Abstract
The MS ring of the flagellar basal body of Salmonella is an integral membrane structure consisting of about 26 subunits of a 61-kDa protein, FliF. Out of many nonflagellate fliF mutants tested, three gave rise to intergenic suppressors in flagellar region II. The pseudorevertants swarmed, though poorly; this partial recovery of motile function was shown to be due to partial recovery of export function and flagellar assembly. The three parental mutants were all found to carry the same mutation, a six-base deletion corresponding to loss of Ala-174 and Ser-175 in the predicted periplasmic domain of the FliF protein. The 19 intergenic suppressors identified all lay in flhA, and they consisted of 10 independent examples at the nucleotide level or 9 at the amino acid level. Since two of the nine corresponded to different substitutions at the same amino acid position, only eight positions in the FlhA protein have given rise to suppressors. Thus, FliF-FlhA intergenic suppression is a fairly rare event. FlhA is a component of the flagellar protein export apparatus, with an integral membrane domain encompassing the N-terminal half of the sequence and a cytoplasmic C-terminal domain. All of the suppressing mutations lay within the integral membrane domain. These mutations, when placed in a wild-type fliF background, had no mutant phenotype. In the fliF mutant background, mutant FlhA was dominant, yielding a pseudorevertant phenotype. Wild-type FlhA did not exert significant negative dominance in the pseudorevertant background, indicating that it does not compete effectively with mutant FlhA for interaction with mutant FliF. Mutant FliF was partially dominant over wild-type FliF in both the wild-type and second-site FlhA backgrounds. Membrane fractionation experiments indicated that the fliF mutation, though preventing export, was mild enough to permit assembly of the MS ring itself, and also assembly of the cytoplasmic C ring onto the MS ring. The data from this study provide genetic support for a model in which at least the FlhA component of the export apparatus physically interacts with the MS ring within which it is housed.
The MS ring of the flagellar basal body of Salmonella is usually thought of in terms of its role in motor function: as a mounting plate for the rotor element of the motor/switch, also known as the cytoplasmic C ring. However, with increasing attention being paid to the process of flagellar protein export, which occurs by a type III pathway (7) and entails delivery of the export substrates into the lumen of the nascent structure, a second role for the MS ring is emerging, namely, as the structure that houses the membrane components of the export apparatus.
The MS ring has a complex appearance, with two rings (M and S) and a collar projecting beyond the cytoplasmic membrane into the periplasmic space, yet it is constructed from subunits of a single 61-kDa protein, FliF. It is estimated that there are about 26 subunits arranged as an annulus that has a central pore about 10 nm in diameter (10). The export apparatus has six integral membrane components (FlhA, FlhB, FliO, FliP, FliQ, and FliR) in addition to some soluble components (FliH, the ATPase FliI, and putative chaperones FliJ, FlgN, FliS, and FliT) (1, 2, 4, 16, 18, 19, 22). We have argued (2, 15) that the logical location for the membrane components of the export apparatus is in a patch of specialized membrane within the core of the MS ring, so the apparatus can deliver its substrates into the lumen. Thus far, two of these components (FliP and FliR) have been shown to be associated with the basal body (2); in the case of FliR, immunoelectron microscopy positioned it in the vicinity of the cytoplasmic face of the MS ring.
If the model of the MS ring enclosing the export apparatus is correct, the question arises whether there are specific interactions between the two structures, or whether the export apparatus is better thought of as a floating island. This report describes a number of examples where a specific FliF mutation can be suppressed by mutations in an integral membrane component of the export apparatus, FlhA, suggesting that the MS ring and the export apparatus do in fact interact.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The fliF parental mutant strains are derived from SJW1103 (34). The pseudorevertants derived from these fliF mutants were mapped by P22-mediated transduction to flhA in flagellar region II. The characteristics of the parental strains and their pseudorevertants are listed in Table 1. Chromosomal DNA from the mutant and pseudorevertant strains was prepared by the method of Woo et al. (32). Plasmids containing the fliF mutant insert or the flhA isolated second site mutant inserts were cloned into pTrc99A1de4 (2), using NdeI and BamHI sites created by PCR with mutant chromosomal DNA as target.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli NovaBlue | Recipient for cloning experiments | Novagen |
| Salmonella | ||
| JR501 | For converting plasmid to Salmonella compatibility | 25 |
| SJW1103 | Wild-type strain for motility and chemotaxis | 34 |
| SJW2706 (=SJW2713 = SJW2715) | fliF (deletion of bp 521–526, resulting in loss of A174 and S175) | This study |
| SJW1684 | ΔfliF | 12 |
| MM06xx series | fliF flhA pseudorevertants from SJW2706, SJW2713, or SJW2715 | This study |
| MM16xx series | flhA second-site mutants from corresponding MM06xx strain | This study |
| KK1012 | Deletion of region II | 14 |
| SJW1364 | flhA | 18 |
| SJW156 | flgD | 23 |
| Plasmids | ||
| pMM106 | pET19b / N-His-FLAG-FlhA | This study |
| pMM130 | pTrc99A / FlhA | This study |
| pMMhA0608 | pTrc99A / FlhA of MM0608 | This study |
| pMMhA0609 | pTrc99A / FlhA of MM0609 | This study |
| pMMhA0610 | pTrc99A / FlhA of MM0610 | This study |
| pMMhA0621 | pTrc99A / FlhA of MM0621 | This study |
| pMMhA0635 | pTrc99A / FlhA of MM0635 | This study |
| pMMhA0652 | pTrc99A / FlhA of MM0652 | This study |
| pMMhA0654 | pTrc99A / FlhA of MM0654 | This study |
| pMMiF2706 | pTrc99A / FliF of SJW2706 | This study |
Luria broth and soft tryptone motility plates are described in reference 29. Nutrient gelatin agar is described in reference 33.
DNA techniques.
PCR and cloning were performed as described previously (18) except that the Taq polymerase was from Qiagen (Valencia, Calif.). DNA sequencing was carried out with the modified T7 DNA polymerase Sequenase version 2.0 (USB Corp., Cleveland, Ohio) and various primers synthesized using an ABI model 392 DNA-RNA synthesizer (Perkin-Elmer, Foster City, Calif.).
Preparation of soluble and membrane protein fractions.
The cells were inoculated into 100 ml of Luria broth and grown overnight at 37°C with shaking. After collection by centrifugation, cells were resuspended in 5 ml of 10 mM Tris-HCl (pH 8.0)–200 mM dithiothreitol and sonicated (Branson model 250 Sonifier, Danbury, Conn.). The cell lysates were centrifuged (13,000 × g, 10 min, 4°C) to pellet undisrupted cells; then the cell lysates were centrifuged at 130,000 × g for 1 h. The supernatant and pellet fractions, which contained the soluble proteins and membrane proteins, respectively, were collected separately.
Preparation of periplasmic and culture supernatant fractions.
The periplasmic and culture supernatant fractions from SJW1103 (wild type), SJW2706 (fliF), MM0608 (fliF flhA), and SJW156 (flgD) were prepared as described elsewhere (18).
Preparation of hook-basal bodies.
Hook-basal bodies were prepared from SJW1364 (flhA) carrying pMM106, a pET19b-based plasmid encoding N-His-FLAG-FlhA, as described by Fan et al. (2).
Antibodies.
Monoclonal anti-FliF antibody and polyclonal anti-FlgD antibody are described in references 5 and 23, respectively. Polyclonal antibodies against FliG and FliN were a gift from K. Oosawa and S.-I Aizawa, Teikyo University, Japan. Anti-FLAG M2 monoclonal antibody was obtained from Sigma (St. Louis, Mo.). Antibodies were detected using an ECL (enhanced chemiluminescence) immunoblotting detection kit from Amersham (Little Chalfont, United Kingdom).
RESULTS
Isolation of pseudorevertants of fliF mutants.
Pseudorevertants were isolated from a total of 33 fliF mutants by streaking single colonies out on 8% gelatin agar, incubating them for up to 4 days, and looking for small swarms emerging from the streak. Cells from these swarms were picked and purified as single colonies. The locations of the suppressing mutations were broadly determined in terms of the major flagellar regions: I, II and III, which are at about 26, 42, and 43 centisomes, respectively (26). (Flagellar region III in fact consists of two subregions, IIIa and IIIb, with a region unrelated to flagellar function in between [11].)
None of the fliF mutants yielded suppressor mutations lying in region I. For 30 of them, all of the suppressor mutations lay in region III. Since fliF itself lies in region IIIb, many of these suppressors may have been intragenic, and none were analyzed further in this study. The remaining three fliF mutants (SJW2706, SJW2713, and SJW2715) between them gave rise to a total of 24 examples of intergenic suppressors in region II, in addition to a total of 229 examples of suppressors in region III. Finer deletion mapping of two of the region II suppressors indicated that they lay in or close to flhA, a gene which encodes an integral membrane component of the flagellar export apparatus (18); DNA sequence analysis later confirmed that all of the suppressors we identified lay in flhA (see below).
Motility of SJW2706 (fliF) and its fliF flhA pseudorevertants.
DNA sequence analysis of the three parental fliF strains revealed that they were identical (see below). Strain SJW2706 (fliF) was chosen for further study. SJW2706 swarmed very poorly at 30°C, whereas the pseudorevertants, illustrated by MM0608, swarmed considerably better (Fig. 1), although still only at about 20% of the wild-type rate (estimated after 15 h at 30°C [data not shown]). When examined in liquid culture by dark-field light microscopy, SJW2706 was essentially nonmotile, with only an occasional cell swimming feebly. Few if any flagella could be seen. With MM0608 and the other pseudorevertants that were examined, ca. 20 to 50% of the cells were swimming. Swimming speed was less than that of wild-type cells, and there were only ca. two to three flagella per swimming cell. Wild-type SJW1103, in contrast, was vigorously motile, with well-formed bundles containing ca. five to seven flagella. Thus, the swarming behavior can be simply explained by essentially total failure to assemble flagella in the case of the parental fliF mutant and partial success in assembling flagella in the case of the pseudorevertants.
FIG. 1.
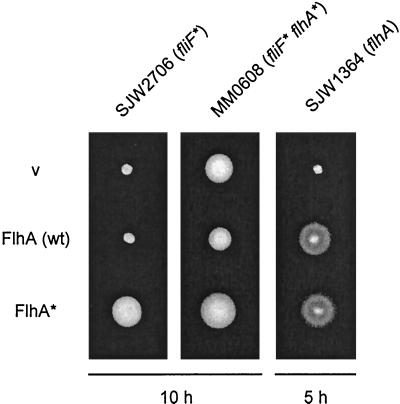
Swarming abilities of SJW1103 (wild type [wt]), SJW2706 (parental fliF mutant, fliF∗), MM0608 (pseudorevertant, fliF∗ flhA∗), and MM1608 (flhA∗ single-site mutant derived from MM0608). The plates were incubated for the times indicated; note the much longer times used for the fliF∗ mutant and the pseudorevertant than for the wild type and the flhA∗ strain.
The parental FliF mutation and the suppressing FlhA mutations.
FliF is a protein containing 560 amino acids, or 559 after cleavage of the N-terminal methionine (9). (In this report, numbering starts with Met-1; the sequence in databases such as SwissProt starts with Ser-2.) The fliF mutation in all three parental strains was found to be the same, an in-frame deletion of six nucleotides (bp 521 to 526) about a third of the way into the gene, resulting in loss of two amino acids, A174 and S175.
Flagellar region II contains a mixture of flagellar, motor, chemotaxis, and receptor genes. Classical genetic deletion mapping had indicated that for at least two of the region II pseudorevertants, the suppressing mutations lay in or near flhA. DNA sequence analysis was carried out on the suppressor alleles of all 24 pseudorevertants and resulted in identification of 19 of the mutations (Table 2). They were all missense mutations, lying within flhA, as the deletion mapping had suggested. Two of the mutations were encountered several times, so that only 10 were distinct. Three of those were different nucleotide changes within the same codon, two of them giving the mutation F138L and the other giving F138C. Thus, amino acid changes were seen at only eight FlhA positions.
TABLE 2.
Mutations in FlhA pseudorevertants
| Strain | Base change | Amino acid change | Predicted locationa |
|---|---|---|---|
| MM0636 | G171T | M57I | TM2 |
| MM0666 | T179A | L60Q | TM2 |
| MM0634 | T353A | L118Q | 2nd periplasmic loop |
| MM0635 | T353A | L118Q | 2nd periplasmic loop |
| MM0640 | T353A | L118Q | 2nd periplasmic loop |
| MM0642 | T353A | L118Q | 2nd periplasmic loop |
| MM0644 | T353A | L118Q | 2nd periplasmic loop |
| MM0646 | T353A | L118Q | 2nd periplasmic loop |
| MM0652 | T353A | L118Q | 2nd periplasmic loop |
| MM0654 | T353A | L118Q | 2nd periplasmic loop |
| MM0610 | T412C | F138L | TM4 |
| MM0674 | C414A | F138L | TM4 |
| MM0670 | T413G | F138C | TM4 |
| MM0624 | T443G | I148S | 2nd cytoplasmic loop |
| MM0625 | G664A | G222S | TM5 |
| MM0608 | G802C | V268L | TM6 |
| MM0618 | G802C | V268L | TM6 |
| MM0620 | G802C | V268L | TM6 |
| MM0663 | G815A | S272N | 3rd cytoplasmic loop |
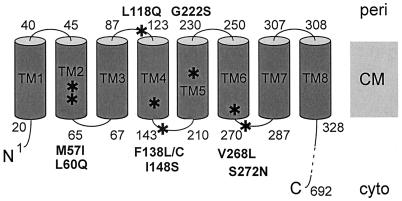
See Fig. 7 for illustration.
FlhA is a large integral membrane protein containing 692 residues (17). It has an N-terminal transmembrane domain extending to about residue 328 and a C-terminal soluble domain comprising the remainder of the protein. All of the mutations lie within the membrane domain and in fact are confined to a subregion of it extending from residues 57 to 272.
Comparison of swarming abilities of the pseudorevertants.
With the knowledge of the second-site flhA mutations responsible for suppression, we examined whether these could be ranked or compared in any useful way in terms of their abilities to suppress. After 15 h at 30°C, among those giving the poorest swarming was MM0663, whose FlhA mutation was S272N. It swarmed at about 75% of the rate of MM0608 (V268L) (whose swarming was shown in comparison with the wild-type strain and the parental fliF mutant in Fig. 1). The best swarming was observed with MM0624 (I148S), which swarmed at about 140% the rate of MM0608. Thus, although there were differences, they were small compared with the roughly fivefold difference between the wild-type strain and the pseudorevertants as a group.
Construction and properties of second-site flhA mutants.
The second-site mutations from a subset of the pseudorevertants (MM0608, MM0609, MM0610, MM0621, MM0635, MM0652, and MM0654) were transferred by phage P22-mediated transduction into KK1012 (lacking region II [14]) to construct strains containing only the second-site flhA alleles: MM1608, MM1609, MM1610, MM1621, MM1635, MM1652, and MM1654, respectively. (The flhA mutations of MM1635, MM1652, and MM1654 subsequently proved to be identical.) These strains, illustrated by MM1608 (Fig. 1), swarmed at essentially wild-type levels, indicating that these second-site flhA mutations by themselves have no phenotype.
Complementation, dominance, and multicopy properties of the suppressor flhA mutations.
To examine the properties of the suppressor flhA alleles expressed in trans, the wild-type flhA gene and several of the suppressor flhA alleles were cloned into pTrc99A. Even without induction, pTrc99A-based plasmids result in expression levels that are considerably higher than those from the chromosome (21). The insert consisted of the entire flhA gene (bases 1 to 2079, with no flanking material from either flhB or flhE [Table 1]). These plasmids were introduced into SJW2706 (fliF), MM0608 (fliF flhA), and a flhA null mutant, SJW1364. The resulting transformants were inoculated onto soft tryptone agar plates and incubated at 30°C (Fig. 2).
FIG. 2.
Swarm test of the complementation and dominance properties of wild-type (wt) FlhA (middle row) and suppressor mutant FlhA (FlhA∗) from pseudorevertant MM0608. pTrc99A-based plasmids producing these proteins were used to transform the strains indicated at the top. SJW2706 is the parental mutant (fliF*), MM0608 is a pseudorevertant (fliF* flhA*), and SJW1364 is a flhA null mutant. v, pTrc99A vector. Transformed cells were incubated on tryptone semisolid agar plates for the times indicated.
Plasmids carrying the second-site suppressor flhA alleles (illustrated in Fig. 2 by pMMhA1608) had the ability to suppress to a considerable degree the FliF defect of SJW2706, giving essentially the pseudorevertant phenotype; thus, overproduced mutant FlhA was dominant over wild type for interaction with mutant FliF. Conversely, pMM130 (wild-type FlhA) only slightly inhibited swarming of MM0608; in other words, even at chromosomal levels of expression, mutant FlhA outcompetes overproduced wild-type FlhA for interaction with mutant FliF. All second-site suppressor FlhA proteins complemented the flhA null mutant, confirming the results obtained with the isolated second-site mutants, namely, that the suppressor flhA mutations by themselves have no phenotype.
Complementation, multicopy, and dominance properties of the parental FliF mutant protein.
We wished to know whether the parental FliF mutant protein would, in high copy number, improve the function of the parental mutant itself, and also whether it would exert negative dominance over wild-type cells (generating poorly motile first-site phenotype) or second-site flhA mutants (generating pseudorevertant phenotype).
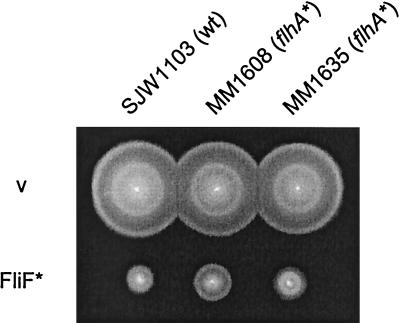
The parental mutant fliF allele from SJW2706 was cloned into pTrc99A to give plasmid pMMiF2706, which was then used to transform various hosts. The swarming of the parental mutant SJW2706 or two pseudorevertants that were tested (MM0608 and MM0635) was not improved by overproduction of mutant FliF (data not shown). In the wild-type and second-site mutant backgrounds, the level of swarming was reduced markedly, to about 40% of that of the same cells transformed with vector (Fig. 3). Thus, although there was clearly dominance, it was not complete, since that would have resulted in the much slower swarming of the parental mutant (in the case of the wild-type host) or the pseudorevertant (in the case of the second-site host).
FIG. 3.
Swarm test of the dominance properties of mutant FliF (FliF∗) from SJW2706. A pTrc99A-based plasmid, pMMiF2706, producing this protein was used to transform the strains indicated at the top. SJW1103 is wild type (wt); MM1608 and MM1635 are second-site mutants (flhA*). v, pTrc99A vector. Transformed cells were incubated on tryptone semisolid agar plates for 5 h at 30°C.
The parental FliF mutation does not affect C-ring assembly.
fliF null mutants fail to assemble the C ring, which is a peripheral membrane structure consisting of FliG, FliM, and FliN; thus, the C ring associates with the membrane only via the MS ring (13). Formation of the C ring is essential not only for motor function but also (for poorly understood reasons) for export and further flagellar assembly. To examine whether the parental FliF mutation affects C-ring formation, cells were lysed and separated into a high-speed pellet fraction (containing membrane and membrane-associated material) and a supernatant fraction (containing soluble cytoplasmic proteins). The fractions were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with anti-FliF, anti-FliG, and anti-FliN antibodies (Fig. 4). SJW1103 (wild type) and null mutants SJW1684 (fliF) and SJW1364 (flhA) were used as controls.
FIG. 4.
Distribution of the MS-ring protein FliF and the C-ring motor/switch proteins FliG and FliN between the high-speed pellet, or membrane, fraction (m) and the supernatant fraction (s) of cells of wild-type SJW1103 (wild type [wt]), a fliF null mutant SJW1684 (fliF), the first-site fliF mutant SJW2706 (fliF∗), and a flhA null mutant SJW1364 (flhA). The positions of molecular mass markers (in kilodaltons) are shown on the left.
FliF was detected in the membrane fractions of wild-type cells (lane 1), the parental fliF mutant (lane 5), and the flhA null mutant (lane 7) but not in that of the fliF null mutant (lane 3). Both FliG and FliN were detected predominantly in the membrane fractions of wild-type cells (lane 1), the parental fliF mutant (lane 5), and the flhA null mutant (lane 7). Only in the case of the fliF null mutant were FliG and FliN found mostly in the soluble fraction (lane 4). Thus, the parental FliF mutation does not appear to significantly affect either assembly of the MS ring itself or assembly of the C ring onto the MS ring. This result and the fact that the suppressor mutations lie in the flhA gene suggest that the parental FliF mutation directly impairs the assembly or function of the export apparatus within the central pore of the MS ring.
The parental fliF mutant is defective in export and second-site flhA mutations partially suppress the defect.
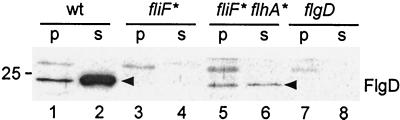
We next examined whether the failure of the parental fliF mutant to swarm is a result of failure to export substrates and whether the second-site suppressor flhA mutations restore the ability to export. FlgD, the hook-capping protein, was chosen as a test substrate. Periplasmic and culture supernatant fractions of SJW2706 and MM0608 were prepared as described before (18), and immunoblotting was carried out with polyclonal anti-FlgD antibody. SJW1103 (wild type) and SJW156 (flgD) were used as positive and negative controls, respectively.
FlgD was not detected in either the periplasmic or the culture supernatant fractions of the parental fliF mutant (Fig. 5, lanes 3 and 4). We conclude that its poor flagellation and motility result directly from poor flagellar protein export. FlgD was exported into both fractions by the intergenic fliF suppressor mutant (lanes 5 and 6), indicating that the second-site flhA mutation can suppress the defect in flagellar protein export of the parental fliF mutant. However, suppression was not complete: both the overall level of FlgD export and the amount found in the supernatant (lane 6) versus the periplasm (lane 5) were much lower than with wild-type cells (lanes 2 and 1). The latter aspect can be explained by the fact that impaired export of periplasmic components (the rod proteins) results in doubly impaired export of external components like FlgD.
FIG. 5.
Export of the hook-capping protein FlgD to the periplasm (p) and culture supernatant (s) of cells of wild-type (wt) SJW1103, the first-site fliF mutant SJW2706 (fliF∗), the pseudorevertant (fliF∗ flhA∗), and the flgD null mutant SJW156 (flgD). Samples were subjected to SDS-PAGE and immunoblotted with polyclonal anti-FlgD antibody. Size in kilodaltons is indicated at the left.
Attempt to obtain biochemical evidence for FlhA association with the basal body.
Two membrane components of the export apparatus (FliP and FliR) have been shown previously to be associated with the basal body (2). The genetic evidence described above provides strong reason to believe that FlhA interacts with the basal-body MS-ring protein FliF. We sought biochemical evidence for such an interaction, by preparing hook-basal bodies from a flhA mutant transformed with a plasmid producing FlhA tagged with the FLAG epitope (6) and, as a control, wild-type cells transformed with vector. The samples were then subjected to SDS-PAGE and immunoblotting. With monoclonal anti-FliF antibody, a strong band at the position expected for FliF was seen with both hook-basal body samples. With monoclonal anti-FLAG antibody, there was a weak signal at the position expected for FLAG-tagged FlhA in the case of the sample from the mutant transformed with the plasmid producing FLAG-tagged FlhA and no signal with the control (data not shown). Thus, we have obtained weak biochemical evidence for FlhA being in physical association with the basal body.
DISCUSSION
Both the first-site FliF mutation and its suppressors are rare.
In our initial survey, we found that the majority of fliF mutants giving rise to suppressors produced ones that were in flagellar region III and may well have been intragenic. Only 3 out of 33 fliF mutants gave suppressors that were in a different flagellar region and therefore necessarily were intergenic, and even here the intergenic suppressors were much less common than ones in the same region. Since these three mutants proved to be identical, the true numbers we obtained are 1 out of 31. Of the 19 suppressor mutations identified, two were encountered several times (Table 2). Also, three different mutations were found within a particular codon (number 138), but these gave rise to only two distinct amino acid changes. The end result is that we found nine distinct amino acid changes, involving eight positions. Thus, both the parental and the suppressing mutations appear to be fairly rare events, indicating that the interaction between the MS ring and the export apparatus may be quite a restricted one.
Position of the parental FliF mutation.
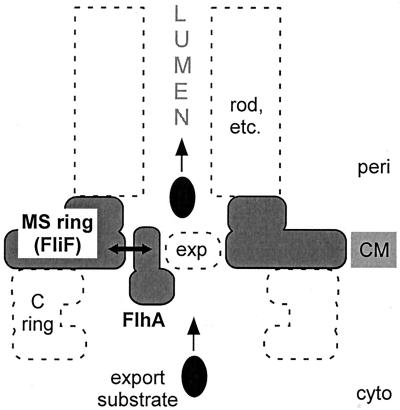
As is evident from the complex shape of the MS ring (30), the FliF protein subunits from which it is built must themselves have a complex architecture. This makes it difficult to predict where the two-amino-acid deletion (A174 and S175) in the mutant FliF protein is located within its three-dimensional structure. However, on functional grounds (suppression by a mutation in FlhA), it seems reasonable to propose that the deletion may be on the inner surface of the annular pore of the MS ring (Fig. 6). Apparently, it does not greatly affect the intersubunit interface within the MS ring, or the interface between the MS ring and the C ring, since both of these structures assemble in the FliF mutant (Fig. 4).
FIG. 6.
Cartoon interpreting the suppression of a mutational defect in the MS-ring protein FliF by a mutation in one of the membrane components of the flagellar protein export apparatus, FlhA. Other membrane components of the export apparatus are indicated by exp and a dashed oval. The export apparatus, which translocates export substrates from the cytoplasm into the lumen of the nascent flagellar structure (rod, etc.), is believed to be located in a patch of membrane within the pore that exists within the MS ring. Suppression is postulated to be a result of physical interaction (double-headed arrow) between the inner annular surface of the MS ring and the transmembrane region of FlhA or, in one case, near the interface between the transmembrane region and the soluble domain. The C ring is a part of the motor that is mounted onto the MS ring. CM, cytoplasmic membrane; cyto, cytoplasm; peri, periplasm.
Since a mutant producing an essentially full-length fusion between FliF and the motor/switch protein FliG (a known cytoplasmic protein) has close to wild-type function (3), it is clear that the C terminus of FliF lies in the cytoplasm. The protein must therefore cross the membrane an even number of times. From topology prediction programs (e.g., TopPred2 [31]), FliF is predicted to have either two or three transmembrane segments, centered at about positions 37, 199, and 467 of the 560-residue protein (9); the first of these is much more strongly predicted to exist than the other two. Ueno et al. (30), in a study with trypsin-digested FliF and C-terminally truncated FliF, assumed that the only two spans were those at 37 and 467 but gave no clear justification for ignoring the possibility of a span at position 199. FliF of Caulobacter crescentus (24) is 26% identical to FliF of Salmonella and can be aligned with reasonable confidence throughout most of its sequence. Jenal and Shapiro (8) have analyzed the topology of Caulobacter FliF by β-lactamase and β-galactosidase fusions and concluded that Q129 (equivalent to Salmonella E137) and E332 (equivalent to Salmonella E367) lie in the periplasm. The result with E332 would seem to preclude a second span as early in the Salmonella sequence as position 199. However, given the complex shape of the FliF subunit which gives rise to the quite bulky M-ring feature of the MS ring, it may be that FliF has an unusual transmembrane structure and crosses the membrane more often than the two times indicated by computer algorithms. With this reservation, we tentatively conclude that the parental mutation (deletion of amino acids 174 and 175) lies in the periplasmic domain of FliF.
Conservation of FliF sequence among species is generally quite weak, based on CLUSTAL W alignments (28) of the 11 FliF sequences available in the SwissProt database (version 39). However, the parental FliF deletion (A174 and S175) belongs in one of the regions that is most strongly conserved, and within this fairly conserved region it contributes to a short conserved motif A-S(A)-V(I)-X-V(L/I), where A is conserved in all 11 sequences, S and the first V are both conserved in 10 of the 11 sequences, and the second V is conserved in 3 of the 11 sequences. Thus, it appears to represent an important part of the FliF sequence, such that deletion could cause a significant conformational change and so affect the interface with FlhA.
Positions of the suppressor FlhA mutations.
FlhA is a 692-amino-acid integral membrane protein with a large soluble domain encompassing the C-terminal half of the protein (residues 328 to 692) (Fig. 7). Within the N-terminal half, the membrane protein topology algorithm TopPred2, using a span length of 19 amino acids, predicts eight α-helical transmembrane spans (TM1 to TM8) at the following approximate residue positions: TM1, 21 to 39; TM2, 46 to 64; TM3, 68 to 86; TM4, 124 to 142; TM5, 211 to 229; TM6, 251 to 269; TM7, 288 to 306; and TM8, 309 to 327. TM7 is less strongly predicted than the others and is predicted weakly or not at all by some other algorithms (e.g., TMHMM [27]). Two experimental arguments, however, support its existence, which would place the C-terminal domain in the cytoplasm rather than in the periplasm.
FIG. 7.
Schematic illustration of the predicted transmembrane organization of FlhA, the integral membrane component of the export apparatus that gave rise to suppression of mutations in the MS-ring protein FliF. Positions of residues at the beginning and end of predicted terminal and loop regions are indicated. Mutations identified in this study are indicated by boldface letters and asterisks. CM, cytoplasmic membrane; cyto, cytoplasm; peri, periplasm.
First, PhoA fusion analysis of the FlhA homolog for export of virulence factors, Salmonella InvA (which is 34% identical overall to FlhA and 47% identical in the transmembrane region), supports the existence of eight transmembrane spans (Jorge Galán, personal communication); specifically, three fusions at positions corresponding to predicted periplasmic loops 1, 2, and 3 gave a phosphatase-positive result (indicating they are located in the periplasm), while two fusions following predicted TM8 (and therefore lying beyond the transmembrane region) gave a phosphatase-negative result (indicating they are located in the cytoplasm).
Second, in a previous analysis of interactions among components of the export apparatus, the cloned soluble C-terminal domain of FlhA was found to interact with known cytoplasmic components, namely, FliH, FliI, and FliJ (20).
As might have been expected from the fact that they are suppressing a defect in a largely transmembrane structure, the MS ring, all of the FlhA mutations identified in this study lie within its N-terminal membrane-spanning domain (Table 2 and Fig. 7), extending from residue 57 (in predicted TM2) through residue 272 (in the cytoplasmic loop following predicted TM6). Thus, of the eight predicted transmembrane spans of FlhA, only TM2 to TM6 and the loops associated with them have thus far provided examples of suppression. A reasonable hypothesis would be that these spans form a surface that is exposed to the inner surface of the MS-ring annulus.
The FlhA mutations themselves (Table 2) are conservative or moderately so. Several of them involve replacement of one hydrophobic residue by another. Some involve replacement of a hydrophobic residue by a polar one. None involve replacement or introduction of a charged residue. Also, they typically reflect the degree of conservation that exists at the corresponding positions (using CLUSTAL W alignment) in the 10 FlhA sequences in the SwissProt database (version 39). This situation seems consistent with the need to suppress a defect in the FliF-FlhA interaction without severely disrupting the role of FlhA in export. This latter point is reinforced by the fact that the second-site FlhA mutations themselves (in a wild-type FliF background) have wild-type motility (Fig. 1).
Since the export apparatus is believed to be located in a membrane patch within the annular structure of the MS ring, it seems likely that the pseudorevertants with a second mutation in FlhA are compensating for the FliF mutation by altering a physical interface between the two proteins (Fig. 6). However, the situation is unusual in that the MS ring consists of ca. 26 subunits of FliF, presumably all with equivalent quaternary interactions. On steric grounds, it is impossible for all 26 FliF subunits to be simultaneously in pairwise interaction with 26 FlhA molecules, since it has been estimated that the pore of the MS ring annulus can accommodate only about 70 membrane-spanning segments (2) and each FlhA subunit is predicted to contain 8 membrane-spanning segments (and there are five other membrane components of the export apparatus to be accounted for). It seems likely that there will be a single copy of the export complex, containing some low number of FlhA subunits—perhaps just one. Probably because of a quite tight geometry, a FlhA subunit will interact with whichever FliF subunit it happens to be close to.
The single parental FliF mutation has given rise to suppressors in predicted periplasmic loops, transmembrane spans, and cytoplasmic loops of FlhA, and so it is clear that they cannot all correspond to immediately proximal interactions. Rather, we imagine that the FliF mutation causes a displacement that can be compensated for in various ways by the FlhA mutations in its N-terminal transmembrane domain. The absence of any examples in the C-terminal cytoplasmic sequence suggests it may constitute a distinct domain that is insensitive to the detailed state of the MS ring.
A cartoon illustrating our overall interpretation of the FliF-FlhA suppression data is given in Fig. 6.
Interpretation of multicopy and dominance effects.
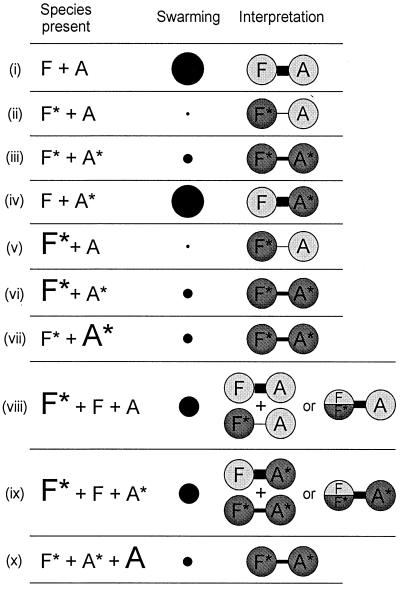
The multicopy and dominance effects observed, and our interpretation of them, are illustrated in cartoon form in Fig. 8. For convenience, mutant FliF and FlhA will be referred to as FliF∗ and FlhA∗, respectively. We observed no examples of simple multicopy effects. In other words, overproduction of FliF∗ in the absence of FliF had no effect on swarming [cf. cases (v) and (ii) or cases (vi) and (iii)]. The same was true of overproduction of FlhA∗ in the absence of FlhA [cf. cases (vii) and (iii)].
FIG. 8.
Schematic illustration of effects of FliF and FlhA overproduction (as a result of expression from pTrc99A-based plasmids) on swarming in various host backgrounds. (i) Wild-type, untransformed or transformed with vector; (ii) parental FliF∗ mutant, untransformed or transformed with vector; (iii) FliF∗ FlhA∗ pseudorevertant, untransformed or transformed with vector; (iv) second-site FlhA∗ mutant, untransformed or transformed with vector; (v) parental FliF∗ mutant with FliF∗ overexpressed; (vi) FliF∗ FlhA∗ pseudorevertant with FliF∗ overexpressed; (vii) FliF∗ FlhA∗ pseudorevertant with FlhA∗ overexpressed; (viii) wild-type with FliF∗ overexpressed; (ix) second-site FlhA∗ mutant with FliF∗ overexpressed; (x) FliF∗ FlhA∗ pseudorevertant with FlhA overexpressed. F, wild-type FliF; F∗, mutant FliF; A, wild-type FlhA; A∗, mutant FlhA. Chromosomal expression is indicated by the smaller font, while plasmid expression is indicated by the larger font. Swarming ability is indicated qualitatively by the diameter of the black circles, categorized by four levels: wild-type, intermediate, pseudorevertant, and parental. The data are interpreted in terms of a physical interaction between FliF and FlhA subunits, where the strength of the interaction is indicated by the thickness of the connecting bar. Wild-type proteins are indicated by light shading, and mutant proteins are indicated by dark shading. In cases (viii) and (ix), the possibility of a mixed (FliF|FliF∗) multimer is indicated (see text).
There were, however, two interesting examples with regard to dominance. The first was where FliF∗ was overproduced in either a wild-type background [case (viii)] or a FliF FlhA∗ (second-site mutant) background [case (ix)]. In both cases, the wild-type level of swarming of the host was considerably reduced, but not to the level expected if FliF∗ - FlhA [case (ii)] or FliF∗-FlhA∗ [case (iii)] interactions were dominant. Thus, the result was intermediate between dominant negative and recessive, with copy number effects offsetting interaction effects.
The second was where FlhA was overproduced in a FliF∗ FlhA∗ (pseudorevertant) background [case (x)]. If FlhA had been dominant negative, this should have produced a FliF∗ FlhA, or parental mutant, phenotype [case (i)]. In fact, the pseudorevertant phenotype was retained; thus, the FliF∗-FlhA∗ interaction proved resistant to the elevated FlhA levels.
The result of case (x) suggests that FliF∗ and FlhA interact with each other to a negligible extent (a result also supported by the parental mutant phenotype with respect to export and motility). The result from case (viii), where FliF∗ has a marked negative effect on the strong positive interaction between FliF and FlhA, might appear to contradict this. We think the resolution of this apparent contradiction may lie in the fact that FliF is a homomultimer. Both FliF and FliF∗ can form this multimer; thus, if both proteins are being produced, a mixed multimer could form, with an intermediate level of interaction with FlhA [case (viii)] or FlhA∗ (case (ix)].
An alternative explanation would be that FliF∗ sequesters other components with which it interacts, such as the C ring, the export apparatus, or the rod proteins. However, since we know that FliF∗ permits C-ring assembly (Fig. 4), and also that overproduction of FliF∗ does not inhibit the motility of pseudorevertants, we think this explanation is less likely.
Thus, although we have not demonstrated conclusively that FliF and FlhA physically interact, all of the above results are consistent with such an interaction, whose strength can be ranked as follows (Fig. 8): FliF-FlhA = FliF-FlhA∗ > (FliF |FliF∗)-FlhA = (FliF|FliF∗)-FlhA∗ > FliF∗-FlhA∗ > FliF∗-FlhA.
Is FlhA unique in suppressing mutation in FliF?
There are six integral membrane proteins (FlhA, FlhB, FliO, FliP, FliQ, and FliR) that are associated with flagellar protein export. Why has only FlhA emerged from this suppression study? To avoid analyzing potential examples of intragenic suppression, we set aside the many suppressor mutations that were in the same flagellar region (region III) as the parental fliF one. However, this also meant that we eliminated the possibility of encountering suppressors in the four export genes that lie in region III: fliO, fliP, fliQ, and fliR. Thus, of the export genes, only suppression by flhA and flhB could have been detected by our strategy. It is still noteworthy, however, that of the region II suppressors, no examples were found in flhB.
In independent studies using a different parental FliF mutant, H. Komatsu and K. Oosawa (personal communication) have found examples of intergenic suppression involving the Mot and switch proteins. Taken together, the data suggest that the inner pore of the MS ring interacts with the export apparatus, while its outer circumference and its cytoplasmic face interact with the motor.
ACKNOWLEDGMENTS
We thank Hitomi Komatsu and Kenji Oosawa for sharing data prior to publication on suppression of FliF mutations by mutations in the Mot and switch proteins, and we thank Jorge Galán for providing unpublished data on the topology of Salmonella InvA.
This work has been supported by USPHS grants GM40355 and AI12202.
REFERENCES
- 1.Fan F, Macnab R M. Enzymatic characterization of FliI: an ATPase involved in flagellar assembly in Salmonella typhimurium. J Biol Chem. 1996;271:31981–31988. doi: 10.1074/jbc.271.50.31981. [DOI] [PubMed] [Google Scholar]
- 2.Fan F, Ohnishi K, Francis N R, Macnab R M. The FliP and FliR proteins of Salmonella typhimurium, putative components of the type III flagellar export apparatus, are located in the flagellar basal body. Mol Microbiol. 1997;26:1035–1046. doi: 10.1046/j.1365-2958.1997.6412010.x. [DOI] [PubMed] [Google Scholar]
- 3.Francis N R, Irikura V M, Yamaguchi S, DeRosier D J, Macnab R M. Localization of the Salmonella typhimurium flagellar switch protein FliG to the cytoplasmic M-ring face of the basal body. Proc Natl Acad Sci USA. 1992;89:6304–6308. doi: 10.1073/pnas.89.14.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser G M, Bennett J C Q, Hughes C. Substrate-specific binding of hook-associated proteins by FlgN and FliT, putative chaperones for flagellum assembly. Mol Microbiol. 1999;32:569–580. doi: 10.1046/j.1365-2958.1999.01372.x. [DOI] [PubMed] [Google Scholar]
- 5.Homma M, Aizawa S-I, Dean G E, Macnab R M. Identification of the M-ring protein of the flagellar motor of Salmonella typhimurium. Proc Natl Acad Sci USA. 1987;84:7483–7487. doi: 10.1073/pnas.84.21.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopp T P, Prickett K S, Price V L, Libby R T, March C J, Cerretti D P, Urdal D L, Conlon P J. A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio/Technology. 1988;6:1204–1210. [Google Scholar]
- 7.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenal U, Shapiro L. Cell cycle-controlled proteolysis of a flagellar motor protein that is asymmetrically distributed in the Caulobacter predivisional cell. EMBO J. 1996;15:2393–2406. [PMC free article] [PubMed] [Google Scholar]
- 9.Jones C J, Homma M, Macnab R M. L-, P-, and M-ring proteins of the flagellar basal body of Salmonella typhimurium: gene sequences and deduced protein sequences. J Bacteriol. 1989;171:3890–3900. doi: 10.1128/jb.171.7.3890-3900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama E, Shiraishi T, Oosawa K, Baba N, Aizawa S-I. Geometry of the flagellar motor in the cytoplasmic membrane of Salmonella typhimurium as determined by stereo-photogrammetry of quick-freeze deep-etch replica images. J Mol Biol. 1996;255:458–475. doi: 10.1006/jmbi.1996.0038. [DOI] [PubMed] [Google Scholar]
- 11.Kawagishi I, Müller V, Williams A W, Irikura V M, Macnab R M. Subdivision of flagellar region III of the Escherichia coli and Salmonella typhimurium chromosomes and identification of two additional flagellar genes. J Gen Microbiol. 1992;138:1051–1065. doi: 10.1099/00221287-138-6-1051. [DOI] [PubMed] [Google Scholar]
- 12.Kubori T, Shimamoto N, Yamaguchi S, Namba K, Aizawa S-I. Morphological pathway of flagellar assembly in Salmonella typhimurium. J Mol Biol. 1992;226:433–446. doi: 10.1016/0022-2836(92)90958-m. [DOI] [PubMed] [Google Scholar]
- 13.Kubori T, Yamaguchi S, Aizawa S-I. Assembly of the switch complex onto the MS ring complex of Salmonella typhimurium does not require any other flagellar proteins. J Bacteriol. 1997;179:813–817. doi: 10.1128/jb.179.3.813-817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutsukake K, Ohya Y, Yamaguchi S, Iino T. Operon structure of flagellar genes in Salmonella typhimurium. Mol Gen Genet. 1988;214:11–15. doi: 10.1007/BF00340172. [DOI] [PubMed] [Google Scholar]
- 15.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 16.Minamino T, Chu R, Yamaguchi S, Macnab R M. Role of FliJ in flagellar protein export in Salmonella. J Bacteriol. 2000;182:4207–4215. doi: 10.1128/jb.182.15.4207-4215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamino T, Iino T, Kutsukake K. Molecular characterization of the Salmonella typhimurium flhB operon and its protein products. J Bacteriol. 1994;176:7630–7637. doi: 10.1128/jb.176.24.7630-7637.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minamino T, Macnab R M. Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol. 1999;181:1388–1394. doi: 10.1128/jb.181.5.1388-1394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minamino T, Macnab R M. FliH, a soluble component of the type III flagellar export apparatus of Salmonella, forms a complex with FliI and inhibits its ATPase activity. Mol Microbiol. 2000;37:1494–1503. doi: 10.1046/j.1365-2958.2000.02106.x. [DOI] [PubMed] [Google Scholar]
- 20.Minamino T, Macnab R M. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol Microbiol. 2000;35:1052–1064. doi: 10.1046/j.1365-2958.2000.01771.x. [DOI] [PubMed] [Google Scholar]
- 21.Muramoto K, Makishima S, Aizawa S-I, Macnab R M. Effect of hook subunit concentration on assembly and control of length of the flagellar hook of Salmonella. J Bacteriol. 1999;181:5808–5813. doi: 10.1128/jb.181.18.5808-5813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohnishi K, Fan F, Schoenhals G J, Kihara M, Macnab R M. The FliO, FliP, FliQ, and FliR proteins of Salmonella typhimurium: putative components for flagellar assembly. J Bacteriol. 1997;179:6092–6099. doi: 10.1128/jb.179.19.6092-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohnishi K, Ohto Y, Aizawa S-I, Macnab R M, Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishnan G, Zhao J-L, Newton A. Multiple structural proteins are required for both transcriptional activation and negative autoregulation of Caulobacter crescentus flagellar genes. J Bacteriol. 1994;176:7587–7600. doi: 10.1128/jb.176.24.7587-7600.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu J, Hartin R J. Quick transformation in Salmonella typhimurium LT2. BioTechniques. 1990;8:43–44. [PubMed] [Google Scholar]
- 26.Sanderson K E, Hessel A, Liu S-L, Rudd K E. The genetic map of Salmonella typhimurium, edition VIII. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: ASM Press; 1996. pp. 1903–1999. [Google Scholar]
- 27.Sonnhammer E L L, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. In: Glasgow J, Littlejohn T, Major F, Lathrop R, Sankoff D, Sensen C, editors. Intelligent systems for molecular biology. Menlo Park, Calif: AAAI Press; 1998. pp. 175–182. [PubMed] [Google Scholar]
- 28.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toker A S, Kihara M, Macnab R M. Deletion analysis of the FliM flagellar switch protein of Salmonella typhimurium. J Bacteriol. 1996;178:7069–7079. doi: 10.1128/jb.178.24.7069-7079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno T, Oosawa K, Aizawa S-I. Domain structures of the MS ring component protein (FliF) of the flagellar basal body of Salmonella typhimurium. J Mol Biol. 1994;236:546–555. doi: 10.1006/jmbi.1994.1164. [DOI] [PubMed] [Google Scholar]
- 31.von Heijne G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992;225:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- 32.Woo T H S, Cheng A F, Ling J M. An application of a simple method for the preparation of bacterial DNA. BioTechniques. 1992;13:696–698. [PubMed] [Google Scholar]
- 33.Yamaguchi S, Fujita H, Ishihara A, Aizawa S-I, Macnab R M. Subdivision of flagellar genes of Salmonella typhimurium into regions responsible for assembly, rotation, and switching. J Bacteriol. 1986;166:187–193. doi: 10.1128/jb.166.1.187-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi S, Fujita H, Sugata K, Taira T, Iino T. Genetic analysis of H2, the structural gene for phase-2 flagellin in Salmonella. J Gen Microbiol. 1984;130:255–265. doi: 10.1099/00221287-130-2-255. [DOI] [PubMed] [Google Scholar]