Abstract
Bacillus stearothermophilus PV72 expresses different S-layer genes (sbsA and sbsB) under different growth conditions. No stretches of significant sequence identity between sbsA and sbsB were detected. In order to investigate S-layer gene regulation in B. stearothermophilus PV72, we characterized the upstream regulatory region of sbsA and sbsB by sequencing and primer extension analysis. Both genes are transcribed from unique but different promoters, independently of the growth phase. Localization of sbsB in the sbsA-expressing strain PV72/p6 revealed that the coding region of the second S-layer gene sbsB is located not on the chromosome but on a natural megaplasmid of the strain, whereas the upstream regulatory region of sbsB was exclusively detected on the chromosome of PV72/p6. For sbsB expression, the coding region has to be integrated into the chromosomally located expression site. After the switch to sbsB expression, the sbsA coding region was removed from the chromosome but could still be detected on the plasmid of the sbsB-expressing strain PV72/p2. The sbsA upstream regulatory region, however, remained on the chromosome. This is the first report of S-layer variation not caused by intrachromosomal DNA rearrangements, but where variant formation depends on recombinational events between the plasmid and the chromosome.
Bacterial surface layers (S-layers) have been found as the outermost cell envelope component on over 200 different species belonging to nearly every taxonomic group of the bacterial phylum (for a review, see reference 29a). The morphological and chemical properties of many different S-layers have been studied in detail. In recent years more attention was drawn to the field of S-layer gene regulation and variation. For several individual strains, S-layer variation has been observed (5, 7, 9, 13). In pathogenic microorganisms, S-layer variation is a strategy to escape an effective immune response of the infected host (4, 11, 22). Genetically, S-layer variation was shown to be based on chromosomal DNA rearrangements, like the inversion of promoter sequences (6) and entire gene cassettes (8), as well as recombination between variable and constant gene segments (5). Here we report another type of S-layer variation depending on recombinational events between the natural megaplasmid and chromosomal DNA of the organism.
Bacillus stearothermophilus PV72 wild-type cells (PV72/p6) are covered with the S-layer protein SbsA. The molecular mass of the subunits is 130 kDa, and they form a hexagonal (p6) array on the surface. SbsA is stably synthesized on complex medium under glucose and oxygen double limitation at a growth temperature of 57°C. S-layer protein synthesis in B. stearothermophilus PV72, however, is highly associated with the physiological state of the cell and can be influenced by a number of different parameters (25, 30, 31, 32). One important factor of S-layer synthesis in B. stearothermophilus PV72 is the dissolved oxygen concentration in the medium. When the dissolved oxygen concentration is increased from 20 or 30% to 50% during continuous cultivation on synthetic growth medium, SbsA becomes rapidly replaced by the second S-layer protein SbsB (21, 30, 31, 32). In comparison to SbsA, SbsB monomers are smaller (97 kDa) and assemble into an S-layer lattice with oblique (p2) symmetry. During the switch, both S-layer proteins can be visualized on a single cell by electron microscopy (31). This indicates that no selection on existing, sbsB-expressing cells takes place, but variation is induced in single cells. Furthermore, no cells covered with SbsB are detected in electron microscopy during sbsA expression. In addition to S-layer variation, the secondary cell wall polymer that is responsible for binding of the SbsA subunits to the cell wall is replaced. This polymer now specifically binds SbsB subunits (16). This suggests complex regulation of S-layer protein variation in B. stearothermophilus PV72.
Both S-layer-encoding genes sbsA and sbsB have been cloned and sequenced (19, 21). In contrast to other S-layer genes, which are involved in S-layer protein variation (5, 7), nucleotide sequence comparison of sbsA and sbsB revealed no significant stretches of sequence identity within their coding regions (21). To gain closer insight into S-layer protein variation of B. stearothermophilus PV72 induced by increased oxygen concentration, both S-layer-encoding genes and their upstream regulatory regions were investigated. Since preliminary experiments demonstrated that sbsB is not located on the chromosome of PV72/p6 during sbsA expression, natural megaplasmid DNA from this strain was used to detect sbsB. Here we report a new type of S-layer protein variation in B. stearothermophilus PV72, based on recombinational events between the chromosome and the natural megaplasmid of this strain.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
Bacillus strains were grown in SVIII medium (2) at 57°C. B. stearothermophilus PV72/p6 (wild type) expresses the S-layer protein SbsA (19, 29). The variant, B. stearothermophilus PV72/p2, expressing sbsB, was isolated from a continuous culture of strain PV72/p6 when cells were grown at a dissolved oxygen concentration of 50% instead of 20 to 30% (29, 33). Escherichia coli DH5α was grown in Luria-Bertani medium at 37°C (27). For selecting transformants harboring the cloning vector pKK232-8 and clones with the inserted sbsB (pHS-p2u) and sbsA (pHS-p6u) upstream regions, ampicillin or chloramphenicol (or both) was added to a final concentration of 100 μg/ml each. The promoter-probing vector pKK232-8 (Pharmacia) was used for the cloning of both upstream regulatory regions.
Oligonucleotides used for PCR, primer extension, and hybridization analyses.
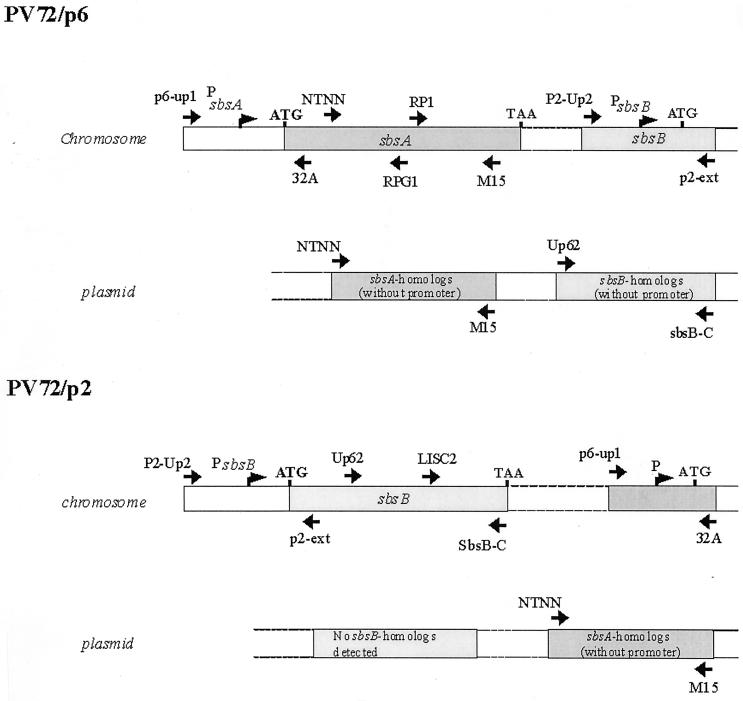
Oligonucleotides used for the amplification of sbsA and sbsB nucleotide sequences as well as for the generation of specific hybridization probes are given in Table 1. The positions of the oligonucleotides and the sequences detected either on the chromosome or on the plasmids of PV72/p2 and PV72/p6 are illustrated in Fig. 5.
TABLE 1.
Oligonucleotides used in PCR, primer extension, and hybridization analyses
| Specificity | Oligonucleotide | Sequence (5′ → 3′) |
|---|---|---|
| sbsA | T7-S | AATAAGGATCCGATGAAGCTGCGCTTACTC |
| 32A | GCTGTTGCTAGTTTCACA | |
| 32A-S | TATAAAGCTTGCTGTTGCTAGTTTCACA | |
| E1 | CCTTGCGCGGTGACAAACACC | |
| H4i | TACCAACCAAACAGGC | |
| M15 | TGTTCCATTCCATGAACC | |
| NTG | AACTGTGCTTTTGCTTGG | |
| NTGi1 | ATTTTTTTCAGAGATTTCGTCAATACTTT | |
| NTNN | GCTACAGATGTAGCAACAGT | |
| RP1 | GTCTTCAATTCACAACAACG | |
| P6-UP1 | ATCGGAATTCAGGCGAACCGTTCGCCAT | |
| RPG1 | AGTAGGAGCGGTAGCAAC | |
| sbsB | LISC2 | AAAATATATCAACTCTG |
| LIS-iG | TGCACTATTGCCGCTATC | |
| NIS3A | GCGGTTAATTCGACTA | |
| NIS1A-G | CTGCGCATCTAGACGAGT | |
| P2-ext | ATAGGACTTAGGTTGAT | |
| P2-Up2 | TTGTATAATTTCTTTTATG | |
| SbsB-C | ATGCACCTCTCAACAATTC | |
| UP62 | CTAAAGTTACAGCTGTAAAACCG |
FIG. 5.
Schematic illustration of the localization of sbsA- and sbsB-specific oligonucleotides used for PCR and hybridization analyses to detect sbsA and sbsB on the chromosome and the plasmids of PV72/p2 and PV72/p6. The sequences detected are shown as boxed areas.
DNA manipulations.
Chromosomal DNA from B. stearothermophilus PV72/p2 and PV72/p6 was prepared as described by Ausubel et al. (1). Restriction endonuclease digestion, DNA analysis, transformation procedures, agarose gel electrophoresis, and cloning of DNA fragments were carried out as described by Sambrook et al. (27). All enzymes and restriction endonucleases required were obtained from Boehringer Mannheim or New England Biolabs.
Isolation of megaplasmids from B. stearothermophilus PV72/p6 and PV72/p2.
Plasmid DNA from cultures of B. stearothermophilus PV72/p2 and PV72/p6 was isolated by a slightly modified alkaline lysis method (31) described by Birnboim and Doly (3), followed by twofold cesium chloride-ethidium bromide density gradient centrifugation. To enhance cell lysis and to separate cell wall-associated proteins and nucleases, cells were repeatedly resuspended in a high-salt buffer (10 mM Tris-HCl [pH 8.0], 20 mM EDTA [pH 8.0], 50 mM NaCl). As plasmid DNA was unstable in solution after isolation (Tris-HCl or Tris-HCl/EDTA), it was kept stable as a dry pellet at room temperature and dissolved in Tris-EDTA buffer (pH 8.0) just before use.
Isolation of RNA.
Total RNA from cells of B. stearothermophilus PV72/p2 and PV72/p6 was isolated at the beginning (optical density at 600 nm [OD600] of 0.35), during mid-exponential growth (OD600 of 0.7), and in stationary growth phase. Samples of cells (1.2 ml) were harvested by centrifugation (10,000 × g for 3 min at 4°C) and suspended in 100 μl of Tris-EDTA (pH 8.0) containing lysozyme (1 mg/ml). After 5 min of incubation at room temperature, 250 μl of RLT lysis buffer (Qiagen) and 30 μl of diethyl pyrocarbonate (DEPC)-treated 2 M sodium acetate were added. To separate chromosomal DNA from the RNA, samples were extracted two times with water-saturated, acidic phenol-chloroform. RNA was precipitated with 1% (vol/vol) isopropanol for 30 min at −20°C. Pellets were dissolved in 30 μl of diethyl pyrocarbonate-treated H2O, of which 3 μl was analyzed on a 1% agarose gel (1× Tris-borate-EDTA). For Northern blot and primer extension analyses, RNA was digested with DNase I as recommended by the manufacturer.
Hybridization techniques.
Northern and Southern blotting were carried out as described by Sambrook et al. (27). sbsA- and sbsB-specific DNA fragments derived by PCR from chromosomal DNA were purified with the QIAquick PCR purification kit (Qiagen) and biotinylated by random labeling with the Phototope Star detection kit (New England Biolabs). After labeling, the PCR fragments were again purified with the QIAquick PCR purification kit (Qiagen) and used for Southern and Northern blotting. Oligonucleotides 32A and p2-ext were biotinylated with Biotin-CE Phosphoramidite (Cruachem Ltd., Glasgow, U.K.) using a PolyGen DNA synthesizer (Langen, Munich, Germany) and used for Northern hybridization. For detection, the Phototope Star detection kit was used.
PCR and inverse PCR.
PCR amplifications of sbsA and sbsB upstream regions were carried out in a 100-μl reaction volume containing 10 μl of 2 mM deoxynucleotides, 1 U of Taq DNA polymerase, 1× Taq reaction buffer, 0.1 μM each of primers, and 500 ng of genomic DNA or 200 ng of plasmid DNA of B. stearothermophilus PV72/p2 or PV72/p6. Thirty cycles of amplification were carried out in a thermocycler (Biometra Trio Thermoblock). Each cycle consisted of a 90-s denaturation step at 92°C, a 40-s annealing step with annealing temperatures depending on the calculated Tm of the oligonucleotides used, and a 90-s extension step at 72°C. The PCR products were verified by 0.8% agarose gel electrophoresis and purified with the QIAquick PCR purification kit (Qiagen). Inverse PCR was carried out to amplify the unknown upstream regulatory sbsA and sbsB nucleotide sequence by the method described by Ochman et al. (23). Chromosomal DNA from B. stearothermophilus PV72/p2 and PV72/p6 was digested with ClaI and HindIII, respectively. After heat inactivation of the enzymes, samples were religated (rapid DNA ligation kit; Boehringer Mannheim) and used directly as template for inverse PCR.
Generation of sbsA and sbsB upstream regions from B. stearothermophilus PV72/p6 and PV72/p2.
The upstream region of sbsA from B. stearothermophilus PV72/p6 was generated by inverse PCR using primers T7-S and 32A-S, resulting in a 2,300-bp PCR product. This PCR fragment was cloned as a BamHI-HindIII fragment into the multiple cloning site of the promoter probe vector pKK 232-8, giving rise to pHS-p6u in E. coli DH5α. Positive clones were able to grow on chroramphenicol (100 μg/ml). The insert was sequenced with primers NTG, NTGi1, and H4i. The upstream region of sbsB from B. stearothermophilus PV72/p2 was amplified by inverse PCR using primers NIS3A and NIS1A-G, resulting in a 720-bp PCR product, and cloned as described for the inverse PCR product of sbsA, giving rise to pHS-p2u.
DNA sequencing.
For sequence analysis, PCR fragments were purified as described before. Purification of plasmids pHS-p2u and pHS-p6u was carried out with Talent miniprep columns (Talent, Rome, Italy). The DNA sequence of pHS-p2u, containing the 5′ region of sbsB, and the DNA sequence of pHS-p6u, containing the 5′ region of sbsA, were determined by the dideoxy chain termination method of Sanger et al. (28). PCR product sequencing was carried out with LI-COR DNA sequencer model 4000.
Primer extension analysis.
For mapping the transcriptional start of sbsA and sbsB mRNA, two different methods were applied. For sbsA, an infrared fluorescence-labeled oligonucleotide was used, and for sbsB, a [γ-32P]ATP-labeled oligonucleotide was used for the reaction.
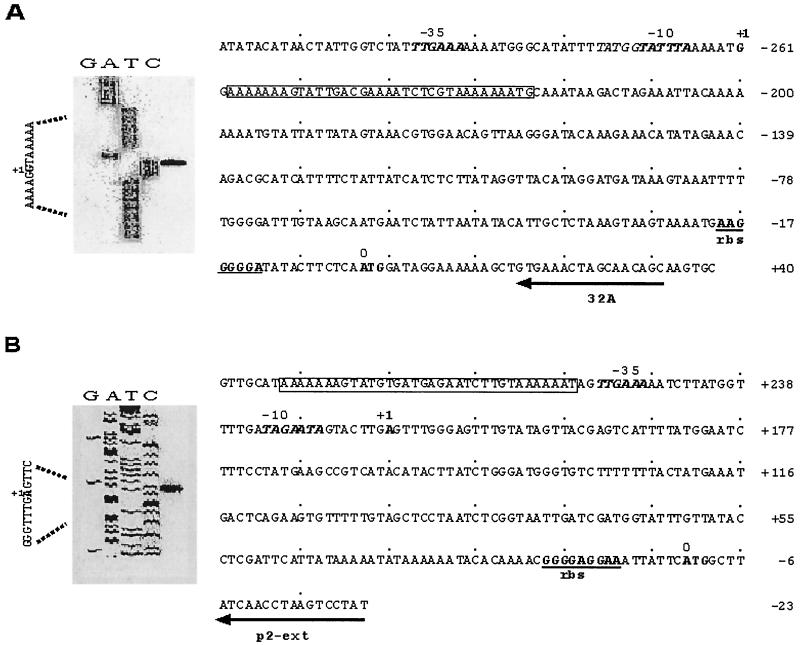
Primer extension analysis was carried out with 7 μg of total RNA isolated from strain PV72/p6 and PV72/p2 in mid-exponential and stationary growth phase. One-tenth femtomole of infrared fluorescence 5′-end-labeled, sbsA-specific primer 32A, and 0.4 fmol of [γ-32P]ATP-end-labeled sbsB-specific primer p2-ext (see Fig. 1, arrows) were used for the annealing reaction in 15 μl of reaction buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl). The reaction mixture was heated to 80°C for 5 min and then rapidly cooled by incubating the samples in a mixture of dry ice and ethanol. After shock freezing, 3 μl of 36 mM MgCl2 was added. For the extension reaction at 37°C for 30 min, 10 U of Moloney murine leukemia virus (M-MLV) reverse transcriptase in 10 μl of reaction mixture containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 10 mM dithiothreitol, 1 mM each of the four deoxynucleoside triphosphates, and 2.5 μl of the annealing mixture were used. Two volumes of M-MLV loading dye were added to the reaction mixture containing the sbsB extension product and heated for 3 min at 95°C, and 3 μl was finally analyzed on a denaturing 8% polyacrylamide gel along with the sequence reaction carried out with the same primer as the extension reaction and pHS-p2u (sbsB) as the template. The fluorescence-labeled primer extension mix was analyzed using a LI-COR DNA sequencer model 4000 by the dideoxy chain termination method (28) and pHS-p6u (sbsA) as the template for the sequencing reaction.
FIG. 1.
Primer extension and nucleotide sequence analysis of the sbsA and sbsB upstream regions. The transcriptional start of both transcripts is indicated by +1. The primers used for the extension reaction (32A for sbsA and p2-ext for sbsB) are underlined and indicated by arrows. The extended products were separated by electrophoresis alongside a dideoxy sequence ladder generated with the same primers. The primer extension signal is indicated for sbsA in panel A and for sbsB in panel B. DNA sequencing lanes are as labeled, and the transcriptional start residues G for sbsA (boldface, indicated with +1) and A for sbsB (boldface, indicated with +1) are labeled on the left. In the nucleotide sequence, the −10 and −35 regions of sbsA and sbsB are shown in boldface, and the ribosome-binding sites (rbs) are underlined. A stretch of 35 nucleotides with 87.7% identity (boxed area) is found close to the promoter of both genes.
Stability of mRNA transcripts.
To a culture of PV72/p2 and PV72/p6 at an OD600 of 0.6, rifampin (Sigma) was added to a final concentration of 0.1 mg/ml. Samples (1.2 ml) were taken at 1-min intervals for the first 5 min and then at intervals of 5 min. To avoid RNA degradation, each sample was shock frozen by incubation in liquid nitrogen. RNA was isolated and purified as described before. An equal amount of RNA was loaded, separated on a denaturing formaldehyde-agarose gel as described by Sambrook et al. (27), and transferred to a Gene Screen Plus membrane (NEN-Dupont). For detection of sbsA- and sbsB-specific transcripts, the same oligonucleotides as for primer extension (32A for sbsA and p2-ext for sbsB) were used.
Nucleotide sequence accession numbers.
The nucleotide sequences of the sbsA and sbsB upstream regulatory regions have been submitted to the EMBL nucleotide database under accession nos. AJ401028 and AJ401027, respectively.
RESULTS
Cloning and sequencing of the sbsA and sbsB upstream regulatory region.
From the available sequence data for sbsA and sbsB, primers for inverse PCR were constructed to amplify both upstream regions. Inverse PCR of the sbsA upstream sequence with primers T7-S and 32A-S resulted in a PCR product of 2,300 bp, whereas inverse PCR of the sbsB upstream region, carried out with primer pair NIS3A and NIS1A-G, resulted in a PCR fragment of 720 bp. By this procedure, 643 additional base pairs of sbsB and 1,600 bp of the sbsA upstream sequence were generated. Since cloning of the corresponding DNA fragments into common vector systems like pUC and pKS failed, the fragments were cloned into the promoter probing vector pKK232-8 and transformed into E. coli DH5α. This vector allows the cloning of strong promoters and the selection of promoter-carrying clones on chloramphenicol. Positive clones of either sbsA (pHS-p6u) or sbsB (pHS-p2u) were able to grow at a high concentration of chloramphenicol (200 μg/ml), indicating that the sbsA as well as the sbsB promoter is active in E. coli. Nucleotide comparison of 1,372 bp of the sbsA and 637 bp of the sbsB upstream region revealed different upstream regions (Fig. 1). A stretch of 35 bp with 87.8% identity was found within both upstream regions close to the transcriptional start, embedded in an AT-rich region of low complexity (Fig. 1, boxed area). No similarity of the sbsA and sbsB upstream regions to other known genes was detected by databank analyses. Four direct repeats (A4–8TG) spaced by 19 and 20 nucleotides are located in the upstream region of sbsA (Fig. 1A, positions −295 to −286, −267 to −261, −232 to −224, and −203 to −194). No direct repeats are found within the upstream region of sbsB.
Primer extension analysis and promoter activity.
Total RNA from both strains at an OD600 of 0.7 (mid-exponential growth) was used for primer extension analysis to map the transcriptional start of sbsA and sbsB mRNA. Primer extension analysis was carried out with oligonucleotide 32A for sbsA mRNA and p2-ext for sbsB mRNA (Fig. 1, arrows). Single signals with regard to the ATG start codon were detected at position −261 for sbsA mRNA from PV72/p6 (Fig. 1A, +1), and at position −217 (Fig. 1B, +1) for sbsB mRNA from PV72/p2. This indicated that both genes are transcribed from a single promoter. With RNA isolated from the early exponential growth phase (OD600 = 0.35) or stationary phase, identical transcriptional starts were mapped for both mRNAs (data not shown). The −35 regions of both genes are identical (TTGAAA), while the −10 regions of sbsA (TATTTA) and sbsB (TAGAAT) are different (Fig. 1).
Transcription of sbsA and sbsB and mRNA stability.
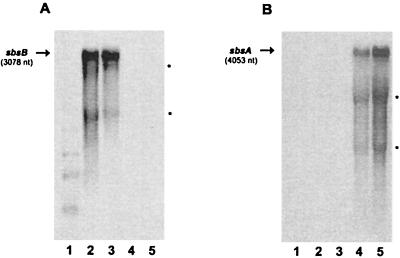
To investigate sbsA and sbsB transcripts, 250 μl of overnight cultures of PV72/p6 and PV72/p2 was inoculated into 25 ml of fresh SVIII medium, and growth was monitored by measuring the OD600. Previous Southern blot analysis (data not shown) revealed chromosomal DNA rearrangements of the sbsA gene between an OD of 0.35 (early exponential growth) and 0.7 (mid-exponential growth phase). In order to detect possible differences in sbsA or sbsB transcription correlated to these rearrangements, total mRNA was investigated at both OD values. The same oligonucleotides as for primer extension analysis were used (p2-ext for sbsB and 32A for sbsA mRNA) in Northern blotting. Independent of the OD value, a single transcript was detected for both mRNAs, which are indicated by arrows in Fig. 2 A and B. Two additional signals appeared close to the positions of the 23S and 16S rRNAs (indicated by asterisks). These signals did not disappear when different sbsA-specific oligonucleotides or randomly labeled sbsA-specific probes were used and might reflect degradation and/or termination products of the transcripts. For both mRNAs, the stability of the transcripts was analyzed. A half-life of 5 min was detected for both mRNA transcripts (data not shown).
FIG. 2.
Northern blot analysis of total RNA from B. stearothermophilus PV72 isolated at different growth phases. Lane 1, molecular size standards. Lanes 2 and 3, total RNA from B. stearothermophilus PV72/p2 isolated at an OD600 of 0.35 and 0.7, respectively; lanes 4 and 5, total RNA from B. stearothermophilus PV72/p6 isolated at an OD600 of 0.35 and 0.7, respectively. Total RNA from B. stearothermophilus PV72/p2 and PV72/p6 was probed with oligonucleotide 32A (sbsA) and p2-ext (sbsB), respectively. Arrows indicate the signals corresponding to the full-length transcripts of sbsA and sbsB. Additional signals at the size of the 23S and 16S rRNAs are indicated by asterisks.
Localization of sbsB in the sbsA-expressing strain PV72/p6.
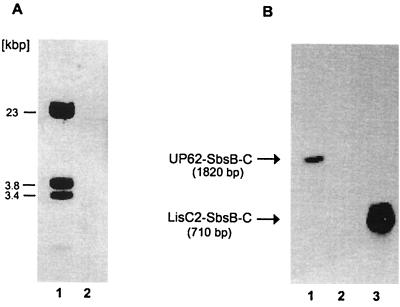
The entire coding and upstream regulatory region of sbsB can be detected on the chromosome of the sbsB-expressing strain PV72/p2. From previous experiments, it was known that the sbsB-encoding sequence could not be detected on the chromosome of the sbsA-expressing strain PV72/p6 by hybridization and PCR analysis (20, 30, 31). Based on the knowledge that B. stearothermophilus PV72/p6 carries natural megaplasmids (31), we suggested that the sbsB coding region might be located on the plasmid and integrate into the chromosome during the switch from sbsA to sbsB expression. To confirm this hypothesis, PCR and hybridization analyses were carried out to detect sbsB on the plasmid of PV72/p6. In hybridization experiments, an sbsB-specific probe of 1,820 nucleotides (Up62-SbsB-C) that covers the middle and 3′ region of sbsB (see Fig. 5), generated with primer pair Up62 and SbsB-C, was used. This probe clearly hybridized with BamHI (which does not cut sbsB)-cleaved plasmid DNA of PV72/p6 (Fig. 3A, lane 1), but not with chromosomal DNA of this strain (Fig. 3A, lane 2). Three hybridization signals were detected. Since BamHI does not cut within the sbsB coding region, it might be suggested that more than one sbsB-homologous copy is present on the plasmid. Although in previous PCR analysis (31) we were not able to detect the sbsB gene on the plasmid of PV72/p6, this new finding indicated that sbsB or sbsB-homologous sequences are located on the plasmid of the sbsA-expressing strain PV72/p6. To confirm the data obtained by Southern hybridization, PCR analyses were carried out under optimized conditions (optimized purification of plasmid DNA and altered PCR conditions). Primer combinations specific for the upstream regulatory region (P2-Up2–p2-ext), internal region (UP62–SbsB-C), and the very C-terminal region (LISC2–SbsB-C) of sbsB were used with plasmid DNA from PV72/p6. With primer pairs UP62–SbsB-C and LISC2–SbsB-C, amplification products of the correct lengths (1,820 and 680 bp, respectively), as calculated from the nucleotide sequence of sbsB, were obtained (data not shown). The upstream regulatory region could not be amplified with plasmid DNA. To confirm the accuracy of the amplified PCR products, the corresponding agarose gel was blotted on a nylon membrane and hybridized with a probe (P2-Up2–SbsB-C) specific for the entire sbsB gene (see Fig. 5), including its upstream regulatory region, in a Southern blot (Fig. 3). Specific signals corresponding to the amplified PCR products were obtained (Fig. 3B, lanes 1 and 3), whereas no signal was detected with the upstream region of sbsB (Fig. 3B, lane 2).
FIG. 3.
(A) Southern blot of BamHI-digested megaplasmid DNA (lane 1) and chromosomal DNA (lane 2) of the sbsA-expressing strain B. stearothermophilus PV72/p6. Membrane-bound restriction fragments that hybridized with probe UP62-SbsB-C, targeted to the middle and 3′ region of sbsB, are indicated by their molecular masses. No hybridization signal was detected with chromosomal DNA (lane 2). (B) Southern blot of sbsB-specific PCR products amplified from plasmid DNA of PV72/p6. Membrane-bound PCR products were detected by chemiluminescence. A probe targeted to the entire sbsB coding and upstream regulatory region was used for hybridization. The signals corresponding to the PCR products UP62–SbsB-C (lane 1) and LISC2–SbsB-C (lane 3) are indicated by arrows on the left. The upstream regulatory region of sbsB was not amplified in the PCR and did not hybridize (lane 2).
The results obtained by Southern hybridization and PCR analysis suggested that in the sbsA-expressing strain, the sbsB coding region is located on the plasmid, not on the chromosome, whereas the regulatory region is not located on the plasmid. However, the sbsB regulatory region could be mapped on the chromosome of PV72/p6 by PCR and Southern blotting (data not shown).
Localization of sbsA in the sbsB-expressing strain PV72/p2.
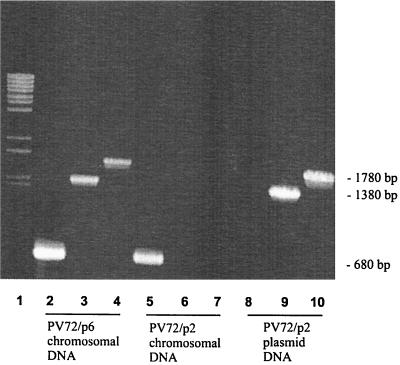
The entire sbsA coding and upstream regulatory region can be detected on the chromosome of PV72/p6 before the switch to sbsB expression. Previous hybridization experiments indicated that the coding region of sbsB has been removed from the chromosome of PV72/p6. To answer the question of which parts of the sbsA gene remain on the chromosome of PV72/p2 or whether plasmid DNA is involved, chromosomal and plasmid DNA from PV72/p2 was used to amplify the sbsA gene. As a positive control, chromosomal DNA from PV72/p6 was used. In the control, each sbsA-specific primer pair resulted in amplification of the expected product (Fig. 4, lanes 2, 3, and 4). With sbsA-specific primers, using PV72/p2 chromosomal DNA as the template, the upstream regulatory region (Fig. 4, lane 5) but not the coding region (Fig. 4, lanes 6 and 7) of sbsA was amplified. The localization of the primers is shown in Fig. 5. The results suggest that the upstream regulatory region of sbsA but not the coding region is present on the chromosome of PV72/p2. In contrast to plasmid DNA from PV72/p2, the coding region of sbsA could be detected (Fig. 4, lanes 9 and 10). The upstream regulatory region of sbsA was not amplified when plasmid DNA was used (Fig. 4, lane 8). Therefore it might be suggested that the sbsA coding region is removed from the chromosome of PV72/p2 during the switch to sbsB expression. Whether the sbsA coding region is integrated into the plasmid of PV72/p2 or deleted from the chromosome has to be determined in further experiments.
FIG. 4.
PCR analysis to detect sbsA in the sbsB-expressing strain PV72/p2. Chromosomal DNA of the sbsA-expressing strain PV72/p6 was used as a positive control. Reactions were carried out with primer combinations specific for either the upstream regulatory region of sbsA (P6up1-32A) (lanes 2, 5, and 8), the internal region (NTNN-RPG1) (lanes 3, 6, and 9), or the C terminus (RP1-M15) (lanes 4, 7, and 10). PCR products were separated on a 0.8% agarose gel and stained with ethidium bromide. Lane 1, molecular size standards. Lanes 2, 3, and 4, PV72/p6 chromosomal DNA. Lanes 5, 6, and 7, PV72/p2 chromosomal DNA. Lanes 8, 9, and 10, PV72/p2 plasmid DNA.
DISCUSSION
S-layer gene expression in B. stearothermophilus PV72 is tightly linked to the physiological state of the organism and can be influenced by various factors, such as temperature, glucose concentration, amino acid composition, and dissolved oxygen concentration (25, 30, 31). The finding that the synchronized switch from sbsA to sbsB expression is correlated with the exchange of the second cell wall polymer that binds the S-layer subunits to the bacterial membrane (16) suggests complex regulation of the S-layer genes and other surface structures. In Lactobacillus acidophilus, both S-layer proteins (encoded by slpA and slpB) are located on the chromosome, using a single expression site via a flip mechanism (7). In Campylobacter fetus, the expression of different S-layer genes depends on the presence of homologous, silent copies on the chromosome (34) that recombine in a recA-dependent manner (14). The S-layer genes sbsA and sbsB of B. stearothermophilus PV72 have no regions of significant sequence identity. This suggests that no homologous recombination between sbsA and sbsB occurs. Only a single copy of sbsA in PV72/p6 and of sbsB in PV72/p2 can be detected on the chromosome. One major question was the localization of the second S-layer gene sbsB in the sbsA-expressing strain PV72/p6. Since sbsB cannot be detected on the chromosome of this strain, we followed the hypothesis that sbsB might be located on the plasmid of PV72/p6. In previous studies (31), we were not able to detect sbsB on the plasmid by PCR analysis. However, the occurrence of three hybridization signals of the sbsB gene on the plasmid (complete digestion) of the sbsA-expressing strain (Fig. 3A, lane 1) indicated the presence of at least three sbsB homologues. By optimizing the conditions for PCR and plasmid DNA purification, the sbsB coding region could be detected on the plasmid of PV72/p6, confirming the results obtained by Southern blot analysis. After the switch to sbsB expression (PV72/p2), sbsB was detected exclusively on the chromosome (Fig. 5). The number of sbsB homologue copies on the plasmid of PV72/p6 and their exact locations (flanking regions) are currently being investigated.
The presence of multiple sbsA (31) and sbsB (this study) homologue copies on the plasmids of PV72/p6 and the presence of the sbsA coding region on the plasmid of PV72/p2 emphasize the role of plasmid DNA in S-layer gene variation in B. stearothermophilus PV72. Furthermore, the plasmids in B. stearothermophilus PV72 might play an essential role in the survival of the organism. Efforts to cure the strains of the plasmids failed (data not shown). Integration of plasmid DNA into the chromosome is considered a dynamic event, contributing to the flexibility of bacterial genomes (24). Plasmid-encoded surface proteins have been reported for several different bacteria. In many cases they play an essential role in virulence and adhesion. In Yersinia pestis, the causative agent of plague, the integration of plasmid-encoded surface proteins into the chromosome, including the capsular antigen and murine toxin as well as the entire calcium dependence plasmid that is common to pathogenic yersiniae, has been demonstrated (26). The localization of the S-layer gene sbsB on a large plasmid of PV72/p6 and its integration into the chromosome are in agreement with these findings, but they differ from S-layer variation in other bacteria. So far, no switch back from sbsB to sbsA expression was observed, indicating that switchback to sbsA expression is a rare or even impossible event.
As described for other S-layer mRNAs, long untranslated leader sequences (UTRs) are found in both the sbsA and sbsB transcripts, which can form stable stem-loop structures. Secondary structures within the UTRs are supposed to prolong the half-life of mRNAs. For other S-layer mRNAs harboring extended 5′-UTRs, long half-lives of up to 20 min were reported (10, 12, 17). In the case of sbsA and sbsB, a half-life of 5 min was detected for the transcripts. Both promoters are located within an AT-rich region of low complexity. For Bacillus subtilis, it was demonstrated that transcription from the flagellin promoter is stimulated 20-fold by an A+T-rich region termed the upstream promoter element (18). Whether transcription of sbsA and sbsB is enhanced by the A+T-rich region has to be determined in further experiments. The results of this study show that S-layer variation in B. stearothermophilus PV72 differs from S-layer variation described for other bacteria. Recombinational events between the chromosome and the natural megaplasmids seem to be crucial for a change in the expression of S-layer genes in B. stearothermophilus PV72. The molecular mechanism of the recombinations as well as the factors that are involved in the regulation of sbsA and sbsB expression still have to be determined.
ACKNOWLEDGMENTS
We thank Birgit Dalheimer for critically reading the manuscript.
This work was supported by the Fonds zur Förderung der Wissenschaftlichen Forschung (FWF, project number S72/08).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1987. [Google Scholar]
- 2.Bartelmus W, Perschak F. Schnellmethode zur Keimzahlbestimmung in der Zuckerindustrie. Z Zuckerind. 1957;7:276–281. [Google Scholar]
- 3.Birnboim H C, Doly J A. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;6:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M J, Smith P F, Repine J E, Joiner K A. Pathogenesis of Campylobacter fetus infections: failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Investig. 1988;5:1434–1444. doi: 10.1172/JCI113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser M J, Wang E, Tummuru M K, Washburn R, Fuimoto S, Labigne A. High frequency S-layer protein variation in Campylobacter fetus revealed by sapA mutagenesis. Mol Microbiol. 1994;14:521–532. doi: 10.1111/j.1365-2958.1994.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 6.Blaser M, Dworkin J. Generation of Campylobacter fetus S-layer protein diversity utilizes a single promoter on an invertible DNA segment. Mol Microbiol. 1996;19:1241–1253. doi: 10.1111/j.1365-2958.1996.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 7.Boot H J, Kolen C P, Pouwels P H. Identification, cloning, and nucleotide sequence of a silent S-layer protein gene of Lactobacillus acidophilus ATCC 4356 which has extensive similarity with the S-layer protein gene of this species. J Bacteriol. 1995;177:7222–7230. doi: 10.1128/jb.177.24.7222-7230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boot H J, Kolen C P, Pouwels P H. Interchange of the silent and active S-layer protein genes of Lactobacillus acidophilus by inversion of the chromosomal segment. Mol Microbiol. 1996;21:799–809. doi: 10.1046/j.1365-2958.1996.401406.x. [DOI] [PubMed] [Google Scholar]
- 9.Boot H J, Powels P H. Expression, secretion and antigenic variation of bacterial S-layer proteins. Mol Microbiol. 1996;21:1117–1123. doi: 10.1046/j.1365-2958.1996.711442.x. [DOI] [PubMed] [Google Scholar]
- 10.Boot H J, Kolen C P, Aandreadaki F J, Lee R J, Pouwels P H. The Lactobacillus acidophilus S-layer protein gene expression site comprises two consensus promoter sequences, one of which directs transcription of stable mRNA. J Bacteriol. 1996;178:5388–5394. doi: 10.1128/jb.178.18.5388-5394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ching W M, Carl M, Dasch G A. Mapping of monoclonal antibody binding sites on CNBr fragments of the S-layer protein antigens of Rickettsia typhi and Rickettsia prowazekii. Mol Immunol. 1992;29:95–105. doi: 10.1016/0161-5890(92)90161-p. [DOI] [PubMed] [Google Scholar]
- 12.Chu S, Gustafson C E, Feutrier J, Cavaignac S, Trust T J. Transcriptional analysis of the Aeromonas salmonicida S-layer protein gene vapA. J Bacteriol. 1993;175:7968–7975. doi: 10.1128/jb.175.24.7968-7975.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubreuil J D, Kostrzynska M, Austin J W, Trust T J. Antigenic differences among Campylobacter fetus S-layer proteins. J Bacteriol. 1990;172:5035–5043. doi: 10.1128/jb.172.9.5035-5043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dworkin J, Shedd O L, Blaser M J. Nested DNA inversion of Campylobacter fetus S-layer genes is recA dependent. J Bacteriol. 1997;23:7523–7529. doi: 10.1128/jb.179.23.7523-7529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eder J. Versuche zur Aufklärung der Funktion parakristalliner Proteinmembranen bei Bacillus stearothermophilus. Ph.D. thesis. Vienna, Austria: University of Agriculture; 1983. [Google Scholar]
- 16.Egelseer E M, Leitner K, Jarosch M, Hotzy C, Zayni S, Sleytr U B, Sára M. The S-layer proteins of two Bacillus stearothermophilus wild-type strains are bound via their N-terminal region to a secondary cell wall polymer of identical chemical composition. J Bacteriol. 1998;180:1488–1495. doi: 10.1128/jb.180.6.1488-1495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer J A, Smit J, Agabian N. Transcriptional analysis of the major surface array gene of Caulobacter crescentus. J Bacteriol. 1988;170:4706–4713. doi: 10.1128/jb.170.10.4706-4713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredrick K, Caramori T, Chen Y F, Galizzi A, Helmann J D. Promoter architecture in the flagellar regulon of Bacillus subtilis: high-level expression of flagellin by the sigma D RNA polymerase requires an upstream promoter element. Proc Natl Acad Sci USA. 1995;92:2582–2586. doi: 10.1073/pnas.92.7.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuen B, Sleytr U B, Lubitz W. Sequence analysis of the sbsA gene encoding the 130-kDa surface-layer protein of Bacillus stearothermophilus strain PV72. Gene. 1994;45:115–120. doi: 10.1016/0378-1119(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 20.Kuen B, Sára M, Lubitz W. Heterologous expression and self-assembly of the S-layer protein SbsA of Bacillus stearothermophilus in Escherichia coli. Mol Microbiol. 1996;19:495–503. doi: 10.1046/j.1365-2958.1996.386918.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuen B, Koch A, Asenbauer E, Sára M, Lubitz W. Molecular characterization of the Bacillus stearothermophilus PV72 S-layer gene sbsB induced by oxidative stress. J Bacteriol. 1997;179:1664–1670. doi: 10.1128/jb.179.5.1664-1670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munn C B, Ishiguro E E, Kay W W, Trust T J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982;36:1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochman H, Medhora M, Garza D, Hartl D L. Amplification of flanking sequences by inverse PCR. In: Innis M, Gelfand D, Sninsky J, White T, editors. PCR: application & protocols. New York, N.Y: Academic Press; 1989. pp. 219–227. [Google Scholar]
- 24.Ott M. Dynamics of the bacterial genome: deletions and integrations as mechanisms of bacterial virulence modulation. Zentralbl Bakteriol. 1993;278:457–468. doi: 10.1016/s0934-8840(11)80817-0. [DOI] [PubMed] [Google Scholar]
- 25.Pink T, Langer K, Hotzy C, Sára M. Regulation of S-layer protein synthesis of Bacillus stearothermophilus PV72 through variation of continuous cultivation conditions. J Biotechnol. 1996;50:189–200. [Google Scholar]
- 26.Protsenko O A, Filippov A A, Kutyrev V V. Integration of the plasmid encoding the synthesis of capsular antigen and murine toxin into Yersinia pestis chromosome. Microb Pathog. 1991;11:123–128. doi: 10.1016/0882-4010(91)90005-u. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain termination inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sára M, Sleytr U B. Comparatative studies of S-layer proteins from Bacillus stearothermophilus strains expressed during growth in continuous culture under oxygen-limited and non-oxygen-limited conditions. J Bacteriol. 1994;176:7182–7189. doi: 10.1128/jb.176.23.7182-7189.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Sára M, Sleytr U B. S-layer proteins. J Bacteriol. 2000;182:859–868. doi: 10.1128/jb.182.4.859-868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sára M, Kuen B, Mayer H F, Mandl F, Schuster K C, Sleytr U B. Dynamics in oxygen-induced changes in S-layer protein synthesis from Bacillus stearothermophilus PV72 and the S-layer-deficient variant T5 in continuous culture and studies of the cell wall composition. J Bacteriol. 1996;178:2108–2117. doi: 10.1128/jb.178.7.2108-2117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholz H, Kuen B, Lubitz W, Sára M. S-layer variation in Bacillus stearothermophilus. FEMS Microbiol Rev. 1997;20:69–78. [Google Scholar]
- 32.Scholz H, Hummel S, Witte A, Lubitz W, Kuen B. The transposable element IS4712 prevents S-layer gene (sbsA) expression in Bacillus stearothermophilus, and also affects the synthesis of altered surface layer proteins. Arch Microbiol. 2000;174:97–103. doi: 10.1007/s002030000181. [DOI] [PubMed] [Google Scholar]
- 33.Schuster K C, Mayer H F, Kieweg R, Sleytr U B, Sára M. A synthetic medium for continuous culture of the S-layer-carrying Bacillus stearothermophilus PV72 and studies of the influence of growth conditions on cell wall properties. Biotechnol Bioeng. 1995;48:66–77. doi: 10.1002/bit.260480110. [DOI] [PubMed] [Google Scholar]
- 34.Tummuru M K, Blaser M J. Rearrangement of sapA homologues with conserved and variable regions in Campylobacter fetus. Proc Natl Acad Sci USA. 1993;90:7265–7269. doi: 10.1073/pnas.90.15.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]