Abstract
This review discusses the formation of hypochlorous acid HOCl and the role of reactive chlorinated species (RCS), which are catalysed by the enzyme myeloperoxidase MPO, mainly located in leukocytes and which in turn contribute to cellular oxidative stress. The reactions of RCS with various organic molecules such as amines, amino acids, proteins, lipids, carbohydrates, nucleic acids, and DNA are described, and an attempt is made to explain the chemical mechanisms of the formation of the various chlorinated derivatives and the data available so far on the effects of MPO, RCS and halogenative stress. Their presence in numerous pathologies such as atherosclerosis, arthritis, neurological and renal diseases, diabetes, and obesity is reviewed and were found to be a feature of debilitating diseases.
Keywords: hypochlorous acid HOCl, halogenative stress, myeloperoxidase MPO, pathologies
1. Introduction
Bacteria are constantly confronted with environmental stressors, such as pH and temperature extremes, osmotic pressure, antimicrobial agents, and oxidants, which can induce irreversible damage. Among the most widespread reactive agents are reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide and hydroxyl radical, as well as reactive chlorine species (RCS), such as hypochlorous acid [1]. Neutrophils destroy pathogenic invaders by ingesting them through phagocytosis and by releasing antimicrobial proteins, oxidants, and digestive enzymes. In addition, stimulated neutrophils also participate in the respiratory burst, which is accompanied by an increase in oxygen consumption and the consequent production of ROS by the enzyme NADPH-oxidase. This enzyme complex assembles at the plasma membrane and transfers electrons from NADPH to molecular oxygen, forming the superoxide anion •O2−. Then, the enzyme superoxide dismutase (SOD) converts •O2− into hydrogen peroxide H2O2, a precursor of several toxic species [2] and, on the other hand, according to Winterbourn et al., 2006, MPO converts •O2− into hydrogen peroxide H2O2 [2]. In the cell, there are different defence mechanisms to prevent damage induced by these species, including antioxidants (such as ascorbic acid and β-carotene), and enzymes (catalase, superoxide dismutase, and glutathione peroxidase). However, under conditions of oxidative stress, due to the overproduction of oxidative species, or when antioxidant levels are severely depleted, these species become detrimental, causing the oxidation of biomolecules, and may ultimately lead to cell death [3].
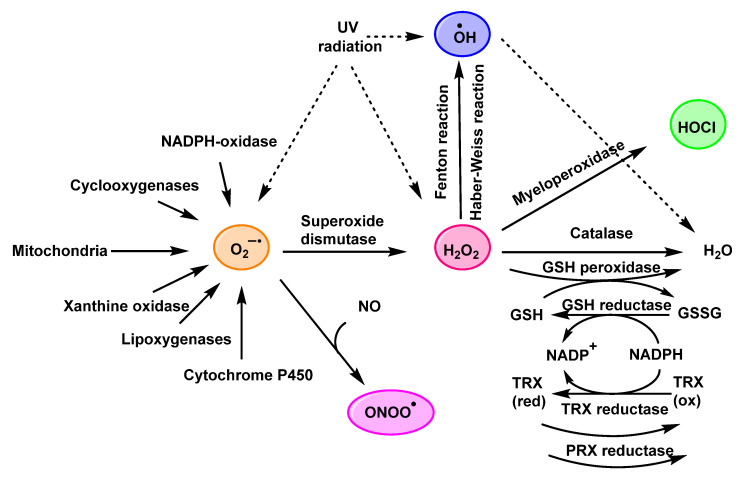
In the 1970s and 1980s, the term “oxidative stress” was used to describe this process. Later, the German biochemist Helmut Sies defined it as an imbalance between oxidants and antioxidants in favour of oxidants [4]. The formation of the different reactive species is shown in Figure 1.
Figure 1.
Chemical and enzymatic reactions generating ROS and main antioxidant systems.
2. Enzyme Myeloperoxidase (MPO) and Its Catalytic Cycle
Myeloperoxidase (MPO), also called hydrogen peroxide oxidoreductase with a particular (EC 1.11.1.7), is an enzyme found in the primary granules of granulocytic cells (neutrophils, eosinophils, and, to a lesser extent, monocytes). Lymphocytes lack MPO enzyme activity. However, the most common sources are neutrophils, where the enzyme is located at the lysosomal level in the azurophil granules [5].
MPO is the major component of white blood cells in humans [6]. These leukocytes recognise microorganisms through various receptors that act by stimulating the migration of the cells to the site of infection, promoting the phagocytosis of the microorganisms and stimulating the production of biocidal substances that destroy the microorganisms. A second microbicidal mechanism used by activated leukocytes occurs during the respiratory burst, which involves reducing molecular oxygen to reactive oxygen intermediates (ROS), such as superoxide radicals •O2−, using the reduced form of NADPH [7]. The superoxide undergoes a disproportionate reaction to give oxygen (O2) and hydrogen peroxide (H2O2), and the latter is used by the MPO enzyme to convert the normally very unreactive halide ions into hypohalous acids that are relatively strong oxidising agents and toxic to bacteria [8]. When intense leukocyte activation occurs, ROS, nitric oxide, and lysosomal enzymes are released, which can injure normal host tissues [9].
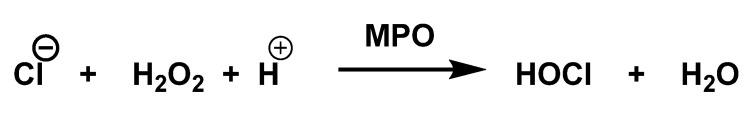
The main reaction catalysed by MPO, under physiological conditions, is the oxidation of the Cl− anion by H2O2 to give hypochlorous acid HOCl, a very reactive oxidising agent, which can also act as a chlorinating agent [8,10,11,12,13], Figure 2.
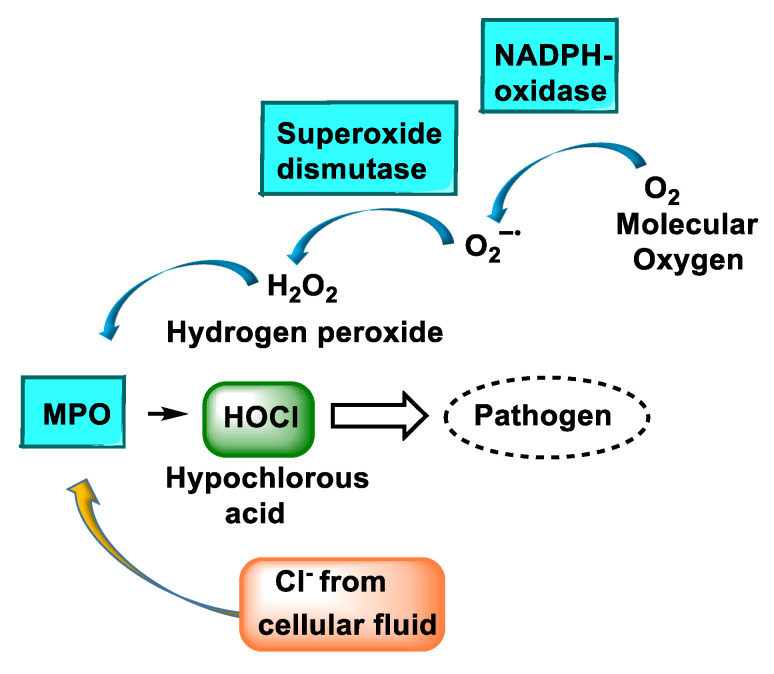
Figure 2.
Schematic representation of HOCl production during the respiratory burst. The cell uses molecular oxygen (O2) to produce H2O2, using the enzyme NADPH oxidase. MPO deposited on the azurophilic granules catalyses the reaction of H2O2 and the chloride anion Cl− to form hypochlorous acid HOCl.
Myeloperoxidase plays a key role in the production of oxidants by neutrophils. This heme-enzyme uses hydrogen peroxide and chloride to catalyse the production of hypochlorous acid, the main strong oxidant generated by neutrophils in appreciable quantities. In addition to chlorination, myeloperoxidase exhibits other activities. It readily oxidises thiocyanate to hypothiocyanite, converts a variety of organic substrates to reactive free radicals, and hydroxylates aromatic compounds. Depending on the concentration of its competing substrates and local environmental conditions, myeloperoxidase may substantially affect the production of oxidants by neutrophils. MPO is the only peroxidase that catalyses the conversion of H2O2 and chloride to produce hypochlorous acid (HOCl) [14]. This is a potent oxidising agent that contributes to the defence mechanism against infectious agents; however, it may be capable of acting on the body’s own cells in the case of prolonged and extended activation of this enzyme. In these cases, the high oxidative power of HOCl is involved in the mismatch of the redox state and can consequently lead to systemic oxidative stress. The MPO system is also involved in leukocyte functions such as the chemical modifications of plasma and tissue components, the elimination of abnormal host cells and inflammatory processes [15].
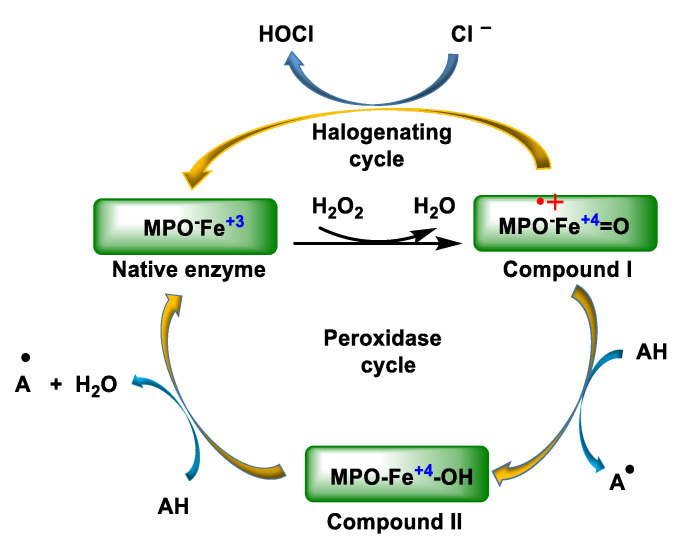
The native enzyme MPO is a protein with a central iron in the heme and MPO-Fe3+ can be at various oxidation states, as shown in Figure 3. MPO-Fe+3 reacts rapidly and irreversibly with hydrogen peroxide from phagocytic cells activated by contact with foreign particles, forming an enzyme–substrate complex with a strong oxidative capacity. Compound I can be reduced to compound II by an excess of H2O2 or by the presence of a reducing agent. This compound II, although quite stable, can be reduced to ferric MPO in the presence of a reducing agent or the superoxide radical or it can also be oxidised to compound III under high concentrations of H2O2. On the other hand, native MPO reacts with superoxide to give compound III.
Figure 3.
Normal catalytic cycling of myeloperoxidase. The native state of the enzyme, ferric MPO, reacts with hydrogen peroxide to form the redox intermediate Compound I. Compound I oxidises chloride to regenerate ferric MPO via the halogenation cycle.
The MPO enzyme uses H2O2 to oxidise chloride, but it also oxidises bromide Br−, iodide I−, and thiocyanate SCN−, taking them to their respective hypohalogenated acids. At neutral pH values, physiological plasma ion concentrations are ~100 mM Cl−, 20–100 µM Br−, <1 µM I−, 20–100 µM SCN− [16], and MPO primarily generates HOCl and HOSCN (which can be a significant product due to its rapid oxidation by Compound I), and significant levels of this anion are found in smokers and in vegetarians and vegans who consume diets rich in plants and fruits [17]. HOI formation is usually insignificant, although it is generated rapidly, due to the low plasma levels of I−, varying significantly with diet (lower levels are detected in vegans than vegetarians). HOBr is usually formed at low concentrations, it is pH dependent, and can also be improved by means of food supplements [18].
The relative specificity constants for chloride, bromide and thiocyanate are 1:60:730, respectively, with thiocyanate being the most favoured substrate for MPO. In the presence of 100 mM Cl−, MPO catalyses the hypothiocyanite production at thiocyanate concentrations of 25 µM. At 100 µM thiocyanate, roughly 50% of peroxide is transformed into hypothiocyanite, and the quantity of hypohalogenic acid production was equal to the sum of the individual rates obtained when each of these negative ions was present alone. The percentage of MPO-induced H2O2 loss in the presence of 100 mM Cl− doubled when 100 µM thiocyanate was added and was maximal at 1 mM thiocyanate. This indicates that at plasma concentrations of thiocyanate and chloride, myeloperoxidase is far from saturated. Thus, thiocyanate is an important physiological substrate of MPO [19].
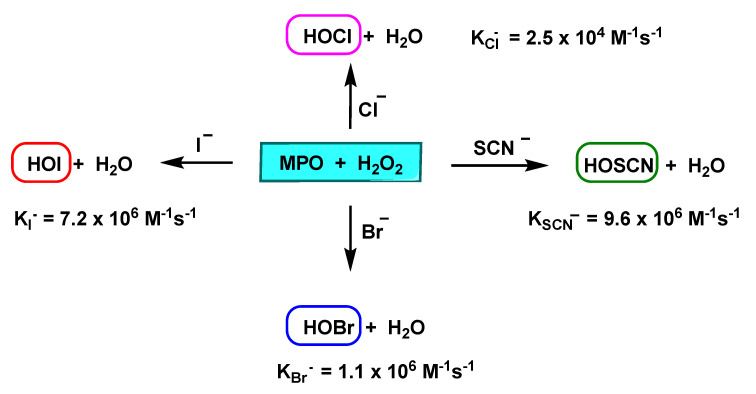
In general, SCN− is not considered a biologically functional ion, however, it plays an important role as a substrate for peroxidase enzymes, which form an essential part of innate defence [20]. Peroxidases catalyse the oxidation of halides (Cl−, Br−, I−) and pseudohalides (SCN−) by H2O2, resulting in potent oxidising agents that exhibit antimicrobial activity in vitro and in vivo, as they damage the vital structural and functional components of microorganisms. They oxidised acceptor molecules, e.g., bromide Br− to hypobromite BrO−, iodide I− to hypoiodite IO−, and thiocyanate SCN− to hypothiocyanite OSCN−, as shown in Figure 4.
Figure 4.
Catalysed conversion of Cl−, Br−, I−, and SCN− by MPO, with second-order constants K.
MPO oxidises chloride, bromide, iodide, and thiocyanate ions (H=Cl−, Br−, I−, and SCN−), to the respective HOX acids. With a 100 mM concentration of Cl−, MPO catalyses OSCN− production at SCN− concentrations of 25 µM, and at 100 µM of SCN−, approximately 50% of H2O2 is converted to OSCN− [19]. The loss of H2O2 induced by MPO in the presence of 100 mM Cl− doubled when 100 µM of SCN− was added and was maximal at 1 mM of SCN−. It is concluded that SCN− is an important physiological substrate of MPO [19]. The transition from SCN− to HOSCN by MPO is an important detoxification mechanism by removing the oxidant H2O2 and subsequently HOCl [21].
The pKa values of the resulting hypohalous acids are: 7.59 for HOCl, 8.7 for HOBr, 10.4 for HOI, and 4.85 for HOSCN [22]. The hypohalous acids HOX are in equilibrium with their anions OX−. At physiological pH values, OSCN− predominates over HOSCN, HOBr over OBr− and HOI over OI−, in nearly equal mixtures of HOCl and OCl−.
The MPO enzyme plays an important defensive role in innate immunity, as the HOX acids it produces facilitate the destruction of invading bacteria and pathogens. HOCl is the main strong oxidant generated by neutrophils and a powerful microbicidal mediator. In vitro, one million stimulated neutrophils can produce 0.1 µM of HOCl and this concentration can kill 15 million Escherichia coli bacteria in less than 5 min [23]. HOCl reacts quickly with some biological molecules, mostly those with thiols, thioethers, heme proteins, and amino groups, triggering tissue injury [24]. Taurine, at a concentration of 15 mM, is found naturally in cells and acts as a scavenger of HOCl, safeguarding collateral damage to cellular macromolecules (proteins, lipids, and DNA). Taurine present in human plasma and cells and HOCl form taurine chloramine (–O3SCH2CH2NHCl), a weaker oxidant with greater stability [25]. Tau-NHCl accumulates in the extracellular medium and does not inhibit neutrophil functions, may moderate neutrophil cytotoxic activity long after HOCI has been eliminated.
Endogenic hypohalous acids can also provoke tissue damage at the sites of inflammation, an area of research in neurodegenerative disorders, Alzheimer’s and Parkinson’s diseases, metabolic and cardiovascular dysfunction (atherosclerosis, diabetes), autoimmune dysregulation and ageing, among other conditions. MPO is associated with various skin pathologies: such as hypersensitivity and contact irritation, psoriasis, UV damage, photoaging or melanoma [26].
3. Formation and Features of Hypochlorous Acid HOCl
Biologically, HOCl is classified within a group of molecules known as reactive oxygen species (ROS) synthesised by cells of the immune system (neutrophils and macrophages). This agent can participate in subsequent non-enzymatic reactions such as the oxidation and chlorination of cellular components and can also react with substances present in the environment that modulate its biological effects [15], as shown in Figure 5.
Figure 5.
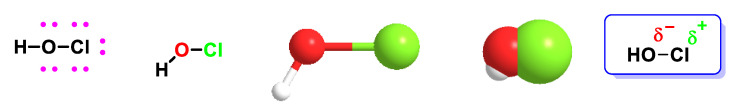
In hypochlorous acid, the chlorine–oxygen bond is polarised as shown in the figure, so Cl+ will act as an electrophilic reactant.
The usual behaviour of hypochlorous acid with the carbon and nitrogen of the amino group is that of an electrophilic agent, in which the chlorine atom combines with a pair of electrons from the substrate, while the hydroxide ion is simultaneously or subsequently separated, often assisted by a proton from the solvent or from a reactive centre elsewhere in the substrate. This feature mainly occurs in reactions with ammonia nitrogen, amides, amino acids, and peptides, with phenolic and other aromatic compounds. Cl+ transfer is favoured by the negative charge, basicity, and nucleophilicity of the receiving acid [27].
Although H2O2 is a strong oxidising agent, it does not readily react with biological molecules in the absence of catalysts. In addition, leukocytes and most cells possess enzymes that rapidly remove H2O2 by converting it into O2 and/or H2O. In contrast, HOCl and chloramines react rapidly with biological molecules, and no specific enzymatic mechanism is known to remove these oxidants.
Hypochlorous acid is the main strong oxidant generated by neutrophils. The enzyme MPO catalyses the production of hypochlorous acid from hydrogen peroxide and chloride [28], as shown in Figure 6.
Figure 6.
Production of hypochlorous acid from hydrogen peroxide and chloride.
HOCl and the associated hypochlorite anion are present in the same concentrations, because the pKa of HOCl is ~7.5 in aqueous media at physiological pH values [29,30].
Approximately 70% of the H2O2 produced in neutrophils is converted into HOCl [31]. Excessive HOCl generation can cause tissue damage and is thought to be important in the progression of several diseases including atherosclerosis, chronic inflammation, and some cancers. When the human body is invaded by bacteria or viruses, our immune system responds immediately by sending an increased number of white blood cells called neutrophils to the site of invasion. Once activated, neutrophils produce large amounts of HOCl through the enzyme myeloperoxidase, which is effective in killing all pathogenic invading microbes [1].
HOCl is considered an extremely powerful oxidant. It is estimated that 106 stimulated neutrophils can produce approximately 0.2 µmol of HOCl during 2 h of incubation [1]. In a matter of milliseconds, 0.2 µmol of HOCl is sufficient to destroy 150 million E. coli cells [32]. HOCl is involved in the destruction of bacterial cells [32], penetrating through cell walls with a water-like ease, mainly due to its low molecular weight and its electroneutrality [33]. Once inside the cell, thanks to its strong oxidising power, it can react with a large number of biological molecules, especially DNA, RNA, thiols, heme-proteins, amino groups, carbohydrates, and lipids [34].
Although HOCl has potent bactericidal properties and plays an important role in the human immune system, this oxidant also causes tissue damage, particularly under inflammatory conditions. There is a strong link between chronic inflammation and the incidence of many types of cancer, which may be associated with the ability of HOCl and related oxidants, such as N-chloramines, to damage DNA [35].
HOCl is part of a new group of microbicidal substances known as “non-antibiotic antimicrobial molecules” which, due to their broad spectrum, fast action, wide safety margin, concentration, and form of stabilisation, can be used in disinfection processes and/or to control and prevent a wide range of skin and mucosal infections [36].
HOCl plays a powerful microbicidal role in the innate immune system. The regulated production of microbicidal HOCl is required for the host to control invading microorganisms. However, due to the highly reactive nature and diffusibility of HOCl, its uncontrolled production may trigger undesirable consequences on mammal physiology [37]. For HOCl, the pKa value is 7.59 and at physiological pH, there is nearly equal mixtures of HOCl and OCl−. After formation, the ClO− diffuses away the enzyme cavity, and it is rapidly protonated according to ClO−/HOCl equilibrium.
The harmful actions induced by these species persist for long periods, even after the cessation of its production. Possibly, much of the secondary damage arises from the reaction of long-lived chloramines/chloramides and bromamines/bromamides, through HOCl/HOBr reaction with amines and amides. The longer lifetimes of these species allow their diffusion out of the site of formation, across cell membranes, unleashing damage in remote places. These species, which are generated extracellularly, can exert intracellular effects, and the degree of penetration into the cell is dependent on the structure of the halogenated species [8].
4. HOCl Reaction with Lipids
Phospholipids, fatty acids, sterols, and sphingolipids are susceptible to HOCl oxidation. Alkenes in the aliphatic residues of esterified and non-esterified fatty acids as well as the double bond in cholesterol are oxidised to chlorohydrins. Another lipid target of HOCl is the primary amino groups present in ethanolamine and serine glycerophospholipids. The main targets of reactive chlorine species RCS in LDL lipids are unsaturated –CH=CH– bonds of fatty acids and cholesterol and NH2 groups of polar heads of some phospholipids [38].
4.1. HOCl Reaction with Unsaturated Bonds
Chlorohydrins are formed by adding HOCl to the double bonds of unsaturated fatty acids and cholesterol, and these can be readily detected by gas chromatography–mass spec GC-MS [39]. These products can have physiological effects, including destabilisation of membrane structures and cytotoxicity, but the rate of formation of these materials is relatively slow and they are therefore relatively minor products in most situations [40].
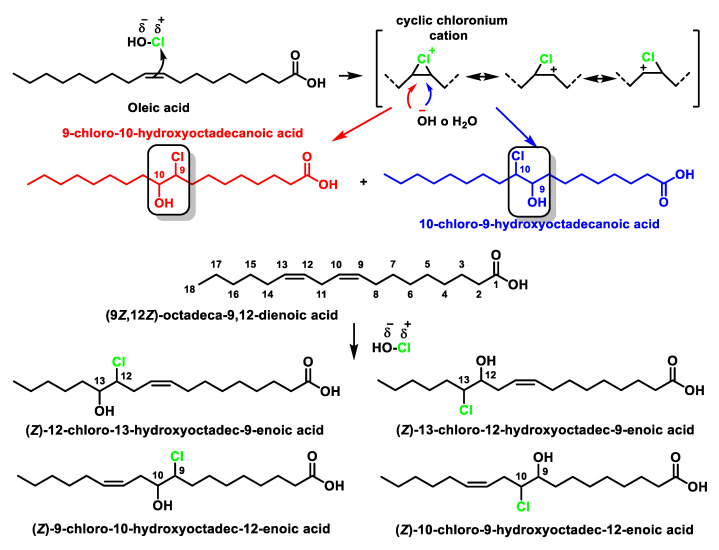
Hypochlorous acid reacts with unsaturated bonds in lipids, but not with saturated bonds, and the ClO− ion does not participate in this reaction. It is an electrophilic addition reaction of a strongly polarised asymmetric reactant, HO–Cl, to alkenes which may be asymmetrically substituted, in this case, giving two isomeric products. The formation of chlorohydrin results from the electrophilic attack of the pi bond to give an intermediate chloronium ion. The opening of this cyclic ion by the dorsal attack of the hydroxyl or water gives as the final product the addition of chlorine to one of the carbons and a hydroxyl to the other carbon. The resulting compound is a chlorohydrin.
Oleic acid, an 18-carbon unsaturated fatty acid, adds chlorine to the C=C double bond to rapidly form two isomeric chlorohydrins, as shown in Figure 7.
Figure 7.
Electrophilic addition of HOCl to unsaturated fatty acids.
If a fatty acid chain contains several unsaturated bonds, any of them can be attacked by HOCl. In the presence of excess hypochlorous acids, bis-chlorohydrins and monochlorohydrins [41] are formed on all the double bonds of the acids [42]. This gives rise to a complex pattern of positioning and stereoisomers, which can be characterised by their fragmentation patterns in mass spectrometry and by nuclear magnetic resonance NMR. Chlorohydrins by HCl elimination form epoxides under slightly alkaline conditions, but otherwise chlorohydrins are not highly reactive compounds [42].
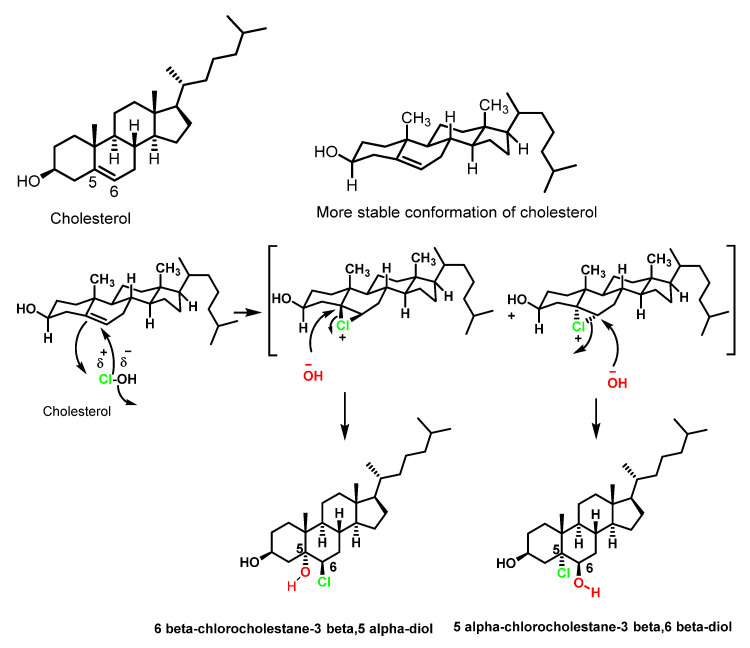
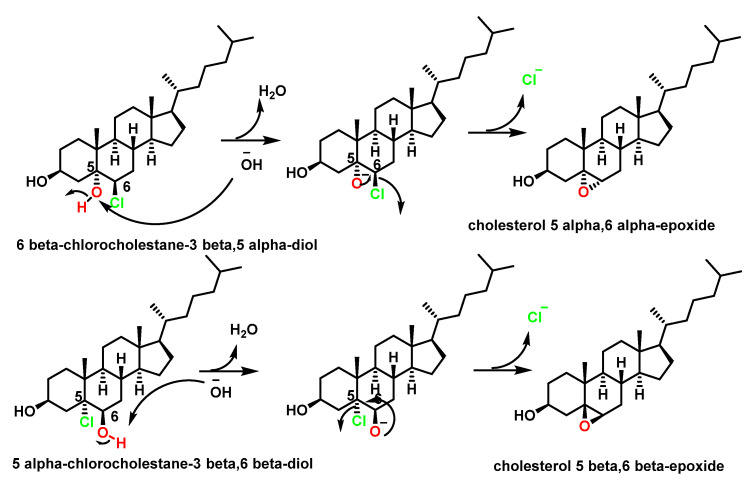
Cholesterol is one of the most abundant steroids, present in almost all animal tissues, especially in the brain and spinal cord. The human body contains 200–300 g of cholesterol. In cholesterol, the sole target of hypochlorous acid is the double bond at the 5, 6-position of the steroid nucleus mainly to lead to the formation of the α- and β-isomers of chlorohydrins [43]. The same products are formed in LDL treated with HOCl or in the presence of MPO/H2O2/Cl− [44,45].
Studies of hypercholesterolaemia and hypertriglyceridemia indicate that elevated levels of low-density lipoproteins LDL are the main cause of cardiovascular disease. Apolipoprotein B is part of lipoproteins that are considered atherogenic and promoters that develop atherosclerosis. There are two forms of apolipoprotein B100 (ApoB100) and B48 (ApoB48). ApoB100 is present in very low-density lipoproteins (VLDL), intermediate density lipoproteins IDL, and low-density lipoproteins LDL.
The products were identified by gas chromatography–mass spectrometry as cholesterol α- and β-chlorohydrins (6β-cholesterol-3β, 5α-diol and 5α-cholesterol-3β, 6β-diol), cholesterol α- and β-epoxides (cholesterol 5α, 6β-epoxide and cholesterol 5β, 6β-epoxide), and a new cholesterol hydrochloride. The conversion of cholesterol into the oxidation products required active myeloperoxidase, hydrogen peroxide, and halide, and could be blocked by catalase or by HOCl scavengers [46]. Furthermore, in the absence of the enzyme system, the HOCl reagent generated the same product distribution. These results indicate that myeloperoxidase can convert cholesterol into chlorohydrins and epoxides via a reaction involving HOCl. Other oxygenated sterols are cytotoxic and mutagenic and are potent regulators of cholesterol homeostasis in cultured mammalian cells [47], Figure 8.
Figure 8.
Electrophilic addition of HOCl to cholesterol. Mechanism of formation of cholesterol chlorohydrins.
The formation of cholesterol chlorohydrin by HOCl has been observed in several cell types including erythrocytes, neutrophils, and mammary carcinoma cells [48]. Unlike aliphatic hydrocarbon chlorohydrins, cholesterol chlorohydrins are unstable and are transformed into epoxides by dehydrochlorination, the mechanism of this reaction being the formation of a chloroalkoxide intermediate by the rapid transfer of a proton to the −OH base, followed by the closure of the cycle with Cl− exit to give the three-linked cyclic ether. Initial deprotonation of the alcohol and the subsequent step are much faster than the attack of the hydroxyl on the chlorine-bonded carbon to the alkanediol, Figure 9.
Figure 9.
Synthesis of epoxy-cholesterol by intramolecular Williamson reaction via an intramolecular SN2 reaction.
Chlorohydrins from both unsaturated fatty acids and cholesterol can cause the lysis of target cells, possibly by altering membrane structures. Unlike N-chloroderivatives, C-chloroderivatives are not oxidising and chlorinating agents.
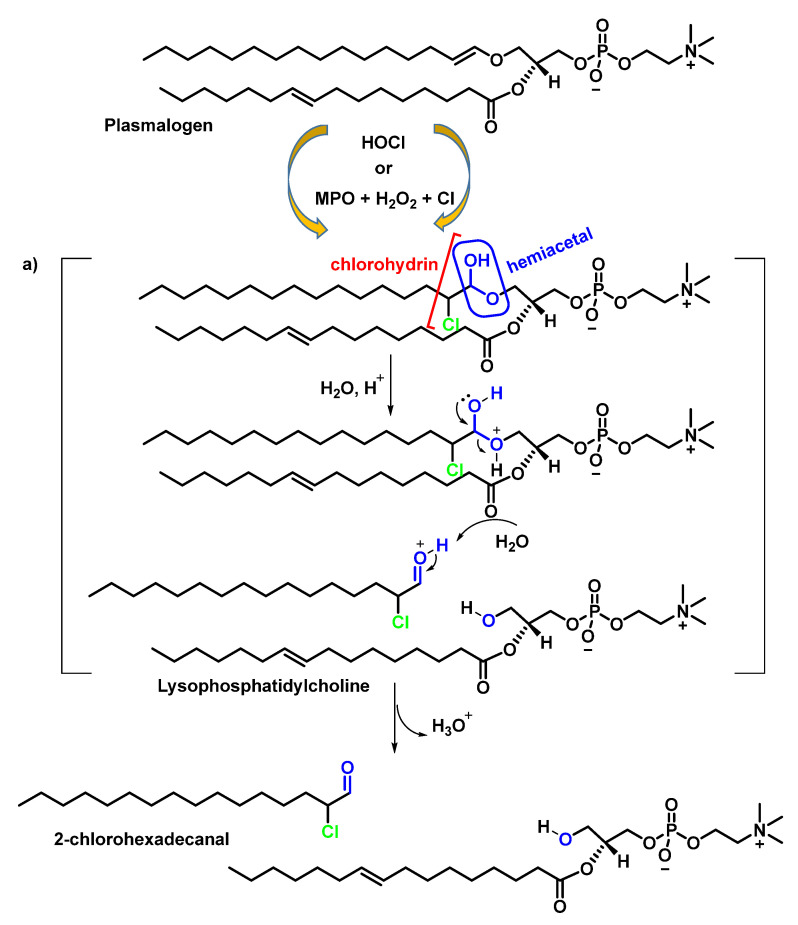
Plasmalogens are phospholipids containing an aliphatic chain linked to a vinyl ether attached to the sn-1 position of glycerol and are abundant in tissues of the cardiovascular system [49]. Chemically, this vinyl ether is a masked aldehyde that is acid labile (pH ≤ 2). The HOCl oxidation products of plasmalogen derivatives are α-chloro aldehyde and unsaturated molecular species of lysophosphatidylcholine. The α-chloro aldehyde is the precursor of both α-chloro fatty alcohol and α-chloro fatty acid. Both aldehyde and α-chloro-fatty acid accumulate in activated neutrophils. The production of the alpha-chlorinated aldehyde can be explained by the formation of chlorohydrin, preferentially at the vinyl double bond (C=C–O), which has a higher electron density than C=C. A hemiacetal is obtained which, by acid hydrolysis, fragments and leads to the formation of the aldehyde and the lysophosphatidylcholine alcohol, as shown in Figure 10.
Figure 10.
Plasmalogen modification by HOCl or MPO/H2O2/Cl−. (a) Acid-catalysed hydrolysis of hemiacetal. The vinyl ether bond of plasmalogens is targeted by HOCl resulting in the release of the masked aldehyde as an α-chloro-fatty aldehyde. The other product of this reaction is an unsaturated molecular species of lysophosphatidylcholine.
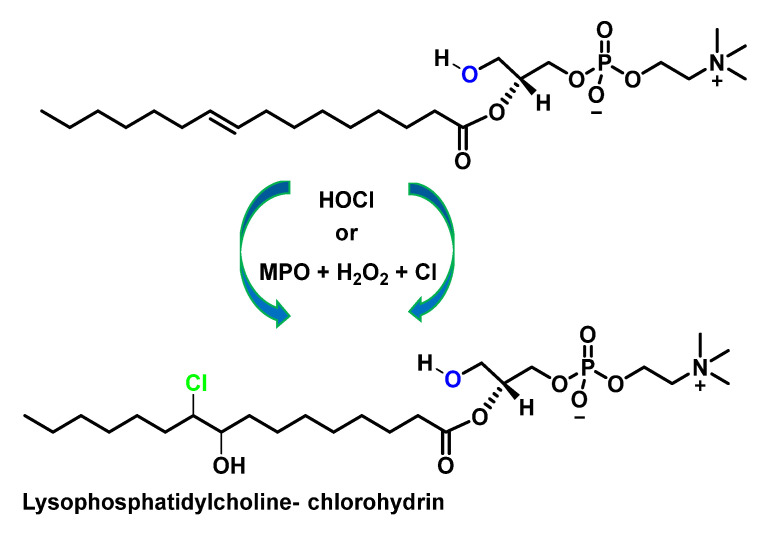
The reaction continues towards the formation of chlorohydrin from lysophosphatidylcholine, as can be shown in Figure 11.
Figure 11.
The alkene bond in the unsaturated molecular species of lysophosphatidylcholine is targeted by HOCl to yield a chlorohydrin molecular species.
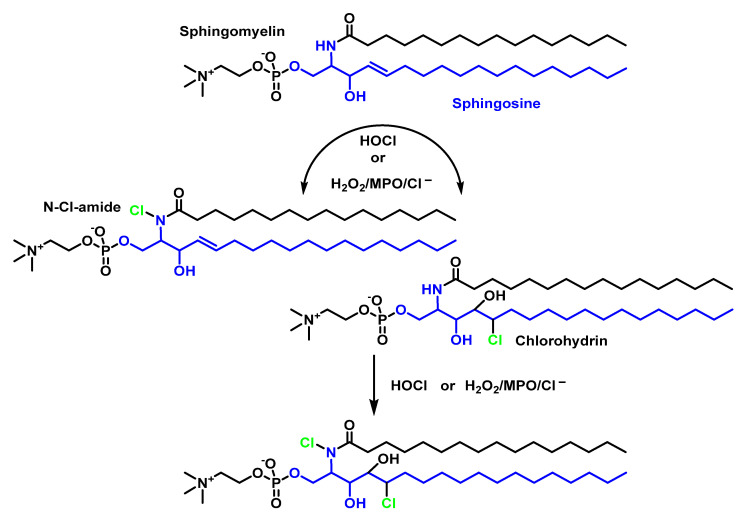
The second most abundant phospholipid in LDL after phosphatidylcholine (~900 µg per mg of protein) is sphingomyelin [50] (~400 µg per mg protein). Sphingomyelin is the only human phospholipid that does not contain glycerol but has the amino alcohol sphingosine. The fatty acid of sphingomyelin is bound to sphingosine by the amino group, while the polar group of phosphocholine is bound to the hydroxyl group of sphingosine. The HOCl targets on the sphingomyelin molecule are the sphingosine double bonds and the hydrogen atom of the amide. The rate constant of the reaction of HOCl with sphingomyelin was estimated to be 18.7 ± 3.05 M−1 s−1 [51], which is faster than the reaction between HOCl and unsaturated acyl chain bonds (0.56 M−1-s−1) [52], as shown in Figure 12.
Figure 12.
Sphingomyelin modification by HOCl or H2O2/MPO/Cl−. Chlorinated sphingomyelin derivatives produce significant deleterious effects on cells, including apoptosis and DNA damage.
4.2. HOCl Reaction with Phospholipids
The polar heads of phospholipids in LDL are mainly choline phosphate, ethanolamine phosphate, and serine phosphate [53]. HOCl does not react with the quaternary nitrogen of phosphatidylcholine [47].
The unsaturated fatty acids that form phosphatidylcholine, a naturally occurring phospholipid and a major component of the basic structure of biological membranes [54], when chlorinated, lead to the formation of chlorohydrin, which is significantly toxic and can damage cellular structures [40], Figure 13.
Figure 13.
Scheme of reaction of phosphatidylcholine with HOCl.
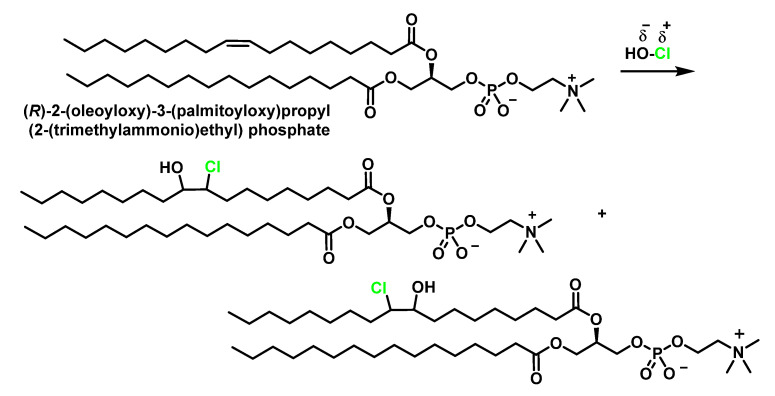
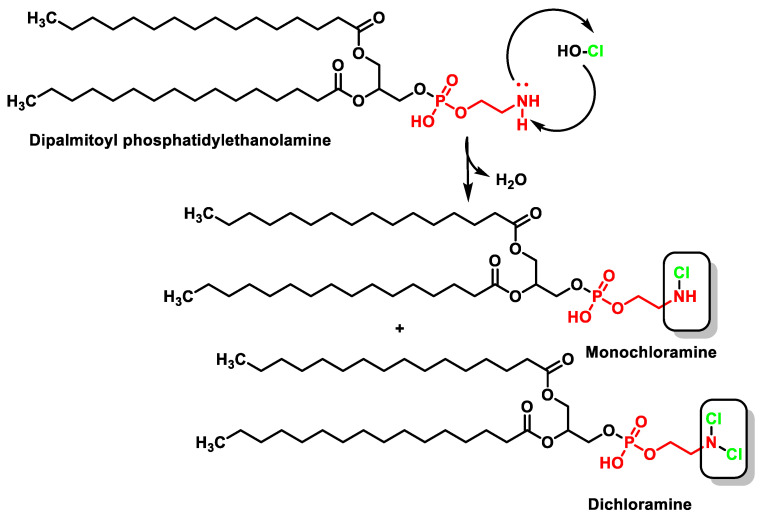
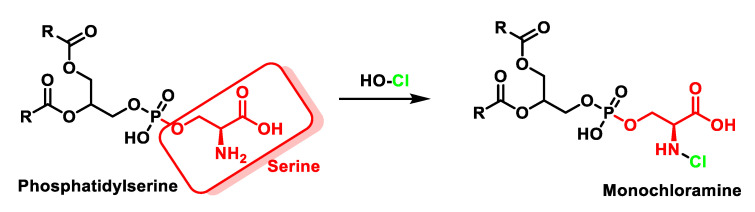
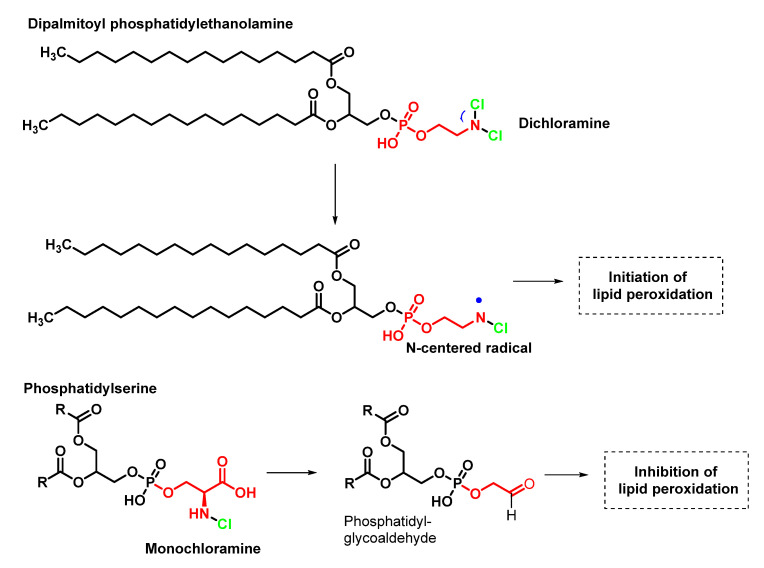
In phospholipids bearing a primary amino group, such as phosphatidylethanolamine, which is found in all living cells, the formation of chloramines is diminished compared to chlorohydrin, even when there are C=C double bonds in the carbon chains. HOCl transforms phosphatidylethanolamine into mono- and then dichloramine [55], as shown in Figure 14. The oxidation of phosphatidylserine by HOCl is represented in Figure 15.
Figure 14.
Formation of monochloramine and dichloramine from the oxidation of dipalmitoyl phosphatidylethanolamine by hypochlorous acid.
Figure 15.
Formation of monochloramine from the oxidation of phosphatidylserine by HOCl.
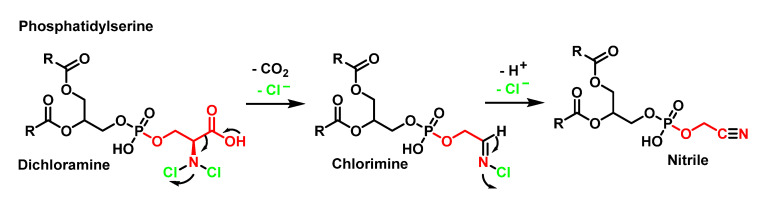
The decomposition of dichloroamine with the loss of Cl− and decarboxylation gives the formation of a transient (chloro)imine which loses Cl− and a proton to give the nitrile. These modifications by HOCl coincide well with the known pathways for amino acids, as shown in Figure 16.
Figure 16.
Decomposition of dichloroamine, formation of a transient (chloro)imine.
Phosphatidylethanolamine and phosphatidylserine chloramines differ considerably in their stability due to the presence of the carboxyl group in the position close to the NH2 group on the serine phosphate. The dichloramine of phosphatidylethanolamine undergoes dehydrochlorination accompanied by the homolytic cleavage of the N–Cl bond with the formation of the N-centred radical. The chloramine of serine phosphate rapidly decomposes with the formation of aldehydes [55], as shown in Figure 17.
Figure 17.
Conversion of the amino group of phosphatidylethanolamine and phosphatidylserine by reaction with HOCl.
Kawai et al., 2006, indicated that although phosphatidylserine is also chlorinated upon reaction with HOCl, the chloramine formed is rapidly decomposed into phosphatidylglycolaldehyde, a new class of lipid aldehyde. Phosphatidylglycolaldehyde formation is also confirmed in porcine brain PS and in the cell membrane ghost of erythrocytes exposed to HOCl [55]. It is most likely that the rapid formation of the aldehyde, which is stable, prevents the formation of the N radical and thus prevents peroxidation.
5. Reaction of HOCl with Ammonia and Aliphatic Amines
The usual behaviour of hypochlorous acid towards carbon and nitrogen in organic substrates is as an electrophilic agent, where chlorine takes on the partial characteristics of the “Cl+” ion and combines with the electronic pair of the substrate, simultaneously releasing the OH− ion, often assisted by the H+ of the solvent or by other reactive centres of the substrate [56].
One type of HOCl capture compounds of great utility in MPO studies, and which may be the most important under physiological conditions, are nitrogen compounds, particularly ammonia and primary amines. HOCl reacts with these compounds to give the corresponding N-chloramines, which also have oxidising and chlorinating activity. The chloramines act as carriers and reservoirs for the “Cl+” formed in the MPO-catalysed oxidation of Cl− ions. The most important role of HOCl and chloramines in leukocytes is to mediate the toxicity of the MPO/H2O2/Cl− system on the target cells of these oxidising agents, usually micro-organisms that have invaded the tissues [57].
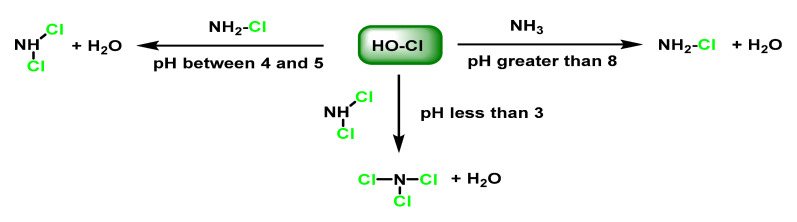
The chlorination of ammonia gives monochloramine NH2Cl and aliphatic amines give the derived monochloramines RNHCl. N-chloramines are reactive and have a long half-life and are strong oxidisers and chlorinating agents, although they are less reactive than HOCl and are not reduced by H2O2 [58]. Ammonia and amines protect MPO from inactivation, indicating that chloramines do not attack the enzyme. With ammonia, for example, NH2Cl, NHCl2, and NCl3 are sequentially formed via the reactions, as shown in Figure 18.
Figure 18.
Synthesis of mono, di, and trichloro ammonia.
The formation of the different species is conditioned by the ratio of chlorine/ammonia concentrations, when the concentration of ammonia and amines is higher than the amount of HOCl, the formation of chloramines with a single N–Cl bond is favoured. The reaction time, the temperature as well as the pH of the reaction medium also play a role. Thus, at pH values below 3, mainly tri-chloramine is obtained, and at pH values between 4 and 5, dichloramine is formed and the product that is mainly generated at pH > 8 is monochloramine [59,60].
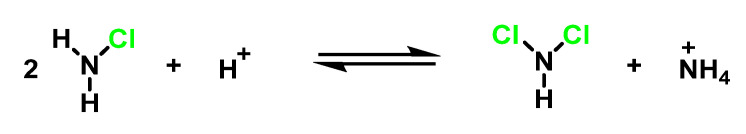
Dihaloamines can also be obtained by the disproportionation reaction of the corresponding N-monohaloamine in an acid medium, through the reaction of Figure 19.
Figure 19.
Dihaloamines obtained by the disproportionation reaction of the corresponding N-monohaloamine in an acid medium.
For reactions of HOCl with the N atom of amines and similar compounds, the electrophilic chlorine atom could be visualised as trapping the free electron pair of the N atom releasing the H+ of the amine and the OH− of HOCl. Alternatively, the reaction could be interpreted as a nucleophilic displacement of the OH− from the HOCl by the amine in which case the nucleophilicity of the amine will compete with that of the OH− ion. According to either reaction model, the specific chlorination rates on amines vary directly with the basicity (or nucleophilic character) of the nitrogenous substrate.
In the case of primary aliphatic amines, mono- and di-chloramines RNHCl and RNCl2, respectively) are formed, while in the case of secondary aliphatic amines, only monochloramine R1R2NCl formation is possible. The reaction rate is also fast and increases with the nucleophilic character of the amine attacking the chlorine atom of the HOCl. The chlorination of tertiary amines is at least three orders of magnitude slower than the chlorination of secondary and primary amines of similar basicity. This behaviour is explained based on the unavailability of hydrogens of these amines.
A recent theoretical study on the chlorination of N-compounds indicates that the transition state involves three water molecules to give rise to a concerted cyclic transition state [61,62,63], Figure 20.
Figure 20.
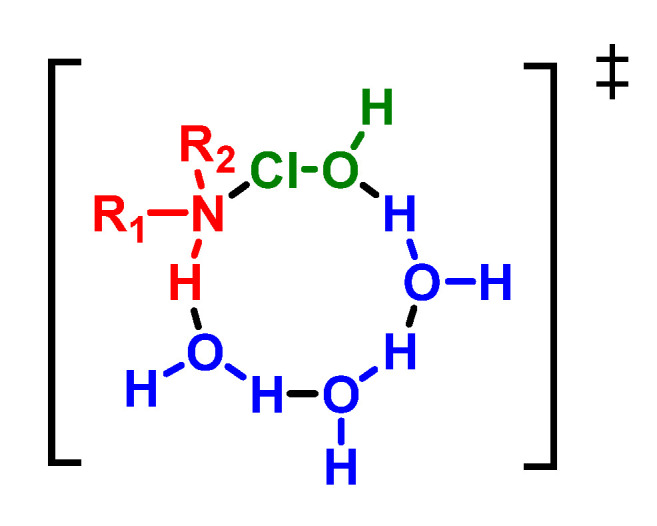
Transition state of the amine chlorination reaction.
Chloramines represent an important class of leukocyte oxidants and contribute to microbicidal, cytotoxic, and cytolytic oxidative activities, the chemical modification of regulatory substances, and the uptake and metabolism of nitrogenous compounds [64]. The most abundant N-residues available for reaction with HOCl in biological systems are primary amino groups, such as taurine, polyamines, amino sugars, lysine, and extreme residues of proteins.
6. Reactions of HOCl with Amino Acids
Hypochlorous acid is a weak acid and its pKa is 7.6 [65]; however, it is an extremely strong oxidant that reacts indiscriminately with a wide variety of functional groups in proteins, nucleotides, and lipid membrane, and selectively oxidises thiols [66]. In the case of proteins, the oxidising HOCl most rapidly reacts with sulphur containing residues such as cysteine and methionine, followed by protein side chains containing lysine, histidine, tryptophan, and tyrosine [67].
6.1. Reaction of HOCl with Nitrogen-Containing Compounds
HOCl rapidly reacts with nitrogen-containing compounds (particularly amines and, to a lesser extent, amides), producing chlorinated derivatives, including chloramines (R–N(R′)–Cl) and chloramides (R–C(O)–N(R′)–Cl). These compounds can be further oxidised to form dichlorinated species (R–N–Cl2).
In addition, nitrogen chlorination can occur at the α-amino group of amino acids, at the N-terminus of peptides and proteins, and at nucleophilic centres on the side of protein chains (e.g., lysine, arginine). On the other hand, chloramides can be generated at the peptide bonds of proteins and, in excess of HOCl, at the side chains of asparagine and glutamine, with amide groups. The formation of chloramines interferes with protein folding and leads to protein aggregation.
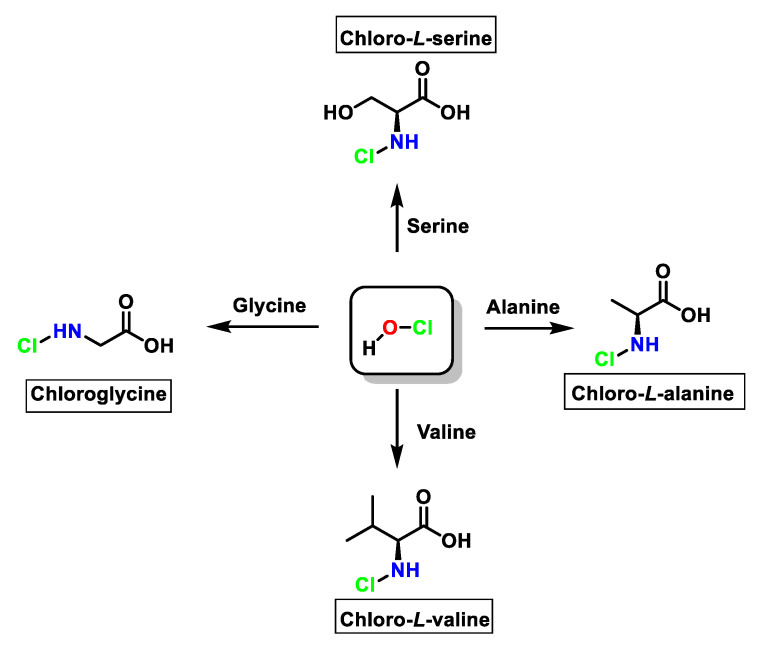
For amino acids without a functional group in the chain that can react with HOCl (Gly, Ala, Val, Ser), the reaction proceeds only with the amino group with monochloramine formation, and dichloramine is formed in excess of HOCl, as shown in Figure 21.
Figure 21.
Schematic the mechanism of formation of N-chloro-α-amino acids from amino acids includes an electrophilic attack via the Cl+ of HOCl on the nitrogen atom together with a proton transfer from the nitrogen atom to HOCl to release a water molecule.
6.2. Reaction of HOCl with Aromatic Amino Acids
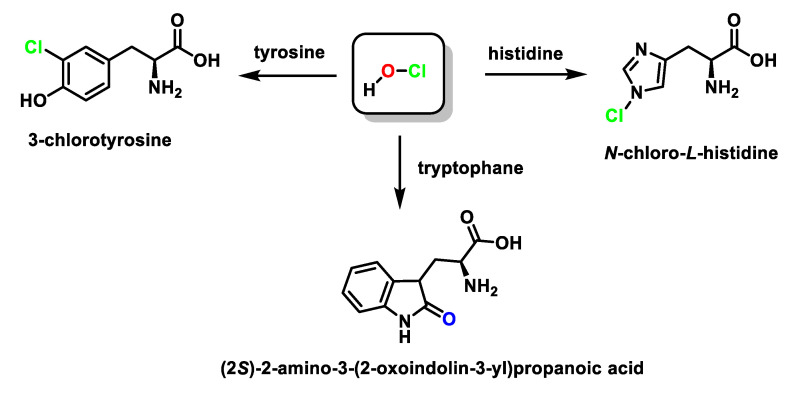
The imidazole ring of histidine reacts with HOCl to form a short-lived chloro-amine, which rapidly transfers its chlorine group into another amine [68]. Tryptophan reacts with HOCl to form 2-oxindole, while the reaction of HOCl with tyrosine forms 3-chlorotyrosine and 3,5-dichlorotyrosine, Figure 22.
Figure 22.
Products obtained in the reaction of aromatic amino acids with HOCl.
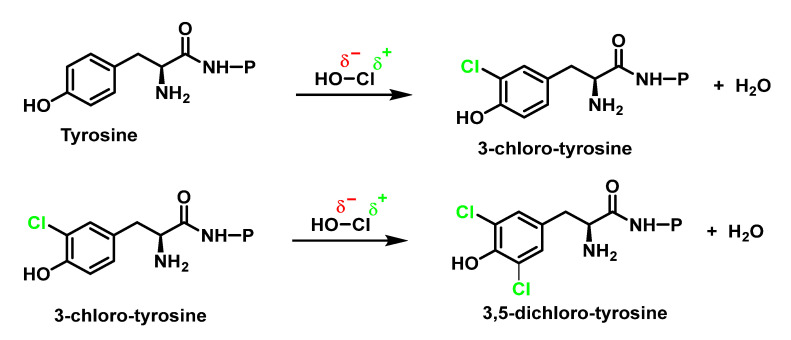
3-Chlorotyrosine is a chemically stable product formed when tyrosine reacts with HOCl, or a chloramine generated by HOCl. In particular, it has been observed that this aromatic amino acid (both free and in protein) undergoes the rapid chlorination of the aromatic ring, through an electrophilic aromatic substitution reaction of a hydrogen for a chlorine to give 3-chlorotyrosine with excess HOCl to form 3,5-dichlorotyrosine [69,70,71,72,73,74], as shown in Figure 23.
Figure 23.
Reaction of P-protected tyrosine with HOCl and formation of 3-chloro- and 3,5-dichlorotyrosine derivatives.
Direct HOCl attack and a chlorine transfer from the chloramine initially produced in the amino group of the amino acid are the two mechanisms proposed for the chlorination of the phenolic side chain of Tyr [75].
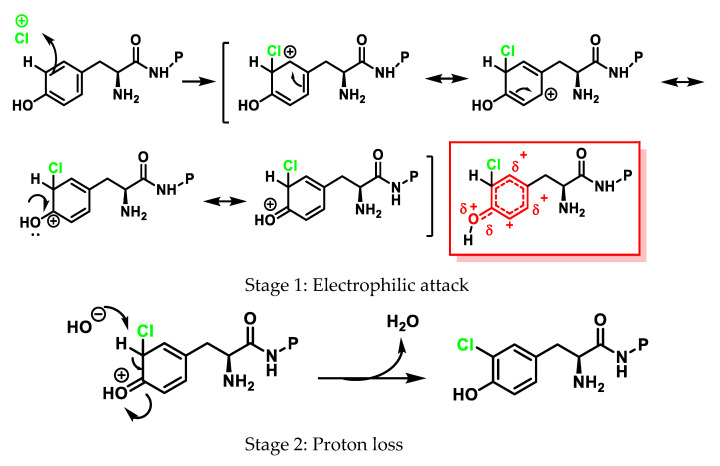
The general mechanism of electrophilic aromatic substitution begins with the electrophilic attack of “Cl+” to give an intermediate hexadienyl cation with delocalised charge, in a step that is the rate-determining Stage 1, as shown in Figure 20. The subsequent rapid loss of protons regenerates the aromatic ring (now substituted), as shown in Stage 2 of Figure 24.
Figure 24.
Mechanism of the electrophilic substitution in tyrosine and the formation of 3-chlorotyrosine. Stage 1: electrophilic attack; Stage 2: proton loss.
Hypochlorous acid and chloramines result in the chlorination of tyrosine residues to form 3-chlorotyrosine [76]. This halogenated amino acid is a specific biomarker for HOCl-mediated damage [39].
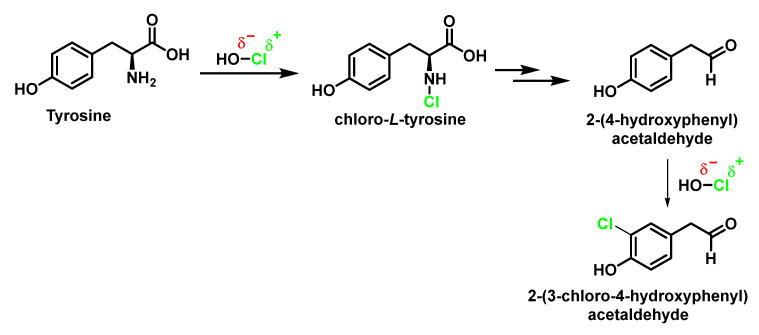
With the free amino acid, in addition to the reaction with the aromatic ring to give 3-chlorotyrosine, a reaction can occur at the amino group and the resulting formation of p-hydroxyphenylacetaldehyde [77]. Both products can continue to react to give, respectively, 3-chloro-4-hydroxyphenylacetaldehyde and 3,5-dichlorotyrosine [73], Figure 25.
Figure 25.
Formation of p-hydroxyphenylacetaldehyde [77] and 3-chloro-4-hydroxyphenylacetaldehyde.
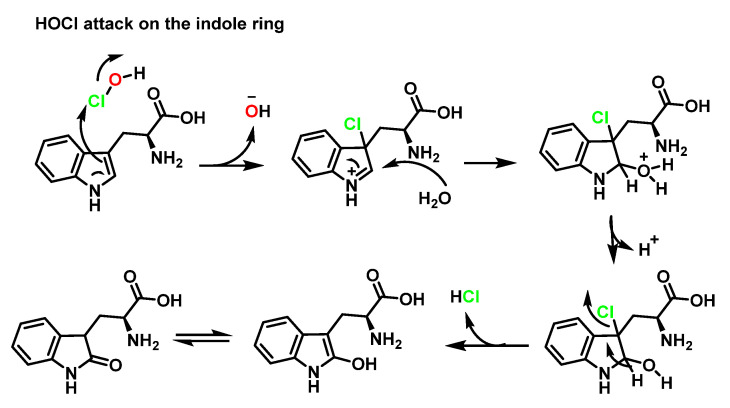
HOCl-mediated oxidation of free tryptophan can proceed via direct attack by HOCI on the indole ring, for example, in a classical electrophilic aromatic substitution mechanism such as that of tyrosine. Chlorine enters the C–3 position of the indole ring forming an iminium cation, which is attacked at C–2 by a water molecule, after the removal of HCl, aromaticity is restored and the enol is obtained, which is in equilibrium with the carbonyl, as shown in Figure 26.
Figure 26.
Hypochlorous acid could directly attack the free amino group of the amino acid Trip, with the consequent formation of a chloramine, in an electrophilic substitution mechanism [78].
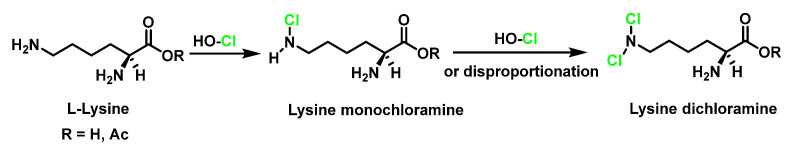
Lysine and arginine are the second targets of HOCl in proteins, which chlorinates them to form chloramines. Lys and Arg are chlorinated by HOCl or MPO/H2O2/Cl− at the N of the amino acid chain, as shown in Figure 27.
Figure 27.
HOCl reaction with Lys and formation of monochloroamine and dichloroamine.
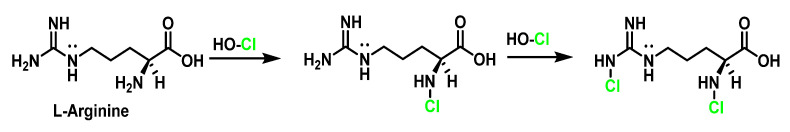
Using spectrophotometric techniques, HOCl and L-Arg were found to react rapidly (k = 7.1 × 105 m−1 s−1) to form two main products which were identified by mass spectrometry as monochlorinated and dichlorinated adducts of L-Arg [79], as shown in Figure 28.
Figure 28.
Proposed structures of chlorinate L-arginine products.
6.3. Reaction of HOCl with SULFUR-Containing Amino Acids
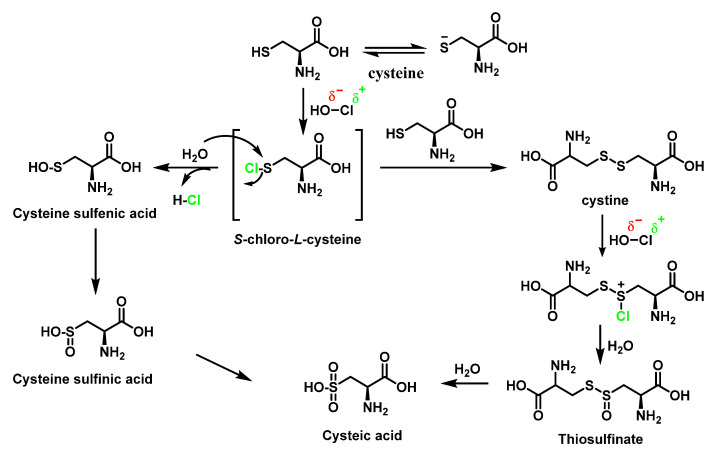
HOCl and reacts extremely rapidly (k ≈ 3107 M−1 s−1) with sulphur-containing residues (cysteine and methionine) [75,80]. The thiol functional group of the amino acid cysteine can undergo a wide range of oxidative modifications. The thiol group of cysteine reacts with HOCl and through the formation of an unstable sulphenyl chloride which undergoes various reactions with nucleophilic reagents can react with water to form a sulphenic acid Cys-SOH [73,78,81]. Most sulphenic acids are highly unstable, with a half-life of minutes and that react with a cysteine thiol present in the vicinity to form a disulphide or are further oxidised to sulphinic Cys-SO2H and sulphenic Cys-SO3H acids [82]. Sulphenyl chloride can react with the thiol group of a second cysteine molecule to generate the disulphide, cystine. Cystine can also be oxidised by HOCl, possibly via an intermediate sulfenyl chloride CysS+(Cl)SCys, to finally give rise to more cysteic acid by hydrolysis, as shown in Figure 29.
Figure 29.
Summary of products of oxidation of Cys and Cystine residues by HOCl.
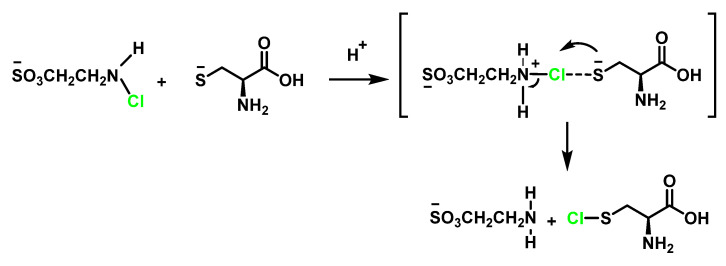
Cysteine can also react with N-Cl-taurine to generate sulphenyl chloride, as shown in Figure 30.
Figure 30.
Formation of S-chloro-L-cysteine.
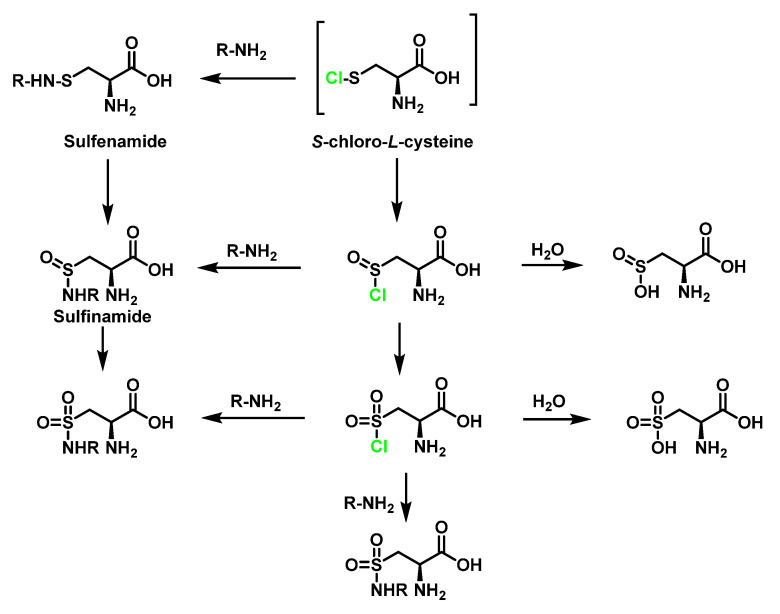
Sulphenyl chloride intermediates can react with nitrogen derivatives [83]. The guanidine groups of the Arg side chains, and the side-chain amines of Lys are all capable of reacting with sulphenyl chlorides to form sulphenamides RSN-. Other reactions of sulphenamides with oxygen produce sulphinamides Cys-SO-N-R and sulphonamides Cys-SO2-N-R. Chloride Cys-S-Cl is converted into sulphonyl chloride Cys-SO2-Cl, in excess of HOCl. Sulphonyl chloride Cys-SO2Cl can be converted to thiosulphonates Cys-SO2-S-Cys on reaction with thiols Cys-SH. Both sulfonyl chloride Cys-SO2Cl and thiosulfonates Cys-SO2-S-Cys can be hydrolysed to form sulfonic acid Cys-SO3H. Generally, the irreversible formation of sulphonic acid Cys-SO3H inhibits protein formation or degrades proteins. In addition to the aforementioned reactions, sulfonyl Cys-SO2Cl and sulfenyl Cys-S-Cl chlorides can also react with amino R-NH2 compounds to form sulphenamide Cys-S-NH-R, sulphinamide Cys-SO-NH-R, as well as sulphonamide Cys-SO2-NH-R. In addition, sulphenyl chlorides Cys-S-Cl can decompose to thiyl radicals Cys-S at a high temperature, in the presence of metal ions or ultraviolet light [84]. This process can compete with the other processes described above, particularly when the thiol group is isolated or within a protein, as shown in Figure 31.
Figure 31.
Proposed reaction pathways for S–N cross-linking and oxidation by HOCl of Cys.
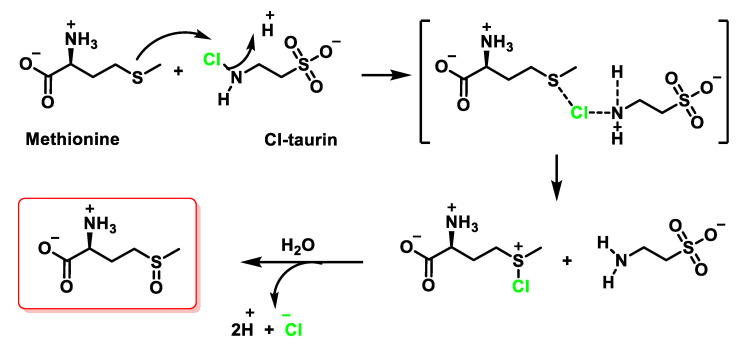
The oxidation of the Met side chain by HOCl is less complex, where methionine sulfoxide is the only product observed with even a 2.5-fold molar excess of HOCl over N-acetyl-Met [85]. Methionine sulfoxide is a stable oxidation product of Met, and it is likely that this oxidation plays a critical role in the bactericidal action of HOCl. The reaction of methionine with HOCl initially produces a sulphonium chloride, which is subsequently hydrolysed to give sulfoxide as the final product. Assuming that the same product is formed with chloramines, then a mechanism can be proposed in which hydrogen ions promote the transfer of Cl+ to sulphur [86], as shown in Figure 32.
Figure 32.
Mechanism of Met oxidation mediated by Cl–taurine to yield methionine sulfoxide.
Proteins, the most abundant cellular molecules, are the main target of HOCl. In this context, sulphur-containing compounds such as cysteine, methionine and glutathione have the highest degree of reactivity with HOCl compared to other biological molecules. Thiols are cell crucial targets for oxidation.
Second-order rate constants were determined by Pattison and Davies, 2001, for the reactions of HOCl with amino acids side chain groups, α-amino groups and backbone amides, Table 1, at physiological pH, 7.4, in aqueous solution. The order of reactivity of the various side chains was Met > Cys > Cystine > His > Trp > Lys > Tyr > Arg > Gln > Asn [80]. Constants are second-order determined for the reactions of HOCl with amino acids’ side-chain groups, α-amino groups, and backbone amides [79].
Table 1.
Second-order rate constant for backbone amides varies over several orders of magnitude, depending on the environment. This value is a maxima estimate based on studies with cyclic dipeptides.
| Residue | K2 M−1 s−1 | Residue | K2 M−1 s−1 |
|---|---|---|---|
| Met | 3.8 × 107 | Lys | 5.0 × 103 |
| Cys | 3.0 × 107 | Tyr | 44 |
| Cistine | 1.6 × 105 | Arg | 26 |
| His | 1.0 × 105 | Backbone amides | <10 |
| α-amino | 1.0 × 105 | Asn | 0.03 |
| Trp | 1.1 × 104 | Gln | 0.03 |
6.4. Reaction of HOCl with Taurine
Taurine, 2-aminoethane sulphonic acid, was first isolated in 1827 by Friedrich Tiedemann and Leopold Gmelin [87] in ox (bull) bile, hence its name. Taurine, 2-aminoethanesulphonic acid, is a β-amino acid containing sulphur which is not involved in protein synthesis, but is abundant in the cytoplasm of most cells [88]. Taurine is involved in the elimination of foreign micro-organisms by the immune system [89]. Its other functions include its role as a neurotransmitter, protector of the photoreceptors of the retina, antioxidant, etc.
Taurine is an amino acid found in high concentration in cells, acting as a trap for HOCI generated by the MPO/H2O2/Cl− pathway to form N-Cl-taurine, which can still be an oxidant with much lower reactivity and toxicity than HOCl. Taurine chloramine possesses antimicrobial and anti-inflammatory activity [90]. Taurine is present in human plasma and cells, acts as a major HOCl-attractant forming taurine chloramine (–O3SCH2CH2NHCl), is more stable and a weaker oxidant. These species exert a protective effect on neutrophils and help retain bactericidal potential for prolonged periods. Recent studies have suggested that chloro-taurine may act as a physiological modulator of inflammation, inhibiting the production of pro-inflammatory agents [91].
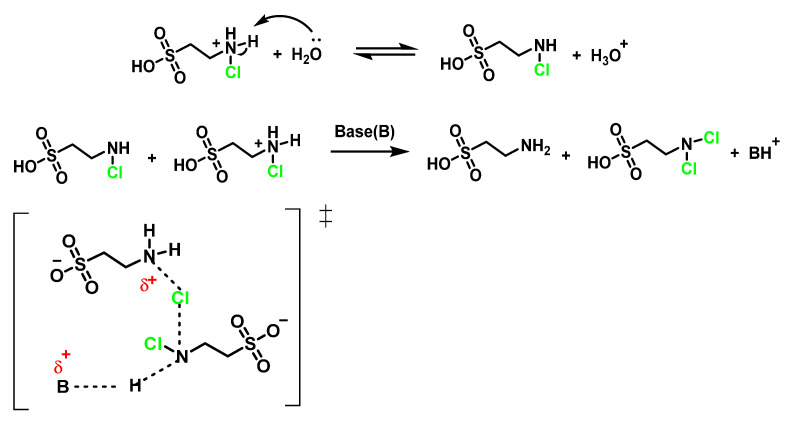
Tau-NHCl, which accumulates in the extracellular medium and does not inhibit neutrophil functions, may continue to moderate neutrophil cytotoxic activity long after HOCI has been eliminated. Antelo et al., 2000, have studied the disproportionation of N-chlorotaurin Tau-NHCl to give rise to N, N-dichlorotaurin Tau-NCl2. These authors suggest that the abstraction of a proton for a base takes place in the slow stage and propose a concerted mechanism in which deprotonation and chlorine transfer occur simultaneously in the transition state [92], as shown in Figure 33.
Figure 33.
Proposed transition state for the N-chlorotaurin disproportionation reaction.
Chlorinated taurine derivatives are more hydrophilic and do not pass through biological membranes, so they have very low toxicity [93]. Taurine-chloramine TauCl also appears to play a chlorinated oxidant scavenger role. Taurine chloramines react slowly with bactericidal components, and taurine obstructs the bactericidal activity of the MPO/H2O2/Cl− pathway [94]. Leukocytes contain 20–30 mM taurine in the cytosol [95], and it is proposed that leukocytes use taurine to trap HOCl from phagolysosomes [90]. Taurine derivatives, N-chlorotaurine TauNHCl, and N, N-dichlorotaurine TauNCl2, are the most important components formed by the reaction of chlorine generated by the MPO/H2O2/Cl− pathway with taurine, an amino acid found in the body in higher concentrations than other amino acids.
6.5. Reaction of HOCl with Peptides and Proteins
The above reactions of HOCl with functional groups on amino acid chains can also occur in the case of proteins and polypeptides. Proteins are likely to be the main targets for HOCl reaction within a cell due to their abundance and high reactivity with HOCl, although little information is available on the oxidation mechanisms [80].
Within proteins, the sulphur of cysteine Cys and methionine Met react rapidly with HOCl, but also the side chains of lysine (Lys), histidine (His), tryptophan (Trp), and tyrosine (Tyr), and the -amino groups of amino acids and peptides are oxidised and/or chlorinated. However, the reactivity of functional groups in amino acid side chains and the same groups in the structure of a polypeptide chain can vary significantly. The treatment of small globular proteins, such as insulin (5.7 kDa) and lysozyme (14.4 kDa), with increasing concentrations of HOCl, leads to modification of several amino acid residues (His, Lys, Arg, Tyr, etc.). The tertiary structure of the protein influences the reactivity of the amino acid residue side chains and/or their availability for HOCl.
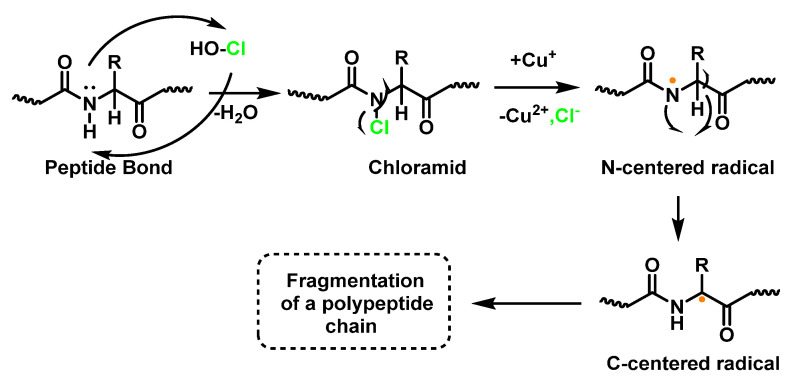
The result of the HOCl-protein interaction is fragmentation, which is mainly due to the cleavage of a peptide bond. When HOCl interacts with a peptide bond, the chloramide is formed, as can be seen in the scheme. It should be noted that the reaction rate with the amide of the peptide bond is much slower than with an amino group. The reaction rate constant depends to a large extent on the chemical structure of the compound. In aqueous media, chloramines hydrolysis slowly with peptide bond breakage and protein fragmentation [96]. In addition, the N–Cl bond can undergo homolytic cleavage with the formation of an N-centred radical, e.g., in the presence of transition metal ions. The N-centred radical is short-lived, these species undergo rapid migration reactions of 1,2 hydrogen atoms to give α-carbon centred radicals stabilised by C=O conjugation with most peptides, leading to the fragmentation of the polypeptide chain [97], as shown in Figure 34.
Figure 34.
Formation of chloramide.
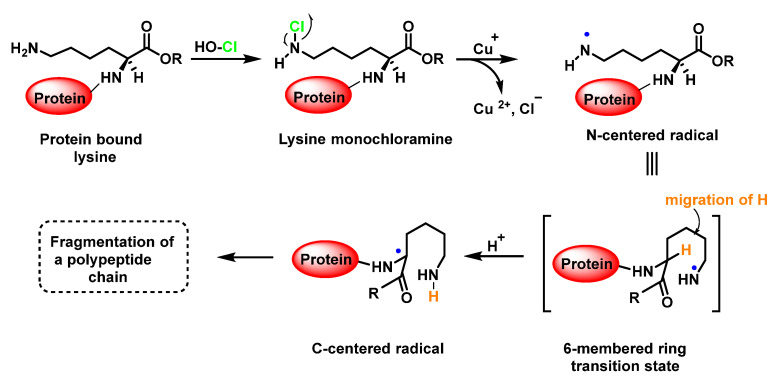
Another reason for the cleavage of the peptide bond may be the formation of chloramide or chloramine in the reaction of HOCl with the side-chain amide group of Glutamine or with the amino group of Lysine [98], as shown in Figure 31, in the case of Lysine, with intramolecular reordination via a six-link cyclic intermediate, transformed into a radical centred on the C atom of the peptide bond. Under aerobic conditions, such a C-centred radical is rapidly converted into a peroxyl radical with subsequent degradation of the polypeptide chain, Figure 35.
Figure 35.
Formation of the radical centred on the N of Lys with subsequent transformation to a C-centred radical.
6.6. Reaction of HOCl with Glutathione
Glutathione GSH is a water-soluble, low-molecular-weight tripeptide that is present in millimolar concentrations in almost all eukaryotic cells and in many bacteria, most of which are Gram-negative. Glutathione is synthesised by the consecutive action of two ATP-dependent enzymes.
While glutathione serves as a redox buffer, the oxidation of the sulphydryl groups on proteins can affect their functional properties. The formation of protein di-sulphides, mixed disulphides with glutathione or sulphenic acids can lead to changes in enzymatic activity, conformation or affinity towards other molecules. Such changes contribute to cell damage caused by oxidative stress. The modulation of the thiol redox state also provides a sensitive mechanism for the regulation of metabolic processes of the thiolate group [99], RS-, which is more reactive as a nucleophile than the corresponding protonated group RSH.
Glutathione GSH is a classic example of a scavenger antioxidant. It forms the first line of defence and efficiently removes reactive species, e.g., hypochlorous acid, before they cause damage to biomolecules.
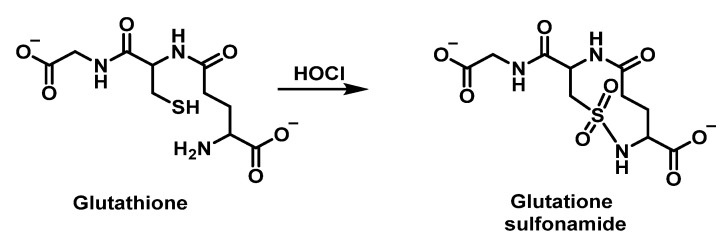
Winterbourn et al., 2002, studied the chlorination of GSH by HOCl and found glutathione thiosulphonate GSO2SG and the corresponding sulphonamide as oxidation products alongside GSSG. In this case, intramolecular condensation is thought to occur between a sulphonyl chloride intermediate RSO2Cl of Cys and the amino group of the glutamyl side chain. It has been proposed that glutathione sulphonamide may be a specific marker of HOCl oxidation in biological systems. In addition to understanding the impact of reactive oxidants on cellular function, it is important to have reliable biomarkers to detect oxidant formation and its consequences in vivo. Glutathione sulphonamide is a promising candidate for a biomarker of HOCl production by neutrophils in vivo. The reaction with GSH is one of the fastest reactions observed for HOCl, which makes GSH likely to be an important target for HOCl in vivo, as shown in Figure 36.
Figure 36.
Reaction of GSH with HOCl and formation of glutathione sulphonamide.
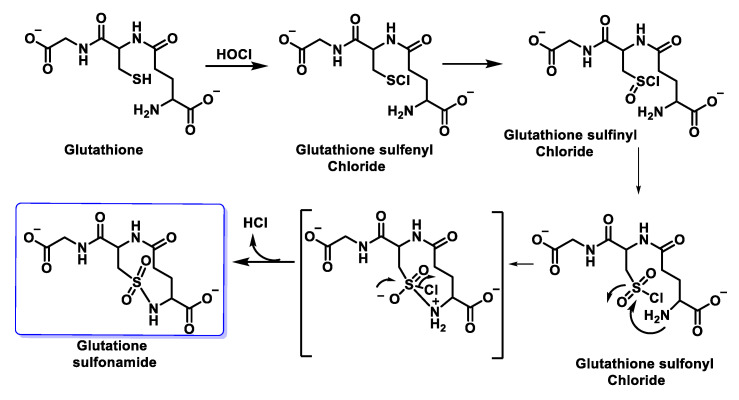
The possible mechanism for this transformation is shown in Figure 37.
Figure 37.
Intramolecular cyclisation of the RSCl intermediate with the terminal amino group on oxidation by hypochlorous acid. The proposed mechanism of formation is through the condensation of the sulphonyl chloride with the amino group of the glutamyl residue of glutathione.
In human red cells, GSH is oxidised to glutathione disulphide GSSG; therefore, GSH probably acts as a protector of cells against HOCl toxicity [100]. Vissers et al., 1995, exposed human red blood cells to low doses of hypochlorous acid, resulting in the loss of intracellular GSH. The disappearance of GSH precedes the oxidation of membrane thiols and the formation of chloramines. The lost GSH is converted into GSSG. These authors indicate that in metabolically active cells, GSH is recovered, so it may act as a protector of cell components against HOCl damage [101].
Other more complex oxidation products involving the intramolecular reaction of the RSCl intermediate with adjacent primary or secondary amines to form sulphonamide bonds have also been detected [102].
Sulphenyl chloride GSCI reacts preferentially with more GSH to give rise to GSSG [103]. In a later work, Pullar et al., 2000, working with endothelial cells, obtained GSCI as a major product of GSH oxidation by HOCl. The corresponding cyclic sulphonamide, observing a very small increase in GSSG and glutathione sulphonic acid, both in the extra- and intracellular medium [104].
Following Winterbourn and Brennan, 1997, with the proposal of sulphonamide formation, Haenen et al., 2014, proposed that glutathione has a total scavenging capacity of four HOCl molecules. It appears that one molecule of GSH is rapidly consumed by the reaction with one molecule of HOCl, but the total HOCl scavenging capacity of GSH amounts to four, i.e., four molecules of HOCl are removed in total by one molecule of GSH. GSH is not only a good biological antioxidant because of its reaction rate with HOCl, but because the products generated are also effective antioxidants, as shown in Figure 38.
Figure 38.
Cyclic amides formed in the reaction of GSH with HOCl.
6.7. Degradation of Amines, N-Monochloro-α-Amino Acids, and Lys
Once formed, N-halo-derivatives can undergo different processes depending on the conditions of the medium: disproportionation, fragmentation, and elimination. Mono- and dichloramines are unstable and are mild oxidisers that can transfer chlorine to other substrates, regenerating the original amine in the process [102,105]. Reactions of amines, such as Lys side chain or amino terminal groups, with HOCl lead to the formation of unstable mono- and dichloramines (with high excesses of HOCl). These products are mild oxidants and can transfer chlorine to other substrates, regenerating the original amine in the process [106].
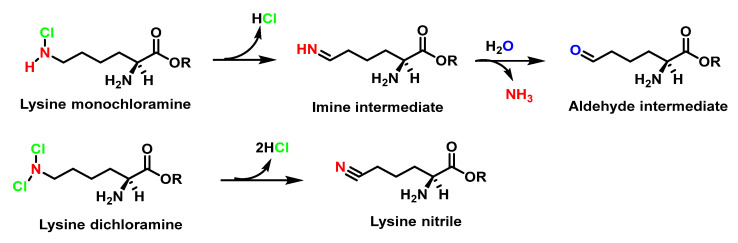
It has also been proposed that Lys-derived chloramines are transformed into aldehydes by the loss of hydrochloric acid and ammonia, as shown in Figure 39.
Figure 39.
Decomposition of lysine monochloramide with formation of aldehyde and nitrile from dichlorinated Lys.
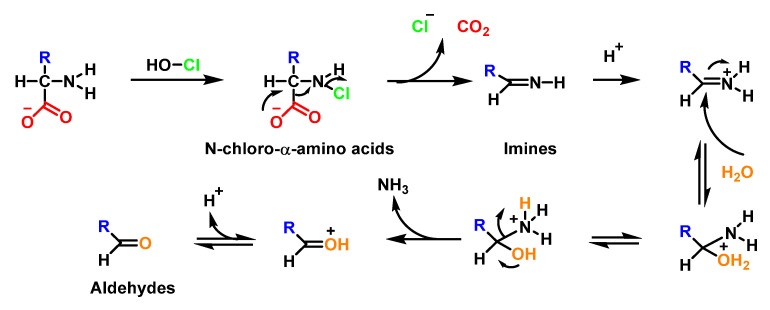
In the absence of oxidisable substrates, the chloramines formed in amino-acids decompose to produce aldehydes and amines under neutral conditions [73]. This is believed to occur through decarboxylation and the loss of chloride from chloramines to form unstable imines which undergo acid hydrolysis to generate aldehydes with the loss of ammonia [107], as shown in Figure 40.
Figure 40.
Degradation mechanisms of N-chloro-α-amino acid anions under neutral conditions.
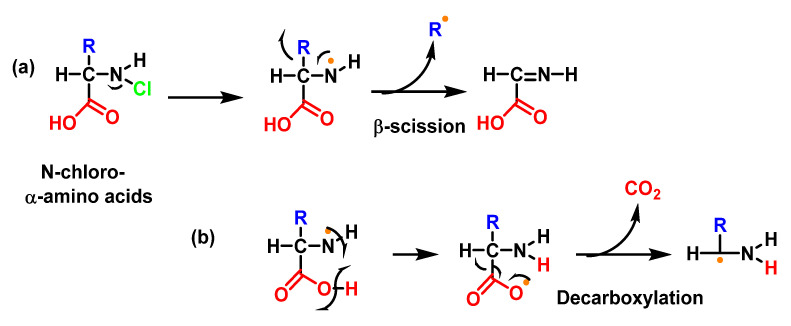
Studies with Lys and other amino acids have revealed that the initial nitrogen-centred radicals can undergo a number of additional rapid rearrangements, e.g., for example, cleavage processes with a loss of the side chain or carboxyl groups, as shown in Figure 41.
Figure 41.
(a) β-fragmentation reaction by homolytic cleavage of the C-C bond in position to an N-centred radical, giving rise to an iminic derivative and a C-radical R; and (b) β-fragmentation by homolytic cleavage with the loss of CO2 and a C-centred radical.
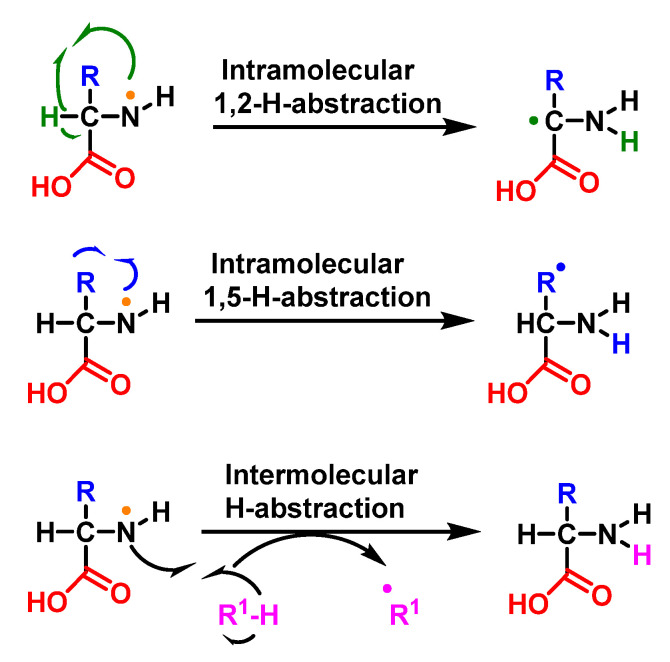
Another reaction is that of the intra- or intermolecular hydrogen abstraction, a 1,2, 1,5 (and sometimes 1,6) transfer of a hydrogen atom from a delta or epsilon carbon to the amine radical or other species capable of readily transferring an H, giving rise to a C-radical, as shown in Figure 42.
Figure 42.
Intra- or intermolecular hydrogen abstraction reactions.
7. Reactions of HOCl with Nucleotides and Nucleic Acids
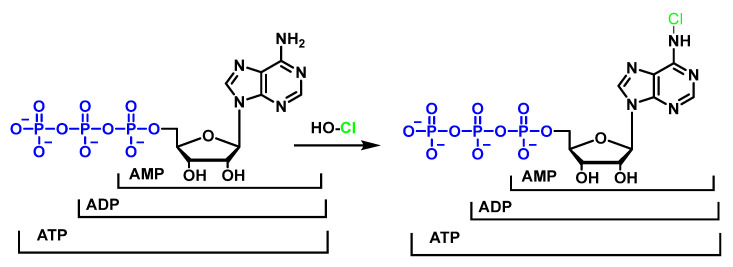
Studies on the reactions of HOCl with nucleotides and nucleic acid bases indicate that the most favoured reactions are with amino groups to give chloramines [108,109]. Investigations of the reactions between HOCl and nucleotides have led to the proposal that HOCl exerts its toxic action by destroying the electron transport chain and the adenosine nucleotide pool AMP/ADP/ATP [110,111], as shown in Figure 43.
Figure 43.
Reaction of AMP/ADP/ATP with HOCl and chlorination at the primary amino group of the adenosine derivative.
HOCl does not react with ribose and therefore the most likely target for HOCl on nucleotides is the nitrogen in the nitrogenous bases. It has been shown that the HOCl-induced chlorination of the –NH2 substituents of nucleotides available on adenosine, cytidine and guanosine is much slower, in neutral solution, than the chlorination of the heterocyclic –NH groups available on guanosine, inosine, thymidine, and uridine. These secondary chloramines, –NCl, are particularly reactive, for example, with GSH, disulphide, NADH, and aliphatic amines, as shown in Figure 44.
Figure 44.
(a) Faster NH-chlorination of thymidine, uridine, inosine, and guanosine; and (b) slower –NH2 chlorination of adenosine, cytidine, and guanosine.
Experiments with nucleoside mixtures show that the propensity for radical formation is cytidine > adenosine = guanosine > uridine = thymidine heterocyclic chloramines are themselves reactive and capable of acting as chlorine donors. Therefore, these products are not suitable biomarkers. However, the C5-chlorination of cytosine has recently been described for deoxycytidine and nucleic acids exposed to myeloperoxidase. 5-chlorocytosine appears to be a stable, albeit minority, end product, particularly at near-neutral pH. However, with suitably sensitive detection methods, 5-chlorocytosine may also prove useful as a biomarker for HOCl.
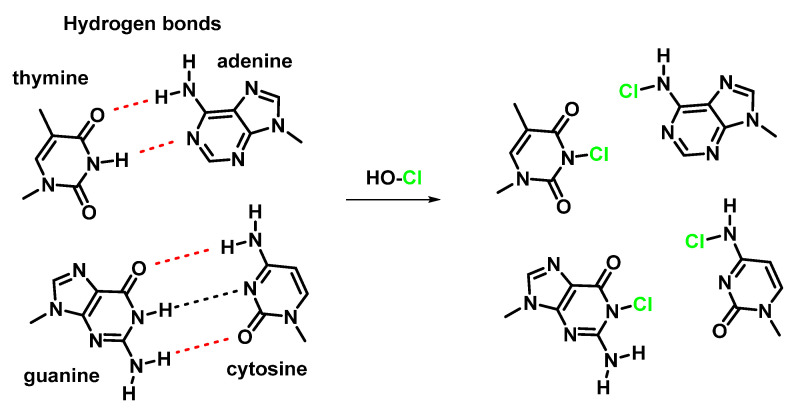
Both amino functions are involved in base pairing, so it is not surprising that HOCl− induced base chlorination leads to efficient DNA denaturation. Another example of this is found in NADH where it is observed that, in the reaction with HOCl, there is a fast primary reaction and a slow secondary reaction, when there is excess HOCl, probably in this case destroying the nicotinamide ring.
The reaction between HOCl with DNA can lead to structural changes, and chemical modification, with the heterocyclic –NH groups R-NHR’ of guanosine and thymidine derivatives more reactive than the exocyclic NH2 groups R-NH2 of guanosine, adenosine, and cytidine derivatives [108,109,112,113]. The reaction of HOCl with these groups results in the formation of semi-stable chloramines R-NHCl and RR′NCl [114,115,116,117], which can lead to the dissociation of the DNA double strand due to the disruption of hydrogen bonds [111] and the formation of nitrogen-centred radicals [118], as shown in Figure 45.
Figure 45.
Formation of chloroamines and the breaking of the hydrogen bond.
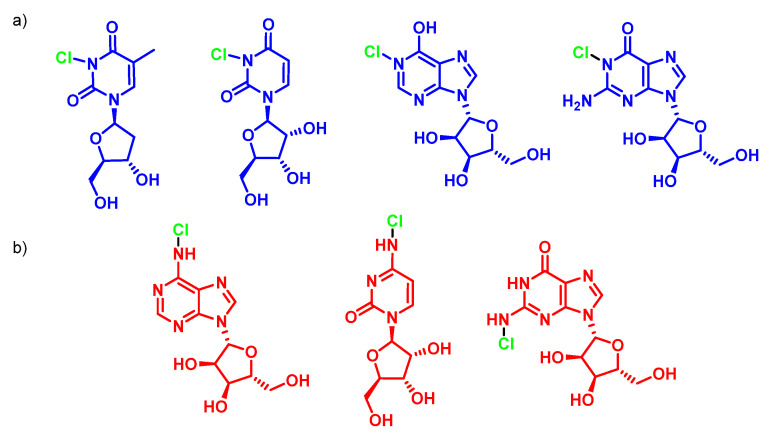
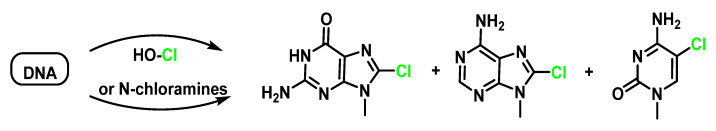
Thymidine is the initial site of chloramine formation, from which there is a rapid transfer of chlorine atoms to the exocyclic NH2 groups of cytidine and adenosine. Chloramine decomposition can be accelerated by ultraviolet light, metal ions, and by thermal decomposition, giving rise to nitrogen-centred radicals derived from nucleosides and DNA. The treatment of DNA with HOCl or N-chloroamines gives 5-chloro-2′-deoxycytidine, 8-chloro-2′-deoxyadenosine and 8-chloro-2′-deoxyguanosine as end products, as shown in Figure 46.
Figure 46.
Formation of 8-chloro-2′-deoxyguanosine, 8-chloro-2′-deoxyadenosine, and 5-chloro-2′-deoxycytidine by the reaction of DNA with HOCl or N-chloramines.
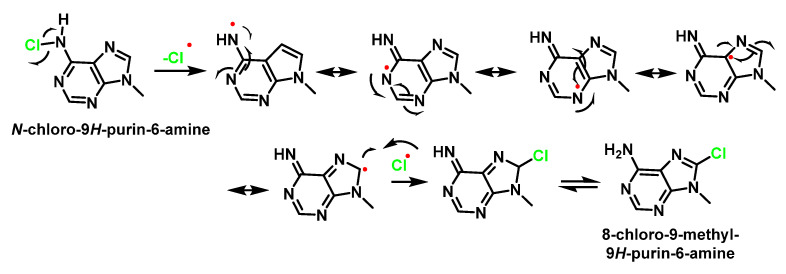
A possible mechanism of formation of 8-chloro-9-methyl-9H-purin-6-amine from the N-chloro-9H-purin-6-amine derivative by homolytic cleavage of the N–Cl bond and the formation of the nitrogen-centred radical is that of it being stabilised by the delocalisation of the odd electron through the double bonds of the two cycles, as shown in Figure 47.
Figure 47.
Possible mechanism of formation of 8-chloro-9-methyl-9H-purin-6-amine from the N-chloro-9H-purin-6-amine derivative and formation of the N-centred radical.
Chlorination of cellular genetic material may play an important role in the development of several inflammatory cancers [35].
8. Reaction of HOCl with Carbohydrates
Neutrophils cause the degradation of proteoglycans, an important component of polysaccharide chains, and this process also involves MPO [119]. The main target for HOCl in carbohydrates is the nitrogen in amino sugars (D-glucosamine, D-galactosamine, D-mannosamine, etc.), in which the hydroxyl group on the 2-C atom is replaced by a primary amino group [120]. The reaction of HOCl with the free NH2 group of the unsubstituted glucosamine proceeds rapidly, usually even faster than with the NH2 group of amino acids. The formation of the dichloro amine only occurs with a large excess of HOCl.
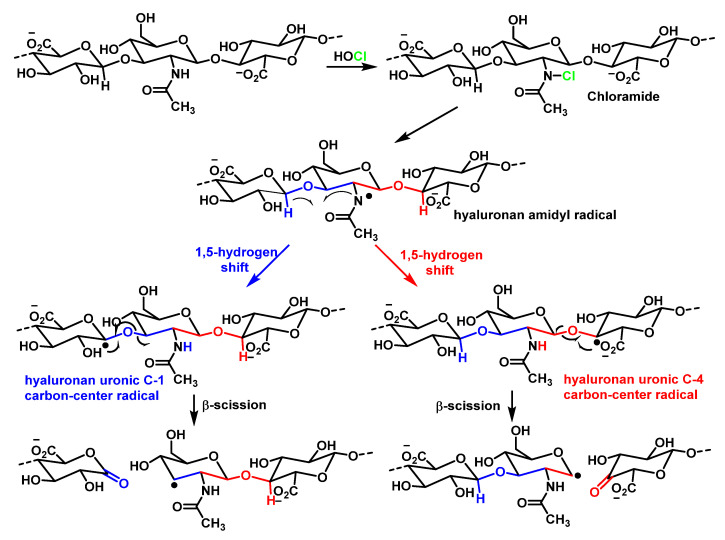
The reaction of HOCl with homopolysaccharides proceeds more slowly than with the corresponding monosaccharides. This can probably be explained by steric inter-action and the influence of negatively charged groups on the polysaccharides. Myeloperoxidase in the presence of H2O2 and physiological levels of chloride ion also generates chloramides from glycosaminoglycans. Chloro-derivatives of amino sugars and their substituted analogues are not stable and decompose gradually with formation of free radical intermediates [121]. One of the mechanisms of decomposition, with the example of hyaluronic acid, is shown in Figure 48.
Figure 48.
Scheme of N-centred amidyl-radical formation and transformation into C-centred radical with the fragmentation of the polysaccharide chain during the reaction of HOCl with the acetylamide-group of hyaluronic acid.
The chloramide, which is formed in the first stage of the reaction with HOCl, is transformed into an N-centred amidyl radical as a result of the homolytic cleavage of N–Cl bonds in the presence of transition metal ions. The N-centred radical undergoes rearrangement and a C-centred radical is formed. This eventually leads to the fragmentation of the polysaccharide chain [122,123].
The main components of the extracellular matrix are proteoglycans, which contain many negatively charged groups on the carbohydrate side. MPO, a polycationic protein secreted by leukocytes into the extracellular space, can bind to the glycosaminoglycans of the proteoglycans by electrostatic interactions. The functioning of the enzyme in the vicinity of proteoglycans, which is accompanied by the production of HOCl, leads to the formation of carbohydrate chloramines and chloramides which decompose by a radical mechanism, as shown in Figure 44, and which is accompanied by the fragmentation of glycosaminoglycans and extracellular matrix in general [120,124].
The degradation of proteoglycans should significantly affect cell adhesion, migration, proliferation, growth, and phenotype. Free radical formation may be the cause of cellular dysfunction and the development of diseases such as atherosclerosis, rheumatoid arthritis, asthma and other diseases in which intensive MPO-dependent oxidant generation (including HOCl) is observed [125,126,127].
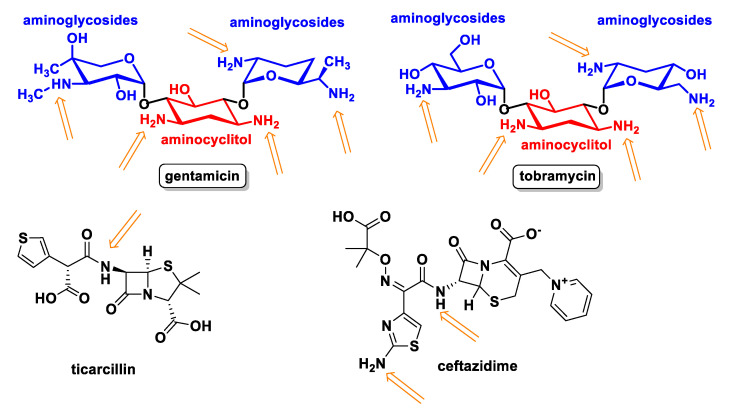
The aminoglycosides gentamicin and tobramycin convert HOCl into non-cytotoxic chloramines and protect lung epithelial cells against oxidative damage by the action of MPO. The aminoglycoside-aminocyclitols antimicrobials constitute one of the families of antibacterial agents with the highest activity on aerobic Gram-negative bacilli. These compounds are formed by the combination of a cyclic amino alcohol (aminocyclitol) and amino sugars (aminoglycosides) linked by glycosidic bonds. Antibiotics such as Ticarcillin (a beta-lactam antibiotic belonging to the carboxypenicillin group, one of the penicillin subgroups) and Ceftazidime (a third-generation cephalosporin), and probably other penicillin and cephalosporins, are potent HOCI scavengers and protect lung epithelial cells, specifically the sulphydryl and methionine groups, from oxidation by HOCl. These antioxidant properties of antibiotics may contribute to tissue protection in patients with cystic fibrosis [128], as shown in Figure 49.
Figure 49.
Arrows indicate possible chlorination sites with HOCl.
9. Role of MPO in Human Diseases and Inflammation
MPO is a marker and mediator of inflammation and oxidative stress. An elevated myeloperoxidase level is associated with increased risk, prevalence, and severity, and predicts a poor prognosis in patients with cardiovascular disease. The most common CVD-related actions of MPO are: (i) generation of dysfunctional atherogenic lipoproteins; (ii) reduced NO availability; (iii) endothelial dysfunction; (iv) impaired vasoreactivity; and (v) the instability of the atherosclerotic plaque. This links the MPO levels to the pathophysiology of CVD. Thus, MPO can be considered a mediator or a tool through which inflammation promotes CVD at the molecular and cellular level. MPO can damage host tissue by generating reactive halogenating and nitrating agents [129].
MPO has a detrimental effect during chronic inflammation, and inflammation is reduced in conditions of MPO deficiency. Indeed, this has been observed in many acute and chronic inflammatory diseases. In cases of inflammatory response to non-infectious stimuli (in the absence of pathogens), several recent studies have shown an increase in the inflammatory process associated with the level of MPO.
Neutrophils and macrophages contribute to myocardial and other tissue injury resulting from post-ischemic reperfusion and inflammation [130]. These actions are mainly due to the production by these cells of HOCl/ClO−, superoxide •O2−, and derivatives of •NO [131,132,133,134].
MPO also has other catalytic activities [2]: (a) it reacts with the superoxide •O2− generating the oxo-myeloperoxidase which, in the presence of H2O2, forms “Compound II”; (b) it complexes •NO and catalyses the production of the •NO2 radical [2,133,134], using the nitrite ion as a substrate; (c) it peroxidises different oxidisable organic molecules, forming the corresponding free radicals [135,136,137]; and (d) it hydroxylates aromatic molecules [138,139].
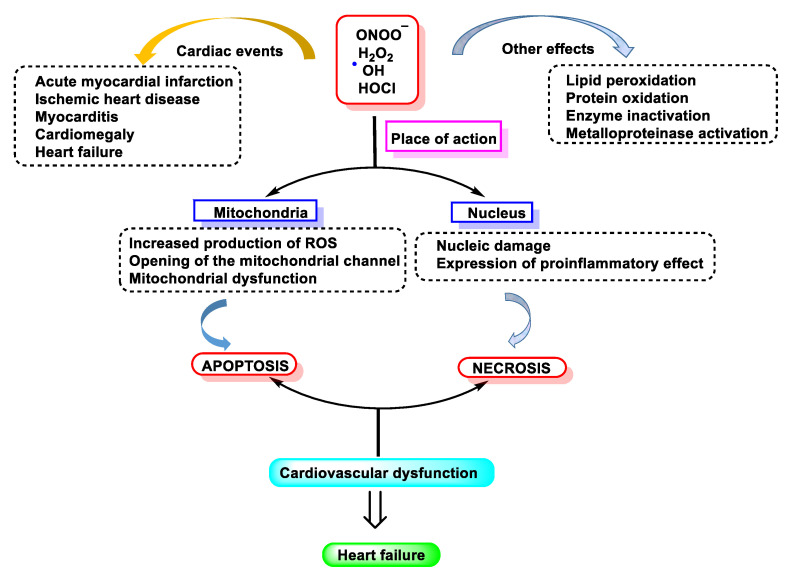
Lipoamide dehydrogenase (LADH) is a mitochondrial enzyme that is very important for energy generation in cardiac muscle. It is inhibited by oxygen free radicals, making it an optimal target for the active species generated by MPO [138,139,140,141]. These species inactivate LADH and thus may contribute to tissue damage by other oxy-radicals (Fenton reaction) as a consequence of post-ischemic reperfusion or inflammation. Compounds with thiol groups, some of which are used therapeutically, counteract the pro-oxidant action of MPO-dependent systems, as shown in Figure 50.
Figure 50.
Free radical-induced damage to the cardiovascular system.
9.1. MPO in Human Diseases
In this section, we selected a number of diseases that are known to be positively related to the MPO enzyme and HOCl. This topic is more extensively reflected in Table 1, specifically in Section 9.3.
MPO is linked to many aspects of human cardiovascular disease and it is believed that this enzyme acts on both the initiation and propagation of cardiac pathologies [142]. The latter findings agree with results of many other studies that support the concept that MPO plays an important role in the pathogenesis of atherosclerosis and cardiovascular disease. Evidence supports the fact that MPO plays a very important role in the pathogenesis of atherosclerosis because it contributes to vascular dysfunction during acute inflammation by modulating the endothelial NO bioavailability [143]. A recent animal model study of ischaemia-related myocardial damage revealed increases in MPO in arrhythmogenic left ventricular remodelling, as manifested by connexin 43 rupture due to MMP-7 activation and increased post-ischaemic ventricular fibrosis [144]. Thus, MPO contributes to vascular dysfunction by virtue of its capacity to generate potent ROS and to promote the activity of matrix metalloproteinase MMPs [145]
The accumulation of LDL-derived cholesterol in the arterial wall triggers atherosclerosis, the main cause of cardiovascular disease. HDL, on the other hand, delays atherosclerosis by promoting cholesterol efflux. It has been proposed that HDL loses its cardioprotective effects in patients suffering from atherosclerosis [146]. One potential pathway involves oxidative damage by MPO; Baohai Shao et al., 2010, demonstrated that HDL from patients with cardiovascular disease contains high levels of 3-chlorotyrosine and 3-nitrotyrosine, two characteristic products of MPO [46].
MPO and HOCl− modified proteins have been detected in diseased renal tissue. Mollenhauer et al., 2017, observed a significant reduction in renal function loss after the reperfusion of chemically damaged kidneys in MPO-KO mice compared to WT mice, demonstrating a contribution of MPO in the induction of organ damage after renal ischaemia-reperfusion by influencing critical factors such as neutrophil extravasation [144].
Chronic inflammation plays a key role in tumour promotion in lung cancer. Amy L. Rymaszewski et al., 2014, in mouse studies demonstrated that neutrophils are critical mediators of tumour promotion in methylcholanthrene (MCA)-initiated and butylated hydroxytoluene (BHT)-promoted lung carcinogenesis and subsequently, they investigated the role of neutrophil MPO activity in the inflammation-promoted model, observing increased protein levels and MPO activity in the lungs of mice-administered BHT. An MPO inhibitor reduced tumour burden.
Rotem Volkman et al., 2019, reviewed the body of evidence linking neutrophil-derived MPO in the pathogenesis of Alzheimer’s disease (AD), verifying this role in an animal model. They reproduced haematological chimerism in the 5XFAD mouse model of AD with MPO-deficient mice, resulting in 5XFAD with haematological MPO deficiency (5XFAD-MPO KO). Behavioural examinations of 5XFAD-MPO KO mice showed a significantly superior performance in spatial learning and memory, associative learning and anxiety/risk assessment behaviour compared to 5XFAD mice transplanted with WT cells (5XFAD-WT). Immunohistochemical and hippocampal mRNA expression analyses showed significantly reduced levels of inflammatory mediators in 5XFAD-MPO KO mice, with no apparent difference in the number of amyloid-β plaques. In addition, immunoblotting and mRNA analyses showed significantly reduced levels of APOE in 5XFAD-MPO KO mice.
Taken together, their analyses indicate a substantial involvement of neutrophil-derived MPO in the pathogenesis of the 5XFAD model of AD and suggest that MPO is a potential therapeutic target in AD.
In recent years, a significant amount of evidence has implicated a role of MPO in the pathogenesis of atherosclerosis. MPO is an enzymatic source of eicosanoids and bioactive lipids and generates atherogenic forms of low- and high-density lipoproteins. These factors demonstrate that increased systemic levels of MPO and its oxidation products predict increased cardiovascular risk. Consequently, interest has focused on the potential of MPO for the development of new mechanisms to analyse its presence as risk markers, as well as therapies to prevent cardiovascular events. Rachel J. Roth Flach et al., 2019, examined the role of MPO inhibitors in the treatment of heart failure and acute coronary syndrome in humans. The results obtained in their study suggest that MPO inhibition does not alter the atherosclerotic plaque area or leukocyte uptake, but rather alters the inflammatory tone of atherosclerotic lesions; therefore, MPO inhibition has utility in promoting atherosclerotic lesion stabilisation and preventing atherosclerotic plaque rupture [147].
9.2. MPO and Organ Inflammation
MPO can damage the host tissue by generating reactive halogenating and nitrating agents. Lower levels of 3-chlorotyrosine, 3-bromotyrosine, 3-nitrotyrosine, and protein carbamylation are observed at sites of inflammation in MPO-KO mice compared to WT mice. Therefore, since MPO has a detrimental effect during chronic inflammation, it is to be expected that inflammation is reduced under conditions of MPO deficiency. Indeed, this has been observed in many acute and chronic inflammatory diseases [148].
Recent observations extend this perspective and deeply implicate MPO in the regulation of cellular homeostasis, playing a central role in the initiation and propagation of acute and chronic vascular inflammatory disease. Thus, low levels of HOCl interfere with intracellular signalling, as MPO-dependent lipoprotein oxidation modulates its affinity for macrophages and the vascular wall. Simultaneously, MPO-mediated endothelial NO depletion impairs vasodilation and nitrotyrosine (NO2Tyr) formation in the vascular wall may affect the structure and function of matrix proteins [149].
The development of imaging techniques to accurately identify MPO localisation and molecular targets of HOCl in vivo is an important advance. Until recently, the involvement of MPO in inflammatory disease has been inferred by its presence, together with the detection of HOCl biomarkers, in biological fluids or diseased tissues. These results provide valuable information regarding the cell types responsible for MPO release in vivo, along with new insight into potential therapeutic opportunities [16].
9.3. Summary Table of the Relationship between MPO and Different Diseases
As follows, Table 2 summarises the relationship between MPO and disease (including recent research findings), citing diseases and references.
Table 2.
Occurrence of MPO in various diseases.
| Disease Classification | Disease and References |
|---|---|
| Autoimmune Disease | Inflammatory bowel disease/colitis [150,151,152] Rheumatoid arthritis [153,154] Systemic lupus erythematosus [155,156,157] |
| Neuronal Pathology | Alzheimer’s disease [158,159,160] Multiple sclerosis [161,162,163] Neurodegenerative disease [164,165,166] Parkinson’s disease [167,168] Stroke [169,170,171] |
| Cardiovascular Pathology | Atrial fibrillation [172,173] Cardiovascular disease/atherosclerosis [174,175,176] Hypertension [177,178,179] Myocardial infarction [180,181,182] Vascular dysfunction [183,184,185] Asthma [186,187,188] |
| Pulmonary Pathology | Chronic obstructive pulmonary disease [189,190,191] Cystic fibrosis [192,193,194] |
| Miscellaneous | Ageing [195,196,197] Cancer [198,199,200] Chronic kidney disease [201,202,203] Inflammation [16,129,204] Lipoprotein modification [151,182,183] Metabolic syndrome/obesity [205,206,207] Type 2 diabetes [207,208,209] |
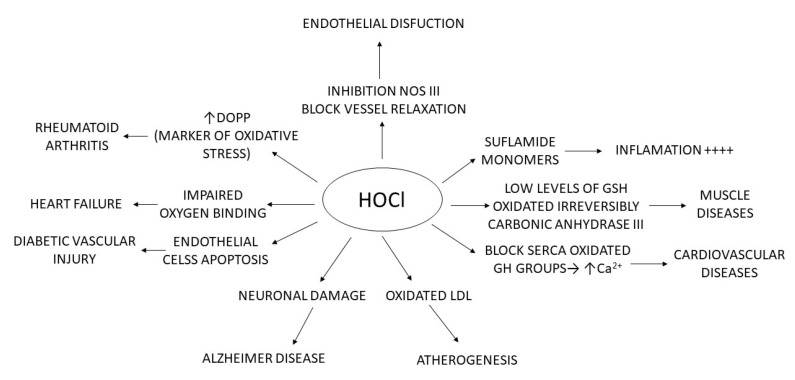
Figure 51, below, summarises what has been explained throughout the manuscript: chlorinated species have mainly been related to pathologies where the cause is linked to molecular alterations and their effect on inflammatory processes, tissue damage, damage to genetic material, apoptosis, etc.
Figure 51.
Relationship of HOCl with different pathologies.
9.4. Myeloperoxidase as a Disease Biomarker
The antibacterial activity of MPO, through the production of HOCl and its controlled release at the site of infection, is of vital importance for its activity to be effective. Its uncontrolled expression overstates inflammation and can lead to tissue damage, even if inflammation does not occur. Several tissue lesions and the pathogenesis of diseases such as rheumatoid arthritis, cardiovascular and liver diseases, diabetes and cancer are linked to MPO-derived HOCl. Therefore, increased MPO activity is a good diagnostic tool for biomarkers of inflammation and oxidative stress [6].
Coordination between several biochemical pathways, including neutrophil activation, •O2− production by NADPH oxidase, and MPO release by exocytosis, leads to clearance of bacterial infection [57], as invading bacteria induce increased H2O2 production by the enzyme superoxide dismutase SOD, whereby MPO produces HOCl. Both products, H2O2 and HOCl, are particularly toxic to invading bacteria. This biochemical phenomenon is called the respiratory burst [210]. When bacterial infection occurs, one of the important mediators of this cascade is formylated peptide, which also acts as a chemoattractant, activating neutrophils via the formylated peptide receptor fPR, a G protein-coupled receptor [211]. The release of H2O2 oxidises various substrates, such as halides (Cl−, Br−), and pseudohalides (thiocyanate SCN—) [8,212]. These oxidant species, under normal physiological circumstances, are toxic to several micro-organisms and play an important role in the immune system. Their excessive or deregulated production of oxidants can cause damage to host cells and lead to various diseases [213,214]. The polycationic character of MPO allows it to bind to negatively charged surfaces of pathogens and causes the destruction of their cell membrane, inducing lysis of the bacteria. This enzyme can also bind to other cell surfaces, such as epithelial cells, macrophages, fibroblasts, endothelial cells, platelets, neutrophils, low-density lipoproteins (LDL), and very low-density lipoproteins (VLDL) [215,216].
9.5. Measurement of MPO Activity
Currently, there is considerable interest in developing “biomarkers” of MPO activity that can be applied to humans. These involve measuring the end products of oxidative damage in different classes of biomolecules or directly determining the production of HOCl. Different types of equipment are currently available on the market. The most common method of measuring MPO is through enzyme-linked immunosorbent assay ELISA kits. Among the different devices, the following are worth mentioning:
Arigo Biolaboratories Corporation (Hsinchu City, Taiwan), developed a MPO/Myeloperoxidase Activity Assay Kit (Colorimetric) that can be used to measure myeloperoxidase activity in whole neutrophils, neutrophil lysates, tissue homogenates, and plasma (EDTA). This colorimetric device bases its principle of operation on the fact that HOCl rapidly reacts with taurine to produce a stable taurine chloramine product. This step neutralises HOCl, which would otherwise accumulate and inactivate MPO. A stop solution containing catalase is added to stop the catalysis of MPO, removing the hydrogen peroxide. Finally, taurine chloramine reacts with the yellow chromogenic probe TNB, with a decrease in colour indicating increased MPO activity. The concentration of MPO in the samples is then determined by comparing the absorbance of the samples at 405–412 nm with the standard curve.
Abcam, at the Cambridge Biomedical Campus, Cambridge, UK, markets a colorimetric kit with similar characteristics to that described above, notably that it can be used to detect MPO as low as 0.05 mU per well.
Cayman Chemical Company, in Ann Arbor, MI, USA, markets a complete assay for the isolation of neutrophils and the measurement of MPO activity that analyses the release of MPO by activated phagocytes and uses TMB as a chromogenic substrate for MPO, including a specific inhibitor of MPO function to verify specificity. This also includes the reagents necessary to isolate neutrophils from human whole blood.
Celltechgen Laboratory, in Houston, TX, USA, is a state-of-the-art medical testing laboratory service that provides a complete range of tests for the diagnosis, screening, or evaluation of diseases and health conditions and markets an MPO Peroxidation Activity Assay Kit, suitable for use as a high-throughput MPO activity assay. The assay kit oxidises a substrate to generate fluorescence (Ex/Em = 571/585 nm), directly proportional to total peroxidase activity in the sample. The assay is high-throughput adaptable and can detect less than 2 µU of MPO activity. This kit can be used to detect MPO activity as low as 0.5 µU per well.
9.6. Inhibitors of MPO
The microbicidal activity of the MPO enzyme is due to its ability to oxidise halide or pseudohalide ions (X = Cl−, Br−, I− and SCN−) producing the respective HOX acids. During the phagocytosis of pathogens, MPO is released by azurophilic granules into phagolysosomes but can also be discharged outside phagocytes. Tissue damage that occurs during inflammation is largely due to MPO-derived oxidants. As referenced in previous chapters, this enzyme is a key factor in several conditions, including cardiovascular, inflammatory, neurodegenerative, renal, immune-mediated, and neurodegenerative diseases. Therefore, MPO is an attractive target for therapeutic intervention in the prophylaxis of the aforementioned disorders. The only negative effect that can be expected from MPO inhibitors is a decrease in neutrophil activity against pathogens. MPO is an immunological enzyme that acts in neutrophils. However, MPO is located in azurophil granules, which protect this enzyme from changes in the extracellular environment. Therefore, it is believed that the effect of inhibitors on MPO activity can be easily attenuated by exclusively focusing on extracellular enzymes that are not critical for pathogen eradication but rather are involved in host damage.
Relatively polar MPO inhibitors cannot penetrate neutrophils and are thought to inhibit extracellular enzymes exclusively. The structure and reaction mechanism of MPO is known, allowing a rational strategy for the development of specific inhibitors, with the intention of preserving its activity against bacteria, but hindering its pathophysiological persistent activation during the course of the diseases [217].
There are three approaches to discover and develop such inhibitors:
The first approach is to prepare tiny compounds with a reduction potential of 0.97 V E°′ (A-/AH) 1.35 V. MPO compound I can easily oxidise these molecules, leading to the inactive state MPO compound II. At the same time, they cannot degrade the MPO compound II and cause this inactive form of MPO to build up. The main problem with these inhibitors is that when they are used in living organisms, several biomolecules can act as substrates for MPO and degrade the MPO compound II to restore the original enzyme. As a result, many inhibitors lose their efficacy in vivo.
The second approach focuses on tiny molecules that bind with high affinity to the active site of MPO. These chemicals are designed to strongly interact with the active site residues of the enzyme. The key amino acid that forms a salt bridge or hydrogen bond with the inhibitor is Glu102. In addition, to convert the MPO compound I into the MPO compound II, the inhibitor must have a reduction potential E°′ (A-/AH) of less than 1.35 V. When this type of molecule induces the inactive state of the enzyme, it maintains its contacts with the active site of MPO and prevents additional substrates from entering the active site, leading to the accumulation of MPO compound II by competitive inhibition. Several effective MPO inhibitors, such as aminoalkyl-indole compounds and aryl hydroxamic acid derivatives [47,48,49,50], were prepared using this strategy.
The third approach is to prepare tiny molecules that form covalent bonds after oxidation by MPO and have a relatively high affinity for the enzyme. These compounds are irreversible MPO inhibitors that act by degrading the heme group. Inhibitors that rely on overriding this mechanism are irreversible and form a strong covalent bond with the Fe of the haem centre, blocking the access of H2O2 to the active site, and thus inactivating the enzyme. The second mechanism is based on inducing competition between the inhibitor compound and the enzyme substrate. In this case, the inhibitor either forms a complex with MPO preventing further cycling or acts as a substrate for MPO by forming and accumulating compound II. An alternative approach based on the design of HOCl scavenging compounds can be considered in order to avoid the induced oxidative damage. However, this mechanism will not prevent the peroxidation cycle and the formation of superoxide and hydroxyl radical, which are involved in oxidative stress and tissue injury. Due to the complexity of the MPO catalytic mechanism, the search for an effective inhibitor is still under development [218].
Lifestyle factors are important drivers of chronic diseases such as cardiovascular syndrome, with inflammation being a key factor. MPO is an inflammatory enzyme associated with obesity, hypertension, and heart failure, so its attenuation could have protective effects on multiple organs. Arnold Piek et al., 2019, tested the effects of a novel oral MPO inhibitor AZM198 in an obese/hypertensive mouse model with a cardiac phenotype Treated animals showed therapeutic AZM198 levels of 2.1 µM, corresponding to a 95% inhibition of MPO. AZM198 reduced elevated circulating MPO levels in HFD/AngII mice to normal values. Independently of food intake, body weight gain and fat accumulation were attenuated, along with reduction in visceral adipose tissue (VAT) inflammation and the attenuation of the severity of non-alcoholic steatohepatitis [219].
Most peroxidase enzymes are inhibited by benzoic acid hydrazide (BAH)-containing compounds, but the inhibition mechanism by BAH compounds is unknown. Jiansheng Huang et al., 2015, reported that the MPO inhibition by BAH and 4-(trifluoromethyl)-BAH due to hydrolysis of the ester bond between the MPO heavy chain glutamate 242 ((HC)Glu(242)) residue and the heme pyrrole A ring. In their manuscript, they provide evidence that the destruction of the heme ring does not occur by heme prosthetic group tracking and provides indications that the mechanism of hydrolysis follows a potential attack of the carbonyl of (HC)Glu(242), leading to a rearrangement that causes the release of the vinyl-sulphonium bond between (HC)Met(243) and the pyrrole A-ring [220].
Clinical studies have been only conducted on AZD5904, AZD3241, AZD4831, and PF06282999. The first three chemicals are thioxanthine derivatives, while the fourth is a thiopyrimidinone. The common mechanism of action of these drugs is a thioether bridge between the heme group of the enzyme and the oxidised thioxantine or thiopyrimidinone. Thioxanthins are compounds known as MPO inhibitors and derivatives with the 2-thioxanthin group show the highest activity. Thioxanthine is oxidised by compound I, forming a highly reactive free radical. This radical readily transfers electrons to the heme group of compound II and forms a covalent bond across the sulphur atom to one of the heme’s pyrrole rings [221].
Several naturally occurring compounds possess inhibitory activities against MPO, including polyphenols, melatonin, and flavonoids. Seeds and aerial parts of Peganum harmala L. are widely used in Algeria as anti-inflammatory remedies. Sihem Bensalem et al. evaluated in their study the alkaloids and pure β-carboline compounds present, as well as their possible anti-inflammatory and MPO inhibitory action, concluding that the total alkaloids from seeds and aerial parts strongly inhibited MPO at 20 µg/mL (97 ± 5% and 43 ± 4%, respectively) while, at the same concentration, those from the roots showed very low inhibition (15 ± 6%) [222].
On the other hand, ceruloplasmin is a plasma protein produced by activated hepatocytes and macrophages and is involved in the physiological clearance and inactivation of MPO [223]. In addition to these, some naturally occurring compounds, such as polyphenols, with pronounced antioxidant and anti-inflammatory characteristics, have inhibitory activities against MPO. These compounds include ferulic acid, caffeic acid, resveratrol, chalcones, and gallic acid. [224,225]. Yuko Shiba et al., 2008, examined the MPO inhibitory effects of dietary flavonoids, using a combination of biological assays and theoretical computational studies. Quercetin and the plasma metabolites inhibited the formation of dityrosine catalysed by the MPO enzyme and HL-60 cells in a dose-dependent manner [226].
10. Conclusions
HOCl is the most important reactive form of chlorine formed in living organisms in the MPO halogenation cycle. HOCl biologically falls into a group of small molecules known as reactive oxygen species (ROS) synthesised by the cells of the immune system (neutrophils and macrophages). HOCl-dependent reactions play a dual role: on the one hand, it performs a bactericidal function against infections, and on the other hand, it can cause damage to supramolecular structures and cells of the host organism. In this review, HOCl is presented as a powerful antimicrobial oxidant, capable of modifying DNA, lipids, and lipoproteins, rapidly reacting with nucleophiles such as the sulphur atom present in thiols and thioethers (cysteine and methionine). Modification of amines and amides by reaction with HOCl generate chloramines and chloramides, respectively, in virtually all organic biological compounds, which could have serious cellular implications. It is also important to note that the oxidation of tyrosine residues by HOCl leads to the formation of 3-chlorotyrosine, which is considered a marker of MPO activity and halogenative stress.
Author Contributions
Conceptualization, C.M.C.A. and C.A.J.; investigation, C.M.C.A. and C.A.J.; writing—original draft preparation, C.A.J. and E.P.-L.; writing—review and editing, C.M.C.A., C.A.J., J.M.P.d.l.L., E.P.-L. and F.J.P.; supervision, C.M.C.A., C.A.J. and J.M.P.d.l.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was funded by project “Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI) del Gobierno de Canarias”, project ProID2020010134 and Spanish Ministry of Economy and Competitiveness (Grant PID2019-105838RB-C31).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.da Cruz Nizer W.S., Inkovskiy V., Overhage J. Surviving reactive chlorine stress: Responses of gram-negative bacteria to hypochlorous acid. Microorganisms. 2020;8:1220. doi: 10.3390/microorganisms8081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kettle A., Winterbourn C. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997;3:3–15. doi: 10.1080/13510002.1997.11747085. [DOI] [PubMed] [Google Scholar]
- 3.Juan C.A., Pérez de la Lastra J.M., Plou F.J., Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021;22:4642. doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sies H. Oxidative stress: Concept and some practical aspects. Antioxidants. 2020;9:852. doi: 10.3390/antiox9090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malle E., Furtmüller P., Sattler W., Obinger C. Myeloperoxidase: A target for new drug development? Br. J. Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan A.A., Alsahli M.A., Rahmani A.H. Myeloperoxidase as an active disease biomarker: Recent biochemical and pathological perspectives. Med. Sci. 2018;6:33. doi: 10.3390/medsci6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurst J.K. What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 2012;53:508–520. doi: 10.1016/j.freeradbiomed.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2010;48:8–19. doi: 10.3164/jcbn.11-006FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankar S., Mahadevan A., Satishchandra P., Uday Kumar R., Yasha T., Santosh V., Chandramuki A., Ravi V., Nath A. Neuropathology of HIV/AIDS with an overview of the Indian scene. Indian J. Med. Res. 2005;121:468–488. [PubMed] [Google Scholar]
- 10.Hampton M.B., Kettle A.J., Winterbourn C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood J. Am. Soc. Hematol. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 11.Hawkins C.L., Morgan P.E., Davies M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009;46:965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Lane A.E., Tan J.T., Hawkins C.L., Heather A.K., Davies M.J. The myeloperoxidase-derived oxidant HOSCN inhibits protein tyrosine phosphatases and modulates cell signalling via the mitogen-activated protein kinase (MAPK) pathway in macrophages. Biochem. J. 2010;430:161–169. doi: 10.1042/BJ20100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos D.R., García M.V., Canle M., Santaballa J.A., Furtmüller P.G., Obinger C. Myeloperoxidase-catalyzed chlorination: The quest for the active species. J. Inorg. Biochem. 2008;102:1300–1311. doi: 10.1016/j.jinorgbio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Winterbourn C.C., Hampton M.B., Livesey J.H., Kettle A.J. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: Implications for microbial killing. J. Biol. Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 15.Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Davies M.J., Hawkins C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020;32:957–981. doi: 10.1089/ars.2020.8030. [DOI] [PubMed] [Google Scholar]
- 17.Leung A.M., LaMar A., He X., Braverman L.E., Pearce E.N. Iodine status and thyroid function of Boston-area vegetarians and vegans. J. Clin. Endocrinol. Metab. 2011;96:E1303–E1307. doi: 10.1210/jc.2011-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senthilmohan R., Kettle A.J. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch. Biochem. Biophys. 2006;445:235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 19.van Dalen C.J., Whitehouse M.W., Winterbourn C.C., Kettle A.J. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997;327:487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zámocký M., Hofbauer S., Schaffner I., Gasselhuber B., Nicolussi A., Soudi M., Pirker K.F., Furtmüller P.G., Obinger C. Independent evolution of four heme peroxidase superfamilies. Arch Biochem. Biophys. 2015;574:108–119. doi: 10.1016/j.abb.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Love D.T., Barrett T.J., White M.Y., Cordwell S.J., Davies M.J., Hawkins C.L. Cellular targets of the myeloperoxidase-derived oxidant hypothiocyanous acid (HOSCN) and its role in the inhibition of glycolysis in macrophages. Free Radic. Biol. Med. 2016;94:88–98. doi: 10.1016/j.freeradbiomed.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Dunford H.B., Araiso T., Job D., Ricard J., Rutter R., Hager L.P., Wever R., Kast W.M., Boelens R., Ellfolk N., et al. Peroxidases. Springer; Dordrecht, The Netherlands: 1982. pp. 337–355. [Google Scholar]
- 23.McKenna S.M., Davies K.J. The inhibition of bacterial growth by hypochlorous acid. Possible role in the bactericidal activity of phagocytes. Biochem. J. 1988;254:685–692. doi: 10.1042/bj2540685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storkey C., Davies M.J., Pattison D.I. Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach. Free Radic. Biol. Med. 2014;73:60–66. doi: 10.1016/j.freeradbiomed.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Schuller-Levis G.B., Park E. Taurine and its chloramine: Modulators of immunity. Neurochem. Res. 2004;29:117–126. doi: 10.1023/B:NERE.0000010440.37629.17. [DOI] [PubMed] [Google Scholar]
- 26.Roh J.S., Sohn D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018;18:e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouellette R.J., Rawn J.D. Organic Chemistry Study Guide: Key Concepts, Problems, and Solutions. Elsevier; Amsterdam, The Netherlands: 2015. [Google Scholar]
- 28.Winterbourn C.C. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology. 2002;181:223–227. doi: 10.1016/S0300-483X(02)00286-X. [DOI] [PubMed] [Google Scholar]
- 29.Morris J.C. The acid ionization constant of HOCl from 5 to 35. J. Phys. Chem. 1966;70:3798–3805. doi: 10.1021/j100884a007. [DOI] [Google Scholar]
- 30.Wang L., Bassiri M., Najafi R., Najafi K., Yang J., Khosrovi B., Hwong W., Barati E., Belisle B., Celeri C. Hypochlorous acid as a potential wound care agent: Part I. Stabilized hypochlorous acid: A component of the inorganic armamentarium of innate immunity. J. Burn. Wounds. 2007;6:e5. [PMC free article] [PubMed] [Google Scholar]
- 31.Mütze S., Hebling U., Stremmel W., Wang J., Arnhold J.r., Pantopoulos K., Mueller S. Myeloperoxidase-derived hypochlorous acid antagonizes the oxidative stress-mediated activation of iron regulatory protein 1. J. Biol. Chem. 2003;278:40542–40549. doi: 10.1074/jbc.M307159200. [DOI] [PubMed] [Google Scholar]
- 32.Weiss S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 33.White G.C. The Handbook of Chlorination and Alternative Disinfectant. Van Nostrand Reinhold International; London, UK: 1992. [Google Scholar]
- 34.Ward J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- 35.Stanley N.R., Pattison D.I., Hawkins C.L. Ability of hypochlorous acid and N-chloramines to chlorinate DNA and its constituents. Chem. Res. Toxicol. 2010;23:1293–1302. doi: 10.1021/tx100188b. [DOI] [PubMed] [Google Scholar]
- 36.Del Rosso J.Q., Bhatia N. Status report on topical hypochlorous acid: Clinical relevance of specific formulations, potential modes of action, and study outcomes. J. Clin. Aesthetic Dermatol. 2018;11:36. [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Q., Lee K.A., Lee S., Lee K.M., Lee W.J., Yoon J. A highly specific fluorescent probe for hypochlorous acid and its application in imaging microbe-induced HOCl production. J. Am. Chem. Soc. 2013;135:9944–9949. doi: 10.1021/ja404649m. [DOI] [PubMed] [Google Scholar]
- 38.Ford D.A. Lipid oxidation by hypochlorous acid: Chlorinated lipids in atherosclerosis and myocardial ischemia. Clin. Lipidol. 2010;5:835–852. doi: 10.2217/clp.10.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winterbourn C.C., Kettle A.J. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 2000;29:403–409. doi: 10.1016/S0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 40.Schröter J., Schiller J. Chlorinated phospholipids and fatty acids:(patho) physiological relevance, potential toxicity, and analysis of lipid chlorohydrins. Oxidative Med. Cell. Longev. 2016;2016:8386362. doi: 10.1155/2016/8386362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnhold J., Osipov A.N., Spalteholz H., Panasenko O.M., Schiller J. Formation of lysophospholipids from unsaturated phosphatidylcholines under the influence of hypochlorous acid. Biochim. Biophys. Acta BBA Gen. Subj. 2002;1572:91–100. doi: 10.1016/S0304-4165(02)00271-4. [DOI] [PubMed] [Google Scholar]
- 42.Winterbourn C.C., van den Berg J.J., Roitman E., Kuypers F.A. Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch. Biochem. Biophys. 1992;296:547–555. doi: 10.1016/0003-9861(92)90609-Z. [DOI] [PubMed] [Google Scholar]
- 43.Heinecke J.W., Li W., Mueller D.M., Bohrer A., Turk J. Cholesterol chlorohydrin synthesis by the myeloperoxidase-hydrogen peroxide-chloride system: Potential markers for lipoproteins oxidatively damaged by phagocytes. Biochemistry. 1994;33:10127–10136. doi: 10.1021/bi00199a041. [DOI] [PubMed] [Google Scholar]
- 44.Carr A.C., van den Berg J.J., Winterbourn C.C. Chlorination of cholesterol in cell membranes by hypochlorous acid. Arch. Biochem. Biophys. 1996;332:63–69. doi: 10.1006/abbi.1996.0317. [DOI] [PubMed] [Google Scholar]
- 45.Hazen S.L., Hsu F.F., Duffin K., Heinecke J.W. Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. J. Biol. Chem. 1996;271:23080–23088. doi: 10.1074/jbc.271.38.23080. [DOI] [PubMed] [Google Scholar]
- 46.Shao B., Oda M.N., Oram J.F., Heinecke J.W. Myeloperoxidase: An oxidative pathway for generating dysfunctional high-density lipoprotein. Chem. Res. Toxicol. 2010;23:447–454. doi: 10.1021/tx9003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panasenko O., Torkhovskaya T., Gorudko I., Sokolov A. The role of halogenative stress in atherogenic modification of low-density lipoproteins. Biochemistry. 2020;85:34–55. doi: 10.1134/S0006297920140035. [DOI] [PubMed] [Google Scholar]
- 48.Spickett C.M., Jerlich A., Panasenko O.M., Arnhold J., Pitt A.R., Stelmaszyńska T., Schaur R.J. The reactions of hypochlorous acid, the reactive oxygen species produced by myeloperoxidase, with lipids. Acta Biochim. Pol. 2000;47:889–899. doi: 10.18388/abp.2000_3944. [DOI] [PubMed] [Google Scholar]
- 49.Braverman N.E., Moser A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Yang P., Subbaiah P.V. Regulation of hepatic lipase activity by sphingomyelin in plasma lipoproteins. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids. 2015;1851:1327–1336. doi: 10.1016/j.bbalip.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nusshold C., Kollroser M., Köfeler H., Rechberger G., Reicher H., Üllen A., Bernhart E., Waltl S., Kratzer I., Hermetter A. Hypochlorite modification of sphingomyelin generates chlorinated lipid species that induce apoptosis and proteome alterations in dopaminergic PC12 neurons in vitro. Free Radic. Biol. Med. 2010;48:1588–1600. doi: 10.1016/j.freeradbiomed.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spalteholz H., Wenske K., Arnhold J. Interaction of hypohalous acids and heme peroxidases with unsaturated phosphatidylcholines. Biofactors. 2005;24:67–76. doi: 10.1002/biof.5520240108. [DOI] [PubMed] [Google Scholar]
- 53.Greig F.H., Kennedy S., Spickett C.M. Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation. Free Radic. Biol. Med. 2012;52:266–280. doi: 10.1016/j.freeradbiomed.2011.10.481. [DOI] [PubMed] [Google Scholar]
- 54.Kanno K., Wu M.K., Scapa E.F., Roderick S.L., Cohen D.E. Structure and function of phosphatidylcholine transfer protein (PC-TP)/StarD2. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids. 2007;1771:654–662. doi: 10.1016/j.bbalip.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawai Y., Kiyokawa H., Kimura Y., Kato Y., Tsuchiya K., Terao J. Hypochlorous acid-derived modification of phospholipids: Characterization of aminophospholipids as regulatory molecules for lipid peroxidation. Biochemistry. 2006;45:14201–14211. doi: 10.1021/bi0610909. [DOI] [PubMed] [Google Scholar]
- 56.Villamena F.A. Reactive Species Detection in Biology. Elsevier; Amsterdam, The Netherlands: 2017. [Google Scholar]
- 57.Nauseef W.M. Myeloperoxidase in human neutrophil host defence. Cell. Microbiol. 2014;16:1146–1155. doi: 10.1111/cmi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas E.L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: Nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect. Immun. 1979;23:522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corbett R., Metcalf W., Soper F. 395. Studies of N-halogeno-compounds. Part IV. The reaction between ammonia and chlorine in aqueous solution, and the hydrolysis constants of chloroamines. J. Chem. Soc. Resumed. 1953:1927–1929. doi: 10.1039/jr9530001927. [DOI] [Google Scholar]
- 60.Chapin R.M. Dichloro-amine. J. Am. Chem. Soc. 1929;51:2112–2117. doi: 10.1021/ja01382a018. [DOI] [Google Scholar]
- 61.Abia L., Armesto X., Canle M., Garcia M., Santaballa J. Oxidation of aliphatic amines by aqueous chlorine. Tetrahedron. 1998;54:521–530. doi: 10.1016/S0040-4020(97)10312-X. [DOI] [Google Scholar]
- 62.Armesto X., García M., Santaballa J. Aqueous chemistry of N-halo-compounds. Chem. Soc. Rev. 1998;27:453–460. doi: 10.1039/a827453z. [DOI] [Google Scholar]
- 63.Fernández-Pérez M.-I. Ph.D. Thesis. University of Coruña; A Coruña, Spain: 2002. [(accessed on 4 August 2022)]. Mecanismos de Oxidación y Fotooxidación de Compuestos de Relevancia Bioquímica y Medioambiental: 1. Halogenación de Aminoácidos Azufrados. 2. Fotodegradación de Biocidas Tipo S. Triazina. Available online: https://ruc.udc.es/dspace/handle/2183/11706. [Google Scholar]
- 64.Bochkov V.N., Oskolkova O.V., Birukov K.G., Levonen A.-L., Binder C.J., Stöckl J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davies M.J., Hawkins C.L., Pattison D.I., Rees M.D. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 66.Lloyd M.M., van Reyk D.M., Davies M.J., Hawkins C.L. Hypothiocyanous acid is a more potent inducer of apoptosis and protein thiol depletion in murine macrophage cells than hypochlorous acid or hypobromous acid. Biochem. J. 2008;414:271–280. doi: 10.1042/BJ20080468. [DOI] [PubMed] [Google Scholar]
- 67.Pattison D., Davies M. Reactions of myeloperoxidase-derived oxidants with biological substrates: Gaining chemical insight into human inflammatory diseases. Curr. Med. Chem. 2006;13:3271–3290. doi: 10.2174/092986706778773095. [DOI] [PubMed] [Google Scholar]
- 68.Ulfig A., Leichert L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 2021;78:385–414. doi: 10.1007/s00018-020-03591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kettle A.J. Methods in Enzymology. Volume 300. Elsevier; Amsterdam, The Netherlands: 1999. Detection of 3-chlorotyrosine in proteins exposed to neutrophil oxidants; pp. 111–120. [DOI] [PubMed] [Google Scholar]
- 70.Olszowski S., Olszowska E., Stelmaszyńska T., Krawczyk A., Marcinkiewicz J., Baczek N. Oxidative modification of ovalbumin. Acta Biochim. Pol. 1996;43:661–672. doi: 10.18388/abp.1996_4462. [DOI] [PubMed] [Google Scholar]
- 71.Domigan N.M., Charlton T.S., Duncan M.W., Winterbourn C.C., Kettle A.J. Chlorination of tyrosyl residues in peptides by myeloperoxidase and human neutrophils. J. Biol. Chem. 1995;270:16542–16548. doi: 10.1074/jbc.270.28.16542. [DOI] [PubMed] [Google Scholar]
- 72.Hazen S.L., Hsu F.F., Mueller D.M., Crowley J.R., Heinecke J.W. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J. Clin. Investig. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davies M., Fu S., Wang H., Dean R. Reactions of hypochlorous acid with tyrosine and peptidyl-tyrosyl residues give dichlorinated and aldehydic products in addition to 3-chlorotyrosine. J. Biol. Chem. 2000;275:10851–10858. doi: 10.1074/jbc.275.15.10851. [DOI] [PubMed] [Google Scholar]
- 74.Kang J., Joseph I., Neidigh J.W. Hypochlorous acid damages histone proteins forming 3-chlorotyrosine and 3, 5-dichlorotyrosine. Chem. Res. Toxicol. 2008;21:1028–1038. doi: 10.1021/tx7003486. [DOI] [PubMed] [Google Scholar]
- 75.Hawkins C., Pattison D., Davies M. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 76.Kettle A.J. Neutrophils convert tyrosyl residues in albumin to chlorotyrosine. FEBS Lett. 1996;379:103–106. doi: 10.1016/0014-5793(95)01494-2. [DOI] [PubMed] [Google Scholar]
- 77.Hazen S.L., Hsu F.F., Heinecke J.W. p-Hydroxyphenylacetaldehyde is the major product of L-tyrosine oxidation by activated human phagocytes: A chloride-dependent mechanism for the conversion of free amino acids into reactive aldehydes by myeloperoxidase. J. Biol. Chem. 1996;271:1861–1867. doi: 10.1074/jbc.271.4.1861. [DOI] [PubMed] [Google Scholar]
- 78.Pereira W., Hoyano Y., Summons R., Bacon V., Duffield A. Chlorination studies II. The reaction of aqueous hypochlorous acid with α-amino acids and dipeptides. Biochim. Biophys. Acta BBA Gen. Subj. 1973;313:170–180. doi: 10.1016/0304-4165(73)90198-0. [DOI] [PubMed] [Google Scholar]
- 79.Zhang C., Reiter C., Eiserich J.P., Boersma B., Parks D.A., Beckman J.S., Barnes S., Kirk M., Baldus S., Darley-Usmar V.M. L-arginine chlorination products inhibit endothelial nitric oxide production. J. Biol. Chem. 2001;276:27159–27165. doi: 10.1074/jbc.M100191200. [DOI] [PubMed] [Google Scholar]
- 80.Pattison D.I., Davies M.J. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- 81.DrozdŹ R., Naskalski J.W., Sznajd J. Oxidation of amino acids and peptides in reaction with myeloperoxidase, chloride and hydrogen peroxide. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1988;957:47–52. doi: 10.1016/0167-4838(88)90155-0. [DOI] [PubMed] [Google Scholar]
- 82.Gupta V., Carroll K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Biophys. Acta BBA Gen. Subj. 2014;1840:847–875. doi: 10.1016/j.bbagen.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fu X., Mueller D.M., Heinecke J.W. Generation of intramolecular and intermolecular sulfenamides, sulfinamides, and sulfonamides by hypochlorous acid: A potential pathway for oxidative cross-linking of low-density lipoprotein by myeloperoxidase. Biochemistry. 2002;41:1293–1301. doi: 10.1021/bi015777z. [DOI] [PubMed] [Google Scholar]
- 84.Davies M.J., Hawkins C.L. Hypochlorite-induced oxidation of thiols: Formation of thiyl radicals and the role of sulfenyl chlorides as intermediates. Free Radic. Res. 2000;33:719–729. doi: 10.1080/10715760000301241. [DOI] [PubMed] [Google Scholar]
- 85.Naskalski J. Oxidative modification of protein structures under the action of myeloperoxidase and the hydrogen peroxide and chloride system. Ann. Biol. Clin. 1994;52:451–456. [PubMed] [Google Scholar]
- 86.Hawkins C.L. Hypochlorous acid-mediated modification of proteins and its consequences. Essays Biochem. 2020;64:75–86. doi: 10.1042/EBC20190045. [DOI] [PubMed] [Google Scholar]
- 87.Tiedemann F., Gmelin L. Einige neue Bestandtheile der Galle des Ochsen. Ann. Phys. 1827;85:326–337. doi: 10.1002/andp.18270850214. [DOI] [Google Scholar]
- 88.Merckx C., De Paepe B. The Role of Taurine in Skeletal Muscle Functioning and Its Potential as a Supportive Treatment for Duchenne Muscular Dystrophy. Metabolites. 2022;12:193. doi: 10.3390/metabo12020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marcinkiewicz J. Taurine bromamine (TauBr)-its role in immunity and new perspectives for clinical use. J. Biomed. Sci. 2010;17:S3. doi: 10.1186/1423-0127-17-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marcinkiewicz J., Kontny E. Taurine and inflammatory diseases. Amino Acids. 2014;46:7–20. doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marcinkiewicz J., Walczewska M. Neutrophils as sentinel cells of the immune system: A role of the MPO-halide-system in innate and adaptive immunity. Curr. Med. Chem. 2020;27:2840–2851. doi: 10.2174/0929867326666190819123300. [DOI] [PubMed] [Google Scholar]
- 92.Antelo J.M., Arce F., Calvo P., Crugeiras J., Ríos A. General acid–base catalysis in the reversible disproportionation reaction of N-chlorotaurine. J. Chem. Soc. Perkin Trans. 2. 2000:2109–2114. doi: 10.1039/b003929m. [DOI] [Google Scholar]
- 93.Gottardi W., Nagl M. N-chlorotaurine, a natural antiseptic with outstanding tolerability. J. Antimicrob. Chemother. 2010;65:399–409. doi: 10.1093/jac/dkp466. [DOI] [PubMed] [Google Scholar]
- 94.Gottardi W., Hagleitner M., Nagl M. N, N-Dichlorotaurine: Chemical and Bactericidal Properties. Arch. Pharm. Int. J. Pharm. Med. Chem. 2005;338:473–483. doi: 10.1002/ardp.200500146. [DOI] [PubMed] [Google Scholar]
- 95.Seol S.-I., Kim H.J., Choi E.B., Kang I.S., Lee H.-K., Lee J.-K., Kim C. Taurine protects against postischemic brain injury via the antioxidant activity of taurine chloramine. Antioxidants. 2021;10:372. doi: 10.3390/antiox10030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hawkins C.L., Davies M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019;294:19683–19708. doi: 10.1074/jbc.REV119.006217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davies M.J. Protein oxidation and peroxidation. Biochem. J. 2016;473:805–825. doi: 10.1042/BJ20151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hawkins C.L., Davies M.J. The role of reactive N-bromo species and radical intermediates in hypobromous acid-induced protein oxidation. Free Radic. Biol. Med. 2005;39:900–912. doi: 10.1016/j.freeradbiomed.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 99.Peskin A.V., Winterbourn C.C. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic. Biol. Med. 2001;30:572–579. doi: 10.1016/S0891-5849(00)00506-2. [DOI] [PubMed] [Google Scholar]
- 100.Harlan J., Levine J., Callahan K., Schwartz B., Harker L. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J. Clin. Investig. 1984;73:706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vissers M.C., Winterbourn C.C. Oxidation of intracellular glutathione after exposure of human red blood cells to hypochlorous acid. Biochem. J. 1995;307:57–62. doi: 10.1042/bj3070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Folkes L.K., Candeias L.P., Wardman P. Kinetics and mechanisms of hypochlorous acid reactions. Arch. Biochem. Biophys. 1995;323:120–126. doi: 10.1006/abbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 103.Winterbourn C.C., Brennan S.O. Characterization of the oxidation products of the reaction between reduced glutathione and hypochlorous acid. Biochem. J. 1997;326:87–92. doi: 10.1042/bj3260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haenen G.R., Bast A. Glutathione revisited: A better scavenger than previously thought. Front. Pharmacol. 2014;5:260. doi: 10.3389/fphar.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hawkins C.L., Davies M.J. Hypochlorite-induced damage to proteins: Formation of nitrogen-centred radicals from lysine residues and their role in protein fragmentation. Biochem. J. 1998;332:617–625. doi: 10.1042/bj3320617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thomas E.L., Grisham M.B., Jefferson M.M. Methods in Enzymology. Volume 132. Elsevier; Amsterdam, The Netherlands: 1986. Preparation and characterization of chloramines; pp. 569–585. [DOI] [PubMed] [Google Scholar]
- 107.Zhao H., Zhou Y., Han C., Liu Y.D., Zhong R. Degradation mechanisms and substituent effects of N-chloro-α-amino acids: A computational study. Environ. Sci. Technol. 2020;54:2635–2645. doi: 10.1021/acs.est.9b04440. [DOI] [PubMed] [Google Scholar]
- 108.Prütz W.A. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch. Biochem. Biophys. 1996;332:110–120. doi: 10.1006/abbi.1996.0322. [DOI] [PubMed] [Google Scholar]
- 109.Prütz W. Interactions of hypochlorous acid with pyrimidine nucleotides, and secondary reactions of chlorinated pyrimidines with GSH, NADH, and other substrates. Arch. Biochem. Biophys. 1998;349:183–191. doi: 10.1006/abbi.1997.0440. [DOI] [PubMed] [Google Scholar]
- 110.Albrich J.M., McCarthy C.A., Hurst J.K. Biological reactivity of hypochlorous acid: Implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. USA. 1981;78:210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bernofsky C. Nucleotide chloramines and neutrophil-mediated cytotoxicity. FASEB J. 1991;5:295–300. doi: 10.1096/fasebj.5.3.1848195. [DOI] [PubMed] [Google Scholar]
- 112.Prütz W. Consecutive halogen transfer between various functional groups induced by reaction of hypohalous acids: NADH oxidation by halogenated amide groups. Arch. Biochem. Biophys. 1999;371:107–114. doi: 10.1006/abbi.1999.1377. [DOI] [PubMed] [Google Scholar]
- 113.Prütz W.A., Kissner R., Koppenol W.H., Rüegger H. On the irreversible destruction of reduced nicotinamide nucleotides by hypohalous acids. Arch. Biochem. Biophys. 2000;380:181–191. doi: 10.1006/abbi.2000.1914. [DOI] [PubMed] [Google Scholar]
- 114.Hayatsu H., Pan S., Ukita T. Reaction of sodium hypochlorite with nucleic acids and their constituents. Chem. Pharm. Bull. 1971;19:2189–2192. doi: 10.1248/cpb.19.2189. [DOI] [PubMed] [Google Scholar]
- 115.Patton W., Bacon V., Duffield A., Halpern B., Hoyano Y., Pereira W., Lederberg J. Chlorination studies. I. The reaction of aqueous hypochlorous acid with cytosine. Biochem. Biophys. Res. Commun. 1972;48:880–884. doi: 10.1016/0006-291X(72)90690-0. [DOI] [PubMed] [Google Scholar]
- 116.Gould J., Richards J., Miles M. The formation of stable organic chloramines during the aqueous chlorination of cytosine and 5-methylcytosine. Water Res. 1984;18:991–999. doi: 10.1016/0043-1354(84)90250-1. [DOI] [Google Scholar]
- 117.Gould J., Richards J., Miles M. The kinetics and primary products of uracil chlorination. Water Res. 1984;18:205–212. doi: 10.1016/0043-1354(84)90070-8. [DOI] [Google Scholar]
- 118.Hawkins C.L., Davies M.J. Hypochlorite-induced damage to nucleosides: Formation of chloramines and nitrogen-centered radicals. Chem. Res. Toxicol. 2001;14:1071–1081. doi: 10.1021/tx010071r. [DOI] [PubMed] [Google Scholar]
- 119.McGowan S.E., Thompson R.J. Extracellular matrix proteoglycan degradation by human alveolar macrophages and neutrophils. J. Appl. Physiol. 1989;66:400–409. doi: 10.1152/jappl.1989.66.1.400. [DOI] [PubMed] [Google Scholar]
- 120.McGowan S.E. Mechanisms of extracellular matrix proteoglycan degradation by human neutrophils. Am. J. Respir. Cell Mol. Biol. 1990;2:271–279. doi: 10.1165/ajrcmb/2.3.271. [DOI] [PubMed] [Google Scholar]
- 121.Panasenko O., Gorudko I., Sokolov A. Hypochlorous acid as a precursor of free radicals in living systems. Biochemistry. 2013;78:1466–1489. doi: 10.1134/S0006297913130075. [DOI] [PubMed] [Google Scholar]
- 122.Hawkins C.L., Davies M.J. Degradation of hyaluronic acid, poly-and mono-saccharides, and model compounds by hypochlorite: Evidence for radical intermediates and fragmentation. Free Radic. Biol. Med. 1998;24:1396–1410. doi: 10.1016/S0891-5849(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 123.Rees M.D., Hawkins C.L., Davies M.J. Hypochlorite-mediated fragmentation of hyaluronan, chondroitin sulfates, and related N-acetyl glycosamines: Evidence for chloramide intermediates, free radical transfer reactions, and site-specific fragmentation. J. Am. Chem. Soc. 2003;125:13719–13733. doi: 10.1021/ja0370591. [DOI] [PubMed] [Google Scholar]
- 124.Woods A.A., Davies M.J. Fragmentation of extracellular matrix by hypochlorous acid. Biochem. J. 2003;376:219–227. doi: 10.1042/bj20030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heinecke J.W. Mechanisms of oxidative damage by myeloperoxidase in atherosclerosis and other inflammatory disorders. J. Lab. Clin. Med. 1999;133:321–325. doi: 10.1016/S0022-2143(99)90061-6. [DOI] [PubMed] [Google Scholar]
- 126.Baskol G., Demir H., Baskol M., Kilic E., Ates F., Karakukcu C., Ustdal M. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem. Funct. Cell. Biochem. Modul. Act. Agents Dis. 2006;24:307–311. doi: 10.1002/cbf.1257. [DOI] [PubMed] [Google Scholar]
- 127.Andreadis A.A., Hazen S.L., Comhair S.A., Erzurum S.C. Oxidative and nitrosative events in asthma. Free Radic. Biol. Med. 2003;35:213–225. doi: 10.1016/S0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 128.Cantin A., Woods D.E. Protection by antibiotics against myeloperoxidase-dependent cytotoxicity to lung epithelial cells in vitro. J. Clin. Investig. 1993;91:38–45. doi: 10.1172/JCI116196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ndrepepa G. Myeloperoxidase–A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta. 2019;493:36–51. doi: 10.1016/j.cca.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 130.Kuzuya T., Fuji H., Hoshida S., Nishida M., Goshima K., Hori M., Kamada T., Tada M. Polymorphonuclear leukocytes-induced injury in hypoxic cardiac myocytes. Free Radic. Biol. Med. 1994;17:501–510. doi: 10.1016/0891-5849(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 131.Leone A., Palmer R., Knowles R., Francis P., Ashton D., Moncada S. Constitutive and inducible nitric oxide synthases incorporate molecular oxygen into both nitric oxide and citrulline. J. Biol. Chem. 1991;266:23790–23795. doi: 10.1016/S0021-9258(18)54352-0. [DOI] [PubMed] [Google Scholar]
- 132.Cooper C.E., Odell E. Interaction of human myeloperoxidase with nitrite. FEBS Lett. 1992;314:58–60. doi: 10.1016/0014-5793(92)81461-T. [DOI] [PubMed] [Google Scholar]
- 133.Klebanoff S.J. Reactive nitrogen intermediates and antimicrobial activity: Role of nitrite. Free Radic. Biol. Med. 1993;14:351–360. doi: 10.1016/0891-5849(93)90084-8. [DOI] [PubMed] [Google Scholar]
- 134.Van Der Vliet A., Eiserich J.P., Halliwell B., Cross C.E. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite: A potential additional mechanism of nitric oxide-dependent toxicity. J. Biol. Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 135.O’Brien P.J. Radical formation during the peroxidase catalyzed metabolism of carcinogens and xenobiotics: The reactivity of these radicals with GSH, DNA, and unsaturated lipid. Free Radic. Biol. Med. 1988;4:169–183. doi: 10.1016/0891-5849(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 136.Eastmond D., Smith M., Ruzo L., Ross D. Metabolic activation of phenol by human myeloperoxidase and horseradish peroxidase. Mol. Pharmacol. 1986;30:674–679. [PubMed] [Google Scholar]
- 137.Frimat B., Gressier B., Odou P., Brunet C., Dine T., Luycky M., Cazin M., Cazin J. Metabolism of clozapine by human neutrophils: Evidence for a specific oxidation of clozapine by the myeloperoxidase system with inhibition of enzymatic chlorination cycle. Fundam. Clin. Pharmacol. 1997;11:267–274. doi: 10.1111/j.1472-8206.1997.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 138.Gutierrez-Correa J., Stoppani A. Inactivation of heart dihydrolipoamide dehydrogenase by copper Fenton systems. Effect of thiol compounds and metal chelators. Free Radic. Res. 1995;22:239–250. doi: 10.3109/10715769509147543. [DOI] [PubMed] [Google Scholar]
- 139.Correa J.G., Stoppani A. Catecholamines enhance dihydrolipoamide dehydrogenase inactivation by the copper Fenton system. Enzyme protection by copper chelators. Free Radic. Res. 1996;24:311–322. doi: 10.3109/10715769609088028. [DOI] [PubMed] [Google Scholar]
- 140.Correa J.G., Stoppani A. Inactivation of lipoamide dehydrogenase by cobalt (II) and iron (II) Fenton systems: Effect of metal chelators, thiol compounds and adenine nucleotides. Free Radic. Res. Commun. 1993;19:303–314. doi: 10.3109/10715769309056519. [DOI] [PubMed] [Google Scholar]
- 141.Gutierrez Correa J., Biscardi A.M., Stoppani A.O. Inactivación de la lipoamida deshidrogenasa de miocardio por catecolaminas: Protección por captopril y otros tioles. Med. B. Aires. 1995;55:397–407. [PubMed] [Google Scholar]
- 142.Anatoliotakis N., Deftereos S., Bouras G., Giannopoulos G., Tsounis D., Angelidis C., Kaoukis A., Stefanadis C. Myeloperoxidase: Expressing inflammation and oxidative stress in cardiovascular disease. Curr. Top. Med. Chem. 2013;13:115–138. doi: 10.2174/1568026611313020004. [DOI] [PubMed] [Google Scholar]
- 143.Eiserich J.P., Baldus S., Brennan M.-L., Ma W., Zhang C., Tousson A., Castro L., Lusis A.J., Nauseef W.M., White C.R. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 144.Mollenhauer M., Friedrichs K., Lange M., Gesenberg J., Remane L., Kerkenpaß C., Krause J., Schneider J., Ravekes T., Maass M. Myeloperoxidase mediates postischemic arrhythmogenic ventricular remodeling. Circ. Res. 2017;121:56–70. doi: 10.1161/CIRCRESAHA.117.310870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kessenbrock K., Plaks V., Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carr A.C., McCall M.R., Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: Reaction pathways and antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2000;20:1716–1723. doi: 10.1161/01.ATV.20.7.1716. [DOI] [PubMed] [Google Scholar]
- 147.Roth Flach R.J., Su C., Bollinger E., Cortes C., Robertson A.W., Opsahl A.C., Coskran T.M., Maresca K.P., Keliher E.J., Yates P.D. Myeloperoxidase inhibition in mice alters atherosclerotic lesion composition. PLoS ONE. 2019;14:e0214150. doi: 10.1371/journal.pone.0214150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Z., Nicholls S.J., Rodriguez E.R., Kummu O., Hörkkö S., Barnard J., Reynolds W.F., Topol E.J., DiDonato J.A., Hazen S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 149.Pham P., Pham P., Pham P. Patients with diabetes mellitus type 2 and hypomagnesemia may have enhanced glomerular filtration via hypocalcemia. Clin. Nephrol. 2012;78:442–448. doi: 10.5414/CN107525. [DOI] [PubMed] [Google Scholar]
- 150.Chami B., Martin N.J., Dennis J.M., Witting P.K. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch. Biochem. Biophys. 2018;645:61–71. doi: 10.1016/j.abb.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 151.Garrity-Park M., Loftus E.V., Jr., Sandborn W.J., Smyrk T.C. Myeloperoxidase immunohistochemistry as a measure of disease activity in ulcerative colitis: Association with ulcerative colitis-colorectal cancer, tumor necrosis factor polymorphism and RUNX3 methylation. Inflamm. Bowel Dis. 2012;18:275–283. doi: 10.1002/ibd.21681. [DOI] [PubMed] [Google Scholar]
- 152.Chami B., Ahmad G., Schroder A., San Gabriel P., Witting P. The role of myeloperoxidase and neutrophil extracellular traps in the pathogenesis of inflammatory bowel disease. Gastroenterology. 2021;160:S5–S6. doi: 10.1053/j.gastro.2021.01.044. [DOI] [Google Scholar]
- 153.Wang W., Jian Z., Guo J., Ning X. Increased levels of serum myeloperoxidase in patients with active rheumatoid arthritis. Life Sci. 2014;117:19–23. doi: 10.1016/j.lfs.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 154.Fernandes R.M.S.N., Silva N.P.d., Sato E.I. Increased myeloperoxidase plasma levels in rheumatoid arthritis. Rheumatol. Int. 2012;32:1605–1609. doi: 10.1007/s00296-011-1810-5. [DOI] [PubMed] [Google Scholar]
- 155.Telles R.W., Ferreira G.A., Silva N.P.d., Sato E.I. Increased plasma myeloperoxidase levels in systemic lupus erythematosus. Rheumatol. Int. 2010;30:779–784. doi: 10.1007/s00296-009-1067-4. [DOI] [PubMed] [Google Scholar]
- 156.Olson S., Lee J., Poirier M., Little D., Prince L., Baker T., Edison J., Abbott K. Anti-myeloperoxidase antibodies associate with future proliferative lupus nephritis. Autoimmune Dis. 2017;2017:1872846. doi: 10.1155/2017/1872846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Carrillo-Vázquez D.A., Jardón-Valadez E., Torres-Ruiz J., Juárez-Vega G., Maravillas-Montero J.L., Meza-Sánchez D.E., Domínguez-López M.L., Varela J.C.A., Gómez-Martín D. Conformational changes in myeloperoxidase induced by ubiquitin and NETs containing free ISG15 from systemic lupus erythematosus patients promote a pro-inflammatory cytokine response in CD4+ T cells. J. Transl. Med. 2020;18:429. doi: 10.1186/s12967-020-02604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Green P.S., Mendez A.J., Jacob J.S., Crowley J.R., Growdon W., Hyman B.T., Heinecke J.W. Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. J. Neurochem. 2004;90:724–733. doi: 10.1111/j.1471-4159.2004.02527.x. [DOI] [PubMed] [Google Scholar]
- 159.Tzikas S., Schlak D., Sopova K., Gatsiou A., Stakos D., Stamatelopoulos K., Stellos K., Laske C. Increased myeloperoxidase plasma levels in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2014;39:557–564. doi: 10.3233/JAD-131469. [DOI] [PubMed] [Google Scholar]
- 160.Volkman R., Ben-Zur T., Kahana A., Garty B.Z., Offen D. Myeloperoxidase deficiency inhibits cognitive decline in the 5XFAD mouse model of Alzheimer’s disease. Front. Neurosci. 2019;13:990. doi: 10.3389/fnins.2019.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pulli B., Bure L., Wojtkiewicz G.R., Iwamoto Y., Ali M., Li D., Schob S., Hsieh K.L.-C., Jacobs A.H., Chen J.W. Multiple sclerosis: Myeloperoxidase immunoradiology improves detection of acute and chronic disease in experimental model. Radiology. 2015;275:480. doi: 10.1148/radiol.14141495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gray E., Thomas T.L., Betmouni S., Scolding N., Love S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci. Lett. 2008;444:195–198. doi: 10.1016/j.neulet.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 163.Nagra R.M., Becher B., Tourtellotte W.W., Antel J.P., Gold D., Paladino T., Smith R.A., Nelson J.R., Reynolds W.F. Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J. Neuroimmunol. 1997;78:97–107. doi: 10.1016/S0165-5728(97)00089-1. [DOI] [PubMed] [Google Scholar]
- 164.Pravalika K., Sarmah D., Kaur H., Wanve M., Saraf J., Kalia K., Borah A., Yavagal D.R., Dave K.R., Bhattacharya P. Myeloperoxidase and neurological disorder: A crosstalk. ACS Chem. Neurosci. 2018;9:421–430. doi: 10.1021/acschemneuro.7b00462. [DOI] [PubMed] [Google Scholar]
- 165.Ray R., Katyal A. Myeloperoxidase: Bridging the gap in neurodegeneration. Neurosci. Biobehav. Rev. 2016;68:611–620. doi: 10.1016/j.neubiorev.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 166.Gellhaar S., Sunnemark D., Eriksson H., Olson L., Galter D. Myeloperoxidase-immunoreactive cells are significantly increased in brain areas affected by neurodegeneration in Parkinson’s and Alzheimer’s disease. Cell Tissue Res. 2017;369:445–454. doi: 10.1007/s00441-017-2626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Choi D.-K., Pennathur S., Perier C., Tieu K., Teismann P., Wu D.-C., Jackson-Lewis V., Vila M., Vonsattel J.-P., Heinecke J.W. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J. Neurosci. 2005;25:6594–6600. doi: 10.1523/JNEUROSCI.0970-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jucaite A., Svenningsson P., Rinne J.O., Cselenyi Z., Varnäs K., Johnström P., Amini N., Kirjavainen A., Helin S., Minkwitz M. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: A PET study in Parkinson’s disease. Brain. 2015;138:2687–2700. doi: 10.1093/brain/awv184. [DOI] [PubMed] [Google Scholar]
- 169.Cojocaru I.M., Cojocaru M., Iliescu I., Botnaru L., Gurban C.V., Sfrijan F., Tanasescu R. Plasma myeloperoxidase levels in patients with acute ischemic stroke. Rom. J. Intern. Med. 2010;48:101–104. [PubMed] [Google Scholar]
- 170.Tay A., Tamam Y., Yokus B., Ustundag M., Orak M. Serum myeloperoxidase levels in predicting the severity of stroke and mortality in acute ischemic stroke patients. Eur. Rev. Med. Pharm. Sci. 2015;19:1983–1988. [PubMed] [Google Scholar]
- 171.Yu G., Liang Y., Huang Z., Jones D.W., Pritchard K.A., Zhang H. Inhibition of myeloperoxidase oxidant production by N-acetyl lysyltyrosylcysteine amide reduces brain damage in a murine model of stroke. J. Neuroinflamm. 2016;13:119. doi: 10.1186/s12974-016-0583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li S.-B., Yang F., Jing L., Ma J., Jia Y.-D., Dong S.-Y., Zheng W.-F., Zhao L.-S. Myeloperoxidase and risk of recurrence of atrial fibrillation after catheter ablation. J. Investig. Med. 2013;61:722–727. doi: 10.2310/JIM.0b013e3182857fa0. [DOI] [PubMed] [Google Scholar]
- 173.Rudolph V., Andrié R.P., Rudolph T.K., Friedrichs K., Klinke A., Hirsch-Hoffmann B., Schwoerer A.P., Lau D., Fu X., Klingel K. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 2010;16:470–474. doi: 10.1038/nm.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nicholls S.J., Hazen S.L. Myeloperoxidase, modified lipoproteins, and atherogenesis. J. Lipid Res. 2009;50:S346–S351. doi: 10.1194/jlr.R800086-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tsimikas S. Oxidative biomarkers in the diagnosis and prognosis of cardiovascular disease. Am. J. Cardiol. 2006;98:S9–S17. doi: 10.1016/j.amjcard.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 176.Zhang R., Brennan M.-L., Fu X., Aviles R.J., Pearce G.L., Penn M.S., Topol E.J., Sprecher D.L., Hazen S.L. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 177.Klinke A., Berghausen E., Friedrichs K., Molz S., Lau D., Remane L., Berlin M., Kaltwasser C., Adam M., Mehrkens D. Myeloperoxidase aggravates pulmonary arterial hypertension by activation of vascular Rho-kinase. JCI Insight. 2018;3:e97530. doi: 10.1172/jci.insight.97530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Van der Zwan L.P., Scheffer P.G., Dekker J.M., Stehouwer C.D., Heine R.J., Teerlink T. Hyperglycemia and oxidative stress strengthen the association between myeloperoxidase and blood pressure. Hypertension. 2010;55:1366–1372. doi: 10.1161/HYPERTENSIONAHA.109.147231. [DOI] [PubMed] [Google Scholar]
- 179.Rocha-Penha L., Caldeira-Dias M., Tanus-Santos J.E., de Carvalho Cavalli R., Sandrim V.C. Myeloperoxidase in hypertensive disorders of pregnancy and its relation with nitric oxide. Hypertension. 2017;69:1173–1180. doi: 10.1161/HYPERTENSIONAHA.116.08854. [DOI] [PubMed] [Google Scholar]
- 180.Mocatta T.J., Pilbrow A.P., Cameron V.A., Senthilmohan R., Frampton C.M., Richards A.M., Winterbourn C.C. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J. Am. Coll. Cardiol. 2007;49:1993–2000. doi: 10.1016/j.jacc.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 181.Rees M.D., Pattison D.I., Davies M.J. Oxidation of heparan sulphate by hypochlorite: Role of N-chloro derivatives and dichloramine-dependent fragmentation. Biochem. J. 2005;391:125–134. doi: 10.1042/BJ20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Omran M.M., Zahran F.M., Kadry M., Belal A.A., Emran T.M. Role of myeloperoxidase in early diagnosis of acute myocardial infarction in patients admitted with chest pain. J. Immunoass. Immunochem. 2018;39:337–347. doi: 10.1080/15321819.2018.1492423. [DOI] [PubMed] [Google Scholar]
- 183.Abdo A.I., Rayner B.S., van Reyk D.M., Hawkins C.L. Low-density lipoprotein modified by myeloperoxidase oxidants induces endothelial dysfunction. Redox Biol. 2017;13:623–632. doi: 10.1016/j.redox.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Manchanda K., Kolarova H., Kerkenpaß C., Mollenhauer M., Vitecek J., Rudolph V., Kubala L., Baldus S., Adam M., Klinke A. MPO (myeloperoxidase) reduces endothelial glycocalyx thickness dependent on its cationic charge. Arterioscler. Thromb. Vasc. Biol. 2018;38:1859–1867. doi: 10.1161/ATVBAHA.118.311143. [DOI] [PubMed] [Google Scholar]
- 185.Vita J.A., Brennan M.-L., Gokce N., Mann S.A., Goormastic M., Shishehbor M.H., Penn M.S., Keaney J.F., Jr., Hazen S.L. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Caramori G., Papi A. Oxidants and asthma. Thorax. 2004;59:170–173. doi: 10.1136/thorax.2002.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ekmekci O., Donma O., Sardoğan E., Yildirim N., Uysal O., Demirel H., Demir T. Iron, nitric oxide, and myeloperoxidase in asthmatic patients. Biochemistry. 2004;69:462–467. doi: 10.1023/B:BIRY.0000026205.89894.25. [DOI] [PubMed] [Google Scholar]
- 188.Jatakanon A., Uasuf C., Maziak W., Lim S., Chung K.F., Barnes P.J. Neutrophilic inflammation in severe persistent asthma. Am. J. Respir. Crit. Care Med. 1999;160:1532–1539. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 189.O’Donnell C., Newbold P., White P., Thong B., Stone H., Stockley R.A. 3-Chlorotyrosine in sputum of COPD patients: Relationship with airway inflammation. COPD J. Chronic Obstr. Pulm. Dis. 2010;7:411–417. doi: 10.3109/15412555.2010.528086. [DOI] [PubMed] [Google Scholar]
- 190.Zhu A., Ge D., Zhang J., Teng Y., Yuan C., Huang M., Adcock I.M., Barnes P.J., Yao X. Sputum myeloperoxidase in chronic obstructive pulmonary disease. Eur. J. Med. Res. 2014;19:12. doi: 10.1186/2047-783X-19-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andelid K., Glader P., Jirholt P., Gjertsson I., Jansson A.E., Lindén A. Systemic myeloperoxidase in COPD. Respir. Med. 2013;107:S4. doi: 10.1016/j.rmed.2013.08.022. [DOI] [Google Scholar]
- 192.Kettle A.J., Chan T., Osberg I., Senthilmohan R., Chapman A.L., Mocatta T.J., Wagener J.S. Myeloperoxidase and protein oxidation in the airways of young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2004;170:1317–1323. doi: 10.1164/rccm.200311-1516OC. [DOI] [PubMed] [Google Scholar]
- 193.Van der Vliet A., Nguyen M.N., Shigenaga M.K., Eiserich J.P., Marelich G.P., Cross C.E. Myeloperoxidase and protein oxidation in cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L537–L546. doi: 10.1152/ajplung.2000.279.3.L537. [DOI] [PubMed] [Google Scholar]
- 194.Vasu V.T., De Cruz S.J., Houghton J.S., Hayakawa K.A., Morrissey B.M., Cross C.E., Eiserich J.P. Evaluation of thiol-based antioxidant therapeutics in cystic fibrosis sputum: Focus on myeloperoxidase. Free Radic. Res. 2011;45:165–176. doi: 10.3109/10715762.2010.521154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Casciaro M., Di Salvo E., Pace E., Ventura-Spagnolo E., Navarra M., Gangemi S. Chlorinative stress in age-related diseases: A literature review. Immun. Ageing. 2017;14:21. doi: 10.1186/s12979-017-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Alikhan M.A., Jaw J., Shochet L.R., Robson K.J., Ooi J.D., Brouwer E., Heeringa P., Holdsworth S.R., Kitching A.R. Ageing enhances cellular immunity to myeloperoxidase and experimental anti-myeloperoxidase glomerulonephritis. Rheumatology. 2022;61:2132–2143. doi: 10.1093/rheumatology/keab682. [DOI] [PubMed] [Google Scholar]
- 197.Lu H., Xu S., Liang X., Dai Y., Huang Z., Ren Y., Lin J., Liu X. Advanced glycated end products alter neutrophil effect on regulation of CD4+ T cell differentiation through induction of myeloperoxidase and neutrophil elastase activities. Inflammation. 2019;42:559–571. doi: 10.1007/s10753-018-0913-5. [DOI] [PubMed] [Google Scholar]
- 198.Fedeles B.I., Freudenthal B.D., Yau E., Singh V., Chang S.-c., Li D., Delaney J.C., Wilson S.H., Essigmann J.M. Intrinsic mutagenic properties of 5-chlorocytosine: A mechanistic connection between chronic inflammation and cancer. Proc. Natl. Acad. Sci. USA. 2015;112:E4571–E4580. doi: 10.1073/pnas.1507709112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rymaszewski A.L., Tate E., Yimbesalu J.P., Gelman A.E., Jarzembowski J.A., Zhang H., Pritchard K.A., Jr., Vikis H.G. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers. 2014;6:1111–1127. doi: 10.3390/cancers6021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Weitzman S.A., Gordon L.I. Inflammation and cancer: Role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990;76:655–663. doi: 10.1182/blood.V76.4.655.655. [DOI] [PubMed] [Google Scholar]
- 201.Kisic B., Miric D., Dragojevic I., Rasic J., Popovic L. Role of myeloperoxidase in patients with chronic kidney disease. Oxidative Med. Cell. Longev. 2016;2016:1069743. doi: 10.1155/2016/1069743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lehners A., Lange S., Niemann G., Rosendahl A., Meyer-Schwesinger C., Oh J., Stahl R., Ehmke H., Benndorf R., Klinke A. Myeloperoxidase deficiency ameliorates progression of chronic kidney disease in mice. Am. J. Physiol. Ren. Physiol. 2014;307:F407–F417. doi: 10.1152/ajprenal.00262.2014. [DOI] [PubMed] [Google Scholar]
- 203.Madhusudhana Rao A., Anand U., Anand C. Myeloperoxidase in chronic kidney disease. Indian J. Clin. Biochem. 2011;26:28–31. doi: 10.1007/s12291-010-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen S., Chen H., Du Q., Shen J. Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain ischemia injury: Potential application of natural compounds. Front. Physiol. 2020;11:433. doi: 10.3389/fphys.2020.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Q., Xie Z., Zhang W., Zhou J., Wu Y., Zhang M., Zhu H., Zou M.-H. Myeloperoxidase deletion prevents high-fat diet–induced obesity and insulin resistance. Diabetes. 2014;63:4172–4185. doi: 10.2337/db14-0026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 206.Zaki M., Basha W., Reyad H., Mohamed R., Hassan N., Kholousi S. Association between myeloperoxidase levels and risk of insulin resistance in Egyptian obese women. Open Access Maced. J. Med. Sci. 2018;6:629. doi: 10.3889/oamjms.2018.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qaddoumi M.G., Alanbaei M., Hammad M.M., Al Khairi I., Cherian P., Channanath A., Thanaraj T.A., Al-Mulla F., Abu-Farha M., Abubaker J. Investigating the role of myeloperoxidase and angiopoietin-like protein 6 in obesity and diabetes. Sci. Rep. 2020;10:6170. doi: 10.1038/s41598-020-63149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.García A.G., Rodríguez M.R., Alonso C.G., Ochoa D.Y.R., Aguilar C.A. Myeloperoxidase is associated with insulin resistance and inflammation in overweight subjects with first-degree relatives with type 2 diabetes mellitus. Diabetes Metab. J. 2015;39:59–65. doi: 10.4093/dmj.2015.39.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Heinecke J.W., Goldberg I.J. Myeloperoxidase: A therapeutic target for preventing insulin resistance and the metabolic sequelae of obesity? Diabetes. 2014;63:4001–4003. doi: 10.2337/db14-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.El Kebir D., József L., Pan W., Filep J.n.G. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ. Res. 2008;103:352–359. doi: 10.1161/01.RES.0000326772.76822.7a. [DOI] [PubMed] [Google Scholar]
- 211.Dorward D.A., Lucas C.D., Chapman G.B., Haslett C., Dhaliwal K., Rossi A.G. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am. J. Pathol. 2015;185:1172–1184. doi: 10.1016/j.ajpath.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Futosi K., Fodor S., Mócsai A. Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013;17:1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 213.Hawkins C.L., Pattison D.I., Stanley N.R., Davies M.J. Tryptophan residues are targets in hypothiocyanous acid-mediated protein oxidation. Biochem. J. 2008;416:441–452. doi: 10.1042/BJ20070941. [DOI] [PubMed] [Google Scholar]
- 214.Britigan B.E., Ratcliffe H.R., Buettner G.R., Rosen G.M. Binding of myeloperoxidase to bacteria: Effect on hydroxyl radical formation and susceptibility to oxidant-mediated killing. Biochim. Biophys. Acta BBA Gen. Subj. 1996;1290:231–240. doi: 10.1016/0304-4165(96)00014-1. [DOI] [PubMed] [Google Scholar]
- 215.Haegens A., Vernooy J.H., Heeringa P., Mossman B.T., Wouters E.F. Myeloperoxidase modulates lung epithelial responses to pro-inflammatory agents. Eur. Respir. J. 2008;31:252–260. doi: 10.1183/09031936.00029307. [DOI] [PubMed] [Google Scholar]
- 216.Klinke A., Nussbaum C., Kubala L., Friedrichs K., Rudolph T.K., Rudolph V., Paust H.-J., Schröder C., Benten D., Lau D. Myeloperoxidase attracts neutrophils by physical forces. Blood J. Am. Soc. Hematol. 2011;117:1350–1358. doi: 10.1182/blood-2010-05-284513. [DOI] [PubMed] [Google Scholar]
- 217.Lazarevic-Pasti T., Leskovac A., Vasic V. Myeloperoxidase inhibitors as potential drugs. Curr. Drug Metab. 2015;16:168–190. doi: 10.2174/138920021603150812120640. [DOI] [PubMed] [Google Scholar]
- 218.Galijasevic S. The development of myeloperoxidase inhibitors. Bioorg. Med. Chem. Lett. 2019;29:1–7. doi: 10.1016/j.bmcl.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 219.Piek A., Koonen D.P., Schouten E.-M., Lindtstedt E.L., Michaëlsson E., de Boer R.A., Silljé H.H. Pharmacological myeloperoxidase (MPO) inhibition in an obese/hypertensive mouse model attenuates obesity and liver damage, but not cardiac remodeling. Sci. Rep. 2019;9:18765. doi: 10.1038/s41598-019-55263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Huang J., Smith F., Panizzi J.R., Goodwin D.C., Panizzi P. Inactivation of myeloperoxidase by benzoic acid hydrazide. Arch. Biochem. Biophys. 2015;570:14–22. doi: 10.1016/j.abb.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Furtmüller P.G., Arnhold J., Jantschko W., Pichler H., Obinger C. Redox properties of the couples compound I/compound II and compound II/native enzyme of human myeloperoxidase. Biochem. Biophys. Res. Commun. 2003;301:551–557. doi: 10.1016/S0006-291X(02)03075-9. [DOI] [PubMed] [Google Scholar]
- 222.Bensalem S., Soubhye J., Aldib I., Bournine L., Nguyen A.T., Vanhaeverbeek M., Rousseau A., Boudjeltia K.Z., Sarakbi A., Kauffmann J.M. Inhibition of myeloperoxidase activity by the alkaloids of Peganum harmala L.(Zygophyllaceae) J. Ethnopharmacol. 2014;154:361–369. doi: 10.1016/j.jep.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 223.Segelmark M., Persson B., Hellmark T., Wieslander J. Binding and inhibition of myeloperoxidase (MPO): A major function of ceruloplasmin? Clin. Exp. Immunol. 1997;108:167–174. doi: 10.1046/j.1365-2249.1997.d01-992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kohnen S., Franck T., Van Antwerpen P., Zouaoui Boudjeltia K., Mouithys-Mickalad A., Deby C., Moguilevsky N., Deby-Dupont G., Lamy M., Serteyn D. Resveratrol inhibits the activity of equine neutrophil myeloperoxidase by a direct interaction with the enzyme. J. Agric. Food Chem. 2007;55:8080–8087. doi: 10.1021/jf071741n. [DOI] [PubMed] [Google Scholar]
- 225.Zeraik M.L., Ximenes V.F., Regasini L.O., Dutra L., Silva D.H.S., Fonseca L., Coelho D., Machado S., Bolzani V.d.S. 4′-Aminochalcones as novel inhibitors of the chlorinating activity of myeloperoxidase. Curr. Med. Chem. 2012;19:5405–5413. doi: 10.2174/092986712803833344. [DOI] [PubMed] [Google Scholar]
- 226.Shiba Y., Kinoshita T., Chuman H., Taketani Y., Takeda E., Kato Y., Naito M., Kawabata K., Ishisaka A., Terao J. Flavonoids as substrates and inhibitors of myeloperoxidase: Molecular actions of aglycone and metabolites. Chem. Res. Toxicol. 2008;21:1600–1609. doi: 10.1021/tx8000835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.