidime, Gentamicin | Ciprofloxacin | Cefazolin Followed by ceftriaxone | Full recovery |
| Ruziaki and Hashami, 2017 [82] | 2017 | F/1 yr old | Oman | None | Sepsis | Ceftriaxone, Ceftazidime, Cefipime, Ciprofloxacin, Gentamicin | N/A | Iv Ceftriaxone (80 mg per kg per dose once a day for 14 days | Full recovery |
| Yasayancan and Koseoglu, 2017 [57] | 2017 | M/68 yr old | Turkey | Lung cancer, adrenal metastasis | Polymicrobial Bacteremia | Cefepime, Colistin, Levofloxacin, Tigecycline | Gentamycin, Imipenem, Meropenem, Piperacillin–Tazobactam | Piperacillin–Tazobactam and ciprofloxacin iv, then Cefepime Teicoplanin, then Tigecycline/Colistin | Died |
| Tartar and Tartar, 2020 [83] | 2017 | M/14 yr old | Turkey | None | Perforated appendicitis | Amikacin, Ampicillin–Sulbactam, Ceftazidime, Cefazolin, Ciprofloxacin, Gentamicin, Imipenem, Piperacillin, SXT | N/A | Surgery, IV Cefazolin (100 mg/kg), Amikacin (15 mg/kg), Metronidazole (30 mg/kg). | Full recovery |
| Tartar and Tartar, 2020 [83] | 2017 | F/5 yr old | Turkey | None | Acute appendicitis | Amikacin, Ertapenem, Ciprofloxacin, Gentamicin, Imipenem, Piperacillin, SXT | Ampicillin–Sulbactam, Ceftazidime, Cefuroxime | Surgery, IV Cefazolin (100 mg/kg), Amikacin (15 mg/kg), Metronidazole (30 mg/kg) | Full recovery |
| Farooq et al., 2017 [20] |
2017 | F/65 yr old | India | Colostomy | Gastroenteritis | Amikacin, Cefepime, Cefoperazone/Salbactam, Ceftazidime, Colistin, Gentamicin, Imipenem Cotrimoxazole, Minocycline, Meropenem, Piperacillin/Tazobactam, Tigecycline | Aztreonam, Ciprofloxacin, Levofloxacin | Oral Ciprofloxacin (500 mg for 3 days), probiotics | Full recovery |
| Cetin et al., 2018 [57] | 2018 | M/10 yr old | Turkey | Cerebral palsy, scoliosis, supported with long-term home mechanical ventilation | Pneumonia | Amikacin, Ceftazidime, Cefepime, Imipenem, Levofloxacin, Meropenem, Netilmicin, Piperacillin, Piperacillin-Tazobactam, Tigecycline, SXT | Aztreonam, Ciprofloxacin, Colistin, Gentamicin, Tetracycline | Amikacin (1 × 225 mg), Piperacillin-Tazobactam (3 × 1.5 g) Vancomycin (4 × 150 mg), | Died |
| Lovell and Forde, 2019 [84] | 2019 | M/39 yr old | Barbados | Alcoholism, asthma, pancreatitis | Bacteremia | Cefepime, Cefotaxime, Ceftriaxone, Ciprofloxacin, Levofloxacin, Meropenem, Piperacillin-Tazobactam, SXT | Cefazolin, Ertapenem, Gentamicin | Initially Meropenem 1 g IV every 8 h, Fluconazole 800 mg IV, a 21-day course of Meropenem and a 14-day course of Fluconazole (unsuccessfully), then SXT | Full recovery |
| Tiwari and Nanda, 2019 [85] | 2019 | F/46 yr old | India | None | Bacteremia | Amikacin, Cefuroxime, Ciprofloxacin, Colistin Gentamicin, Imipenem, Meropenem, Tigecycline, Cotrimoxazole | Piperacillin-Tazobactam | Initially Piperacillin-Tazobactam, Vancomycin, then changed for Gentamicin (4 mg/kg/daily) and Imipenem (25 mg/kg 8 hourly) for 10 days | Full recovery |
| Buyukberber et al., 2021 [86] | 2020 | F/4yr old | Turkey | Previous urinary surgery | Urinary tract infection | Ceftazidime, Ciprofloxacin; Meropenem Piperacillin/tazobactam | Amikacin, Gentamicin, Imipenem, SXT | Amikacin Followed by Ceftazidime | Full recovery |
| Miloudi et al., 2021 [87] | 2020 | N/A/12 | Morocco | None | Acute appendicitis | Aminoglycosides, Amoxicillin/Clavulanic acid, 2nd, and 3rd generation Cephalosporins, Carbapenems, Colistin, Ticarcillin | Ciprofloxacin, Norfloxacin, SXT | Appendectomy and surgical drainage, Amoxicillin/Clavulanic acid (3 g/24 h for 15 days) | Full recovery |
| Ayhancı et al., 2021 [88] | 2021 | M/51 yr old | Turkey | None | Bacteriemia | Amikacin, Ciprofloxacin Gentamicin, Levofloxacin, Imipenem, Meropenem | N/A | Levofloxacin 500 mg/day w | Full recovery |
| Sammoni et al., 2022 [89] | 2022 | M/16 yr old | Syria | Burn victim | Sepsis | Colistin | N/A | Cefazolin and Ceftriaxone Followed by Colistin-amikacin for 14 days | Full recovery |
F—Female, M—Male, N/A—Not Available, SXT sulfamethoxazole-Trimethoprim. * Antibiotic susceptibility testing was carried out using a variety of methods, including disk diffusion testing, agar and broth dilution testing and E-testing methods.
Table 3.
Incidences of Comamonas kerstersii infection from 2013 to 2022. Main characteristics of the case reports.
| Author (Ref.) | Year | Sex/Age | Country | Co-Morbidity | Type of Infection | Susceptible to * | Resistance to * | Antibiotic Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Almuzara et al., 2013 [90] | 2013 | F/43 yr old | Argentina | Ovarian tumor with peritoneal metastases | Sigmoid perforation by foreign body (biliary stent), rectovaginal fistula, and colostomy | Amikacin, Ampicillin, Ampicillin-Sulbactam, Cephalothin, Cefoxitin, Cefotaxime, Ceftazidime, Cefepime, Colistin, Gentamicin, Imipenem, Meropenem, Piperacillin-Tazobactam, SXT | Ciprofloxacin | Ampicillin-Sulbactam, Piperacillin-Tazobactam, Ertapenem | Full recovery |
| Almuzara et al., 2013 [90] | 2013 | M/48 yr old | Argentina | None | Perforated appendix | Amikacin, Ampicillin, Ampicillin-Sulbactam, Cephalothin, Cefoxitin, Cefotaxime, Ceftazidime, Cefepime, Ciprofloxacin, Colistin, Gentamicin, Imipenem, Meropenem, Piperacillin-Tazobactam, SXT | N/A | Ampicillin-Sulbactam, Ciprofloxacin, Amoxicillin-Clavulanic acid | Full recovery |
| Almuzara et al., 2013 [90] | 2013 | F/10 yr old | Argentina | None | Perforated gangrenous appendix | Amikacin, Ampicillin, Ampicillin-Sulbactam, Cephalothin, Cefoxitin, Cefotaxime, Ceftazidime, Cefepime, Colistin, Gentamicin, Imipenem, Meropenem, Piperacillin-Tazobactam, SXT, Ciprofloxacin, Colistin, SXT | Ciprofloxacin | Ampicillin, Metronidazole, Gentamicin, and then Amoxicillin-Clavulanic acid | Full recovery |
| Almuzara et al., 2013 [90] | 2013 | F/21 yr old | Argentina | None | Perforated gangrenous appendix | Amikacin, Ampicillin, Ampicillin-Sulbactam, Cephalothin, Cefoxitin, Cefotaxime, Ceftazidime, Cefepime, Colistin, Gentamicin, Imipenem, Meropenem, Piperacillin-Tazobactam, SXT | Ciprofloxacin | Ampicillin, Metronidazole, Gentamicin | Full recovery |
| Biswas et al., 2014 [91] | 2014 | M/10 yr old | United Kingdom | None | Perforated appendix | Amikacin, Ceftazidime, Ciprofloxacin, Colistin, Gentamicin, Meropenem, Piperacillin-Tazobactam | N/A | Open appendectomy, Piperacillin-Tazobactam (5 days), Amoxicillin-Clavulanic acid, Ciprofloxacin | Full recovery |
| Biswas et al., 2014 [91] | 2014 | M/9 yr old | United Kingdom | None | Septic shock (due to perforated appendix) | Amoxicillin-clavulanic acid, Ceftazidime, Colistin, Gentamicin, Meropenem, Piperacillin-Tazobactam | Ciprofloxacin | Surgery, Amoxicillin-Clavulanic acid, Gentamicin, Metronidazole (intravenously, 3 days), Amoxicillin-Clavulanic acid (orally) | Full recovery |
| Opota et al., 2014 [92] | 2014 | M/65 yr old | Switzerland | Diabetes | Bacteremia with sign of diverticulosis | Ceftazidime, Ciprofloxacin, Meropenem, Imipenem, Minocycline, Levofloxacin, SXT | N/A | Imipenem-Cilastatin (10 days) | Full recovery |
| Almuzara et al., 2017 [93] | 2017 | F/54 yr old | Argentina | Obesity, hypertension, diabetes | Septic shock | SXT, Metronidazole | Piperacillin/Tazobactam, Vancomycin | SXT 15 mg/kg (intravenously every 12 h) and Metronidazole 500 mg (intravenously every 8 h), 30 days | Full recovery |
| Almuzara et al., 2017 [93] | 2017 | F/15 yr old | Argentina | None | Pelvic peritonitis due to genital tract infection | N/A | N/A | Ceftriaxone (intravenously 2 g/day, 6 days), Metronidazole (orally 500 mg/12 h, 8 days), Doxycycline (orally 100 mg/12 h, 8 days), Amoxicillin/Clavulanic acid (orally 500 mg/8 h, 14 days) | Full recovery |
| Almuzara et al., 2018 [94] | 2018 | F/5 yr old | Argentina | None | Urinary tract infection | Amikacin, Ampicillin, Ampicillin/Sulbactam, Cephalothin, Colistin, Cefotaxime, Ceftazidime, Cefepime, Ciprofloxacin, Gentamycin, Imipenem, Meropenem, Piperacillin-Tazobactam, SXT | Ceftriaxone | Piperacillin/Tazobactam (intravenously 200 mg/kg per day, every 8 h, 10-days), Amoxicillin/Clavulanic (orally 50 mg/kg per day, 14 days) | Full recovery |
| Zhou et al., 2018 [95] | 2018 | M/31 yr old | China | None | Acute peritonitis, perforated appendix (with abdominal abscess) | All except Ciprofloxacin Levofloxacin, SXT | Ciprofloxacin Levofloxacin, SXT | Exploratory laparotomy, appendectomy, tube drainage, Cefuroxime and metronidazole (14 days) | Full recovery |
| Liu et al., 2020 [96] | 2020 | M/62 yr old | China | None | Intra-abdominal infection due to perforated colon | Amikacin, Ceftazidime, Cefepime, Ciprofloxacin, Colistin Imipenem, Levofloxacin, Meropenem, Minocycline, Piperacillin-Tazobactam, SXT | Cephalothin, Cefotaxime, Ciprofloxacin, Gentamicin |
Surgery (left thoracotomy exploration, repair of oesophageal hiatal hernia, laparotomy exploration, partial colectomy, colostomy), Piperacillin-Tazobactam (Intravenously 4.5 g, every 8 h, 14 days) | Full recovery |
| Palacio et al., 2020 [97] | 2020 | M/16 yr old | Uruguay | None | Acute appendicitis | Amikacin, Ampicillin Sulbactam, Ceftazidime, Cefepime, Gentamicin, Piperacillin/Tazobactam, Meropenem, Imipenem, Cotrimoxazole | N/A | Laparoscopic surgery, Piperacillin/Tazobactam (intravenously, 4.5 g every 6 h, 10 days) | Full recovery |
| Farfán-Cano et al., 2020 [98] | 2020 | M/14 yr old | Ecuador | None | Perforated appendicitis | N/A | N/A | Piperacillin/Tazobactam (14 days) | Full recovery |
| Farfán-Cano et al., 2021 [99] | 2020 | F/27 yr old | Ecuador | Obesity and being on lactation period | Acute appendicitis | N/A | N/A | Ciprofloxacin and Metronidazole IV for 10 days | Full recovery |
| Farfán-Cano et al., 2021 [99] | 2020 | M/29 yr old | Ecuador | None | Acute appendicitis | N/A | N/A | Conventional Appendectomy, Ciprofloxacin, and Metronidazole | Full recovery |
| Farfán-Cano et al., 2021 [99] | 2020 | M/68 yr old | Ecuador | None | Acute appendicitis | N/A | N/A | Laparoscopic appendectomy | Full recovery |
| Farfán-Cano et al., 2021 [99] | 2020 | F/16 yr old | Ecuador | None | Acute appendicitis | N/A | N/A | Conventional appendectomy, Ampicillin/Sulbactam + Metronidazole | Full recovery |
| Farfán-Cano et al., 2021 [99] | 2020 | F/16 yr old | Ecuador | Psoriasis | Acute appendicitis | N/A | N/A | Conventional appendectomy, Ampicillin/ Sulbactam | Full recovery |
| Rong et al., 2022 [100] | 2022 | M/82 yr old | Canada | Type 2 diabetes | Bacteremia | Ceftazidime, Gentamicin, Imipenem, Meropenem, Piperacillin/tazobactam, Tobramycin | Ciprofloxacin | Ppiperacillin-tazobactam Followed by intravenous Ceftriaxone (1 g/day) | Full recovery |
| Bennani et al., 2022 [101] | 2002 | M/8 yr old | Morocco | None | Acute appendicitis | N/A | N/A | Intravenous Amoxicillin-clavulanic acid, Gentamicin, and Metronidazole Followed by oral Amoxicillin-Clavulanic acid. |
Full recovery |
F—Female, M—Male, N/A—Not Available, SXT sulfamethoxazole-trimethoprim. * Antibiotic susceptibility testing was carried out using a variety of methods, including disk diffusion testing, agar and broth dilution testing and E-testing methods.
Table 4.
Incidences of Comamonas spp. infection from 2000 to 2022. Main characteristics of the case reports.
| Author (Ref.) | Year | Sex/Age | Country | Co-Morbidity | Type of Infection | Susceptible to * | Resistance to * | Antibiotic Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Sonnenwirth, 1970 [102] | 1970 | F/71 yr old | USA | Rheumatic heart disease | Endocarditis | Chloramphenicol, Oxytetracycline Tetracycline |
Ampicillin, Cephalothin, Colistin, Penicillin, Streptomycin | Penicillin | Full recovery |
| Isotalo et al., 2000 [103] Comamonas spp. | 2000 | M/35 yr old | Canada | None | Tenosynovitis (From an animal bite) | N/A | N/A | Intravenous (IV) cefazolin at 1 g/8 h and gentamicin 80 mg/8 h for a total of 72 h | Full recovery |
| Kaeuffer et al., 2018 [104] Comamonas aquatica | 2017 | M/66 yr old | France | Diabetes, ischemic heart disease, removed sigmoid polyps | Bacteremia and septic shock | Amoxicillin-Clavulanic acid, Ceftazidime, Cefepime, Ciprofloxacin, Imipenem, Piperacillin-Tazobactam | N/A | Norepinephrine, Cefotaxime, Ciprofloxacin (10 days) | Full recovery |
| Guo et al., 2021 [105] Comamonas thiooxydans | 2021 | F/60 yr old | China | Kidney stones. | Urinary Tract Infection | Chloramphenicol, Imipenem, SXT | Amikacin, Aztreonam, Ceftazidime, Cefepime Ciprofloxacin, Gentamicin, Levofloxacin | Imipenem-cilastatin 1 g IV for 1 month to fight | Full recovery |
F—Female, M—Male, N/A—Not Available, SXT sulfamethoxazole-trimethoprim. * Antibiotic susceptibility testing was carried out using a variety of methods including disk diffusion testing, agar and broth dilution testing and E-testing methods.
Table 2, Table 3 and Table 4 illustrate 77 instances of infection caused by Comamonas spp. that were found in literature sources. It was found that only five Comamonas species (out of 24 species so far identified) have caused infections in humans. Most of these infections were caused by Comamonas testosteroni (50 instances—65.3%), other infections were due to Comamonas kerstersii (23 instances—29.8%), Comamonas aquatica (1 instance—1.3%), Comamonas thiooxydans (1 instance—1.3%), and Comamonas terrigena (1 instance—1.3%). In 47 instances (61%) out of 76, the patients had underlying conditions. Twenty different types of infection were caused by the different Comamonas species. These included pneumonia, polymicrobial bacteremia, bacteremia/septic shock, purulent meningitis, and sepsis.
Most patients had one underlying condition, seven had patients with two underlying conditions, and eight had patients with multiple underlying conditions (for example, obesity and diabetes). The most abundant of these underlying conditions were diabetes (in 8 patients—10.3%), various types of cancer (in 5 patients—6.5%) and alcoholism (in 4 patients—5.2%). Other major underlying conditions included obesity (in 3 patients—3.9%), hypertension (in 4 patients—10.9%), and renal failure (in 3 patients—3.9%). A full list of underlying conditions can be seen in Table 2, Table 3 and Table 4. A total of 70 patients (92.1%) were treated successfully and recovered fully, and 6 patients (7.8%) died. All patients who died due to Comamonas spp. infection suffered from one or more underlying conditions. These cases are discussed in more detail below. Surprisingly, to date, no pseudo-outbreaks have been found associated with Comamonas spp.
Most of the reported infections caused by Comamonas spp. appear to be community-acquired [22].
Death Associated with Comamonas spp. Infection
Six instances of death associated with Comamonas spp. infection have been reported. All six cases were linked to C. testosterone (Table 2). The first two instances were reported by Barbaro et al. [54]. In one of these instances, a mother who was an intravenous drug abuser gave birth to a premature baby, and this newborn baby died of sepsis caused by C. testosteroni infection 24 h after he was born. The second instance was very similar as it was also associated with sepsis due to C. testosteroni infection in a premature baby who was stillborn by an intravenous drug abuser mother. The third instance of death was reported in 2008 by Jin et al. [55]. In this case, a 54-year-old homeless man alcoholic was hit by a car, he received multiple fractures of the facial bones and was hospitalized. He was diagnosed with multiple cerebral and cerebellar infarcts, which resulted in changed mental status. He died 15 days after the injury. An autopsy revealed diffuse purulent meningitis due to C. testosteroni infection. In the fourth instance reported by Swain and Rout, a 50-year-old woman who suffered from diabetes and had a chronic renal disease was hospitalized for bacteremia and septic shock [56]. She was treated with piperacillin-tazobactam antibiotics until C. testosteroni was identified. The microorganism was found to be resistant to piperacillin–tazobactam, so treatment was then changed to cefoperazone–sulbactam. However, despite this, the woman died due to septic shock. The fifth instance of death associated with Comamonas spp. was reported in 2017 by Yasayancan and Koseoglu [57]. A 68-year-old man with lung cancer and adrenal metastasis was diagnosed with polymicrobial bacteremia due to C. testosteroni, Staphylococcus haemolyticus, and Acinetobacter baumannii infection. The patient died on the 16th day, despite suitable treatments against these pathogens. The last reported instance of death due to C. testosteroni infection was reported in 2018 by Cetin et al. A 10-year-old boy with serious underlying conditions (cerebral palsy, scoliosis, and long-term support with home mechanical ventilation) was diagnosed with pneumonia due to C testosteroni infection [58]. The patient was treated with appropriate antimicrobial therapy, and after 21 days of treatment infection was cured but due to the patient’s poor health conditions, he died on day 50 of hospitalization. No deaths have been associated with C. kerstersii or any other Comamonas spp (Table 3 and Table 4).
6. Treatment of Comamonas spp. Infections
Antibiotic treatment of Comamonas spp. infections can be difficult. Comamonas spp. can be resistance to various antibiotic families including β-lactams (penicillins, cephalosporins and the development of resistance to carbapenems). To date, no controlled trials of antimicrobial therapies for Comamonas spp. infections in humans have taken place; consequently, antibiotic treatment ought to be based upon results of in vitro susceptibility testing on isolates. A variety of different antibiotics have been employed to treat Comamonas spp. infections found in the literature and, in most cases, they are susceptible to aminoglycosides, fluoroquinolones, carbapenems, piperacillin-tazobactam, trimethoprim-sulfamethoxazole, and cephalosporins (Table 2, Table 3 and Table 4).
Resistance to β-lactams class antimicrobials can be due to the possession of several genes by Comamonas spp. C. testosteroni S44 possesses a three-gene operon that codes for a Class A β-lactamases (resistance to benzylpenicillin, ampicillin, cefalexin, cefazolin, cefuroxime, ceftriaxone, and cefepime). These genes are CzoA (Class A β-lactamase encoding gene)—inhibits β-lactams antibiotics, CzoR (LysR type transcriptional regulator)—positively affects the expression of CzoA, and the IscR gene—enhances the regulatory effect of CzoR when bounded to its promoter region [106]. Several resistance genes were found in C. kerstersii 8943, including tetA, strB, sul1, blaOXA-1, strA, sul2, catB3 and floR. The blaIMP–8 gene (giving resistance to β-lactam antibiotics) has been found in a Comamonas thiooxydans isolate, which caused a urinary tract infection. This isolate also had a novel class D beta-lactamase gene blaOXA and a aac(6′)-Ib-c gene (resistance to aminoglycoside antibiotics). A variety of efflux pumps were also identified in the genomes of this bacterial isolate. [105]. A study in 2022 found another Comamonas thiooxydans isolate with a plasmid-based blaIMP–1 gene [107]. In a study by Hem et al., 2022, 32 Comamonas. denitrificans and 5 C. testosteroni from wastewater, 1 C. denitrificans from a wetland, and 1 C. aquatica from a lake with public access were sequenced. All were found to be resistant to carbapenem antibiotics. However, only 13 C. denitrificans isolates were found to have an identifiable carbapenemase blaGES-5. No identifiable carbapenemase genes were found in the other isolates. Other C. denitrificans isolates carried extended-spectrum b-lactamase (ESBL) blaOXA genes. This was the first report of resistance to carbapenem antibiotics in both C. denitrificans and C. aquatica; however, carbapenem-resistance was previously reported in a C. testosteroni infection in Turkey in 2015 [77,108].
7. Conclusions
Comamonas spp. are not currently considered important pathogens and are thought of as being of low virulence and of being a lesser danger in comparison to other non-fermenting Gram-negative bacteria such as Pseudomonas aeruginosa. Nevertheless, in this review, fifty-five separate outbreaks of Comamonas spp. infections have been identified from the scientific literature not taking into account unreported/undocumented cases. It must be recommended that the scientific community acknowledge the ability of this organism to elude antimicrobials and thus the potential for antimicrobial resistance transference between organisms, particularly in an era of growing antimicrobial susceptibility concerns.
Author Contributions
Conceptualization, M.P.R. methodology, M.P.R. and L.S.; investigation, M.P.R. and L.S.; data curation, M.P.R. and L.S. writing—original draft preparation, M.P.R., L.S., R.G. and S.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ryan M.P., Adley C.C. Ralstonia spp.: Emerging global opportunistic pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:291–304. doi: 10.1007/s10096-013-1975-9. [DOI] [PubMed] [Google Scholar]
- 2.Ryan M.P., Pembroke J.T., Adley C.C. Ralstonia pickettii: A persistent Gram-negative nosocomial infectious organism. J. Hosp. Infect. 2006;62:278–284. doi: 10.1016/j.jhin.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Ryan M.P., Adley C.C. Sphingomonas paucimobilis: A persistent Gram-negative nosocomial infectious organism. J. Hosp. Infect. 2010;75:153–157. doi: 10.1016/j.jhin.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Coughlan A., Ryan M.P., Cummins N.M., Towler M.R. The response of Pseudomonas aeruginosa biofilm to the presence of a glass polyalkenoate cement formulated from a silver containing glass. J. Mater. Sci. 2011;46:285–287. doi: 10.1007/s10853-010-4945-y. [DOI] [Google Scholar]
- 5.Ryan M.P., Pembroke J.T. Brevundimonas spp: Emerging global opportunistic pathogens. Virulence. 2018;9:480–493. doi: 10.1080/21505594.2017.1419116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan M.P., Pembroke J.T. The Genus Ochrobactrum as major opportunistic pathogens. Microorganisms. 2020;8:1797. doi: 10.3390/microorganisms8111797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham J.E.M., Simon G.L. Comamonas testosteroni bacteremia: A case report and review of the literature. Infect. Dis. Clin. Pract. 2007;15:272–273. doi: 10.1097/IPC.0b013e31802ce475. [DOI] [Google Scholar]
- 8.Zhong F., Wu J., Dai Y., Yang L., Zhang Z., Cheng S., Zhang Q. Bacterial community analysis by PCR-DGGE and 454-pyrosequencing of horizontal subsurface flow constructed wetlands with front aeration. Appl. Microbiol. Biotechnol. 2015;99:1499–1512. doi: 10.1007/s00253-014-6063-2. [DOI] [PubMed] [Google Scholar]
- 9.Handschuh H., Ryan M.P., O’Dwyer J., Adley C.C. Assessment of the bacterial diversity of aircraft water: Identification of the frequent fliers. PLoS ONE. 2017;12:e0170567. doi: 10.1371/journal.pone.0170567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong J., Li D., Li H., He M., Miller S.J., Yu L., Rensing C., Wang G. Genome analysis and characterization of zinc efflux systems of a highly zinc-resistant bacterium, Comamonas testosteroni S44. Res. Microbiol. 2011;162:671–679. doi: 10.1016/j.resmic.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Andrade G., Esteban E., Velasco L., Lorite M.J., Bedmar E.J. Isolation andiIdentification of N2-fixing microorganisms from the Rhizosphere of Capparis spinosa (L.) Plant Soil. 1997;197:19–23. doi: 10.1023/A:1004211909641. [DOI] [Google Scholar]
- 12.Pavone S., Rinoldo R., Albini E., Fiorucci A., Caponi B., Fratto A., Manuali E., Papa P., Magistrali C.F. First report of urinary tract infection caused by Comamonas kerstersii in a goat. BMC Vet. Res. 2021;17:1–5. doi: 10.1186/s12917-021-02840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y.H., Huang Z., Liu S.J. Chemotaxis towards aromatic compounds: Insights from Comamonas testosteroni. Int. J. Mol. Sci. 2019;20:2701. doi: 10.3390/ijms20112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boon N., Goris J., De Vos P., Verstraete W., Top E.M. Bioaugmentation of activated sludge by an indigenous 3-Chloroaniline- Degrading Comamonas testosteroni Strain, I2gfp. Appl. Environ. Microbiol. 2000;66:2906–2913. doi: 10.1128/AEM.66.7.2906-2913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L., Jiang C.Y., Liu X.Y., Wu J.F., Han J.G., Liu S.J. Plant-microbe association for rhizoremediation of chloronitroaromatic pollutants with Comamonas sp. strain CNB-1. Environ. Microbiol. 2007;9:465–473. doi: 10.1111/j.1462-2920.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- 16.Oppermann U.C.T., Belai I., Maser E. Antibiotic resistance and enhanced insecticide catabolism as consequences ofsSteroid induction in the gram-negative bacterium Comamonas testosteroni. J. Steroid Biochem. Mol. Biol. 1996;58:217–223. doi: 10.1016/0960-0760(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 17.Wu J.F., Jiang C.Y., Wang B.J., Ma Y.F., Liu Z.P., Liu S.J. Novel partial reductive pathway for 4-Chloronitrobenzene and Nitrobenzene degradation in Comamonas sp. strain CNB-1. Appl. Environ. Microbiol. 2006;72:1759–1765. doi: 10.1128/AEM.72.3.1759-1765.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Z., Qi X., Zeng X.A., Lu Y., Zhou J., Cui K., Zhang L. A newly isolated bacterium Comamonas Sp. XL8 alleviates the toxicity of cadmium exposure in rice seedlings by accumulating cadmium. J. Hazard. Mater. 2021;403:123824. doi: 10.1016/j.jhazmat.2020.123824. [DOI] [PubMed] [Google Scholar]
- 19.Staniland S., Coppock M., Tuffin M., van Zyl L., Roychoudhury A.N., Cowan D. Cobalt uptake and resistance to trace metals in Comamonas testosteroni isolated from a heavy-metal contaminated site in the Zambian copperbelt. Geomicrobiol. J. 2010;27:656–668. doi: 10.1080/01490450903527994. [DOI] [Google Scholar]
- 20.Farooq S., Farooq R., Nahvi N. Comamonas testosteroni: Is it still a rare human pathogen. Case Rep. Gastroenterol. 2017;11:42–47. doi: 10.1159/000452197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H.J., Lee Y., Oh K., Choi S.-H., Sung H., Huh J.W. Septic shock due to unusual pathogens, Comamonas testosteroni and Acinetobacter guillouiae in an immune competent patient. Korean J. Crit. Care Med. 2015;30:180–183. doi: 10.4266/kjccm.2015.30.3.180. [DOI] [Google Scholar]
- 22.Cooper G.R., Staples E.D., Iczkowski K.A., Clancy C.J. Comamonas (Pseudomonas) testosteroni endocarditis. Cardiovasc. Pathol. 2005;14:145–149. doi: 10.1016/j.carpath.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Davis G.H., Park R.W. A taxonomic study of certain bacteria currently classified as Vibrio species. J. Gen. Microbiol. 1962;27:101–119. doi: 10.1099/00221287-27-1-101. [DOI] [PubMed] [Google Scholar]
- 24.De Vos P., Kersters K., Falsen E. Comamonas Davis and Park 1962 Gen. Nov., Nom. Rev. Emend., and Comamonas terrigena Hugh 1962 Sp. Nov., Nom. Rev. Int. J. Syst. Bacteriol. 1985;35:443–453. doi: 10.1099/00207713-35-4-443. [DOI] [Google Scholar]
- 25.Wen A., Fegan M., Hayward C., Chakraborty S., Sly L.I. Phylogenetic Relationships among Members of the Comamonadaceae, and Description of Delftia acidovorans (Den Dooren de Jong 1926 and Tamaoka et Al. 1987) Gen. Nov., Comb. Nov. Int. J. Syst. Bacteriol. 1999;49:567–576. doi: 10.1099/00207713-49-2-567. [DOI] [PubMed] [Google Scholar]
- 26.Tamura K., Stecher G., Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan M.P., Adley C.C., Pembroke J.T. The use of MEGA as an educational tool for examining the phylogeny of antibiotic resistance genes. In: Méndez-Vilas A., editor. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education. Volume 4. Formatex; Badajoz, Spain: 2013. pp. 736–743. [Google Scholar]
- 28.Wauters G., De Baere T., Willems A., Falsen E., Vaneechoutte M. Description of Comamonas aquatica Comb. Nov. and Comamonas kerstersii sp. nov. for two subgroups of Comamonas terrigena and emended description of Comamonas terrigena. Int. J. Syst. Evol. Microbiol. 2003;53:859–862. doi: 10.1099/ijs.0.02450-0. [DOI] [PubMed] [Google Scholar]
- 29.Kämpfer P., Busse H.J., Baars S., Wilharm G., Glaeser S.P. Comamonas aquatilis sp. Nov., isolated from a garden pond. Int. J. Syst. Evol. Microbiol. 2018;68:1210–1214. doi: 10.1099/ijsem.0.002652. [DOI] [PubMed] [Google Scholar]
- 30.Tago Y., Yokota A. Comamonas badia sp. nov., A floc-forming bacterium isolated from Activated Sludge. J. Gen. Appl. Microbiol. 2004;50:243–248. doi: 10.2323/jgam.50.243. [DOI] [PubMed] [Google Scholar]
- 31.Young C.C., Chou J.H., Arun A.B., Yen W.S., Sheu S.Y., Shen F.T., Lai W.A., Rekha P.D., Chen W.M. Comamonas composti sp. nov., isolated from food waste compost. Int. J. Syst. Evol. Microbiol. 2008;58:251–256. doi: 10.1099/ijs.0.65277-0. [DOI] [PubMed] [Google Scholar]
- 32.Gumaelius L., Magnusson G., Pettersson B., Dalhammar G. Comamonas denitrificans sp. nov., an efficient denitrifying bacterium isolated from Activated Sludge. Int. J. Syst. Evol. Microbiol. 2001;51:999–1006. doi: 10.1099/00207713-51-3-999. [DOI] [PubMed] [Google Scholar]
- 33.Park E.H., Kim Y.S., Cha C.J. Comamonas fluminis sp. nov., isolated from the Han River Republic of Korea. Int. J. Syst. Evol. Microbiol. 2022;72:3. doi: 10.1099/ijsem.0.005287. [DOI] [PubMed] [Google Scholar]
- 34.Kim K.H., Ten L.N., Liu Q.M., Im W.T., Lee S.T. Comamonas granuli sp. nov., isolated from granules used in a Wastewater Treatment Plant. J. Microbiol. 2008;46:390–395. doi: 10.1007/s12275-008-0019-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., Wang Y., Zhou S., Wu C., He J., Li F. Comamonas guangdongensis sp. nov., isolated from subterranean forest sediment, and emended description of the Genus Comamonas. Int. J. Syst. Evol. Microbiol. 2013;63:809–814. doi: 10.1099/ijs.0.040188-0. [DOI] [PubMed] [Google Scholar]
- 36.Hatayama K. Comamonas humi sp. nov., Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2014;64:3976–3982. doi: 10.1099/ijs.0.067439-0. [DOI] [PubMed] [Google Scholar]
- 37.Sun L.N., Zhang J., Chen Q., He J., Li Q.F., Li S.P. Comamonas jiangduensis Sp. Nov., a biosurfactant producing bacterium isolated from agricultural soil. Int. J. Syst. Evol. Microbiol. 2013;63:2168–2173. doi: 10.1099/ijs.0.045716-0. [DOI] [PubMed] [Google Scholar]
- 38.Chang Y.H., Han J.-I., Chun J., Lee K.C., Rhee M.S., Kim Y.B., Bae K.S. Comamonas koreensis sp. nov., a non-motile species from wetland in Woopo, Korea. Int. J. Syst. Evol. Microbiol. 2002;52:377–381. doi: 10.1099/00207713-52-2-377. [DOI] [PubMed] [Google Scholar]
- 39.Etchebehere C., Errazquin M.I., Dabert P., Moletta R., Muxí L. Comamonas nitrativorans sp. nov., a novel denitrifier isolated from a denitrifying reactor treating landfill leachate. Int. J. Syst. Evol. Microbiol. 2001;51:977–983. doi: 10.1099/00207713-51-3-977. [DOI] [PubMed] [Google Scholar]
- 40.Chou J.H., Sheu S.Y., Lin K.Y., Chen W.M., Arun A.B., Young C.C. Comamonas odontotermitis sp. nov., isolated from the gut of the termite Odontotermes formosanus. Int. J. Syst. Evol. Microbiol. 2007;57:887–891. doi: 10.1099/ijs.0.64551-0. [DOI] [PubMed] [Google Scholar]
- 41.Xie F., Ma H., Quan S., Liu D., Chen G. Comamonas phosphati Nov., sp. nov., isolated from a phosphate mine. Int. J. Syst. Evol. Microbiol. 2016;66:456–461. doi: 10.1099/ijsem.0.000742. [DOI] [PubMed] [Google Scholar]
- 42.Kang W., Kim P.S., Hyun D.W., Lee J.Y., Kim H.S., Oh S.J., Shin N.R., Bae J.W. Comamonas piscis sp. nov., isolated from the intestine of a Korean rockfish, Sebastes schlegelii. Int. J. Syst. Evol. Microbiol. 2016;66:780–785. doi: 10.1099/ijsem.0.000790. [DOI] [PubMed] [Google Scholar]
- 43.Subhash Y., Bang J.J., You T.H., Lee S.S. Description of Comamonas sediminis sp. nov., isolated from lagoon sediments. Int. J. Syst. Evol. Microbiol. 2016;66:2735–2739. doi: 10.1099/ijsem.0.001115. [DOI] [PubMed] [Google Scholar]
- 44.Zhu D., Xie C., Huang Y., Sun J., Zhang W. Description of Comamonas serinivorans sp. nov., isolated from wheat straw compost. Int. J. Syst. Evol. Microbiol. 2014;64:4141–4146. doi: 10.1099/ijs.0.066688-0. [DOI] [PubMed] [Google Scholar]
- 45.Park K.H., Yu Z., Dong K., Lee S.S. Comamonas suwonensis sp. nov., isolated from stream water in the Republic of Korea. Int. J. Syst. Evol. Microbiol. 2021;71:4. doi: 10.1099/ijsem.0.004681. [DOI] [PubMed] [Google Scholar]
- 46.Chitpirom K., Tanasupawat S., Akaracharanya A., Leepepatpiboon N., Prange A., Kim K.W., Lee K.C., Lee J.S. Comamonas terrae sp. nov., an arsenite-oxidizing bacterium isolated from agricultural soil in Thailand. J. Gen. Appl. Microbiol. 2012;58:245–251. doi: 10.2323/jgam.58.245. [DOI] [PubMed] [Google Scholar]
- 47.Tamaoka J., Ha D.M., Komagata K. Reclassification of Pseudomonas acidovorans Den Dooren de Jong 1926 and Pseudomonas testosteroni Marcus and Talalay 1956 as Comamonas acidovorans comb. nov. and Comamonas testosteroni comb. nov., with an Emended Description of the Genus Comamonas. Int. J. Syst. Bacteriol. 1987;37:52–59. doi: 10.1099/00207713-37-1-52. [DOI] [Google Scholar]
- 48.Narayan K.D., Pandey S.K., Das S.K. Characterization of Comamonas thiooxidans sp. nov., and comparison of thiosulfate oxidation with Comamonas testosteroni and Comamonas composti. Curr. Microbiol. 2010;61:248–253. doi: 10.1007/s00284-010-9602-9. [DOI] [PubMed] [Google Scholar]
- 49.Yu X.Y., Li Y.F., Zheng J.W., Li Y., Li L., He J., Li S.P. Comamonas zonglianii sp. nov., isolated from phenol contaminated soil. Int. J. Syst. Evol. Microbiol. 2011;61:255–258. doi: 10.1099/ijs.0.019612-0. [DOI] [PubMed] [Google Scholar]
- 50.Willems A., Gillis M. Bergey’s Manual of Systematics of Archaea and Bacteria. Major Reference Works; Wiley; Hoboken, NJ, USA: 2015. Comamonas; pp. 1–17. [Google Scholar]
- 51.Willems A., De Vos P. Comamonas. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E., editors. The Prokaryotes. Springer; New York, NY, USA: 2006. pp. 723–736. [Google Scholar]
- 52.Public Health England UK Standards for Microbiology Investigations Identification of Pseudomonas species and other Non-Glucose Fermenters. Issued by the Standards Unit, Public Health England. [(accessed on 31 May 2022)];2015 Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/422699/ID_17i3.pdf.
- 53.Wu Y., Zaiden N., Cao B. The Core- and Pan-Genomic Analyses of the Genus Comamonas: From environmental adaptation to potential virulence. Front. Microbiol. 2018;9:3096. doi: 10.3389/fmicb.2018.03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbaro D.J., Mackowiak P.A., Barth S.S., Southern P.M. Pseudomonas testosteroni Infections: Eighteen Recent Cases and a Review of the Literature. Rev. Infect. Dis. 1987;9:124–129. doi: 10.1093/clinids/9.1.124. [DOI] [PubMed] [Google Scholar]
- 55.Jin L., Perper J.A., Cina S.J. Comamonas testosteroni meningitis in a homeless man. J. Forensic Sci. 2008;53:1198–1199. doi: 10.1111/j.1556-4029.2008.00810.x. [DOI] [PubMed] [Google Scholar]
- 56.Swain B., Rout S. Comamonas testosteroni bacteraemia in a Tertiary Care Hospital. Indian J. Med. Microbiol. 2015;33:602–603. doi: 10.4103/0255-0857.167325. [DOI] [PubMed] [Google Scholar]
- 57.Yasayancan N., Koseoglu H.I. The 20th Comamonas testosteroni bacteremia case in the Literature from Turkey: Mortal and polymicrobial a case report and literature review. Eurasian J. Med. Oncol. 2017;1:168–171. doi: 10.14744/ejmo.2017.16878. [DOI] [Google Scholar]
- 58.Çetin Ş., Baslarli S., Celik B., Celik I. Pneumonia case by caused Comamonas testosteroni in pediatric intensive care unit. Eurasian J. Med. Oncol. 2018;2:251–253. doi: 10.14744/ejmo.2018.73745. [DOI] [Google Scholar]
- 59.Atkinson B.E., Smith D.L., Lockwood W.R. Pseudomonas testosteroni septicemia. Ann. Intern. Med. 1975;83:369–370. doi: 10.7326/0003-4819-83-3-369. [DOI] [PubMed] [Google Scholar]
- 60.Smith E.G. Pseudomonas testosteroni pyarthrosis and septicemia. Clin. Microbiol. Newsl. 1979;1:4. doi: 10.1016/S0196-4399(79)80108-7. [DOI] [Google Scholar]
- 61.Franzetti F., Cernuschi M., Esposito R., Moroni M. Pseudomonas Infections in Patients with AIDS and AIDS-related Complex. J. Intern. Med. 1992;231:437–443. doi: 10.1111/j.1365-2796.1992.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 62.Le Moal G., Paccalin M., Breux J.P., Roblot F., Roblot P., Becq-Giraudon B. Central venous catheter-related infection due to Comamonas testosteroni in a woman with breast cancer. Scand. J. Infect. Dis. 2001;33:627–628. doi: 10.1080/00365540110026827. [DOI] [PubMed] [Google Scholar]
- 63.Arda B., Aydemir S., Yamazhan T., Hassan A., Tünger A., Serter D. Comamonas testosteroni meningitis in a patient with recurrent cholesteatoma. APMIS. 2003;111:474–476. doi: 10.1034/j.1600-0463.2003.1110404.x. [DOI] [PubMed] [Google Scholar]
- 64.Smith M.D., Gradon J.D. Bacteremia due to Comamonas species possibly associated with exposure to tropical fish. South. Med. J. 2003;96:815–817. doi: 10.1097/01.SMJ.0000051869.86765.D9. [DOI] [PubMed] [Google Scholar]
- 65.Gul M., Ciragil P., Bulbuloglu E., Aral M., Alkis S., Ezberci F. Comamonas testosteroni bacteremia in a patient with perforated acute appendicitis. Acta Microbiol. Immunol. Hung. 2007;54:317–321. doi: 10.1556/amicr.54.2007.3.6. [DOI] [PubMed] [Google Scholar]
- 66.Carolo G., Ganau M., De Micheli E., Gerosa M., Solbiati M. P1466 Comamonas testosteroni Spondylodiscitis. Int. J. Antimicrob. Agents. 2007;29:S410. doi: 10.1016/S0924-8579(07)71305-5. [DOI] [Google Scholar]
- 67.Reddy A.K., Murthy S.I., Jalali S., Gopinathan U. Post-Operative endophthalmitis due to an unusual pathogen, Comamonas testosteroni. J. Med. Microbiol. 2009;58:374–375. doi: 10.1099/jmm.0.006072-0. [DOI] [PubMed] [Google Scholar]
- 68.Katircioǧlu K., Özkalkanli M.Y., Yurtsever S.G., Savaci S. Yoǧun Bakim Hastasinda Comamonas testosteroni Enfeksiyonu. Turk Anesteziyoloji Reanimasyon Dern. Derg. 2010;38:129–132. [Google Scholar]
- 69.Nseir W., Khateeb J., Awawdeh M., Ghali M. Catheter-related bacteremia caused by Comamonas testosteroni in a hemodialysis patient. Hemodial. Int. 2011;15:293–296. doi: 10.1111/j.1542-4758.2010.00524.x. [DOI] [PubMed] [Google Scholar]
- 70.Ozden S., Kocturk S.A., Guler S., Kilinc D. Comamonas testoster Noni Infection in Intensive Care Patient. 1st National Congress of Clinical Microbiology; Antalya, Turkey: 2011. p. 107. [Google Scholar]
- 71.Tsui T.L., Tsao S.M., Liu K., Chen T.Y., Wang Y.L., Teng Y.H., Lee Y.T. Comamonas testosteroni infection in Taiwan: Reported Two cases and literature review. J. Microbiol. Immunol. Infect. 2011;44:67–71. doi: 10.1016/j.jmii.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 72.Farshad S., Norouzi F., Aminshahidi M., Heidari B., Alborzi A. Two Cases of Bacteremia Due to an Unusual Pathogen, Comamonas testosteroni in Iran and a Review Literature. J. Infect. Dev. Ctries. 2012;6:521–525. doi: 10.3855/jidc.2215. [DOI] [PubMed] [Google Scholar]
- 73.Al Ramahi J.W., Rumoh S.A., Khali B.W. Comamonas testosteroni Blood Stream Infection in a patient with end-stage renal failure on hemodialysis. Int. Arab. J. Antimicrob. Agents. 2013;3:4. [Google Scholar]
- 74.Bayhan G.I., Tanir G., Karaman I., Özkan Ş. Comamonas testosteroni: An unusual bacteria associated with acute appendicitis. Balkan Med. J. 2013;30:447–448. doi: 10.5152/balkanmedj.2013.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altun E., Kaya B., Taktakoǧlu O., Karaer R., Paydas S., Balal M., Seyrek N. Comamonas testosteroni peritonitis secondary to dislocated intrauterine device and laparoscopic intervention in a continuous ambulatory peritoneal dialysis patient. Perit. Dial. Int. 2013;33:576–578. doi: 10.3747/pdi.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orsini J., Tam E., Hauser N., Rajayer S. Polymicrobial bacteremia involving Comamonas testosteroni. Case Rep. Med. 2014;2014:578127. doi: 10.1155/2014/578127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duran A., Okur F., Sahin V., Uyar I., Abacilar A., Akpinar M., Alayunt E., Ates M. Comamonas testosteroni endocarditis in Turkey: A case report and review of the literature. Int. Med. J. Sifa Univ. 2015;2:44. doi: 10.4103/2148-7731.152117. [DOI] [Google Scholar]
- 78.Khalki H., Deham H., Taghouti A., Yahyaoui G., Mahmoud M. Appendicite à Comamonas testosteroni. Med. Mal. Infect. 2016;46:168–170. doi: 10.1016/j.medmal.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 79.Pekintürk N., Akgüneş A. Nadir Bir Patojen Comamonas testosteronı: Olgu Sunumu Ve Literatürün Gözden Geçirilmesi. Kocaeli Üniversitesi Sağlık Bilim. Derg. 2016;2:7–10. doi: 10.30934/kusbed.358629. [DOI] [Google Scholar]
- 80.Parolin M., Baraldi M., Valentini E., Murer L., Vidal E. Comamonas testosteroni-associated peritonitis in a pediatric peritoneal dialysis patient. World J. Nephrol. 2016;5:220. doi: 10.5527/wjn.v5.i2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hung Y.M., Chang Y.T., Kao C.H. Polymicrobial Bacteremia Involving Comamonas testosteroni in a Patient on Dialysis with Acute Appendicitis. Ther. Apher. Dial. 2017;21:637–638. doi: 10.1111/1744-9987.12583. [DOI] [PubMed] [Google Scholar]
- 82.Ruziaki W., Hashami H. Unusual pathogen Comamonas testosteroni sepsis following gastroenteritis in a 12 months old child: Case report and literature review. Am. J. Med. Case Reports. 2017;5:148–150. doi: 10.12691/ajmcr-5-6-4. [DOI] [Google Scholar]
- 83.Tartar A.S., Tartar T. A rare pathogen in acute appendicitis: Two cases with Comamonas testosteroni infection and literature review. J. Pediatr. Infect. Dis. 2020;15:110–112. doi: 10.1055/s-0038-1641604. [DOI] [Google Scholar]
- 84.Lovell A.R.O., Forde C.A. Comamonas testosteroni bacteremia in a young male with mancreatitis: A case peport. J. Clin. Case Rep. 2019;9:10001260. [Google Scholar]
- 85.Tiwari S., Nanda M. Bacteremia caused by Comamonas testosteroni an unusual pathogen. J. Lab. Physicians. 2019;11:87–90. doi: 10.4103/JLP.JLP_116_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buyukberber S.G., Mumcuoglu I., Ozbay B.O., Aypak A., Dinc B. A Rare Pathogen Comamonas Testosteroni: A Case Report and Review of the Literature, 14 July 2021, Preprint (Version 1) [(accessed on 31 May 2022)]. Available online: [DOI]
- 87.Miloudi M., El Kamouni Y., Oulhadj H., Arsalane L., Zouhair S. Comamonas testosteroni appendicitis: About a case and review of the Llterature. Infect. Dis. Now. 2021;51:395–397. doi: 10.1016/j.medmal.2020.09.023. [DOI] [PubMed] [Google Scholar]
- 88.Ayhanci T., Demiray T., Özmen E., İnce B., Sadeq M., Aydin A., Yaylaci S. A rare case of bacteriemia due to Comamonas testosteroni. J. Biotechnol. Strateg. Heal. Res. 2021;5:85–89. doi: 10.34084/bshr.898874. [DOI] [Google Scholar]
- 89.Sammoni A., Abdalah A., Al-Aissami M. Comamonas testosteroni bacteremia: A rare unusual pathogen detected in a burned patient: Case report and literature review. Ann. Med. Surg. 2022;75:103371. doi: 10.1016/j.amsu.2022.103371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Almuzara M.N., Cittadini R., Ocampo C.V., Bakai R., Traglia G., Ramirez M.S., Del Castillo M., Vay C.A. Intra-abdominal infections due to Comamonas kerstersii. J. Clin. Microbiol. 2013;51:1998–2000. doi: 10.1128/JCM.00659-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biswas J.S., Fitchett J., O’Hara G. Comamonas kerstersii and the Perforated Appendix. J. Clin. Microbiol. 2014;52:3134. doi: 10.1128/JCM.00909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Opota O., Ney B., Zanetti G., Jaton K., Greub G., Prod’hom G. Bacteremia caused by Comamonas kerstersii in a Patient with diverticulosis. J. Clin. Microbiol. 2014;52:1009–1012. doi: 10.1128/JCM.02942-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Almuzara M., Barberis C., Veiga F., Bakai R., Cittadini R., Vera Ocampo C., Alonso Serena M., Cohen E., Ramirez M.S., Famiglietti A., et al. Unusual presentations of Comamonas kerstersii infection. New Microbes New Infect. 2017;19:91–95. doi: 10.1016/j.nmni.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Almuzara M., Cittadini R., Estraviz M.L., Ellis A., Vay C. First Report of Comamonas kerstersii causing urinary tract infection. New Microbes New Infect. 2018;24:4–7. doi: 10.1016/j.nmni.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y.H., Ma H.X., Dong Z.Y., Shen M.H. Comamonas kerstersii bacteremia in a patient with acute perforated appendicitis. Medicine. 2018;97:e9296. doi: 10.1097/MD.0000000000009296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X.J., Qiao X.W., Huang T.M., Li L., Jiang S.P. Comamonas kerstersii Bacteremia. Med. Mal. Infect. 2020;50:288–290. doi: 10.1016/j.medmal.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Palacio R., Cabezas L., Cornejo C., Seija V. Comamonas kerstersii bacteremia in a young man with acute appendicitis. Rev. Chil. Infectol. 2020;37:182–185. doi: 10.4067/s0716-10182020000200182. [DOI] [PubMed] [Google Scholar]
- 98.Farfán-Cano G., Parra-Vera H., Ávila-Choez A., Silva-Rojas G., Farfán-Cano S. First identification in Ecuador of Comamonas kerstersii as an infectious agent. Rev. Chil. Infectol. 2020;37:179–181. doi: 10.4067/s0716-10182020000200179. [DOI] [PubMed] [Google Scholar]
- 99.Farfán-Cano G.G., Sarmiento-Bobadilla J.A., León E.A.J., Crespo-Díaz C.M., Silva-Rojas G.A., Parra-Vera H.J., Solórzano-Bravo M.T., Chantong-Villacres L.A., Silva-Rojas K.J. Comamonas kerstersii Strains on in patients with acute Appendicitis: Review of literature and case report. Interam. J. Med. Heal. 2021;4:e202101016. doi: 10.31005/iajmh.v4i.165. [DOI] [Google Scholar]
- 100.Rong K., Delport J., AlMutawa F. Comamonas kerstersii bacteremia of unknown origin. Case Rep. Infect. Dis. 2022;2022:1–3. doi: 10.1155/2022/1129832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bennani H., El Ouarradi A., Hanchi A.L., Soraa N. A young child with acute perforated appendicitis due to Comamonas kerstersii: A rare case report. Pan Afr. Med. J. 2022;41:186. doi: 10.11604/pamj.2022.41.186.29615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sonnenwirth A.C. Bacteremia with and without meningitis due to Yersinia enterocolitica, Edwardsiella tarda, Comamonas terrigena, and Pseudomonas maltophilia. Ann. N. Y. Acad. Sci. 1970;174:488–502. doi: 10.1111/j.1749-6632.1970.tb45575.x. [DOI] [PubMed] [Google Scholar]
- 103.Isotalo P.A., Edgar D., Toye B. Polymicrobial tenosynovitis with Pasteurella multocida and other gram-negative Bacilli after a Siberian Tiger bite. J. Clin. Pathol. 2000;53:871–872. doi: 10.1136/jcp.53.11.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaeuffer C., Schramm F., Meyer A., Hansmann Y., Guffroy A., Argemi X. First case of Comamonas aquatica bacteremia complicated by septic shock. Médecine Mal. Infect. 2018;48:540–542. doi: 10.1016/j.medmal.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Guo X., Wang Q., Xu H., He X., Guo L., Liu S., Wen P., Gou J. Emergence of IMP-8-Producing Comamonas thiooxydans causing Urinary Tract Infection in China. Front. Microbiol. 2021;12:585716. doi: 10.3389/fmicb.2021.585716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhuang W., Liu H., Li J., Chen L., Wang G. Regulation of Class A β-Lactamase czoA by czoR and iscR in Comamonas testosteroni S44. Front. Microbiol. 2017;8:2573. doi: 10.3389/fmicb.2017.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzuki Y., Nakano R., Nakano A., Tasaki H., Asada T., Horiuchi S., Saito K., Watanabe M., Nomura Y., Kitagawa D., et al. Comamonas thiooxydans expressing a plasmid encoded IMP-1 Carbapenemase isolated from continuous ambulatory peritoneal dialysis of an inpatient in Japan. Front. Microbiol. 2022;13:808993. doi: 10.3389/fmicb.2022.808993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hem S., Wyrsch E.R., Drigo B., Baker D.J., Charles I.G., Donner E., Jarocki V.M., Djordjevic S.P. Genomic Analysis of Carbapenem-Resistant Comamonas in Water Matrices: Implications for Public Health and Wastewater Treatments. Appl. Environ. Microbiol. 2022;88:e00646-22. doi: 10.1128/aem.00646-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.